Abstract
There is substantial evidence indicating that disruption of Ca2+ homeostasis and activation of cytosolic proteases play a key role in the pathogenesis and progression of Duchenne Muscular Dystrophy (DMD). However, the exact nature of the Ca2+ deregulation and the Ca2+ signaling pathways that are altered in dystrophic muscles have not yet been resolved. Here we examined the contribution of the store-operated Ca2+ entry (SOCE) for the pathogenesis of DMD. RT-PCR and Western blot found that the expression level of Orai1, the pore-forming unit of SOCE, was significantly elevated in the dystrophic muscles, while parallel increases in SOCE activity and SR Ca2+ storage were detected in adult mdx muscles using Fura-2 fluorescence measurements. High-efficient shRNA probes against Orai1 were delivered into the flexor digitorum brevis muscle in live mice and knockdown of Orai1 eliminated the differences in SOCE activity and SR Ca2+ storage between the mdx and wild type muscle fibers. SOCE activity was repressed by intraperitoneal injection of BTP-2, an Orai1 inhibitor, and cytosolic calpain1 activity in single muscle fibers was measured by a membrane-permeable calpain substrate. We found that BTP-2 injection for 2 weeks significantly reduced the cytosolic calpain1 activity in mdx muscle fibers. Additionally, ultrastructural changes were observed by EM as an increase in the number of triad junctions was identified in dystrophic muscles. Compensatory changes in protein levels of SERCA1, TRP and NCX3 appeared in the mdx muscles, suggesting that comprehensive adaptations occur following altered Ca2+ homeostasis in mdx muscles. Our data indicates that upregulation of the Orai1-mediated SOCE pathway and an overloaded SR Ca2+ store contributes to the disrupted Ca2+ homeostasis in mdx muscles and is linked to elevated proteolytic activity, suggesting that targeting Orai1 activity may be a promising therapeutic approach for the prevention and treatment of muscular dystrophy.
Introduction
Muscular dystrophy is characterized by muscle degeneration and reduced contractile function due to the death of skeletal muscle fibers. The most common type, Duchenne muscular dystrophy (DMD), results from a loss of function of the dystrophin gene [1]. Dystrophin is a high molecular weight structural protein that stabilizes the sarcolemma of muscle fibers by linking cytoskeletal actin to laminin in the extracellular matrix through the dystroglycan complex [2], protecting the muscle against various mechanical stresses to maintain sarcolemmal integrity [3]. Additional studies indicate a role for dystrophin in modulating a number of different cellular processes and signaling events [4]. While the exact cause of muscle fiber death is not clearly established, there is an increasing body of evidence showing that a defect in Ca2+ homeostasis is a causal factor for the progressive damage observed in muscular dystrophy [5]. One of the early cellular defects observed in DMD muscle biopsies was an increase in the number of fibers positive for a histochemical Ca2+ staining [6], and later efforts established that DMD may be associated with increased influx of Ca2+ [7], [8]. However, the identity of the Ca2+ influx pathways that are altered in dystrophic muscle fibers has not yet been clearly resolved [9]–[14].
Store-operated Ca2+ entry (SOCE), or capacitative Ca2+ entry, was originally observed in non-excitable cells as a Ca2+ influx pathway stimulated by reduction of intracellular Ca2+ stores [15]. Previous studies from various investigators demonstrate that SOCE is present in skeletal muscle cells [16]–[18], and that SOCE plays an important function during stress conditions such as strenuous exercise and fatigue [19]–[22]. The molecular components of the SOCE machinery include stromal interaction molecule 1 (STIM1) as an endoplasmic/sarcoplasmic reticulum (ER/SR) Ca2+ sensor [23], [24] that translocates from the ER/SR membrane to regions close to the plasma membrane following depletion of the intracellular Ca2+ stores [25]. This movement of STIM1 activates Orai, a pore-forming unit that allows permeation of Ca2+ through the plasma membrane [26], [27] into the cytosol [28]. Recent studies indicate that Orai1 [29], [30] and STIM1 [31] comprise the principal isoforms composing the SOCE machinery in cultured myotubes and adult muscle fibers. In our previous study, we examined the mRNA expression levels of all known Orai isoforms and STIM1 and confirmed that Orai1 is the major isoform expressed in muscle cells while STIM1 is also abundantly expressed in muscle cells [32].
In this study, we compared the expression levels of SOCE components in wild type (wt) and mdx muscles using real-time PCR and western blotting. Our results showed that Orai1 was significantly upregulated in mdx muscle, while STIM1 levels remained largely unchanged. This observation was accompanied by a significant increase in SOCE activity and an elevated caffeine-sensitive SR Ca2+ store in the mdx muscle fibers. The contribution of Orai1 to aggravated SOCE in mdx fibers was confirmed by specific knockdown of Orai1 expression in adult skeletal muscle by a small hairpin (sh) RNA probe. Furthermore, treatment by BTP2, a specific SOCE inhibitor, significantly reduced the cytosolic calpain activity in dystrophic fibers. Our data establishes that Orai1 is an essential component of SOCE machinery in adult skeletal muscle and provides evidence to support that Orai1-mediated SOCE is a major pathway contributing to the elevated Ca2+ entry and increased proteolytic activity in dystrophic muscles.
Results
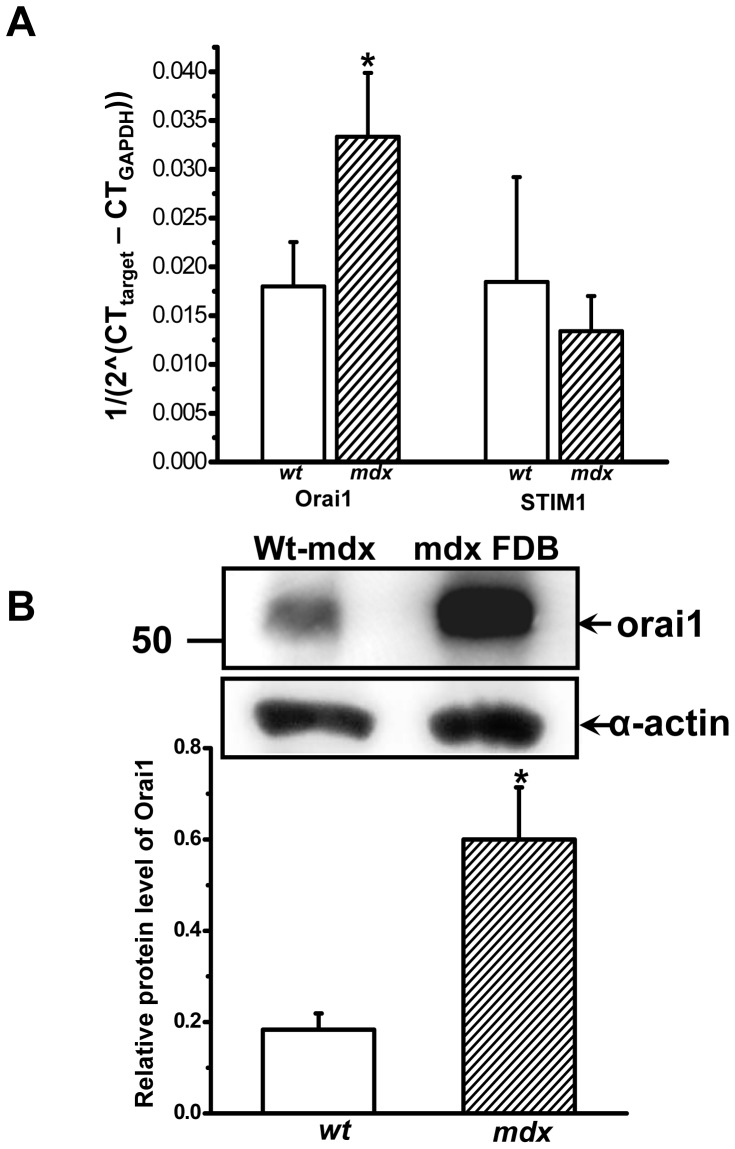
Previous studies suggest a correlation between excessive Ca2+ entry and pathology in dystrophic muscle [13], [33]. However, the source of the aberrant Ca2+ entry in mdx muscle fibers remains unclear. SOCE has been shown to present in skeletal muscle cells [34], and previous studies identified the major components of SOCE as Orai1 [26] and STIM1 [24] in immune cells. Here we examined the expression levels of Orai1 and STIM1 in adult skeletal muscle from the mdx and strain- and age-matched wt mice (8 to 10 weeks old) using real-time PCR and western blot assays. Our initial screening of mRNA expression in gastrocnemius muscle shows the expression level of Orai1 was significantly higher in mdx muscle ( Fig. 1A ). Further studies show that the protein level of Orai1 is increased in flexor digitorum brevis (FDB) muscle ( Fig. 1B ) as well as gastrocnemius and extensor digitorum longus (EDL) muscle (Fig. S1), supporting the possibly that Orai1-mediated Ca2+ entry may be elevated in mdx muscle fibers.
