Abstract
Background
Coenzyme Q10 (CoQ10) is a common antioxidant supplement with known cardioprotective effects and potential anticancer benefits.
Objective
We performed a randomized, double-blind, placebo-controlled study of oral CoQ10 in female breast cancer patients with the primary objective of determining CoQ10's effects on self-reported fatigue, depression, and quality of life (QOL).
Methods
Eligible women with newly diagnosed breast cancer and planned adjuvant chemotherapy were randomized to oral supplements of 300-mg CoQ10 or placebo, each combined with 300-IU vitamin E, divided into 3 daily doses. Treatment was continued for 24 weeks. Blood tests, quality of life (QOL) measures, and levels of plasma CoQ10 and vitamin E were obtained at baseline and at 8, 16, and 24 weeks. Mixed-effects models were used to assess treatment differences in outcomes over time.
Results
Between September 2004 and March 2009, 236 women were enrolled. Treatment arms were well balanced with respect to age (range, 28 to 85 years), pathologic stage (stage 0, 91%; stage I, 8%; stage II, 1%), ethnicity (white, 87%; black, 11%; Hispanic, 2%), and planned therapy. Baseline CoQ10 levels in the CoQ10 and placebo arms were 0.70 μg/mL and 0.73 μg/mL, respectively; the 24-week CoQ10 levels were 1.83 μg/mL and 0.79 μg/mL, respectively. There were no significant differences between the CoQ10 and placebo arms at 24 weeks for scores on the Profile of Mood States–Fatigue (POMS-F) questionnaire (least squares means, 7.08 vs 8.24; P = .257), the Functional Assessment of Chronic Illness Therapy–Fatigue (FACIT-F) tool (37.6 vs 37.6; P = .965), the Functional Assessment of Cancer Therapy–Breast Cancer (FACT-B) instrument (111.9 vs 110.4; P = .577), or the Center for Epidemiologic Studies–Depression (CES-D) scale (11.6 vs 12.3; P = .632).
Conclusions
Supplementation with conventional doses of CoQ10 led to sustained increases in plasma CoQ10 levels, but did not result in improved self-reported fatigue or QOL after 24 weeks of treatment.
Keywords: Coenzyme Q10, CoQ10, Cancer-Related Fatigue, Treatment-Related Fatigue, Breast Cancer, Randomized Clinical Trial
Published data suggest that at least 80% of cancer patients who are undergoing treatment, especially multimodality therapy, experience a significant degree of fatigue that may negatively impact their quality of life (QOL), emotional well-being, and treatment tolerance.1-11 Compared with the fatigue experienced by those without cancer, cancer-related fatigue is typically more severe and not reliably relieved by rest.12 Up to 80% of women receiving adjuvant breast cancer therapy may experience significant cancer and treatment-related fatigue.13 Persistent fatigue may affect a significant number of these women; published data suggest that it may last for months or years after the completion of therapy in at least 30% of patients with solid tumors or hematologic malignancies.14-18 For example, 81% of 1,372 women with breast cancer who had completed primary treatment described fatigue during or following their therapy.19
Patients perceive fatigue to be the most distressing symptom associated with their cancer experience, even worse than pain or nausea and vomiting.4 However, studies suggest that fatigue usually does not exist in isolation, but rather as part of a symptom cluster that often includes depression, difficulty sleeping, and pain.13,20 Current clinical practice guidelines recommend a regular assessment of fatigue in all cancer patients during and following their treatment,21,22 and several validated self-assessment tools for fatigue are available.23
Multiple recent reviews summarizing pharmacologic and nonpharmacologic approaches to treating cancer-related fatigue have been published24-27; unfortunately, relatively few pharmacologic interventions have been efficacious. Coenzyme Q10 (also known as ubiquinone) is a fat-soluble quinine with properties similar to vitamins.28 It is an antioxidant and a redox coenzyme of the respiratory chain29-31 that occurs naturally in the organs of most animal species,32 as well as in relatively high levels in the heart, liver, kidney and pancreas of humans.28 Biochemically, CoQ10 works by (1) having a direct regulatory role on succinyl and the reduced form of nicotinamide adenine dinucleotide (NADH) dehydrogenases, (2) acting as a catalyst and playing an integral role in regulating the cytochrome bc1 complex, and (3) possibly having direct membrane-stabilizing properties that are separate from its role in oxidative phosphorylation.28-33 Thus, CoQ10 works within human cells to create energy for cell growth and maintenance.30,34,35
Oral CoQ10 is well absorbed, although rather slowly, with peak plasma levels occurring 5 to 10 hours after ingestion.36 Normal plasma levels of CoQ10 range between 0.64 and1.06 µg/mL plasma.37-45 Males have higher levels than do females; older adults have lower levels of CoQ10 than do younger adults.46 The typical US diet provides approximately 5 mg to10 mg of CoQ10 per day. Side effects of CoQ10 may include insomnia, elevated liver enzymes, rash, nausea, epigastric pain, dizziness, photophobia, irritability, headache, and heartburn47,48; however, regardless of the dosage used, few untoward effects have been observed.49
Although CoQ10 has been used for several decades as a dietary supplement for general health maintenance, the benefits of its administration have been most extensively evaluated in a variety of cardiovascular and neurodegenerative conditions. In patients with congestive heart failure, CoQ10 supplementation to standard medical therapy improved QOL, New York Heart Association classification, and congestive symptoms including shortness of breath and edema.47,50-53 Similar benefits were seen in a study of patients with hypertrophic cardiomyopathy.54 Ongoing CoQ10 administration has led to sustained decreases in both systolic and diastolic blood pressure.55 Finally, data suggest that high-dose CoQ10 administration may slow the functional decline experienced by patients with early-stage Parkinson's disease.41
In light of its role in mitochondrial energy generation, CoQ10 supplementation has been evaluated in a variety of patient populations with fatigue. It has been clearly demonstrated to improve the symptoms of weakness and fatigue in the rare patient with inherited defects in CoQ10 biosynthesis.56,57 CoQ10 administration also has beneficial effects on dyspnea and exercise tolerance—cardiac fatigue—in patients with congestive heart failure and/or cardiomyopathy.29,39,47 However, conflicting data exist regarding the effect of CoQ10 on fatigue in a normal population. Cooke et al58 described a trend toward an increased time to exhaustion following 2 weeks of CoQ10 intake. A number of other placebo-controlled studies failed to demonstrate an improvement in physical functioning in similar trained and untrained populations.59-63
Clinical and epidemiologic investigations of CoQ10 in cancer are limited, and the few small studies that have been reported have evaluated the ability of CoQ10 supplementation to ameliorate or prevent cardiotoxicity in patients receiving anthracycline chemotherapy.64,65 CoQ10 is a common supplement used by patients with breast and other cancers; its purported benefits include improved cancer- and treatment-related fatigue.66 However, no prospective data have been published on the efficacy of this supplement in the fatigued cancer population. As a result, we performed a randomized, double-blind, placebo controlled study of CoQ10 in women with breast cancer who were beginning adjuvant chemotherapy. The primary aim of this trial was to assess the effect of CoQ10 supplementation on treatment-induced fatigue in these women; secondary goals were to assess the compound's effects on overall QOL and depression.
METHODS
Patient Population
Women with newly diagnosed breast cancer who were scheduled to receive adjuvant chemotherapy were eligible for this randomized, placebo controlled, double-blinded trial. Additional eligibility criteria included an Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2; ability to provide written informed consent; a hemoglobin level ≥ 11 g/dL; a total cholesterol level ≥ 160 mg/dL; a bilirubin level ≤ 1.5 × upper limit of normal (ULN); a plasma glutamic oxaloacetic transaminase (SGOT) level ≤ 2.5 × ULN; and plasma glutamate pyruvate transaminase (SGPT) ≤ 2.5 × ULN. Additional ineligibility criteria included an involuntary loss of greater than 5% of body weight in the previous 3 months; current or planned statin therapy; current or planned use of medications for fatigue, including corticosteroids (other than an allowable intermittent use as part of a chemotherapy regimen), amphetamines, or other stimulants including methylphenidate or modafinil; uncontrolled hypertension; pregnancy; uncontrolled thyroid dysfunction; and current or planned anticoagulant therapy (except for maintenance of catheter patency). The study was approved by the Institutional Review Board (IRB) of the Wake Forest School of Medicine and by each participating site's IRB. All study participants provided written informed consent.
