Abstract
Epithelial ovarian cancer (EOC) ranks first as the cause of death for gynecological cancers in the United States. SUZ12 is a component of the polycomb repressive complex 2 (PRC2) and is essential for PRC2-mediated gene silencing by generating trimethylation on lysine 27 residue of histone H3 (H3K27Me3). The role of SUZ12 in EOC has never been investigated. Here we show that SUZ12 is expressed at significantly higher levels in human EOC (n=117) compared with either normal human ovarian surface epithelium (n=35, p<0.001) or fallopian tube epithelium (n=15, p<0.001). There is a positive correlation between expression of SUZ12 and EZH2 in human EOC (p<0.001). In addition, expression of SUZ12 positively correlates with Ki67, a marker of cell proliferation (p<0.001), and predicts shorter overall survival (p=0.0078). Notably, knockdown of SUZ12 suppresses the growth of human EOC cells in vitro and in vivo in both orthotopic and subcutaneous xenograft EOC models. In addition, SUZ12 knockdown decreases the levels of H3K27Me3 and triggers apoptosis of human EOC cells. Mechanistically, we identified HRK, a pro-apoptotic gene, as a novel SUZ12 target gene, and demonstrated that HRK upregulation mediates apoptosis induced by SUZ12 knockdown in human EOC cells. In summary, we show that SUZ12 promotes the proliferation of human EOC cells by inhibiting apoptosis and HRK is a novel SUZ12 target gene whose upregulation contributes to apoptosis induced by SUZ12 knockdown.
Keywords: epithelial ovarian cancer, SUZ12, EZH2, HRK, apoptosis
Introduction
Over 85% of ovarian cancers are of epithelial origin (1). Epithelial ovarian cancers (EOC) are classified into distinct histological types including serous, mucinous, endometrioid, and clear cell (1). The most common histology of EOC is serous (~60% of all cancers) and less common histologies include endometrioid, clear cell, and mucinous (2). Recently, an alternative classification has been proposed, in which EOC is broadly divided into two types (3). Type I EOC includes mucinous, low-grade serous, low-grade endometrioid and clear cell carcinomas, and type II EOC includes high-grade serous and high-grade endometrioid carcinomas (3). EOC remains the most lethal gynecological malignancy in the United States (4). Thus, there is an urgent need to better understand the etiology of EOC in order to develop novel therapeutics for this disease.
SUZ12 is essential for polycomb repressive complex 2 (PRC2) mediated gene silencing (5–8). In addition to SUZ12, PRC2 also contains the catalytic subunit EZH2 (9). PRC2 epigenetically silences gene transcription by generating trimethylation on lysine 27 residue of histone H3 (H3K27Me3). EZH2 lacks methyltransferase enzyme activity on its own, and has to complex with SUZ12 to attain histone methyltransferase activity (9, 10).
EZH2 is overexpressed in several types of cancers, including prostate and breast cancers (11–15). Its overexpression correlates with the aggressiveness and poor prognosis in breast and prostate cancers (11–13). Indeed, EZH2 is often overexpressed in human EOC and its knockdown triggers apoptosis of human EOC cells (16, 17). By contrast, the role SUZ12 in EOC remains poorly understood.
Here we examined the expression of SUZ12 in human EOC specimens and discovered that SUZ12 is expressed at significantly higher levels in human EOCs compared with either normal human ovarian surface epithelium or fallopian tube epithelium. In addition, we demonstrated that SUZ12 expression positively correlates with expression of EZH2 and Ki67, a cell proliferation marker. Conversely, SUZ12 knockdown suppresses the growth of human EOC cells in vitro and in vivo in xenograft EOC models. Consistently, SUZ12 knockdown induces apoptosis of human EOC cells. Mechanistically, we identified HRK, a pro-apoptotic gene, as a novel SUZ12 target gene whose upregulation contributes to apoptosis induced by SUZ12 knockdown in human EOC cells.
Materials and Methods
Cell culture
Human EOC cell lines SKOV3, PEO1 and OVCAR10 were cultured according to American Type Culture Collection (ATCC) and as we have previously described (16, 18). The cell line identification was confirmed by DNA Diagnostic Center (www.dnacenter.com).
FACS, immunoflurescence staining, and immunoblot analysis
FACS and indirect immunoflurescence (IF) staining were performed as described previously (19). The following antibodies were used for IF: rabbit anti-H3K27Me3 (Cell Signaling, 1:1,000), and rabbit anti-H3K9Me2 (Abcam, 1:500). The antibodies used for immunoblot were from indicated suppliers: rabbit anti-H3K27Me3 (Cell signaling, 1:1,000), rabbit anti-H3K9Me3 (Abcam, 1:2,000), mouse anti-histone H3 (Millipore, 1:10,000), mouse anti-GAPDH (Millipore, 1:10,000), rabbit anti-PARP p85 fragment (Promega, 1:1,000), rabbit anti-cleaved caspase 3 (Cell Signaling, 1:1,000), and rabbit anti-cleaved Lamin A (Cell signaling, 1:1,000) and mouse anti-HA (Cell signaling, 1:1,000). Mouse anti-SUZ12 (220A) was as described previously (20).
siRNA, shRNA, lentivirus packaging, and infection
The sense sequences of 2 individual shRNA to the human SUZ12 gene (shSUZ12) are: 5′-GCTTACGTTTACTGGTTTCTT-3′ and 5′-CGGAATCTCATAGCACCAATA -3′, respectively. Lentivirus packaging was performed using virapower system (Invitrogen) according to manufacturer’s instruction. PEO1 and SKOV3 at 40% to 50% confluence were infected with lentivirus expressing shSUZ12 or vector control. The infected cells were selected with 1 μg/mL (for PEO1) or 3 μg/mL (for SKOV3) of puromycin, respectively. siHRK was purchased from Dharmacon (Cat: L-008216-00-0005) and transfection was performed following the manufacturer’s instruction. A siRNA to luciferase (siGL2) was used as a negative control.
Inducible expression of shRNA resistant SUZ12
To generate shRNA resistant SUZ12 expression construct that do not affect the protein sequence, but resistant to the shSUZ12 #1, 3 rounds of mutagenesis were carried out to mutate every third base of the coding region in SUZ12 open reading frame (ORF) targeted by shSUZ12 #1 using Quikchange II XL Site-Directed Mutagenesis kit (Stratagene, Cat. No: 200521). Mutagenic primers are as the following: Round 1 : forward: 5′-GTCAGCTCATTTGCAACTCACATTCACGGGTTTCTTCCAC-3′ and reverse: 5′-GTGGAAGAAACCCGTGAATGTGAGTTGCAAATGAGCTGAC-3′; Round 2 forward: 5′-CTCACATTCACGGGCTTTTTCCACAAAAATGATAAGC-3′ and reverse: 5′-GCTTATCATTTTTGTGGAAAAAGCCCGTGAATGTGAG-3′; and Round 3 forward: 5′-GTCAGCTCATTTGCAATTGACATTCACGGGCTTTTTCC-3′ and reverse: 5′-GGAAAAAGCCCGTGAATGTCAATTGCAAATGAGCTGAC-3′. The shRNA resistant SUZ12 was then sub-cloned into an inducible retroviral vector pRetroXTight-Pur (Retro-X Tet-On, Invitrogen) and the inducible SUZ12 SKOV3 cell line was generated following manufacturer’s instruction. Twenty-four hours after infection with shSUZ12 #1 virus, shRNA resistant SUZ12 was induced by DOX (Clontech, 500 ng/ml) following manufacturer’s instruction.