Figure 1. Up-regulation of Orai1 in mdx muscle.
(A) mRNA expression levels of Orai1 and STIM1 in gastrocnemius muscles from wt C57BL/10ScSnJ and dystrophic C57BL/10ScSn-Dmdmdx/J mice were detected by real-time PCR. Relative mRNA copy numbers were shown, n = 6–8 for Orai1 and n = 4 for STIM1, * P<0.05. (B) Western blot showed upregulation of Orai1 protein (∼50 kD) in wt and mdx FDB muscles. The observed molecular size of Orai1 protein was higher than the predicted molecular weight of 33 kDa, possibly due to post-translational modification, splice variations or changes in relative charges of the amino acid of the protein. Sarcomeric α-actin (42 kD) was used as a loading control and for normalization of densitometry, n = 6∼7, * P<0.05.
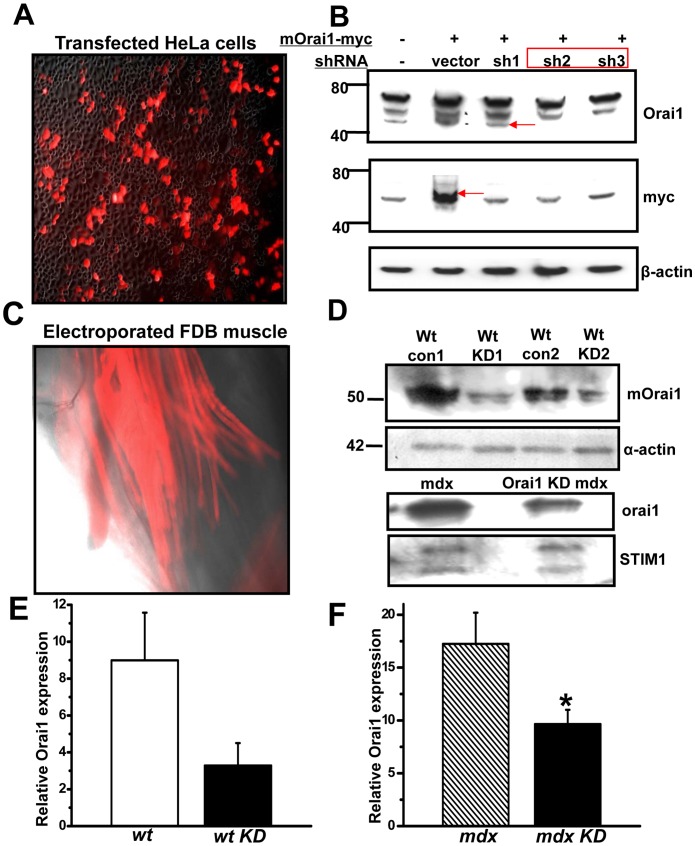
To establish the direct link between Orai1 and store-operated Ca2+ entry in skeletal muscle, and to examine the status of Orai1-mediated SOCE in mdx muscle, we attempted to knock down Orai1 expression in native skeletal muscles. Three shRNA probes targeting the coding region of the full length mouse Orai1 cDNA (shOrai1) were designed and cloned into a vector containing a RFP marker cassette driven under a separate promoter. Efficacy of these shRNA probes were first tested in HeLa cells co-transfected with a myc-tagged full-length Orai1 cDNA ( Fig. 2A ). Our results revealed that all shRNA probes could effectively knock down the ectopic expression of mouse Orai1 with a myc tag ( Fig. 2B ). Two probes (sh2 and sh3) were chosen for subsequent functional studies. Blank vector (con) and shOrai1 plasmids were introduced into FDB muscle of the living mice using an electroporation method [35]. As shown in Fig. 2C, 2 weeks after gene delivery, more than 50% of the muscle fibers in the FDB were RFP-positive. Individual muscle fibers were isolated and RFP-positive fibers were picked using a capillary tube for western blot analysis [36] of Orai1 protein expression. These experiments revealed significant knock down of the endogenous Orai1 by the shOrai1 probes in transfected wt and mdx fibers ( Fig. 2D, 2E and 2F ), proving the in vivo efficacy of the shRNA probes. Furthermore, this shRNA probe had no effect on the expression of STIM1, thus any effects on SOCE could not be a result of off-target knockdown of other major component of the SOCE molecular machinery. The efficiency of our shRNA probes against orai1 had recently been confirmed in a separate study to knock down Orai1 in cultured HL-1 cardiomyocytes [37].
Figure 2. Effective knockdown of endogenous Orai1 gene in both wt and mdx FDB fibers by shRNA probe.
(A) Superimposed transmission light and red fluorescent images (100x) of Hela cells after 24 h transfection with shOrai1 with a RFP marker and mouse full length Orai1 with a myc tag (mOrai1-myc). (B) Western blot showing effective knockdown of the exogenous mOrai1 gene by shOrai1 probes (sh1∼3) No. 2 and No. 3. β-actin was used as a loading control. (C) Fluorescence image (100x) showing the successful transfection of FDB muscle 2 weeks after electroporation of shOrai1 probe. (D) Single fiber western blots confirmed the effectiveness of shOrai1 in knocking down the endogenous mOrai1 in wt mice (upper panel) and mdx mice (lower panel). Pooled extracts from 15 individual FDB fibers were loaded per lane. The exposure used to generate these images was adjusted to produce a robust signal that could be used to assess the level of Orai1 knockdown for both wt and mdx mice. (E) Densitometry of Orai1 Western blot in wild type muscle transfected with control or shOrai1 vectors using NIH image J, n = 2. (F) Densitometry of Orai1 Western blot in mdx muscle transfected with control or shOrai1 vector, n = 3, *P<0.10.
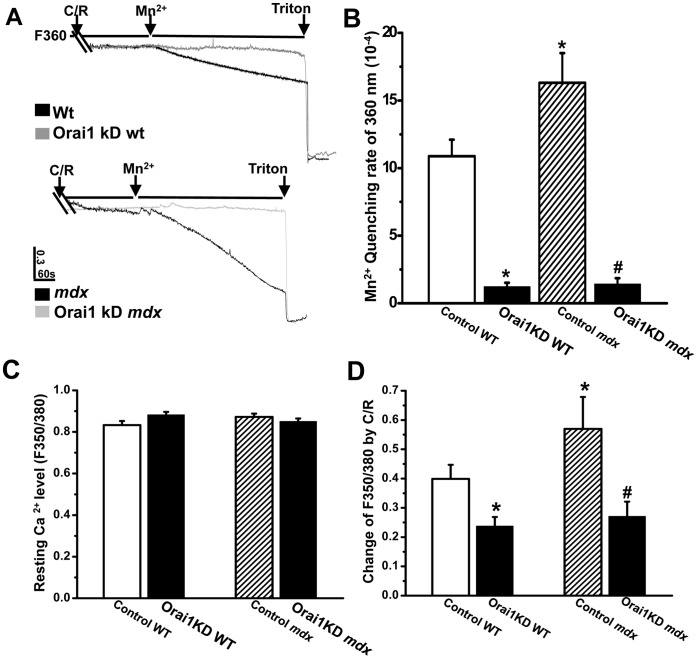
The functional consequences of reduced Orai1 expression was assessed by measuring Mn2+ quenching of Fura-2 fluorescence to detect SOCE activity in intact adult skeletal muscle fibers [38]. As shown in Fig. 3A and 3B , Ca2+ entry following SR store depletion by caffeine plus ryanodine was significantly higher in the control mdx muscle fibers than the control wt FDB fibers. Knock down of Orai1 abolished majority of the SOCE activity in both wt and mdx muscle fibers. These findings indicate that the Orai1 pore-conducting unit constitutes a major source of Mn2+-sensitive SOCE in adult skeletal muscle fiber despite distinct Ca2+ entry kinetics between the excitable and non excitable cells [34]. Furthermore, a significant portion of the excessive Ca2+ entry apparent in mdx fibers may pass through the amplified SOCE pathway since Orai1 knockdown reduced the excessive Ca2+ entry in mdx fiber to a level comparable to that of the wt fiber. However, our results cannot exclude the possibility that Ca2+ entry from other sources, including Ca2+ leak channels, receptor activated Ca2+ entry and additional Ca2+ entry pathways, may also contribute to the Ca2+ deregulation in dystrophic muscles [13], [39].
Figure 3. Elevation of Orai1-mediated SOCE activity and SR Ca2+ store overload in adult mdx muscles.