Treatment
Eligible patients were stratified by type of chemotherapy (anthracycline vs no anthracycline) and whether or not they received radiation as part of their treatment course. Participants were then randomized to receive daily oral supplements of either 300-mg CoQ10 (Soft Gel Technologies, Los Angeles, California) per day or placebo—each combined with 300-IU vitamin E (Soft Gel Technologies, Los Angeles, California)—divided into 3 doses daily of either 100-mg CoQ10 or placebo, plus 100-IU vitamin E. Vitamin E served as a lipid carrier to improve absorption of the lipophilic CoQ10 molecule. CoQ10 or placebo supplements were begun no later than 4 days after chemotherapy initiation; they were taken 3 times daily with food for 24 weeks. Participants were instructed to avoid taking any additional supplements containing CoQ10 or vitamin E for the duration of the study. Adherence to study medications was assessed by serial measures of serum CoQ10 and vitamin E levels at baseline and following 8, 16, and 24 weeks of therapy.
Outcome Measures
Several QOL instruments were used to provide data on the primary outcome of fatigue, as well as secondary end points including overall QOL, depression, and social support, all of which could affect fatigue in a given patient. Fatigue was measured via the POMS-F (a 7-question fatigue subscale of the Profile of Mood States assessment tool, in which items are rated for the past week on a 5-point scale ranging from 0 [not at all] to 4 [extremely])67-70; the FACIT-F (a 13-item fatigue scale from the Functional Assessment of Chronic Illness Therapy Measurement System, in which items are rated from 0 [not at all] to 4 [very much])71,72; and a self-reported Linear Analog Scale Assessment - Fatigue (LASA-Fatigue) in which patients were asked to rate their overall level of fatigue using a 100 millimeter line with the anchors of “absolutely no fatigue” at 0 and “the worst possible fatigue imaginable” at 10.
QOL was assessed via the Functional Assessment of Cancer Therapy–Breast Cancer (FACT-B) instrument, which provided an assessment of the patients’ health status in addition to specific breast cancer–related concerns.73 Depressive symptomatology was assessed by the short form (8-item) Center for Epidemiologic Studies–Depression scale (CES-D).74 The social support of participants was measured, as a control variable, using the 20-item Medical Outcomes Study (MOS) Social Support Survey.75 All symptom and QOL assessments were measured at baseline and again following 8, 16, and 24 weeks of study treatment.
Analytical Method for α-Tocopherol (Vitamin E)
α-Tocopherol was quantified by reverse-phase, high-performance liquid chromatography (HPLC) via a modification of the method reported by Hess et al.76 All sample-handling steps were performed under subdued amber lighting. Patient plasma samples were collected from October 2004 to September 2009 and were stored at –80° C until they were analyzed. CoQ10 is stable for several years when it is stored at –80° C.77 Prior to extraction, 50 μL of 25 μg/mL vitamin K (used as an internal standard in place of the tocol used by Hess et al76) was added to 200-μL plasma and 1-mL ethanol containing 30-μM butylatedhydroxytoluene (BHT). The solution was extracted twice with 2-mL aliquots of hexane. The combined hexane extracts were evaporated to dryness under nitrogen, then reconstituted with 200-μL ethanol containing 30-μM BHT. Duplicate 35-μL aliquots were subjected to reverse-phase HPLC using a Beckman Ultrasphere C18 (4.6 × 250 mm) analytical column at 25° C. The isocratic mobile phase was composed of acetonitrile/tetrahydrofuran/methanol/1% ammonium acetate (684:220:68:28, by volume) at a flow rate of 1.5 mL/min. The eluted components were detected at 290 nm (vitamin E) and 269 nm (vitamin K) via a variable wavelength detector programmed to monitor the 2 wavelengths during different time segments of separation.
Analytical Method for CoQ10
CoQ10 was quantified following complete oxidation to ubiquinone with CuCl2 using a modification of the method reported by Kaikkonen.77 Plasma samples (200 μL) were mixed with 1-mL ethanol (containing no BHT) and 50 μL of 25-μg/mL vitamin K. Oxidation was performed by the addition of 200 μL of 2-mM CuCl2 at room temperature in the dark for 30 minutes. The reaction mixture was then rapidly extracted twice with 4-mL portions of hexane. The combined hexane extracts were evaporated to dryness under nitrogen, and then reconstituted with 200 μL of ethanol. Duplicate 35-μL injections were subjected to reverse-phase HPLC under the same conditions used for vitamin E. The column eluent was monitored at 325 nm (vitamin K) and 270 nm (CoQ10).
Statistical Considerations
The primary objective of this randomized trial was to assess the effect of CoQ10 on self-reported cancer treatment–related fatigue in breast cancer patients following 24 weeks of therapy. Secondary objectives were to assess the effect of CoQ10 on overall QOL and depression. Patients were stratified by planned radiation therapy (yes/no) and type of chemotherapy (anthracycline vs no anthracycline) and were assigned within strata to receive CoQ10 or a placebo with equal probability via variably sized permuted block randomization. The study was powered to detect a 30% relative difference in the POMS-F subscale (ie, 9.9 vs 6.9) between the 2 groups with 90% power at the 5% 2-sided level of significance, with assumptions of an adjusted standard deviation (SD) of 5.9 for the POMS-F subscale, and a dropout rate of approximately 40%, and an allowance for 1 interim look. The required sample size was 118 per group.
Chi-square, Fisher exact, and Wilcoxon rank-sum tests were used to assess baseline group differences in categorical and continuous variables. Repeated measures analysis of variance (RM-ANOVA) was used to assess the effect of CoQ10 on each outcome over time. Models were constrained to have equal group means at baseline, as proposed by Fitzmaurice et al.78 This approach gives the same estimate of treatment effect as does an RM-ANCOVA model (ie, the baseline measure of the outcome is used as a covariate) when there are no missing data, but our approach uses all the data, even data on participants who are missing at baseline and those who have only baseline observations, which allows us to use more data. Various covariance structures were considered for each model, including unstructured, compound symmetry, autoregressive, and Toeplitz, and the Bayesian Information Criterion was used to choose the most appropriate covariance structure for each outcome. Age, race, body mass index (BMI), and strata were included as covariates in separate models. The primary interest was in the effect of CoQ10 at 24 weeks, and this effect was assessed by using a linear contrast within the RM-ANOVA. The same modeling strategy was used to assess the effect of CoQ10 on the secondary outcome measures.
