Abstract
20-Hydroxyvitamin D3 (20(OH)D3), the major product of CYP11A1 action on vitamin D3, is biologically active and is produced in vivo. As well as potentially having important physiological actions, it is of interest as a therapeutic agent due to its lack of calcemic activity. In the current study we have examined the ability of CYP24A1, the enzyme that inactivates 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), to metabolize 20(OH)D3. Rat CYP24A1 was expressed in Escherichia coli, purified by Ni-affinity chromatography and assayed with substrates incorporated into phospholipid vesicles which served as a model of the inner mitochondrial membrane. In this system CYP24A1 metabolized 1,25(OH)2D3 with a catalytic efficiency 1.4-fold higher than that seen for 25-hydroxyvitamin D3 (25(OH)D3). CYP24A1 hydroxylated 20(OH)D3 to several dihydroxy-derivatives with the major two identified by NMR as 20,24-dihydroxyvitamin D3 (20,24(OH)2D3) and 20,25-dihydroxyvitamin D3 (20,25(OH)2D3). The catalytic efficiency of CYP24A1 for 20(OH)D3 metabolism was more than 10-fold lower than for either 25(OH)D3 or 1,25(OH)2D3 and no secondary metabolites were produced. The two major products, 20,24(OH)2D3 and 20,25(OH)2D3, caused significantly greater inhibition of colony formation by SKMEL-188 melanoma cells than either 1,25(OH)2D3 or the parent 20(OH)D3, showing that CYP24A1 plays an activating, rather than an inactivating role on 20(OH)D3.
Keywords: CYP24A1, vitamin D metabolism, 20-hydroxyvitamin D, phospholipid vesicles, melanoma
1. Introduction
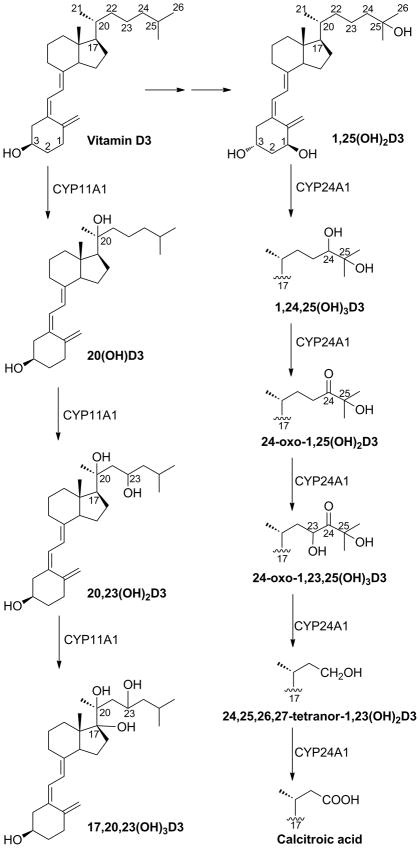
CYP24A1 catalyzes the inactivation of 1,25-dihydroxyvitamin D3 (1,25(OH)2D3) with initial production of 1,24,25-trihydroxyvitamin D3 (1,24,25(OH)3D3), as illustrated in Fig. 1 [1–4]. This product can undergo further CYP24A1-catalyzed oxidations resulting in cleavage of the side chain between C23 and C24 to produce calcitroic acid, the final product which is excreted (Fig. 1) [3, 5, 6]. Some mammalian species including the human also display a CYP24A1-catalyzed C23 oxidation pathway where initial hydroxylation is at C23 and the final product is 1,25-dihydroxyvitamin D3-26,23-lactone [6–9]. The rat enzyme almost exclusively catalyzes the C24 oxidation pathway, while this pathway accounts for 79% of the metabolism of 1,25(OH)2D3 by the human enzyme [7]. CYP24A1 also catalyzes the hydroxylation of 25-hydroxyvitamin D3 (25(OH)D3) [1, 2, 9, 10] by the C24 pathway with some of the primary product, 24,25-dihydroxyvitamin D3 (24,25(OH)2D3), escaping from the enzyme and being one of the major dihydroxyvitamin D metabolites seen in human serum [11]. While this product lacks the typical activity seen for 1,25(OH)2D3, it has been reported to be active in stimulating cartilage development and bone fracture repair [12].
Figure 1.
Pathways of vitamin D3 metabolism by CYP11A1 and CYP24A1. CYP11A1 catalyzes the conversion of vitamin D3 to 20(OH)D3, 20,23(OH)2D3 and 17,20,23(OH)3D3. Vitamin D3 is activated by other P450s to produce the biologically active hormone, 1,25(OH)2D3. This hormone is inactivated by CYP24A1 through sequential oxidation reactions which convert 1,25(OH)2D3 to the excretory product, calcitroic acid.
