Abstract
Splenic filtration of Plasmodium falciparum infected red blood cells has been hypothesized to influence malaria pathogenesis. We have developed a minimum cylindrical diameter (MCD) filtration model which estimates physical splenic filtration during malaria infection. The key parameter in the model is the minimum cylindrical diameter (MCD), the smallest tube or cylinder that a red blood cell (RBC) can traverse without lysing. The MCD is defined by a relationship between the RBC surface area and volume. In the MCD filtration model, the MCD filtration function represents the probability of a cell becoming physically removed from circulation. This modeling approach was implemented at a field site in Blantyre, Malawi. We analyzed peripheral blood samples from 120 study participants in 4 clinically defined groups (30 subjects each): cerebral malaria, uncomplicated malaria, aparasitemic coma, and healthy controls. We found statistically significant differences in the surface area and volumes of uninfected RBCs when healthy controls were compared to malaria patients. The estimated filtration rates generated by the MCD model corresponded to previous observations in ex vivo spleen experiments and models of red blood cell loss during acute malaria anemia. There were no differences in the estimated splenic filtration rates between cerebral malaria and uncomplicated malaria patients. The MCD filtration model estimates that at time of admission, 1 ring-stage infected RBC is physically filtered by the spleen for each parasite that remains in peripheral circulation. This field study is the first to use microfluidic devices to identify rheological diversity in RBC populations associated with malaria infection and illness in well characterized groups of children living in a malaria endemic area.
Introduction
Plasmodium falciparum parasites physically and biochemically alter the host red blood cells (RBCs) throughout their 48 hour asexual lifecycle (Miller et al., 2002). These alterations may contribute to the wide range of clinical syndromes associated with malaria infection. The spleen filters infected RBCs from circulation by physical selection and an immune-mediated recognition of the infected RBCs followed by phagocytosis (Groom et al., 1991). Retention of infected RBCs has been directly observed to occur in the splenic region called the red pulp (Safeukui et al., 2008). The red pulp contains a structure called the reticular mesh, a fibrous network of cells and fibrils that create a tortuous path for RBCs. An additional mechanical challenge for passage of cells occurs at the interendothelial slits which are the narrowest constrictions in the body (Groom et al., 1991). These splenic structures are where mechanical filtration of damaged, aged, and infected RBCs have been observed to occur (Mebius et al., 2005, Safeukui et al., 2008).
As malaria parasites remodel the host RBC, the infected RBC becomes less deformable; the relationship between surface area and volume is altered, and the shear elastic modulus of the plasma membrane and the viscosity of the cell are increased (Herricks et al., 2009, Glenister et al., 2002). The altered physique of the P. falciparum-infected RBC may result in increased retention rates in the spleen. The rate of splenic mechanical filtration may be one factor affecting an individual’s total parasite burden and potentially influencing the pathogenesis of malaria (Buffet et al., 2009, Langreth et al., 1985, Chotivanich et al., 2002, Garnham, 1970).
Splenectomized individuals in malaria-endemic regions are more likely to have parasites in circulation and are also observed to have some late-stage parasites present in the peripheral blood (Bach et al., 2005). In contrast, individuals infected with P. falciparum malaria who have intact spleens, have ring-stage infected RBCs present in the peripheral blood; late stage infected RBCs are rarely observed. In studies of P. falciparum in Columbian Owl monkeys, splenectomized monkeys were observed to be susceptible to virulent infections while intact monkeys were not (Langreth et al., 1985). When monkeys were infected with knob-less non-cytoadhering strains of P. falciparum, splenectomized monkeys were observed to have all stages of infected RBCs in circulation where monkeys with intact spleens did not develop significant parasitemia (Langreth et al., 1985). The different disease outcomes in the two groups were thought to be due to the spleen eliminating the less deformable, non-cytoadhering parasites from circulation, thereby thwarting the development of severe disease (Langreth et al., 1985).
One hypothesis is that less selective filtration of ring-stage infected RBCs permits a rapid increase in total body parasite load and could be associated with more severe disease (Buffet et al., 2009). Despite extensive studies characterizing the biochemical and physical changes to the host RBCs, development of complicated malaria infection is not well understood. In particular, the role of RBC deformability remains of interest. There is little direct evidence that patient-to-patient variation in splenic filtration contributes to the wide spectrum of clinical syndromes seen in individuals infected with P. falciparum. Tools to investigate how RBC deformability might affect splenic filtration continue to improve in terms of approximating the physiological process (Deplaine et al., 2011). RBC deformability measurements such as micropipette aspiration and ektacytometry have shown associations between red blood cell deformability and severity of malaria infection (Glenister et al., 2002, Dondorp et al., 1999, Dondorp et al., 1997). More recently, finite element models of RBC membranes have been utilized to simulate the behavior and morphology of RBCs cytoadhering and traversing capillaries, but these models have not generated quantitative estimates of how reduced deformability of the asexual blood stage might translate to increased splenic retention of infected RBCs (Fedosov et al., 2011a, Fedosov et al., 2011b).
The RBC cell membrane, while very flexible, cannot stretch. A characteristic of lipid bilayer membranes are that they can only experience strains of less than 10% in response to in-plane stresses without fracturing (Rand et al., 1964). This characteristic of RBC membranes means that the cell deforms at a constant surface area and also restricts the cell to deformations which do not increase its surface area, otherwise the cell will lyse. The surface area and volume of RBCs in circulation exhibit a linear relationship (Canham et al., 1968, Gifford et al., 2006, Gifford et al., 2003, Herricks et al., 2009). Canham and Burton proposed that if a cell is deformed to fit inside a cylindrical tube, the smallest diameter tube that the cell could fit, without increasing its surface area or subsequently lysing, is defined by a linear relationship between surface area and volume (Supplemental Figure 1). This diameter for a cell they termed the minimum cylindrical diameter (MCD).
Canham and Burton observed shifts in the MCD of the erythrocytes of patients before and after splenectomy. Following these observations, they hypothesized that the linear relationship between surface area and volume of circulating RBCs was likely the outcome of splenic filtration on the basis of the MCD. If RBCs were filtered according to a different geometric parameter, then the relationship between surface area and volume of the circulating RBCs would fit that particular geometric parameter. It is important to note that no aspect of the splenic interendothelial slits or reticular mesh appear structurally similar to cylindrical pores. Based on the fact that the properties of the cellular membrane limit the amount a cell can deform while traversing a constriction or a tortuous path and the fact that RBCs in circulation have a linear relationship between surface area and volume; Canham and Burton made the following conjecture, that the constrictions encountered in the spleen, regardless of shape, have an equivalent cylindrical diameter or pore size.