RESULTS
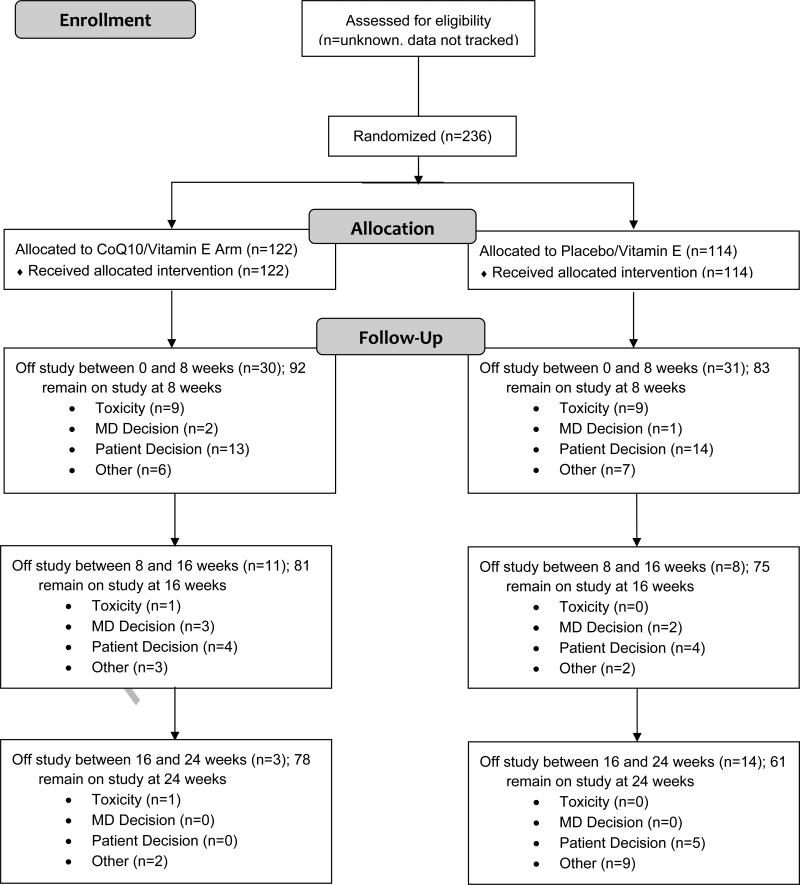
In all, 236 patients were enrolled between August 2004 and March 2009 (Figure 1). Baseline characteristics for all participants are summarized in Table 1. Ages ranged from 28 to 85 years, with a median of 51 years. Most patients were non-Hispanic whites (87%); 2% were Hispanic, and 11% were non-Hispanic blacks. Most patients were receiving anthracycline chemotherapy (84%); 61% also received radiation therapy. In all, 91% of the patients had an Eastern Cooperative Oncology Group (ECOG) performance status of 0. CoQ10 levels ranged from 0.13 to 3.4 μg/mL, with a median of 0.67 μg/mL; 44% of the patients had CoQ10 levels that were lower than normal (< 0.64 μg/mL). Patients reported low levels of fatigue at study initiation. Patient characteristics did not differ significantly between the treatment groups.
Figure 1.
Consort Flow Diagram of Patients Enrolled on This Trial
Table 1.
Patient Characteristics
| Characteristic | CoQ10 | Control (Vitamin E) | P Value |
|---|---|---|---|
| Total no. (%) | 122 (100) | 114 (100) | |
| Age | .239 | ||
| Median, y (range) | 52 (31-85) | 50 (28-72) | |
| ≥ 50 years, no. (%) | 72 (59) | 60 (53) | |
| BMI | .133 | ||
| Median BMI (range) | 27.3 (18.8-53.2) | 29.7 (18.4-50.8) | |
| Underweight-normal (BMI < 25), no. (%) | 38 (31) | 35 (31) | |
| Overweight (BMI 25-30), no. (%) | 46 (38) | 25 (22) | |
| Obese (BMI ≥ 30), no. (%) | 37 (31) | 54 (47) | |
| Race/ethnicity | .505 | ||
| Hispanic, no. (%) | 3 (2) | 1 (1) | |
| Black, no. (%) | 15 (12) | 11 (10) | |
| White, no. (%) | 104 (85) | 102 (89) | |
| ECOG Performance status | .815 | ||
| 0, no. (%) | 112 (92) | 103 (90) | |
| 1, no. (%) | 10 (8) | 10 (9) | |
| 2, no. (%) | 0 (0) | 1 (1) | |
| Stratum | — | ||
| Anthracycline + radiotherapy, no. (%) | 61 (50) | 59 (52) | |
| Anthracycline & no radiotherapy, no. (%) | 40 (33) | 38 (33) | |
| Nonanthracycline + radiotherapy, no. (%) | 14 (11) | 11 (10) | |
| Nonanthracycline & no radiotherapy, no. (%) | 7 (6) | 6 (5) | |
| Plasma CoQ10 level (μg/mL), median (range) | 0.63 (0.13-3.40) | 0.72 (0.23-1.73) | .152 |
| Plasma Vitamin E level (μg/mL), median (range) | 11.1 (0.6-52.2) | 11.3 (3.1-51.1) | .890 |
| POMS-F score, median (range) | 4.0 (0-27) | 4.0 (0-28) | .949 |
| FACIT-F score, median (range) | 44.0 (10-52) | 42.0 (11-52) | .141 |
| LASA-Fatigue score, median (range) | 2.0 (0-9) | 2.0 (0-8) | .808 |
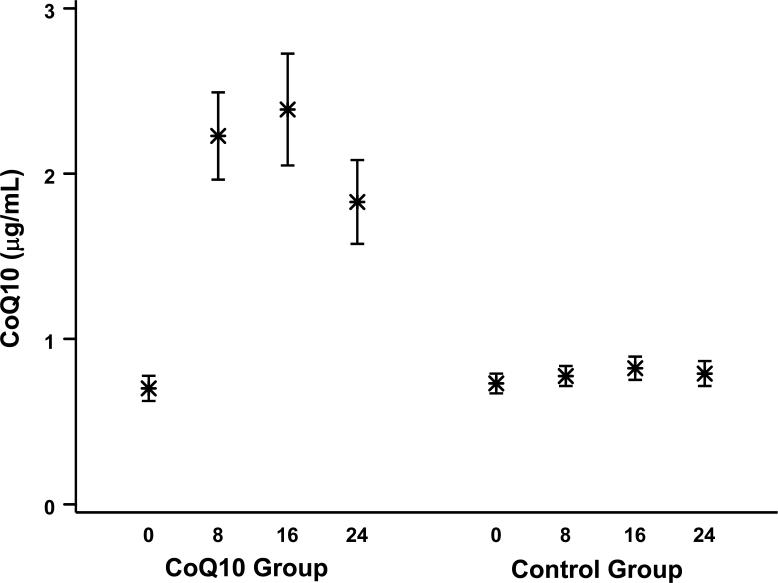
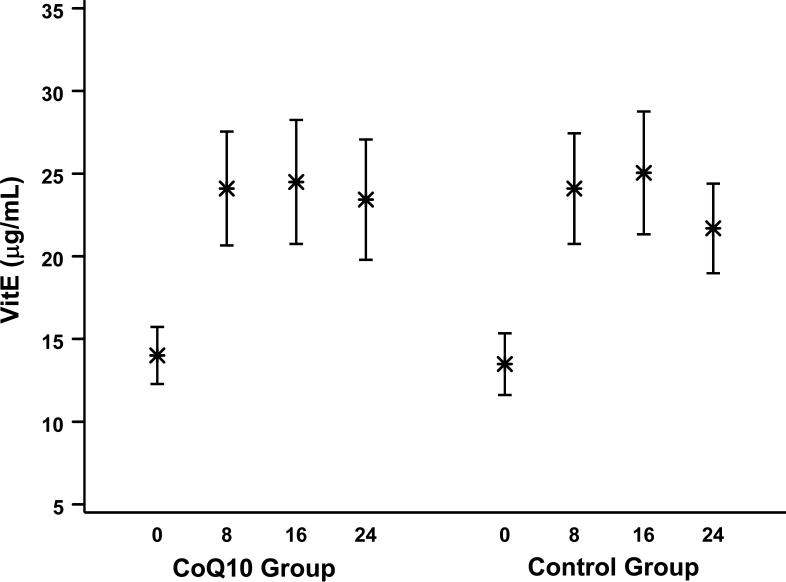
CoQ10 and vitamin E plasma levels were measured at baseline and following 8, 16, and 24 weeks of therapy. These data are summarized in Figures 2 and 3 for all samples collected at each visit. On average, CoQ10 supplementation resulted in an approximate 3-fold increase in plasma levels of CoQ10, from a mean (SD) of 0.7 (0.4) μg/mL at baseline to 2.2 (1.2) μg/mL at 8 weeks. However, there was much variability in postrandomization levels, and several patients failed to have noticeable increases in their levels; the average posttreatment CoQ10 levels were lower than the baseline levels for 12% of the participants on the CoQ10 arm. All patients received vitamin E supplementation, and levels almost doubled from 13.8 (8.7) μg/mL at baseline to 24.1 (15.1) μg/mL at 8 weeks. Average posttreatment vitamin E levels were lower than baseline levels for 11% of the participants.