Human ovarian tissue microarrays and specimens
Tissue microarrays, including core samples from 117 primary human EOCs, 35 cases of normal ovary tissues and 15 cases of fallopian tube tissues were obtained from FCCC Biosample Repository Core Facility. Use of these human specimens was approved by the Institutional Review Board.
Immunohistochemical staining and scoring
The expression of SUZ12 was detected using avidin–biotin–peroxidase methods and as previously described (18). Briefly, tissue sections were subjected to antigen retrieval by steaming in 0.01 mol/L of sodium citrate buffer (pH 6.0) for 30 minutes. After quenching endogenous peroxidase activity with 3% hydrogen peroxide and blocking nonspecific protein binding with 1% bovine serum albumin, sections were incubated overnight with primary monoclonal SUZ12 antibody (220A 1:40) at 4°C, followed by biotinylated goat anti-mouse IgG (DAKO, 1:400) for 1 hour, detecting the antibody complexes with the labeled streptavidin-biotin system (DAKO), and visualizing them with the chromogen 3,30-diaminobenzidine. Sections were lightly counterstained with hematoxylin. In addition, anti-EZH2 (Millipore; 1:100) and anti-Ki67 (Dako, 1:100) antibodies were used on consecutive sections as we have previously described (16).
RNA isolation, qRT-PCR and PCR array
RNA was isolated using Trizol (Invitrogen) according to manufacturer’s instruction. For qRT-PCR, Trizol-isolated RNA was further purified using an RNeasy kit (Qiagen) following manufacture’s instruction. The primers for HRK genes used for qRT-PCR are: forward: 5′-GCAACAGGTTGGTGAAAACCCT-3′ and reverse: 5′-ATTGGGGTGTCTGTTTCTGCAGC-3′. Expression of the housekeeping gene β-2-microglobulin was used to normalize mRNA expression. Apoptosis genes PCR array was purchased from SABiosciences (Cat: PAHS-012A) and the analysis was performed following the manufacturer’s instruction. The real-time PCR data was analyzed by using RT2 Profiler PCR Array Data Analysis version 3.4. For survival analysis, RNA was isolated and amplified from microdissected high-grade serous primary ovarian tumor specimens as described previously (21). All specimens and their corresponding clinical information were obtained under IRB-approved protocols. qRT-PCR for SUZ12 was performed using the following primers: forward 5′-CCGAGCACTGTGGTTGAGTA-3′; and reverse 5′-AACTGCATCTGATGGTGGTG-3′ using a SYBR Green One-Step qRT-PCR kit accordingly to the manufacturer’s instructions (Invitrogen). Expression of the housekeeping gene GAPDH was used to normalize mRNA expression.
Soft agar colony formation assay
A total of 5000 cells were inoculated in a 6-well plate in 1.5 ml of RPMI 1640 medium supplemented with 10% FBS and 0.35% agar on a base layer of 1.5 mL of the same medium containing 0.6% agar as previously described. Three weeks after plating, the cells were stained with 0.005% crystal violet (Sigma) in PBS to visualize the colonies.
Annexin V staining for detecting apoptotic cells
Phosphatidylserine externalization was detected using an Annexin V staining kit (Millipore) following manufacture’s instruction. Annexin V positive cells were detected by Guava system and analyzed with Guava Nexin software Module (Millipore).
In vivo tumorigenicity assay
The protocol was approved by Institutional Animal Care and Use Committee (IACUC). For orthotopic transplantation, SKOV3 cells were infected with a luciferase encoding retrovirus (hygro-pWZL-Luciferase) and infected cells were selected with 50 μg/mL hygromycin as previously described (18). Drug-selected cells were then infected with control or shSUZ12 encoding lentivirus and subsequently selected with 3 μg/mL puromycin and 50 μg/mL hygromycin. 3 × 105 drug-selected cells were unilaterally injected into ovarian bursa sac of immunocompromised mice with 9 mice in each group. From week 2 post infection, tumors were visualized by injecting luciferin (i.p.; 4mg/mice) resuspended in PBS and imaged with an IVIS Spectrum imaging system every two weeks until week 8. Images were analyzed using Live Imaging 4.0 software. At week 8, tumors were surgically dissected and fixed in 10% formalin. For subcutaneous transplantation, SKOV3 cells were infected with control or shSUZ12 encoding lentivirus and subsequently selected with 3μg/mL puromycin. 1×106 drug-selected cells were subcutanously injected into flank of immunocompromised mice with 5 mice in each group (16). Tumor growth was monitored every two weeks. Tumor volume was calculated by the equation V (mm3) = (axb2)/2, where a is the largest diameter and b is the perpendicular diameter. At week 5, tumors were surgically dissected and fixed in 10% formalin.
Chromatin Immunoprecipitation (ChIP)
SKOV3 cells were fixed with 1% formaldehyde for 15 min and quenched with 0.125 M glycine at room temperature as we have previously described (22). Chromatin was isolated by adding cell lysis buffer (1% SDS, 10mM EDTA, 50mM Tris-HCl, pH 8.1, 1mM PMSF) followed by sonication to shear the DNA to an average length of 300–500 bp. Lysates were pre-cleared with Salmon Sperm DNA/Protein A Agarose (Millipore, Cat. No:16-157) for 1–2 hr. The following antibodies were used to perform ChIP: anti-H3K27Me3 (C36B11, Cell signaling), anti-histone H3 (05-928, Millipore) or anti-SUZ12 (220A). An isotype matched IgG was used a negative control. ChIP DNA was purified by phenol-chloroform extraction and ethanol precipitation and analyzed with PCR against the genomic locus of the human HRK gene (Forward: 5′-GAA ACA AAA ACC CAA GAC TAA G-3′ and Reverse: 5′-TGA CTA CCT TGG GAA AGA ATG ATA G-3′). PCR products were visualized on a 2% agarose gel.
Statistical analysis
Quantitative data were expressed as mean ± SD, unless otherwise stated. Analysis of variance (ANOVA) with Fisher’s Least Significant Difference (LSD) was used to identify significant differences in multiple comparisons. The χ2 test was used to analyze the relationship between categorical variables. Overall survival was defined as the time elapsed from the date of diagnosis and the date of death from ovarian cancer, otherwise were considered as censored observations. Disease-free survival was defined as the time elapsed from the date of surgery and the date of the first recurrence. Kaplan–Meier survival plots were generated and comparisons made using the log-rank statistic. For all statistical analyses, the level of significance was set at 0.05.
