Abstract
Background and Purpose
Examining participant-perceived change in walking provides insight into whether changes were meaningful for participants. This study examined the relationships between change scores in standardized walking outcomes and ratings of perceived change following exercise post-stroke.
Methods
Self- and fast-paced gait speed and 6-Minute Walk Test (6MWT) distance were assessed in 22 participants (age 67±10.3 years, 1.8±0.9 years post-stroke) before and after a 3-month exercise program. Perceived changes were evaluated using a 15-point Likert scale. Correlation analyses between measured and perceived change were performed. Subgroups of low and high baseline scores were compared for differences in measured and perceived change.
Results
6MWT change was correlated with perceived change (ρ=0.52, P=0.01), greater change was demonstrated among participants who perceived improvement relative to those who did not (difference 34.4 meters (95% CI 17.2, 51.6), P=0.04). After controlling for measured change, participants with low baseline 6MWT distances perceived less change compared to those who walked high distances at baseline (P=0.006).
Discussion and Conclusions
A global rating scale using meaningful and context-specific questions was used to determine the relationship between measured and participant-perceived change in 6MWT distance. A meaningful difference in 6MWT change was observed between participants who did and those who did not perceive improvement. Individuals with lower baseline scores may require larger changes in walking distance to perceive that a change has occurred. This study contributes to a growing body of evidence about the relationships between perceived and measured change in function, and is a step in determining thresholds for perceived change in walking after stroke.
Introduction
The most important goal cited by people living with stroke is to improve walking function.1, 2 Mobility interventions, including community exercise programs for people with stroke, are effective in improving walking ability, as demonstrated through randomized controlled trials using standardized outcome measures. In meta-analyses of overground physical therapy gait training3 and intensive gait and mobility training after stroke,4 such interventions are effective in improving walking speed and endurance (measured with the Six-Minute Walk Test, 6MWT). Additionally, in an 8-week non-controlled trial, community exercise for people with stroke was effective in improving gait speed.5 Most recently, results from the Locomotor Experience Applied Post-Stroke (LEAPS) trial demonstrated that both body-weight supported treadmill training and traditional physical therapy intervention resulted in positive benefit to walking speed and endurance.6
While these studies have demonstrated significant intervention-related benefits, Beaton and colleagues7 emphasized that statistical significance may not necessarily result in clinically relevant or important changes. Duncan and colleagues6 did report that more than 50% of study participants in the LEAPS trial exceeded thresholds for meaningful change in these areas. Although there are different approaches to identifying clinically important change, the participants’ perspectives are considered paramount as indicators of whether or not interventions were meaningful to the consumer. Whether measured changes in walking are perceived by study participants are not well understood.
Broad-based, health-related quality of life questionnaires have been used to evaluate self-reported change after exercise, but often in the form of summary scores for overall physical function rather than dedicated questions that specifically relate to measures of walking. Global rating scales may offer a reasonable alternative to health-related quality of life questionnaires. These are anchor-based methods for evaluating change in an individual’s health status over time, whereby the degree of change corresponds to an external criterion, or anchor.8 They are easy to administer and interpret9 and have been used to determine minimal clinically important change in symptom severity for other populations, including chronic obstructive pulmonary disease,8, 10 carpal tunnel syndrome11 and low back pain populations.12
A commonly-used global rating scale is the 15-point Likert scale, wherein 0 represents the middle anchor of “No change” and the scale spans from +7 “A very great deal better” to −7 “A very great deal worse”.8 This scale was used to establish small and meaningful changes post-exercise in older adults (0.05 m/s for gait speed and 20 m for 6MWT distance).13 While this study included a cohort of participants with stroke, global rating scale data for perceived change were not available for this subgroup.
