Abstract
Background
We developed and validated a new and simple metric, the Programmatic Compliance Score (PCS), based on the IAS-USA antiretroviral therapy management guidelines for HIV-infected adults, as a predictor of all-cause mortality, at a program-wide level. We hypothesized that non-compliance would be associated with the highest probability of mortality.
Methods and Findings
3543 antiretroviral-naive HIV-infected patients aged ≥19 years who initiated antiretroviral therapy between January 1, 2000 and August 31, 2009 in British Columbia (BC), Canada, were followed until August 31, 2010. The PCS is composed by six non-performance indicators based on the IAS-USA guidelines: (1) having <3 CD4 count tests in the first year after starting antiretroviral therapy; (2) having <3 plasma viral load tests in the first year after starting antiretroviral therapy; (3) not having drug resistance testing done prior to starting antiretroviral therapy; (4) starting on a non-recommended antiretroviral therapy regimen; (5) starting therapy with CD4 <200 cells/mm3; and (6) not achieving viral suppression within 6 months since antiretroviral therapy initiation. The sum of these six indicators was used to develop the PCS score - higher score indicates poorer performance. The main outcome was all-cause mortality. Each PCS component was independently associated with mortality. In the mortality analysis, the odds ratio (OR) for PCS ≥4 versus 0 was 22.37 (95% CI 10.46–47.84).
Conclusions
PCS was strongly associated with all-cause mortality. These results lend independent validation to the IAS-USA treatment guidelines for HIV-infected adults. Further efforts are warranted to enhance the PCS as a means to further improve clinical outcomes. These should be specifically evaluated and targeted at healthcare providers and patients.
Introduction
HIV treatment has evolved tremendously since the advent of highly active antiretroviral therapy (HAART) in 1996 [1]–[9]. The goal of HAART is to decrease HIV-related morbidity and mortality through sustained full suppression of viral replication (i.e. plasma viral load <50 copies/mL). More recently, HAART has also been recognized as a highly effective strategy to prevent HIV transmission [10]. Definitive confirmation of the efficacy of HIV treatment as prevention was provided by the recent results of the HPTN 052 trial [11]. In this study, immediate use of HAART was shown to decrease HIV transmission by 96%. This has generated a renewed enthusiasm in the global roll out of HAART [12], [13]. However, it is still not clear which metrics, at the individual and the population levels, should be used to monitor and evaluate the impact of this powerful intervention.
HIV treatment guidelines provide evidence-based standards aimed to optimize the management of HIV infected individuals. However, for a variety of reasons, not all patients are fully adherent to these guidelines. Here, we developed and validated a composite metric, the Programmatic Compliance Score, to assess the impact of non-compliance with HIV treatment guidelines on all-cause mortality, among antiretroviral therapy-treated HIV-infected individuals within a fully subsidized, population-based antiretroviral therapy program. We hypothesized that non-compliance would be associated with the highest chance of dying prematurely.
Methods
Ethical Approval
The Centre's HIV/AIDS Drug Treatment program has received ethical approval from the University of British Columbia Ethics Review Committee at its St. Paul's Hospital site. The program also conforms with the province's Freedom of Information and Protection of Privacy Act.
HIV Patients on Treatment in British Columbia
This study was conducted using population data from the British Columbia (BC) Centre for Excellence in HIV/AIDS (the Centre) [7]. The Centre's guidelines have remained consistent with recommendations of the IAS-USA since 1996 and up to the latest guidelines in 2010 [1]–[9]. The details of this program have been described elsewhere [14]. In BC, medical and laboratory monitoring, including specialized tests such as CD4 cell counts, plasma viral load, genotypic resistance testing are all fully subsidized. The Centre has received ethical approval from the University of British Columbia Ethics Review Committee at the St Paul's Hospital site.
Study Population
Eligible study participants were ≥19 years old, naïve to antiretroviral therapy when they started treatment between January 1, 2000 and August 31, 2009. These patients were followed until death due to any cause, or if alive, until the last contact date or August 31, 2010, whichever came first. Finally, to be eligible for this analysis, participants were required to have at least one baseline CD4 cell count and a plasma viral load measurement available within six months prior to the antiretroviral starting date.