Results
SUZ12 is expressed at significantly higher levels in human EOCs compared with either normal human ovarian surface epithelium or fallopian tube epithelium
We sought to determine the expression of SUZ12 protein in human EOCs by IHC. The specificity of the anti-SUZ12 antibody was confirmed in our study. A single band at the predicted molecular weight (~83kDa) was detected in human SKOV3 cells and was absent after expression of a short hairpin RNA to the human SUZ12 gene (shSUZ12) that effectively knocked down SUZ12 mRNA expression (Figure S1A and data not shown). In addition, SUZ12 staining was lost when primary anti-SUZ12 antibody was replaced with an isotype-matched IgG control (Figure S1B).
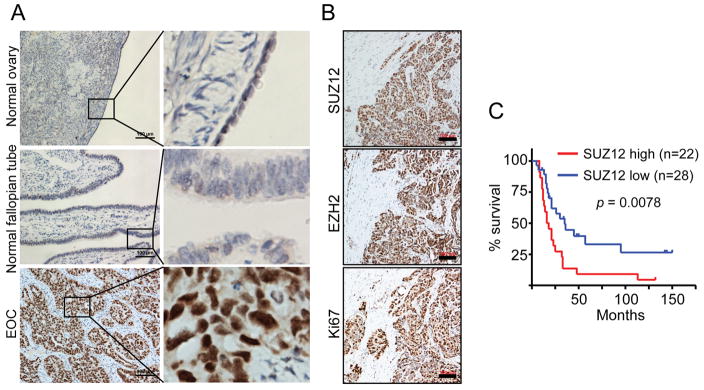
We next examined SUZ12 expression in 117 cases of human EOCs and 35 cases of normal human ovary with surface epithelium. Notably, there is recent evidence to suggest that a proportion of EOC may arise from distant fallopian tube epithelium (23, 24). Thus, we also included 15 cases of normal human fallopian tube specimens in our study. As shown in Figure 1A and S1C, in all four major histosubtypes of human EOCs, the nuclei was positive for SUZ12 IHC staining. In contrast, SUZ12 staining in normal human ovarian surface epithelial cells or fallopian tube epithelial cells was markedly weaker (Figure 1A). We scored expression of SUZ12 as high (H score ≥50) or low (H score <50) based on a histological score as previously described (25, 26). SUZ12 was scored as high in 76.9% (90/117) of human EOCs. In contrast, SUZ12 was scored high in 20% (7/35) and 13.3% (2/15) of normal human ovarian surface epithelium and fallopian tube epithelium, respectively (Table 1). Statistical analysis revealed that SUZ12 was expressed at significantly higher levels in human EOCs compared with either normal human ovarian surface epithelium (p<0.001) or fallopian tube epithelium (p<0.001) (Table 1). Consistently, SUZ12 was expressed at higher levels in human EOC cell lines compared with normal human ovarian surface epithelial cells (Figure S1D). On the basis of these studies, we conclude that SUZ12 is expressed at significantly higher level in human EOCs compared with either normal human ovarian surface epithelium or fallopian tube epithelium.
Figure 1. SUZ12 is expressed at significantly higher levels in human EOCs compared with normal human ovarian surface epithelium or fallopian tube epithelium, and predicts poor overall survival in EOC patients.
A, examples of SUZ12 IHC staining in normal human ovarian surface epithelium, fallopian tube epithelium and human EOC (shown is an example of high-grade serous histosubtype EOC specimen). Bar = 100 μm. B, representative images from consecutive, serial sections of the same EOC specimen depicting the positive correlation between expression of SUZ12 and expression of EZH2 and Ki67, a cell proliferation marker. Bar = 100 μm. C, A higher SUZ12 expression correlates with a shorter overall survival in EOC patients. The univariate overall survival curve (Kaplan-Meier method) for EOC patients with high (n = 22) or low (n = 28) SUZ12 expression based on median levels of mRNA expression determined by qRT-PCR in a cohort of 50 EOC patients (p = 0.0078). The p value was calculated based on Log-rank (Mantel-Cox) test using GraphPad Prism version 5.0 software.
Table 1.
SUZ12 expression in human EOCs and its correlation with clinicopathological variables or expression of EZH2 and Ki67.
| Patient characteristics | SUZ12 protein expression
|
p | |||
|---|---|---|---|---|---|
| low (n) | high (n) | total (n) | high (%) | ||
| Normal tissue | |||||
| normal ovary epithelial | 28 | 7 | 35 | 20.0% | <0.001* |
| Fallopian tube | 13 | 2 | 15 | 13.3% | <0.001** |
| Epithelial ovarian cancer | 27 | 90 | 117 | 76.9% | |
| Type I | 7 | 17 | 24 | 70.8% | |
| low grade serous | 4 | 1 | 5 | ||
| mucinous | 1 | 2 | 3 | ||
| low grade Endometrioid | 2 | 5 | 7 | ||
| clear cell | 0 | 8 | 8 | ||
| other | 0 | 1 | 1 | ||
| Type II | 20 | 73 | 93 | 78.5% | |
| high grade serous | 16 | 71 | 87 | ||
| other(high grade endometrioid) | 4 | 2 | 6 | ||
| age | |||||
| ≤55 | 11 | 33 | 44 | 75.0% | |
| >55 | 16 | 57 | 73 | 78.1% | 0.701 |
| Laterality | |||||
| Left | 8 | 25 | 33 | 75.8% | |
| Right | 5 | 17 | 22 | 77.3% | |
| Bilaterality | 14 | 47 | 61 | 77.1% | 0.988 |
| undetermined | 0 | 1 | 1 | ||
| Ki67# | |||||
| 0–10% | 16 | 9 | 25 | 36.0% | |
| 10%–40% | 6 | 18 | 24 | 75.0% | |
| 40%–100% | 5 | 63 | 68 | 92.7% | <0.001 |
| EZH2 expression## | |||||
| low | 19 | 22 | 41 | 53.7% | |
| high | 8 | 68 | 76 | 89.5% | <0.001 |
| Tumor stage | |||||
| Stage 1/2 | 10 | 20 | 30 | 66.7% | |
| Stage 3/4 | 17 | 70 | 87 | 80.5% | 0.122 |
Compared with epithelial ovarian cancer, p<0.001;
compared with serous epithelial ovarian cancer, p<0.001;
Spearman’s Rank Order Correlation also showed SUZ12 positively corelated with KI67 (p<0.001), and rs is 0.465;
Spearman’s Rank Order Correlation also showed SUZ12 positively corelated with EZH2 (p<0.001), and rs is 0.515.
