Abstract
The association between gestational exposure to ethanol and adolescent ethanol abuse is well established. Recent animal studies support the role of fetal ethanol experience-induced chemosensory plasticity as contributing to this observation. Previously, we established that fetal ethanol exposure, delivered through a dam’s diet throughout gestation, tuned the neural response of the peripheral olfactory system of early postnatal rats to the odor of ethanol. This occurred in conjunction with a loss of responsiveness to other odorants. The instinctive behavioral response to the odor of ethanol was also enhanced. Importantly, there was a significant contributory link between the altered response to the odor of ethanol and increased ethanol avidity when assessed in the same animals. Here, we tested whether the neural and behavioral olfactory plasticity, and their relationship to enhanced ethanol intake, is a result of the mere exposure to ethanol or whether it requires the animal to associate ethanol’s reinforcing properties with its odor attributes. In this later respect, the opioid system is important in the mediation (or modulation) of the reinforcing aspects of ethanol. To block endogenous opiates during prenatal life, pregnant rats received daily intraperitoneal administration of the opiate antagonist naltrexone from gestational day 6–21 jointly with ethanol delivered via diet. Relative to control progeny, we found that gestational exposure to naltrexone ameliorated the enhanced postnatal behavioral response to the odor of ethanol and postnatal drug avidity. Our findings support the proposition that in utero ethanol-induced olfactory plasticity (and its relationship to postnatal intake) requires, at least in part, the associative pairing between ethanol’s odor quality and its reinforcing aspects. We also found suggestive evidence that fetal naltrexone ameliorated the untoward effects of gestational ethanol exposure on the neural response to non-fetal-exposure odorants. Thus, gestational naltrexone may also have a neuroprotective and/or neuroproliferative impact on olfactory development.
Keywords: naltrexone, fetal ethanol exposure, ethanol intake, olfactory plasticity, odor preference, flavor perception
Introduction
Gestational ethanol can have considerable untoward effects for the human fetus during development that is both extensive and enduring. The timing of ethanol exposure during fetal development, and the duration and dose are important factors in determining the myriad sequelae which can include, for example, cranial and facial malformations, reduced nursing reflexes, decreased birth weight1–5 and developmental defects in the nervous system.6 Fetal ethanol exposure also results in substantive behavioral changes.7–9
Despite these profound consequences, there are other clinically important, yet understated, negative long-term consequences. Adolescent and adult ethanol addiction is prominently associated with fetal ethanol exposure. Human studies reveal: (a) that fetal ethanol exposure is the number one predictor of later misuse10–13 and (b) that the younger the experience with ethanol the greater the likelihood of persistent misuse into adulthood.12 There have been emerging data outlining how fetal exposure enhances ethanol avidity.
An extensive literature has addressed the issue regarding the information the developing fetus can acquire behaviorally about odorant stimuli present in the fetal milieu. Indeed, the developing fetus can respond to chemical stimuli present in the amniotic surroundings and ‘remember’ the information gained by this experience into postnatal life.14–20 In this respect, ethanol, while being both potentially neurotoxic and teratogenic during fetal development, is also, nonetheless, a chemosensory stimulus. Studies show that maternal ethanol intake during pregnancy can alter the subsequent predilection and avidity for ethanol by a mother’s progeny. Indeed, both human21 and animal studies demonstrate that in utero experience alters the responsiveness to particular chemosensory qualities of ethanol (see reviews22,23). Importantly, the smell and taste qualities of ethanol are considered to be key determinants of ethanol intake.24–26
Recent evidence supports the role of fetal ethanol experience-induced chemosensory plasticity as a key contributor to the foregoing observations and, in turn, the progressive epidemiological pattern of ethanol abuse noted above. The prevailing data indicate that there are epigenetic chemosensory mechanisms (i.e. smell, taste and orosomato-sensory) by which maternal ethanol use can be conveyed to offspring.24,27 With specific regard to olfaction, animal studies have shown an important relationship between the neural responses of the peripheral olfactory system, the reaction to ethanol odor and enhanced postnatal ethanol avidity, stemming from fetal drug experience. Fetal exposure, delivered via a dam’s diet throughout gestation, tuned both the neural response of the olfactory epithelium (OE) as well as the innate behavioral response to the odor of ethanol in infant rats.28 Moreover, the initial behavioral response was attributable to the neural effect. Ethanol intake was also enhanced in these animals.29 Notably, the magnitude of the fetal experience on the enhanced odor response predicted the extent of the prenatal effect on ethanol avidity when assessed in the same animals, and there was a significant causal relationship.24 This collection of behavioral findings persisted into adolescence.24,30,31 Continuation of the increased response to the odor of ethanol, however, does not appear to be dependent on the founding peripheral neural effect, which ameliorates by adolescence.31 This latter finding suggested that more central mechanisms, for instance changes in olfactory bulb (OB) circuitry, underlie the later increased ethanol odor response.32 In short, the preceding studies demonstrated that in utero experience increases ethanol affinity by enhancing the encoding and odor qualities that are important for ethanol odor preference and flavor.24
Regarding the above, a fundamental mechanistic question is whether the founding neural and behavioral olfactory plasticity, and their relationship to increases in the affinity for ethanol is a function of the simple experience with ethanol (i.e. stimulus-induced plasticity) and/or does it require the animal to associate ethanol’s pharmacological properties with its odor qualities. There is evidence that supports the potential for both possibilities. On the one hand, passive odorant exposure in neonatal animals alters the peripheral neural and behavioral response to the exposure odorant.33,34 Moreover, animals exposed in utero to odorants without a known reinforcing property, delivered via the dam’s diet, develop enhanced peripheral neurophysiological sensitivity35,36 and increased preference for the exposure odorant.36 On the other hand, evidence shows that the opioid system is important in the mediation or modulation of the reinforcing aspects of ethanol consumption both in general37,38 and as an effect of fetal exposure, as well. In this latter respect, Chotro and Arias39 demonstrated that ethanol exposure during the last four gestational days enhanced postnatal drug affinity through a conditioned response that required the postabsorptive properties of ethanol. In terms of olfactory function, at a minimum, opioid inputs to the OB40–44 have, along with other centrifugal connections,8–42,42–51 been shown to be important to bulbar effects that can encode the associative significance of an odor.
In the present study, we took advantage of the ability to block endogenous opiates during prenatal life.52,53 Throughout gestation, pregnant rats received daily intraperitoneal administration of an opiate antagonist jointly with ethanol delivered via their diet. Relative to the progeny of control treated dams, we determined whether and to what degree gestational exposure to an opiate antagonist alters the known enhanced: (1) olfactory neural and behavioral odor response to ethanol (Experiment 1) and (2) postnatal affinity for ethanol (Experiment 2) that stems from in utero ethanol exposure.
Materials and methods
Upstate Medical University’s Institutional Animal Care and Use Committee approved all methods used in this study.
Dietary treatment of pregnant dams
Preliminary study
On gestational (G) day 4, pregnant rats (Long-Evans; Harlan-Sprague Dawley, Indianapolis, IN, USA) were initially separated into weight-matched pairs. The members of each pair, in turn, were randomly assigned to two maternal treatment groups. The groups, relative to their maternal dietary and drug treatments were designated as CTL/NTX (free-choice control liquid diet and naltrexone injection) and CTL/H2O (free-choice control liquid diet and control injection) (see description of control diet delivery below).
Experiments 1 and 2
Similar to the above, the members of weight-matched triads of rat dams (Long-Evans; Harlan-Sprague Dawley) were randomly assigned to one of three maternal treatment groups on gestational (G) day 4, as per previously established protocol.28,29 The groups, relative to their combined maternal dietary and drug treatments, were designated as ET/NTX (ethanol-containing diet and naltrexone injection), ET/H2O (ethanol-containing diet and control injection) and CTL/H2O (free-choice control liquid diet and control injection) (see rationale for treatment groups below).
From G4 to 5, all dams, independent of maternal treatment group, were fed a free access control liquid diet (L100252; Research Diets, New Brunswick, NJ, USA) prior to the start of maternal treatment on G6. Following this diet familiarization process, ET/H2O and ET/NTX dams received a free-choice liquid diet (L10251; Research Diets) that contained ethanol (6.7% v/v) during G11–20.28–30 Animals were introduced to the diet from G6 to 10 with increasing amounts of ethanol.28–30 This previously established method yields a rather consistent amount of exposure that models moderate to high intake54,55 during a time period of olfactory development in which olfactory neurons begin responding to stimuli and their axons connect with the OB.56,57 The weight-matched free-choice liquid diet dams (CTL/H2O) had continuous access to water and the same liquid diet (L100252; Research Diets) they received during the familiarization period.
Drug treatment
Previous work has demonstrated that a single daily intraperitoneal injection of naltrexone given at a dose of 50 mg/kg is sufficient to prevent endogenous opioids from binding with their receptors for 24 h.52,53 Moreover, these same studies showed there were no untoward consequences on the duration or progress of pregnancy, the viability of dams or their offspring or the general development of the fetus. Consequently, from G6 to 21 (note that naltrexone was given one day longer than the ethanol-containing and control diets), dams receiving NTX treatment (i.e. ET/NTX or CTL/NTX) were given a daily intraperitoneal injection of 50 mg/kg naltrexone hydrochloride (NTX; Sigma Chemical, St Louis, MO, USA). Injections occurred at 16:00 hours each day (i.e. the time the liquid diet was changed in the cages). In contrast, dams receiving control injections (i.e. ET/H2O or CTL/H2O) were injected with an equal quantity of sterile water vehicle (H2O). Each day the dams were weighed and the NTX dose or H2O volume modified appropriately. NTX was made weekly and kept in the dark at 4°C.