20S-Hydroxyvitamin D3 (20(OH)D3) is the major product of CYP11A1 action on vitamin D3 and can be further metabolized by purified CYP11A1 to a number of products with the main ones being 20,23-dihydroxyvitamin D3 (20,23(OH)2D3) and 17,20,23-trihydroxyvitamin D3, as illustrated in Fig. 1 [13–17]. Our most recent studies have shown that 20(OH)D3 can be produced by human placentae and rat adrenal glands fragments ex-vivo through a reaction catalyzed by CYP11A1 [18, 19]. Furthermore, we have shown that this secosteroid can be produced by cultured keratinocytes both with and without the addition of vitamin D3 to the culture media, and have provided data indicating that 20(OH)D3 is present in human serum [18, 19]. Thus, there is now compelling evidence that CYP11A1 provides an alternative pathway for vitamin D3 activation via production of 20(OH)D3, separate from the classical pathway producing 1,25(OH)2D3. 20(OH)D3 shares many, but not all of the actions of 1,25(OH)2D3. It inhibits the proliferation and stimulates the differentiation of a range of normal and cancer cells including keratinocytes, melanoma and leukemia cells [20–25]. Similar properties are seen for 20-hydroxyvitamin D2, the main product of CYP11A1-mediated metabolism of vitamin D2 [26–28]. 20(OH)D3 inhibits NF-κB activity by stimulating the expression of inhibitory IκBα protein [29, 30] and therefore serves as an excellent candidate for treatment of inflammatory diseases. Knockdown of the vitamin D receptor inhibits the effects of 20(OH)D3 indicating that like 1,25(OH)2D3, it works via the vitamin D receptor [24, 28, 30]. 20(OH)D3 also shows comparable activity to 1,25(OH)2D3 in stimulating translocation of the VDR coupled to green fluorescent protein from the cytoplasm to the nucleus [15, 28, 31]. However, unlike 1,25(OH)2D3, high concentrations of 20(OH)D3 (up to 30 μg/kg) do not raise serum calcium levels in rodents [22, 23]. Therefore, 20(OH)D3 has the potential to serve as a relatively non-toxic drug for treatment of hyperproliferative and inflammatory disorders. While 20(OH)D3 is a relatively poor substrate for 1α-hydroxylation by CYP27B1 [32], the product 1,20-dihydroxyvitamin D3, has calcemic activity although lower than that seen for 1,25(OH)2D3 [22]. Another important difference to 1,25(OH)2D3 is that 20(OH)D3 is a very poor inducer of CYP24A1 expression in a number of cell systems [15, 21, 22, 24, 28]. Therefore, if used therapeutically it may not be subject to the same rapid inactivation that occurs with 1,25(OH)2D3 and many vitamin D analogs when its levels are elevated [4].
Metabolism of 20(OH)D3 to 20,23(OH)2D3 and other products by CYP11A1 occurs with low catalytic efficiency and the metabolites retain strong biological activity [15, 22, 25, 29, 31]. Since CYP24A1 is the enzyme that inactivates 1,25(OH)2D3, it is important to know whether CYP24A1 can metabolize 20(OH)D3 and whether the resulting products are also biologically inactive. We have addressed these questions in the current study using purified rat CYP24A1 expressed in E. coli. The rat enzyme was chosen for these studies because it is easier to express and purify than the less stable human enzyme [33], plus we have used rodents to test the toxicity and other effects of 20(OH)D3 [22, 23] where potential metabolism by CYP24A1 is of importance. The rat and human enzymes share 90% amino acid sequence identity [34] and both metabolize 25(OH)D3 and 1,25(OH)2D3 predominantly by the C24 oxidation pathway, therefore data for 20(OH)D3 metabolism for the rat enzyme should provide a useful model for 20(OH)D3 metabolism by human CYP24A1.
The anti-melanoma activity of 1,25(OH)2D3 is well established (reviewed in [35–37], but its clinical use is limited by its calcemic activity. Since 20(OH)D3 is non-calcemic and non-toxic at high concentrations [22, 23], it is a good candidate for treatment of melanoma with comparable anti-melanoma activity to 1,25(OH)2D3, mediated via binding to the vitamin D receptor [20, 25, 28]. These effects of 20(OH)D3 include differential inhibition of melanoma growth compared to melanocytes [25]. Since inhibition of melanoma colony formation in soft agar by 20(OH)D3 is well characterized and represents a good in vitro measure of anti-tumorigenic activity, we have chosen this system for the preliminary biological testing of the products of CYP24A1 action on 20(OH)D3 and have shown that both of the major products, 20,24(OH)2D3 and 20,25(OH)2D3, display enhanced, rather than reduced, anti-melanoma activity.
2. Materials and Methods
2.1. Materials
20(OH)D3 was synthesized enzymatically from the action of CYP11A1 on vitamin D3 and was purified by TLC and reverse phase HPLC as before [15, 16]. 20,26-Dihydroxyvitamin D3 (20,26(OH)2D3) was produced by the action of human CYP27A1 on 20(OH)D3 [38]. 25(OH)D3 and NADPH were purchased from Merck (Darmstadt, Germany). Vitamin D3, dioleoyl phosphatidylcholine, bovine heart cardiolipin, cyclodextrin, glucose-6-phosphate were purchased from Sigma (St Louis, MO, U.S.A.). Glucose-6-phosphate dehydrogenase was from Roche (Mannheim, Germany). All solvents were of HPLC grade and were purchased from Merck (Darmstadt, Germany). The MTT reagent was from Promega (Madison, WI, U.S.A.). The charcoal-stripped serum was from HyClone (Logan, UT, U.S.A.).