SUZ12 expression positively correlates with expression of EZH2 and Ki67, and predicts shorter overall survival
We next sought to determine the correlation between expression of SUZ12 and EZH2, or Ki67, a marker of cell proliferation. We examined the expression of EZH2 and Ki67 by IHC in the same set of human EOC specimens (Figure 1B and Table 1). There was a significant positively correlation between expression of SUZ12 and EZH2 (p<0.001) or Ki67 (p<0.001) (Table 1). We next examined the correlation between SUZ12 expression and clinical and pathological features of human EOCs. There is a trend towards significance between expression of SUZ12 and tumor stage (p=0.122) (Table 1). Notably, the majority of the examined EOC cases are type II high-grade serous subtypes that are typically of stage III/IV (Table 1). Further, we assessed the correlation between SUZ12 expression and prognosis of EOC patients (n=50), for which long-term follow-up data were available. There was significant correlation between expression of SUZ12 and overall survival in EOC patients (p=0.0078) (Figure 1C). Based on these results, we conclude that SUZ12 expression positively correlates with expression of EZH2 and Ki67, and predicts poor overall survival in EOC patients.
SUZ12 knockdown suppresses the growth of human EOC cells in vitro and in vivo
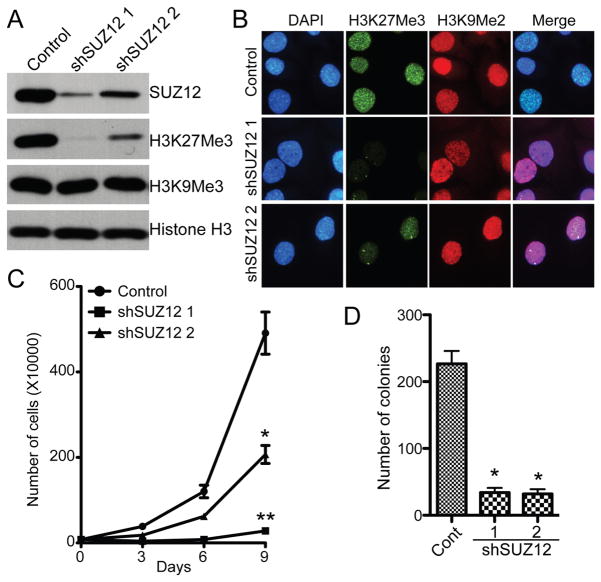
Since SUZ12 expression correlates with expression of a cell proliferation marker Ki67, we sough to determine the effects of SUZ12 knockdown on proliferation of human EOC cells. Toward this goal, we utilized 2 individual shRNAs to the human SUZ12 gene (shSUZ12). The knockdown efficacy of shSUZ12 in SKOV3 human cells was confirmed by immunoblot (Figure 2A). Notably, the level of H3K27Me3 was significantly reduced in shSUZ12 expressing SKOV3 cells as determined by both immunoblot and immunofluorescence staining (Figure 2A–B). By contrast, shSUZ12 has no effects on the level of trimethylated lysine 9 histone H3 (H3K9Me3) that is generated by the histone methyltransferases such as Suv39H1, Suv39H2 and SETDB1 (Figure 2A–B) (27). Together, these data support the premise that SUZ12 is necessary for H3K27Me3 epigenetic modification in human EOC cells.
Figure 2. SUZ12 knockdown inhibits the growth of human EOC cells in vitro.
A, SKOV3 cells were infected with 2 individual lentivirus encoded shSUZ12 or control. 3 days post drug-selection, cells were examined for expression of SUZ12, H3K27Me3, H3K9Me3 and histone H3 by immunoblot. B, same as A, but examined for expression of H3K27Me3 and H3K9Me2 by immunofluorescence staining. DAPI counterstaining was used to visualize the cell nuclei. C, same as A, but equal number of drug-selected cells were seeded and counted at indicated time points using trypan blue exclusion assay that only counts for the viable cells. In addition, the cells were ensured to be in log phase throughout the experiments by splitting them before they reached confluence. Mean of 3 independent experiments with SD. * p=0.001 and ** p<0.001 compared with controls. D, same as A, 5000 drug-selected cells were seeded in soft agar and the number of colonies were counted after 3 weeks of culture. Mean of 3 independent experiments with SD. * p<0.001 compared with controls.
Compared with controls, SUZ12 knockdown significantly inhibited both anchorage-dependent and -independent growth in soft agar in SKOV3 cells (p <0.001) (Figure 2C-D). In addition, SUZ12 knockdown also decreased the expression levels of H3K27Me3 and suppressed both anchorage-dependent and -independent growth in PEO1 cells (Figure S2), suggesting that the observed growth inhibitory effects are not cell line specific.
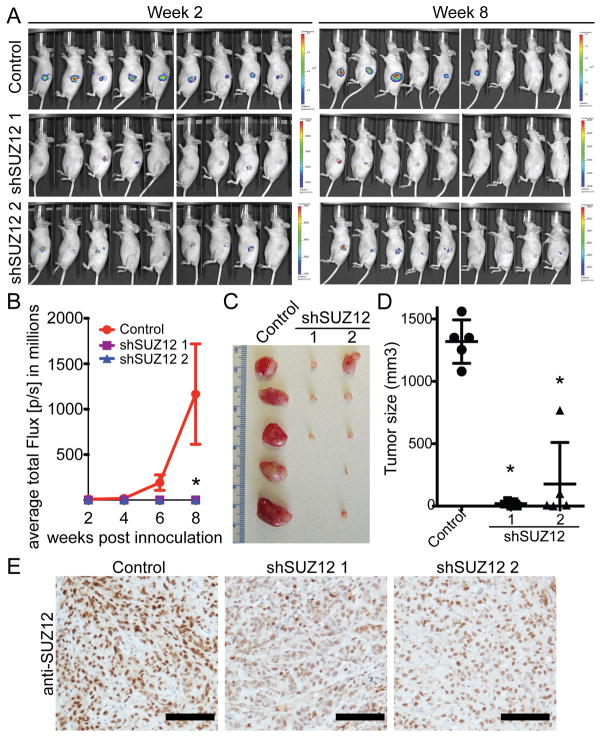
We next sought to determine the effects of SUZ12 knockdown on the growth of SKOV3 cells in vivo in immunocompromised mice. Toward this goal, a luciferase gene was retrovirally transduced into control or shSUZ12-expressing SKOV3 cells to monitor the cell growth in vivo via noninvasive imaging as previously described (18). These cells were injected unilaterally into the bursa sac covering the ovary in female immunocompromised mice (n=9 for each of the groups). Tumor growth was monitored by measuring luciferase activity every 2 weeks starting at week 2 after injection, and the tumor growth was followed for a total of 8 weeks. Indeed, SUZ12 knockdown significantly suppressed the orthotopically xenografted SKOV3 human EOC cells compared with controls (Figure 3A–B). In addition, we also examined the growth of control and shSUZ12-expressing SKOV3 cells in a subcutaneous (s.c.) xenograft model in immunocompromised mice. The same growth inhibitory effects of shSUZ12 were also observed in the s.c. xenograft EOC model (Figure 3C–D). SUZ12 knockdown in the surgically dissected xenografted tumors was confirmed by IHC staining (Figure 3E). Based on these results, we conclude that SUZ12 knockdown suppresses the growth of human EOC cells in vivo in both orthotopic and s.c. xenograft EOC models.