Filters of all types tend not to have a strict cutoff of particle sizes they trap, but attenuate particles over a finite range of particle size: larger particles don’t pass through a filter as often as smaller particles. Using the Canham and Burton conjecture, we developed a MCD filtration model based on the assumption that the spleen mechanically filters cells as a function of MCD: cells with a larger MCD have a greater chance of being trapped by the spleen than cells with a smaller MCDs. The resulting filtration function describes the probability of a cell becoming trapped by the spleen as a function of the MCD.
If the Canham and Burton conjecture is true and if all RBCs in peripheral circulation are under constant filtration pressure, then both infected RBCs and uninfected RBCs should have the same upper limit to their MCD. RBC dimensions such as mean corpuscular volume vary between individuals and may vary between those with malaria infection and healthy individuals. Since parasites must invade and then remodel uninfected RBCs in circulation, the surface area and volume of infected RBCs is altered from the uninfected RBCs which they invaded the relative increase in MCD of the infected RBCs over that of uninfected RBCs may differ from individual to individual, depending on the characteristics of the uninfected RBCs (Supplemental Figure 1B–E). In order to understand how malaria parasites are filtered from circulation on the basis of MCD, the surface area and volume of uninfected RBCs in malaria patients and healthy individuals must be well characterized.
We developed a microfluidic device capable of measuring the surface area and volume of thousands of individual infected and uninfected RBCs (Gifford et al., 2003, Herricks et al., 2009). Here we describe the use of this microfluidic device at a clinical field site, analyzing blood samples collected directly from study participants. We utilized these samples to develop an MCD filtration model which estimates the filtration rates of infected RBCs at the time of admission to the hospital.
We describe the development of the MCD filtration model in a stepwise process. First, we demonstrate the difference in surface area, volume and MCD in cultured schizont-stage RBCs and ring-stage parasites from a sample taken directly from a patient. The schizont-stage RBCs are maintained in culture and therefore not under filtration pressure, while the rings-stage RBCs are presumably under constant filtration pressure from the spleen. Secondly, we study ring-stage infected RBCs collected directly from a patient and compare them with ring-stage RBCs from the same patient, but maintained in culture for 48 hours. We then use geometric operations to characterize the difference between the patient’s uninfected erythrocytes and the infected RBC population observed in culture. The same geometric operations applied to uninfected RBCs does not replicate the population of infected RBCs in samples taken directly from a patient; an additional filtration operation is required to recreate the characteristics of the infected RBCs that are observed in circulation.
Following demonstration of the MCD filtration model on a single patient, we compare the uninfected RBCs of four patient groups to determine if differences in the uninfected RBC population are associated with malaria infection or clinical syndrome. Finally, we use the MCD filtration model to estimate the filtration rate of parasites in malaria patients and compare filtration rates of cerebral malaria patients to those of patients with uncomplicated malaria. The MCD filtration model, based solely on the geometry of cells in circulation, agrees partially with ex vivo splenic filtration studies, and may be useful in studies of the impact of acquired immunity, individual susceptibility to infection and disease, and drug effects.
Results
Comparing cultured schizont-stage infected RBCs to ex vivo ring-stage infected RBCs
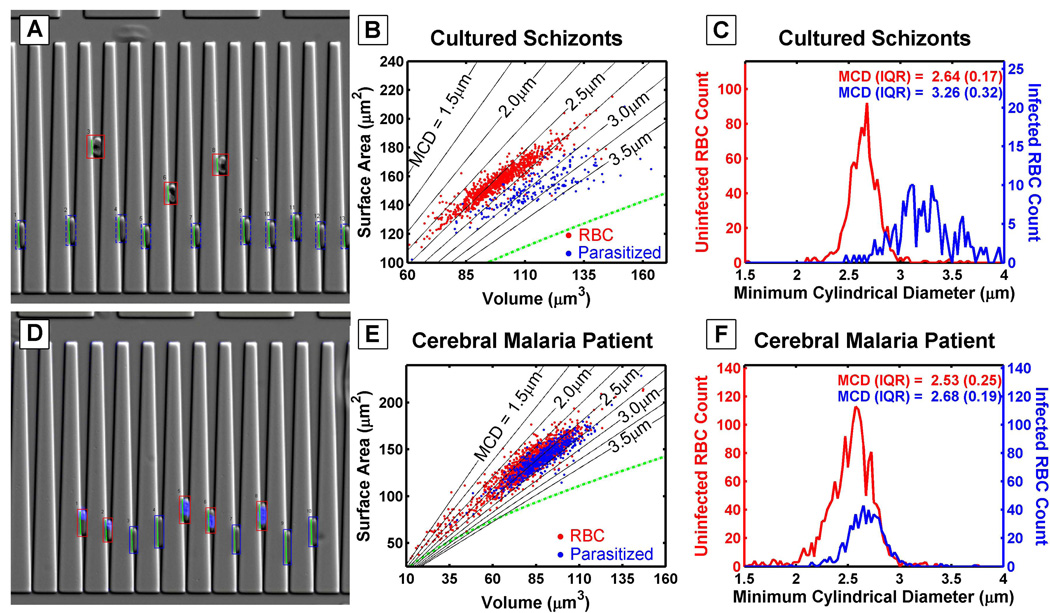
The microfluidic devices utilized in this study; measure surface area and volume by trapping RBCs in v-shaped constrictions (Herricks et al., 2009, Gifford et al., 2003). The surface area and volume of individual RBCs is calculated by their position and length in the wedge shaped constriction. For example, these devices can detect differences between infected RBCs at different stages of the life cycle. Figures 1A and 1D compare cultured schizont-stage infected RBCs and ring-stage infected RBCs taken directly from a malaria patient. In general, cultured schizont infected RBCs have a larger volume and smaller surface area than ring-stage infected RBCs taken directly from a cerebral malaria patient (Figure 1B and 1E). The ring-stage infected RBCs are identified with a fluorescent nuclear stain HOECHST (Figure 1D). The MCD population histogram is generated by calculating the MCD for each RBC, and the population distribution width is described by the inter quartile range (IQR) (Figure 1C and 1F). In the patient sample, the range of the ring-stage infected RBC MCD distribution is within that of the uninfected RBC MCD population distribution, while in the cultured schizonts, the MCD distribution is largely beyond that of the uninfected RBC population.
Figure 1. Cultured Schizonts Compared To A Cerebral Malaria Patients Ring Stage Parasites.
Diagram of how cells are trapped in a wedge, where infected RBCs are identified visually (A) and by using fluorescence (D). The surface area and volume plots are determined by measuring the length of cells and depth of cells penetrating the wedge (B and E). Lines of constant MCD are shown. The cultured schizonts have MCDs larger than 2.75 µm which is larger than the majority of uninfected RBCs (Herricks et al., 2009). In a sample taken from an individual cerebral malaria patient and analyzed immediately, the majority of both uninfected RBCs (red) and infected RBCs (blue) have an MCD of less than 3µm. The MCD is calculated from the surface area and volume and the populations are compared in a histogram (C and F). Please note the differing axes scales between the cultured parasite blood and the blood taken from the malaria patient.