(A) Representative trace of Mn2+ quenching of Fura-2 fluorescence at 360 nm (F360) wavelength. Lines and arrows designate perfusion of muscle fiber by 20 mM caffeine plus 5 uM ryanodine, MnCl2 and TritonX-100. Upper panel: black trace is FDB fiber from wt mice transfected with empty vector (control WT) and grey trace is with shOrai1 (Orai1KD WT); lower panel: black trace is FDB fiber from mdx mice transfected with empty vector (Control mdx) and grey trace is with Orai1 (Orai1KD mdx). (B) Statistical summarization of the data in (A), n = 11–19, * P<0.05 compared to Control WT; # P<0.05 compared to Control mdx. (C) Statistical results of resting intracellular Ca2+ levels in Control WT (open bar), Orai1KD WT (black bar), Control mdx (hatched bar) and Orai1KD mdx (black bar). (D) Statistical results of caffeine-sensitive SR Ca2+ store in the four groups. n = 11–19, * P<0.05 compared to Control WT; # P<0.05 compared to Control mdx.
Early studies detected a greater level of global Ca2+ concentration in muscle biopsies from DMD patients than that seen in healthy volunteers [40], but the level of [Ca2+]i detected in mdx muscle fibers has varied in different studies [41]. In these preparations we did not resolve a significant difference in the resting Ca2+ level between the wt and mdx fibers using radiometric measurement of Fura-2 fluorescence ( Fig. 3C ), suggesting dystrophic fibers maintain normal control of [Ca2+]i by the SR Ca2+ uptake and sarcolemmal extrusion systems [42]. This may be a distinct aspect of mdx muscle fibers that contributes to the relatively mild symptoms of the mdx mice when compared to human DMD patients. Additionally, it is possible our use of Fura-2 fluorescent dye may not detect changes in certain microdomains of the fiber or subtle alteration to overall [Ca2+]i levels since the dye itself provides significant buffering of free Ca2+ in the cytosol.
Other investigators previously showed that the action potential-induced Ca2+ transient is significantly lower in mdx muscle fiber while others found that the SR Ca2+ store in mdx muscle is significantly higher than in controls [43]. In this study, we applied combination of caffeine and ryanodine to clamp the RyR channel in its open state, so the total SR Ca2+ store can be assessed. Our result found that the C/R-releasable SR Ca2+ store was significantly higher in the mdx muscle than in wt muscle ( Fig. 3D ), possibly as a result of elevated SOCE. This combination of increased SOCE and an overloaded SR Ca2+ store in mdx results in an increased overall Ca2+ burden in the dystrophic fiber, which may be close to the maximal compensatory capacity of the muscle that will eventually result in death of the muscle fiber. The SR Ca2+ overload in mdx muscle can be rescued by knock down of Orai1 ( Fig. 3D ), suggesting that upregulated SOCE machinery likely is the causative factor for the Ca2+ store overload in mdx muscle. Furthermore, knocking down of Orai1 in wt control also reduces the SR Ca2+ store in transfected muscle fibers ( Fig. 3D ), confirming the role of Orai1-mediated SOCE in replenishing the SR Ca2+ store as part of normal muscle physiology [44].
We previously examined the osmotic-shock induced Ca2+ spark activity in mdx muscles [45], where the Ca2+ sparks localized at sarcolemmal periphery in wt fibers are found within the fiber center and also appear at higher frequency in mdx muscle. These results have been confirmed by studies from other investigators [46], [47]. Our findings here suggest that overloading of the SR Ca2+ store in the mdx muscle fibers may provide the source for the increased Ca2+ spark activity in these fibers. Taken together, our data showed that dystrophic muscle fibers of 8 to10 weeks are facing an increased Ca2+ burden throughout different cellular compartments yet the cytosolic Ca2+ level is still tightly maintained.
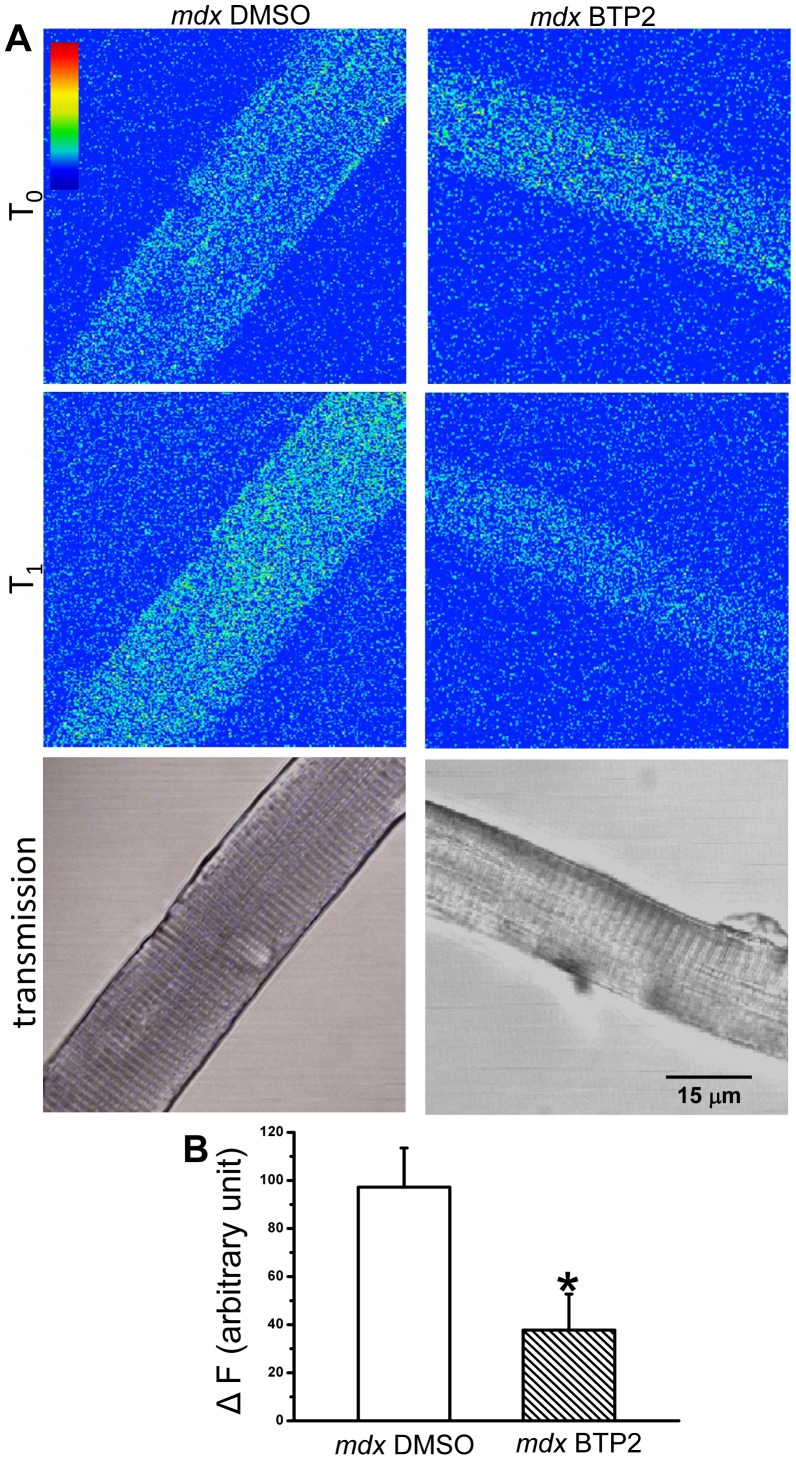
The enzymatic activity of calpain is significantly increased in mouse and human dystrophic muscle, which may be related to the increased proteolysis in dystrophic muscle and progresses of muscular dystrophy [33], [48]–[50]. To probe the contribution of Orai1-mediated Ca2+ entry in calpain activation, we adopted a method using a fluorogenic, membrane-permeable peptide, Suc-LLVY-AMC to measure the relative enzymatic activity of calpain. As shown in Figure 4 , intraperitoneal injection of BTP-2, a specific SOCE inhibitor, for 2 weeks significantly reduced the calpain activity in mdx muscle fiber, as compared to the mice injected with vehicle control. This indicates that even without elevation in the steady state cytosolic Ca2+ concentration, increase in Ca2+ influx through SOCE can activate calpain in dystrophic muscle fibers.
Figure 4. SOCE inhibitor reduces calpain activation in dystrophic fibers.
(A) Confocal images of FDB fibers from DMSO-injected mdx mice and BTP2 injected mdx mice at T0 (addition of 50 µM fluorogenic, membrane-permeable peptide, Suc-LLVY-AMC) and T1 (25 min later to allow for penetration of Suc-LLVY-AMC into FDB fiber and cleavage by cytosolic calpain) at excitation wavelength of 360 nm and emission wavelength of 460 nm. The lower transmission panel is to show that only healthy muscle fibers with clear striation pattern were chosen for the experiment; (B) Statistical analysis of changes in the fluorescence signals (ΔF) generated by Suc-LLVY-AMC cleavage in control mdx fiber (open bar) and BTP2-treated mdx fiber (hatched bar), n = 8 (BTP2) and 14 (DMSO), * P<0.05.
