Abstract
Background & Aims
Porphyria cutanea tarda (PCT) is an iron-related disorder caused by reduced activity of hepatic uroporphyrinogen decarboxylase (UROD); it can be treated by phlebotomy or low doses of hydroxychloroquine. We performed a prospective pilot study to compare the efficacy and safety of these therapies.
Methods
We analyzed data from 48 consecutive patients with well-documented PCT to characterize susceptibility factors; patients were treated with phlebotomy (450 mL, every 2 weeks until they had serum ferritin levels of 20 ng/mL) or low-dose hydroxychloroquine (100 mg orally, twice weekly, until at least 1 month after they had normal plasma levels of porphyrin). We compared the time required to achieve a normal plasma porphyrin concentration (remission, the primary outcome) for 17 patients treated with phlebotomy and 13 treated with hydroxychloroquine.
Results
The time to remission was a median 6.9 months for patients that received phlebotomy and 6.1 months for patients treated with hydroxychloroquine treatment (6.7 and 6.5 months for randomized patients), a difference that was not significant (Log Rank P=.06 and P=.95, respectively). The sample size was insufficient to confirm noninferiority of hydroxychloroquine treatment (hazard ratio [HR], 2.19; 95% confidence interval [CI], 0.95–5.06) for all patients. Patients that received hydroxychloroquine had substantially better compliance. There were no significant side effects of either treatment.
Conclusions
Hydroxychloroquine, 100 mg twice weekly, is as effective and safe as phlebotomy in patients with PCT, although noninferiority was not established. Given these results, higher-dose regimens of hydroxychloroquine, which have more side effects, do not seem justified. Compliance was better and projected costs were lower for hydroxychloroquine than phlebotomy treatment. Long-term studies are needed to compare durability of response.
Keywords: 4-aminoquinolines, clinical trial, drug, liver disease
INTRODUCTION
Porphyria cutanea tarda (PCT), the most common porphyria, is due to inhibition in the liver of, uroporphyrinogen decarboxylase (UROD), the fifth enzyme in the heme biosynthetic pathway, to less than ~20% of normal, and accumulation of highly carboxylated porphyrinogens as the corresponding oxidized porphyrins.1 These are photoactivated with production of reactive oxygen species that damage the skin, causing increased friability and blistering. A uroporphomethene inhibitor of UROD has been isolated from the liver of mice with biochemical features of PCT.2 Hepatic siderosis is found in up to 90% of cases, and a normal or increased amount of iron is necessary for PCT to develop in humans or laboratory rodents.1, 3–8 PCT is heterogeneous in terms of multiple susceptibility factors found in individual patients.9
PCT responds readily to treatment either by repeated phlebotomy or a low dose regimen of a 4-aminoquinoline antimalarial – either hydroxychloroquine or chloroquine.1 Many uncontrolled studies have demonstrated efficacy for phlebotomies.10–16 The recommended approach is to remove a unit of whole blood (450 ml) at 2-week intervals until the ferritin level reaches ~20 ng/mL;17, 18 with most patients requiring 5–8 phlebotomies to achieve remission.10, 19 Disadvantages include expense, discomfort, inconvenience, syncope, venous access problems and the need to monitor serum ferritin to avoid iron deficiency anemia from too many phlebotomies or incomplete remission from too few.
4-Aminoquinoline antimalarials were found to cause hepatotoxicity and increases in photosensitivity and porphyrin excretion in PCT, followed by complete remission.20–23 Low-dose regimens, usually 125–250 mg chloroquine or 100–200 mg of hydroxychloroquine twice weekly, were found to be effective and avoided these transient adverse effects.19, 24–32 Advantages include low cost, convenient oral dosing and fewer clinic visits. However, the rationale and mechanism of action of these drugs are not established, their use for this indication is off-label, suitable dosage forms are not available, tablets are not scored for division, and it is not established how treatment should be monitored and when it should be stopped. Potential hepatotoxicity and retinal damage with prolonged use and hemolysis particularly in patients with glucose-6- phosphate dehydrogenase (G6PD) deficiency are also concerns.1
These drugs may interact with the large amounts of porphyrins stored in acidic hepatocyte organelles such as lysosomes resulting in their release into plasma.33 Other postulated mechanisms such as inhibition of hepatic δ-aminolevulinate synthase activity and increased urinary iron excretion34–36 are less plausible. These drugs are avoided in patients with severe liver damage or advanced renal insufficiency, in the latter because the excess porphyrins released into plasma are not effectively dialyzed. Hydroxychloroquine may have fewer side effects than chloroquine and is more widely used for other treatment indications.37
Few studies have compared the safety and effectiveness of these treatments prospectively,19, 26 and none have compared compliance, to our knowledge. We designed an unblinded pragmatic trial comparing these two therapies in achieving the clear biochemical endpoint of a normal plasma porphyrin concentration. The study enrolled all patients seen with PCT at this center over a 4 year period, with randomization when appropriate criteria were met.
METHODS
Study design
This unblinded study was approved by the Institutional Review Board and conducted at the Institute for Translational Science Clinical Research Center (ITS-CRC) at UTMB, with written informed consent from all subjects. All authors had access to the study data and reviewed and approved the final manuscript. Diagnostic criteria were: a) typical friability and blistering lesions affecting sun-exposed areas, b) elevated plasma porphyrins (>0.9 mcg/dL) and a fluorescence emission peak at 618–620 nm in plasma diluted at neutral pH,38 c) elevated urinary porphyrins (>300 nmol/L, nmol/24h or nmol/g creatinine) and d) increases in highly carboxylated porphyrins in urine or plasma. Other porphyrias were excluded. Biochemical testing was carried out by the Porphyria Laboratory at the University of Texas Medical Branch (UTMB).39
Subjects were assessed for the following susceptibility factors: a) alcohol use b) smoking, c) use of estrogens, d) HCV infection, e) HFE mutations, f) HIV infection and g) UROD mutations (determined by the Porphyria Center at Mount Sinai Medical Center, New York, NY).40
Randomization
Eligible patients were randomized to treatment in blocks of 6. Contraindications to randomization included refusal, or a contraindication or poor tolerance to either treatment in the past. Contraindications for hydroxychloroquine including those found in product labeling were: lactation, pregnancy, psoriasis, retinal disease, G6PD deficiency, recent and continued use of hepatotoxic drugs, serum bilirubin >3mg/dL, serum alanine aminotransferase >200 U/L, or institutional normalized ratio (INR) >1.4, serum creatinine #x0003E;3 mg/dL, serum ferritin >600 ng/mL, or greater 0than the upper limit of normal in the presence of homozygous or compound heterozygous HFE mutations. PCT patients with hemochromatosis are reportedly resistant to chloroquine,41 and are more appropriately treated by phlebotomy, considering other adverse effects of iron overload. Specific contraindications for phlebotomy included: hemoglobin <10 g/dL or hematocrit of <33, and poor venous access. Nonrandomized patients were assigned to the more appropriate of the two treatments. Patients were advised to avoid alcohol, smoking, iron supplements and estrogens according to standard care for PCT.