Figure 2.
Mean Plasma CoQ10 Levels (With 95% Confidence Interval [CI]) at Baseline and 8, 16, and 24 Weeks by Treatment Group
Figure 3.
Mean Vitamin E Levels (95% CI) at Baseline and 8, 16, and 24 Weeks by Treatment Group
The primary objective of this study was to assess the effect of CoQ10 on patients’ self-reported fatigue levels at 24 weeks post randomization. Fatigue was quantified primarily by the POMS-F subscale. The FACIT-F subscale and a LASA-Fatigue were also used to quantify fatigue. Higher values for the FACIT-F subscale and lower values for the POMS-F subscale and the LASA-Fatigue indicate less fatigue. The raw fatigue measures over time are summarized in Table 2, and the least squares means adjusted for covariates are shown in Table 3. As expected, fatigue increased significantly with the onset of chemotherapy (P < .001) for patients in both groups, and gradually lessened thereafter, although never to pretreatment levels. In comparison to the placebo arm, CoQ10 supplementation was not significantly associated with changes in any of the fatigue measures at 24 weeks or at any time during this study. The interaction between baseline CoQ10 levels and treatment was not significant and the interaction between baseline fatigue and treatment was not significant in separate RM-ANCOVA models indicating that the treatment effect did not differ depending on initial fatigue or CoQ10 levels. Separate models that were examined for the subgroup of patients whose CoQ10 levels were below normal at baseline also failed to show any treatment benefit for CoQ10, as did models that were run on the patients in the lowest quartile of fatigue (worst fatigue) at baseline. We also examined the treatment effect in patients who were somewhat compliant (defined here as having a 20% increase in vitamin E levels from baseline to posttreatment assessments). Again, there was no significant benefit to CoQ10 in this group of patients.
Table 2.
Summary of Fatigue Measures Over Time
| Outcome | Week | CoQ10 | Control (Vitamin E) | ||||
|---|---|---|---|---|---|---|---|
| No. | Mean | SD | No. | Mean | SD | ||
| POMS-F (range, 0-28) | 0 | 120 | 5.59 | 6.06 | 113 | 5.69 | 6.09 |
| 8 | 92 | 9.92 | 7.05 | 81 | 9.32 | 7.61 | |
| 16 | 79 | 8.62 | 7.05 | 72 | 8.89 | 7.87 | |
| 24 | 78 | 6.65 | 6.52 | 61 | 8.43 | 7.66 | |
| FACIT-F (range, 0-52) | 0 | 120 | 41.0 | 9.69 | 113 | 38.9 | 10.6 |
| 8 | 92 | 34.1 | 12.1 | 81 | 34.4 | 11.8 | |
| 16 | 80 | 35.3 | 11.6 | 72 | 35.9 | 12.1 | |
| 24 | 76 | 38.9 | 11.3 | 61 | 36.9 | 12.0 | |
| LASA-Fatigue (range, 0-10) | 0 | 118 | 2.36 | 2.14 | 112 | 2.54 | 2.34 |
| 8 | 90 | 3.91 | 2.58 | 80 | 4.19 | 2.67 | |
| 16 | 80 | 3.93 | 2.53 | 73 | 3.71 | 2.62 | |
| 24 | 77 | 2.92 | 2.36 | 61 | 3.52 | 2.49 | |
This table contains data from all completed Fatigue Measurement instruments at each timepoint.
Table 3.
Fatigue Measures: Least Squares Means (Standard-Error)a
| Outcome | Baseline | 8 Weeks | 16 Weeks | 24 Weeks |
P Valuesb |
|
|---|---|---|---|---|---|---|
| Overall | 24 Weeks | |||||
| POMS-F score | ||||||
| CoQ10 | 5.61 (0.45) | 9.92 (0.67) | 8.68 (0.71) | 7.08 (0.71) | ||
| Control | 5.61 (0.45) | 9.24 (0.70) | 8.70 (0.74) | 8.24 (0.79) | ||
| Group difference | — | 0.68 (0.93) | –0.02 (0.99) | –1.17 (1.03) | .473 | .257 |
| FACIT-F score | ||||||
| CoQ10 | 40.0 (0.72) | 33.7 (1.05) | 34.7 (1.11) | 37.6 (1.13) | ||
| Control | 40.0 (0.72) | 34.9 (1.10) | 36.6 (1.16) | 37.6 (1.23) | ||
| Group difference | — | –1.27 (1.43) | –1.91 (1.51) | 0.07 (1.58) | .529 | .965 |
| LASA-Fatigue score | ||||||
| CoQ10 | 2.44 (0.16) | 3.92 (0.24) | 3.96 (0.25) | 3.08 (0.26) | ||
| Control | 2.44 (0.16) | 4.12 (0.25) | 3.69 (0.26) | 3.48 (0.28) | ||
| Group difference | — | –0.21 (0.33) | 0.27 (0.35) | –0.41 (0.37) | .404 | .267 |
Least squares means are calculated at the mean level of each covariate.
P values are for differences between treatment groups; overall P value assesses differences at any time; 24-week P value assesses differences at 24 weeks.
As expected, patients in both treatment groups experienced decreases in their overall QOL (P < .001), as seen in Table 4. Treatment with CoQ10, however, did not significantly improve the patients’ QOL at 24 weeks, as measured by the FACT-B total score (P = .764 overall; P = .577 at 24 weeks). Nonsignificant increases in depressive symptoms after the initiation of adjuvant therapy were noted, and these symptoms were also not significantly different between the CoQ10 and the placebo groups (P = .697 overall; P = .632 at 24 weeks).
Table 4.
Quality of Life and Depression, LS Means (SE)a
| Outcome | Baseline | 8 Weeks | 16 Weeks | 24 Weeks | P Valuesb | |
|---|---|---|---|---|---|---|
| Overall | 24 Weeks | |||||
| Fact-B score | .764 | .577 | ||||
| CoQ10 | 111.3 (1.29) | 105.0 (1.67) | 108.3 (1.85) | 111.9 (1.92) | ||
| Placebo | 111.3 (1.29) | 105.2 (1.73) | 106.2 (1.89) | 110.4 (2.07) | ||
| Group difference | — | –0.16 (1.98) | 2.10 (2.36) | 1.46 (2.61) | ||
| CES-D score | .697 | .632 | ||||
| CoQ10 | 12.0 (0.65) | 13.0 (0.89) | 11.6 (0.96) | 11.6 (1.02) | ||
| Placebo | 12.0 (0.65) | 13.0 (0.93) | 13.0 (1.01) | 12.3 (1.11) | ||
| Group difference | — | –0.05 (1.15) | –1.42 (1.28) | –0.69 (1.45) | ||
Least squares means are calculated at the mean level of each covariate.
P values are for differences between treatment groups; overall P value assesses differences at any time; 24-week P value assesses difference at 24 weeks.
Grade 3 and 4 toxicities that were experienced by patients in this trial are summarized in Table 5. No severe drug-related toxicity was assessed as possibly, probably, or definitely attributed to CoQ10. CoQ10 and placebo patients did not differ significantly in the incidence of grade 3 and 4 toxicities (P = .301) or any toxicity (all grades) (P = .430).
Table 5.