Estimating splenic filtration by comparing cultured ring-stage infected RBCs to circulating infected RBCs
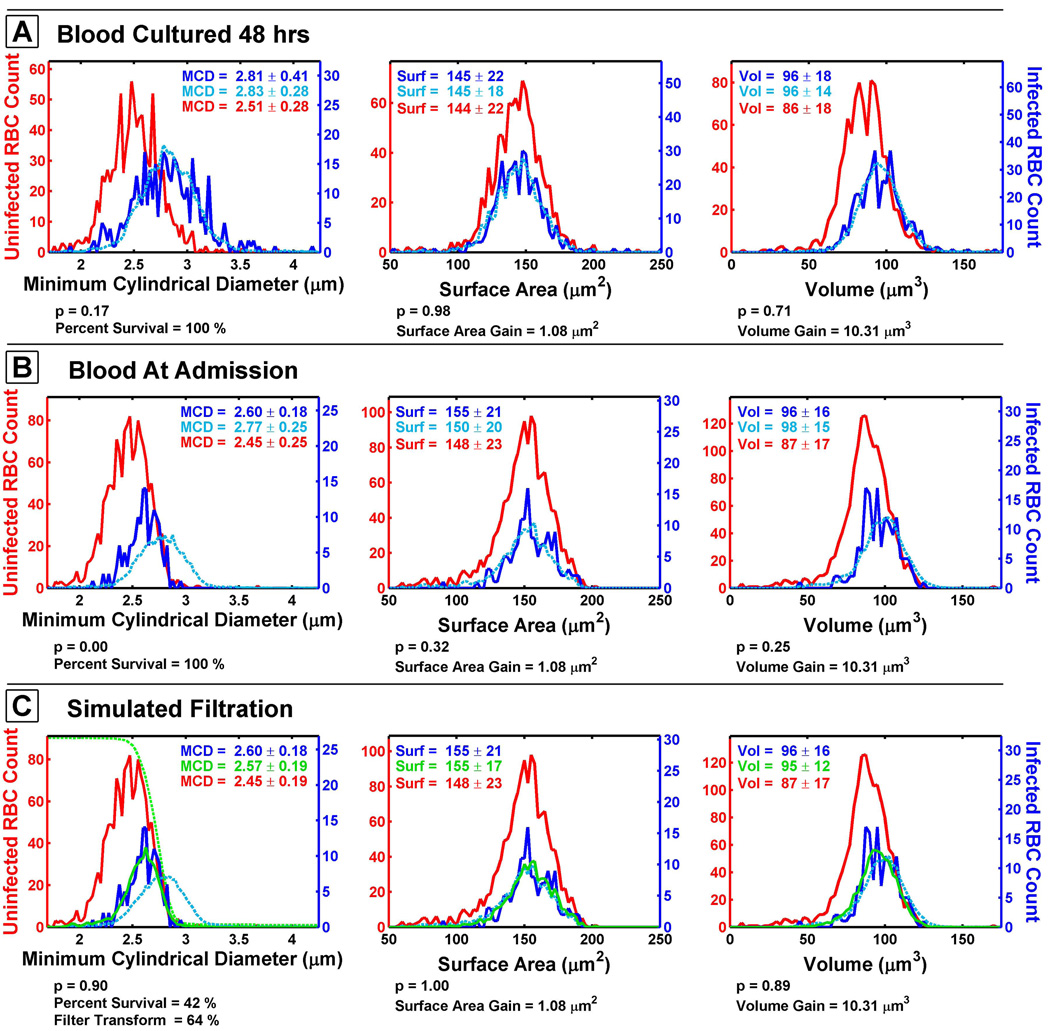
A demonstration of how the MCD filtration model was developed is depicted by comparing cultured ring-stage infected RBCs to a blood sample taken directly from a malaria patient (Figure 2). For one study subject, a blood sample was immediately analyzed using a microfluidic device. The remainder of the sample was placed into culture for 48 hours. This experiment generated new ring-stage infected RBCs in the patient’s original blood, but the malaria blood culture had been free of filtration selection for 48 hours. The MCD distribution of the cultured ring-stage infected RBCs (dark blue line) is distinctly different from what is observed in circulation (Figure 2A and 2B). This difference is assumed to be due, in part, to the effect of splenic filtration on the in vivo infected RBC population. The cultured ring-stage infected RBCs have a larger mean MCD of 2.81 µm compared to a mean MCD of 2.60 µm for a patient’s sample collected at admission.
Figure 2. Comparison of Cultured Parasites and Simulated Filtration.
The surface area and volume populations of a patient’s blood cultured for 48 hours so at the ring stage (A). Performing operations on the surface area and volume to create a simulated invaded RBC population (light blue line) from uninfected RBC (red lines) generates a good estimate of the infected RBCs (dark blue lines) observed in in vitro culture of the patient blood. When the same operations are performed on blood drawn from the patient, the MCD of the simulated invaded RBC population does not match that of the infected RBCs observed in the patient’s blood sample (B). Attenuating the MCD population by a filtration function (green dashed line) to create a simulated and filtered population (solid green line) generates a population similar to the observed infected cell population (C). The p-value below each plot is the result of the Kolmogorov-Smirnov test and is used as a fitting parameter to rank distributions of similarity of the simulated invaded RBC population to the infected RBC population. Note that the y-axis scales differ between graphs.
The parasite remodeling of the host RBCs was estimated by randomly sampling the uninfected RBC population and then changing the volume and surface area of these RBCs to create a simulated invaded RBC population. The simulated invaded RBCs were then compared to measurements made on infected RBCs from the same patient sample. The infected RBC population for the cultured parasites could be simulated by increasing uninfected RBC volume by 10.31 µm3 and the surface area by 1.08 µm2 (light blue dashed line). These simulated invaded RBCs gave a good approximation of the infected RBCs grown in vitro for 48 hours (Figure 2A). When the same operations were performed on the sample taken from the patient at the time of admission, the MCD for the simulated invaded RBCs exceeded that of the actual infected RBC population taken directly from the patient (Figure 2B).
When simulated invaded RBCs are placed through a Monte Carlo simulation designed to filter cells as a function of MCD (solid green line), the resulting population distributions of MCD, surface area, and volume all closely resemble the infected RBC population observed in this patient (Figure 2C). The MCD filtration model is a series of operations which alter the surface area and volume of randomly selected uninfected RBCs and then attenuate the population of these “invaded” RBCs as a function of MCD. The model effectively relates the uninfected RBC population to the infected RBC population through a series of geometric operations (See Methods and Supplemental Information).