Laboratory Data
All plasma viral load measurements in BC are centrally done at the St Paul's Hospital virology laboratory. The quantification range of plasma viral load assays has evolved over time, as previously described. Thus, for analytical purposes, we truncated our measurements to range from <50 to >100,000 copies/mL. CD4 cell counts are measured by flow cytometry, followed by fluorescent monoclonal antibody analysis (Beckman Coulter, Inc., Mississauga, Ontario, Canada). The CD4 data come from different laboratories across BC, and, in our database, we capture >80% of all CD4 tests done in the province. HIV genotypic resistance testing is performed centrally at the Centre's laboratory. Samples have been assigned to one of four resistance categories based on a modification of the 2011 IAS-USA list of mutations [15].
Antiretroviral Regimen
The recommended antiretroviral therapy regimens have changed since 2000, based on the BC guidelines for treating HIV-positive adults, as in the IAS-USA guidelines [4]–[9]. Therefore, we developed rules to classify regimes as contemporary appropriate or not, as described in the Supplementary text.
The Programmatic Compliance Score
We developed six performance indicators based on the IAS-USA guideline recommendations during 2000–2010 [4]–[9]: (1) Having <3 (coded as 1) or ≥3 (coded as 0) CD4 cell count measurements in the first year after starting antiretroviral therapy; (2) Having <3 (coded as 1) or ≥3 (coded as 0) plasma viral load measurements in the first year after starting antiretroviral therapy; (3) Having a genotypic resistance test performed (coded as 0) or not (coded as 1) at baseline; (4) Initiating antiretroviral therapy with baseline CD4 cell count with <200 cells/mm3 (coded as 1) or ≥200 cells/mm3 (coded as 0); (5) Initiating antiretroviral therapy on a combination regimen recommended by contemporary guidelines (coded as 0) or not (coded as 1); and (6) Achieving viral suppression within 6 months of initiating antiretroviral therapy (coded as 0) or not (coded as 1). Viral suppression was defined by two consecutive plasma viral loads <50 copies/mL. Our main measure of exposure, the programmatic compliance score (PCS), was then obtained by adding the values for indicators 1 to 6, which provided a range from 0 (least compliance) to 6 (most non-compliance).
Outcome Measure and Statistical Analyses
The primary outcome in this study was all-cause mortality. Deaths occurring during the follow-up period were identified on a continuous basis through record linkages carried out with the BC Division of Vital Statistics and enhanced by direct physician reports to the program.
Further, we considered the following baseline explanatory variables: age, gender, history of injection drug use (IDU), year of antiretroviral therapy initiation, plasma viral load, follow-up time in months and place of residence at the start of antiretroviral therapy. Place of residence was used to control for the heterogeneity in patient treatment access, care and socio-demographic factors not previously defined. We also considered adherence to antiretroviral therapy measured at 12 months from antiretroviral therapy initiation, since the calculation of adherence at 6 months in our database can yield less precise estimates. Adherence was estimated by dividing the number of months of medications dispensed by the number of months of follow-up. We have previously shown that this measure of adherence is independently associated with HIV viral suppression and survival [16]–[18]. Adherence was categorized as either <95% or ≥95%.
We run two sets of analysis. The first set included data from patients who have started antiretroviral therapy between 2000 and 2009. The second analysis, recognizing that some of the variables were not collected systematically before the year 2006, we restricted our analysis from patients who have started antiretroviral therapy between 2006 and 2009.
In bivariable analyses, categorical variables were compared using the Fisher's exact test, and continuous variables were compared using the Wilcoxon rank-sum test. The mortality probability model was built in terms of finding the best predictive probabilistic model of mortality, using logistic regression [19]. First, we drew a random sample without replacement from the original data, splitting the original data in half. One half was used as a training dataset, in which we built the multivariable logistic model and assessed its fit by calculating the area under the receiver operating characteristic curve. This area was used to assess the model's ability to discriminate between those who died versus those who did not. A backward stepwise technique was used in the selection of covariates to build this model. The selection of variables was based on two criteria: Akaike Information Criterion (AIC) and Type III p-values. These two criteria balance the model choice by finding the best explanatory model (Type III p-values based on the Type III Sum of Squares, with lower p-values indicate more significance) and at the same time a model with the best goodness-of-fit statistic (AIC – lower values indicate better fit). At each step of this process, the AIC value and the Type III p-values of each variable were recorded, and the variable with the highest Type III p-value was dropped, until there are no more variables left. The final model has the lowest AIC. The second half of the data was used to assess whether the predictions based on the coefficients obtained from the analysis on the training dataset were valid or not. For goodness of fit, we calculated the mean squared error, the mean absolute error and the Wilcoxon rank-sum test. Kaplan-Meier method with log-rank test was used for the comparison of the unadjusted survival rates. All analyses were performed using SAS software version 9.2 (SAS, Cary, NC).