Observed Grade 3 and 4 Toxicities
| Toxicities | CoQ10 | Placebo | Total | ||
|---|---|---|---|---|---|
| Grade 3 | Grade 4 | Grade 3 | Grade 4 | ||
| Allergic reaction | 1 | 0 | 0 | 0 | 1 |
| Anemia | 1 | 0 | 0 | 0 | 1 |
| Constipation | 1 | 0 | 1 | 0 | 2 |
| Dehydration | 3 | 0 | 2 | 0 | 5 |
| Diarrhea | 3 | 0 | 2 | 0 | 5 |
| Dizziness | 1 | 0 | 0 | 0 | 1 |
| Fatigue | 1 | 0 | 3 | 0 | 4 |
| Fever | 2 | 0 | 1 | 0 | 3 |
| Hot flashes | 0 | 0 | 2 | 0 | 2 |
| Hypotension | 1 | 0 | 0 | 0 | 1 |
| Ileus | 1 | 0 | 0 | 0 | 1 |
| Infection | 5 | 0 | 2 | 1 | 8 |
| Left ventricle dysfunction | 0 | 0 | 1 | 0 | 1 |
| Leukopenia | 2 | 1 | 2 | 0 | 5 |
| Neutropenia | 6 | 6 | 2 | 2 | 16 |
| Pain | 1 | 0 | 4 | 0 | 5 |
| Vomiting | 1 | 0 | 1 | 0 | 2 |
| Total | 30 | 7 | 23 | 3 | 63 |
Each cell within this table contains the number of individual patients who experienced the listed Grade 3 or 4 toxicity while enrolled on this trial. Row totals in the last column represent the number of unique patients who experienced each listed toxicity. Column totals given in the last row of the table represent the total number of occurrences of Grade 3 and 4 toxicities and includes patients who experienced more than one Grade 3 or 4 toxicity.
DISCUSSION
The primary aim of this randomized, double-blind, placebo-controlled trial was to determine the effect of CoQ10 supplementation on self-reported fatigue in women who had breast cancer and were beginning adjuvant chemotherapy. Despite a host of clinical trials, relatively few pharmacologic interventions for cancer-related fatigue have been effective. Erythropoiesis-stimulating agents have improved fatigue in a number of prospective, randomized, phase III clinical trials of anemic cancer patients receiving chemotherapy79; however, recent concerns over tumor stimulation and decreased survival following the use of these agents have significantly curtailed their use. A subset of patients who had more severe fatigue and/or advanced disease benefited somewhat from treatment with the psychostimulant methylphenidate in a phase III trial reported by Moraska et al,80 although no benefit was seen in the study population as a whole. Similar results were seen in more than 800 patients who were treated on a phase III trial of modafinil, a nonamphetamine psychostimulant, in which only those patients with severe baseline fatigue seemed to benefit.81,82
In this trial, despite serologic evidence of an average 3-fold increase in CoQ10 levels in women on the treatment arm, no difference in fatigue between CoQ10 supplemented and placebo-treated patients was seen according to 3 separate, validated measures. This was true for the patients with below-normal CoQ10 levels at baseline and for patients with worse fatigue at baseline, subgroups that might be more likely to benefit from CoQ10 supplementation. The fatigue trajectory seen in these patients (ie, fatigue that worsened with treatment and only gradually returned toward baseline after almost 6 months) mirrors the observations seen in previously published trials of fatigue in newly diagnosed women with breast cancer. Although not efficacious, CoQ10 supplementation was devoid of significant toxicity; adverse events seen in these patients represented the side effects of chemotherapy with or without radiation therapy.
One reason for the widespread use of CoQ10 supplementation by patients with breast and other cancers has been to correct a perceived CoQ10 deficiency that is thought to predispose patients to an increase in treatment-related toxicity. CoQ10 deficiency has been described in a cohort of 200 women who were hospitalized for breast surgery for both malignant and nonmalignant lesions.83 In our study, CoQ10 levels at baseline were below the lower limit of normal38-40, 84 for approximately 44% of enrolled women. Supplementation with standard doses of CoQ10 led to a significant and sustained increase in plasma CoQ10 levels in treated patients on this trial. Although a variety of factors can influence plasma CoQ10 levels (eg, age, race, plasma lipid levels, and use of concurrent medications such as statins), the steady-state plasma CoQ10 levels that were seen in treated women on this trial mirror those described in other patient populations that were supplemented with similar amounts of CoQ10.39-41,84
This trial did not address the benefits, if any, of CoQ10 dose escalation. The safety of escalated doses of CoQ10 has been evaluated in a randomized, placebo-controlled trial in patients with early Parkinson's disease. A total of 80 patients received doses of 300 mg to 1,200 mg per day of CoQ10 for up to 16 months, and there was no difference in the incidence of drug-related toxicities between the placebo and treatment arms.41 Doses of up to 3,000 mg/day for up to 8 months have also been well tolerated in cohorts of patients with Parkinson's disease and amyotrophic lateral sclerosis.85,86 Although treated patients in all of these trials experienced a low incidence of gastrointestinal side effects (including nausea, vomiting, diarrhea, and abdominal discomfort), these symptoms did not appear to be dose related, and they occurred at identical rates in both treatment and placebo arms.41,47,87,88 In this trial, dose escalation of CoQ10 was not attempted. However, no evidence of an improvement in fatigue was seen in the patients with the highest sustained levels of CoQ10 following supplementation (100% increase or more). Although this result does not rule out a possible benefit of higher doses, it provides no suggestion of a “dose response” in the population of women enrolled in this trial.
A large number of patients dropped out of this study before the 24-week study end point. However, the causes for study discontinuation were not different between the 2 treatment arms. Of the 97 patients who withdrew before the scheduled final study assessment, 21% did so for toxicities related to their primary anticancer therapy. Another 41% of these patients discontinued therapy following prolonged periods of inability to reliably tolerate oral medications. Again, this was primarily related to treatment-induced toxicities, particularly hospitalization, nausea, and/or vomiting, as well as an unwillingness to comply with a 3-times-a-day dosing regimen of study medications in the face of a perceived daunting schedule of antitumor therapy. In light of this dropout rate, we also analyzed all primary and secondary end points at the interim 8- and 16-week time points. This analysis again failed to reveal any indication of a CoQ10 effect on fatigue, depression, and QOL.
CONCLUSIONS
Based on our data, there is no evidence to support the use of standard-dose CoQ10 supplementation to ameliorate treatment-related fatigue in newly diagnosed women with breast cancer. Although fatigued patients without breast cancer were not specifically included in this study, there are no compelling mechanistic data to suggest that these patients would respond differently to CoQ10. In addition, this study was designed to limit and/or prevent fatigue among patients who were initiating adjuvant therapy. It was not targeted toward already-fatigued patients to try to reduce their symptoms. Given the results of this study, however, it does not seem likely that using CoQ10 supplementation to target fatigued patients would result in better outcomes.
Evaluation of dose-escalated CoQ10 could be attempted, with an expectation of higher steady-state plasma CoQ10 levels. However, the absence of any suggested benefit for the supplement in any of the patient subgroups examined in this trial indicates that newer approaches utilizing conventional or complementary agents would probably be a better use of limited clinical research resources.
Acknowledgments
Funding/Support: This trial was supported by the National Cancer Institute Division of Cancer Prevention Grant U10 CA81851; National Clinical Trials Registration No. NCT00096356.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr. Shaw reported receiving compensation from defendants’ attorneys for medicolegal expert testimony and payment for lectures as a visiting professor. All other authors have no conflicts of interest to disclose.
Previous Presentation: Presented in part at the American Society of Clinical Oncology Annual Meeting; June 4-8, 2010; Chicago, Illinois.
Additional Contributions: We would like to thank the following research base staff (Lisa Autry; Gina Enevold, MSN, GNP; June Fletcher-Steede, BS, RT; Rhonda Kimball; Robin Rosdhal, RN; Cissy Yates, MT), laboratory technician (Mark Morris), institutions (Alamance Regional Medical Center; East Carolina University), and CCOPs (Christiana Care Health Services CCOP; Louisiana State University–Shreveport MBCCOP; Northern Indiana Cancer Research Consortium CCOP; Ozarks Regional CCOP; Santa Rosa Memorial CCOP; Southeast Cancer Control Consortium and Western Regional CCOP) for their help with and participation in this clinical trial.