Experimental subjects
Following birth, all litters were surrogate-fostered to a set of control dams fed lab chow and water during gestation. Two days after fostering, litter size was reduced to a maximum of 10 pups per litter with as close a 50/50 balance of male and females as possible.
For each experiment, we tested animals between the ages of postnatal (P) day 12–14. We selected this time window because it represents preweanling ages that can recall the in utero experience with ethanol.57–62 Furthermore, previous work has shown an association between fetal exposure, olfactory neural and behavioral function, and the affinity for ethanol in early postnatal rats.24,28,29
In each of our experiments, only one male and one female per litter were assigned to a specific experiment. This procedure was followed in order to avoid inflating the likelihood of type I error by treating animals from the same litter as independent observations.
Olfactory behavioral and neural response
We evaluated the unconditioned innate sniffing response to the odor of ethanol and mapped the neurophysiological response of the OE, using whole-body plethysmography and optical recording methods, respectively.
Behavior
An animal’s sniffing behavior is the act of moving air through the nose. Important to our purpose, animals are known to reflexively change their odor sampling behavior in response to odors in their surroundings.63 In this respect, the assessment of sniffing provides a method for quantifying the attentiveness/responsivity to odorant stimuli.24,28,64 Therefore, in keeping with our previous work studying the effect of in utero ethanol exposure on the innate odor-mediated responses to ethanol,24,28 we used our procedure for evaluating olfactory function that is based on an animal’s instinctive odorant-induced sniffing response.
Briefly, using whole-body plethysmography and computer controlled olfactometry, we recorded changes in an animal’s breathing patterns in response to ethanol odor.28,45 Stimuli were presented into a testing chamber through which airflow was continuously delivered. Animals were first habituated to the testing chamber. Odor and air stimuli were presented randomly in groups of 20 trials (10 air and 10 odorant). A trial lasted six seconds. Five different concentrations of ethanol were used (expressed as the fraction of vapor saturation at 20°C: 3.125 × 10−3, 6.25 × 10−3, 1.25 × 10−2, 2.5 × 10−2 and 5 × 10−2). Each concentration was used for a single group of 20 trials. Concentration/block was tested from the lowest to highest concentration.
For each stimulus presented, the recorded sniffing pattern was analyzed with respect to 14 response variables.28,45 These variables were: the sniffing frequency for a stimulus trial; the number of inspirations and expirations; the duration, volume, average flow rate, and peak flow rate of an inspiration and expiration; the total inspiratory and expiratory volume; and the total apneic duration.
As we have previously described, an animal’s sniffing responses are multifaceted and vary with odorant stimuli (both concentration and quality).65 Furthermore, despite the finding that these responses can be dissected into many descriptive parameters (e.g. flow rate, volume), data obtained from a single variable is inadequate to assess a differential reflexive behavioral response to odorant stimuli.65 Therefore, we used our established approach and created an ‘index’ that incorporated the descriptive variables of the animal’s reflexive responses to odor stimulation into a single primary measure.24,31 Importantly, for our purposes, fetal ethanol-induced changes in ethanol intake is causally linked to the fetal exposure effect on this primary odor response measure.24
To generate the behavioral index, we took the following approach. Each animal provided a 14 × 5 data matrix to the complete data-set (i.e. 14 response pattern variables for each concentration of ethanol odorant stimulation). The 14 response pattern variables (that is to say, dimensions of behavioral response) of the entire experimental data-set were condensed into two orthogonal dimensions (namely, Factors 1 and 2) by principle components analysis (PCA). The calculated values of the two resultant PCA factors (at each odorant concentration) defined the behavioral response of an individual animal to a specific stimulus concentration. This process reduced each animal’s 14 × 5 data matrix to 2 × 5: two response variables for every concentration of ethanol evaluated. These data were used in a preliminary analysis to test the consistency of the experimental differences related to main effects based on the observational error.
To begin making inferences of a causal nature related to treatment effects (using appropriately adjusted error terms), we specifically examined the effect of fetal naltrexone on the overall behavioral response. As such, to express each animal’s 2 × 5 matrix as a 2 × 1 matrix that integrated the animal’s responses over all odorant concentrations in each uncorrelated dimension (viz, Factor 1 and Factor 2), we calculated the average response across the five concentrations of odorant. This resulted in x and y pairs that located each ET/NTX, ET/H2O and CTL/H2O animal in an ethanol odor-mediated behavioral response space. The x and y data pairs were subsequently used to determine the impact of naltrexone treatment on the innate response to the odor of ethanol.
Neurophysiology
For every behaviorally tested animal, we recorded the odorant-induced responses of their OE, using our standard optical methods and a voltage-sensitive dye.28,66–69 We recorded from a 3.5 × 3.5 mm expanse of the OE on the flat surface of the turbinates, using a 12-bit CCD camera (array size: 120 × 120 pixels; Dalsa, Waterloo, ON, Canada). To accomplish this, rats were anesthetized, killed by decapitation and the right nasal cavity split along the long axis. The tissue was then immersed in the voltage-sensitive dye di-4-ANEPPS and processed, following established procedures.28,66–69 Just before testing, the exposed half of the nasal cavity was mounted in a chamber that had a clear plastic cover and an input and output port.70 Odorant was drawn across the OE by a vacuum applied to the output port. During a trial, computer-activated valves changed the flow from blank to odorized air.
In addition to the odor of ethanol, we assessed the OE’s response to carvone, citral, propyl acetate and ethylacetoacetate. With respect to these latter four stimuli, we previously found that fetal ethanol exposure had an untoward effect on the neural response.28
For each tissue evaluated, we randomly presented each of the five odorants. An amyl acetate standard was also presented as the first and last stimulus. For each odorant presentation, we measured the magnitude of neural response by determining the peak response height.
Voluntary ethanol intake
Early preweanling rats do not ingest fluid from a sipper tube. Thus, we assessed the affinity for ethanol by directly infusing an appropriate solution into the mouth using a cannula inserted through the animal’s cheek.24,29,61,62,71 Briefly, three hours prior to testing, an individual pup was placed in a plastic cage. Pups were kept warm with a heat lamp. After one hour, the rat had a flanged polyethylene tube (PE10 tubing; Clay Adams, Parsippany, NJ, USA) inserted into the center of its cheek. Because of the position of the cannula, the pups had the opportunity to either swallow or reject the infused solution.
Immediately prior to intake testing, the anogenital region of each animal was stimulated in order to initiate bowel and bladder emptying. The rats were then weighed, placed in another heated open plastic chamber (15 × 7 × 15 cm) and the cannula connected to an infusion pump that delivered a 5.0% v/v solution of ethanol.
Each test was preceded by a 10-minute habituation period in the chamber. The ethanol solution was then infused over 15 min with a 3.0 s ‘on’ and 10 s ‘off’ duty cycle. The infusion rate for an individual animal provided a volume equivalent to 5.5% of the preinfusion body weight. The affinity for ethanol was defined by the grams of absolute ethanol ingested per kilograms of body weight. The amount ingested was determined by the difference in the measured pre- and postinfusion body weights.
Results
Preliminary study
We initially performed a study to specifically evaluate whether gestational naltrexone, per se, altered the behavioral and/or neurophysiological response to odorant stimulation (see Experiment 1, below, for analytic details). In this study, we used the offspring of dams that were fed: (1) a liquid control diet and control injection (CTL/H2O, N = 8 male and 8 female) and (2) a liquid control diet and naltrexone injection (CTL/NTX, N = 8 male and 8 female). The experimental subjects represent one randomly selected male and female animal from each of eight CTL/H2O and eight CTL/NTX dams.
In keeping with previous reports that neonates exposed to naltrexone during fetal development are larger in body weight compared with control offspring,52 we also found that CTL/NTX animals were larger in terms of body weight than CTL/H2O animals (mean ± SEM: 27.73 ± 0.742 and 24.01 ± 0.548, respectively). ANOVA (analysis of variance) demonstrated a significant main effect of treatment and sex (F [1, 20] = 69.93; P = nil and F [1, 20] = 9.93; P = 0.005). There was no sex by treatment interaction (F [1, 20] = 0.332; P = 0.570).
Reflexive sniffing response to ethanol odor
MANOVA (multivariate analysis of variance: Wilk’s Lambda criterion) using weight as a conditional covariate, revealed no strong evidence for an overall differential main effect of treatment (F [2, 141] = 1.25; P = 0.142) on the behavioral response to the odor of ethanol. While, on average, there was a significant main effect of sex (F [2, 141] = 3.62; P = 0.029), importantly, there was no sex by treatment interaction (F [2, 141] = 2.01; P = 0.136).
Optical recording of odorant-induced mucosal activity
In keeping with prior studies of the odor response of the OE, there was a highly significant differential response to different odorants (F [4, 136] = 19.40; P = nil).28,66–69 Nonetheless, there was no evidence for an effect of maternal treatment (F [1, 140] = 0.743; P = 0.39), sex (F [1, 136] = 0.049; P = 0.825), sex by treatment (F [1, 136] = 0.020; P = 0.887) or treatment by odor interactions (F [4, 136] = 0.010; P = 0.999).
Experiment 1: Does gestational naltrexone ameliorate the fetal ethanol-induced enhanced behavioral and neural response to the odor of ethanol?