Hydroxychloroquine treatment
Patients were instructed to take 100 mg hydroxychloroquine twice weekly, and record doses on an intake sheet. Hydroxychloroquine sulfate (Plaquenil®) 200 mg tablets (the smallest available dose form) were halved by the UTMB Pharmacy and verified to be within a range of 85–115% of the intended 100 mg. The intake sheet and remaining half tablets were examined at each visit.
Repeated phlebotomy
At 2-week intervals, ~450 ml of whole blood were removed at the UTMB Blood Bank or sometimes at other sites for patient convenience. Hemoglobin or hematocrit was measured prior to each phlebotomy, and phlebotomy postponed if the hemoglobin level was <10 g/dL or hematocrit was less than 33. Compliance was determined at each visit and from Blood Bank reports.
Study visits
Patients were asked to return every 2 weeks at least until biochemical remission. Symptoms and physical findings were recorded and blood and urine samples obtained for plasma and urine porphyrins, blood counts, metabolic panel, ferritin, and a pregnancy test (monthly for women with reproductive potential treated with hydroxychloroquine). Serum HCV RNA (IU/L) and urinary iron excretion (collected in metal-free plastic tubes) were measured in some patients; it has been suggested that hydroxychloroquine might mobilize iron and increase its urinary excretion.35
Treatment outcomes
The primary outcome was time to achieving a biochemical remission of PCT, defined as a normal total plasma porphyrin concentration (<0.9 mcg/dL). Secondary outcomes included times to intermediate (50% and 75%) reductions in plasma porphyrins, and to normal urine porphyrins, proportion of patients achieving biochemical remission at 3, 6, and 12 months, and tolerability and safety of phlebotomy and hydroxychloroquine. Time to clinical remission (absence of new skin lesions) was quite variable and difficult to assess because skin friability predisposing to blister formation recovers slowly after porphyrins become normal. Hydroxychloroquine was discontinued when the plasma porphyrin concentration remained normal for at least one month. The endpoint for phlebotomy was achieving a serum ferritin of 20 ng/mL.
Safety assessments
Safety evaluations at each study visit included questions for known side effects per hydroxychloroquine product labeling including sensory changes, weakness and visual symptoms, and laboratory assessments noted above. Ophthalomogical examations were scheduled before, at 3 and 6 months and at the end of hydroxychloroquine treatment.42
Data analysis
Demographic, clinical characteristics and susceptibility factors were compared in randomized and nonrandomized patients and the 2 treatment groups. Continuous and categorical variables were assessed using Student’s t and chi-square tests respectively. Kaplan-Meier survival curves were compared for time to primary and secondary outcomes. Unadjusted Cox proportional hazard models were built to compare the times to treatment outcomes. Cox proportional hazard regression analyses models were built to study effects of demographics, susceptibility factors, baseline ferritin and plasma and urine porphyrins on the unadjusted HR on the comparison of the two treatments. Primary and secondary survival outcomes as well as categorical secondary outcomes were analyzed statistically using tests of non-inferiority.
Sample Size and Power
We assessed power for a noninferiority study with ~30 patients per group using nQuery Advisor® Version 7.0. We estimated about 95% of patients treated with phlebotomy would achieve remission within 4 months, and considered that hydroxychloroquine (test treatment) would be noninferior to phlebotomy (standard treatment) if at least 85% of hydroxychloroquine-treated patients achieved remission within 5 months. With 30 subjects in each group, the observed two-sided 95.0% confidence interval is expected to lie between −0.35 and 0.20 with 82% power when the phlebotomy proportion is 0.95 and the test treatment expected proportion is 0.85; results are based on 2000 simulations using the Newcombe-Wilson score method to construct the confidence interval.43
RESULTS
Treatment assignment
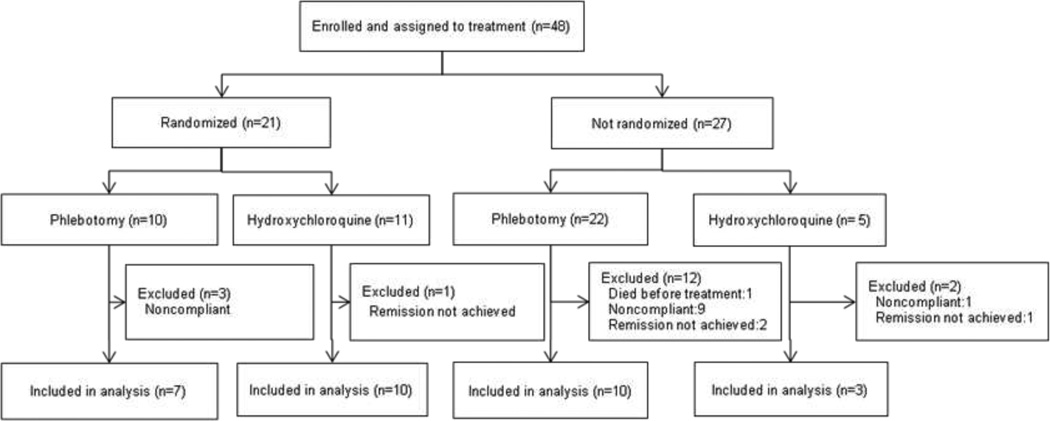
Forty-eight patients who met the inclusion criteria were enrolled and assigned to treatment. Twenty-one patients, a smaller number than expected, were eligible for randomization; 10 were randomized to phlebotomy and 11 to hydroxychloroquine (Figure 1). Twenty-seven patients did not meet criteria for randomization; 22 were assigned to phlebotomy and 5 to hydroxychloroquine (Figure 1). Reasons for assignment to phlebotomy were high serum ferritin (12 patients), preference (6 patients), already started phlebotomies (3 patients) and advanced renal disease (1 patient). Reasons for hydroxychloroquine rather than phlebotomy were anemia due to HIV or treatment of hepatitis C (started before onset of PCT), poor venous access, dislike of blood draws and living at a distance where phlebotomies were not easily arranged (1 patient for each).