Comparing dimensions of uninfected RBCs in clinically defined groups
We used microfluidic devices to compare the uninfected RBCs in peripheral circulation between individuals with and without malaria infection. The lines of constant MCD are not parallel and converge towards smaller volumes and surface areas (Supplemental Figure 1C). The relative increase in MCD is larger for RBCs with smaller surface area and volume. Since malaria parasites invade and grow within RBCs from the peripheral circulation, the baseline parameters of those RBCs should be described in order to determine the contribution of innate RBC parameters to filtration rates.
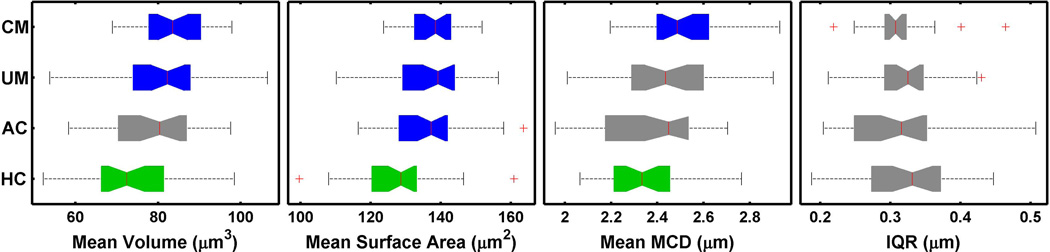
Blood was collected from individuals following previously described protocols from March through July of 2010 at the Blantyre Malaria Project, Malawi-Liverpool-Wellcome Trust Clinical Research Programme located at Queen Elizabeth Central Hospital in Blantyre, Malawi (Taylor et al., 2004). Samples from 30 cerebral malaria patients, 30 uncomplicated malaria patients, 30 aparasitemic coma patients, and 30 healthy controls were included in the analysis (see “Methods” for the clinical criteria associated with each category). Of the 30 cerebral malaria patients, 6 died. All patients were 3 to 161 months of age. All samples were drawn on admission to the hospital. The uninfected RBC population mean volume, mean surface area, mean MCD and IQR is provided (Table 1). Healthy controls have a statistically significantly smaller mean volume and smaller mean surface area than malaria patients (Figure 3). The difference in the mean MCD was statistically significant and smaller for the healthy control population when compared to either the uncomplicated malaria or the cerebral malaria population. In aparasitemic coma patients the mean surface area of uninfected RBCs was statistically different from healthy controls, but the mean volume, MCD, and IQR were not statistically different from either healthy controls or malaria patients.
Table 1.
Population parameters of mean RBC volume (MCV), mean surface area, and mean MCD. Std stands for standard deviation.
| Uninfected RBC Population Statistics |
RBC Population Volume Mean (Std) [Range] |
RBC Population Surface Area Mean (Std) [Range] |
RBC Population MCD Mean (Std) [Range] |
|---|---|---|---|
| Cerebral malaria N=30 |
83.8 (7.74) [69.0 – 97.9] |
138.8 (7.57) [123.7 – 151.8] |
2.51 (0.170) [2.20 – 2.93] |
| Uncomplicated malaria N=30 |
80.9 (12.9) [53.8 – 106.5] |
137.0 (10.9) [110.2 – 156.5] |
2.43 (0.241) [2.01 – 2.90] |
| Aparasitemic Coma N=30 |
78.8 (11.2) [58.4 – 97.6] |
136.0 (11.0) [116.5 – 163.7] |
2.39 (0.220) [1.96 – 2.70] |
| Healthy Control N=30 |
73.1 (10.9) [52.2 – 98.5] |
127.6 (12.4) [99.6 – 160.9] |
2.36 (0.162) [2.07 – 2.76] |
Figure 3. Comparison of Population Metrics.
Uninfected RBC population comparisons of the cerebral malaria (CM), uncomplicated malaria (UM), aparasitemic coma (AC), and healthy controls. The colors green and blue indicate that these populations are significantly different by a T-test with a p<0.05. Grey indicates that the populations are not significantly different from either green or the blue. The horizontal breadth of each colored bar represents the range between the 25th and 75th percentiles. The notches in the colored bars indicate the 95% confidence interval around the mean (the red line). The whiskers indicate values within 2.7 and 99.3 percentiles, while the crosses are outliers.
Comparing patients with cerebral malaria and uncomplicated malaria using the MCD model
We used the MCD filtration model to approximate the change in surface area, the change in volume and the splenic filtration of infected RBCs in patients with different presentations of malaria infection. One requirement of the simulation algorithm is a sufficient number of infected RBCs to compare with the simulated “invaded” RBCs; in our populations, this corresponded to a peripheral parasitemia of 1% (see Methods). Nineteen cerebral malaria and 19 uncomplicated malaria met this criterion, and were included in the analysis. Since the mean surface area and volume of uninfected RBCs varies between individuals (Figure 3), the MCD model estimates the change in volume and surface area relative to that of the patients own uninfected RBCs, rather than the absolute values of volume and surface area. The estimated gain in volume and surface area expected for infected RBCs after merozoite invasion are 4.70 µm3 and −1.39 µm2. The increase in mean volume and surface area of infected RBCs in the CM patients was 9.47±3.08 µm3 and 2.37±4.50 µm2 when compared to uninfected RBCs (Table 2). In patients with uncomplicated malaria, the mean gain in volume and surface area was 9.45±4.97 µm3 and −0.34±5.07 µm2, respectively. These differences between the cerebral malaria and uncomplicated malaria were not statistically significant.
Table 2.
No statistically significant difference was observed in the volume gained and surface area gained when Cerebral Malaria and Uncomplicated Malaria patient populations were compared.
| Infected RBC Population Statistics |
Volume Gain Mean (Std) µm3 |
Surface Area Gain Mean (Std) µm2 |
Filtration Transform Mean (Std) % |
Percent Survive Mean (Std) % |
| Cerebral Malaria N = 19 |
9.47 (3.08) | 2.37 (4.50) | 70.7 (22.5) | 55.9 (26.6) |
| Uncomplicated Malaria N = 19 |
9.45 (4.97) | −0.34 (5.07) | 68.0 (29.7) | 45.5 (28.7) |
| Combined Populations N=38 |
9.46 (4.08) | 1.01 (4.92) | 69.4 (26.0) | 50.7 (27.8) |
The MCD filtration model estimated the mean filtration transform function, a gauge of how aggressively the filtration function filters cells (Supplemental Materials), was 70.7% for cerebral malaria patients, and 68.0% in patients with uncomplicated malaria. The average rate of infected RBCs surviving simulated filtration were 55.9% and 45.5% for the cerebral malaria and uncomplicated malaria patients respectively. Neither measure of estimated filtration was significantly different between cerebral malaria and uncomplicated malaria patients. When the cerebral malaria and uncomplicated malaria populations are combined, the MCD filtration model estimates that, on average, 50% of red cells infected with ring state parasites are not filtered by the spleen and thus could survive in circulation (Table 2). These values correspond to an average of 1 infected RBC being trapped by the spleen for every parasite observed in circulation.