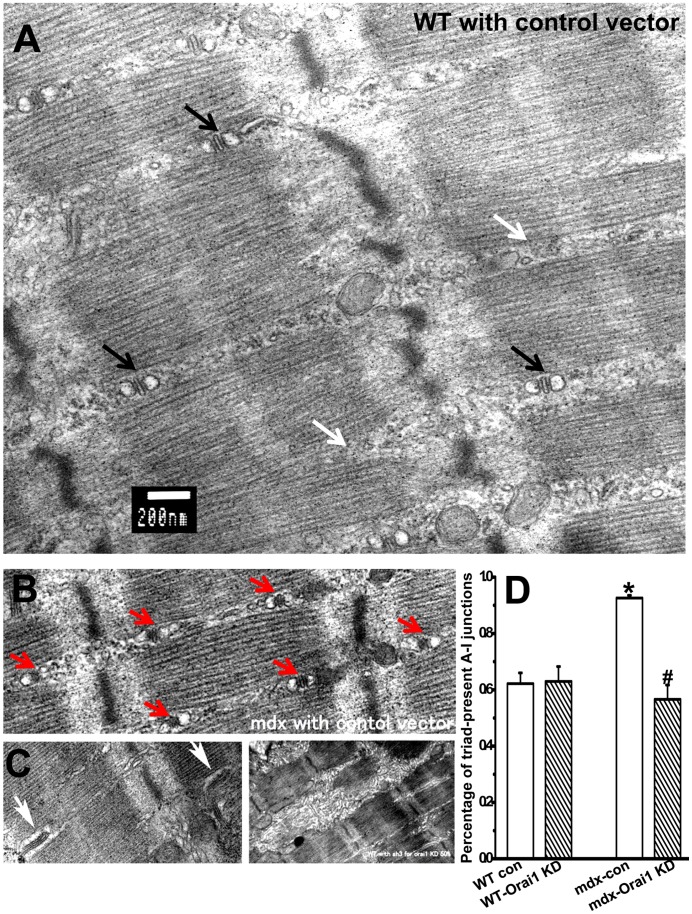
We next examined the ultrastructural alterations of the wt and mdx muscle with Orai1-knockdown using electron microscopy (EM). As shown in Fig. 5A , longitudinal sections of the wt muscle reveal ordered sarcomeres and clear triad structure at A-I junctions. The structure of SR is apparently abnormal in wt muscle with Orai1 knockdown ( Fig. 5C ), showing thick and rough reticulation and disoriented terminal cisterna structures. This suggests that Orai1 expression can influence SR ultrastructure in skeletal muscle, either through direct interaction with STIM1 in the SR [51], [52] or because that reduced SR Ca2+ storage following Orai1 knockdown compromises SR morphology. Our data is the first piece of evidence to show the ultrastructural changes that result from decreased Orai1 expression in muscle. The overall ultrastructure of the observed mdx muscle fibers appears generally normal ( Fig. 5B ), consistent with previous studies [53], [54]. However, upon careful quantification, we found the number of triads at A-I junctions was significantly increased in the mdx muscles ( Fig. 5D ). It is possible that the increased triad junctions may reflect a compensatory mechanism to manage the elevated SR Ca2+ load in mdx muscle or an indication of muscle regeneration.
Figure 5. Changes in muscle ultrastructure after Orai1 knockdown.
Longitudinal section of FDB muscles under transmission EM of the (A) wt muscle transfected with control vector. Scale bar is 200 nm. Black arrows designate triads at A-I junctions and white arrows designate triad-defect A-I junctions; (B) mdx muscle transfected with control vector. Red arrows designate the dense triads at A-I junctions; (C) abnormal features observed in muscles with Orai1 knockdown. Left: disoriented triad junctional structure (white arrows); right: thick and rough reticulation as compared to the fine SR network structure in wt (A) and mdx control (B) muscle; (D) Statistical results summarize the percentage of triad-containing A-I junctions in muscle bundles from wt control, wt Orai1 knockdown, mdx control and mdx Orai1 knockdown. Data are mean ± S.E.M. *P<0.05 compared to wt control and # P<0.05 compared to mdx control.
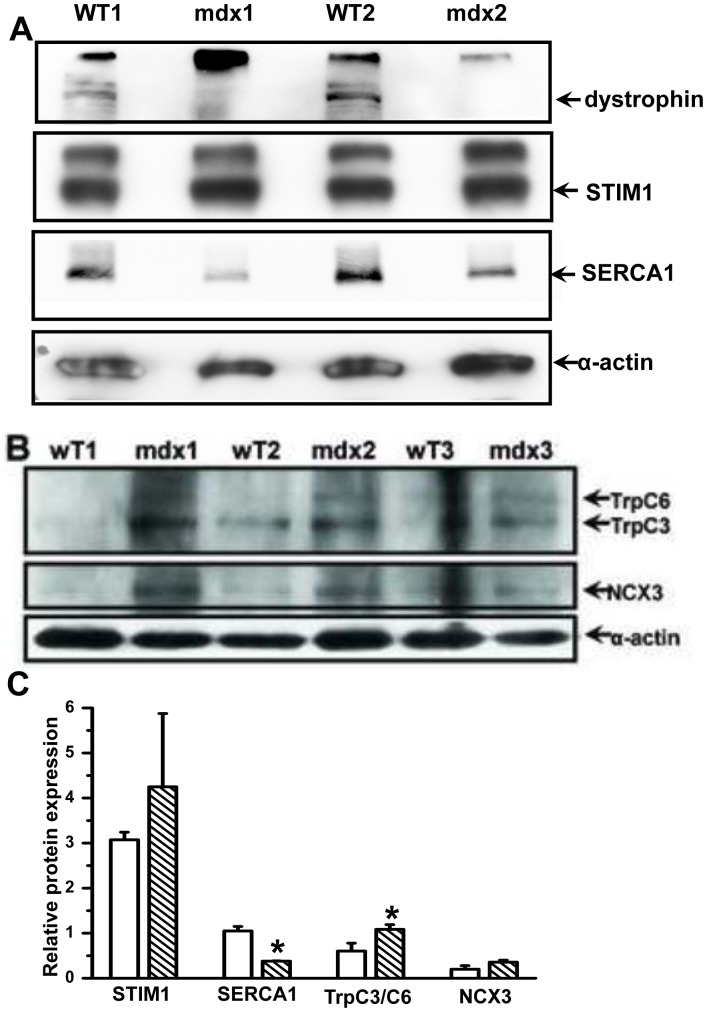
To further elucidate additional adaptive or compensative responses of the dystrophic muscle to the increased Ca2+ burden following elevated SOCE we assessed the protein levels of other essential Ca2+ signaling proteins in skeletal muscle by performing western blots for STIM1 and SR/ER Ca2+ ATPase (SERCA) 1. As shown in Fig. 6A , we frequently detected two bands by the STIM1 antibody, one at the predicted size for STIM1 (80 kDa) and another at 100 kDa, possibly representing a post-translational modification of the STIM1 protein. The wt and mdx muscles displayed similar levels of STIM1 expression, indicating that skeletal muscle fibers contain a surplus of STIM1 beyond that which is necessary to activate Orai1. This idea is supported by a recent study showing multiple functions for STIM1 in skeletal muscles, including activation of SOCE and inhibition L-type Ca2+ channel function simultaneously [55]. In addition, the protein expression level of SERCA1 was significantly reduced in mdx muscle as compared to that of the control muscle (SERCA1/α-actin densitometry value of 0.65±0.08 vs. 0.16±0.04, n = 6∼7 and P<0.001). It is possible that the altered SERCA expression may be an adaptive change to the increased SR Ca2+ load in the mdx muscle fiber. The expression level of transient receptor potential channel (TRP) C3/6, the molecular components for another potential Ca2+ entry pathway in skeletal muscle [39], [56], was also tested here ( Fig. 6B ). Our antibody detected a TRPC6 band at ∼111 kDa and a TRPC3 band at ∼97 kDa. The endogenous expression level of either isoform of TRPC appears to be relatively low in skeletal muscles, consistent with previous reports [39], [56]. However, there appears to be an increase in TRPC3/6 expression in the mdx muscle preparation ( Fig. 6C ), suggesting possible additional source for the increase Ca2+ entry in mdx skeletal muscles. In addition, we probed the expression of the skeletal muscle isoform sodium-calcium exchanger, i.e., NCX3 and our data showed slight upregulation in NCX3 expression ( Fig. 6B and 6C ). However, the difference in NCX3 expression was not statistically significant between the wt and the mdx skeletal muscle.
Figure 6. Compensatory change in protein expression of other Ca2+ shuttling pathways in mdx muscles.
(A) Absence of the 427 kDa dystrophin in mdx muscles was confirmed and expression levels of STIM1 and SERCA1 in FDB muscles from the wt C57BL/10ScSnJ and dystrophic C57BL/10ScSn-Dmdmdx/J mice were tested by western blot. Sarcomeric a-actin was used as a loading control. (B) In a separate study, the levels of TRPC3/6 and NCX3 were tested in wt and mdx muscles. (C) Densitometry of detected protein expression relative to α-actin, n = 2 for STIM1 and SERCA1 and n = 3 for TrpC3/C6 and NCX, *P<0.10.