Given the foregoing findings, we limited the subsequent design of Experiment 1 to an analysis that included only a CTL/H2O control group. We did so in order to more tightly focus the test of our a priori primary hypothesis.
We used the progeny of dams that were fed: (1) a liquid diet containing ethanol and received naltrexone injections throughout gestation (ET/NTX, N = 10 male and 10 female); (2) a liquid diet containing ethanol and received control injection (ET/H2O, N = 10 male and 10 female) and (3) a liquid control diet and control injection (CTL/H2O, N = 10 male and 10 female). The experimental subjects represent one randomly selected male and female animal from each of 10 ET/NTX, 10 ET/H2O and 10 CTL/H2O dams. The ET/H2O dams served to control for the injection procedure. More importantly, these animals represented our standard gestational exposure procedure that results in an enhanced response to the odor of ethanol.24,28 The CTL/H2O animals, although also controlling for the effect of experimental handling and injection, set the standard by which to judge the effects of gestational naltrexone treatment. That is, whether and to what degree naltrexone treatment throughout gestation ameliorates the enhanced ethanol odor-mediated response in the offspring of the ET/NTX-treated dams.
Reflexive sniffing response to ethanol odor
For the ET/NTX, ET/H2O and CTL/H2O controls, ANOVA showed that, on average, body weight significantly varied with maternal treatment (F [2, 45] = 5.86; P = 0.005). There was no differential effect of sex (F [1, 45] = 1.53; P = 0.223) or sex by treatment interaction (F [2, 45] = 0.334; P = 0.718). Thus, as above, in subsequent behavioral analyses, the animals’ weights were used as a conditional covariate.
In the first step of our analysis, the 2 × 5 behavioral response data (i.e. two PCA variables at each odorant concentration) were evaluated to test the reliability of observed differences related to main effects. This test of effects was based on observational error. MANOVA (with odorant concentration, treatment and sex as main effects) demonstrated a significant effect of odorant concentration (F [8, 558] = 21.22; P = nil) and treatment (F [4, 558] = 5.46; P = 0.0003) on the innate response to the odor of ethanol. There was no evidence of a sex effect (F [2, 279] = 1.278; P = 0.280). However, there was a significant sex by treatment interaction (F [4, 558] = 5.27; P = 0.0004).
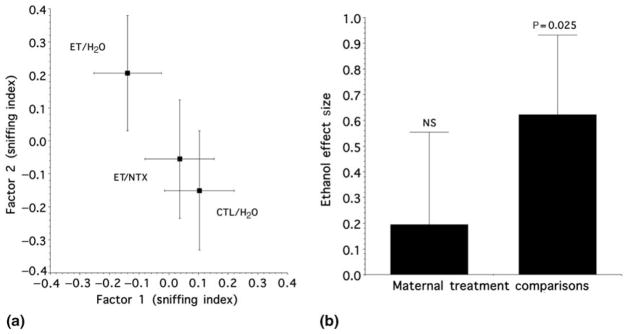
The central element of our study was to test whether the known enhanced responsiveness to the odor of ethanol (stemming from prior fetal exposure) requires the animal to associate ethanol’s reinforcing properties with its odor attributes. Thus, the subsequent behavioral analysis was directed toward testing our a priori hypothesis that gestational naltrexone would ameliorate the known consequences of in utero ethanol exposure. As described above, each animal provided a pair of x and y values that located its comparative position in an ethanol odor-mediated behavioral response space (Figure 1a). As qualitatively illustrated in this figure, on average, there was an unambiguous division between the ET/H2O animals and the ET/NTX and CTL/H2O groups in the behavioral response space.
Figure 1.
(a) Relative position of the maternal treatment groups in an odor-mediated behavioral response space. For any two groups, the degree to which their responses were similar maps as the degree of closeness in the space. The data are expressed as the mean (± 2D SEM) composite sniffing indexes for the maternal treatment groups. (b) ‘Ethanol Effect Size’ (mean ± SEM from the weighted effect size analysis) as a function of maternal treatment comparison. The left column quantifies the degree of difference between ET/NTX versus CTL/H2O in (a). The right column quantifies the magnitude of the effect of our standard fetal ethanol exposure paradigm (i.e. ET/H2O) relative to the average effects (in two dimensions) of the ET/NTX and CTL/H2O exposures. ET/H2O, ethanol-containing diet and control injection; ET/NTX, ethanol-containing diet and naltrexone injection; CTL/H2O, free-choice control liquid diet and control injection; NS, not significant
Given the above, to more formally evaluate our hypothesis (based upon appropriately adjusted error terms), the two-dimensional response data were first used to quantify and assess the degree of difference between ET/NTX versus CTL/H2O. To accomplish this, we tested the hypothesis that the weighted city-block distance of sized differences between two treatment groups was zero. The weighted city-block distance, in terms of the effect sizes for the two indexes, was defined as the combination of absolute values of the individual effect sizes. The weighting scheme for the city-block distance was based on the a priori conjecture that any true effect size on an individual principal component factor is reflected by the excess of its Eigen value above the Kaiser criterion of 1 used in the PCA. As illustrated in Figure 1 (right panel b, left column), the ET/NTX versus CTL/H2O comparison quantified the extent to which naltrexone treatment altered the consequences of fetal ethanol exposure. Importantly, we found no strong evidence for a differential effect between these two maternal treatments (mean ± SEM: 0.195 ± 0.36) (one-tailed t [56] = 0.54; P = 0.296).
On the basis of the above finding, we next quantified and tested the magnitude of the effect of our standard fetal ethanol exposure paradigm (i.e. ET/H2O) relative to the average effects (in two dimensions) of the ET/NTX and CTL/H2O exposures. In this latter respect, the average effect of these two maternal treatment groups contains elements of both NTX and CTL exposures. As such, a formal test of this contrast represented a conservative comparator for evaluation of our hypothesis. In other words, if there was a significant difference between ET/H2O animals versus the average effect of the ET/NTX and CTL/H2O groups and there was no evidence of a difference between ET/NTX versus CTL/H2O animals, then given the finding of our preliminary study (i.e. no difference between CTL/NTX versus CTL/H2O), it would be parsimonious to conclude that NTX treatment ameliorated the known consequences of in utero ethanol exposure on the response to ethanol odor. Indeed, in keeping with our prior studies and a priori prediction,24,28 the altered behavioral response to the odor of ethanol was significant for the ET/H2O versus the average effect of the ET/NTX and CTL/H2O maternal treatment groups (mean ± SEM: 0.622 ± 0.31) (one-tailed t [57] = 2.0; P = 0.025) (Figure 1b, right column). Importantly, comparison of the two columns illustrated in Figure 1b show that, relative to the average effects of ET/NTX and CTL/H2O exposures, gestational naltrexone decreased the enhanced behavioral response to the odor of ethanol by approximately 3.2-fold.
Optical recording of odorant-induced mucosal activity
Previously, we observed that in utero experience with ethanol resulted in a neural tuning to the exposure odorant.28 That is, relative to control, fetal ethanol-exposed animals showed a control level of neural responsiveness to the odor of ethanol in the face of a decrease in response magnitude to stimulation with non-fetal-exposure odorants. We also found that gestational ethanol exposure did not alter the unique differential patterns of neural response for different odorants.28,68,72 Thus, our present analysis focused on exploring the specificity of these same foregoing effects, as a consequence of combined gestational ethanol and naltrexone.
To this end, we first performed an overall analysis to test the null hypothesis that there were no observed differences related to main effects on the response of the OE (ANOVA: odorant, sex, treatment, sex by treatment or odor by treatment effects). As expected,67 there was a significant differential odorant effect on the peak magnitude of the response (F [4, 263] = 9.57; P = nil). Importantly, there was strong evidence for a maternal treatment effect (F [2, 263] = 5.94; P = 0.003). There was no evidence of an average main effect of sex, sex by treatment or odor by treatment interactions (all Ps >0.2).
We next performed additional analyses to examine whether there were specific differential effects between maternal treatment and the response to the odor of ethanol, as opposed to maternal treatment and the effect on the neural response to the non-fetal-exposure odorants. In keeping with our prior study on the effect of gestational ethanol exposure on the neurophysiological olfactory response to ethanol odor in early postnatal animals, there was no evidence of a maternal treatment effect28 (F [2,43] = 0.77; P = 0.467). Moreover, there was no sex (F [1,43] = 0.002; P = 0.959) or sex by treatment interaction (F [2,43] = 0.22; P = 0.799).