2.2. Preparation of enzymes
Mouse adrenodoxin and human adrenodoxin reductase were expressed in Escherichia coli and purified as before [15, 39, 40]. The cDNA for rat CYP24A1 was synthesized by GenScript Corporation (Piscataway, NJ) according to the published sequence with the N-terminal mitochondrial target sequence removed [41] and the new N-terminus enriched with purines as described by Annalora et al. [3]. The cDNA was designed to encode a 6-histidine tag at the C-terminus of the CYP24A1. The construct was ligated to pTrc99A via NcoI (5′ end) and HindIII (3′ end) restriction sites. Competent E. coli (JM109) cells were transformed with the pTrc99A-CYP24A1 construct and the CYP24A1 expressed and purified using Ni-affinity chromatography as described for CYP27B1 [42]. The expression level measured after the Ni-affinity chromatography was 230 nmol/L culture.
2.3. Measurement of 20(OH)D3 metabolism by CYP24A1 in phospholipid vesicles
Dioleoyl phosphatidylcholine (1.08 μmol), bovine heart cardiolipin (0.19 μmol) and secosteroid substrate (as required) were placed in glass tubes and the ethanol solvent removed under nitrogen. Buffer (0.5 mL) comprising 20 mM HEPES pH 7.4, 100 mM NaCl, 0.1 mM DTT and 0.1 mM EDTA was added and the tubes sonicated for 10 min in a bath-type sonicator [32, 43]. The incubation mixture contained vesicles (510 μM phospholipid), CYP24A1 (0.15–0.5 μM), mouse adrenodoxin (15 μM), human adrenodoxin reductase (0.4 μM), glucose-6-phosphate (2 mM), glucose-6-phosphate dehydrogenase (2 U/mL) and NADPH (50 μM), in the same buffer used for vesicle preparation. Adrenodoxin and adrenodoxin reductase served to transport electrons from NADPH to the CYP24A1 [6]. Samples (typically 0.25–1.0 mL) were incubated at 37°C, with shaking (see Section 3.1. for incubation times), then reactions terminated by the addition of 2.5 mL ice-cold dichloromethane and vortexing. After phase separation aided by centrifugation, the lower organic phase was retained and the upper aqueous phase was extracted twice more with 2.5 mL aliquots of dichloromethane. The dichloromethane was removed under nitrogen and the residual sample dissolved in solvent for HPLC analysis. Reverse phase HPLC on a C18 column was carried out as described before [32] to measure product formation by CYP24A1. Kinetic parameters were determined by fitting the Michaelis-Menten equation to the experimental data using Kaleidagraph 3.6 (Synergy Software) [17].
2.4. Large-scale preparation of 20,24(OH)2D3 and 20,25(OH)2D3
Rat CYP24A1 (1.0 μM) was incubated with 50 μM 20(OH)D3 (added from a 0.75 mM stock in 4.5% cyclodextrin) in 40 mL buffer comprising 20 mM HEPES pH 7.4, 100 mM NaCl, 0.1 mM DTT, 0.1 mM EDTA, 15 μM mouse adrenodoxin, 0.4 μM human adrenodoxin reductase, 2 mM glucose-6-phosphate, 2 U/mL glucose-6-phosphate dehydrogenase and 50 μM NADPH, for 2 h at 37°C. The reaction was stopped with 80 mL ice-cold dichloromethane and products were extracted in a scaled up version of that described above (Section 2.3.) incubations with phospholipid vesicles.
The 20,24(OH)2D3 and 20,25(OH)2D3 products were purified by reverse phase HPLC using a Grace Smart column (15 cm × 4.6 mm, particle size 7 μm) with a gradient of 50 to 65% acetonitrile in water for 45 min at a flow rate of 0.5 mL/min. This gave baseline separation of 20,24(OH)2D3 and 20,25(OH)2D3. These products were further purified on the same column using isocratic conditions, 71% methanol in water for 20,25(OH)2D3 and 73% methanol in water for 20,24(OH)2D3, at a flow rate of 0.5 mL/min for 1 h. The yield of product, determined spectrophotometrically at 263 nm using an extinction coefficient of 18,000 M−1cm−1[44], was 80 nmol 20,25(OH) 2D3and 280 nmol 20,24(OH) 2D3, enough for structure determination by mass spectrometry and NMR, and biological testing.
2.5. Mass Spectrometry
Mass spectra of the dihydroxy metabolites were measured using a Bruker Esquire IonTrap-LC/MS system (Bruker Daltonics, Billerica, MA, U.S.A.). The measurements were performed on an electrospray ionization (ESI) source with nitrogen as the nebulizing gas. Data were transferred to an offline PC and processed with ACD software (Advanced Chemistry Development, Toronto, Canada).