Figure 1.
Enrollment, assignment to treatment with or without randomization of 48 study patients with porphyria cutanea tarda, and inclusion of the 30 patients who achieved a normal plasma porphyrin concentration in the analysis comparing time to remission with phlebotomy and low dose hydroxychloroquine.
Characteristics of evaluable patients
Treatment efficacy in terms of time to achieving remission, defined as a normal plasma porphyrin concentration, could be assessed in 30 patients (17 randomized and 13 not randomized, Figure 1). Time to remission could not be determined in 18 patients, one of whom died of heart disease before treatment was started, 13 were insufficiently compliant in providing samples, and 4 had incomplete remissions (Figure 1).
Demographic features, clinical characteristics and susceptibility factors for the 30 evaluable patients (Table 1) were not significantly different from the 16 patients who were not evaluable (not shown). Further, baseline characteristics among the 30 evaluable patients were not significantly different in those randomized and not randomized. Most patients were middle aged Caucasian males. Duration of current symptoms and number of prior episodes of PCT were similar in patients treated by phlebotomy and hydroxychloroquine. Ten (6 randomized, 4 nonrandomized) had previous episodes of PCT (1 in 6 patients and 2 or more in 4; all previously treated by phlebotomy.
Table 1.
Demographic and clinical characteristics including susceptibility factors in 30 patients with porphyria cutanea tarda treated by phlebotomy or low-dose hydroxychloroquine who achieved remission and in the 17 who were randomized to treatment.
| Characteristics | All patients | P | Randomized patients | P | ||
|---|---|---|---|---|---|---|
| Phlebotomy (N=17) |
Hydroxychloroquine (N=13) |
Phlebotomy (N=7) |
Hydroxychloroquine (N=10) |
|||
| Age (Years, mean±SD) | 51.3±5.0 | 50.3±6.0 | 0.61 | 50.7±5.4 | 49±6.3 | 0.66 |
| Sex (% males) | 88 | 38 | 0.004 | 86 | 30 | 0.024 |
| Race (% Caucasians) | 94 | 92 | 0.84 | 86 | 90 | 0.78 |
| Alcohol use* (% of cases) | 100 | 92 | 0.24 | 100 | 90 | 0.39 |
| Smoking* (% of cases) | 88 | 92 | 0.71 | 86 | 90 | 0.79 |
| Estrogen use* (% of cases) | 0 | 46 | 0.002 | 0 | 50 | 0.026 |
| Hepatitis C (% of cases) | 88 | 92 | 0.71 | 100 | 90 | 0.39 |
| HIV (% of cases) | 0 | 0 | NA | 0 | 0 | NA |
| HFE mutations (% of cases) | 58 | 77 | 0.30 | 43 | 70 | 0.26 |
| UROD mutations (% of cases) | 6 | 8 | 0.84 | 14 | 10 | 0.79 |
| No. of prior PCT episodes (mean±SD) | 0.47±0.80 | 0.54±0.88 | 0.83 | 0.43±0.92 | 0.5±0.85 | 0.85 |
| Current episode in mo (mean±SD) | 12±4 | 27±10 | 0.14 | 13±5 | 32±13 | 0.28 |
| Ferritin† (nmol/ml, mean±SD, range) |
482±272 101–1251 |
311±200 113–858 |
0.07 | 344±159 101–579 |
334±221 113–858 |
0.91 |
| Plasma Porphyrins† (mcg/dL, mean±SD (range) |
7.2±6.4 1.5–25.6 |
7.8±7.2 1.1–25.5 |
0.78 | 4.6±2.7 1.5–8.2 |
8.2±7.9 1.1–25.5 |
0.27 |
| Urine porphyrins† (nmol/L, mean±SD (range) |
1958±2484 310–10,317 |
2509±2801 471–9703 |
0.57 | 1451±1173 347–3336 |
2963±3064 471–9703 |
0.24 |
Abbreviations: HFE, hemochromatosis gene; UROD, uroporphyrinogen decarboxlase.
Within the previous year
Pretreatment
Multiple susceptibility factors for PCT were present in these 30 evaluable patients; alcohol use, smoking, and hepatitis C (26 genotype 1 and one genotype 2) were the most common (Table 1). Among 20 patients with HFE mutations, 4 were C282Y heterozygotes, 9 were H63D heterozygotes, 1 was a S65C heterozygote, 4 were C282Y/H63D compound heterozygotes, 2 were H63D homozygotes and none were C282Y homozygotes. Two patients had UROD mutations, and therefore had type 2 (familial) PCT.