For the four non-fetal-exposure odorants, as expected, ANOVA illustrated a significant main effect of odorant stimuli on the neural response (F [3, 208] = 7.59; P = 0.0001). Furthermore, there was strong evidence of a maternal treatment effect (F [2, 208] = 5.09; P = 0.007) with no main effect of sex (F [1, 208] = 0.086; P = 0.77), sex by treatment (F [2, 208] = 0.22; P = 0.799) or odorant by treatment (F [6, 208] = 0.259; P = 0.954) interactions. In this latter respect, the clear absence of an odorant by treatment interaction emphasized that the differential responses due to treatment were uniform across the non-fetal exposure odorants. Such a finding was consistent with our prior observations.28
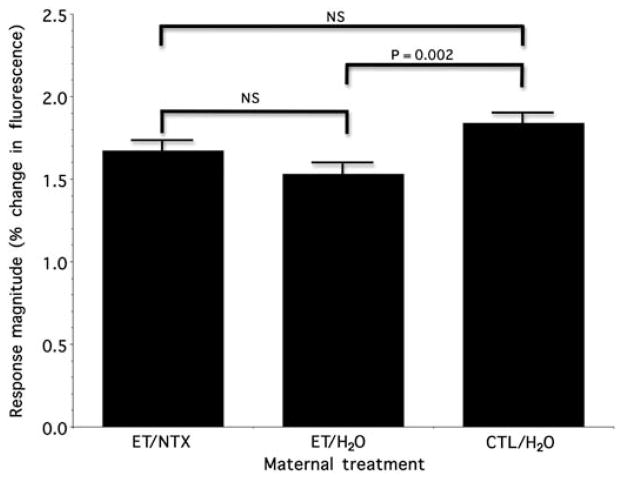
Despite the observation of an overall maternal treatment effect, as illustrated in Figure 2, on average, there was the impression that naltrexone treatment improved the untoward impact of in utero ethanol on the response of the OE to the non-fetal-exposure odorants. Post hoc analysis (using Fisher’s least significant difference criterion) for multiple pair-wise comparisons of our a priori contrast of treatment means between ET/H2O versus CTL/H2O showed, as expected,28 a significant negative impact (mean difference = 0.30; P = 0.002). Importantly, comparison of ET/NTX and CTL/H2O animals revealed no strong evidence for a difference (mean difference = 0.13; P = 0.063). Nonetheless, there was also no difference between ET/H2O versus ET/NTX animals (P = 0.175).
Figure 2.
Neural response to the non-fetal-exposure odorants as a function of maternal treatment group. The figure illustrates the peak height of the neural response (height of the bars: mean ± SEM) averaged across the four non-fetal-exposure odorants. The data are the means (± SEM) for each maternal treatment group derived from the analysis of variance. The response is expressed as the percent change in optical fluorescence from baseline activity. See text for details (NS, not significant). ET/H2O, ethanol-containing diet and control injection; ET/NTX, ethanol-containing diet and naltrexone injection; CTL/H2O, free-choice control liquid diet and control injection
Experiment 2: Does gestational naltrexone ameliorate fetal ethanol-induced increases in ethanol intake?
We used ET/NTX (N = 14 male and 14 female), ET/H2O (N = 14 male and 14 female) and CTL/H2O (N = 14 male and 14 female) exposed progeny. The experimental subjects represent one randomly selected male and female animal from each of 14 ET/NTX, 14 ET/H2O and 14 CTL/H2O dams.
In keeping with the animals evaluated for their behavioral response to the odor of ethanol, ANOVA again indicated that prenatal treatment significantly affected body weight (F [2, 65] = 10.79; P = nil); however, there was no effect of sex (F [1, 65] = 0.166; P = 0.685) or sex by treatment interaction (F [2, 65] = 1.824; P = 0.169).
The focus of Experiment 2 was to examine the relationship between gestational ET/NTX exposure and postnatal ethanol avidity. Given the finding of a treatment effect on the weights of the animals, we examined the conditional effect of maternal treatment by including weight as a covariate in our analysis.29 That is, we adjusted for the possible contributory mediating consequence of the maternal treatment, per se, on the animals’ weights. As such, the analysis tested whether, for rats of a particular weight, ethanol intake per unit weight differs as a result of fetal exposure. The analysis showed a significant treatment effect (F [2, 64] = 4.157; P = 0.02) on ethanol avidity. There was no evidence of an effect of sex (F [1, 64] = 0.606; P = 0.439) or sex by treatment interaction (F [2, 64] = 0.047; P = 0.954).
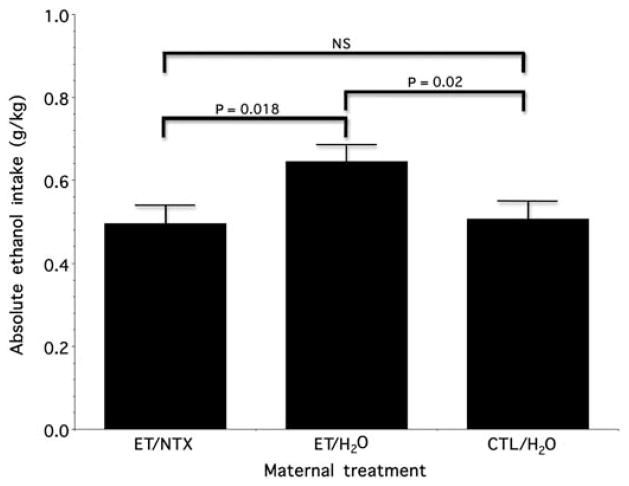
Figure 3 qualitatively illustrates how absolute ethanol intake (grams of ethanol per kilogram body weight) varied with the dam’s treatment during pregnancy. In keeping with our own findings,24,29 and those of others (e.g. see refs.22,23), we again found that fetal ethanol exposure (i.e. evaluation of the progeny of ET/H2O-treated dams) increased postnatal ethanol intake relative to control exposure (i.e. CTL/H2O treated dams). More importantly, however, naltrexone treatment ameliorated the enhancement effect of fetal exposure on drug avidity. Post hoc analysis (using Fisher’s least-significant-difference criteria) for pair-wise comparisons of our a priori contrast of treatment means between ET/NTX and ET/H2O animals revealed a significant impact of gestational naltrexone treatment on reducing the effect of prenatal ethanol exposure on subsequent ethanol avidity (mean difference = 0.15; P = 0.018). Importantly, paired comparison of ET/NTX and CTL/H2O animals revealed no evidence of an effect (mean difference = 0.011; P = 0.861), whereas comparison of ET/H2O vs CTL/H2O also showed significant evidence of increased postnatal avidity for ethanol (mean difference = 0.139; P = 0.020).
Figure 3.
Ethanol acceptance as a function of maternal treatment. The data show grams of absolute ethanol ingested per kilogram of body weight as a function of prenatal exposure to ethanol and naltrexone. The data are expressed as the mean ± SEM. See text for details (NS, not significant). ET/H2O, ethanol-containing diet and control injection; ET/NTX, ethanol-containing diet and naltrexone injection; CTL/H2O, free-choice control liquid diet and control injection
Discussion
A variety of studies reveal an association between fetal exposure to ethanol and the likelihood for ethanol abuse in later life.10–13 The precursors contributing to this observation are likely many. Nevertheless, there is a rich experimental literature that specifically speaks to the impact fetal experience with ethanol’s chemosensory qualities has on subsequent predilection for and intake of the drug.22,39,61,62,73–79 In this respect, we previously reported that fetal ethanol experience-induced olfactory plasticity developmentally altered the olfactory system, enhancing the odor ‘qualities’ of ethanol that are central to both odor preference, intake and flavor (the integration of odor, taste and orosomatosensation). We found that rats exposed to gestational ethanol showed evidence of a neural and behavioral tuning of the olfactory response to the odor of ethanol and enhanced ethanol intake when tested in pre-weanling animals.28,29 More importantly, we showed that while a significant part of the postnatal odor-mediated behavioral effect was attributable to the impact of ethanol exposure on the neural response,28 elevated ethanol intake, in turn, was coupled to the enhanced behavioral odor response.24
Mechanistically, the present work addressed whether the previously observed neural and behavioral olfactory plasticity, and their relationship to enhanced ethanol intake results from the simple exposure to ethanol, i.e. stimulus-activated plasticity, or does it potentially require the pairing of ethanol’s odor quality with its pharmacological effects (i.e. associative conditioning). In so doing, this study extends upon previous work by others examining the potential mechanisms underlying the manifestation of prenatal ethanol-related enhancements in postnatal drug intake.8–24,24–29,39,45,80,81
Using behavioral and neurophysiological methods, the results of Experiment 1 confirmed previous observations that gestational ethanol exposure through diet results in an enhanced responsiveness to the odor of ethanol in early postnatal rats.24,28 More specifically, compared with control animals (CTL/H2O), fetal ethanol exposure (ET/H2O): (a) altered the innate behavioral response to the odor of ethanol and (b) tuned the ethanol odor response of the OE in conjunction with an untoward effect in responsiveness to the other non-fetal exposure odorants (for details see ref.28). The replication of these previous findings was important not only in their own right, but, importantly, they set the standard by which to evaluate whether and to what extent the endogenous opioid system may be important to these observed effects.
Relative to our a priori hypothesis, in Experiment 1, we also found that, compared with control exposure (CTL/H2O), administration of gestational naltrexone to pregnant rats in conjunction with an ethanol-containing diet (ET/NTX) ameliorated the enhanced behaviorally mediated ethanol odor effect in their progeny (Figure 1a and b). We come to this interpretation based on several important observations. (1) We found no significant difference in the behavioral response to ethanol odor between ET/NTX versus CTL/H2O animals. (2) There was strong evidence for the conservative comparison between ET/H2O animals versus the average effect of the ET/NTX and CTL/H2O groups. Indeed, naltrexone treatment concomitant with gestational ethanol exposure resulted in a 3.2-fold reduction in the behavioral effect observed in the foregoing comparison. (3) Finally, in our initial study, we found no strong evidence for a differential behavioral response to ethanol odor between CTL/NTX versus CTL/H2O animals. Importantly, the lack of a behavioral difference between the CTL/NTX versus CTL/H2O animals highlight the specificity of the interaction between fetal ethanol and fetal naltrexone exposures in the ET/NTX group rather than our observations resulting from more general effects of naltrexone.
