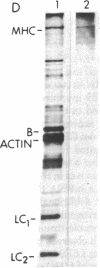
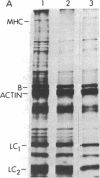
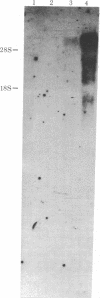
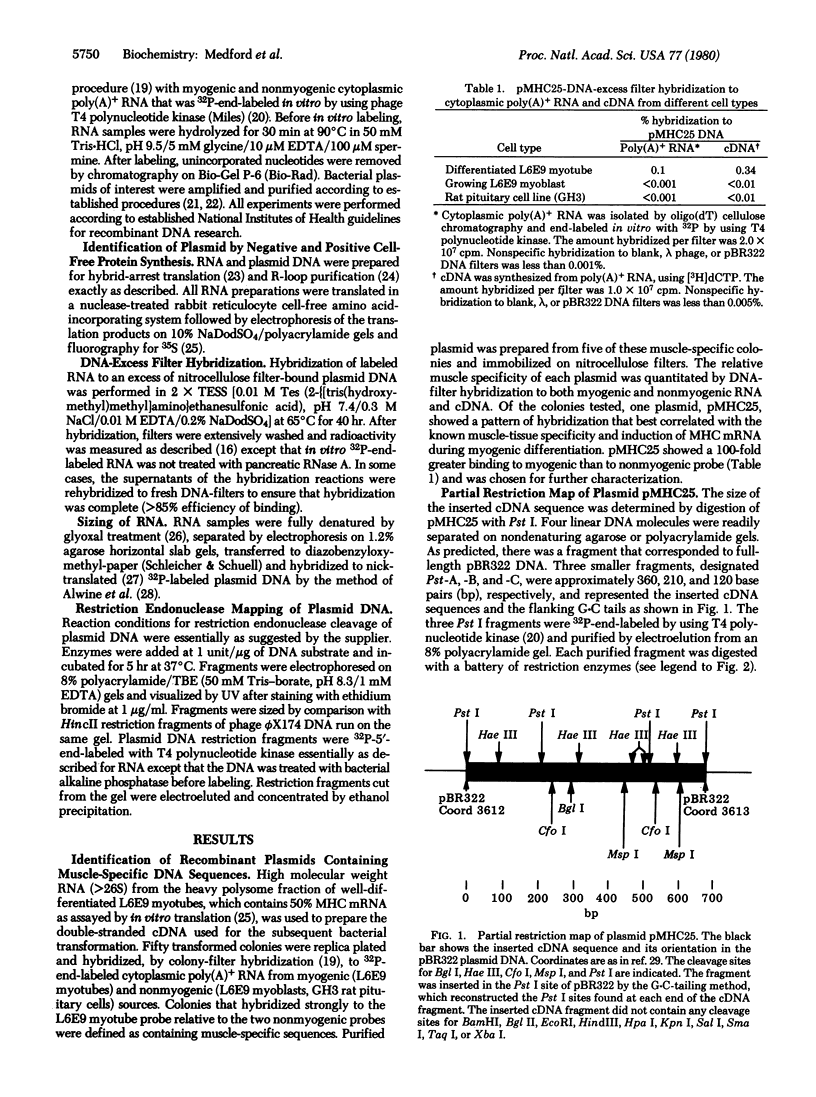
Abstract
A recombinant DNA plasmid, designated pMHC25, has been constructed that contains structural gene sequences for rat skeletal muscle myosin heavy chain (MHC). The identity of the MHC sequence insert in pMHC25 was determined by muscle-tissue specificity, inhibition of MHC protein synthesis in vitro by hybrid-arrested translation, purification of mRNA that directs the synthesis of MHC protein in vitro, and hybridization to a 33S cytoplasmic mRNA found only in differentiated muscle cells. pMHC25-DNA-excess filter hybridizations were used to show that more than 90% of the newly synthesized MHC mRNA that appears in the cytoplasm of differentiated L6E9 myotubes contains a long 3' poly(A) tail. In contrast, 90% of the MHC mRNA that accumulates in the cytoplasm of these same cells during myogenic differentiation lacks this long 3' poly(A) tail. These results suggest the occurrence of a posttranscriptional event in differentiated L6E9 myotubes that involves the cytoplasmic processing of poly(A)+ MHC mRNA to poly(A)- or poly(A)-short MHC mRNA.