Results
Cohort Characteristics
A total of 3543 antiretroviral naïve adults (79% males) were eligible to participate in this study. At baseline, the median age was 42 years (25th to 75th percentile range [Q1–Q3]: 35–48 years), CD4 cell count was 190 cells/mm3 (Q1–Q3: 100–280 cells/mm3), and plasma viral load was 4.9 log10 copies/mL (Q1–Q3: 4.4–5.0 log10 copies/mL). The median follow-up was 44 months (Q1–Q3: 22–77 months). Of these patients, 39% had a history of IDU, 52% started antiretroviral therapy before 2006, and 63% were more than 95% adherent during the first year of follow-up.
Programmatic Compliance Score (PCS)
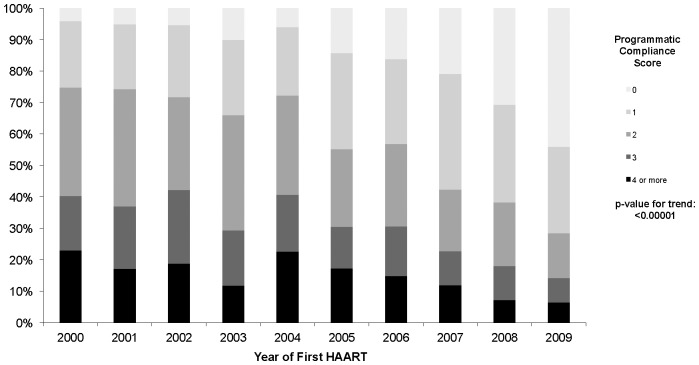
At baseline, 42% of patients did not receive a genotypic resistance test before therapy initiation, 11% of patients received a non-recommended antiretroviral therapy regimen, and 52% of patients had a baseline CD4 count <200 cells/mm3. During the first year of follow-up, 23% of patients had fewer than 3 CD4 count tests done, 16% had <3 plasma viral load tests done, and 46% did not achieve viral suppression within 6 months of beginning treatment. After several exploratory analyses, we decided to group the PCS as 0, 1, 2, 3 and 4 or more. The distribution of PCS was, then: 16% for a 0 score, 26% for a score of 1, 27% for a score of 2, 16% for a score of 3 and 14% for scores of 4 or more. Figure 1 shows the distribution of PCS over time, and as we moved from 2000 to 2009, individuals starting antiretroviral therapy on the later years were more likely to have a PCS score of 0 (p-value for trend <0.0001).
Figure 1. Distribution of the programmatic compliance score over time.
Predictive Mortality Probability Model (Follow-Up from 2000 to 2010)
At the end of follow-up, 499 (14%) deaths were recorded, producing an overall crude death rate of 33 per 1000 person-years. Table 1 shows the bivariable association of our main exposure, indicators and baseline factors with all cause mortality. Those who have died were more likely to be older (44 versus 41 years; p-value<0.0001), to have a shorter follow-up (23 versus 47 months; p-value<0.0001), to have a history of IDU (18% versus 11%; p-value<0.0001), to have adherence <95% during the first year on antiretroviral therapy (21% versus 10%; p-value<0.0001), and to have started antiretroviral therapy during 2000–2005 (21% versus 7% p-value<0.0001). All six indicators were strongly associated with all cause mortality, and therefore, those with a PCS ≥4 were more likely to have died in this study (38% versus 20%, 12%, 7% and 2% for scores 3, 2, 1 and 0, respectively; p-value<0.0001).
Table 1. Descriptive statistics by mortality status at the end of follow-up.