Reference List
- 1.Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. J Natl Cancer Inst Monogr. 2004;(32):40–50. doi: 10.1093/jncimonographs/lgh027. [DOI] [PubMed] [Google Scholar]
- 2.Campos MP, Hassan BJ, Riechelmann R, Del Giglio A. Cancer-related fatigue: a practical review. Ann Oncol. 2011;22(6):1273–1279. doi: 10.1093/annonc/mdq458. [DOI] [PubMed] [Google Scholar]
- 3.Curt GA, Breitbart W, Cella D, et al. Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 2000;5(5):353–360. doi: 10.1634/theoncologist.5-5-353. [DOI] [PubMed] [Google Scholar]
- 4.Vogelzang NJ, Breitbart W, Cella D, et al. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: results of a tripart assessment survey. The Fatigue Coalition. Semin Hematol. 1997;34(3 suppl 2):4–12. [PubMed] [Google Scholar]
- 5.Stasi R, Abriani L, Beccaglia P, Terzoli E, Amadori S. Cancer-related fatigue: evolving concepts in evaluation and treatment. Cancer. 2003;98(9):1786–1801. doi: 10.1002/cncr.11742. [DOI] [PubMed] [Google Scholar]
- 6.Flechtner H, Bottomley A. Fatigue and quality of life: lessons from the real world. Oncologist. 2003;8(suppl 1):5–9. doi: 10.1634/theoncologist.8-suppl_1-5. [DOI] [PubMed] [Google Scholar]
- 7.Wang XS, Giralt SA, Mendoza TR, et al. Clinical factors associated with cancer-related fatigue in patients being treated for leukemia and non-Hodgkin's lymphoma. J Clin Oncol. 2002;20(5):1319–1328. doi: 10.1200/JCO.2002.20.5.1319. [DOI] [PubMed] [Google Scholar]
- 8.Irvine D, Vincent L, Graydon JE, Bubela N, Thompson L. The prevalence and correlates of fatigue in patients receiving treatment with chemotherapy and radiotherapy: a comparison with the fatigue experienced by healthy individuals. Cancer Nurs. 1994;17(5):367–378. [PubMed] [Google Scholar]
- 9.Jacobsen PB, Hann DM, Azzarello LM, Horton J, Balducci L, Lyman GH. Fatigue in women receiving adjuvant chemotherapy for breast cancer: characteristics, course, and correlates. J Pain Symptom Manage. 1999;18(4):233–242. doi: 10.1016/s0885-3924(99)00082-2. [DOI] [PubMed] [Google Scholar]
- 10.Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: the scale of the problem. Oncologist. 2007;12(suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- 11.Henry DH, Viswanathan HN, Elkin EP, Traina S, Wade S, Cella D. Symptoms and treatment burden associated with cancer treatment: results from a cross-sectional national survey in the U.S. Support Care Cancer. 2008;16(7):791–801. doi: 10.1007/s00520-007-0380-2. [DOI] [PubMed] [Google Scholar]
- 12.Glaus A, Crow R, Hammond S. A qualitative study to explore the concept of fatigue/tiredness in cancer patients and in healthy individuals. Eur J Cancer Care (Engl) 1996;5(2 suppl):8–23. doi: 10.1111/j.1365-2354.1996.tb00247.x. [DOI] [PubMed] [Google Scholar]
- 13.So WK, Marsh G, Ling WM, et al. The symptom cluster of fatigue, pain, anxiety, and depression and the effect on the quality of life of women receiving treatment for breast cancer: a multicenter study. Oncol Nurs Forum. 2009;36(4):E205–E214. doi: 10.1188/09.ONF.E205-E214. [DOI] [PubMed] [Google Scholar]
- 14.Bower JE, Ganz PA, Desmond KA, Rowland JH, Meyerowitz BE, Belin TR. Fatigue in breast cancer survivors: occurrence, correlates, and impact on quality of life. J Clin Oncol. 2000;18(4):743–753. doi: 10.1200/JCO.2000.18.4.743. [DOI] [PubMed] [Google Scholar]
- 15.Bower JE, Ganz PA, Desmond KA, et al. Fatigue in long-term breast carcinoma survivors: a longitudinal investigation. Cancer. 2006;106(4):751–758. doi: 10.1002/cncr.21671. [DOI] [PubMed] [Google Scholar]
- 16.Cella D, Davis K, Breitbart W, Curt G, for the Fatigue Coalition Cancer-related fatigue: prevalence of proposed diagnostic criteria in a United States sample of cancer survivors. J Clin Oncol. 2001;19(14):3385–3391. doi: 10.1200/JCO.2001.19.14.3385. [DOI] [PubMed] [Google Scholar]
- 17.Baker F, Denniston M, Smith T, West MM. Adult cancer survivors: how are they faring?. Cancer. 2005;104(11 suppl):2565–2576. doi: 10.1002/cncr.21488. [DOI] [PubMed] [Google Scholar]
- 18.Andrykowski MA, Curran SL, Lightner R. Off-treatment fatigue in breast cancer survivors: a controlled comparison. J Behav Med. 1998;21(1):1–18. doi: 10.1023/a:1018700303959. [DOI] [PubMed] [Google Scholar]
- 19.Janz NK, Mujahid M, Chung LK, et al. Symptom experience and quality of life of women following breast cancer treatment. J Womens Health (Larchmt) 2007;16(9):1348–1361. doi: 10.1089/jwh.2006.0255. [DOI] [PubMed] [Google Scholar]
- 20.Loscalzo MJ, Clark KL. Problem-related distress in cancer patients drives requests for help: a prospective study. Oncology (Williston Park) 2007;21(9):1133–1138. [PubMed] [Google Scholar]
- 21.Berger A, Abernethy A, Atkinson A. [July 4, 2011];National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Cancer-Related Fatigue. Version 1. 2011 www.nccn.org/professionals/physician_gls/pdf/fatigue.pdf.