Comparison of times to remission
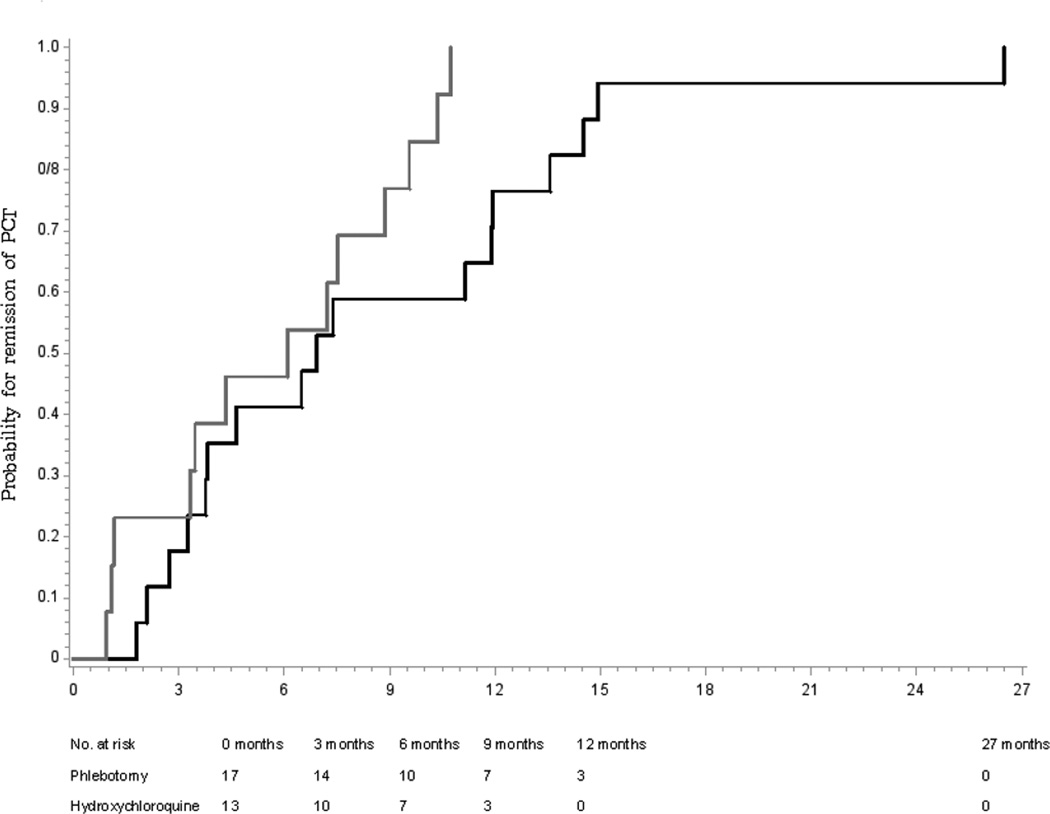
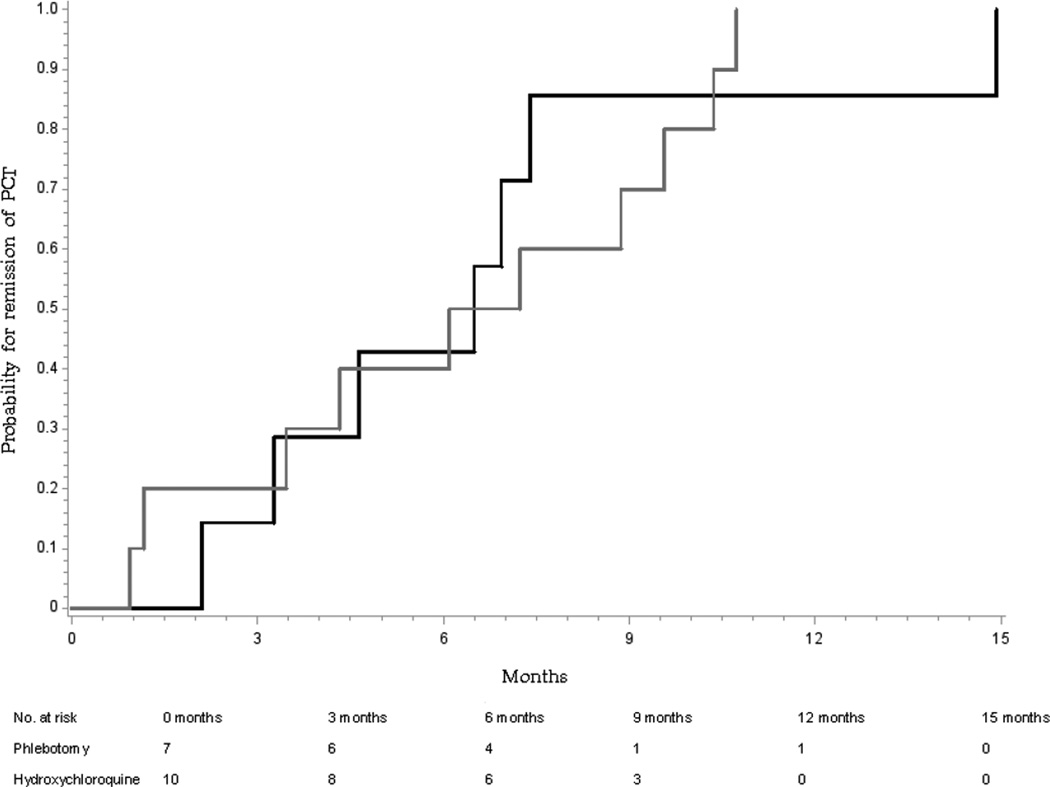
Median time to remission in all 30 evaluable patients (Figure 1) was somewhat shorter with hydroxychloroquine than phlebotomy (6.1 vs. 6.9 months; Log rank P=0.06; Figure 2), and similar in the 17 randomized patients (6.5 vs. 6.7 months; Log Rank P=0.95, Figure 3). Because the differences were not within the prespecified noninferiority margins for the CI on the HRs of 0.67 and 1.50, noninferiority could not be confirmed. Differences in times to intermediate endpoints (50 or 75% reduction in plasma porphyrins or normalization of total urinary porphyrin concentrations (Table 2), and the proportion achieving normal plasma porphyrin concentrations at 3, 6, and 12 months (not shown) were also not significant.
Figure 2.
Cumulative probability of time to remission, defined as achieving a normal plasma porphyrin concentration, of 30 patients with porphyria cutanea tarda treated by phlebotomy (n=17, black line) or low-dose hydroxychloroquine (n=13, gray line). The median time to remission was somewhat shorter with hydroxychloroquine than with phlebotomy [6.1 vs. 6.9 months; Log Rank P=0.06]
Figure 3.
Cumulative probability of time to remission of 17 patients with porphyria cutanea tarda randomized to treatment by phlebotomy (n=7, black line) or low-dose hydroxychloroquine (n=10, gray line). The median time to remission was similar with hydroxychloroquine and with phlebotomy [6.5 vs. 6.7 months; Log Rank P=0.95].
Table 2.
Times to remission and to intermediate reductions of plasma porphyrins in 30 patients with porphyria cutanea tarda treated by phlebotomy or low-dose hydroxychloroquine and in the 17 patients randomized to treatment.
| All patients | Randomized patients | |||||
|---|---|---|---|---|---|---|
| Phlebotomy (N=17) |
Hydroxychloroquine (N=13) |
HR [95% CI] | Phlebotomy (N=7) |
Hydroxychloroquine (N=10) |
HR [95% CI] | |
| Months | Months | |||||
| 50% reduction in Plasma Porphyrins |
2.4 (N=17) |
2.6 (N=13) |
1.4 [0.7–3.1] | 2.1 (N=7) |
2.5 (N=10) |
1.2 [0.4–3.3] |
| 75% reduction in Plasma Porphyrins |
4.8 (N=17) |
4.1 (N=11) |
1.32 [0.6–2.9] | 6 (N=7) |
4.2 (N=9) |
1.2 [0.4–3.3] |
| Normal Plasma Porphyrins |
6.9 (N=17) |
6.1 (N=13) |
2.2 [0.9–5.1] | 6.5 (N=7) |
6.7 (N=10) |
1.0 [0.4–2.9] |
| Normal Urinary Porphyrins |
6.9 (N=17) |
4 (N=11) |
0.9 [0.4–2.0] | 3.3 (N=7) |
4.1 (N=8) |
0.6 [0.2–1.7] |
Abbreviations: HR: hazard ratio; CI: confidence interval
Duration of treatment
Treatment consisted of a median number of 6 phlebotomies per patient (range 3–18 for 17 patients) and a median dose of 6.7 grams (67 twice-weekly 100 mg doses) of hydroxychloroquine per patient (range: 1.77–10.7 grams for 13 patients). Because hydroxychloroquine continued for at least one month after normalization of plasma porphyrin concentration whereas phlebotomies were stopped before plasma porphyrins became normal, it is not surprising that the duration of treatment was more than twice as long with hydroxychloroquine (median 8.3 vs. 2.8 months for all and 8.7 vs. 2.8 months for randomized patients).