With respect to the neurophysiological response of the OE, recall that compared with CTL/H2O animals, ET/H2O progeny demonstrated a maintained neural response to the odor of ethanol along with a highly significant loss of responsivity to the non-fetal-exposure odorants (Figure 2). In short, consistent with our prior work,28 the responsive neurons to ethanol odor were stabilized against a landscape of ethanol’s toxic effects on OE neurons responsive to the non-exposure odorants. Interestingly, for these same odorants, there was no strong evidence for a difference in response between the ET/NTX versus ET/H2O and ET/NTX versus CTL/H2O comparisons. Taken together, these findings are consistent with the notion of an intermediate effect of combined ET/NTX exposure.
As outlined below, there are two possible interpretations to the overall findings of Experiment 1 which are not necessarily mutually exclusive, namely (1) that naltrexone treatment blocked the reinforcing or pharmacological properties of ethanol and (2) that, perhaps, gestational naltrexone had a neuroprotective and/or neuroproliferative impact on olfactory development.
With regards to the first alternative, specifically where and how in the olfactory pathway the fetus develops a connection between ethanol’s odor quality and its pharmacological consequence is not definitively known at this time. However, one interesting possibility is the OB. Data suggest that the OB can be considered a key odor processing structure,82 so as to encode that a stimulus has gained associative significance.44,83–90 To this point, there are important descending central influences on the OB that can alter its responsiveness to odor based on prior experience. In infant and adult animals, for example, central descending monoaminergic and noradrenergic activity onto the OB that signal ‘reward’ are needed to alter the bulb’s responses to odors that have gained biological significance.47–51 There are also studies showing that the OB receives inputs, for example, from the mesolimbic dopamine system:91 an area thought to be important in the development and maintenance of ethanol reinforcement92–94 and dopamine has also been implicated in associative modulation of the OB.95,96 Importantly, ethanol administration differentially modulates opiod receptors in the mesolimbic pathways.92 Finally, endogenous opiates appear to play a role in early associative OB effects,40–44 and the opioid systems are believed to mediate (or modulate) the reinforcing effects of ethanol37,97–99 through a variety of structures.100 Some of these structures are intimately related with olfaction (olfactory tubercle and amygdala101) and connected to the foregoing pathways. Thus, there is ample prospect for the developing fetus to encode both the odor quality of ethanol and acquire associative pairing. In short, pairing the activation of olfactory sensory neurons that are responding to ethanol with descending ethanol-related influences on OB circuits may serve as an encoding mechanism that can be blocked by the non-selective opioid antagonist, naltrexone.
The suggestive finding that ET/NTX animals were intermediate in terms of the untoward consequences of in utero exposure on the OE’s response to the non-fetal exposure odorants indicates a possible neuroprotective effect on peripheral olfactory development and even, perhaps, olfactory system development, in general. Indeed, naltrexone has been shown as a neuroprotectant in experimental models of neural damage102 and studies have clearly demonstrated the general damaging impact of fetal exposure on pre- and postnatal olfactory development.103–105
Naltrexone can also be neuroproliferative. Systemic doses like those used in the present study are known to accelerate cell proliferation and growth.106 Taken together, therefore, the potential neuroprotective and neuroproliferative effects of naltrexone might be expected to compromise the general development of fetal ethanol-induced olfactory plasticity. Recall that the behavioral effect observed in early postnatal animals is causally mediated, in part, by the initial founding outcome of fetal exposure on the OE.28 These fetal exposure effects, however, wane over time absent any further experience with the drug.28,31 Thus, it is intriguing to speculate that fetal naltrexone exposure might contribute to the amelioration of the early founding process, not only by blocking the associative pairing of ascending and descending inputs to the OB, but also by interfering with the stabilization and tuning of ethanol stimulus-activated channels. In other words, naltrexone-induced neural protection and the enhancement of normally occurring neurogenesis at the level of the OE107,108 and OB109,110 might accelerate the process of ethanol odor returning to a biologically ‘neutral’ status (the lack of biological significance).111
A note of caution must be raised regarding our discussion of the potential neuroprotective effects of naltrexone in our study. In this respect, we do no wish to suggest that prenatal naltrexone might be useful as a neuroprotective agent and/or a method for reducing prenatal alcohol effects. To this point, there are many studies describing the potential untoward effects of opiate blockade in utero, as endogenous opioids serve a number of developmental processes in addition to neuromodulation.
Indeed, continuous opiate blockage like that used in the present study has a significant impact on both pre-and postnatal physical development52 as well as behavioral development during postnatal life.112 For example, fetal naltrexone-exposed rats have larger wet weights of brain, heart, lungs and kidneys. Behaviorally, weanling animals have reduced motor activity, and the incidence of activities such as grooming and face washing, to name a few.
In Experiment 2, we again found that gestational exposure to ethanol (at a dose approximating moderate–high daily ethanol ingestion55) significantly increased ethanol intake in the preweanling ET/H2O animals.24,29 These results further add to the general animal literature demonstrating that fetal exposures ranging from the chronic intake mimicked with the present maternal feeding paradigm to limited exposure during the last few days of gestation22,23 result in enhanced postnatal avidity.
Critical to the goals of this study, however, was the finding that gestational naltrexone in conjunction with chronic maternal ethanol exposure (ET/NTX animals), indeed, blocked enhanced postnatal drug avidity. Given the foregoing odor-mediated behavioral and neurophysiological results, and our previous observation of a causal link between postnatal ethanol avidity and the enhanced behavioral response to the odor of ethanol,24 such a finding is important to the fundamental interpretation of the overall study. That is, despite the possible neuroprotective and neuroproliferative effects of naltrexone, our results are consistent with the notion that fetal ethanol experience-induced olfactory plasticity (and its relationship to enhanced post-natal intake) requires, at least in part, the paired association between ethanol’s odor quality and its reinforcing aspects. In this respect, our observation that naltrexone reduced ethanol intake is consistent with previous studies, using short prenatal ethanol exposures (i.e. single intragastric infusions during the last four days of gestation)39,80,81 and another non-specific opioid antagonist, naloxone. Given the consistency of findings across the two widely differing fetal exposure paradigms and the short time course of ethanol exposure during late gestation in the naloxone studies, it is difficult to reconcile a highly prominent role for the potential neuroprotective and neuroproliferative effects of naltrexone.
Finally, the flavor of ethanol is the integration of odor, gustation and orosomatosensation and, as we have noted, in utero exposure increases ethanol intake, in part, by enhancing its olfactory qualities.24 As such, the results of our study also add to the proposition that fetal exposure alters the flavor profile of ethanol39,79 and that the opioid system mediates this effect.80
Acknowledgments
The work was supported by NIH-NIAAA Grants AA014871 and AA017823 to SL Youngentob. The authors would like to thank Dr Paul Sheehe for his statistical advice and consultation.
Footnotes
Author contributions: SLY designed the study, analyzed and interpreted the data and wrote the initial draft of the manuscript. PFK wrote the data acquisition and analysis programs for the neurophysiology, contributed to the interpretation of the data and made editorial comments on the manuscript. LMY developed, piloted and conducted the combined injection/feeding paradigm, ran the behavioral experiments and made editorial comments on the manuscript.