- 22.Dy SM, Lorenz KA, Naeim A, Sanati H, Walling A, Asch SM. Evidence-based recommendations for cancer fatigue, anorexia, depression, and dyspnea. J Clin Oncol. 2008;26(23):3886–3895. doi: 10.1200/JCO.2007.15.9525. [DOI] [PubMed] [Google Scholar]
- 23.Mortimer JE, Barsevick AM, Bennett CL, et al. Studying cancer-related fatigue: report of the NCCN scientific research committee. J Natl Compr Canc Netw. 2010;8(12):1331–1339. doi: 10.6004/jnccn.2010.0101. [DOI] [PubMed] [Google Scholar]
- 24.Wagner LI, Cella D. Fatigue and cancer: causes, prevalence and treatment approaches. Br J Cancer. 2004;91(5):822–828. doi: 10.1038/sj.bjc.6602012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Escalante CP, Manzullo EF. Cancer-related fatigue: the approach and treatment. J Gen Intern Med. 2009;24(suppl 2):S412–S416. doi: 10.1007/s11606-009-1056-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. A systematic review and meta-analysis of the pharmacological treatment of cancer-related fatigue. J Natl Cancer Inst. 2008;100(16):1155–1166. doi: 10.1093/jnci/djn250. [DOI] [PubMed] [Google Scholar]
- 27.Stone PC, Minton O. Cancer-related fatigue. Eur J Cancer. 2008;44(8):1097–1104. doi: 10.1016/j.ejca.2008.02.037. [DOI] [PubMed] [Google Scholar]
- 28.Greenberg SM, Frishman WH. Coenzyme Q10: a new drug for myocardial ischemia? Med Clin North Am. 1988;72(1):243–258. doi: 10.1016/s0025-7125(16)30792-1. [DOI] [PubMed] [Google Scholar]
- 29.Langsjoen PH, Folkers K, Lyson K, Muratsu K, Lyson T, Langsjoen P. Effective and safe therapy with coenzyme Q10 for cardiomyopathy. Klin Wochenschr. 1988;66(13):583–590. doi: 10.1007/BF01720833. [DOI] [PubMed] [Google Scholar]
- 30.Overvad K, Diamant B, Holm L, Holmer G, Mortensen SA, Stender S. Coenzyme Q10 in health and disease. Eur J Clin Nutr. 1999;53(10):764–770. doi: 10.1038/sj.ejcn.1600880. [DOI] [PubMed] [Google Scholar]
- 31.Forsmark-Andrée P, Lee CP, Dallner G, Ernster L. Lipid peroxidation and changes in the ubiquinone content and the respiratory chain enzymes of submitochondrial particles. Free Radic Biol Med. 1997;22(3):391–400. doi: 10.1016/s0891-5849(96)00330-9. [DOI] [PubMed] [Google Scholar]
- 32.Crane FL, Hatefi Y, Lester RL, Widmer C. Isolation of a quinone from beef heart mitochondria. Biochim Biophys Acta. 1957;25(1):220–221. doi: 10.1016/0006-3002(57)90457-2. [DOI] [PubMed] [Google Scholar]
- 33.Crane FL, Navas P. The diversity of coenzyme Q function. Mol Aspects Med. 1997;18(suppl):S1–S6. doi: 10.1016/s0098-2997(97)00016-2. [DOI] [PubMed] [Google Scholar]
- 34.Crane FL, Sun IL, Sun EE. The essential functions of coenzyme Q. Clin Investig. 1993;71(8 suppl):S55–S59. doi: 10.1007/BF00226841. [DOI] [PubMed] [Google Scholar]
- 35.Pepping J. Coenzyme Q10. Am J Health Syst Pharm. 1999;56(6):519–521. doi: 10.1093/ajhp/56.6.519. [DOI] [PubMed] [Google Scholar]
- 36.Crane FL. Biochemical functions of coenzyme Q10. J Am Coll Nutr. 2001;20(6):591–598. doi: 10.1080/07315724.2001.10719063. [DOI] [PubMed] [Google Scholar]
- 37.Miles MV, Horn PS, Tang PH, et al. Age-related changes in plasma coenzyme Q10 concentrations and redox state in apparently healthy children and adults. Clin Chim Acta. 2004;347(1-2):139–144. doi: 10.1016/j.cccn.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 38.Miles MV, Horn PS, Morrison JA, Tang PH, DeGrauw T, Pesce AJ. Plasma coenzyme Q10 reference intervals, but not redox status, are affected by gender and race in self-reported healthy adults. Clin Chim Acta. 2003;332(1-2):123–132. doi: 10.1016/s0009-8981(03)00137-2. [DOI] [PubMed] [Google Scholar]
- 39.Khatta M, Alexander BS, Krichten CM, et al. The effect of coenzyme Q10 in patients with congestive heart failure. Ann Intern Med. 2000;132(8):636–640. doi: 10.7326/0003-4819-132-8-200004180-00006. [DOI] [PubMed] [Google Scholar]
- 40.Burke BE, Neuenschwander R, Olson RD. Randomized, double-blind, placebo-controlled trial of coenzyme Q10 in isolated systolic hypertension. South Med J. 2001;94(11):1112–1117. doi: 10.1097/00007611-200111000-00015. [DOI] [PubMed] [Google Scholar]
- 41.Shults CW, Oakes D, Kieburtz K, et al. Effects of coenzyme Q10 in early Parkinson disease: evidence of slowing of the functional decline. Arch Neurol. 2002;59(10):1541–1550. doi: 10.1001/archneur.59.10.1541. [DOI] [PubMed] [Google Scholar]
- 42.Rusciani L, Proietti I, Rusciani A, et al. Low plasma coenzyme Q10 levels as an independent prognostic factor for melanoma progression. J Am Acad Dermatol. 2006;54(2):234–241. doi: 10.1016/j.jaad.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 43.Yamamoto Y, Yamashita S, Fujisawa A, Kokura S, Yoshikawa T. Oxidative stress in patients with hepatitis, cirrhosis, and hepatoma evaluated by plasma antioxidants. Biochem Biophys Res Commun. 1998;247(1):166–170. doi: 10.1006/bbrc.1998.8752. [DOI] [PubMed] [Google Scholar]
- 44.Yamashita S, Yamamoto Y. Simultaneous detection of ubiquinol and ubiquinone in human plasma as a marker of oxidative stress. Anal Biochem. 1997;250(1):66–73. doi: 10.1006/abio.1997.2187. [DOI] [PubMed] [Google Scholar]
- 45.Lagendijk J, Ubbink JB, Vermaak WJ. Measurement of the ratio between the reduced and oxidized forms of coenzyme Q10 in human plasma as a possible marker of oxidative stress. J Lipid Res. 1996;37(1):67–75. [PubMed] [Google Scholar]
- 46.Ernster L, Forsmark-Andrée P. Ubiquinol: an endogenous antioxidant in aerobic organisms. Clin Investig. 1993;71(8 suppl):S60–S65. doi: 10.1007/BF00226842. [DOI] [PubMed] [Google Scholar]
- 47.Baggio E, Gandini R, Plancher AC, Passeri M, Carmosino G. Italian multicenter study on the safety and efficacy of coenzyme Q10 as adjunctive therapy in heart failure: CoQ10 Drug Surveillance Investigators. Mol Aspects Med. 1994;15(suppl):S287–S294. doi: 10.1016/0098-2997(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 48.Feigin A, Kieburtz K, Como P, et al. Assessment of coenzyme Q10 tolerability in Huntington's disease. Mov Disord. 1996;11(3):321–323. doi: 10.1002/mds.870110317. [DOI] [PubMed] [Google Scholar]
- 49.Langsjoen PH, Langsjoen PH, Folkers K. A six-year clinical study of therapy of cardiomyopathy with coenzyme Q10. Int J Tissue React. 1990;12(3):169–171. [PubMed] [Google Scholar]
- 50.Morisco C, Trimarco B, Condorelli M. Effect of coenzyme Q10 therapy in patients with congestive heart failure: a long-term multicenter randomized study. Clin Investig. 1993;71(8 suppl):S134–S136. doi: 10.1007/BF00226854. [DOI] [PubMed] [Google Scholar]
- 51.Hofman-Bang C, Rehnqvist N, Swedberg K, Wiklund I, Aström H. Coenzyme Q10 as an adjunctive in the treatment of chronic congestive heart failure: the Q10 Study Group. J Card Fail. 1995;1(2):101–107. doi: 10.1016/1071-9164(95)90011-x. [DOI] [PubMed] [Google Scholar]
- 52.Soja AM, Mortensen SA. Treatment of congestive heart failure with coenzyme Q10 illuminated by meta-analyses of clinical trials. Mol Aspects Med. 1997;18(suppl):S159–S168. doi: 10.1016/s0098-2997(97)00042-3. [DOI] [PubMed] [Google Scholar]
- 53.Berman M, Erman A, Ben-Gal T, et al. Coenzyme Q10 in patients with end-stage heart failure awaiting cardiac transplantation: a randomized, placebo-controlled study. Clin Cardiol. 2004;27(5):295–299. doi: 10.1002/clc.4960270512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Adarsh K, Kaur H, Mohan V. Coenzyme Q10 (CoQ10) in isolated diastolic heart failure in hypertrophic cardiomyopathy (HCM). Biofactors. 2008;32(1-4):145–149. doi: 10.1002/biof.5520320117. [DOI] [PubMed] [Google Scholar]