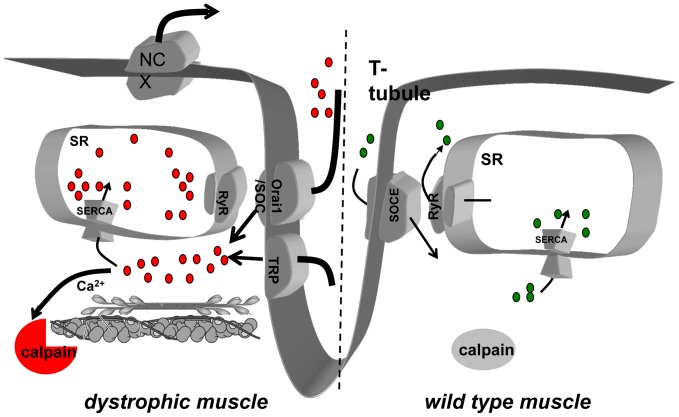
Overall, our results indicate that in dystrophic muscle fibers, upregulation in Orai1 expression is one pathway leading to excessive Ca2+ entry that causes overloading of the SR Ca2+ store and an accompanying reduction in SERCA1 expression. The elevated Ca2+ entry in dystrophic muscle is linked to an increased in cytosolic calpain activity, which may contribute to the progression of muscular dystrophy ( Fig. 7 ).
Figure 7. In mdx dystrophin null muscle fibers elevated Ca2+ entry is at least partially mediated by Orai1 upregulation, leading to an increased SR Ca2+ store.
The excessive Ca2+ entry contributes to activate calpain in the cytosol and possibly leads to increased proteolytic activity. An adaptive downregulation in SERCA1 expression then occurs to accommodate overloaded SR Ca2+ store and upregulation in NCX expression to maintain the low level of resting cytosolic Ca2+ level.
Discussion
A significant body of evidence indicates that dysregulation of Ca2+ influx plays a key role in the pathogenesis and progression of DMD [57] and many candidates have been proposed to mediate the elevated Ca2+ entry in dystrophic fibers, such as stretch-activated channels (SACNSC) [58] and several members of the TRP channel family [39]. Nearly 10 different TRP channels isoforms have been detected in skeletal muscle by RT-PCR, western blot or immunohistochemistry [59], and here we confirm that TRPC3 and TRPC6 were expressed in skeletal muscle ( Fig. 6B ). TRPC function may contribute to the progression of muscular dystrophy as previous studies showed that by expressing a dominant negative TRPC6 could reduce the pathology in mdx and sarcoglycan null mice [39]. Here we detected that the expression level of Orai1 protein expression in dystrophic muscle was increased by ∼2-fold while the ER Ca2+ sensor STIM1 remain unchanged ( Fig. 6A and Fig. 1 ). We also found that the expression level of TRPC3/6 was increased in dystrophic muscle ( Fig. 6B and 6C ). Given previous studies showing that several isoforms of TRP channel form hetero-multimers with Orai1, including TRPC1, 3 and 4, to modulate Orai1 function in SOCE [60], we speculate the excessive TRPC expression may contribute to the activation of Orai1-depedent SOCE independent of the SR store status. This could contribute to the elevated Ca2+ entry observed in mdx muscle even though we determined the Ca2+ store was overloaded in these fibers ( Fig. 3D ). Under these conditions, the Ca2+-sensing mechanism of the SOCE pathway may be disengaged and the elevated TRPC levels could directly activate Orai1 function. This idea is generally supported by previous immunohistochemical data by Krüger et al. [61] that shows TRPC6 localized at the sarcolemma and TRPC3 staining in intracellular patches preferentially in mdx muscle [61], suggesting that different TRPC isoforms may have various functions in the regulation of Orai1 function.
We have shown here that the Orai1 expression level was up-regulated in dystrophic muscles, consistent with a report by Edwards et al. [62]. The functional analysis performed by this group showed an ultra-fast activation and deactivation of SOCE induced by change in Mg2+ concentration in skinned muscle preparations, a response with kinetic properties in excess of that previously observed in intact muscle preparations [63], [64]. In this study, we systematically studied the contribution of Orai1-mediated Ca2+ entry in the progression of muscular dystrophy by applying a shRNA targeting Orai1 and systematic inhibition of SOCE using BTP2. Our results suggest that Orai1 upregulation causes gain of function in SOCE in mdx muscle fiber, leading to overload of the SR Ca2+ store. Treatment by BTP2 also significantly reduced the calpain enzymatic activity in dystrophic muscle fibers, which was linked to the increased proteolytic events that eventually result in progression of the dystrophic phenotype [41], [65]–[67]. Thus, the current data suggest that dystrophic muscle fibers of 8 to 10 weeks are facing an increased Ca2+ burden throughout different cellular compartments although the cytosolic Ca2+ level is still tightly maintained. Even without elevation in resting cytosolic Ca2+ concentration, increase in Ca2+ influx through SOCE can still activate calpain that would result in degradation of contractile proteins in the dystrophic muscle fiber. We have also recently found Orai1 can contribute to Ca2+ entry in cultured HL-1 cardiomyocytes [37], suggesting that Orai1 could potentially contribute to physiology and pathophysiology in other striated muscle tissue.
The typical histological alterations of muscular dystrophy includes reduced cross-section area, increased central nucleus and isolated fibrosis, indicating the constant process of muscle damage and regeneration [68]. In mdx mice, the pathology of affected muscles varies with the age of the animal. Such mice display dystrophic symptoms after the first few weeks of life and then recover through fiber regeneration over the next few weeks. Therefore, we examined mdx mice at 8 to 10 weeks of age for our study to minimize the heterogeneity of the structural alterations [68]. Our EM data showed that even during this stage, the overall ultrastructure of non-regenerating dystrophic muscle fiber is largely intact. However, upon careful analysis of the SR presented at A-I junctions, we found that dystrophic muscle had significantly increased quantity of SR ( Fig. 5D ). It is possible that the increased triad junctions may reflect compensation to manage the elevated SR Ca2+ load in mdx muscle or be one of the subtle indications for muscle regeneration. The shRNA probe used in our study could effectively knockdown the expression of Orail ( Fig. 2 ), which led to disoriented triad structure and rough SR network of the skeletal muscle ( Fig. 5C ), indicating that Orai1 expression is required to maintain the structural integrity of the muscle triad junctional complex. Our data is the first piece of evidence to show the ultrastructural changes that result from decreased Orai1 expression in muscle.
In the current study we also explored the expression level of other Ca2+ handling proteins in mdx muscle. A significant decrease in protein expression of SERCA1 was detected in skeletal muscles from the mdx mice. However, since no increase in resting cytosolic Ca2+ levels was seen in the dystrophic fibers the remaining SERCA must be sufficient to sequester the excessive cytosolic Ca2+ produced by SOCE into the SR and overload the Ca2+ store ( Fig. 3C ). Our findings here indicate that recent studies showing partial rescue of the phenotype of mdx mice by overexpressing SERCA1 specifically in the skeletal muscle may involve replacing lost SERCA1 expression [69]. It is also possible that the decrease in SERCA1 expression may be an adaptive change to the overloaded SR Ca2+ store in mdx fibers. A recent report by Robin et al. revealing increased SERCA1 activity in mdx muscle fibers is consistent with the idea of a compensatory changes in SERCA function in dystrophic muscle [70].
In summary, our results suggest that elevated Ca2+ entry follows Orai1 upregulation in mdx dystrophic muscle fibers, leading to an overfilled SR Ca2+ store ( Fig. 7 ). The excessive Ca2+ entry activates calpain in the cytosol and possibly leads to increased proteolytic activity. An adaptive downregulation in SERCA1 expression then occurs to accommodate overloaded SR Ca2+ store and slight upregulation in NCX expression to maintain normal cytosolic Ca2+ levels. Although a previous studies suggests a link between extracellular Ca2+ entry and the cytosolic [Ca2+] level [71], we cannot detect a change in [Ca2+]i under these experimental conditions. Our study indicates that excessive Ca2+ entry through Orai1 may underlie Ca2+-mediated muscle damage during DMD progression and could represent a potential therapeutic target for the treatment of DMD. Additional studies to investigate the potential mechanisms for increased Orai1 expression following absence of dystrophin are of high interest to elucidate the underlying mechanism for the progression of DMD.