Treatment compliance
Noncompliance in the 30 evaluable patients, as measured by days of interruption of the treatment schedule, was 3.4±6.7 (range 0–25) days for hydroxychloroquine and 30.4±51.3 (range 0–155) days for phlebotomy; P=0.08. Treatment duration was longer in the 6 patients with more than 20 days of noncompliance than in the 24 with better compliance (8.8±3.3 vs. 5.0±3.4 months; P=0.023) indicating, as would be expected, that noncompliance lengthens the duration of treatment needed to achieve remission.
Effects on serum ferritin and iron excretion
Serum ferritin decreased significantly with phlebotomy, as expected, but not in patients treated with hydroxychloroquine (not shown). Urinary iron excretion was assessed at each visit in 22 patients, with particular attention to the 14 day visit, which was after the first phlebotomy and 1–3 days after the fourth hydroxychloroquine doses. For 10 patients treated with hydroxychloroquine, urinary iron was 0.047±0.028 mcg/mg of creatinine (mean±SD) before and 0.043±0.045 during treatment. For 12 patients treated with phlebotomy, urinary iron was 0.231±0.305 mcg/mg of creatinine before and 0.247±0.421 during treatment. Repeated measures analysis did not show statistically significant effects of either treatment.
Effects on hepatitis C virus RNA levels
No patients were started on treatment for hepatitis C during the study, since initial treatment of PCT is preferred. But hepatitis C treatment had been initiated elsewhere in one patient before he developed skin lesions. He was not randomized, due to anemia, and was treated successfully for PCT with hydroxychloroquine while treatment for hepatitis C continued. HCV RNA was measured at baseline and during the first 110 days of starting treatment in 10 patients treated with hydroxychloroquine and 13 treated with phlebotomy. In the hydroxychloroquine patients these titers decreased from 891,938±13,578 (mean±SD) to 481,540±459,642 IU/l (P=0.07 by repeated measures ANOVA adjusting for treatment duration – median 60 days, range 30–76 days) and in the phlebotomy patients from 2,035,289±2,275,985 to 1,482,932±1,254,800 IU/l (P=0.19, median duration 43 days, range 21–110 days,).
Safety
No patient required discontinuation of treatment due to adverse effects, intolerance of treatment, development of an exclusion criterion, or withdrawal of consent. One patient died of an AIDS-related infection during treatment with hydroxychloroquine, a second died of heart disease before treatment was started, and 3 patients were hospitalized for unrelated conditions. Light flashes were more common with hydroxychloroquine among all patients, but not among randomized patients; multiple comparisons of other symptoms were not significantly different (not shown). ALT increased more than 50% above baseline in one patient on each treatment. Baseline and follow up retinal examinations in 13 patients assigned to hydroxychloroquine showed no abnormal findings.
DISCUSSION
This pragmatic clinical trial compared two standard therapies for PCT and enrolled all patients seen at this center over a 4-year period. Patients were randomized if appropriate criteria were met. Susceptibility factors and demographic characteristics (Table 1) were balanced in the two treatment groups and in those randomized and not randomized. Treatment procedures were the same as in standard management of PCT at this center, except that clinic visits were scheduled at regular 2-week intervals, treatment and visits were at no cost, and travel costs were reimbursed.
Twenty-one of the 48 patients were randomized (Figure 1). The other 27 had specified contraindications to either therapy or declined randomization (see Results for details). This experience suggests that approximately half of PCT patients in current clinical practice may be eligible for and be willing to consent to either of these standard therapies, and half are likely to have a medical indication or preference for one therapy, most commonly phlebotomy.
Time to achieving a normal plasma porphyrin concentration was evaluable in 30 patients and this measure of biochemical remission was not significantly different for the two treatments, considering all patients (Figure 2) or the 17 randomized patients (Figure 3). Times to other endpoints (see Results) were also not significantly different (Table 2). Therefore, a clinically significant difference in time to remission with these treatments is unlikely, but larger studies will be needed to confirm noninferiority.
Overall treatment outcomes were consistent with many previous reports supporting efficacy of both treatments (see Introduction). Thirty patients achieved a normal plasma porphyrin concentration, and only 4 patients with adequate numbers of observations (2 on each therapy) did not (Figure 1). Substantial but partial reductions in plasma porphyrins occurred in 3 of these patients. Incomplete remissions were associated with continued estrogen, a known susceptibility factor44 (1 patient treated with hydroxychloroquine), or carisoprodol, an inducer of hepatic heme (and porphyrin) synthesis,45 for chronic back pain (2 patients treated by phlebotomy). The fourth patient with HIV infection, anemia and concurrent treatment with antiretroviral drugs, did not improve with hydroxychloroquine and died of an AIDS-related infection. We could not assess effects of iron overload because a serum ferritin >600 ng/mL excluded patients from hydroxychloroquine and, by chance, none of our patients were homozygous for the C282Y HFE mutation, which is reported to confer poor response to hydroxychloroquine.41 Both treatments were found to be safe, with no significant treatment-related adverse effects (see Results).
Eighteen patients were excluded from the time to remission analysis, due to noncompliance with visits (13 patients), because remission was not achieved (4 patients, discussed above) or death (before treatment, 1 patient). Exclusion for noncompliance was more common with phlebotomy (12 of 22 patients) than hydroxychloroquine (1 of 16 patients, Figure 1). Even in the 30 evaluable patients, noncompliance during treatment was much greater with phlebotomy than hydroxychloroquine (30.4±51.3 and 3.4±6.7 days per patient, respectively, P=0.08), and lengthened treatment duration (see Results). Therefore, compliance with regularly scheduled phlebotomies was often difficult, even when provided at no expense and with reimbursements for travel.