| Deceased at the End of Follow-up | |||
| List of Variables | No | Yes | p-value |
| N = 3044 | N = 499 | ||
| Programmatic Compliance Score | |||
| 0 | 565 (98%) | 13 (2%) | <0.0001 |
| 1 | 868 (93%) | 68 (7%) | |
| 2 | 845 (88%) | 111 (12%) | |
| 3 | 449 (80%) | 114 (20%) | |
| 4 or more | 317 (62%) | 193 (38%) | |
| Age | |||
| Median | 41 | 44 | <0.0001 |
| Q1–Q3 | 35–48 | 37–51 | |
| Follow-up (in months) | |||
| Median | 47 | 23 | <0.0001 |
| Q1–Q3 | 25–82 | 6–48 | |
| Gender | |||
| Male | 2431 (86%) | 385 (14%) | 0.1692 |
| Female | 613 (84%) | 114 (16%) | |
| Injection drug use history | |||
| No | 1918 (89%) | 248 (11%) | <0.0001 |
| Yes | 1126 (82%) | 251 (18%) | |
| Adherence during first year | |||
| ≥95% | 2008 (90%) | 216 (10%) | <0.0001 |
| <95% | 1036 (78%) | 283 (21%) | |
| Year of first ARV | |||
| 2000–2005 | 1455 (79%) | 381 (21%) | <0.0001 |
| 2006–2010 | 1589 (93%) | 118 (7%) | |
| Number of CD4 cell count measurements (1st year) | |||
| ≥3 | 2412 (89%) | 309 (11%) | <0.0001 |
| <3 | 632 (77%) | 190 (23%) | |
| Number of plasma HIV-1 RNA level measurements (1st year) | |||
| ≥3 | 2665 (90%) | 303 (10%) | <0.0001 |
| <3 | 379 (66%) | 196 (34%) | |
| Baseline resistance test | |||
| Yes | 1871 (91%) | 184 (9%) | <0.0001 |
| No | 1173 (79%) | 315 (21%) | |
| Baseline CD4 cell count (cells/mm3) | |||
| ≥200 | 1535 (91%) | 157 (9%) | <0.0001 |
| <200 | 1509 (81%) | 342 (18%) | |
| Recommended HAART regimen | |||
| Yes | 2718 (87%) | 422 (13%) | <0.0001 |
| No | 326 (81%) | 77 (19%) | |
| Suppression at 6 month | |||
| Yes | 1789 (93%) | 127 (7%) | <0.0001 |
| No | 1255 (77%) | 372 (23%) | |
| Health Region | |||
| Vancouver Coastal HA - City Center | 1961 (88%) | 273 (12%) | <0.0001 |
| Vancouver Coastal HA - DTES | 196 (78%) | 55 (22%) | |
| Vancouver Coastal HA - Other | 265 (90%) | 31 (10%) | |
| Interior HA | 118 (83%) | 25 (17%) | |
| Fraser HA | 170 (80%) | 42 (20%) | |
| Vancouver Island HA | 266 (82%) | 60 (18%) | |
| Northern HA | 68 (84%) | 13 (16%) | |
Notes: HA: Health Authority, Q1: 25th percentile; Q3: 75th percentile.
Table 2 shows the Kaplan Meier estimates for the mortality probability by levels of the PCS score. As expected, those individuals with a PCS ≥4 were at a much higher probability of mortality throughout the study period (log-rank test p-value<0.0001), with the crude mortality estimate ranging from 0% (SE ±0%) for PCS = 0 to 20.7% (SE ±1.8%) for PCS ≥4 at 6 months to 1.1% (SE ±0.5%) for PCS = 0 to 30.2% (SE ±2.2%) for PCS ≥4 at 30 months. To predict the probability of mortality for each patient in our study, we present the coefficients in Table 3. The measures used to assess the goodness of fit of our predictive model indicated that we have obtained a really well fitted model. Thus, the odds ratio (OR) for PCS from 1, 2, 3, 4 or more versus 0 were, respectively, 3.81 (95% CI 1.73–8.42), 7.97 (95% CI 3.70–17.18), 11.51 (95% CI 5.28–25.08), and 22.37 (95% CI 10.46–47.84). We were also interested in assessing the importance of each component in the PCS score on the probability of mortality and the strongest influence related to failing to suppress plasma viral load at 6 months (OR 4.25; 95% CI 3.15–5.75; area under the curve 0.668), having <3 plasma viral load tests during the first year (OR: 4.68; 95%CI 3.49–6.28; area under the curve 0.638) and having no resistance test done at baseline (OR: 2.40; 95%CI 1.83–3.15; area under the curve 0.608).
Table 2. Kaplan Meier estimates for the probability of mortality by the levels of the programmatic compliance score.