Materials and Methods
Design of shRNA Probes and Construction of Vectors
Three shRNA probes specifically targeting mOrai1 CDs (NM_175423) were designed. The oligonucleotide sequences for sh2 and sh3 were as follows: sh2: sense: 5′-GAT CGT CCT GGC GCA AGC TCT ACT TAA TTC AAG AGA TTA AGT AGA GCT TGC GCC AGG ACT TTT TT-3′; antisense 5′-AAT TAA AAA AG T CCT GGC GCA AGC TCT ACT TAA TCT CTT GAA TTA AGT AGA GCT TGC GCC AGG AC-3′ and sh3: sense: 5′-GAT CGT GCA CCT GTT TGC CCT CAT GAT TTC AAG AGA ATC ATG AGG GCA AAC AGG TGC ACT TTT TT-3′, and antisense 5′-AAT TAA AAA AGT GCA CCT GTT TGC CCT CAT GAT TCT CTT GAA ATC ATG AGG GCA AAC AGG TGC AC-3′. These shOrai1 probes were cloned into a custom-made pU6r-RFP vector with a multi-red fluorescence protein (RFP) expression cassette. Scramble shRNA in pU6r-RFP vector was used as control for all experiments. Full-length mOrai1 cDNA in pcDNA3.1/Myc-His(−) vector (Invitrogen) was co-transfected with sh2 and sh3 into HeLa cells to test the efficiency of Orai1 knockdown.
Cell Culture and Transfection
HeLa cells were cultured in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin and 100 µg/ml streptomycin at 37°C in an incubator suffused with 5% CO2. Three shRNA probes targeting mOrai1 in pU6r-RFP plasmids were co-transfected with full length mOrai1 pcDNA3.1/Myc-His(−) at 9∶1 molar ratio into HeLa cells using GeneJammer transfection reagent (Stratagene, Cedar Creek, TX) (2 µg DNA: 4 µl reagent) according to the manufacturer’s instructions. Experiments were repeated twice. HeLa cells were used for these experiments as these easily transfected cells allows for resolution of the degree of knockdown without complication from the lower levels of transfection seen in some immortalized muscle cell cultures.
Animals
All animal work was conducted according to relevant national and international guidelines. The full details of this study were reviewed and approved by the Robert Wood Johnson IACUC. 6 week old Male C57BL/10ScSnJ (wt) and C57BL/10ScSn-Dmdmdx/J dystrophic mice (mdx) were purchased from Jackson Laboratory (Bar Harbor, ME). Animals were bred and housed in the Robert Wood Johnson Medical School vivarium facility until they were 8∼10 weeks old before use in our experiments.
Electroporation of Flexor Digitorum Brevis (FDB) Muscle Fiber
The control plasmid and shOrai1 probes were introduced into the FDB muscles of 8 weeks old wt or mdx mice using an electroporation procedure as previously described [35]. Briefly, the animal was anaesthetized with ketamine and xylazine before subcutaneous hyaluronidase injection into each rear foot pad. After 1 hour, control or shOrai1 plasmid DNA in sterilized 0.9% saline was injected into muscle and two acupuncture needles (Austin Medical Equipment, Westchester, IL) connected with the anode and cathode of an ECM 830 Electroporator (BTX, Holliston, MA) were inserted subcutaneously into the FDB muscle. A series of 20 ms square wave pulses of 120 V/cm were generated to promote DNA entry into FDB muscle fibers.
Real-time PCR
Total mRNA was extracted from gastrocnemius muscles of the C57BL/10ScSnJ and dystrophic C57BL/10ScSn-Dmdmdx/J mice using a RNAasy kit and on-column genomic DNA clean kit (Qiagen, Valencia, CA). Isolated mRNA was quantified using UV spectrometer and verified by formaldehyde denaturing agarose gel electrophoresis. Real-time PCR for Orai1, STIM1 and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was performed using our previously described primers and protocols [32]. Triplicate wells were used for each sample and the relative number of mRNA copies to GAPDH was calculated using the formula: 1/2Λ (ct value of target gene – ct value of GAPDH).
Western Blot Assay
Effective knock down of Orai1 by shRNA in vivo was confirmed by western blot using extracts from individual FDB fibers [36]. Briefly, electroporated FDB bundles were enzymatically dissociated and individual transfected single muscle fiber was collected by a 0.8–1.1 mm × 100 mm capillary tube (PYREX, Lowell, MA) under Zeiss Axiovert 2000 fluorescence microscope (Zeiss, Thornwood, NY) and pooled for Western blot assay. Cultured cells, intact muscle bundles or pooled single muscle fibers from wt and mdx mice were lysed in a highly reducing RIPA buffer (in mM, 150 NaCl, 20 NaPO4, 50 NaF, 2 EDTA, 30 Na pyrophosphate, 1 phenylmethanesulphonylfluoride, 0.2 Na vanadate, 14 β-mercaptoethanol, 100 dithiothreitol, 0.1% SDS, 1% deoxycholic acid, 1% triton X-100, 100 KIU/ml aprotinin and 1% p8340 protease inhibitor cocktail (Sigma, St. Louis, MO), pH 7.2) and separated by electrophoresis on a 12% SDS gel. After transfer, PVDF membranes were probed with anti-Orai1 polyclonal (1∶1,000 dilution, Millipore, Billerica, MA), anti-myc (1∶1,000), anti-STIM1 (1∶500) monoclonal (BD Bioscience, Franklin Lakes, NJ), anti-dystrophin monoclonal (1∶500, DSHB, Iowa City, Iowa), anti-SERCA1 monoclonal (1∶8,000, ABR, Golden, CO), anti-NCX3 polyclonal (1∶1,000, Santa Cruz, CA), or TRPC3/6 (1∶1,000, generous gift from Dr. Michael Zhu, University of Texas Health Science Center at Houston). Sarcometirc anti-α-actin (1∶8,000) and β-actin antibodies (Sigma, St. Louis, MO) were used as loading controls. Densitometry of target proteins was performed and normalized to loading controls using Genetools software (Syngene, Frederick, MD).
Ca2+ Measurement and SOCE Activity by Mn2+ Entry Assay
The procedure was described in detail previously [38]. Briefly, transfected FDB muscle fibers were enzymatically disassociated and loaded with 10 µM Fura-2 AM (Molecular Probes, Eugene, OR). To prevent motion artifact associated with increase in [Ca2+]i, 30 µM N-benzyl-p-toluene sulphonamide (Sigma, St. Louis, MO) was applied on muscle fiber for 15 min and left on during the experiment. FDB fibers were examined on a PTI spectrofluorometer system (Photon Technology International, Monmouth Junction, NJ) and selected by presence of RFP signal under wavelength of 550 nm. Fura-2 fluorescence ratio of excitation wavelengths at 350 nm (F350) and 380 nm (F380) and emission at 510 nm was recorded for measuring SR Ca2+ store induced by 20 mM caffeine plus 5 µM ryanodine (C/R). Mn2+ quenching of Fura-2 fluorescence was performed at a wavelength of 360 nm following depletion of SR store by C/R dissolved in 0 mM Ca2+. 1% Triton X-100 was added at the end of the experiment for data normalization. All experiments were conducted at room temperature.
Determination of Calpain Activity in situ
To inhibit SOCE activity, mdx mice were first injected with BTP2 (EMD Biosciences, Rockland, Massachusetts), at a dose of 4 mg/kg body weight, daily Intraperitoneal injection for 3 weeks. Then the mice were sacrificed and enzymatic activity of calpain in single intact FDB fibers was measured using a fluorogenic, membrane-permeable calpain substrate, Succinyl-Leu-Leu-Val-Tyr-7-amino-4 methylcoumarin (Suc-LLVY-AMC) (Enzo life sciences, Farmingdale, NY) based on previous publications [72], [73]. Specifically, enzymatically isolated FDB fibers were plated on glass bottom ΔT dish and treated with 10 µM Lactacystin (Boston Biochem, Cambridge, MA) to inhibit proteasome activity. Then 50 µM Suc-LLVY-AMC was added to the medium and fluorescence signal was monitored immediatedly on Zeiss LSM 510 confocal microscope (Carl Zeiss, Thornwood, NY) using 360 nm excitation and 460 nm emission wavelengths. Measurement of fluorescence signal was continued for 25 min and the relative calpain activity (ΔF) was calculated as peak fluorescence activity (F1) substrates the basal fluorescence level (F0).
Transmission Electron Microscopy
FDB muscle preparations were pre-fixed with 2.5% glutaraldehyde and 3% formaldehyde in 0.1 M cacodylate buffer (pH 7.4), and post-fixed with 1% OsO4 in the same buffer. After washing with cacodylate buffer solution, they were dehydrated with ethanol plus acetone and embedded in epoxy resin. Ultrathin sections were cut, double stained with uranyl acetate and lead citrate, and examined under JEM-1010 electron microscope (Jeol Co., Ltd, Tokyo, Japan).
Statistics
Values are mean ± S.E.M. Significance was determined by Student’s t test. A value of P<0.05 was considered to be statistically significant.
Supporting Information
Increased Orai1 expression in various muscles from the mdx mice. Upper panel: Orai1 expression is increased in extensor digitorum longus (EDL) of the mdx mice, a mostly fast glycolytic skeletal muscle. Blot of α-actin was to show the equal loading of these individual muscles; lower panel: Orai1 expression is increased in gastrocnemius (GN) muscle from the mdx mice, a mixed type of skeletal muscle, as compared to the wild type control.