- 55.Rosenfeldt FL, Haas SJ, Krum H, et al. Coenzyme Q10 in the treatment of hypertension: a meta-analysis of the clinical trials. J Hum Hypertens. 2007;21(4):297–306. doi: 10.1038/sj.jhh.1002138. [DOI] [PubMed] [Google Scholar]
- 56.Quinzii CM, López LC, Naini A, DiMauro S, Hirano M. Human CoQ10 deficiencies. Biofactors. 2008;32(1-4):113–118. doi: 10.1002/biof.5520320113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Quinzii CM, López LC, Von-Moltke J, et al. Respiratory chain dysfunction and oxidative stress correlate with severity of primary CoQ10 deficiency. FASEB J. 2008;22(6):1874–1885. doi: 10.1096/fj.07-100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cooke M, Iosia M, Buford T, et al. Effects of acute and 14-day coenzyme Q10 supplementation on exercise performance in both trained and untrained individuals. J Int Soc Sports Nutr. 2008;5:8. doi: 10.1186/1550-2783-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Laaksonen R, Fogelholm M, Himberg JJ, Laakso J, Salorinne Y. Ubiquinone supplementation and exercise capacity in trained young and older men. Eur J Appl Physiol Occup Physiol. 1995;72(1-2):95–100. doi: 10.1007/BF00964121. [DOI] [PubMed] [Google Scholar]
- 60.Malm C, Svensson M, Ekblom B, Sjödin B. Effects of ubiquinone-10 supplementation and high intensity training on physical performance in humans. Acta Physiol Scand. 1997;161(3):379–384. doi: 10.1046/j.1365-201X.1997.00198.x. [DOI] [PubMed] [Google Scholar]
- 61.Weston SB, Zhou S, Weatherby RP, Robson SJ. Does exogenous coenzyme Q10 affect aerobic capacity in endurance athletes? Int J Sport Nutr. 1997;7(3):197–206. doi: 10.1123/ijsn.7.3.197. [DOI] [PubMed] [Google Scholar]
- 62.Porter DA, Costill DL, Zachwieja JJ, et al. The effect of oral coenzyme Q10 on the exercise tolerance of middle-aged, untrained men. Int J Sports Med. 1995;16(7):421–427. doi: 10.1055/s-2007-973031. [DOI] [PubMed] [Google Scholar]
- 63.Braun B, Clarkson PM, Freedson PS, Kohl RL. Effects of coenzyme Q10 supplementation on exercise performance, VO2 max, and lipid peroxidation in trained cyclists. Int J Sport Nutr. 1991;1(4):353–365. doi: 10.1123/ijsn.1.4.353. [DOI] [PubMed] [Google Scholar]
- 64.Iarussi D, Auricchio U, Agretto A, et al. Protective effect of coenzyme Q10 on anthracyclines cardiotoxicity: control study in children with acute lymphoblastic leukemia and non-Hodgkin lymphoma. Mol Aspects Med. 1994;15(suppl):S207–S212. doi: 10.1016/0098-2997(94)90030-2. [DOI] [PubMed] [Google Scholar]
- 65.Okuma K, Furuta I, Ota K. Protective effect of coenzyme Q10 in cardiotoxicity induced by adriamycin [in Japanese]. Gan To Kagaku Ryoho. 1984;11(3):502–508. [PubMed] [Google Scholar]
- 66.Hill GJ, Shriver BJ, Arnett DB. Examining intentions to use CoQ10 amongst breast cancer patients. Am J Health Behav. 2006;30(3):313–321. doi: 10.5555/ajhb.2006.30.3.313. [DOI] [PubMed] [Google Scholar]
- 67.Sadler IJ, Jacobsen PB. Progress in understanding fatigue associated with breast cancer treatment. Cancer Invest. 2001;19(7):723–731. doi: 10.1081/cnv-100106147. [DOI] [PubMed] [Google Scholar]
- 68.Jacobsen PB, Stein K. Is fatigue a long-term side effect of breast cancer treatment? Cancer Control. 1999;6(3):256–263. doi: 10.1177/107327489900600304. [DOI] [PubMed] [Google Scholar]
- 69.McNair DM, Lorr M. An analysis of mood in neurotics. J Abnorm Psychol. 1964;69:620–627. doi: 10.1037/h0040902. [DOI] [PubMed] [Google Scholar]
- 70.McNair DM, Lorr M, Droppleman L. Profile of Mood States Manual. Educational and Industrial Testing Service; San Diego, CA: 1971. [Google Scholar]
- 71.Cella DF, Tulsky DS, Gray G, et al. The functional assessment of cancer therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 72.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia- related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 73.Cella D. Manual of the Functional Assessment of Chronic Illness Therapy (FACIT) Scales. Version 4. Evanston Northwestern Healthcare; Evanston, IL: 1997. [Google Scholar]
- 74.Myers JK, Weissman MM. Use of a self-report symptom scale to detect depression in a community sample. Am J Psychiatry. 1980;137(9):1081–1084. doi: 10.1176/ajp.137.9.1081. [DOI] [PubMed] [Google Scholar]
- 75.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. [DOI] [PubMed] [Google Scholar]
- 76.Hess D, Keller HE, Oberlin B, Bonfanti R, Schüep W. Simultaneous determination of retinol, tocopherols, carotenes and lycopene in plasma by means of high-performance liquid chromatography on reversed phase. Int J Vitam Nutr Res. 1991;61(3):232–238. [PubMed] [Google Scholar]
- 77.Kaikkonen J, Nyyssönen K, Salonen JT. Measurement and stability of plasma reduced, oxidized and total coenzyme Q10 in humans. Scand J Clin Lab Invest. 1999;59(6):457–466. doi: 10.1080/00365519950185481. [DOI] [PubMed] [Google Scholar]
- 78.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. John Wiley & Sons, Inc; New York, NY: 2004. pp. 122–137. [Google Scholar]
- 79.de la Cruz M, Hui D, Parsons HA, Bruera E. Placebo and nocebo effects in randomized double-blind clinical trials of agents for the therapy for fatigue in patients with advanced cancer. Cancer. 2010;116(3):766–774. doi: 10.1002/cncr.24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moraska AR, Sood A, Dakhil SR, et al. Phase III, randomized, double-blind, placebo-controlled study of long-acting methylphenidate for cancer-related fatigue: North Central Cancer Treatment Group NCCTG-N05C7 trial. J Clin Oncol. 2010;28(23):3673–3679. doi: 10.1200/JCO.2010.28.1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jean-Pierre P, Morrow GR, Roscoe JA, et al. A phase 3 randomized, placebo-controlled, double-blind, clinical trial of the effect of modafinil on cancer-related fatigue among 631 patients receiving chemotherapy: a University of Rochester Cancer Center Community Clinical Oncology Program Research base study. Cancer. 2010;116(14):3513–3520. doi: 10.1002/cncr.25083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. Drug therapy for the management of cancer-related fatigue. Cochrane Database Syst Rev. 2010;7(7):CD006704. doi: 10.1002/14651858.CD006704.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jolliet P, Simon N, Barré J, et al. Plasma coenzyme Q10 concentrations in breast cancer: prognosis and therapeutic consequences. Int J Clin Pharmacol Ther. 1998;36(9):506–509. [PubMed] [Google Scholar]
- 84.Storch A, Jost WH, Vieregge P, et al. Randomized, double-blind, placebo-controlled trial on symptomatic effects of coenzyme Q(10) in Parkinson disease. Arch Neurol. 2007;64(7):938–944. doi: 10.1001/archneur.64.7.nct60005. [DOI] [PubMed] [Google Scholar]
- 85.Shults CW, Beal MF, Song D, Fontaine D. Pilot trial of high dosages of coenzyme Q10 in patients with Parkinson's disease. Exp Neurol. 2004;188(2):491–494. doi: 10.1016/j.expneurol.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 86.Ferrante KL, Shefner J, Zhang H, et al. Tolerance of high-dose (3,000 mg/day) coenzyme Q10 in ALS. Neurology. 2005;65(11):1834–1836. doi: 10.1212/01.wnl.0000187070.35365.d7. [DOI] [PubMed] [Google Scholar]
- 87.Ikematsu H, Nakamura K, Harashima S, Fujii K, Fukutomi N. Safety assessment of coenzyme Q10 (Kaneka Q10) in healthy subjects: a double-blind, randomized, placebo-controlled trial. Regul Toxicol Pharmacol. 2006;44(3):212–218. doi: 10.1016/j.yrtph.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 88.Huntington Study Group A randomized, placebo-controlled trial of coenzyme Q10 and remacemide in Huntington's disease. Neurology. 2001;57(3):397–404. doi: 10.1212/wnl.57.3.397. [DOI] [PubMed] [Google Scholar]