| List of Variables | Follow-up | Log-rank Test | |||
| 12 months | 18 months | 24 months | 30 months | p-value | |
| Number of CD4 cell count measurements (1st year) | |||||
| ≥3 | 2.5% (±0.3%) | 3.9% (±0.4%) | 4.8% (±0.4%) | 6.1% (±0.5%) | <0.0001 |
| <3 | 12.9% (±1.1%) | 14.5% (±1.2%) | 15.8% (±1.3%) | 17.0% (±1.3%) | |
| Number of plasma HIV-1 RNA level measurements (1st year) | |||||
| ≥3 | 1.9% (±0.3%) | 3.1% (±0.3%) | 4.1% (±0.4%) | 5.2% (±0.4%) | <0.0001 |
| <3 | 20.4% (±1.6%) | 23.1% (±1.8%) | 24.6% (±1.8%) | 26.4% (±1.9%) | |
| Baseline resistance test | |||||
| Yes | 3.6% (±0.4%) | 4.4% (±0.5%) | 5.0% (±0.5%) | 5.9% (±0.5%) | <0.0001 |
| No | 7.2% (±0.7%) | 9.4% (±0.8%) | 10.9% (±0.8%) | 12.6% (±0.9%) | |
| Baseline CD4 cell count (cells/mm3) | |||||
| ≥200 | 2.2% (±0.4%) | 3.1% (±0.4%) | 3.9% (±0.5%) | 4.9% (±0.6%) | <0.0001 |
| <200 | 7.8% (±0.6%) | 9.8% (±0.7%) | 10.9% (±0.7%) | 12.4% (±0.8%) | |
| Recommended cART regimen | |||||
| Yes | 3.2% (±0.5%) | 4.5% (±0.5%) | 4.6% (±0.6%) | 5.2% (±0.7%) | 0.0125 |
| No | 6.2% (±0.5%) | 8.0% (±0.6%) | 9.2% (±0.6%) | 10.7% (±0.7%) | |
| Suppression at 6 month | |||||
| Yes | 0.9% (±0.2%) | 1.6% (±0.3%) | 2.1% (±0.3%) | 2.8% (±0.4%) | <0.0001 |
| No | 9.9% (±0.7%) | 12.2% (±0.8%) | 13.8% (±0.9%) | 15.7% (±0.9%) | |
| Programmatic Compliance Score | |||||
| 0 | 0.4% (±0.3%) | 0.8% (±0.4%) | 0.8% (±0.4%) | 1.1% (±0.5%) | |
| 1 | 0.7% (±0.3%) | 1.4% (±0.4%) | 1.9% (±0.5%) | 2.6% (±0.6%) | |
| 2 | 1.8% (±0.4%) | 3.0% (±0.6%) | 4.0% (±0.7%) | 5.0% (±0.7%) | <0.0001 |
| 3 | 7.6% (±1.1%) | 10.3% (±1.1%) | 12.3% (±1.4%) | 14.8% (±1.6%) | |
| 4 or more | 23.6% (±1.9%) | 26.7% (±2.0%) | 28.4% (±2.1%) | 30.2% (±2.3%) | |
Table 3. Results from the predictive model for the probability of mortality based on the programmatic compliance score.
| Variables Necessary to Estimate the Probability of Mortality | Maximum Likelihood Estimates | |||
| Coefficient | Standard Deviation | Odds Ratio (95% Confidence Interval) | Type III P-value | |
| Programmatic Compliance Score | ||||
| 0 | 0.0 | 0.0 | 1(-) | <0.0001 |
| 1 | 1.3387 | 0.4042 | 3.81 (1.73–8.42) | |
| 2 | 2.0761 | 0.3916 | 7.97 (3.70–17.18) | |
| 3 | 2.4434 | 0.3974 | 11.51 (5.28–25.08) | |
| 4 or more | 3.1079 | 0.3878 | 22.37 (10.46–47.84) | |
| Also Adjust Model for: | ||||
| Intercept | −4.4132 | 0.5039 | <0.0001 | |
| Age (in years) | 0.0324 | 0.00742 | 1.03 (1.02–1.05) | <0.0001 |
| History of Injection Drug Use (Yes:1; No: 0) | 0.5298 | 0.153 | 1.70 (1.26–2.29) | 0.0005 |
| Follow-up time in Months | −0.0242 | 0.00265 | 0.98 (0.97–0.98) | <0.0001 |
Sensitivity Analysis
This analysis was restricted to the individuals who have started antiretroviral therapy between 2006 and 2009. In total, we observed 118 deaths (7%), producing an overall crude death rate of 30 per 1000 person-years. Table 4A shows the association of each component of the PCS score and all cause-mortality. Differently from the original analysis, the top three PCS components most influential on the probability of mortality were having <3 plasma viral load tests during the first year (OR: 7.54; 95%CI 5.10–11.16; area under the curve 0.691), failing to suppress plasma viral load at 6 months (OR: 4.66; 95%CI 3.04–7.14; area under the curve 0.680) and starting on a non-recommended antiretroviral therapy (OR: 3.78; 95%CI 2.49–5.75; area under the curve 0.657). The multivariable model fitted for this analysis aimed at finding whether PCS explains the risk of mortality, while adjusting for the same covariates as in the case of the original analysis (Table 4B). The OR for PCS from 1, 2, 3, 4 or more versus 0 were, respectively, 3.02 (95% CI 1.16–7.89), 5.01 (95% CI 1.92–13.06), 9.02 (95% CI 3.44–23.64), and 15.77 (95% CI 6.28–39.61).