The relationships between perceived changes using a global rating scale and measured changes in physical function have not been extensively studied. Examining these associations are important for understanding the broad benefits of mobility interventions among various populations – physical and perceived changes – particularly with those with limited walking ability. Fulk and colleagues14 used the 15-point global rating scale to establish clinically important change in gait speed among individuals completing outpatient stroke rehabilitation intervention. A positive correlation was reported between perceived change in physical endurance and change in measures of cardiorespiratory fitness following an exercise program for older adults,15 which suggests that individuals have the capacity to perceive measured changes in function. One study reported a correlation between perceived benefit of peroneal nerve stimulation and measured reduction in walking effort (physiological cost index) among 20 participants with drop-foot (n=13 with stroke),16 which suggests that individuals may be able to relate perceived changes in walking to actual walking effort. In individuals with chronic obstructive pulmonary disease, perceived changes in the endurance shuttle walking test were positively correlated with measured changes in performance.17
To date, only a few studies have examined the relationships between measured and perceived changes in walking ability following exercise interventions for people with stroke. In addition, the majority of previous studies evaluated the perceived change with respect to a laboratory-based walking test and did not attempt to determine the perceived changes in the context of every day walking activities in one’s own environment. By understanding participants’ perceptions of the benefits derived, we may gain important insight into whether interventions result in meaningful benefits to the consumers. Perceived changes in function may have a greater influence on the engagement of physical activity than the actual change measured on performance-based outcome measures. This is important for understanding whether the intended benefits of improved mobility and community participation are truly realized.
The purposes of this study were to 1) establish the relationships between measured change scores in standardized outcome measures of walking ability (gait speed and 6MWT) and self-reported (perceived) change after participating in a community-based stroke exercise program, and 2) examine whether baseline scores influence measured and perceived change. Results from this study add to the understanding of these outcomes measure by determining if a given change is sufficient for participants to notice a change in their abilities.
Methods
This study utilized participants from one site of a larger multi-site trial that examined the effectiveness of a multi-component community-based participation program for individuals with stroke. Study procedures were approved by local university and hospital research ethics boards. Informed written consent was obtained from all participants. The main trial used a randomized lag-control design, where both groups received the same intervention but start points were offset by 3 months. For the purposes of the current study, both groups were collapsed into a single group for analysis of changes before and after exercise.
Participants
Participants were eligible for the study if they were at least 6 months post-stroke, living in the community, had completed rehabilitation and were able to follow 3-step commands. Exclusion criteria were neurological conditions other than stroke, or significant musculoskeletal conditions (e.g. rheumatoid arthritis).
Intervention
The community program was conducted as group classes of 10 to 15 participants. Twice-weekly 2-hour classes were comprised of 1 hour each of Group Exercise and Leisure Activities. Group Exercise consisted of moderate exercise including stretching, walking and other functional activities (such as sit-to-stand, step-ups on low rises, obstacle course) aimed at improving strength, walking and balance. Leisure Activities included socialization, games and conversation.
Assessments
Participant demographics were recorded, including age, sex and details of stroke (time post event, type of stroke, side of hemiparesis). Use of gait aids was noted.
Measured change in walking ability
Assessments conducted before and after 3 months of the community participation program included:
Self- and fast-paced 5-meter gait speed
Comfortable and maximal gait speeds were performed over a 9-meter distance, where the middle 5 meters were timed. Gait aids were permitted. The same gait aids were used at both time points. The average of 2 trials at each pace was determined. High test-retest reliability has been reported for comfortable- and fast-paced gait speed measures among people with stroke.18
6-Minute Walk Test (6MWT)
Standardized instructions19 were given to walk as far as possible in 6 minutes over a 42-meter square course. Gait aids were permitted. The same gait aids were used at both data collection time points. The 6MWT has high test-retest,18, 20, 21 inter- and intra-rater reliability22 and concurrent validity with VO2peak21 among individuals with stroke.
Perceived change in walking ability
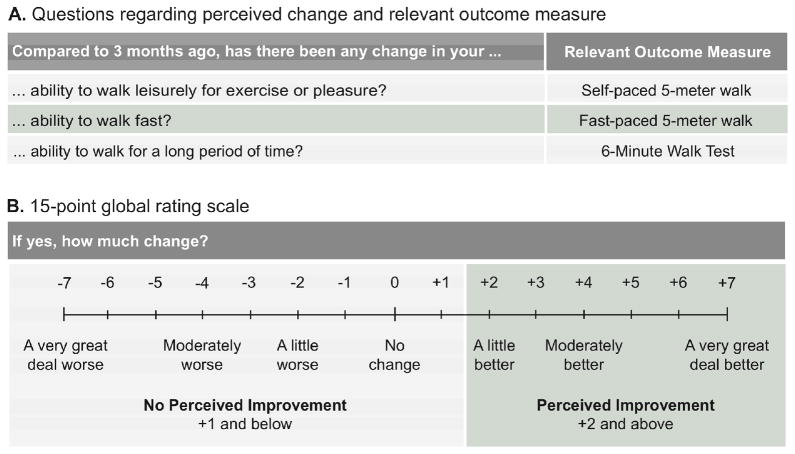
After 3 months of the program, participants were asked to rate perceived changes in walking ability relative to their status at the beginning of the program using a 15-point Likert scale,8 as illustrated in Figure 1 (verbal anchors provided for each point on the Likert scale8 are not shown) Figure 1 also outlines the specific questions posed and corresponding outcome measure. The questions were designed to relate closely with each measure in a functionally relevant context. For example, self-paced gait speed was believed to represent leisurely walking activities, and thus the question was posed accordingly.