(TIF)
Acknowledgments
We thank Dr. Jae-Kyun Ko (Department of Physiology & Biophysics, RWJMS) for his help in designing shOrai1 probes and Dr. Chuanxi Cai (Division of Cardiovascular Medicine, University of Louisville) for constructing the mOrai1-myc expression plasmid.
Funding Statement
This work was supported by a NIH/NIAMS grant (AR054793) to NW and an American Heart Association National scientist development grant to XZ (10SDG2630086). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hoffman EP, Brown RH, Kunkel LM (1992) Dystrophin: the protein product of the Duchene muscular dystrophy locus. 1987. Biotechnology 24: 457–466. [PubMed] [Google Scholar]
- 2. Ibraghimov-Beskrovnaya O, Ervasti JM, Leveille CJ, Slaughter CA, Sernett SW, et al. (1992) Primary structure of dystrophin-associated glycoproteins linking dystrophin to the extracellular matrix. Nature 355: 696–702. [DOI] [PubMed] [Google Scholar]
- 3. Lynch GS, Rafael JA, Chamberlain JS, Faulkner JA (2000) Contraction-induced injury to single permeabilized muscle fibers from mdx, transgenic mdx, and control mice. Am J Physiol Cell Physiol 279: C1290–1294. [DOI] [PubMed] [Google Scholar]
- 4.Perronnet C, Vaillend C (2010) Dystrophins, utrophins, and associated scaffolding complexes: role in mammalian brain and implications for therapeutic strategies. J Biomed Biotechnol: 849426. [DOI] [PMC free article] [PubMed]
- 5. Blake DJ, Weir A, Newey SE, Davies KE (2002) Function and genetics of dystrophin and dystrophin-related proteins in muscle. Physiol Rev 82: 291–329. [DOI] [PubMed] [Google Scholar]
- 6. Bodensteiner JB, Engel AG (1978) Intracellular calcium accumulation in Duchenne dystrophy and other myopathies: a study of 567,000 muscle fibers in 114 biopsies. Neurology 28: 439–446. [DOI] [PubMed] [Google Scholar]
- 7. Alderton JM, Steinhardt RA (2000) How calcium influx through calcium leak channels is responsible for the elevated levels of calcium-dependent proteolysis in dystrophic myotubes. Trends Cardiovasc Med 10: 268–272. [DOI] [PubMed] [Google Scholar]
- 8. Mallouk N, Allard B (2002) Ca(2+) influx and opening of Ca(2+)-activated K(+) channels in muscle fibers from control and mdx mice. Biophys J 82: 3012–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fong PY, Turner PR, Denetclaw WF, Steinhardt RA (1990) Increased activity of calcium leak channels in myotubes of Duchenne human and mdx mouse origin. Science 250: 673–676. [DOI] [PubMed] [Google Scholar]
- 10. Mallouk N, Jacquemond V, Allard B (2000) Elevated subsarcolemmal Ca2+ in mdx mouse skeletal muscle fibers detected with Ca2+-activated K+ channels. Proc Natl Acad Sci U S A 97: 4950–4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takagi A, Kojima S, Ida M, Araki M (1992) Increased leakage of calcium ion from the sarcoplasmic reticulum of the mdx mouse. J Neurol Sci 110: 160–164. [DOI] [PubMed] [Google Scholar]
- 12. Kumar A, Khandelwal N, Malya R, Reid MB, Boriek AM (2004) Loss of dystrophin causes aberrant mechanotransduction in skeletal muscle fibers. Faseb J 18: 102–113. [DOI] [PubMed] [Google Scholar]
- 13. Vandebrouck C, Martin D, Colson-Van Schoor M, Debaix H, Gailly P (2002) Involvement of TRPC in the abnormal calcium influx observed in dystrophic (mdx) mouse skeletal muscle fibers. J Cell Biol 158: 1089–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Phillips MF, Quinlivan R (2008) Calcium antagonists for Duchenne muscular dystrophy. Cochrane Database Syst Rev: CD004571. [DOI] [PMC free article] [PubMed]
- 15. Putney JW Jr (1990) Capacitative calcium entry revisited. Cell Calcium 11: 611–624. [DOI] [PubMed] [Google Scholar]
- 16. Ma J, Pan Z (2003) Retrograde activation of store-operated calcium channel. Cell Calcium 33: 375–384. [DOI] [PubMed] [Google Scholar]
- 17. Kurebayashi N, Ogawa Y (2001) Depletion of Ca2+ in the sarcoplasmic reticulum stimulates Ca2+ entry into mouse skeletal muscle fibres. J Physiol 533: 185–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pan Z, Yang D, Nagaraj RY, Nosek TA, Nishi M, et al. (2002) Dysfunction of store-operated calcium channel in muscle cells lacking mg29. Nat Cell Biol 4: 379–383. [DOI] [PubMed] [Google Scholar]
- 19. Brotto MA, Nagaraj RY, Brotto LS, Takeshima H, Ma JJ, et al. (2004) Defective maintenance of intracellular Ca2+ homeostasis is linked to increased muscle fatigability in the MG29 null mice. Cell Res 14: 373–378. [DOI] [PubMed] [Google Scholar]
- 20. Nagaraj RY, Nosek CM, Brotto MA, Nishi M, Takeshima H, et al. (2000) Increased susceptibility to fatigue of slow- and fast-twitch muscles from mice lacking the MG29 gene. Physiol Genomics 4: 43–49. [DOI] [PubMed] [Google Scholar]
- 21. Zhao X, Yoshida M, Brotto L, Takeshima H, Weisleder N, et al. (2005) Enhanced resistance to fatigue and altered calcium handling properties of sarcalumenin knockout mice. Physiol Genomics 23: 72–78. [DOI] [PubMed] [Google Scholar]
- 22. Thornton AM, Zhao X, Weisleder N, Brotto LS, Bougoin S, et al. (2011) Store-operated Ca(2+) entry (SOCE) contributes to normal skeletal muscle contractility in young but not in aged skeletal muscle. Aging (Albany NY) 3: 621–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liou J, Kim ML, Heo WD, Jones JT, Myers JW, et al. (2005) STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol 15: 1235–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang SL, Yu Y, Roos J, Kozak JA, Deerinck TJ, et al. (2005) STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437: 902–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wu MM, Buchanan J, Luik RM, Lewis RS (2006) Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J Cell Biol 174: 803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, et al. (2006) Orai1 is an essential pore subunit of the CRAC channel. Nature 443: 230–233. [DOI] [PubMed] [Google Scholar]
- 27. Vig M, Beck A, Billingsley JM, Lis A, Parvez S, et al. (2006) CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr Biol 16: 2073–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mercer JC, Dehaven WI, Smyth JT, Wedel B, Boyles RR, et al. (2006) Large store-operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem 281: 24979–24990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lyfenko AD, Dirksen RT (2008) Differential dependence of store-operated and excitation-coupled Ca2+ entry in skeletal muscle on STIM1 and Orai1. J Physiol 586: 4815–4824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Darbellay B, Arnaudeau S, Konig S, Jousset H, Bader C, et al. (2009) STIM1- and Orai1-dependent store-operated calcium entry regulates human myoblast differentiation. J Biol Chem 284: 5370–5380. [DOI] [PubMed] [Google Scholar]
- 31. Stiber J, Hawkins A, Zhang ZS, Wang S, Burch J, et al. (2008) STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat Cell Biol 10: 688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhao X, Weisleder N, Thornton A, Oppong Y, Campbell R, et al. (2008) Compromised store-operated Ca2+ entry in aged skeletal muscle. Aging Cell 7: 561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Alderton JM, Steinhardt RA (2000) Calcium influx through calcium leak channels is responsible for the elevated levels of calcium-dependent proteolysis in dystrophic myotubes. J Biol Chem 275: 9452–9460. [DOI] [PubMed] [Google Scholar]
- 34. Collet C, Ma J (2004) Calcium-dependent facilitation and graded deactivation of store-operated calcium entry in fetal skeletal muscle. Biophys J 87: 268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cai C, Masumiya H, Weisleder N, Matsuda N, Nishi M, et al. (2009) MG53 nucleates assembly of cell membrane repair machinery. Nat Cell Biol 11: 56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao X, Min CK, Ko JK, Parness J, Kim do H, et al. (2010) Increased store-operated Ca2+ entry in skeletal muscle with reduced calsequestrin-1 expression. Biophys J 99: 1556–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Touchberry CD, Elmore CJ, Nguyen TM, Andresen JJ, Zhao X, et al. (2011) Store-operated calcium entry is present in HL-1 cardiomyocytes and contributes to resting calcium. Biochem Biophys Res Commun 416: 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhao X, Weisleder N, Han X, Pan Z, Parness J, et al. (2006) Azumolene inhibits a component of store-operated calcium entry coupled to the skeletal muscle ryanodine receptor. J Biol Chem 281: 33477–33486. [DOI] [PubMed] [Google Scholar]