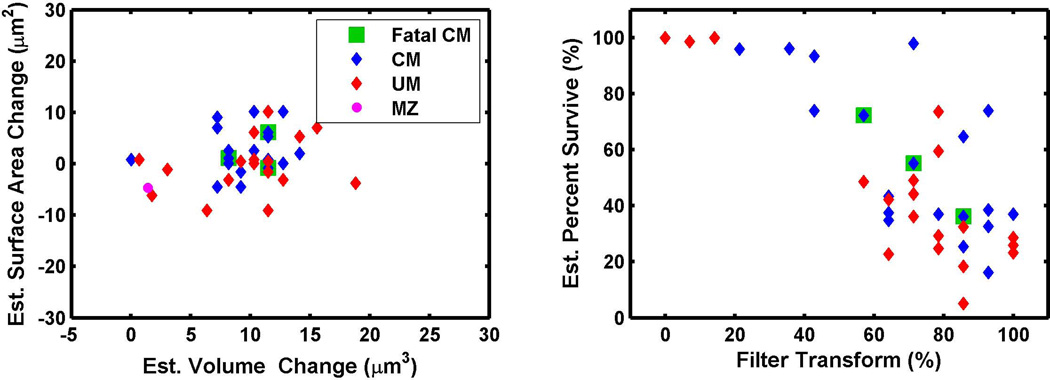
The MCD filtration modeling results for 38 cerebral malaria and uncomplicated malaria patients are plotted and 24 patients had estimates of infected RBC surface area change equal to or greater than zero (Figure 4A). This suggests that parasites had not only replaced all of the membrane surface area lost as a result of invasion, but continued to add surface area to the infected RBC. The algorithm also estimates the splenic attenuation function, expressed as a percentage of the width of the circulating RBC MCD population. The majority of filtration transform functions (31 of 38 patients) were estimated to be between 50 to 100% of the width of the total MCD population, suggesting that the spleen was actively filtering blood during the infection (Figure 4B). As the filter transforms become less stringent (i.e., filtering a smaller proportion of infected cells), there is an increase in percentage of parasites that are predicted to survive in circulation. The majority of subjects, 26 out of 38, had parasite survival rates of less than 60%, but twelve had higher parasite survival rates, suggesting that splenic filtration was less stringent in these patients. While only 3 of the 6 fatal cerebral malaria cases had sufficient parasitemia required for the simulation and estimation, these fatal cerebral malaria cases do not appear to be segregated from the non-fatal cerebral malaria cases or the uncomplicated malaria cases.
Figure 4. Estimated Volume, Surface Area, and Filtration Rates of Malaria Patients.
Since there is a wider variability between individuals in the mean volume and surface area of uninfected RBCs, the amount of volume and surface area gained by infected RBCs relative to an individuals uninfected RBCs is compared (A). Over-all there was no difference between cerebral malaria patients and uncomplicated malaria patients. MZ is the expected volume gained and surface area lost after merozoite invasion. The majority of patients had estimated filtration transforms from 50 to 100% which are predicted to filter parasites so that on average 50% of ring stage RBCs would survive in circulation (B).
Discussion
The study described here was a prospective, observational project designed to determine if the deformability of RBCs, as measured by their surface area, volume, and minimum cylindrical diameter, was a predictor of physical splenic filtration, as estimated by the MCD model.
Surface Area and Volume Findings in Clinically Distinct Groups
We investigated whether variations in uninfected RBC populations are associated with malaria infection or with the severity of malaria disease. The surface area and volume of RBCs from malaria patients are significantly larger than healthy controls, but there was no statistically significant difference between patients with uncomplicated malaria and patients with cerebral malaria (Figure 3). The RBC parameters of aparasitemic coma patients were in between the healthy controls and the uninfected RBCs of malaria patients. We were not able to measure RBC parameters of study subjects before or after their disease episodes, and as a result, we cannot distinguish between the possibility that smaller RBC volume and surface area confers protection against malaria illness or the possibility that malaria infection and illness themselves influence the overall dimensions of circulating RBCs.
MCD Model and Findings
The hypothesis that the MCD filtration model is representative of mechanical splenic filtration is supported by two observations. The first is that surface area and volume of cells are linearly related approximately according to lines of constant MCD as originally observed by Canham and Burton (Figure 1B and 1E). The second is that malaria parasites grown in vitro without splenic selection pressure grow to much larger MCDs than those observed in circulation (Figure 1D and 2B). In all samples taken from malaria patients, the MCD population distribution of ring-stage infected RBCs was within the range of the uninfected RBC (data not shown). Both of these observations offer indirect evidence that the MCD is a good criterion for physical filtration of RBCs from circulation.
The MCD filtration model presented here normalizes variations between and within individuals by using the circulating RBC population to estimate splenic filtration. The model further approximates ”clinical malaria” by deriving invaded RBCs from circulating uninfected RBC population to estimate the expected infected RBC population before filtration. In this manner, invasion and growth of infected RBCs is estimated relative to the individual’s uninfected RBC population, thus normalizing for differences between individuals in the characteristics of their circulating RBCs at the time of sampling. The MCD filtration model estimates that in the majority of individuals, infected RBCs are under some filtration selection (Figure 4B).
The MCD model presented here can distinguish if cell geometry is a factor in splenic filtration by including a case where none of the simulated invaded RBCs are subjected to filtration. If the infected RBC populations could be recreated without subjecting the cells to a simulated filtration, then either the infected RBCs are being filtered by a parameter other than the MCD, or they are not being filtered. To account for the situation where filtration by MCD does not occur, a zero percent filtration function was defined where all simulated “invaded” parasites survive filtration regardless of their MCD. In this case, the simulation results for volume change and surface area change would either not cluster together or the filtration transforms would be zero percent. While this special case appeared in only a single subject, 8 other subjects had estimated filtration rates of 80% parasite survival or higher, suggesting that very little attenuation of the infected RBC population is occurring.
The MCD filtration model estimates that on average 50.7% of ring-stage infected RBCs can survive in circulation (Table 2). This value is larger than studies where ex vivo perfused spleens allowed about 28% of the ring stage parasites to persist in circulation for up to and possibly beyond 2 hours (Safeukui et al., 2008). Another study which compared ex vivo perfused spleens with metal microparticle filters reported infected RBC survival rates of 32.9% (Deplaine et al., 2011). Parasite survival rates as estimated using the MCD filtration model on data from Malawian patients segregated into two groups (Supplemental Figure 6 and Supplemental Table 1). One group had infected RBC survival rates of greater than 60% (n = 12), and the other group (n=26), the infected RBC survival rates were below 60%. In the latter group, survival rates were similar to what has been reported in ex vivo perfused spleens (Safeukui et al., 2008, Deplaine et al., 2011).