Figure 3. SUZ12 knockdown inhibits the growth of human EOC cells in vivo.
A, SKOV3 cells were transduced with luciferase-encoding hygromycin-resistant retrovirus together with a puromycin-resistant shSUZ12 encoding lentivirus or control. 3×105 drug-selected cells were unilaterally injected into the periovarian bursa sac of the female immunocompromised mice (n = 9 for each of the groups.). The radiance of luciferase bioluminescence, an indicator of the rate of tumor growth, was measured every other week from week 2 until week 8 by using the IVIS imaging system. Shown are images taken at week 2 and week 8, respectively. B, quantification of tumor growth from the indicated cells. * p=0.009 compared with controls. C, 1×106 drug-selected control and indicated shSUZ12-expressing SKOV3 cells were inject subcutaneously (s.c.) into immunocompromised mice (n = 5 for each of the groups). 5 weeks postinjection, tumors were surgically removed from mice. D, quantitation of C, the size of tumors was measured. Mean of tumor sizes with SEM. P<0.001 compared with controls. E, xenografted tumors formed by control or indicated shSUZ12-expressing SKOV3 cells were sectioned and stained for SUZ12 expression. Bar = 100 μm.
SUZ12 knockdown triggers apoptosis of human EOC cells
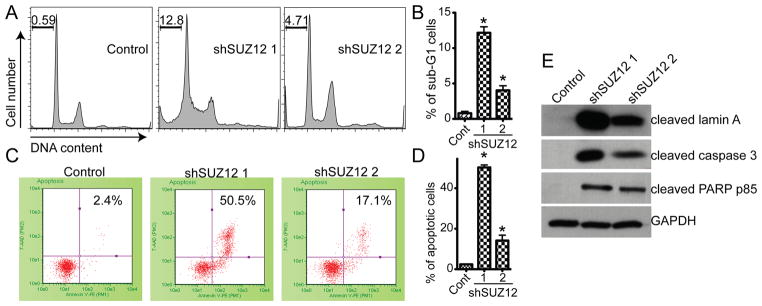
Next, we sought to determine the cellular mechanism by which SUZ12 knockdown inhibits the growth of human EOC cells. DNA content analysis determined by FACS showed that there was an increase in the percentage of the sub-G1 phase in SUZ12 knockdown cells compared with controls (Figure 4A–B), suggesting that these cells may undergo apoptosis. Indeed, markers of apoptosis were significantly induced in SUZ12 knockdown cells compared with controls. Those markers include increased percentage of Annexin V positively stained cells as measured by Guava Nexin assay and upregulation of cleaved Lamin A, PARP p85 and caspase 3 as determined by immunoblot (Figure 4C–E). The degree of apoptosis induced by shSUZ12 correlated with the level of SUZ12 knockdown by 2 individual shSUZ12 (Figure 4), suggesting that the observed apoptosis in SUZ12 knockdown EOC cells was not due to off-target effects.
Figure 4. SUZ12 knockdown triggers apoptosis of human EOC cells.
A, control and shSUZ12 expressing SKOV3 cells were examined for cell cycle distribution by FACS. The percentage of sub-G1 cells was indicated. B, quantitation of A. Mean of three independent experiments with SD. * p<0.001 compared with controls. C, control and indicated shSUZ12-expressing SKOV3 cells were stained for Annexin V, a cell surface marker of apoptosis. Annexin V-positive cells were measured by Guava Nexin assay. The percentage of Annexin V positive cells was indicated. D, quantitation of C. Mean of three independent experiments with SD. * p<0.001 compared with controls. E, same as A and C, but examined for expression of cleaved lamin A, caspase 3 and PARP p85, in control and indicated shEZH2-expressing cells.
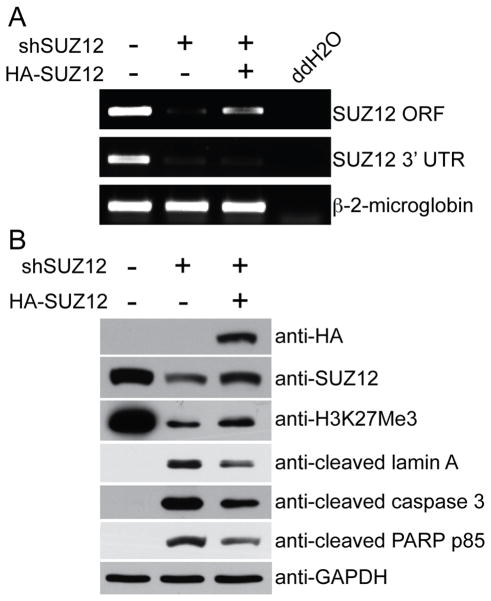
To further limit the potential off-target effect, we performed the rescue experiments. Toward this goal, we generated a shSUZ12 #1 resistant HA-tagged SUZ12-expressing construct that expresses the wild type protein. Expression of shSUZ12 efficiently knocked down the expression of endogenous SUZ12 mRNA as reflected by semi-quantitative RT-PCR using primers targeting the 3′ end UTR (Figure 5A). The expression of shSUZ12 #1 resistant HA-tagged SUZ12 was confirmed at both the mRNA level by semi-quantitative RT-PCR using primers targeting the ORF and at the protein level by immunblot using an anti-HA antibody (Figure 5A–B). Indeed, expression of HA-tagged shSUZ12-resistant wild type SUZ12 protein partially rescued the decrease of H3K27Me3 level and suppressed the expression of markers of apoptosis such as expression of cleaved Lamin A, PARP p85 and caspase 3 in SUZ12 knockdown SKOC3 cells (Figure 5B). Notably, the ectopic HA-tagged shSUZ12 #1 resistant wild type SUZ12 protein is expressed at a level lower than the endogenous SUZ12 level observed in SKOV3 cells (Figure 5A–B), suggesting that the observed effects are not due to supra-physiological levels of ectopic SUZ12 expression.
Figure 5. shSUZ12 resistant HA-tagged SUZ12 rescues apoptosis induced by SUZ12 knockdown.
A, SKOV3 cells were infected with a lentivirus encoding shSUZ12 (#1) together with control or an shSUZ12 resistant SUZ12 that expresses wild type SUZ12 protein. Expression of SUZ12 mRNA was determined by RT-PCR using primers designed to its 3′ UTR (only amplifies endogenous but not ectopic SUZ12 mRNA) or its open reading frame (ORF, amplifies both endogenous and ectopic SUZ12 mRNA). B, same as A, but examined for expression of HA, SUZ12, H3K27Me3, GAPDH and markers of apoptosis including cleaved lamin A, caspase 3 and PARP p85.