Table 4. Relationship between the programmatic compliance score and mortality.
| (A) | ||
| Bivariable Analysis | ||
| List of Variables | Odds Ratio (95% Confidence Interval) | Area Under the Curve |
| Number of CD4 cell count measurements (1st year) | ||
| ≥3 | 1(-) | 0.649 |
| <3 | 3.87 (2.65–5.67) | |
| Number of plasma HIV-1 RNA level measurements (1st year) | ||
| ≥3 | 1(-) | 0.691 |
| <3 | 7.54 (5.10–11.16) | |
| Baseline resistance test | ||
| Yes | 1(-) | 0.594 |
| No | 2.49 (1.69–3.67) | |
| Baseline CD4 cell count (cells/mm3) | ||
| ≥200 | 1(-) | 0.527 |
| <200 | 1.85 (1.04–3.28) | |
| Recommended HAART regimen | ||
| Yes | 1(-) | 0.657 |
| No | 3.78 (2.49–5.75) | |
| Suppression at 6 month | ||
| Yes | 1(-) | 0.680 |
| No | 4.66 (3.04–7.14) | |
(A) Bivariable associations between each of programmatic compliance score components and mortality for individuals who started antiretroviral therapy between 2006 and 2009. (B) Results from the multivariable explanatory model for the probability of mortality based on the programmatic compliance score for individuals who started antiretroviral therapy between 2006 and 2009. Area Under the Curve: 0.896.
Discussion
Using various indicators of non-compliance to treatment guidelines, we developed a simple and highly predictive metric, the Programmatic Compliance Score or PCS, to predict the probability of mortality among HIV-positive individuals starting naïve on antiretroviral therapy. We found that individuals who had a PCS score 4 or higher had a mortality probability 22 times higher than those individuals with PCS score 0. The sensitivity analysis also confirmed these results. The PCS, therefore, may serve as a simple but powerful proxy for the performance of the program as a whole in achieving execution of its accepted guidelines. The PCS incorporates the overall effects of the decisions and social situations of patients, physicians and others in whether or not treatment guidelines are actually implemented.
It is noteworthy and surprising that indicators associated with plasma viral load and resistance were far more impactful than suboptimal CD4 cell count at the start of therapy with respect to the probability of mortality. Low CD4 cell count at the start of antiretroviral therapy has been shown by our group and by others to be highly predictive of adverse treatment outcomes [20]–[22]. Closer monitoring of patients during their first year on antiretroviral therapy, especially plasma viral load, can increase the chances of these individuals to fully benefit from this life-saving therapy at the short- and long terms. The reasons for poor patient monitoring practices are not clear, especially in an environment in which HIV treatment, care and laboratory monitoring are fully subsidized. Further efforts are warranted to explore possible reasons for this at the health care provider and patient levels. This in turn may assist in the development of specific strategies to enhance the PCS as a means to further improve clinical outcomes. These should be specifically evaluated and targeted to health care providers as well as HIV infected clients. Furthermore, our results provide important clues on how to develop effective strategies to improve HIV associated health outcomes not only in BC, but around the world.