The segregation of two groups may be related to differences in surface area of the ring-stage RBCs, where the surface area gain is higher in infected RBCs with the greater survival rates (Supplemental Table 1). The parasite may reduce the chance of splenic entrapment by increasing surface area of the host RBC membrane and subsequently reducing its MCD. Since replacing surface area of the host membrane requires synthesis of material and may take time, this could also indicate a difference in the age of the parasites in circulation or possibly a parasite phenotype. That surface area increases is clear, but the mechanism and composition of the increased surface area are unknown. One possibility is that parasite proteins exported to the surface of the red cell contribute to the increased surface area. The membrane material might be synthesized by the parasite, scavenged from the host, or both. An additional possibility is that in some subjects the infected RBC volume was altered by chemotherapy treatment prior to presenting at the hospital. Additional studies are required to address these possibilities. Overall, the MCD filtration model appears to be in fair agreement with previously published data.
The MCD filtration model presented here strongly suggests that cell geometry, principally the relationship of surface area and volume to the MCD, could account for a large fraction of the infected RBCs retained by the spleen. The estimates of splenic filtration based on the MCD filtration model predict that, on average, 51% or more ring-stage infected RBCs would be removed from circulation. The MCD filtration model suggests that the increased numbers of infected RBCs lost to the spleen could begin to account for the development of anemia during malaria infection. The model predicts that for every ring-stage parasite observed in circulation, an average of 1 infected RBC has been filtered by the spleen. Previous models analyzing anemia during malaria infection of naïve individuals predicted that on average 8.5 RBCs are destroyed for every ring-stage infected RBC observed in circulation (Jakeman et al., 1999). One potential explanation for this discrepancy is that while the MCD filtration model specifically ignores the contributions of membrane stiffness, cell viscosity and immune mediated filtration, these effects are additive components to the splenic filtration rate. In this manner the MCD filtration model may be considered a baseline filtration rate of infected RBCs before other effects such as immune response and dynamic deformation mechanics become the dominant features of infected RBC filtration. Ultimately accounting for these mechanisms of splenic filtration might enable a better description of the development of anemia during malaria infection.
Malaria Pathogenesis and Estimated Filtration
The MCD filtration model appears to account for a portion of anemia development during malaria infection. The filtration attenuation functions do not segregate according to the presentation of uncomplicated or cerebral malaria (Figure 4B). As with all experimental models the MCD filtration model as applied here has some limitations. The observations made in this study are only for a single time point at admission to the hospital and after the development of malaria symptoms. The blood is dynamic and erythrocyte properties may considerably change over the course of malaria infection or non-malaria illness in general. From previous studies of infected RBCs in culture and clearance time of ex vivo perfused spleens, we can estimate these observations are accurate over a 2 to 6 hour time frame (Safeukui et al., 2008, Herricks et al., 2009). Since we do not have a method to gauge the age of the circulating ring-stage RBCs, we cannot determine whether the filtration rates calculated are consistent over the entire 16 to 18 hour time frame that rings-stage RBCs are in circulation. Overall, the MCD filtration model suggests that filtration rates of infected RBCs vary widely between individuals and the influence of mechanical splenic filtration on malaria pathogenesis remains unclear without additional studies. While our MCD filtration model appears to be in good agreement with other studies, the data presented here suggest that the rate of physical splenic filtration, only after the onset of malaria symptoms, does not appear to vary with the severity of malaria disease.
Conclusion
We have successfully developed microfluidic devices designed to inform a model of splenic filtration in malaria infected individuals and applied them in a clinical field setting. We show the first direct demonstration that microfluidic devices can measure differences between cultured parasites and infected RBCs taken ex vivo from patients at a clinical field site. We developed a Monte Carlo based splenic filtration model based on the minimum cylindrical diameter (MCD) of an RBC. This MCD filtration model was in partial agreement with data published on ex vivo perfusion of spleens performed by other researchers. This study also highlights significant variations in RBC surface area and volume values between individuals. The MCD filtration model estimates that approximately half of the infected RBCs in circulation are removed by filtration. The removal of these infected RBCs may contribute, in patients with high-density parasitemias, to the development of anemia. While we cannot show a direct relationship between splenic filtration and the severity of malaria infection, we have developed an important new tool and model for exploring the physical characteristics of infected and uninfected erythrocytes. This tool may be of value in investigations of susceptibility to malaria infections and illness, drug effects, impact of splenectomy, and age-related acquisition of anti-malarial immunity.
Materials and Methods
Sample Collection and Preparation
All sample collection procedures were approved by the Malawi College of Medicine Research and Ethics Committee (COMREC). Samples were collected by hospital staff as part of the routine diagnostic practice. Samples were utilized after the appropriate measurement was performed, but before the sample, which would otherwise be discarded, was actually discarded. All samples were anonymized before the they were retrieved which, in some cases, limited the amount of information collected. Samples were collected by finger-prick into heparinized micro-hematocrit tubes. The blood was removed from the hematocrit tubes and approximately 20–30 µL of blood was diluted in 300 µL RPMI 1640 complete cell culture media with ~50 µg/mL Hoechst 33342 (Invitrogen). The sample was spun down briefly to pellet the RBCs and washed once with complete media. Approximately 100µL of sample was then added to the microfluidic device. The remainder of the blood sample was saved on FTA Elute cards (Whattman) for PCR analysis.
Study Population
Four patient populations were compared. These groups were chosen to determine the differences between the presentation of malaria infection and other forms of illness. Available patient demographic information may be found in Supplemental Table 2
Cerebral malaria
Patients admitted to the Paediatric Research Ward who met a stringent definition cerebral malaria: admission Blantyre Coma Score of ≤ 2, presence of malaria retinopathy, no improvement in coma score after correcting hypoglycemia or within the first two hours of admission, P. falciparum parasitemia of any density, no evidence of meningitis (no pathogens cultured from cerebrospinal fluid collected on admission) and no other identifiable explanation for coma. All patients were managed according to a standard protocol for IV fluids, antipyretics, anticonvulsants, and antibiotics.
Uncomplicated Malaria
Outpatients who were observed for several hours after treatment for blood film-confirmed malaria infection before being discharged. Patients with an identified alternative or additional diagnosis and patients with current or subsequent signs of severe malaria were excluded from this group.
Aparasitemic Coma
Patients admitted to the Paediatric Research Ward with culture-proven meningitis and no evidence of malaria infection on 4 blood films collected at 6-hourly intervals.
Healthy Controls
Aparasitemic and afebrile children who were present at the hospital but not admitted as patients and whose parents consented to provide a finger-prick sample of blood. These served as a healthy control group in a similar age range.