HRK is a SUZ12 target gene that contributes to apoptosis induced by SUZ12 knockdown
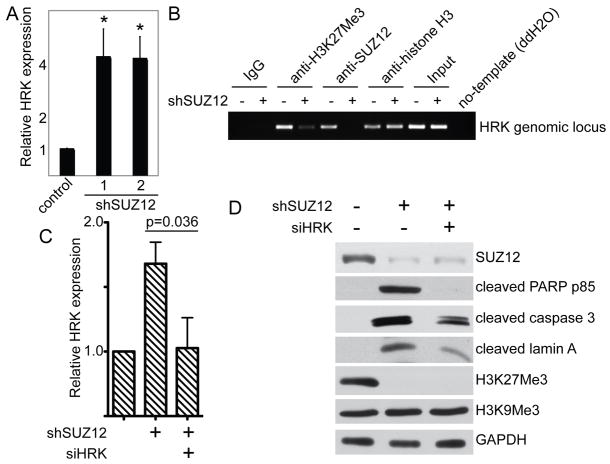
Next, we sought to determine the molecular mechanism by which SUZ12 knockdown induces apoptosis of human EOC cells. Toward this goal, we sought to identify the direct SUZ12 target genes that are implicated in promoting apoptosis observed in SUZ12 knockdown EOC cells. We compared the expression of apoptosis-regulating genes between control and SUZ12 knockdown SKOV3 cells using a qRT-PCR array that consists of 84 apoptosis-regulating genes (Table S1 and Figure S3). We hypothesized that apoptosis-promoting genes are epigenetically silenced by SUZ12 in EOC cells via H3K27Me3 and SUZ12 knockdown induces apoptosis of EOC cells by upregulating the expression of these genes. Thus, we cross-examined the apoptosis-regulating qRT-PCR array data in SKOV3 cells with a H3K27Me3 chromatin immunoprecipitation followed by next generation sequencing (ChIP-seq) dataset in SKOV3 cells (28). This analysis revealed two H3K27Me3 direct target genes that are upregulated >2-fold in SUZ12 knockdown SKOV3 cells, namely, HRK and TNFRSF9.
BH3-only Bcl-2 family member Harakiri (HRK) is a pro-apoptotic gene (29). Notably, HRK has been implicated in regulating apoptosis of human ovarian cancer cells and murine ovarian reserve (30, 31). Thus, we tested the role of HRK in mediating apoptosis induced by SUZ12 knockdown. We confirmed the upregulation of HRK in SUZ12 knockdown SKOV3 cells by qRT-PCR (Figure 6A). Significantly, we demonstrated that SUZ12 directly binds to the HRK genomic locus by ChIP analysis (Figure 6B). Likewise, we showed that H3K27Me3 epigenetic marker is present in the HRK genomic locus (Figure 6B). Importantly, knockdown of SUZ12 severely diminished the association of SUZ12 and H3K27Me3 with the HRK genomic locus (Figure 6B). This result suggests that the presence of H3K27Me3 in HRK genomic locus is dependent upon SUZ12 and the association of SUZ12 to the HRK genomic locus is specific. It has previously been demonstrated that DNA methylation contributes to HRK downregulation in certain types of cancers such as prostate (32). Thus, we treated EOC cells with 5-azacytidine (5-AzaC), a DNA demethylation drug (33), and examined the expression of HRK in these cells. 5-AzaC failed to upregulate HRK expression in EOC cells (Figure S4). This result suggests that HRK expression is not regulated by DNA methylation in human EOC cells. Consistent with our findings, based on the newly released the Cancer Genome Atlas (TCGA) ovarian cancer database (34), HRK gene promoter methylation is very rare in human ovarian cancer (<1% cases show >50% of promoter methylation in human HRK gene promoter; http://cancergenome.nih.gov/) (34).
Figure 6. HRK is a novel SUZ12 target gene whose upregulation contributes to apoptosis induced by SUZ12 knockdown.
A, HRK mRNA expression was determined in SKOV3 cells expressing indicated shSUZ12 or control by qRT-PCR. Expression of the housekeeping gene β-2-microglobulin was used to normalize mRNA expression. * p<0.001 compared with controls. B, SKOV3 cells expressing shSUZ12 (#1) or control were subjected to ChIP analysis using antibodies against H3K27Me3 or SUZ12 using primers targeted to HRK genomic locus. A isotype matched IgG was used as a negative control and an anti-core histone H3 antibody was used as a positive control for ChIP analysis. C, SKOV3 cells express shSUZ12 (#1) with or without transfection with siRNA to the human HRK gene (siHRK). Expression of HRK mRNA in the indicated cells was determined by qRT-PCR. Expression of the housekeeping gene β-2-microglobulin was used to normalize mRNA expression. D, same as A, but examined for expression of SUZ12, H3K27Me3, H3K9Me3, GAPDH and markers of apoptosis including cleaved lamin A, caspase 3 and PARP p85.
Next, we sought to determine whether apoptosis induced by SUZ12 knockdown is mediated, in part, by upregulation of HRK. Toward this goal, we knocked down the HRK expression in SUZ12 knockdown cells. HRK knockdown was confirmed by qRT-PCR (Figure S5). Indeed, HRK knockdown notably suppressed the expression of markers of apoptosis such as the percentage of sub-G1 phase cells and expression of cleaved lamin A, PARP p85 and caspase 3, in SUZ12 knockdown cells (Figure 6C–D and S6). Similar results were also obtained in OVCAR10 human EOC cell line (Figure S7), demonstrating that the observed effects are not cell line specific. Based on these results, we conclude that HRK is a novel SUZ12 target gene whose upregulation contributes to apoptosis induced by SUZ12 knockdown in human EOC cells.
Discussion
Herein, we showed that SUZ12 is expressed at significantly higher levels in human EOCs compared with either normal human ovarian surface epithelium or fallopian tube epithelium. SUZ12 is located at 17q21 and gene amplification has been shown to contribute to SUZ12 upregulation in a number of cancer types (20). However, based on the newly released TCGA ovarian cancer database (34), SUZ12 gene amplification is very rare in human ovarian cancer (<1% cases show >3 copy of SUZ12 gene; http://cancergenome.nih.gov/), suggesting that gene amplification is not a major mechanism that underlies SUZ12 upregulation in human EOCs. Recently, microRNA has also been implicated in regulating the expression of SUZ12 (35). Nevertheless, future studies are warranted to elucidate the mechanisms by which SUZ12 is upregulated in human EOCs.
We demonstrated that SUZ12 expression positively correlates with expression of EZH2 in human EOCs (Figure 1B and Table 1). However, we cannot rule out the possibility that SUZ12 may also have additional EZH2-independent functions in EOC cells. Interestingly, it has previously been reported that H3K27Me3 levels do not correlate with the EZH2 levels in human EOC specimens (36). It is possible that additional factors such as H3K27Me3 demethylase JMJD3 and UTX may also regulate H3K27Me3 levels in EOC cells (37). In addition, SUZ12 expression correlates with expression of a cell proliferation marker, Ki67 (Figure 1B and Table 1). EZH2 has been demonstrated as a poor prognosis marker in a number of cancer types (38). Similarly, we found that a higher level of SUZ12 expression positively correlates with a poor over-all survival in human EOC patients. Collectively, PRC2 could serve as independent prognosis biomarkers for EOCs.