Figure 1.
Determining perceived change using A) questions, and B) a 15-point global rating scale
Analysis
Descriptive statistics were performed. Paired t-tests were used to compare measured changes in walking ability over time.
Relationships between measured and perceived change in walking ability
To determine the relationships between measured change and perceived change, two analyses were performed. Firstly, Spearman correlation analyses quantified the relationships between change scores from the outcome measures (post score minus pre) and participants’ ratings of perceived change. Secondly, the sample was divided into 2 groups based on the lowest threshold for perceiving improvement, where Perceived Improvement group was defined as those whose self-reported change was +2 “A little better” or higher, and the No Perceived Improvement group for those with self-ratings were +1 “Almost the same, hardly any better at all” or lower13 (Figure 1). Then, Mann-Whitney U tests compared change in the performance measures between the Perceived Improvement and No Perceived Improvement groups.
Differences based on baseline scores
To determine whether baseline scores influenced differences in measured or perceived change, the sample was dichotomized into groups of Low or High baseline score. Cutpoints of 0.8 m/s (minimum speed requirement for independent community ambulation after stroke) and 288m (0.8 m/s * 6 minutes) divided low and high baseline groups for self-paced gait speed23 and 6MWT distance, respectively. For fast-paced walk, 1.38 m/s was used to divide low and high baseline groups, based on the minimum speed required to cross the intersection of faster walk signals.24 One-way analyses of covariance were performed to compare between-group differences in perceived change for each variable, while controlling for measured change (covariate).
Statistical Package for the Social Sciences (Version 17.0, Chicago IL) was used with a significance level of P<0.05.
Results
Twenty-two participants (n=13 men, mean ± SD age 67 ± 10.3 years, time post stroke 1.8 ± 0.9 years, Montreal Cognitive Assessment Score 23.7 ± 4.4) completed 3 months of the community participation program. Ten (45%) participants did not use gait aids for walking, 9 (41%) used canes and 3 (14%) used walkers. Walking speeds and 6MWT distances at each time point, and measured and perceived changes are presented in Table 1. Significant improvements were demonstrated in self- and fast-paced walk and 6MWT distance.
Table 1.
Scores from measures of walking ability
| Variable | Baseline | 3 Months | Measured Change scores | P-value for measured change over time* | Perceived change |
|---|---|---|---|---|---|
| Ability to walk leisurely | |||||
| Self-paced 5-m gait speed, m/s | 0.83 ± 0.42 (0.12, 1.52) | 0.89 ± 0.46 (0.14, 1.7) | 0.06 ± 0.13 (−0.22, 0.32) | 0.03** | 1.9 ± 2.2 (−2, +6) |
| Ability to walk fast | |||||
| Fast-paced 5-m gait speed, m/s | 1.16 ± 0.64 (0.16, 2.4) | 1.22 ± 0.71 (0.16, 2.54) | 0.06 ± 0.14 (−0.18, 0.41) | 0.04** | 1.5 ± 2.2 (−2, +7) |