We estimate that hydroxychloroquine treatment is considerably less expensive than phlebotomy (Table 3). Hydroxychloroquine treatment would require fewer visits in clinical practice than during this study, but a pretreatment ophthalmological examination adds to treatment costs. Although costs to the patient might be lower at some other medical facilities, it is likely that costs to all parties are comparable across the U.S.
Table 3.
Estimated average costs for treatment of porphyria cutanea tarda by either phlebotomy or low dose hydroxychloroquine.
| Repeated Phlebotomy1 |
Low-dose hydroxychloroquine2 |
||||
|---|---|---|---|---|---|
| Items | Cost | Number required |
Total cost |
Number required |
Total Cost |
| (USD) | (n) | (USD) | (n) | (USD) | |
| Phlebotomy (450 mL) | 100 | 7 | 700 | - | - |
| Parking and travel | 20 | 7 | 140 | 3 | 60 |
| Time lost from work | 50 | 7 | 350 | 3 | 150 |
| Ophthalmological examination | 180 | - | - | 1 | 180 |
| Hydroxychloroquine (200 mg tablet) | 1.89 | - | - | 24 | 45 |
| Pill cutter | 8 | - | - | 1 | 8 |
| Serum ferritin determination | 85 | 3 | 255 | - | - |
| Plasma porphyrin determination | 50 | 3 | 150 | 3 | 150 |
| Total cost | 1,595 | 593 | |||
The cost of phlebotomy assumes 7 phlebotomies at 2-week intervals with monitoring of ferritin concentration at least 3 times before ending treatment.
The cost of hydroxychloroquine assumes treatment for 6 months with one divided tablet per week and approximately 3 visits at 2-month intervals for monitoring.
The lowest doses of 4-aminoquinolines previously used for PCT have been 125 mg of chloroquine or 100 mg for hydroxychloroquine, as in this study, twice weekly.19, 24–29, 31, 32 Somewhat higher doses 30, 35, 46 are reported as safe and effective and would not require dividing the lowest available dosage forms; much higher doses and dose-escalation regimens have been used 47–51. However, our finding that 100 mg of hydroxychloroquine twice weekly was generally equivalent to phlebotomy in terms of time to remission, and evidence that hepatotoxic and other adverse effects of 4- aminoquinolines are dose-dependent,50, 51 supports the use of this lower dose regimen and avoiding possibly greater risks from higher doses. But measurement intervals were not optimal for detecting short term elevations in liver chemistries. Therefore, further studies assessing short and long term effects of hydroxychloroquine treatment even at this dose level in PCT are needed. Whether relapse rates are different after discontinuing hydroxychloroquine when plasma porphyrin levels are normal for at least one month, as in this study, or after an arbitrary treatment period such as one year26 is also not known.
Strengths of this prospective, pragmatic study include enrollment of a complete cohort of well characterized patients seen at a single center, randomization when specific criteria were met, comparable features of randomized and nonrandomized patients, use of a clear target for comparing efficacy, and consideration of susceptibility factors, compliance, cost and convenience in comparing these treatments. Limitations include fewer than expected patients who could be randomized and more than expected who were noncompliant with visits, which precluded establishing whether hydroxychloroquine is noninferior to phlebotomy. An expanded multicenter study comparing these treatments is planned through the Porphyrias Consortium, which is part of the NIH-funded Rare Disease Clinical Research Network (http://rarediseasesnetwork.epi.usf.edu/index.htm) to establish whether use of hydroxychloroquine is justified for a greater proportion of patients with PCT.
Acknowledgments
Grant support: This study was supported in part by grants R01 FD002604 from the US Food and Drug Administration Office of Rare Diseases, R21 DK073093 from the National Institutes of Health (NIH), a grant (1 U54 DK083909) for the Porphyrias Consortium of the Rare Diseases Clinical Research Network, that includes funding and/or programmatic support from the NIH Office of Rare Disease Research, and was conducted with the support of the Institute for Translational Sciences at the University of Texas Medical Branch, supported in part by a General Clinical Research Center grant MO1-RR000073 and a Clinical and Translational Science Award (UL1RR029876) from the National Center for Research Resources, now at the NIH National Center for Advancing Translational Sciences.
Abbreviations
- UROD
uroporphyrinogen decarboxylase
- PCT
porphyria cutanea tarda
- HCV
hepatitis C virus
- HIV
human immunodeficiency virus
- UTMB
University of Texas Medical Branch
- G6PD
glucose-6-phosphate dehydrogenase
- HR
hazard ratio
- CI
Confidence interval.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: Karl Anderson has received funding for educational programs from Lundbeck and research support from Clinuvel. The remaining authors disclose no conflicts.
Writing Assistance: None
Author Contributions: Dr. Singal was involved with acquisition and interpretation of data, statistical analysis, and drafting and revision of the manuscript. Drs. Hallberg, Lee and Sadagoparamanujam were involved with acquisition and analysis of data and revision of the manuscript. Dr. Grady was involved in study concept and design and obtaining funding. Dr. Freeman was involved in statistical analysis of data and revision of the manuscript. Dr. Anderson was involved with study concept and design, obtained funding, acquisition, analysis and interpretation of data, revision of the manuscript and study supervision.