Device fabrication
Microfluidic devices were fabricated using methods described previously using photolithography and replica molding techniques (McDonald et al., 2000, Shelby et al., 2003). Channel patterns were designed using AutoCAD 2007 (Autodesk) and quartz-chrome photo mask (PhotoSciences) were generated. The channel pattern was transferred from the photo mask to silicon wafers (Montico Silicon) using AZ-1512 photoresist (AZ Materials). The patterns were etched to a depth of about 5 µm using the Bosche deep reactive ion etch process (Oxford Instruments ICP 380). The etch depth and feature size on the silicon masters were characterized using optical profilometery (WYKO NT3300). The wafers were the diced using a dicing saw (Tempress). The silicon masters were vapor primed with the release agent (tridecafluoro-1,1,2,2,-tetrahydrooctly)-trichlorosilane (Gelest) and then mounted in aluminum molds. The microfluidic devices generated by casting polydimethylsiloxane (Dupont Sylguard 184) replicas and cured at 140°C for 90 minutes until the rubber reached a Shore A hardness of 55 or greater. The PDMS device was removed from the silicone/aluminum mold. Tubing connections punched with a blunt 20 gauge needle (SmallParts). The cover slips (Gold Seal #3334) and PDMS devices were then exposed to an oxygen plasma of 10W for 40 seconds (Harrik Plasma Cleaner PDC-001) and then brought into conformal contact. The microfluidic devices were then packaged for transport to the field site in Malawi. Overall 300 microfluidic devices were manufactured and used at the Blantyre field site.
Image Collection and Analysis
Images of the microfluidic devices were recorded on a Nikon TE2000-S (Nikon USA) with a Photometrics CoolSnap EZ camera and a motorized stage (Prior). All bright field DIC images were recorded using a 40X DIC lens through a UV fluorescent filter cube (Croma Tech). Devices were mounted on a custom stage insert so that the devices could be aligned with the X-Y axis. Once the devices were aligned to the stage axis, images were recorded at preset locations using MetaMorph version 7.4 (Molecular Devices). Images were collected automatically using MetaMorph journals to move the stage to each location, autofocus, and then record images. At 200 locations a fluorescent image and a DIC image were collected for a total of 400 images per sample. Using this automation routine, we collected data on 7 to 10 samples per day. Batch image analysis was performed using routines written in MATLAB R2010a (Mathworks) as described previously (Herricks et al., 2009). An additional algorithm was developed to identify fluorescently labeled infected RBCs.
Modeling Filtration
MCD Filtration Model
Simulated RBC populations were generated using a bootstrap methodology. A random uniform distribution was mapped onto the uninfected RBC volume and MCD population. The corresponding surface area was found by solving for the roots of the MCD equation. In this way randomly generated numbers could be utilized to create simulated uninfected RBC population that are independent but with the same population distribution as the patients RBCs. The simulated uninfected RBCs were “remodeled” by adding volume, adding surface area, and recalculating the MCD. Finally, this simulated remodeled population was “filtered” using a Monte Carlo method. A random number was generated for each simulated remodeled RBC. This random number was then compared to the MCD filtration function value at the MCD value for that particular cell. If the random number was greater than the filtration function at the cell’s MCD, then the cell was considered to be filtered. If the random number was less than the MCD filtration function value, then the cell was considered to survive filtration. This process of generating simulated invaded RBCs and filtering them was repeated until the number of cells that survived filtration was equal to the number of infected RBCs observed in the sample. The percent survival was then calculated as the ratio of the number of simulated invaded RBCs that survived filtration to the total number of simulated invaded RBCs generated. The algorithm just described was considered to be one experiment and each experiment was repeated 25 times. The resulting population histograms were averaged to obtain the simulated populations observed in Figure 2A and 2B.
Estimating the volume and surface area gained for the infected RBCs was performed by iterating over a range of values for surface area and volume and MCD attenuation functions. Then the populations for each surface area, volume, and MCD distribution were compared using the Kolmogorov-Smirnov test as a fitting parameter. This process is repeated over a reasonable range of volume, surface area, and filtration attenuation functions. The p-value for each of the three distribution comparisons were averaged together to find the values for each that give the “best average fit” according to the Kolmogorov-Smirnov p-value. This combination of altering the volume, surface area, and filtration function gives an approximation of how much the infected cells in circulation have modified the host RBCs. All simulations were performed in MATLAB using the parallel processing tool box and the statistical tool box. A more detailed explanation may be found in the Supplemental Material.
Construction of Filtration Attenuation Function
The initial model was improved by using the MCD distribution of all circulating RBCs to construct a filtration attenuation function. This filtration function describes the probability of trapping a cell in the spleen as a function of the cell’s MCD. The filtration function was defined as one minus the normalized cumulative sum of the total MCD distribution. This total MCD distribution includes both infected and uninfected RBCs. The initial filtration function was considered to be too stringent filter, so function were put through a transform that reduced the range of MCD over which the filtration function affected. This was accomplished by generating the initial filtration function from a binned histogram. The filter function was transformed by reducing the number of bins in the histogram (Supplemental Material). These transformed filtration attenuation functions are described roughly as the value of the upper MCD quartile where attenuation begins. For instance, an attenuation function described as a filtration transform of 29% attenuates cells with their MCD in the upper 29th percentile of the population. A filter attenuation function of with a transform of 57% attenuates cells with an MCD in the upper 57th percentile of the population.
Filtration Model Assumptions
The splenic filtration model developed here has the following assumptions. 1.) Cells either pass through the spleen or become trapped. 2.) All uninfected RBCs in circulation have an equal chance of invasion. 3.) The parasites in circulation are considered to be synchronous ring-stage infected RBCs. A consequence of these assumptions would be surface area and volume change for each simulated invaded RBC is identical. 4.) The dynamic mechanical features such as cell membrane viscoelasticity and cytoplasm viscosity are not considered in this model. 5.) This model does not consider immune mediated filtration. 6.) Cytoadhesion of ring stage parasites is not considered.
Statistical Analysis
Tukey’s HSD test was performed in MATLAB with statistical tool box. The patient population’s RBC mean volume, mean surface area, mean MCD, and the MCD inner quartile range were compared. All plots were generated in MATLAB.
Malaria Cell culture
Malaria parasites were isolated from patients via venipuncture and then maintained in RPMI 1640 supplemented with 2.5 wt % Albumax II and gentamicin. Malaria parasites were cultured as described previously at 2% hematocrit in O+ blood under a blood gas mixture of 2.5% O2, 5% CO2, and 92.5% N2.(Trager et al., 1976)
Supplementary Material
Acknowledgments
We would specifically like to thank Professor Robert Heyderman for allowing use of the Malawi-Liverpool Wellcome Trust facility. This work was supported by the NIH under the following grants U19AI089688 (P.K.R.), R21 AI081234 (P.K.R.), K23AI079402 (K.B.S), and 5R01AI034969-14 (TT).