2.6. NMR
NMR measurements were performed using an inverse triple-resonance 3 mm probe on a Varian Unity Inova 500 MHz spectrometer (Agilent Technologies, Inc., Santa Clara, CA, U.S.A.). Sample was dissolved in CD3OD and transferred to a 3-mm Shigemi NMR tube (Shigemi Inc., Allison Park, PA). Temperature was regulated at 22°C and was controlled with an accuracy of ± 0.1°C. Chemical shifts were referenced to residual solvent peaks for CD3OD (3.31 ppm for proton and 49.15 ppm for carbon). Standard two-dimensional NMR experiments [1H-1H correlation spectroscopy (COSY), 1H-1H total correlation spectroscopy (TOCSY, mixing time=80 ms), 1H-13C heteronuclear single quantum correlation spectroscopy (HSQC), and 1H-13C heteronuclear multiple bond correlation spectroscopy (HMBC)] were acquired in order to fully elucidate the structures of the metabolites. All data were processed using ACD software (Advanced Chemistry Development, Toronto, ON, Canada), with zero-filling in the direct dimension and linear prediction in the indirect dimension.
2.7. Colony formation assay in soft agar
The SKMEL-188 melanoma cells were cultured as described previously [45]. To measure their tumorigenicity the cells were trypsinized and re-suspended (~1000 cells/well) in 250 μL medium containing 0.4% agarose and 5% charcoal-stripped serum. Cell suspensions were added to a 0.8% agar layer in 4 × 24 well plates. Compounds were added from ethanol stocks (100 μM) to final concentrations of 0.1 nM or 10 nM, in 100 μL media. Each condition was tested in quadruplicate. An ethanol solvent control (amount of ethanol equivalent to test) as well as a media-only control was included in the assay. Cells were allowed to grow at 37°C with 5% CO2 over two weeks with secosteroids in fresh media (100 μL) being added after every 72 h. Soft agar colonies were scored and stained with 0.5 mg/mL MTT reagent, 500 μL/well after two weeks. Colonies were then counted under the microscope. Data are presented as means ± SEM and have been analyzed with Student’s t-test (for unpaired two groups) or one-way analysis of variance, using Prism 4.00 (GraphPad Software, San Diego, CA).
3. Results
3.1. Metabolism of 20(OH)D3 in phospholipid vesicles by rat CYP24A1
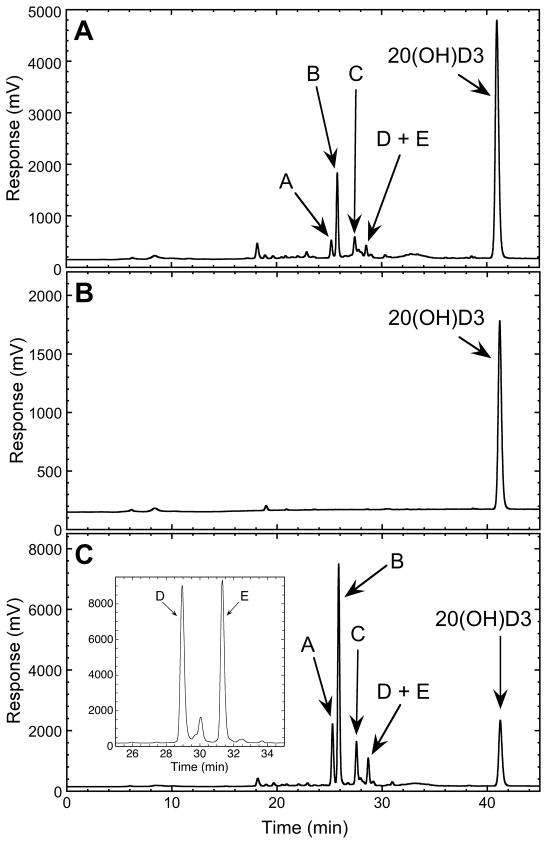
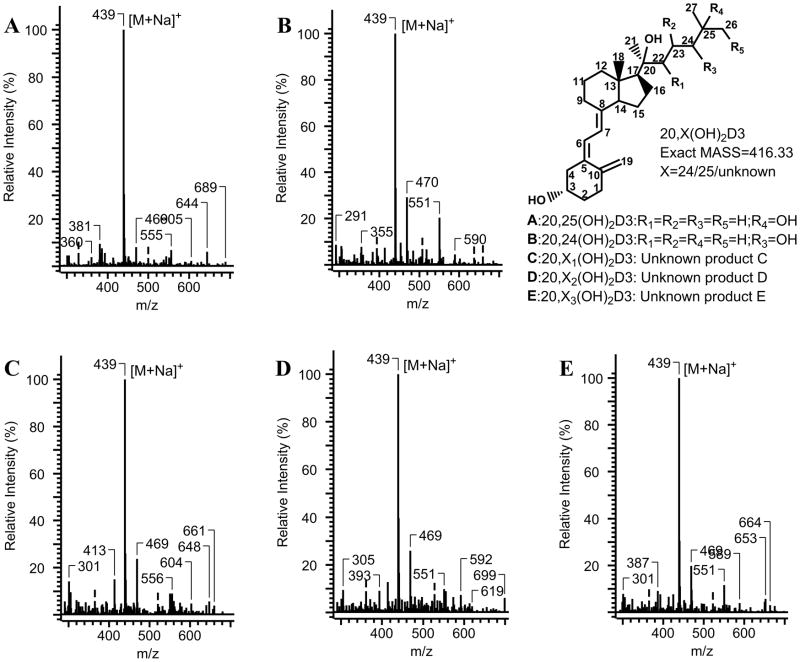
CYP24A1 activity was measured with 20(OH)D3 incorporated into phospholipid vesicles as this system mimics the native environment of the cytochrome in the inner mitochondrial membrane [1, 46] and has been observed to give high catalytic activity for other mitochondrial cytochromes P450 [17, 32, 38, 42, 43, 47, 48]. Incubation of 20(OH)D3 in phospholipid vesicles, with rat CYP24A1, resulted in two major products and several minor ones (Fig. 2). The same products were seen with 20(OH)D3 solubilized in cyclodextrin (Fig. 2), a system often used because of its ability to solubilize a high concentration of secosteroid [15–17, 26, 49]. The amount of total substrate consumed was higher in the cyclodextrin system. A separate HPLC step employing a gradient of methanol in water was required to separate the combined peak D/E obtained with the acetonitrile gradient, into two products of equal proportion plus another very minor product (Fig. 2C, insert). Mass spectra with electrospray ionization of all products (A – E) all showed the same major ion with m/z = 439 (corresponding to M + Na), revealing a molecular weight of 416 and hence that all of these products are dihydroxyvitamin D compounds (Fig. 3). The two major products were identified by NMR as 20,25(OH)2D3 for A and 20,24(OH)2D3 for B, with the full analysis described in Section 3.3. Using authentic standards produced by the actions of CYP11A1 and CYP27A1 on vitamin D3 [15, 38], we were able to exclude that products C, D or E were 20,22(OH)2D3, 20,23(OH)2D3 or 20,26(OH)2D3, or at least the isomeric forms produced by these enzymes.