References
- 1.Clarren SK, Smith DW. The fetal alcohol syndrome. New Eng J Med. 1978;298:1063–7. doi: 10.1056/NEJM197805112981906. [DOI] [PubMed] [Google Scholar]
- 2.Streissguth AP, Landesman-Dwyer S, Martin JC, Smith DW. Teratogenic effects of alcohol in humans and animals. Science. 1980;209:335–61. doi: 10.1126/science.6992275. [DOI] [PubMed] [Google Scholar]
- 3.Astley SJ, Clarren SK, Little RE, Sampson PD, Daling JR. Analysis of facial shape in children gestationally exposed to marijuana, alcohol, and/or cocaine. Pediatrics. 1992;89:67–77. [PubMed] [Google Scholar]
- 4.Osborn JA, Harris SR, Weinberg J. Fetal alcohol syndrome: review of the literature with implication for physical therapists. Phys Ther. 1993;73:599–607. doi: 10.1093/ptj/73.9.599. [DOI] [PubMed] [Google Scholar]
- 5.Abel EL, Hannigan JH. Maternal risk factors in fetal alcohol syndrome: provocative and permissive influences. Neurotoxicol Teratol. 1995;17:448–62. doi: 10.1016/0892-0362(95)98055-6. [DOI] [PubMed] [Google Scholar]
- 6.Hannigan JH, Spear LP, Spear NE, Goodlett CR. Alcohol and Alcoholism: Effects on Brain Development. New Jersey: Lawrence Erlbaum Assoc; 1999. [Google Scholar]
- 7.Abel EL, Sokol RJ. A revised conservative estimate of the incidence of FAS and economic impact. Alcohol Clin Exp Res. 1992;15:514–24. doi: 10.1111/j.1530-0277.1991.tb00553.x. [DOI] [PubMed] [Google Scholar]
- 8.Stratton K, Howe C, Battaglia F. Fetal Alcohol Syndrome. Diagnosis, Epidemiology, Prevention and Treatment. Washington, DC: National Academic Press; 1996. [Google Scholar]
- 9.Sampson PD, Streissguth AP, Bookstein FL, Little RE, Clarren SK, Dehaene P, Hanson JW, Graham JM., Jr Incidence of fetal alcohol syndrome and prevalence of alcohol-related neurodevelopmental disorder. Teratology. 1997;56:317–26. doi: 10.1002/(SICI)1096-9926(199711)56:5<317::AID-TERA5>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 10.Baer JS, Bar HM, Bookstein FL, Sampson PD, Streissguth AP. Prenatal alcohol exposure and family history of alcoholism in the etiology of adolescent alcohol problems. J Stud Alcohol. 1998;59:533–43. doi: 10.15288/jsa.1998.59.533. [DOI] [PubMed] [Google Scholar]
- 11.Baer JS, Sampson PD, Barr HM, Conner PD, Streissguth AP. A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Arch Gen Psychiatry. 2003;60:377–86. doi: 10.1001/archpsyc.60.4.377. [DOI] [PubMed] [Google Scholar]
- 12.Yates WR, Cadoret RJ, Troughton EP, Steward M, Giunta TA. Effect of fetal alcohol exposure on adult symptoms of nicotine, alcohol and drug dependence. Alcohol Clin Exp Res. 1998;22:914–20. [PubMed] [Google Scholar]
- 13.Alati R, AlMamum A, Williams GM, O’Callagham M, Najman JM, Bor W. In utero alcohol exposure and prediction of alcohol disorders in early adulthood: a birth cohort study. Arch Gen Psychiatry. 2006;63:1009–16. doi: 10.1001/archpsyc.63.9.1009. [DOI] [PubMed] [Google Scholar]
- 14.Smotherman WP. In utero chemosensory experience alters taste preferences and corticosterone responsiveness. Behav Neural Biol. 1982a;36:61–8. doi: 10.1016/s0163-1047(82)90245-x. [DOI] [PubMed] [Google Scholar]
- 15.Smotherman WP. Odor aversion learning by the rat fetus. Physiol Behav. 1982b;29:769–71. doi: 10.1016/0031-9384(82)90322-5. [DOI] [PubMed] [Google Scholar]
- 16.Smotherman WP, Robinson SR. The rat fetus in its environment: behavioral adjustments to novel, familiar, aversive, and conditioned stimuli presented in utero. Behav Neurosci. 1985;99:521–30. doi: 10.1037//0735-7044.99.3.521. [DOI] [PubMed] [Google Scholar]
- 17.Smotherman WP, Robinson SR. Psychobiology of fetal experience in the rat. In: Krasnegor NA, Blass EM, Hofer MA, Smotherman WP, editors. Perinatal Development: A Psychobiological Perspective. Orlando, FL: Academic Press; 1987. pp. 39–60. [Google Scholar]
- 18.Smotherman WP, Robinson SR. Behavior of rat fetuses following chemical or tactile stimulation. Behav Neurosci. 1988;102:24–34. doi: 10.1037//0735-7044.102.1.24. [DOI] [PubMed] [Google Scholar]
- 19.Smotherman WP, Robinson SR. Rat fetuses respond to chemical stimuli in gas phase. Physiol Behav. 1990;47:863–8. doi: 10.1016/0031-9384(90)90010-2. [DOI] [PubMed] [Google Scholar]
- 20.Schaal B, Marlier L, Soussignan R. Human fetuses learn odors from their pregnant mother’s diet. Chem Senses. 2000;25:729–37. doi: 10.1093/chemse/25.6.729. [DOI] [PubMed] [Google Scholar]
- 21.Fass AE, Sponton ED, Moya PR, Molina JC. Differential responsiveness to alcohol odor in human neonates: effects of maternal consumption during gestation. Alcohol. 2000;22:7–17. doi: 10.1016/s0741-8329(00)00103-8. [DOI] [PubMed] [Google Scholar]
- 22.Spear N, Molina JC. Fetal or infantile exposure to ethanol promotes ethanol ingestion in adolescence and adulthood: a theoretical review. Alcoholism Clin Exp Res. 2005;29:909–29. doi: 10.1097/01.alc.0000171046.78556.66. [DOI] [PubMed] [Google Scholar]
- 23.Molina JC, Spear NE, Spear LP, Mennella JA, Lewis MJ. Alcohol and development: beyond fetal alcohol syndrome. Dev Psychobiol. 2007;49:227–42. doi: 10.1002/dev.20224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Di Lorenzo PM, Kiefer SW, Rice AG, Garcia J. Neural and behavioral responsivity to ethyl alcohol as a tastant. Alcohol. 1986;3:55–61. doi: 10.1016/0741-8329(86)90071-6. [DOI] [PubMed] [Google Scholar]
- 25.Kiefer SW, Mahadevan RS. The taste of alcohol for rats as revealed by aversion generalization tests. Chem Senses. 1993;18:509–22. [Google Scholar]
- 26.Bachmanov AA, Kiefer SW, Molina WC, Tordoff MG, Duffy VG, Bartoshuk LM, Mennella JA. Chemosensory factors influencing alcohol perception, preferences, and consumption. Alcohol Clin Exp Res. 2003;27:220–31. doi: 10.1097/01.ALC.0000051021.99641.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Glendinning JI, Simons YS, Youngentob L, Youngentob SL. Fetal ethanol exposure attenuates aversive oral effects of TrpV1, but not TrpA1 agonists in rats. Exp Biol Med. 2012;237:236–40. doi: 10.1258/ebm.2011.011345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Youngentob S, Kent P, Sheehe P, Molina J, Spear NE, Youngentob L. Experience-induced fetal plasticity: the effect of gestational ethanol exposure on the behavioral and neurophysiologic olfactory response to ethanol odor in early postnatal and adult rats. Behav Neurosci. 2007a;121:1293–305. doi: 10.1037/0735-7044.121.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Youngentob S, Molina J, Spear NE, Youngentob L. The effect of gestational ethanol exposure on voluntary ethanol intake in early postnatal and adult rats. Behav Neurosci. 2007b:1306–15. doi: 10.1037/0735-7044.121.6.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eade AM, Sheehe PR, Molina JC, Spear NE, Youngentob LM, Youngentob SL. Fetal ethanol-induced olfactory plasticity: the effect of adolescent ethanol re-exposure on the behavioral response to ethanol odor. Behav Brain Funct. 2009;5:3. doi: 10.1186/1744-9081-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eade AM, Sheehe PR, Youngentob SL. Ontogeny of the enhanced fetal-ethanol-induced behavioral and neurophysiologic olfactory response to ethanol odor. Alcohol: Clinc Exp Res. 2010;34:206–13. doi: 10.1111/j.1530-0277.2009.01083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Middleton FA, Carrierfenster K, Mooney SM, Youngentob SL. Experience-induced fetal plasticity: gestational ethanol exposure alters the behavioral response to ethanol odor and the expression of neurotransmission genes in the olfactory bulb of adolescent rats. Brain Res. 2009;1252:105–16. doi: 10.1016/j.brainres.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Panhuber H. The effect of long duration postnatal odour exposure on the development of the rat olfactory bulb. In: Breipohl W, editor. Ontogeny of Olfaction. Berlin: Springer Verlag; 1986. pp. 127–41. [Google Scholar]
- 34.Wang HW, Wysocki CJ, Gold GH. Induction of olfactory receptor sensitivity in mice. Science. 1993;260:998–1000. doi: 10.1126/science.8493539. [DOI] [PubMed] [Google Scholar]
- 35.Semke E, Distel H, Hudson R. Specific enhancement of olfactory receptor sensitivity associated with foetal learning of food odors in the rabbit. Naturwissenschaften. 1995;82:148–9. doi: 10.1007/BF01177279. [DOI] [PubMed] [Google Scholar]
- 36.Hudson R, Distel H. Induced peripheral sensitivity in the developing vertebrate olfactory system. Ann NY Acad Sci. 1998;855:109–15. doi: 10.1111/j.1749-6632.1998.tb10552.x. [DOI] [PubMed] [Google Scholar]
- 37.Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 1997;129:99–111. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- 38.Stromberg MF, Volpicelli JR, O’Brien CP. Effects of naltrexone administered repeatedly across 30 or 60 days on ethanol consumption using a limited access procedure in the rat. Alcohol Clin Exp Res. 1998;22:2186–91. [PubMed] [Google Scholar]