There are several features of our study that should be highlighted. First, this study was based on patients who were naïve to antiretroviral therapy, thus our results were not confounded by previous therapy use. Secondly, despite the potential limitations of using pharmacy refill-based adherence as a surrogate marker of actual pill taking, we have previously shown that this measure of adherence is independently associated with different disease outcomes [16]–[18]. Thirdly, delayed reporting of deaths or incomplete data collection are not likely an issue with this analysis, since all deaths were reported within three months of death through active follow-up with physicians and hospitals and regular linkages to BC Vital Statistics Agency. Fourthly, even though the guidelines prior to 2006 did not explicitly say that baseline resistance should be done prior to starting antiretroviral therapy, we decided to include this indicator in our PCS score because transmitted drug resistance has always been one of the factors that affect future disease outcomes. The PCS score is not an indicator to penalize healthcare providers, but it serves to identify areas in our treatment program that should be improved. It is important to look at temporal trends (2000–2009) so we can identify which indicators have improved and those that have not. Due to missing data in some of the indicators present in the PCS score for patients starting antiretroviral therapy during 2000–2005, we re-run the analysis including only data from patients who started antiretroviral therapy after the year 2006. Baseline resistance testing continued to be an important indicator of programmatic compliance. Fifthly, we acknowledge that the model only uses information collected during the first year on therapy, and not information on the subsequent years to predict the risk of mortality. The first year on antiretroviral therapy is really important in a patient's treatment history. If these individuals are not properly managed in the first year, we believe that it is likely that they will not be managed properly thereafter. Thus, the PCS score was developed so that we want to catch individuals with high risk of mortality due to improper disease management in the beginning of treatment, and we want to identify the areas in which our program that can be improved. Sixthly, one of the indicators in our PCS score is baseline CD4 cell count. Some may argue that is not a measure of non-compliance, but rather a biological measure of disease severity. Based on the cascade of care framework, individuals who have tested positive for HIV should be monitored from the time they get their positive test result until the time they become eligible to receive antiretroviral therapy. In BC, as in many other places around the world, over the years, we have lost many patients in this period of time. These individuals lost to follow-up after they test positive have most often showed up in our emergence departments with, sometimes, a CD4 cell count almost close to zero. This is completely unacceptable. Thus, CD4 cell count at antiretroviral presentation is an indicator of the performance of our system, and not only a biological indicator. Seventhly, although we adjusted our analyses for several demographic and clinical characteristics, as in all observational studies unmeasured differences may exist among study populations, and for this reason, our findings should be interpreted cautiously. Finally, given that this study was conducted at the population-level within a fully subsidized medical system where antiretroviral therapy as well as medical and laboratory monitoring are free of charge to all participants, we are confident that our results are less likely to be biased by direct financial limitations to access to health services, a frequent confounder in cohort and population based studies.
In summary, the Programmatic Compliance Score or PCS metric is highly predictive of all-cause mortality, among HIV infected adults starting antiretroviral therapy. Our results show that individuals with sub-optimal PCS compliance are at a very high probability of premature morbidity and mortality. It is important to mention that the requirement for having a baseline genotypic testing before starting antiretroviral therapy was not explicitly stated in the guidelines prior to 2006, and only emphasized in the guidelines after 2006. However, given that the efficacy of antiretroviral therapy is directly related to having a fully functional regimen, there is no reason for failing to order such an important test, given that it is free for anyone starting antiretroviral therapy in BC. Thus, while these results do not allow us to establish a causal relationship regarding the association between our new metric and survival, this metric highlights the importance of adherence to treatment and monitoring guidelines during the first year on antiretroviral therapy. Despite improvement in the PCS within our cohort over time, it is clear that there is still substantial room for improvement. It should be emphasized that minimizing the occurrence of these non-compliance practices is not solely dependent on the healthcare provider. Health administrative bodies can improve compliance outcomes through regular surveillance and ongoing physician training. Further, the individual patient ultimately bears responsibility for his/her own health; the implications of poor compliance to treatment need to be communicated at the time of initiation. Furthermore, our results provide important clues on how to develop effective strategies to improve HIV associated health outcomes not only in BC, but around the world. Finally, our results also lend independent validation to the most recent IAS-USA antiretroviral therapy management guidelines for HIV infected adults.
Supporting Information
Appropriate Regimens based on the BC guidelines for treating HIV-positive adults between 2000 and 2010.
(DOCX)
Acknowledgments
We thank the patients of the Drug Treatment Program, and the staff at the BC Centre for Excellence for HIV/AIDS for maintaining and updating our database.