Figure 2.
Metabolism of 20(OH)D3 by rat CYP24A1. (A) 20(OH)D3 was incorporated into phospholipid vesicles at a ratio of 0.05 mol/mol phospholipid and was incubated with 1.0 μM CYP24A1 for 30 min at 37°C in a reconstituted system containing adrenodoxin (15 μM) and adrenodoxin reductase (0.4 μM). Samples were extracted using dichloromethane and analyzed by reverse phase HPLC. (B) Control incubation for vesicles without adrenodoxin showing the substrate but the absence of product. (C) CYP24A1 (1.0 μM) was incubated for 30 min with 50 μM 20(OH)D3 dissolved in cyclodextrin (0.45%) in a reconstituted system as for vesicles.
Figure 3.
Mass spectra with electrospray ionization of products A-E of CYP24A1 action on 20(OH)D3.
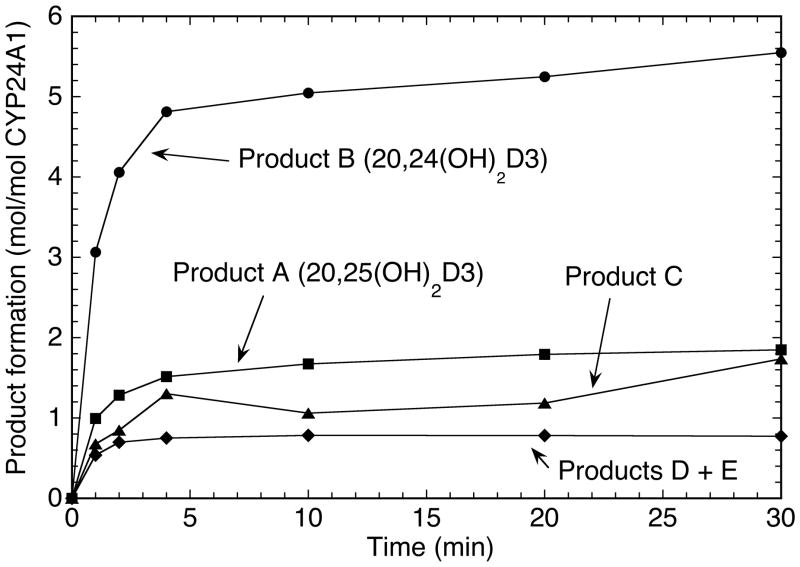
A time course for CYP24A1 activity on 20(OH)D3 in vesicles was carried out at 37°C (Fig. 4). The reaction was largely over by 4 min of incubation despite only 10% of the substrate being used. The proportion of the different products remained reasonably constant throughout the incubation with no lags evident, consistent with the identification of all the products as dihydroxyvitamin D compounds and not secondary metabolites resulting from multiple oxidations.
Figure 4.
Time course for metabolism of 20(OH)D3 by CYP24A1 in phospholipid vesicles. CYP24A1 was incubated with vesicles containing 20(OH)D3 for various times as in Fig. 1.