- 39.Chotro MG, Arias C. Prenatal exposure to ethanol increases ethanol consumption: a conditioned response? Alcohol. 2003;30:19–28. doi: 10.1016/s0741-8329(03)00037-5. [DOI] [PubMed] [Google Scholar]
- 40.Kehoe P, Blass EM. Central nervous system mediation of positive and negative reinforcement in neonatal albino rats. Brain Res. 1986;392:69–75. doi: 10.1016/0165-3806(86)90233-6. [DOI] [PubMed] [Google Scholar]
- 41.Roth TL, Sullivan RM. Endogenous opioids and their role in odor preference acquisition and consolidation following odor-shock conditioning in infant rats. Dev Psychobiol. 2001;39:188–98. doi: 10.1002/dev.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roth TL, Sullivan RM. Consolidation and expression of a shock-induced odor preference in rat pups is facilitated by opioids. Physiol Behav. 2003;78:135–42. doi: 10.1016/s0031-9384(02)00961-7. [DOI] [PubMed] [Google Scholar]
- 43.Roth TL, Sullivan RM. Examining the role of endogenous opioids in learned odor-stroke associations in infant rats. Dev Psychobiol. 2006;48:71–8. doi: 10.1002/dev.20107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roth TL, Moriceau S, Sullivan RM. Opioid modulation of Fos protein expression and olfactory circuitry plays a pivotal role in what neonates remember. Learn Mem. 2006;13:590–8. doi: 10.1101/lm.301206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Youngentob SL, Glendinning JI. Fetal ethanol exposure increases ethanol intake by making it smell and taste better. Proc Natl Acad Sci USA. 2009;106:5359–64. doi: 10.1073/pnas.0809804106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bilko A, Altbacker V, Hudson R. Transmission of food preference in the rabbit: the means of information transfer. Physiol Behav. 1994;56:907–12. doi: 10.1016/0031-9384(94)90322-0. [DOI] [PubMed] [Google Scholar]
- 47.Gervais R, Pager J. Olfactory bulb excitability selectively modified in behaving rats after local 6-hydroxydopamine treatment. Behav Brain Res. 1983;9:165–79. doi: 10.1016/0166-4328(83)90126-2. [DOI] [PubMed] [Google Scholar]
- 48.Gervais R, Holley A, Keverne B. The importance of central noradrenergic influences on the olfactory bulb in the processing of learned olfactory cues. Chem Senses. 1988;13:1–12. [Google Scholar]
- 49.Wilson DA, Leon M. Noradrenergic modulation of olfactory bulb excitability in the postnatal rat. Brain Res. 1988;470:69–75. doi: 10.1016/0165-3806(88)90202-7. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan RM, Wilson DA, Leon M. Norepinephrine and learning-induced plasticity in infant rat olfactory system. J Neurosci. 1989;9:3998–4006. doi: 10.1523/JNEUROSCI.09-11-03998.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sullivan RM, Stackenwalt G, Nasr F, Lemon C, Wilson DA. Association of an odor with activation of olfactory bulb noradrenergic beta-receptors or locus coeruleus stimulation is sufficient to produce learned approach responses to that odor in neonatal rats. Behav Neurosci. 2000;114:957–62. doi: 10.1037/0735-7044.114.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McLaughlin PJ, Tobias SW, Lang CM, Zagon IS. Chronic exposure to the opioid antagonist naltrexone during pregnancy: maternal and offspring effects. Physiol Behav. 1997;62:501–8. doi: 10.1016/s0031-9384(97)00007-3. [DOI] [PubMed] [Google Scholar]
- 53.Zagon IS, Hurst WJ, McLaughlin PJ. Transplacental transfer of naltrexone in rats. Life Sci. 1997;61:1261–7. doi: 10.1016/s0024-3205(97)00671-1. [DOI] [PubMed] [Google Scholar]
- 54.Driscoll CD, Streissguth AP, Riley EP. Prenatal alchol exposure: comparability of effects in human and animal models. Neurotoxicol Teratol. 1990;12:231–7. doi: 10.1016/0892-0362(90)90094-s. [DOI] [PubMed] [Google Scholar]
- 55.Vavrousek-Jakuba EM, Baker RA, Shoemaker WJ. Effect of ethanol on maternal and offspring characteristics: comparison of the formulations fed during gestation. Alcohol Clin Exper Res. 1991;15:129–35. doi: 10.1111/j.1530-0277.1991.tb00530.x. [DOI] [PubMed] [Google Scholar]
- 56.Gesteland RC, Yancey RA, Farbman AI. Development of olfactory receptor neuron selectivity in the rat fetus. Neuroscience. 1982;7:3127–36. doi: 10.1016/0306-4522(82)90235-4. [DOI] [PubMed] [Google Scholar]
- 57.Farbman AI. Developmental neurobiology of the olfactory system. In: Getchell TV, Doty R, Bartoshuk L, Snow J, editors. Smell and Taste in Health and Disease. New York: Raven Press; 1991. pp. 19–33. [Google Scholar]
- 58.Chotro MG, Molina JC. Acute ethanol contamination of the amniotic fluid during gestational day 21: postnatal changes in alcohol responsiveness in rats. Dev Psychobiol. 1990;23:535–47. doi: 10.1002/dev.420230608. [DOI] [PubMed] [Google Scholar]
- 59.Dominguez HD, Chotro MG, Molina JC. Alcohol in the amniotic fluid prior to cesarean delivery: effects of subsequent exposure to the drug’s odor upon alcohol responsiveness. Behav Neural Biol. 1993;60:129–38. doi: 10.1016/0163-1047(93)90229-b. [DOI] [PubMed] [Google Scholar]
- 60.Chotro MG, Kraebel KS, McKinzie DL, Molina JC, Spear N. Prenatal and postnatal ethanol exposure influences preweanling rats’ behavioral and autonomic responding to ethanol odor. Alcohol. 1996;13:377–85. doi: 10.1016/0741-8329(96)00027-4. [DOI] [PubMed] [Google Scholar]
- 61.Abate P, Pepino MY, Dominguez HD, Spear NE, Molina JC. Fetal associative learning mediated through maternal alcohol intoxication. Alcohol Clin Exp Res. 2000;24:39–47. [PubMed] [Google Scholar]
- 62.Abate P, Spear NE, Molina JC. Fetal and infantile alcohol-mediated associative learning in the rat. Alcohol Clin Exp Res. 2001;25:989–98. [PubMed] [Google Scholar]
- 63.Welker WF. Analysis of sniffing in the albino rat. Behavior. 1964;22:223–44. [Google Scholar]
- 64.Youngentob SL. A method for the rapid automated assessment of olfactory function. Chem Senses. 2005;30:219–29. doi: 10.1093/chemse/bji017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Youngentob SL, Mozell MM, Sheehe PR, Hornung DE. A quantitative analysis of sniffing strategies in rats performing odor detection tasks. Physiol Behav. 1987;41:59–69. doi: 10.1016/0031-9384(87)90131-4. [DOI] [PubMed] [Google Scholar]
- 66.Kent PF, Youngentob SL, Sheehe PR. Odorant-specific spatial patterns in mucosal activity predict perceptual differences among odorants. J Neurophysiol. 1995;74:1777–81. doi: 10.1152/jn.1995.74.4.1777. [DOI] [PubMed] [Google Scholar]
- 67.Kent PF, Mozell MM, Youngentob SL, Yurco P. Mucosal activity patterns as a basis for olfactory discrimination: comparing behavior and optical recordings. Brain Res. 2003;981:1–11. doi: 10.1016/s0006-8993(03)02512-5. [DOI] [PubMed] [Google Scholar]
- 68.Youngentob SL, Kent PF, Sheehe PR, Schwob JE, Tzoumaka E. Mucosal inherent activity patterns in the rat: evidence from voltage-sensitive dyes. J Neurophysiol. 1995;73:387–98. doi: 10.1152/jn.1995.73.1.387. [DOI] [PubMed] [Google Scholar]
- 69.Youngentob SL, Kent PF, Margolis FL. OMP gene deletion results in an alteration in odorant-induced mucosal activity patterns. J Neurophysiol. 2003;90:3864–73. doi: 10.1152/jn.00806.2002. [DOI] [PubMed] [Google Scholar]
- 70.Kent PF, Mozell MM, Murphy SJ, Hornung DE. The interaction of imposed and inherent olfactory mucosal activity patterns and their composite representation in a mammalian species using voltage-sensitive dyes. J Neurosci. 1996;16:345–53. doi: 10.1523/JNEUROSCI.16-01-00345.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dominguez HD, Lopez MF, Chotro MG, Molina JC. Perinatal responsiveness to alcohol’s chemosensory cues as a function of prenatal alcohol administration during gestational days 17–20 in the rat. Neurobiol Learn Mem. 1996;65:103–12. doi: 10.1006/nlme.1996.0012. [DOI] [PubMed] [Google Scholar]
- 72.MacKay-Sim A, Shaman P. Topographic coding of odorant quality is maintained at different concentrations in the salamander olfactory epithelium. Brain Res. 1984;297:207–17. doi: 10.1016/0006-8993(84)90562-6. [DOI] [PubMed] [Google Scholar]
- 73.Molina JC, Chotro MG, Dominguez HD. Fetal alcohol learning resulting from alcohol contamination of the prenatal environment. In: Lecanuet JP, Fifer WP, Krasnegor NA, Smotherman WP, editors. Fetal Development: A Psychological Perspective. Hillsdale, NJ: Lawrence Erlbaum Associates; 1995. pp. 419–38. [Google Scholar]
- 74.Molina JC, Dominguez HD, Lopez MF, Pepino MY, Faas AE. The role of fetal and infantile experience with alcohol in later recognition and acceptance patterns of the drug. In: Hannigan JH, Spear LP, Spear NE, Goodlett CR, editors. Alcohol: Effects on Brain and Development. NJ: Erlbaum; 1999. pp. 199–228. [Google Scholar]
- 75.Mennella JA, Beauchamp GK. Infants’ exploration of scented toys: effects of prior experiences. Chem Senses. 1998;23:11–7. doi: 10.1093/chemse/23.1.11. [DOI] [PubMed] [Google Scholar]