Full text
PDF
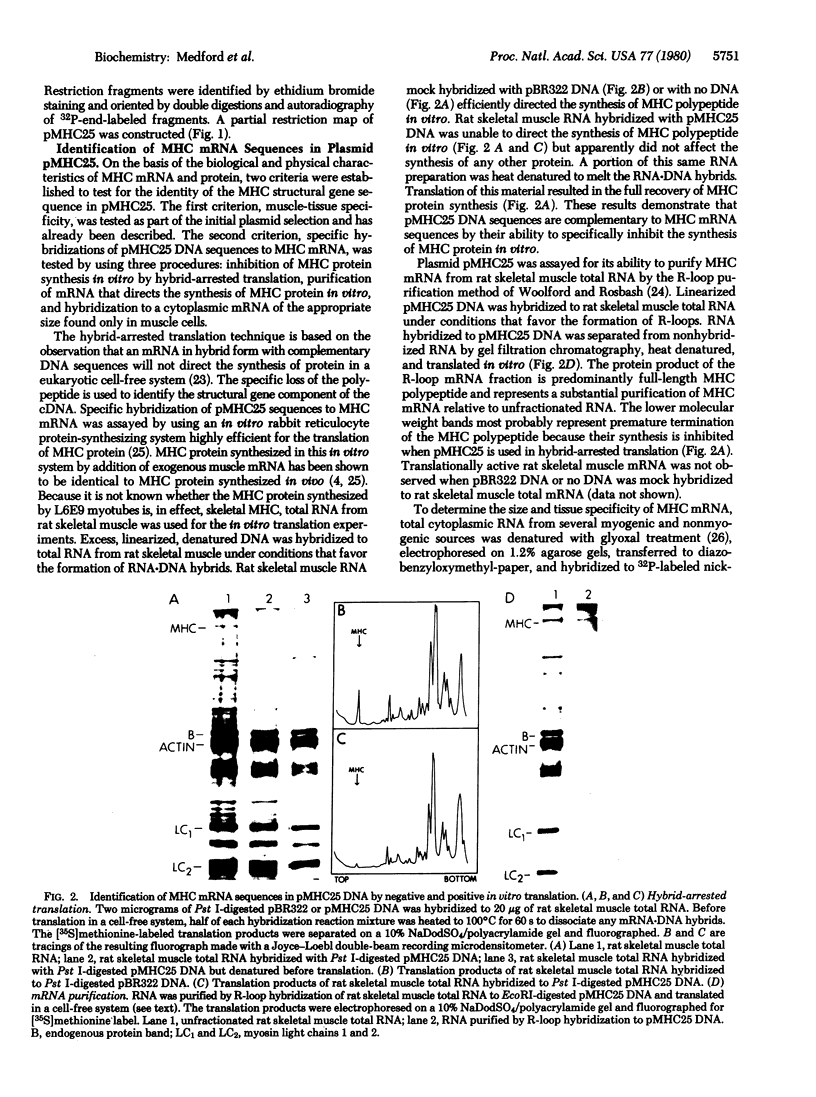
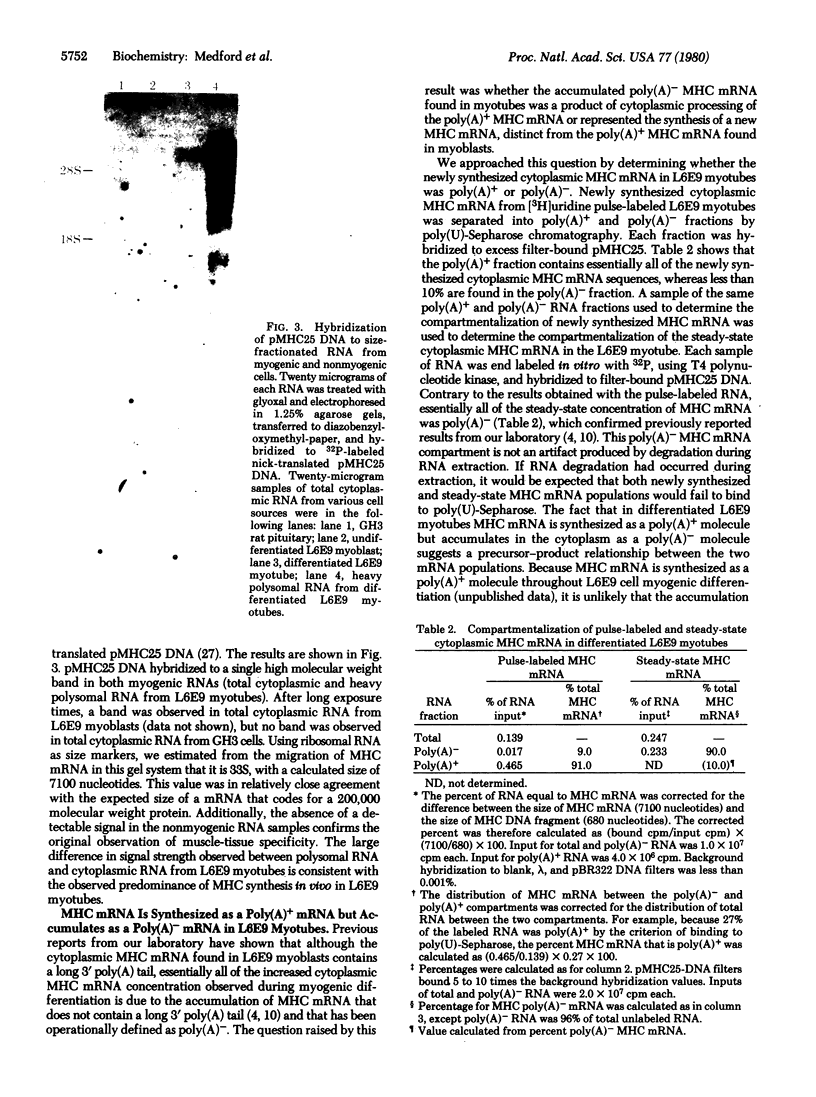

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoff S., Nadal-Ginard B. N. Cell-free translation of mammalian myosin heavy-chain messenger ribonucleic acid from growing and fused-L6E9 myoblasts. Biochemistry. 1979 Feb 6;18(3):494–500. doi: 10.1021/bi00570a019. [DOI] [PubMed] [Google Scholar]
- Benoff S., Nadal-Ginard B. Most myosin heavy chain mRNA in L6E9 rat myotubes has a short poly(A) tail. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1853–1857. doi: 10.1073/pnas.76.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoff S., Nadal-Ginard B. Transient induction of poly(A)-short myosin heavy chain messenger RNA during terminal differentiation of L6E9 myoblasts. J Mol Biol. 1980 Jun 25;140(2):283–298. doi: 10.1016/0022-2836(80)90106-0. [DOI] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Brawerman G. Characteristics and significance of the polyadenylate sequence in mammalian messenger RNA. Prog Nucleic Acid Res Mol Biol. 1976;17:117–148. doi: 10.1016/s0079-6603(08)60068-9. [DOI] [PubMed] [Google Scholar]
- Buckingham M. E., Cohen A., Gros F. Cytoplasmic distribution of pulse-labelled poly(A)-containing RNA, particularly 26 S RNA, during myoblast growth and differentiation. J Mol Biol. 1976 May 25;103(3):611–626. doi: 10.1016/0022-2836(76)90220-5. [DOI] [PubMed] [Google Scholar]
- Chi J. C., Fellini S. A., Holtzer H. Differences among myosins synthesized in non-myogenic cells, presumptive myoblasts, and myoblasts. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4999–5003. doi: 10.1073/pnas.72.12.4999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Miller C. A. Non-chromosomal antibiotic resistance in bacteria. II. Molecular nature of R-factors isolated from Proteus mirabilis and Escherichia coli. J Mol Biol. 1970 Jun 28;50(3):671–687. doi: 10.1016/0022-2836(70)90092-6. [DOI] [PubMed] [Google Scholar]