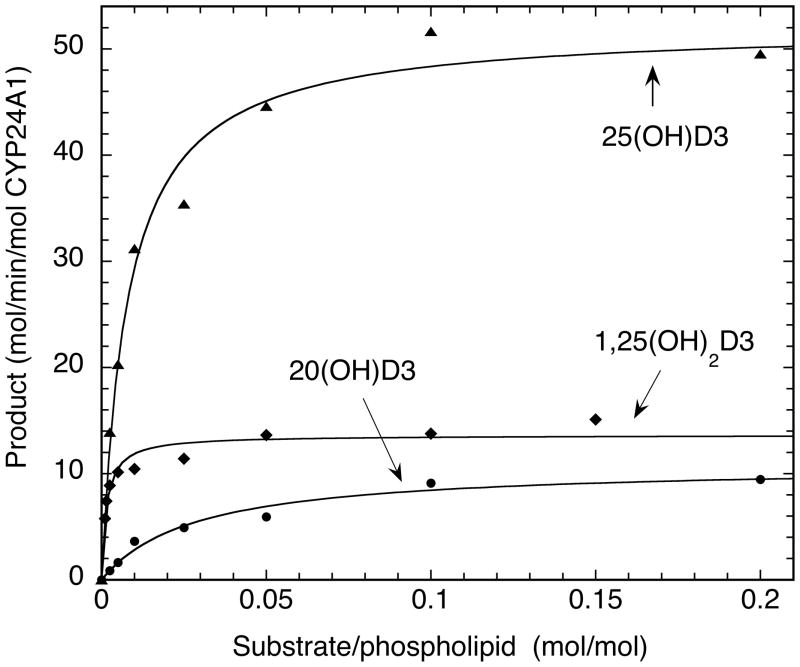
Kinetic experiments were performed with substrates incorporated into phospholipid vesicles to compare the ability of rat CYP24A1 to metabolize 20(OH)D3, 25(OH)D3 and 1,25(OH)2D3. This was also of interest because, despite the substrate access channel for this enzyme being in the hydrophobic domain of the membrane [46], the activity of the purified enzyme has not ever been characterized in a defined membrane reconstituted system. In phospholipid vesicles 20(OH)D3 was metabolized by CYP24A1 with a kcat of 10.8 ± 0.8 mol/min/mol CYP24A1 and a Km of 0.028 ± 0.007 mol substrate/mol phospholipid (Fig. 5). This compares to kcat and Km values for the initial rate of metabolism of 25(OH)D3 of 52.6 ± 2.9 mol/min/mol CYP24A1 and 0.0080 ± 0.0016 mol substrate/mol phospholipid, respectively (Fig. 5) and to 13.60 ± 0.54 mol/min/mol CYP24A1 and 0.0015 ± 0.0003 mol substrate/mol phospholipid for 1,25(OH)2D3, respectively. In the case of 25(OH)D3 the major product of the 1 min incubation was 24,25(OH)2D3 and for 1,25(OH)2D3 was 1,24,25(OH)3D3, as expected. Thus 20(OH)D3 is a relatively poor substrate for CYP24A1 being metabolized with a kcat/Km value of 386 min−1(mol substrate/mol phospholipid)−1 which is 17-fold lower than that for 25(OH)D3 and 24-fold lower than for 1,25(OH)2D3.
Figure 5.
Michaelis-Menten plots for the metabolism of CYP24A1 substrates in phospholipid vesicles. CYP24A1 was incubated with phospholipid vesicles containing 20(OH)D3, 25(OH)D3 or 1,25(OH)2D3 and incubated for 1 min with a reconstituted system containing adrenodoxin and adrenodoxin reductase. Products were extracted and analyzed by reverse phase HPLC. Hyperbolic curves were fitted by non-linear least squares analysis using Kaleidagraph 3.6. The R values for the curve fits were 0.999, 0.992 and 0.949 for 20(OH)D3, 25(OH)D3 and 1,25(OH)2D3, respectively.
3.2. Synthesis of products A and B for NMR analysis
Due to the ability of cyclodextrin to solubilize a high concentration of secosteroid and the greater conversion of substrate to product in this system (Fig. 2), we scaled up an incubation in this system to 40 mL. Following extraction and HPLC purification using two solvent systems (see Section 2.4.), 80 nmol product A and 280 nmol product B were obtained, enough for structure determination by NMR (see Section 3.3.). Insufficient products D-E were produced for NMR so these products were only analyzed by mass spectrometry (see Fig. 3).
3.3. Identification of the two major metabolites produced by CYP24A1 action on 20(OH)D3 as 20,24(OH)2D3 and 20,25(OH)2D3
Analysis of product A by NMR reveals that this metabolite is 20,25(OH)2D3. We have recently shown that 20,25(OH)2D3 is also generated from CYP27A1 action on 20(OH)D3 [38] and product A and CYP27A1-derived 20,25(OH)2D3 displayed identical HPLC retention times. Since the structure elucidation of 20,25(OH)2D3 by NMR has already been presented we will not describe it here for the CYP24A1-derived product.