References
- Bach O, Baier M, Pullwitt A, Fosiko N, Chagaluka G, Kalima M, et al. Falciparum malaria after splenectomy: a prospective controlled study of 33 previously splenectomized Malawian adults. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2005;99:861–867. doi: 10.1016/j.trstmh.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Buffet PA, Safeukui I, Milon G, Mercereau-Puijalon O, David PH. Retention of erythrocytes in the spleen: a double-edged process in human malaria. Current opinion in hematology. 2009;16:157–164. doi: 10.1097/MOH.0b013e32832a1d4b. [DOI] [PubMed] [Google Scholar]
- Canham PB, Burton AC. Distribution of size and shape in populations of normal human red cells. Circ Res. 1968;22:405–422. doi: 10.1161/01.res.22.3.405. [DOI] [PubMed] [Google Scholar]
- Chotivanich K, Udomsangpetch R, McGready R, Proux S, Newton P, Pukrittayakamee S, et al. Central role of the spleen in malaria parasite clearance. The Journal of infectious diseases. 2002;185:1538–1541. doi: 10.1086/340213. [DOI] [PubMed] [Google Scholar]
- Deplaine G, Safeukui I, Jeddi F, Lacoste F, Brousse V, Perrot S, et al. The sensing of poorly deformable red blood cells by the human spleen can be mimicked in vitro. Blood. 2011;117:e88–e95. doi: 10.1182/blood-2010-10-312801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dondorp AM, Angus BJ, Chotivanich K, Silamut K, Ruangveerayuth R, Hardeman MR, et al. Red blood cell deformability as a predictor of anemia in severe falciparum malaria. Am J Trop Med Hyg. 1999;60:733–737. doi: 10.4269/ajtmh.1999.60.733. [DOI] [PubMed] [Google Scholar]
- Dondorp AM, Angus BJ, Hardeman MR, Chotivanich KT, Silamut K, Ruangveerayuth R, et al. Prognostic significance of reduced red blood cell deformability in severe falciparum malaria. Am J Trop Med Hyg. 1997;57:507–511. doi: 10.4269/ajtmh.1997.57.507. [DOI] [PubMed] [Google Scholar]
- Fedosov DA, Caswell B, Karniadakis GE. Wall shear stress-based model for adhesive dynamics of red blood cells in malaria. Biophysical journal. 2011a;100:2084–2093. doi: 10.1016/j.bpj.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedosov DA, Lei H, Caswell B, Suresh S, Karniadakis GE. Multiscale modeling of red blood cell mechanics and blood flow in malaria. PLoS Comput Biol. 2011b;7:e1002270. doi: 10.1371/journal.pcbi.1002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnham PC. The role of the spleen in protozoal infections with special reference to splenectomy. Acta tropica. 1970;27:1–14. [PubMed] [Google Scholar]
- Gifford SC, Derganc J, Shevkoplyas SS, Yoshida T, Bitensky MW. A detailed study of timedependent changes in human red blood cells: from reticulocyte maturation to erythrocyte senescence. Br J Haematol. 2006;135:395–404. doi: 10.1111/j.1365-2141.2006.06279.x. [DOI] [PubMed] [Google Scholar]
- Gifford SC, Frank MG, Derganc J, Gabel C, Austin RH, Yoshida T, Bitensky MW. Parallel microchannel-based measurements of individual erythrocyte areas and volumes. Biophys J. 2003;84:623–633. doi: 10.1016/S0006-3495(03)74882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenister FK, Coppel RL, Cowman AF, Mohandas N, Cooke BM. Contribution of parasite proteins to altered mechanical properties of malaria-infected red blood cells. Blood. 2002;99:1060–1063. doi: 10.1182/blood.v99.3.1060. [DOI] [PubMed] [Google Scholar]
- Groom AC, Schmidt EE, MacDonald IC. Microcirculatory pathways and blood flow in spleen: new insights from washout kinetics, corrosion casts, and quantitative intravital videomicroscopy. Scanning Microsc. 1991;5:159–173. discussion 173-154. [PubMed] [Google Scholar]
- Herricks T, Antia M, Rathod PK. Deformability limits of Plasmodium falciparum-infected red blood cells. Cell Microbiol. 2009;11:1340–1353. doi: 10.1111/j.1462-5822.2009.01334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakeman GN, Saul A, Hogarth WL, Collins WE. Anaemia of acute malaria infections in nonimmune patients primarily results from destruction of uninfected erythrocytes. Parasitology. 1999;119(Pt 2):127–133. doi: 10.1017/s0031182099004564. [DOI] [PubMed] [Google Scholar]
- Langreth SG, Peterson E. Pathogenicity, stability, and immunogenicity of a knobless clone of Plasmodium falciparum in Colombian owl monkeys. Infection and immunity. 1985;47:760–766. doi: 10.1128/iai.47.3.760-766.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JC, Duffy DC, Anderson JR, Chiu DT, Wu H, Schueller OJ, Whitesides GM. Fabrication of microfluidic systems in poly(dimethylsiloxane) Electrophoresis. 2000;21:27–40. doi: 10.1002/(SICI)1522-2683(20000101)21:1<27::AID-ELPS27>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–616. doi: 10.1038/nri1669. [DOI] [PubMed] [Google Scholar]
- Miller LH, Baruch DI, Marsh K, Doumbo OK. The pathogenic basis of malaria. Nature. 2002;415:673–679. doi: 10.1038/415673a. [DOI] [PubMed] [Google Scholar]
- Rand RP, Burton AC. Mechanical Properties of the Red Cell MembraneIMembrane Stiffness and Intracellular Pressure. Biophys J. 1964;4:115–135. doi: 10.1016/s0006-3495(64)86773-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safeukui I, Correas JM, Brousse V, Hirt D, Deplaine G, Mule S, et al. Retention of Plasmodium falciparum ring-infected erythrocytes in the slow, open microcirculation of the human spleen. Blood. 2008;112:2520–2528. doi: 10.1182/blood-2008-03-146779. [DOI] [PubMed] [Google Scholar]
- Shelby JP, White J, Ganesan K, Rathod PK, Chiu DT. A microfluidic model for single-cell capillary obstruction by Plasmodium falciparum-infected erythrocytes. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:14618–14622. doi: 10.1073/pnas.2433968100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor TE, Fu WJ, Carr RA, Whitten RO, Mueller JS, Fosiko NG, et al. Differentiating the pathologies of cerebral malaria by postmortem parasite counts. Nat Med. 2004;10:143–145. doi: 10.1038/nm986. [DOI] [PubMed] [Google Scholar]
- Trager W, Jensen JB. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.