REFERENCES
- 1.Elder GH. Porphyria cutanea tarda and related disorders (Chapter 88) In: Kadish KM, Smith K, Guilard R, editors. Porphyrin Handbook, Part II. Volume 14. San Diego: Academic Press; 2003. pp. 67–92. [Google Scholar]
- 2.Phillips JD, Bergonia HA, Reilly CA, Franklin MR, Kushner JP. A porphomethene inhibitor of uroporphyrinogen decarboxylase causes porphyria cutanea tarda. Proc Natl Acad Sci U S A. 2007;104:5079–5084. doi: 10.1073/pnas.0700547104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lundvall O, Weinfeld A, Lundin P. Iron storage in porphyria cutanea tarda. Acta Med Scand. 1970;188:37–53. doi: 10.1111/j.0954-6820.1970.tb08003.x. [DOI] [PubMed] [Google Scholar]
- 4.Sampietro M, Fiorelli G, Fargion S. Iron overload in porphyria cutanea tarda. Haematologica. 1999;84:248–253. [PubMed] [Google Scholar]
- 5.Bygum A, Brandrup F, Christiansen L, Petersen NE. [Porphyria cutanea tarda] Ugeskr Laeger. 2000;162:2020–2024. [PubMed] [Google Scholar]
- 6.Bonkovsky HL, Lambrecht RW, Shan Y. Iron as a co-morbid factor in nonhemochromatotic liver disease. Alcohol. 2003;30:137–144. doi: 10.1016/s0741-8329(03)00127-7. [DOI] [PubMed] [Google Scholar]
- 7.Gorman N, Zaharia A, Trask HS, Szakacs JG, Jacobs NJ, Jacobs JM, Balestra D, Sinclair JF, Sinclair PR. Effect of an oral iron chelator or iron-deficient diets on uroporphyria in a murine model of porphyria cutanea tarda. Hepatology. 2007;46:1927–1834. doi: 10.1002/hep.21903. [DOI] [PubMed] [Google Scholar]
- 8.Dereure O, Jumez N, Bessis D, Gallix B, Guillot B. Measurement of liver iron content by magnetic resonance imaging in 20 patients with overt porphyria cutanea tarda before phlebotomy therapy: a prospective study. Acta Derm Venereol. 2008;88:341–345. doi: 10.2340/00015555-0472. [DOI] [PubMed] [Google Scholar]
- 9.Jalil S, Grady JJ, Lee C, Anderson KE. Associations among behavior-related susceptibility factors in porphyria cutanea tarda. Clin Gastroenterol Hepatol. 2010;8:297–302. doi: 10.1016/j.cgh.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ippen H. Treatment of porphyria cutanea tarda by phlebotomy. Semin Hematol. 1977;14:253–259. [PubMed] [Google Scholar]
- 11.Epstein JH, Redeker AG. Porphyria cutanea tardo; a study of the effect of phlebotomy. N Engl J Med. 1968;279:1301–1304. doi: 10.1056/NEJM196812122792402. [DOI] [PubMed] [Google Scholar]
- 12.Lundvall O. The effect of phlebotomy therapy in porphyria cutanea tarda Its relation to the phlebotomy-induced reduction of iron stores. Acta Med Scand. 1971;189:33–49. doi: 10.1111/j.0954-6820.1971.tb04337.x. [DOI] [PubMed] [Google Scholar]
- 13.Chlumsky J, Malina L, Chlumská A. The effect of venesection therapy on liver tissue in porphyria cutanea tarda. Acta Hepato-Gastroenterol. 1973;20:124–130. [PubMed] [Google Scholar]
- 14.Ramsay CA, Magnus IA, Turnbull A, Barker H. The treatment or porphyria cutanea tarda by venesection. Q J Med. 1974;43:1–24. [PubMed] [Google Scholar]
- 15.Di Padova C, Marchesi L, Cainelli T, Gori G, Podenzani SA, Rovagnati P, Rizzardini M, Cantoni L. Effects of phlebotomy on urinary porphyrin pattern and liver histology in patients with porphyria cutanea tarda. Am J Med Sci. 1983;285:2–12. doi: 10.1097/00000441-198301000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Adjarov D, Donov M, Ivanov E, Naidenova E. Phlebotomy treatment in porphyria cutanea tarda combined with beta-thalassaemia. Dermatologica. 1984;169:184–187. doi: 10.1159/000249600. [DOI] [PubMed] [Google Scholar]
- 17.Ratnaike S, Blake D, Campbell D, Cowen P, Varigos G. Plasma ferritin levels as a guide to the treatment of porphyria cutanea tarda by venesection. Australas J Dermatol. 1988;29:3–7. doi: 10.1111/j.1440-0960.1988.tb01216.x. [DOI] [PubMed] [Google Scholar]
- 18.Rocchi E, Gibertini P, Cassanelli M, Pietrangelo A, Borghi A, Ventura E. Serum ferritin in the assessment of liver iron overload and iron removal therapy in porphyria cutanea tarda. J Lab Clin Med. 1986;107:36–42. [PubMed] [Google Scholar]
- 19.Malina L, Chlumsky J. A comparative study of the results of phlebotomy therapy and low-dose chloroquine treatment in porphyria cutanea tarda. Acta Derm Venereol. 1981;61:346–350. [PubMed] [Google Scholar]
- 20.Sweeney GD, Saunders SJ, Dowdle EB, Eales L. Effects of chloroquine on patients with cutaneous porphyria of the symptomatic type. Brit Med J. 1965;1:1281–1285. doi: 10.1136/bmj.1.5445.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sweeney GD. Porphyria cutanea tarda, or the uroporphyrinogen decarboxylase deficiency diseases. Clin Biochem. 1986;19:3–15. doi: 10.1016/s0009-9120(86)80064-9. [DOI] [PubMed] [Google Scholar]
- 22.Felsher BF, Redeker AG. Effect of chloroquine on hepatic uroporphyrin metabolism in patients with porphyria cutanea tarda. Medicine. 1966;45:575–583. doi: 10.1097/00005792-196645060-00024. [DOI] [PubMed] [Google Scholar]
- 23.Kowertz MJ. The therapeutic effect of chloroquine: hepatic recovery in porphyria cutanea tarda. JAMA. 1973;223:515–519. [PubMed] [Google Scholar]
- 24.Kordac V, Semradova M. Treatment of porphyria cutanea tarda with chloroquine. Br J Derm. 1974;90:95–100. doi: 10.1111/j.1365-2133.1974.tb06367.x. [DOI] [PubMed] [Google Scholar]
- 25.Kordac V, Papezova R, Semradova M. Chloroquine in the treatment of porphyria cutanea tarda. New Engl J Med. 1977;296:949. [PubMed] [Google Scholar]
- 26.Cainelli T, Padova CD, Marchesi L, Gori G, Rovagnati P, Podenzani SA, Bessone E, Cantoni L. Hydroxychloroquine versus phlebotomy in the treatment of porphyria cutanea tarda. Br J Dermat. 1983;108:593–600. doi: 10.1111/j.1365-2133.1983.tb01062.x. [DOI] [PubMed] [Google Scholar]