| Ability to walk for long period of time | |||||
| 6MWT distance, m | 297.9 ± 175.3 (39, 593) | 320.9 ± 179.8 (44, 628) | 23.0 ± 41.1 (−53, 105) | 0.02** | 1.4 ± 2 (−2, 5) |
Values are mean ± SD (min, max). Abbreviation: 6MWT – 6-Minute Walk Test
Paired t-tests were used to compare the mean baseline scores to mean scores at 3 months
P<0.05
Relationships between measured and perceived change in walking ability
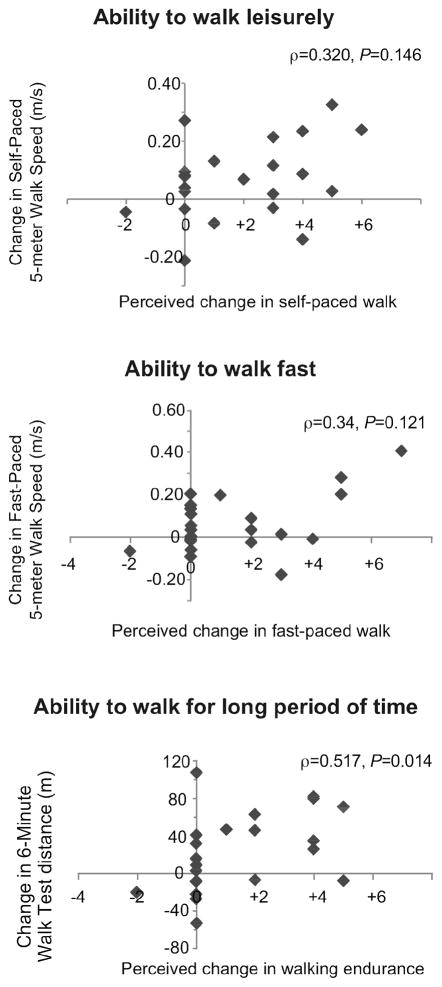
There were no correlations observed between measured and perceived change in self- (ρ=0.32, P=0.15) and fast-paced gait speeds (ρ=0.34, P=0.12). The change in 6MWT distance was moderately correlated with self-reported change in ability to walk for long periods of time (ρ=0.52, P=0.01). The relationship between measured and perceived change is illustrated in Figure 2.
Figure 2.
Relationships between Measured and Perceived change
The positive relationship between measured and perceived change in 6MWT ability was supported by the group comparison between the Perceived Improvement (self-ratings ≥ +2) and No Perceived Improvement (≤ +1) groups, where improvement in 6WMT distance was higher in the Perceived Improvement group by 34.4 meters (95% CI 17.2, 51.6) relative to the No Perceived Improvement group (43.3 ± 34.3 versus 8.9 ± 40.7 meters respectively, Mann-Whitney U=27, P=0.04) (Table 2). There were no group differences in change scores in any of the other domains.
Table 2.
Comparison of “No Perceived Improvement” versus “Perceived Improvement” groups
| No Perceived Improvement Group | Perceived Improvement Group | P-value* | |
|---|---|---|---|
| Ability to walk leisurely | |||
| n | 11 | 11 | |
| Self-paced 5-m walk speed change, m/s | 0.03 ± 0.13 (−0.22, 0.27) | 0.1 ± 0.14 (−0.14, 0.32) | 0.30 |
| Ability to walk fast | |||
| n | 13 | 9 | |
| Fast-paced 5-m walk speed change, m/s | 0.05 ± 0.1 (−0.9, 0.2) | 0.09 ± 0.18 (−0.18, 0.41) | 0.60 |
| Ability to walk long period of time | |||
| n | 13 | 9 | |
| 6MWT distance change, m | 8.9 ± 40.7 (−53, 105) | 43.3 ± 34.3 (−7, 82) | 0.04** |
No Perceived Improvement Group where Self-reported change +1 and below; Perceived Improvement Group where Self-reported change +2 and above. Values are n or mean ± SD (min, max). Abbreviation: 6MWT – 6-Minute Walk Test.
Mann-Whitney U tests were performed to compare measured change in each outcome between the Perceived versus No Perceived Improvement groups
P<0.05
Differences based on baseline scores
The results of subgroup comparisons based on High versus Low baseline scores are given in Table 3. After adjusting for measured change scores in 6MWT, there were group differences in perceived change in ability to walk long distances (F(1,19)=9.61, P=0.006), wherein those exhibiting lower function at baseline perceived less change compared to those starting at higher baseline scores. Notably, the Low baseline group perceived less change despite demonstrating a similar or greater amount of measured change relative to the High baseline group.
Table 3.