- Devlin R. B., Emerson C. P., Jr Coordinate regulation of contractile protein synthesis during myoblast differentiation. Cell. 1978 Apr;13(4):599–611. doi: 10.1016/0092-8674(78)90211-8. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harpold M. M., Dobner P. R., Evans R. M., Bancroft F. C. Construction and identification by positive hybridization-translation of a bacterial plasmid containing a rat growth hormone structural gene sequence. Nucleic Acids Res. 1978 Jun;5(6):2039–2053. doi: 10.1093/nar/5.6.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann Y., Milcarek C., Berissi H., Penman S. HeLa cell poly(A)- mRNA codes for a subset of poly(A)+ mRNA-directed proteins with an actin as a major product. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4801–4805. doi: 10.1073/pnas.74.11.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupersztoch Y. M., Helinski D. R. A catenated DNA molecule as an intermediate in the replication of the resistance transfer factor R6K in Escherichia coli. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1451–1459. doi: 10.1016/0006-291x(73)91149-2. [DOI] [PubMed] [Google Scholar]
- Lowenhaupt K., Lingrel J. B. A change in the stability of globin mRNA during the induction of murine erythroleukemia cells. Cell. 1978 Jun;14(2):337–344. doi: 10.1016/0092-8674(78)90119-8. [DOI] [PubMed] [Google Scholar]
- Masaki T., Yoshizaki C. Differentiation of myosin in chick embryos. J Biochem. 1974 Jul;76(1):123–131. doi: 10.1093/oxfordjournals.jbchem.a130536. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlie J. P., Buckingham M. E., Whalen R. G. Molecular aspects of myogenesis. Curr Top Dev Biol. 1977;11:61–114. doi: 10.1016/s0070-2153(08)60743-7. [DOI] [PubMed] [Google Scholar]
- Nadal-Ginard B. Commitment, fusion and biochemical differentiation of a myogenic cell line in the absence of DNA synthesis. Cell. 1978 Nov;15(3):855–864. doi: 10.1016/0092-8674(78)90270-2. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Kuff E. L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrinou-Georgoulas M., John H. A. The genes and mRNA coding for the heavy chains of chick embryonic skeletal myosin. Cell. 1977 Oct;12(2):491–499. doi: 10.1016/0092-8674(77)90125-8. [DOI] [PubMed] [Google Scholar]