Funding Statement
During this work, VDL was supported by the Canadian Institutes of Health Research and the Michael Smith Foundation for Health Research through Fellowship Awards. BN is a Canadian Institutes of Health Research Bisby Fellow. JSGM is supported by the Ministry of Health Services, Province of British Columbia, through a Knowledge Translation Award from the Canadian Institutes of Health Research and through an Avant-Garde Award (No. 1DP1DA026182-01) from the National Institute on Drug Abuse, at the United States National Institutes of Health. PRH is supported by CIHR/GSK Research Chair in Clinical Virology. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Carpenter CC, Fischl MA, Hammer SM, Hirsch MS, Jacobsen DM, et al. (1996) Antiretroviral therapy for HIV infection in 1996. Recommendations of an international panel. International AIDS Society-USA. JAMA 276: 146–154. [PubMed] [Google Scholar]
- 2. Carpenter CC, Fischl MA, Hammer SM, Hirsch MS, Jacobsen DM, et al. (1997) Antiretroviral therapy for HIV infection in 1997. Updated recommendations of the International AIDS Society-USA panel. JAMA 277: 1962–1969. [PubMed] [Google Scholar]
- 3. Carpenter CC, Fischl MA, Hammer SM, Hirsch MS, Jacobsen DM, et al. (1998) Antiretroviral therapy for HIV infection in 1998: updated recommendations of the International AIDS Society-USA Panel. JAMA 280: 78–86. [DOI] [PubMed] [Google Scholar]
- 4. Carpenter CC, Cooper DA, Fischl MA, Gatell JM, Gazzard BG, et al. (2000) Antiretroviral therapy in adults: updated recommendations of the International AIDS Society-USA Panel. JAMA 283: 381–390. [DOI] [PubMed] [Google Scholar]
- 5. Yeni PG, Hammer SM, Carpenter CC, Cooper DA, Fischl MA, et al. (2002) Antiretroviral treatment for adult HIV infection in 2002: updated recommendations of the International AIDS Society-USA Panel. JAMA 288: 222–235. [DOI] [PubMed] [Google Scholar]
- 6. Yeni PG, Hammer SM, Hirsch MS, Saag MS, Schechter M, et al. (2004) Treatment for adult HIV infection: 2004 recommendations of the International AIDS Society-USA Panel. JAMA 292: 251–265. [DOI] [PubMed] [Google Scholar]
- 7. Hammer SM, Saag MS, Schechter M, Montaner JS, Schooley RT, et al. (2006) Treatment for adult HIV infection: 2006 recommendations of the International AIDS Society-USA panel. JAMA 296: 827–843. [DOI] [PubMed] [Google Scholar]
- 8. Hammer SM, Eron JJ Jr, Reiss P, Schooley RT, Thompson MA, et al. (2008) Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA 300: 555–570. [DOI] [PubMed] [Google Scholar]
- 9. Thompson MA, Aberg JA, Cahn P, Montaner JS, Rizzardini G, et al. (2010) Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA 304: 321–333. [DOI] [PubMed] [Google Scholar]
- 10. Montaner JS, Hogg R, Wood E, Kerr T, Tyndall M, et al. (2006) The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet 368: 531–536. [DOI] [PubMed] [Google Scholar]
- 11. Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, et al. (2011) Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med 365: 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNeil DG Jr (2011) Clinton Aims for ‘AIDS-Free Generation. The New Work Times website. Available: http://www.nytimes.com/2011/11/09/health/policy/hillary-rodham-clinton-aims-for-aids-free-generation.html. Accessed 2012 May 30.
- 13.Office of National AIDS Policy (2012) The White House Website. Available: http://www.whitehouse.gov/administration/eop/onap. Accessed 2012 May 30.
- 14. Montaner JS, Lima VD, Barrios R, Yip B, Wood E, et al. (2010) Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet 376: 532–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson VA, Brun-Vézinet F, Clotet B, Günthard HF, Kuritzkes DR, et al. (2010) Update of the drug resistance mutations in HIV-1: December 2010. Top HIV Med 18: 156–163. [PubMed] [Google Scholar]
- 16. Lima VD, Harrigan R, Bangsberg DR, Hogg RS, Gross R, et al. (2009) The combined effect of modern highly active antiretroviral therapy regimens and adherence on mortality over time. J Acquir Immune Defic Syndr 50: 529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lima VD, Harrigan R, Murray M, Moore DM, Wood E, et al. (2008) Differential impact of adherence on long-term treatment response among naïve HIV-infected individuals. AIDS 22: 2371–2380. [DOI] [PubMed] [Google Scholar]
- 18. Wood E, Hogg RS, Lima VD, Kerr T, Yip B, et al. (2008) Highly active antiretroviral therapy and survival in HIV-infected injection drug users. JAMA 300: 550–554. [DOI] [PubMed] [Google Scholar]
- 19.Steyerberg EW (2010) Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating (Statistics for Biology and Health). Springer Science+Business Media, LLC.
- 20. Cescon AM, Cooper C, Chan K, Palmer AK, Klein MB, et al. (2011) Factors associated with virological suppression among HIV-positive individuals on highly active antiretroviral therapy in a multi-site Canadian cohort. HIV Med 12: 352–360. [DOI] [PubMed] [Google Scholar]
- 21. Moore DM, Harris R, Lima V, Hogg B, May M, et al. (2009) Effect of baseline CD4 cell counts on the clinical significance of short-term immunologic response to antiretroviral therapy in individuals with virologic suppression. J Acquir Immune Defic Syndr 52: 357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sterne JA, May M, Costagliola D, de Wolf F, Phillips AN, et al. (2009) Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet 373: 1352–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appropriate Regimens based on the BC guidelines for treating HIV-positive adults between 2000 and 2010.
(DOCX)