Comparison of Perceived change between groups with Low versus High baseline scores, while controlling for Measured change scores
| Baseline scores | Measured Change | Perceived Change (unadjusted) | Perceived Change (adjusted) | P-value* |
|---|---|---|---|---|
| Ability to walk leisurely | ||||
| Low: Self-paced 5-m gait speed < 0.8 m/s, n=9 | 0.07 ± 0.11 (−0.05, 0.32) | +1.7 ± 2.3 (−2, +5) | 1.62 ± 0.71 | 0.60 |
| High: Self-paced 5-m gait speed ≥ 0.8 m/s, n=13 | 0.06 ± 0.15 (−0.22, 0.27) | +2.1 ± 2.2 (0, +6) | 2.11 ± 0.59 | |
| Ability to walk fast | ||||
| Low: Fast-paced 5-m gait speed < 1.38 m/s, n=14 | 0.03 ± 0.11 (−0.18, 0.28) | +1.1 ± 1.8 (−2, +5) | 1.37 ± 0.55 | 0.81 |
| High: Fast-paced 5-m gait speed ≥ 1.38 m/s, n=8 | 0.13 ± 0.15 (−0.09, 0.41) | +2.1 ± 2.8 (0, +7) | 1.61 ± 0.75 | |
| Ability to walk long period of time | ||||
| Low: 6MWT distance < 288m, n=12 | 20.6 ± 42.3 (−27, 105) | +0.4 ± 1.7 (−2, +5) | 0.47 ± 0.45 | 0.006** |
| High: 6MWT distance ≥ 288m, n=10 | 25.8 ± 41.7 (−53, 82) | +2.6 ± 1.8 (0, +5) | 2.54 ± 0.49 | |
Values are mean ± SD (min, max) for unadjusted means, mean ± SE for adjusted means. Abbreviation: 6MWT – 6-Minute Walk Test
Analyses of covariance were performed to compare perceived changes while controlling for measured change (covariate)
P<0.05
Discussion
A novel feature of this study was the comparison of participant-perceived changes to measured change in walking ability following a community exercise program for people with stroke. Two other studies have examined self-ratings of perceived change in walking ability, but these were performed with a cohort of individuals with chronic obstructive pulmonary disease17 or a mixed sample of participants with drop-foot wherein only a portion were persons with stroke.16 Specific to stroke, earlier studies have examined change from clinicians’ perspectives25 or used distribution-based methods20, 26, 27 to determine minimal detectable change (where statistical significance is used to evaluate change, relative to the probability of observed change by random chance12). The current study contributes to a developing body of evidence examining the relationships between self-perceptions of change and measured changes in walking after stroke. Fulk and colleagues14 used an anchor-based method to establish clinically important change in self-selected gait speed after outpatient stroke rehabilitation. Ours is the first study to use a global rating scale with people with stroke to determine participant-perceived changes in multiple dimensions of walking following a community exercise program.
Using two different methods, we demonstrated a relationship between measured change in 6MWT and perceived ability to walk long distances. Of note, the difference of 34.4 meters (95% CI 17.2, 51.6) in 6MWT distance between the Perceived Improvement and No Perceived Improvement groups exceeds the 20 meters established using anchor-based methods as a small yet meaningful change in 6MWT distance in older adults.13 In contrast, despite demonstrating statistically significant improvements in gait speed, study participants were not able to detect that changes had occurred following participation in the program. Potentially, the measured change in self-selected gait speed (0.06 m/s) may, in fact, not have reached the threshold to be clinically meaningful for this population. Indeed, the change measured is less than the 0.1626 to 0.2027 m/s values reported for clinically detectable change in self-selected gait speed in individuals with stroke.
Our results are aligned with those reported by Pepin and colleagues,17 who also reported a positive association between self-ratings of perceived change and measured change in the endurance shuttle walk test in individuals with chronic obstructive pulmonary disease. A key difference between Pepin and colleagues17 and the current study lies in how perceived change was evaluated: the former study asked how participants rated their perceived performance on a specific outcome measure, whereas we inquired whether participants noted changes in various aspects of walking representative of daily activities. By asking our participants to gauge changes in their “ability to walk for a long period of time”, we evaluated perceived change that reflects the intended aim of the 6MWT, as well as potential meaningful changes in walking that our participants may have experiences in the context of every day activities. Our approach provided an estimate of the functional relevance of intervention-derived benefits to walking.