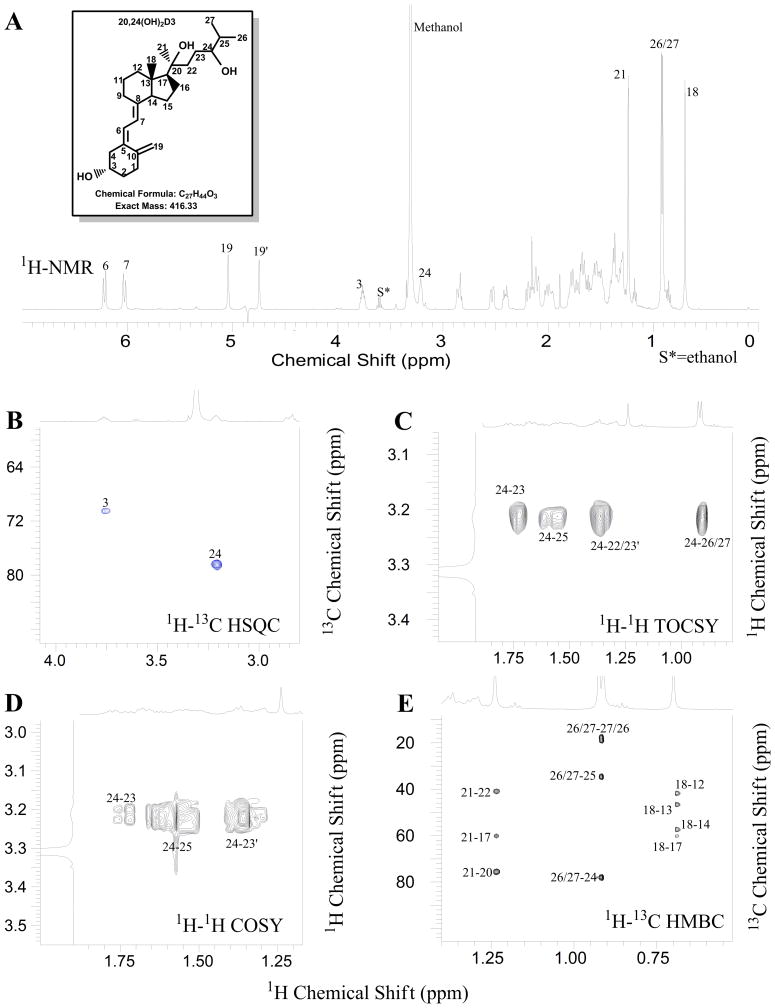
Analysis of product B by mass spectrometry (Fig. 3B) reveals that it is a dihydroxyvitamin D3 derivative. The observed molecular ion had a mass of 439 [M + Na]+ giving a molecular weight of 416. The site of hydroxylation on 20(OH)D3 was unambiguously assigned to be at the 24-position based on the NMR spectra for this metabolite. First, none of the four methyl carbons (C18, C21, C26, C27) are hydroxylated based on 1H NMR (Fig. 6A). 1H-13C HSQC revealed the presence of a new methine group at 3.32 ppm (13C at 78.2 ppm, Fig. 6B). 1H-1H TOCSY (Fig. 6C) clearly showed that this methine is in the same spin system as 26/27-CH3 (1H at 0.92 ppm), indicating the hydroxylation occurred in the side chain. From the 1H-1H COSY (Fig. 6D) spectrum, this methine (1H at 3.32 ppm) showed a strong correlation to 25-CH (1H at 1.62 ppm) and 23-CH2 (1H at 1.74 and 1.39 ppm). From 1H-13C HMBC (Fig. 6E), 26/27-CH3 (1H at 0.92 ppm) showed a strong correlation to the new methine (13C at 78.2 ppm), in addition to the expected correlation to 27/26-CH3 (13C at 18.5/19.3 ppm) and 25-CH (13C at 34.0 ppm). Taken together the above analysis shows that the hydroxylation site can be unambiguously assigned to the 24-position. The full assignments for this metabolite are summarized in Table 1 (for comparison, we also included the assignments for 20(OH)D3).
Figure 6.
NMR spectra of 20,24(OH)2D3. (A) 1D Proton; (B) 1H-13C HSQC; (C) 1H-1H TOCSY; (D) 1H-1H COSY; (E) 1H-13C HMBC.
Table 1.
NMR chemical shift assignments for 20,24(OH)2D3. (Solvent: CD3OD)
| Atom | 20,24(OH)2D3 | 20(OH)D3 | ||
|---|---|---|---|---|
| 1H | 13C | 1H | 13C | |
| 1 | 2.11α, 2.41β | 33.5 | 2.11α, 2.40β | 32.2 |
| 2 | 1.96α, 1.53β | 36.5 | 1.97α, 1.53β | 35.2 |
| 3 | 3.76 | 70.4 | 3.76 | 69.2 |
| 4 | 2.52α, 2.18β | 46.8 | 2.53α, 2.19β | 45.6 |
| 5 | NA | 136.4 | NA | 136.3 |
| 6 | 6.22 | 122.5 | 6.21 | 121.2 |
| 7 | 6.03 | 119.3 | 6.02 | 118.0 |
| 8 | NA | 141.1 | NA | 141.0 |
| 9 | 1.68α, 2.84β | 28.4 | 1.68α, 2.85β | 28.4 |
| 10 | NA | 146.2 | NA | 145.6 |
| 11 | 1.55α, 1.69β | 24.2 | 1.56α, 1.66β | 23.1 |
| 12 | 1.36α, 2.10β | 42.3 | 1.37α, 2.07β | 40.9 |
| 13 | NA | 45.4 | NA | 45.6 |
| 14 | 2.01 | 57.6 | 2.00 | 56.4 |
| 15 | 1.77 | 23.1 | 1.51 | 21.7 |
| 16 | 1.68α, 1.77β | 23.0 | 1.69α, 1.78β | 21.7 |
| 17 | 1.69 | 60.4 | 1.67 | 58.5 |
| 18 | 0.70 | 14.1 | 0.69 | 12.8 |
| 19 | 4.75, 5.04 | 112.4 | 4.74, 5.04 | 111.3 |
| 20 | NA | 74.7 | NA | 74.5 |
| 21 | 1.24 | 25.9 | 1.23 | 24.8 |
| 22 | 1.36 | 39.6 | 1.32, 1.46 | 43.9 |
| 23 | 1.74, 1.38 | 41.0 | 1.33 | 21.7 |
| 24 | 3.22 | 78.2 | 1.16 | 39.6 |
| 25 | 1.62 | 33.5 | 1.55 | 27.8 |
| 26 | 0.93 | 19.3 | 0.89 | 21.7 |
| 27 | 0.92 | 18.5 | 0.89 | 21.7 |
NA – Not applicable (ternary carbons).