- 76.Mennella JA. The transfer of alcohol to human milk: sensory implications and effects on mother-infant interaction. In: Goodlet CR, Hannigan JH, Spear LP, Spear NE, editors. Alcohol: Effects in Brain and Development. New Jersey: Erlbaum; 1999. pp. 177–98. [Google Scholar]
- 77.Mennella JA. Regulation of milk intake after exposure to alcohol in mothers’ milk. Alcohol Clin Exp Res. 2001;25:590–3. [PMC free article] [PubMed] [Google Scholar]
- 78.Spear NE, Molina JC. Consequences of early exposure to alcohol: how animal studies reveal later patterns of use and abuse in humans. In: Carroll M, Overmier B, editors. Animal Research and Human Health. Washington, DC: APA Publishers; 2001. pp. 85–99. [Google Scholar]
- 79.Arias C, Chotro MG. Increased preference for ethanol in the infant rat after prenatal ethanol exposure, expressed on intake and taste reactivity tests. Alcohol Clin Exp Res. 2005a;29:337–46. doi: 10.1097/01.alc.0000156115.35817.21. [DOI] [PubMed] [Google Scholar]
- 80.Arias C, Chotro MG. Increased palatability of ethanol after prenatal ethanol exposure is mediated by the opioid system. Pharmacol Biochem Behav. 2005b;82:434–42. doi: 10.1016/j.pbb.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 81.Miranda-Moralesa RS, Molina JC, Spear NE, Abate P. Participation of the endogenous opioid system in the acquisition of a prenatal ethanol-related memory: effects on neonatal and preweanling responsiveness to ethanol. Physiol Behav. 2010;101:153–60. doi: 10.1016/j.physbeh.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilson DA, Sullivan RM. Neurobiology of associative learning in the neonate: early olfactory learning. Behav Neural Biol. 1994;61:1–18. doi: 10.1016/s0163-1047(05)80039-1. [DOI] [PubMed] [Google Scholar]
- 83.Coopersmith R, Leon M. Enhanced neural response to familiar olfactory cues. Science. 1984;225:849–51. doi: 10.1126/science.6474157. [DOI] [PubMed] [Google Scholar]
- 84.Coopersmith R, Leon M. Enhanced neural response by adult rats to odors experienced early in life. Brain Res. 1986;371:400–3. doi: 10.1016/0006-8993(86)90384-7. [DOI] [PubMed] [Google Scholar]
- 85.Coopersmith R, Henderson SR, Leon M. Odor specificity of the enhanced neural response following early odor experience in rats. Brain Res. 1986;392:191–7. doi: 10.1016/0165-3806(86)90245-2. [DOI] [PubMed] [Google Scholar]
- 86.Brennan PA, Keverne EB. Neural mechanisms of mammalian olfactory learning. Prog Neurobiol. 1997;51:457–81. doi: 10.1016/s0301-0082(96)00069-x. [DOI] [PubMed] [Google Scholar]
- 87.McLean JH, Harley CW, Darby-King A, Yuan Q. pCREB in the neonate rat olfactory bulb is selectively and transiently increased by odor preference-conditioned training. Learn Mem. 1999;6:608–18. doi: 10.1101/lm.6.6.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yuan Q, Harley CW, Bruce JC, McLean JH, Knopfel T. Optical imaging of odor preference memory in the rat olfactory bulb. J Neurophysiol. 2002;87:3156–9. doi: 10.1152/jn.00917.2001. [DOI] [PubMed] [Google Scholar]
- 89.Yuan Q, Harley CW, McLean JH. Mitral cell beta1 and 5-HT2A receptor colocalization and cAMP coregulation: a new model of norepinephrine-induced learning in the olfactory bulb. Learn Mem. 2003;10:5–15. doi: 10.1101/lm.54803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McLean JH, Harley CW. Olfactory learning in the rat pup: a model that may permit visualization of a mammalian memory trace. Neuroreport. 2004;15:1691–7. doi: 10.1097/01.wnr.0000134988.51310.c3. [DOI] [PubMed] [Google Scholar]
- 91.Levy F, Meurisse M, Ferreira G, Thibault J, Tillet Y. Afferents to the rostral olfactory bulb in sheep with special emphasis on the cholinergic, noradrenergic and serotinergic connections. J Chem Neuroanat. 1999;16:245–63. doi: 10.1016/s0891-0618(99)00005-8. [DOI] [PubMed] [Google Scholar]
- 92.Mendez M, Leriche M, Calva JC. Acute ethanol administration differentially modulates mu opiod receptors in mesoaccumbans and mesocortical pathways. Mol Brain Res. 2001;19:148–56. doi: 10.1016/s0169-328x(01)00232-7. [DOI] [PubMed] [Google Scholar]
- 93.Gonzales RA, Job MO, Doyon WM. The role of mesolimbic dopamine in the development and maintenance of ethanol reinforcement. Pharmacol Ther. 2004;103:121–46. doi: 10.1016/j.pharmthera.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 94.Kareken DA, Claus ED, Sabri M, Dzimidzic M, Kosobud AE, Radnovich AJ, Hector D, Ramchandani VA, O’Connor SJ, Lowe M, Li TK. Alcohol-related olfactory cues activate the nucleus accumbens and ventral tegmental area in high-risk drinkers: preliminary findings. Alcohol Cin Exp Res. 2004;28:550–7. doi: 10.1097/01.alc.0000122764.60626.af. [DOI] [PubMed] [Google Scholar]
- 95.Weldon DA, Wool RS, Teicher MH, Shaywitz BA, Cohen DJ, Anderson GM. Effects of apomorphine on appetitive conditioning in 6-hydroxydopamine treated rat pups. Pharmacol Biochem Behav. 1982;17:1281–4. doi: 10.1016/0091-3057(82)90134-4. [DOI] [PubMed] [Google Scholar]
- 96.Weldon DA, Travis ML, Kennedy DA. Posttraining D1 receptor blockade impairs odor conditioning in neonatal rats. Behav Neurosci. 1991;105:450–8. doi: 10.1037//0735-7044.105.3.450. [DOI] [PubMed] [Google Scholar]
- 97.Froehlich JC. Interactions between alchohol and the endogenous opiod systems. In: Zakhari S, editor. Alcohol and the Endocrine System. NIH Publication No. 93-3533. NIAAA Res Monogr 23. Bethesda, MD: NIH; 1993. pp. 31–5. [Google Scholar]
- 98.Froehlich JC. The neurobiology of ethanol-opioid interactions in ethanol reinforcement. Alcohol Clin Exp Res. 1996;20:181A–6A. doi: 10.1111/j.1530-0277.1996.tb01772.x. [DOI] [PubMed] [Google Scholar]
- 99.Froehlich JC, Li TK. Opioid involvement in alcohol drinking. Ann NY Acad Sci. 1994;739:156–67. doi: 10.1111/j.1749-6632.1994.tb19817.x. [DOI] [PubMed] [Google Scholar]
- 100.Oswald LM, Wand GS. Opioids and alcoholism. Physiol Behav. 2004;81:339–58. doi: 10.1016/j.physbeh.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 101.Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depend. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]
- 102.San-Emeterio EP, Hurle MA. Modulation of brain apoptosis-related proteins by the opioid antagonist naltrexone in mice. Neurosci Lett. 2006;403:276–9. doi: 10.1016/j.neulet.2006.04.053. [DOI] [PubMed] [Google Scholar]
- 103.Sulik KK, Johnston MC. Sequence of developmental alterations following acute ethanol exposure in mice: craniofacial features of the fetal alcohol syndrome. Am J Anat. 1983;166:257–9. doi: 10.1002/aja.1001660303. [DOI] [PubMed] [Google Scholar]
- 104.Bonthius DJ, West JR. Acute and long-term neuronal deficits in the rat olfactory bulb following alcohol exposure during the brain growth spurt. Neurotoxicol Teratol. 1991;13:611–9. doi: 10.1016/0892-0362(91)90044-w. [DOI] [PubMed] [Google Scholar]
- 105.Barron S, Riley EP. The effects of prenatal alcohol exposure on behavioral and neuroanatomical components of olfaction. Neurtoxic Teratol. 1992;14:291–7. doi: 10.1016/0892-0362(92)90009-y. [DOI] [PubMed] [Google Scholar]
- 106.Donahue RN, McLaughlin PJ, Zagon IS. Low-dose naltrexone targets the opioid growth factor–opioid growth factor receptor pathway to inhibit cell proliferation: mechanistic evidence from a tissue culture model. Exp Biol Med. 2011;236:1036–50. doi: 10.1258/ebm.2011.011121. [DOI] [PubMed] [Google Scholar]
- 107.Graziadei PPC, Monti Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. I. Morphological aspects of differentiation and structural organization of the olfactory sensory neuron. J Neuroctytol. 1979;8:1–18. doi: 10.1007/BF01206454. [DOI] [PubMed] [Google Scholar]
- 108.Graziadei PPC, Monti Graziadei GA. Neurogenesis and neuron regeneration in the olfactory system of mammals. III. Deafferentation and reinnervation of the olfactory bulb following section of the fila olfactoria. J Neuroctytol. 1980;9:145–62. doi: 10.1007/BF01205155. [DOI] [PubMed] [Google Scholar]
- 109.Rochefort C, Gheusi G, Vincent JD, Lledo PM. Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J Neurosci. 2002;22:2679–89. doi: 10.1523/JNEUROSCI.22-07-02679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rochefort C, Lledo PM. Short-term survival of newborn neurons in the adult olfactory bulb after exposure to a complex odor environment. Eur J Neurosci. 2005;22:2863–70. doi: 10.1111/j.1460-9568.2005.04486.x. [DOI] [PubMed] [Google Scholar]
- 111.Hudson R. From molecule to mind: the role of experience in shaping olfactory function. J Comp Physiol [A] 1999;185:297–304. doi: 10.1007/s003590050390. [DOI] [PubMed] [Google Scholar]
- 112.McLauglin P, Tobias SW, Lang M, Zagon IS. Opioid receptor blockade during prenatal life modifies postnatal behavioral development. Pharm Biochem Behav. 1997;58:1075–82. doi: 10.1016/s0091-3057(97)00307-9. [DOI] [PubMed] [Google Scholar]