Thus, to more accurately evaluate self-reported changes in walking ability following mobility interventions, meaningful and context-specific questions are required that reflect the components of walking that is being evaluated. In the current study, we attempted to achieve this by carefully considering the functional context of each outcome measure examined. Since we did not observe an association between measured changes in gait speed from the 5-meter walk test and perceived changes in the ability to walk leisurely or to walk fast, the relevant questions may require further refinement to better reflect the intention of the test (e.g. “… has there been any change in your ability to walk at a leisurely/fast pace?”). Future research in this area may further elucidate this.
The other key and novel finding from this study was that, after controlling for measured change scores, participants with lower distances walked at baseline on the 6MWT perceived less change compared to those with higher distances walked. These results suggest that, with participants who start at a lower functional level, a greater magnitude of measured change may be required for them to perceive the change. Similarly, in a study by Beninato and colleagues,25 attending physicians on a stroke unit were asked to rate their perceptions of the changes in the Functional Independence Measure demonstrated by their patients, and those with lower admission scores needed to demonstrate larger change scores to be detected. These results are consistent with the current study in that, among individuals starting at lower baseline scores, measured changes in a test of walking endurance may not result in perceived improvements in the ability to walk long distances. Alternatively, it is possible that the community intervention was not sufficient to result in changes in 6MWT performance that exceeded the minimum threshold that would be perceived by the study participants. Secondary analysis revealed that, of the 12 participants who were in the Low baseline group for 6MWT, change scores in 9 (75%) participants were less than the 54m cited as the minimally detectable change among individuals with stroke.20
Study limitations
The limitations of this study must be acknowledged. Firstly, the small sample size increased the risk for failing to observe a change that was present (type II error). A larger pool of participants would also permit greater in-depth analyses, including creating multiple subgroups for comparison. Secondly, there may also be limitations to using global rating scales. The reliability of this measure is not established,28 and there exists a potential influence of recall bias on self-report scores.9, 29 Future work may seek to examine the reliability of using the global rating scale to measure perceived change in walking ability after stroke, and may utilize distribution-based methods to confirm the findings of anchor-based approaches. Additionally, it is possible that the socialization component of the community exercise program may have also affected the participants’ perceptions of their recovery of walking function, in addition to the physical exercise itself. This study, however, sought to establish the relationships between perceived and measured change in walking after stroke over time (irrespective of the intervention), and did not aim to differentiate between the relative contributions of all potentially influential factors. This may be an area for future research, where factors that predict participant-perceived changes are explored. Furthermore, while change scores may be calculated using a number of different methods (such as absolute difference, percent change, or ratios), we chose to use absolute change scores, as these units would be most meaningful to clinicians. We did undertake post-hoc correlation analyses of percent change scores and perceived change and found the results were consistent with the original analyses using absolute change; only percent change and perceived change in 6MWT distance were moderately correlated (ρ=0.43, P=0.049). Finally, factors such as cognitive and emotional status were not examined in this study, but may influence ability to perceive change in function. Future studies may examine the influence of these variables.
Conclusions
Global rating scales are of value to evaluate the perceptions of change in the ability to walk long distances after an exercise program for individuals with stroke. The ability to detect change may be dependent on baseline status. Evaluating perceived change provides important insight for clinicians about whether the intervention results in meaningful benefit from the perspective of participants. This was the first study to evaluate the relationships between measured change from standardized outcome measures and perceived change with questions phrased in functionally relevant contexts. This study is an important step in determining thresholds for perceived change in walking ability after stroke.
Acknowledgments
Financial Support
Project funded by the Canadian Stroke Network and a grant-in-aid from the Heart and Stroke Foundation of BC and Yukon. AT is supported by the Canadian Institutes of Health Research [CIHR MFE-98550] and the Michael Smith Foundation for Health Research (MSFHR) [ST-PDF-03003(11-1)CLIN], JJE is supported by CIHR [CIHR MSH-63617] and the MSFHR, DR was supported by the Heart and Stroke Foundation of Canada, the Canadian Stroke Network, CIHR Institute of Circulatory and Respiratory Health and CIHR Institute of Aging/Rx&D Collaborative Research Program with AstraZeneca Canada Inc.