- 39. Millay DP, Goonasekera SA, Sargent MA, Maillet M, Aronow BJ, et al. (2009) Calcium influx is sufficient to induce muscular dystrophy through a TRPC-dependent mechanism. Proc Natl Acad Sci U S A 106: 19023–19028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oberc MA, Engel WK (1977) Ultrastructural localization of calcium in normal and abnormal skeletal muscle. Lab Invest 36: 566–577. [PubMed] [Google Scholar]
- 41. Whitehead NP, Yeung EW, Allen DG (2006) Muscle damage in mdx (dystrophic) mice: role of calcium and reactive oxygen species. Clin Exp Pharmacol Physiol 33: 657–662. [DOI] [PubMed] [Google Scholar]
- 42. Berchtold MW, Brinkmeier H, Muntener M (2000) Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol Rev 80: 1215–1265. [DOI] [PubMed] [Google Scholar]
- 43. Robert V, Massimino ML, Tosello V, Marsault R, Cantini M, et al. (2001) Alteration in calcium handling at the subcellular level in mdx myotubes. J Biol Chem 276: 4647–4651. [DOI] [PubMed] [Google Scholar]
- 44. Ma J, Pan Z (2003) Junctional membrane structure and store operated calcium entry in muscle cells. Front Biosci 8: d242–255. [DOI] [PubMed] [Google Scholar]
- 45. Wang X, Weisleder N, Collet C, Zhou J, Chu Y, et al. (2005) Uncontrolled calcium sparks act as a dystrophic signal for mammalian skeletal muscle. Nat Cell Biol 7: 525–530. [DOI] [PubMed] [Google Scholar]
- 46. Gailly P (2002) New aspects of calcium signaling in skeletal muscle cells: implications in Duchenne muscular dystrophy. Biochim Biophys Acta 1600: 38–44. [DOI] [PubMed] [Google Scholar]
- 47. Hopf FW, Turner PR, Steinhardt RA (2007) Calcium misregulation and the pathogenesis of muscular dystrophy. Subcell Biochem 45: 429–464. [DOI] [PubMed] [Google Scholar]
- 48. Wadosky KM, Li L, Rodriguez JE, Min JN, Bogan D, et al. (2011) Regulation of the calpain and ubiquitin-proteasome systems in a canine model of muscular dystrophy. Muscle Nerve 44: 553–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Briguet A, Erb M, Courdier-Fruh I, Barzaghi P, Santos G, et al. (2008) Effect of calpain and proteasome inhibition on Ca2+-dependent proteolysis and muscle histopathology in the mdx mouse. FASEB J 22: 4190–4200. [DOI] [PubMed] [Google Scholar]
- 50. Ueyama H, Kumamoto T, Fujimoto S, Murakami T, Tsuda T (1998) Expression of three calpain isoform genes in human skeletal muscles. J Neurol Sci 155: 163–169. [DOI] [PubMed] [Google Scholar]
- 51. Fahrner M, Muik M, Derler I, Schindl R, Fritsch R, et al. (2009) Mechanistic view on domains mediating STIM1-Orai coupling. Immunol Rev 231: 99–112. [DOI] [PubMed] [Google Scholar]
- 52. Schindl R, Muik M, Fahrner M, Derler I, Fritsch R, et al. (2009) Recent progress on STIM1 domains controlling Orai activation. Cell Calcium 46: 227–232. [DOI] [PubMed] [Google Scholar]
- 53. Cullen MJ, Jaros E (1988) Ultrastructure of the skeletal muscle in the × chromosome-linked dystrophic (mdx) mouse. Comparison with Duchenne muscular dystrophy. Acta Neuropathol 77: 69–81. [DOI] [PubMed] [Google Scholar]
- 54. Torres LF, Duchen LW (1987) The mutant mdx: inherited myopathy in the mouse. Morphological studies of nerves, muscles and end-plates. Brain 110 (Pt 2): 269–299. [DOI] [PubMed] [Google Scholar]
- 55. Wang Y, Deng X, Mancarella S, Hendron E, Eguchi S, et al. (2010) The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science 330: 105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kunert-Keil C, Bisping F, Kruger J, Brinkmeier H (2006) Tissue-specific expression of TRP channel genes in the mouse and its variation in three different mouse strains. BMC Genomics 7: 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Constantin B, Sebille S, Cognard C (2006) New insights in the regulation of calcium transfers by muscle dystrophin-based cytoskeleton: implications in DMD. J Muscle Res Cell Motil 27: 375–386. [DOI] [PubMed] [Google Scholar]
- 58. Allen DG, Gervasio OL, Yeung EW, Whitehead NP (2010) Calcium and the damage pathways in muscular dystrophy. Can J Physiol Pharmacol 88: 83–91. [DOI] [PubMed] [Google Scholar]
- 59. Brinkmeier H (2011) TRP Channels in Skeletal Muscle: Gene Expression, Function and Implications for Disease. Adv Exp Med Biol 704: 749–758. [DOI] [PubMed] [Google Scholar]
- 60. Potier M, Trebak M (2008) New developments in the signaling mechanisms of the store-operated calcium entry pathway. Pflugers Arch 457: 405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kruger J, Kunert-Keil C, Bisping F, Brinkmeier H (2008) Transient receptor potential cation channels in normal and dystrophic mdx muscle. Neuromuscul Disord 18: 501–513. [DOI] [PubMed] [Google Scholar]
- 62. Edwards JN, Friedrich O, Cully TR, von Wegner F, Murphy RM, et al. (2010) Upregulation of store-operated Ca2+ entry in dystrophic mdx mouse muscle. Am J Physiol Cell Physiol 299: C42–50. [DOI] [PubMed] [Google Scholar]
- 63. Jacquemond V, Schneider MF (1992) Low myoplasmic Mg2+ potentiates calcium release during depolarization of frog skeletal muscle fibers. J Gen Physiol 100: 137–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jacquemond V, Schneider MF (1992) Effects of low myoplasmic Mg2+ on calcium binding by parvalbumin and calcium uptake by the sarcoplasmic reticulum in frog skeletal muscle. J Gen Physiol 100: 115–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Gailly P, De Backer F, Van Schoor M, Gillis JM (2007) In situ measurements of calpain activity in isolated muscle fibres from normal and dystrophin-lacking mdx mice. J Physiol 582: 1261–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Spencer MJ, Mellgren RL (2002) Overexpression of a calpastatin transgene in mdx muscle reduces dystrophic pathology. Hum Mol Genet 11: 2645–2655. [DOI] [PubMed] [Google Scholar]
- 67. Badalamente MA, Stracher A (2000) Delay of muscle degeneration and necrosis in mdx mice by calpain inhibition. Muscle Nerve 23: 106–111. [DOI] [PubMed] [Google Scholar]
- 68. Briguet A, Courdier-Fruh I, Foster M, Meier T, Magyar JP (2004) Histological parameters for the quantitative assessment of muscular dystrophy in the mdx-mouse. Neuromuscul Disord 14: 675–682. [DOI] [PubMed] [Google Scholar]
- 69.Goonasekera SA, Lam CK, Millay DP, Sargent MA, Hajjar RJ, et al. Mitigation of muscular dystrophy in mice by SERCA overexpression in skeletal muscle. J Clin Invest. [DOI] [PMC free article] [PubMed]
- 70. Robin G, Berthier C, Allard B (2012) Sarcoplasmic reticulum Ca2+ permeation explored from the lumen side in mdx muscle fibers under voltage control. J Gen Physiol 139: 209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Li H, Ding X, Lopez JR, Takeshima H, Ma J, et al. (2010) Impaired Orai1-mediated resting Ca2+ entry reduces the cytosolic [Ca2+] and sarcoplasmic reticulum Ca2+ loading in quiescent junctophilin 1 knock-out myotubes. J Biol Chem 285: 39171–39179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Chen M, Fernandez HL (2004) Stimulation of beta-amyloid precursor protein alpha-processing by phorbol ester involves calcium and calpain activation. Biochem Biophys Res Commun 316: 332–340. [DOI] [PubMed] [Google Scholar]
- 73. Chen M, Fernandez HL (2005) Mu-calpain is functionally required for alpha-processing of Alzheimer’s beta-amyloid precursor protein. Biochem Biophys Res Commun 330: 714–721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Increased Orai1 expression in various muscles from the mdx mice. Upper panel: Orai1 expression is increased in extensor digitorum longus (EDL) of the mdx mice, a mostly fast glycolytic skeletal muscle. Blot of α-actin was to show the equal loading of these individual muscles; lower panel: Orai1 expression is increased in gastrocnemius (GN) muscle from the mdx mice, a mixed type of skeletal muscle, as compared to the wild type control.
(TIF)