The pro-apoptotic HRK gene is located at 12q13.1 and is transcriptionally regulated in several cancer cell types (29, 39, 40). Interestingly, we discovered that HRK is a novel target of SUZ12 mediated gene silencing via H3K27Me3, and demonstrated that its upregulation contributes to apoptosis induced by SUZ12 knockdown in human EOC cells (Figure 6). Notably, SUZ12 knockdown severely diminished the H3K27Me3 level (Figure 2A). Thus, inhibition of SUZ12 by shRNA might affect pathways in addition to HRK in these cells. Consistently, it has been shown that SUZ12 also regulates the expression of genes implicated in cell cycle progression and DNA damage and repair (20). In the future, it will be interesting to investigate additional SUZ12 target genes that contribute to growth inhibition observed in SUZ12 knockdown EOC cells.
We showed that knockdown of SUZ12 is sufficient to decrease the levels of H3K27Me3 in human EOC cells (Figure 2). This is consistent with the premise that SUZ12 is required for PRC2 complex mediated gene silencing via H3K27Me3 epigenetic modification. Indeed, SUZ12 expression positively correlates with the expression of EZH2 (Figure 1B and Table 1). These results suggest that the interaction between SUZ12 and EZH2 may be an alternative target for inactivating PRC2 in addition to the methyltransferase activity of EZH2. Consistently with this idea, phosphorylation of EZH2 by CDK1 kinase at Thr487 is thought to decrease H3K27Me3 levels by disrupting the interaction between SUZ12 and EZH2 (41).
In summary, the data reported here show that SUZ12 is often overexpressed in human EOCs and its expression positively correlates with EZH2, a high proliferation index and a poor overall survival in EOC patients. Knockdown of SUZ12 suppresses the growth of human EOC cells in vitro and in vivo in both orthotopic and s.c xenograft EOC models. Indeed, knockdown of SUZ12 triggers apoptosis of human EOC cells. Further, these observed effects correlate with a decrease in H3K27Me3 level, suggesting that SUZ12 functions via PRC2 to suppress apoptosis of human EOC cells. Mechanistically, we identified the pro-apoptotic HRK gene as a novel SUZ12 target gene whose upregulation contributes to apoptosis induced by SUZ12 knockdown in human EOC cells.
Supplementary Material
Acknowledgments
Grant Support: R.Z. is an Ovarian Cancer Research Fund (OCRF) Liz Tilberis Scholar. This work was supported in part by a DOD ovarian cancer academy award (OC093420 to R.Z.). Support of Core Facilities used in this study was provided by Cancer Center Support Grant (CCSG) CA010815 to The Wistar Institute.
Footnotes
Disclosure of potential conflict of interest: None.
References
- 1.Arulkumaran S, Regan L, Farquharson DIM. Obstetrics and gynaecology. Oxford: Oxford University Press; 2011. [Google Scholar]
- 2.Farley J, Ozbun LL, Birrer MJ. Genomic analysis of epithelial ovarian cancer. Cell Res. 2008;18:538–48. doi: 10.1038/cr.2008.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shih Ie M, Kurman RJ. Ovarian tumorigenesis: a proposed model based on morphological and molecular genetic analysis. Am J Pathol. 2004;164:1511–8. doi: 10.1016/s0002-9440(10)63708-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ozols RF, Bookman MA, Connolly DC, Daly MB, Godwin AK, Schilder RJ, et al. Focus on epithelial ovarian cancer. Cancer Cell. 2004;5:19–24. doi: 10.1016/s1535-6108(04)00002-9. [DOI] [PubMed] [Google Scholar]
- 5.Cao R, Wang L, Wang H, Xia L, Erdjument-Bromage H, Tempst P, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–43. doi: 10.1126/science.1076997. [DOI] [PubMed] [Google Scholar]
- 6.Czermin B, Melfi R, McCabe D, Seitz V, Imhof A, Pirrotta V. Drosophila enhancer of Zeste/ESC complexes have a histone H3 methyltransferase activity that marks chromosomal Polycomb sites. Cell. 2002;111:185–96. doi: 10.1016/s0092-8674(02)00975-3. [DOI] [PubMed] [Google Scholar]
- 7.Kuzmichev A, Nishioka K, Erdjument-Bromage H, Tempst P, Reinberg D. Histone methyltransferase activity associated with a human multiprotein complex containing the Enhancer of Zeste protein. Genes Dev. 2002;16:2893–905. doi: 10.1101/gad.1035902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muller J, Hart CM, Francis NJ, Vargas ML, Sengupta A, Wild B, et al. Histone methyltransferase activity of a Drosophila Polycomb group repressor complex. Cell. 2002;111:197–208. doi: 10.1016/s0092-8674(02)00976-5. [DOI] [PubMed] [Google Scholar]
- 9.Cao R, Zhang Y. SUZ12 is required for both the histone methyltransferase activity and the silencing function of the EED-EZH2 complex. Mol Cell. 2004;15:57–67. doi: 10.1016/j.molcel.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Ketel CS, Andersen EF, Vargas ML, Suh J, Strome S, Simon JA. Subunit contributions to histone methyltransferase activities of fly and worm polycomb group complexes. Mol Cell Biol. 2005;25:6857–68. doi: 10.1128/MCB.25.16.6857-6868.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kleer CG, Cao Q, Varambally S, Shen R, Ota I, Tomlins SA, et al. EZH2 is a marker of aggressive breast cancer and promotes neoplastic transformation of breast epithelial cells. Proc Natl Acad Sci U S A. 2003;100:11606–11. doi: 10.1073/pnas.1933744100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachmann IM, Halvorsen OJ, Collett K, Stefansson IM, Straume O, Haukaas SA, et al. EZH2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J Clin Oncol. 2006;24:268–73. doi: 10.1200/JCO.2005.01.5180. [DOI] [PubMed] [Google Scholar]
- 13.Varambally S, Dhanasekaran SM, Zhou M, Barrette TR, Kumar-Sinha C, Sanda MG, et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature. 2002;419:624–9. doi: 10.1038/nature01075. [DOI] [PubMed] [Google Scholar]
- 14.Saramaki OR, Tammela TL, Martikainen PM, Vessella RL, Visakorpi T. The gene for polycomb group protein enhancer of zeste homolog 2 (EZH2) is amplified in late-stage prostate cancer. Genes Chromosomes Cancer. 2006;45:639–45. doi: 10.1002/gcc.20327. [DOI] [PubMed] [Google Scholar]
- 15.Bryant RJ, Cross NA, Eaton CL, Hamdy FC, Cunliffe VT. EZH2 promotes proliferation and invasiveness of prostate cancer cells. Prostate. 2007;67:547–56. doi: 10.1002/pros.20550. [DOI] [PubMed] [Google Scholar]