Footnotes
This study was conducted at Vancouver Coastal Health, Canada
Presentations at meetings
This work was presented in poster format at the Canadian Stroke Congress, Canadian Stroke Network and Heart and Stroke Foundation of Canada, Québec City QC Canada, June 2010
Author Contributions
AT, as primary author, was responsible for writing the manuscript, data analysis and interpretation. JJE oversaw the data collection, assisted with data analysis and interpretation, and helped draft the manuscript. DR assisted with data analysis and interpretation, and helped with manuscript preparation. All authors have read and approved the final manuscript.
Conflict of interest
None declared
Contributor Information
Ada Tang, Department of Physical Therapy, University of British Columbia, GF Strong Rehabilitation Centre, Rehabilitation Research Lab, 4255 Laurel Street, Vancouver BC Canada V5Z 2G9, Tel (604) 737-6311.
Janice Eng, Department of Physical Therapy, University of British Columbia, GF Strong Rehabilitation Centre, Rehabilitation Research Lab, 4255 Laurel Street, Vancouver BC Canada V5Z 2G9, Tel (604) 714-4105.
Debbie Rand, Department of Occupational Therapy, School of Health Professions, Sackler Faculty of Medicine, Tel Aviv University, Israel.
References
- 1.Bohannon RW, Andrews AW, Smith MB. Rehabilitation goals of patients with hemiplegia. Int J Rehabil Res. 1988;11:181–183. [Google Scholar]
- 2.Harris JE, Eng JJ. Goal priorities identified by individuals with chronic stroke: Implications for rehabilitation professionals. Physiother Can. 2004;56:171–176. doi: 10.2310/6640.2004.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.States RA, Pappas E, Salem Y. Overground physical therapy gait training for chronic stroke patients with mobility deficits. Cochrane Database Syst Rev. 2009;3(3):CD006075. doi: 10.1002/14651858.CD006075.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eng JJ, Tang PF. Gait training strategies to optimize walking ability in people with stroke: A synthesis of the evidence. Expert Rev Neurother. 2007;7(10):1417–1436. doi: 10.1586/14737175.7.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eng JJ, Chu KS, Kim CM, Dawson AS, Carswell A, Hepburn KE. A community-based group exercise program for persons with chronic stroke. Med Sci Sports Exerc. 2003;35(8):1271–1278. doi: 10.1249/01.MSS.0000079079.58477.0B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan PW, Sullivan KJ, Behrman AL, et al. Body-weight-supported treadmill rehabilitation after stroke. N Engl J Med. 2011;364(21):2026–2036. doi: 10.1056/NEJMoa1010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaton DE, Bombardier C, Katz JN, Wright JG. A taxonomy for responsiveness. J Clin Epidemiol. 2001;54(12):1204–1217. doi: 10.1016/s0895-4356(01)00407-3. [DOI] [PubMed] [Google Scholar]
- 8.Jaeschke R, Singer J, Guyatt GH. Measurement of health status. ascertaining the minimal clinically important difference. Control Clin Trials. 1989;10(4):407–415. doi: 10.1016/0197-2456(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 9.Kamper SJ, Maher CG, Mackay G. Global rating of change scales: A review of strengths and weaknesses and considerations for design. J Man Manip Ther. 2009;17(3):163–170. doi: 10.1179/jmt.2009.17.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Redelmeier DA, Guyatt GH, Goldstein RS. Assessing the minimal important difference in symptoms: A comparison of two techniques. J Clin Epidemiol. 1996;49(11):1215–1219. doi: 10.1016/s0895-4356(96)00206-5. [DOI] [PubMed] [Google Scholar]
- 11.Ozyurekoglu T, McCabe SJ, Goldsmith LJ, LaJoie AS. The minimal clinically important difference of the carpal tunnel syndrome symptom severity scale. J Hand Surg Am. 2006;31(5):733–8. doi: 10.1016/j.jhsa.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 12.Stratford PW, Binkley JM, Riddle DL, Guyatt GH. Sensitivity to change of the roland-morris back pain questionnaire: Part 1. Phys Ther. 1998;78(11):1186–1196. doi: 10.1093/ptj/78.11.1186. [DOI] [PubMed] [Google Scholar]