- Perriard J. C., Perriard E. R., Eppenberger H. M. Detection and relative quantitation of mRNA for creatine kinase isoenzymes in mRNA from myogenic cell cultures and embryonic chicken tissues. J Biol Chem. 1978 Sep 25;253(18):6529–6535. [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Messenger RNA turnover in mouse L cells. J Mol Biol. 1973 Oct 5;79(4):681–696. doi: 10.1016/0022-2836(73)90071-5. [DOI] [PubMed] [Google Scholar]
- Revel M., Groner Y. Post-transcriptional and translational controls of gene expression in eukaryotes. Annu Rev Biochem. 1978;47:1079–1126. doi: 10.1146/annurev.bi.47.070178.005243. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Jan;3(1):101–116. doi: 10.1093/nar/3.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiness D., Darnell J. E. Polyadenylic acid segment in mRNA becomes shorter with age. Nat New Biol. 1973 Feb 28;241(113):265–268. doi: 10.1038/newbio241265a0. [DOI] [PubMed] [Google Scholar]
- Soeiro R., Birnboim H. C., Darnell J. E. Rapidly labeled HeLa cell nuclear RNA. II. Base composition and cellular localization of a heterogeneous RNA fraction. J Mol Biol. 1966 Aug;19(2):362–372. doi: 10.1016/s0022-2836(66)80010-4. [DOI] [PubMed] [Google Scholar]
- Sréter F. A., Bálint M., Gergely J. Structural and functional changes of myosin during development: comparison with adult fast, slow and cardiac myosin. Dev Biol. 1975 Oct;46(2):317–325. doi: 10.1016/0012-1606(75)90108-6. [DOI] [PubMed] [Google Scholar]
- Strohman R. C., Moss P. S., Micou-Eastwood J., Spector D., Przybyla A., Paterson B. Messenger RNA for myosin polypeptides: isolation from single myogenic cell cultures. Cell. 1977 Feb;10(2):265–273. doi: 10.1016/0092-8674(77)90220-3. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. pBR322 restriction map derived from the DNA sequence: accurate DNA size markers up to 4361 nucleotide pairs long. Nucleic Acids Res. 1978 Aug;5(8):2721–2728. doi: 10.1093/nar/5.8.2721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolford J. L., Jr, Rosbash M. The use of R-looping for structural gene identification and mRNA purification. Nucleic Acids Res. 1979 Jun 11;6(7):2483–2497. doi: 10.1093/nar/6.7.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]