- 16.Li H, Cai Q, Godwin AK, Zhang R. Enhancer of zeste homolog 2 promotes the proliferation and invasion of epithelial ovarian cancer cells. Mol Cancer Res. 2010;8:1610–8. doi: 10.1158/1541-7786.MCR-10-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lu C, Han HD, Mangala LS, Ali-Fehmi R, Newton CS, Ozbun L, et al. Regulation of tumor angiogenesis by EZH2. Cancer Cell. 2010;18:185–97. doi: 10.1016/j.ccr.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bitler BG, Nicodemus JP, Li H, Cai Q, Wu H, Hua X, et al. Wnt5a suppresses epithelial ovarian cancer by promoting cellular senescence. Cancer Res. 2011;71:6184–94. doi: 10.1158/0008-5472.CAN-11-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nicoletti I, Migliorati G, Pagliacci MC, Grignani F, Riccardi C. A rapid and simple method for measuring thymocyte apoptosis by propidium iodide staining and flow cytometry. J Immunol Methods. 1991;139:271–9. doi: 10.1016/0022-1759(91)90198-o. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Perez D, Sanchez E, Maestre L, Suela J, Vargiu P, Di Lisio L, et al. Deregulated expression of the polycomb-group protein SUZ12 target genes characterizes mantle cell lymphoma. Am J Pathol. 2010;177:930–42. doi: 10.2353/ajpath.2010.090769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, et al. Restoration of p53 function leads to tumour regression in vivo. Nature. 2007;445:661–5. doi: 10.1038/nature05541. [DOI] [PubMed] [Google Scholar]
- 22.Tu Z, Aird KM, Bitler BG, Nicodemus JP, Beeharry N, Xia B, et al. Oncogenic RAS regulates BRIP1 expression to induce dissociation of BRCA1 from chromatin, inhibit DNA repair, and promote senescence. Developmental Cell. 2011 doi: 10.1016/j.devcel.2011.10.010. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurman RJ, Shih Ie M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34:433–43. doi: 10.1097/PAS.0b013e3181cf3d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levanon K, Crum C, Drapkin R. New insights into the pathogenesis of serous ovarian cancer and its clinical impact. J Clin Oncol. 2008;26:5284–93. doi: 10.1200/JCO.2008.18.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarty KS, Jr, Szabo E, Flowers JL, Cox EB, Leight GS, Miller L, et al. Use of a monoclonal anti-estrogen receptor antibody in the immunohistochemical evaluation of human tumors. Cancer Res. 1986;46:4244s–8s. [PubMed] [Google Scholar]
- 26.McCarty KS, Jr, Miller LS, Cox EB, Konrath J, McCarty KS., Sr Estrogen receptor analyses. Correlation of biochemical and immunohistochemical methods using monoclonal antireceptor antibodies. Arch Pathol Lab Med. 1985;109:716–21. [PubMed] [Google Scholar]
- 27.Jenuwein T. The epigenetic magic of histone lysine methylation. FEBS J. 2006;273:3121–35. doi: 10.1111/j.1742-4658.2006.05343.x. [DOI] [PubMed] [Google Scholar]
- 28.Li H, Bitler BG, Maradeo ME, Slifker M, Vathipadiekal V, Creasy CL, et al. Aldh1a1 is a novel EZH2 target gene in epithelial ovarian cancer identified by genome-wide approaches. doi: 10.1158/1940-6207.CAPR-11-0414. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inohara N, Ding L, Chen S, Nunez G. harakiri, a novel regulator of cell death, encodes a protein that activates apoptosis and interacts selectively with survival-promoting proteins Bcl-2 and Bcl-X(L) EMBO J. 1997;16:1686–94. doi: 10.1093/emboj/16.7.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin J, Page C, Jin X, Sethi AO, Patel R, Nunez G. Suppression activity of pro-apoptotic gene products in cancer cells, a potential application for cancer gene therapy. Anticancer Res. 2001;21:831–9. [PubMed] [Google Scholar]
- 31.Jurisicova A, Taniuchi A, Li H, Shang Y, Antenos M, Detmar J, et al. Maternal exposure to polycyclic aromatic hydrocarbons diminishes murine ovarian reserve via induction of Harakiri. J Clin Invest. 2007;117:3971–8. doi: 10.1172/JCI28493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakamura M, Shimada K, Konishi N. The role of HRK gene in human cancer. Oncogene. 2008;27 (Suppl 1):S105–13. doi: 10.1038/onc.2009.48. [DOI] [PubMed] [Google Scholar]
- 33.Baylin SB, Herman JG, Graff JR, Vertino PM, Issa JP. Alterations in DNA methylation: a fundamental aspect of neoplasia. Adv Cancer Res. 1998;72:141–96. [PubMed] [Google Scholar]
- 34.Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–15. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iliopoulos D, Lindahl-Allen M, Polytarchou C, Hirsch HA, Tsichlis PN, Struhl K. Loss of miR-200 inhibition of Suz12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells. Mol Cell. 2010;39:761–72. doi: 10.1016/j.molcel.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei Y, Xia W, Zhang Z, Liu J, Wang H, Adsay NV, et al. Loss of trimethylation at lysine 27 of histone H3 is a predictor of poor outcome in breast, ovarian, and pancreatic cancers. Mol Carcinog. 2008;47:701–6. doi: 10.1002/mc.20413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swigut T, Wysocka J. H3K27 demethylases, at long last. Cell. 2007;131:29–32. doi: 10.1016/j.cell.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 38.Simon JA, Lange CA. Roles of the EZH2 histone methyltransferase in cancer epigenetics. Mutat Res. 2008;647:21–9. doi: 10.1016/j.mrfmmm.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Imaizumi K, Morihara T, Mori Y, Katayama T, Tsuda M, Furuyama T, et al. The cell death-promoting gene DP5, which interacts with the BCL2 family, is induced during neuronal apoptosis following exposure to amyloid beta protein. J Biol Chem. 1999;274:7975–81. doi: 10.1074/jbc.274.12.7975. [DOI] [PubMed] [Google Scholar]
- 40.Sanz C, Benito A, Inohara N, Ekhterae D, Nunez G, Fernandez-Luna JL. Specific and rapid induction of the proapoptotic protein Hrk after growth factor withdrawal in hematopoietic progenitor cells. Blood. 2000;95:2742–7. [PubMed] [Google Scholar]
- 41.Wei Y, Chen YH, Li LY, Lang J, Yeh SP, Shi B, et al. CDK1-dependent phosphorylation of EZH2 suppresses methylation of H3K27 and promotes osteogenic differentiation of human mesenchymal stem cells. Nat Cell Biol. 2011;13:87–94. doi: 10.1038/ncb2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.