- 13.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 14.Fulk GD, Ludwig M, Dunning K, Golden S, Boyne P, West T. Estimating clinically important change in gait speed in people with stroke undergoing outpatient rehabilitation. J Neurol Phys Ther. 2011;35(2):82–89. doi: 10.1097/NPT.0b013e318218e2f2. [DOI] [PubMed] [Google Scholar]
- 15.Emery CF, Blumenthal JA. Perceived change among participants in an exercise program for older adults. Gerontologist. 1990;30(4):516–521. doi: 10.1093/geront/30.4.516. [DOI] [PubMed] [Google Scholar]
- 16.Burridge JH, Elessi K, Pickering RM, Taylor PN. Walking on an uneven surface: The effect of common peroneal stimulation on gait parameters and relationship between perceived and measured benefits in a sample of participants with drop-foot. Neuromodulation. 2007;10(1):59–67. doi: 10.1111/j.1525-1403.2007.00088.x. [DOI] [PubMed] [Google Scholar]
- 17.Pepin V, Laviolette L, Brouillard C, et al. Significance of changes in endurance shuttle walking performance. Thorax. 2011;66(2):115–120. doi: 10.1136/thx.2010.146159. [DOI] [PubMed] [Google Scholar]
- 18.Flansbjer UB, Holmback AM, Downham D, Patten C, Lexell J. Reliability of gait performance tests in men and women with hemiparesis after stroke. J Rehabil Med. 2005;37(2):75–82. doi: 10.1080/16501970410017215. [DOI] [PubMed] [Google Scholar]
- 19.American Thoracic Society. ATS statement: Guidelines for the six-minute walk test. Am J Resp Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 20.Fulk GD, Echternach JL. Test-retest reliability and minimal detectable change of gait speed in individuals undergoing rehabilitation after stroke. J Neurol Phys Ther. 2008;32(1):8–13. doi: 10.1097/NPT0b013e31816593c0. [DOI] [PubMed] [Google Scholar]
- 21.Eng JJ, Dawson AS, Chu KS. Submaximal exercise in persons with stroke: Test-retest reliability and concurrent validity with maximal oxygen consumption. Arch Phys Med Rehabil. 2004;85(1):113–118. doi: 10.1016/s0003-9993(03)00436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kosak M, Smith T. Comparison of the 2-, 6-, and 12-minute walk tests in patients with stroke. J Rehabil Res Dev. 2005;42(1):103–108. doi: 10.1682/jrrd.2003.11.0171. [DOI] [PubMed] [Google Scholar]
- 23.Schmid A, Duncan PW, Studenski S, et al. Improvements in speed-based gait classifications are meaningful. Stroke. 2007;38(7):2096–2100. doi: 10.1161/STROKEAHA.106.475921. [DOI] [PubMed] [Google Scholar]
- 24.Robinett CS, Vondran MA. Functional ambulation velocity and distance requirements in rural and urban communities. A clinical report. Phys Ther. 1988;68(9):1371–1373. doi: 10.1093/ptj/68.9.1371. [DOI] [PubMed] [Google Scholar]
- 25.Beninato M, Gill-Body KM, Salles S, Stark PC, Black-Schaffer RM, Stein J. Determination of the minimal clinically important difference in the FIM instrument in patients with stroke. Arch Phys Med Rehabil. 2006;87(1):32–39. doi: 10.1016/j.apmr.2005.08.130. [DOI] [PubMed] [Google Scholar]
- 26.Tilson JK, Sullivan KJ, Cen SY, et al. Meaningful gait speed improvement during the first 60 days poststroke: Minimal clinically important difference. Phys Ther. 2010;90(2):196–208. doi: 10.2522/ptj.20090079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lewek MD, Randall EP. Reliability of spatiotemporal asymmetry during overground walking for individuals following chronic stroke. J Neurol Phys Ther. 2011;35(3):116–121. doi: 10.1097/NPT.0b013e318227fe70. [DOI] [PubMed] [Google Scholar]
- 28.Crosby RD, Kolotkin RL, Williams GR. Defining clinically meaningful change in health-related quality of life. J Clin Epidemiol. 2003;56(5):395–407. doi: 10.1016/s0895-4356(03)00044-1. [DOI] [PubMed] [Google Scholar]
- 29.Norman GR, Stratford P, Regehr G. Methodological problems in the retrospective computation of responsiveness to change: The lesson of Cronbach. J Clin Epidemiol. 1997;50(8):869–879. doi: 10.1016/s0895-4356(97)00097-8. [DOI] [PubMed] [Google Scholar]