- 27.Marchesi L, Di Padova C, Cainelli T, Reseghetti A, Di Padova F, Rovagnati P, Cantoni L. A comparative trial of desferrioxamine and hydroxychloroquine for treatment of porphyria cutanea tarda in alcoholic patients. Photodermatol. 1984;1:286–292. [PubMed] [Google Scholar]
- 28.Ashton RE, Hawk JLM, Magnus IA. Low-dose oral chloroquine in the treatment of porphyria cutanea tarda. Br J Dermatol. 1984;3:609–613. doi: 10.1111/j.1365-2133.1984.tb06632.x. [DOI] [PubMed] [Google Scholar]
- 29.Seubert S, Seubert A, Stella AM, Guzman H, Batlle A. [Results of treatment of porphyria cutanea tarda with bloodletting and chloroquine] Z Hautkr. 1990;65:223–225. [PubMed] [Google Scholar]
- 30.Valls V, Ena J, Enriquez-De-Salamanca R. Low-dose oral chloroquine in patients with porphyria cutanea tarda and low-moderate iron overload. J Dermatol Sci. 1994;7:169–175. doi: 10.1016/0923-1811(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 31.Freesemann A, Frank M, Sieg I, Doss MO. Treatment of porphyria cutanea tarda by the effect of chloroquine on the liver. Skin Pharmacol. 1995;8:156–161. doi: 10.1159/000211340. [DOI] [PubMed] [Google Scholar]
- 32.Wolff C, Armas R, Krause P, Parraguez A, Ramon Soto J. [Treatment of porphyria cutanea tarda with chloroquine and its effect on associated liver disease: retrospective analysis] Rev Med Chil. 1996;124:456–460. [PubMed] [Google Scholar]
- 33.Scholnick PL, Epstein J, Marver HS. The molecular basis of the action of chloroquine in porphyria cutanea tarda. J Invest Dermatol. 1973;61:226–232. doi: 10.1111/1523-1747.ep12676478. [DOI] [PubMed] [Google Scholar]
- 34.Shanley BC, Taljaard JJF, Deppe WM, Joubert SM. Delta-aminolaevulinic acid in acute porphyria. S A Med J. 1972;46:84. [PubMed] [Google Scholar]
- 35.Malkinson FD, Levitt L. Hydroxychloroquine treatment of porphyria cutanea tarda. Arch Dermat. 1980;116:1147–1150. [PubMed] [Google Scholar]
- 36.Goerz G, Bolsen K, Merk H. Influence of chloroquine on the porphyrin metabolism. Arch Dermatol Res. 1985;277:114–117. doi: 10.1007/BF00414107. [DOI] [PubMed] [Google Scholar]
- 37.Rynes RI. Ophthalmologic considerations in using antimalarials in the United States. Lupus. 1996;5(Suppl 1):S73–S74. [PubMed] [Google Scholar]
- 38.Poh-Fitzpatrick MB, Lamola AA. Direct spectrophotometry of diluted erythrocytes and plasma: a rapid diagnostic method in primary and secondary porphyrinemias. J Lab Clin Med. 1976;87:362–370. [PubMed] [Google Scholar]
- 39.Egger NG, Goeger DE, Payne DA, Miskovsky EP, Weinman SA, Anderson KE. Porphyria cutanea tarda: multiplicity of risk factors including HFE mutations, hepatitis C, inherited uroporphyrinogen decarboxylase deficiency. Dig Dis Sci. 2002;47:419–426. doi: 10.1023/a:1013746828074. [DOI] [PubMed] [Google Scholar]
- 40.Wickliffe JK, Abdel-Rahman SZ, Lee C, Kormos-Hallberg C, Sood G, Rondelli CM, Grady JJ, Desnick RJ, Anderson KE. CYP1A2*1F and GSTM1 alleles are associated with susceptibility to porphyria cutanea tarda. Mol Med. doi: 10.2119/molmed.2010.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stolzel U, Kostler E, Schuppan D, Richter M, Wollina U, Doss MO, Wittekind C, Tannapfel A. Hemochromatosis (HFE) gene mutations and response to chloroquine in porphyria cutanea tarda. Arch Dermatol. 2003;139:309–313. doi: 10.1001/archderm.139.3.309. [DOI] [PubMed] [Google Scholar]
- 42.Marmor MF, Carr RE, Easterbrook M, Farjo AA, Mieler WF. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002;109:1377–1382. doi: 10.1016/s0161-6420(02)01168-5. [DOI] [PubMed] [Google Scholar]
- 43.Newcombe RG. Interval estimation for the difference between independent proportions: comparison of eleven methods. Stat Med. 1998;17:873–890. doi: 10.1002/(sici)1097-0258(19980430)17:8<873::aid-sim779>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 44.Bulaj ZJ, Franklin MR, Phillips JD, Miller KL, Bergonia HA, Ajioka RS, Griffen LM, Guinee DJ, Edwards CQ, Kushner JP. Transdermal estrogen replacement therapy in postmenopausal women previously treated for porphyria cutanea tarda. J Lab Clin Med. 2000;136:482–488. doi: 10.1067/mlc.2000.111024. [DOI] [PubMed] [Google Scholar]
- 45.Anderson KE, Bloomer JR, Bonkovsky HL, Kushner JP, Pierach CA, Pimstone NR, Desnick RJ. Recommendations for the diagnosis and treatment of the acute porphyrias. Ann Intern Med. 2005;142:439–450. doi: 10.7326/0003-4819-142-6-200503150-00010. [DOI] [PubMed] [Google Scholar]
- 46.Taljaard JJ, Shanley BC, Stewart-Wynne EG, Deppe WM, Joubert SM. Studies on low dose chloroquine therapy and the action of chloroquine in symptomatic porphyria. Br J Dermatol. 1972;87:261–269. doi: 10.1111/j.1365-2133.1972.tb00316.x. [DOI] [PubMed] [Google Scholar]
- 47.Vogler WR, Galambos JT, Olansky S. Biochemical effects of chloroquine therapy in porphyria cutanea tarda. Am J Med. 1970;49:316–321. doi: 10.1016/s0002-9343(70)80022-5. [DOI] [PubMed] [Google Scholar]
- 48.Wennersten G, Ros AM. Chloroquine in treatment of porphyria cutanea tarda. Acta Dermatovener. 1982;100:119–123. [PubMed] [Google Scholar]
- 49.Tsega E. Long term effect of high dose, short course chloroquine therapy on porphyria cutanea tarda. Quart J Med. 1987;65:953–957. [PubMed] [Google Scholar]
- 50.Petersen CS, Thomsen K. High-dose hydroxychloroquine treatment of porphyria cutanea tarda. J Am Acad Dermatol. 1992;26:614–619. doi: 10.1016/0190-9622(92)70090-3. [DOI] [PubMed] [Google Scholar]
- 51.Rossmann-Ringdahl I, Olsson R. Porphyria cutanea tarda: effects and risk factors for hepatotoxicity from high-dose chloroquine treatment. Acta Derm Venereol. 2007;87:401–405. doi: 10.2340/00015555-0260. [DOI] [PubMed] [Google Scholar]