3.4. Biological activity of CYP24A1-derived secosteroids on melanoma cells
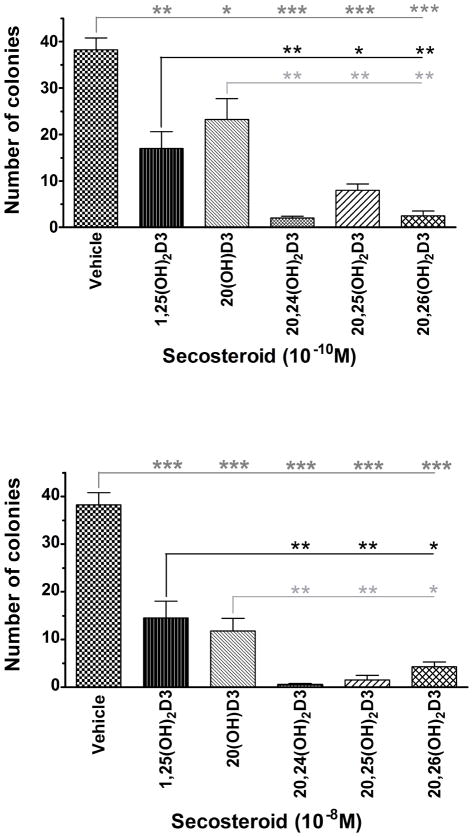
The abilities of 20,24(OH)2D3 and 20,25(OH)2D3 to inhibit melanoma cell proliferation were determined using a soft agar assay, which is regarded as a good measure of tumorigenicity (Fig. 7). We also included 20,26(OH)2D3 in these tests, which like 20,25(OH)2D3, is a product of CYP27A1 action on 20(OH)D3. In agreement with previous studies [25], 20(OH)D3 and 1,25(OH)2D3 significantly inhibited colony formation by melanoma cells. The three new compounds, 20,24(OH)2D3, 20,25(OH)2D3 and 20,26(OH)2D3, at a concentration of 0.1 nM also significantly inhibited colony formation compared to the vehicle control (p<0.0005). Furthermore, they inhibited colony formation significantly more than both 1,25(OH)2D3 and the parent compound, 20(OH)D3. These significant differences were also seen when the secosteroid concentration was increased to 10 nM (Fig. 7).
Figure 7.
The new secosteroids dramatically inhibit colony formation by SKMEL 188 melanoma cells. Colony formation was determined in soft agar. Data represent means ± S.D. (n= 4), and they were analyzed using GraphPad Prism and Student’s t test. Statistical significance: *, p < 0.05; **,p < 0.01; ***,p < 0.001.
4. Discussion
Analysis of the crystal structure of CYP24A1 [46] indicates that substrate enters the active site of CYP24A1 from an access channel open to the hydrophobic domain of the membrane bilayer. Entry of substrate into the active site from the membrane phase is also supported from studies on other mitochondrial P450s [17, 38, 42, 43, 47, 50]. Strong partitioning of the substrates for CYP24A1 such as 25(OH)D3 into the phospholipid bilayer has also been demonstrated [42]. To replicate this in vivo mechanism of substrate access to the active site of CYP24A1, we have analyzed the ability of the expressed rat enzyme to catalyze the hydroxylation of substrates incorporated into phospholipid vesicles. Using this reconstituted system we report the highest kcat values yet described for the metabolism of both 25(OH)D3 and 1,25(OH)2D3. Previously, CYP24A1 purified from isolated rat kidney mitochondria was reported to have a turnover number for 25(OH)D3 of 20 min−1 when assayed in aqueous buffer [1], approximately 40% of the value that we observed. Stimulation by 50% was observed by these authors when phosphatidylcholine was included in the incubation mixture.
It is interesting to compare the kinetic parameters for metabolism of 25(OH)D3 and 1,25(OH)2D3 by expressed rat CYP24A1, as there has been some debate in the past over which is the preferred substrate for the enzyme and over the methodology employed to determine this [51, 52]. For the purified rat enzyme using the phospholipid vesicle system, our data shows that CYP24A1 displays a slightly higher kcat/Km value with 1,25(OH)2D3 (9265 min−1(mol/mol phospholipid)−1) than with 25(OH)D3 (6722 min−1(mol/mol phospholipid)−1), at least for the initial 24-hydroxylation. Product analysis indicated that in the short incubation time used, the 24-hydroxyproduct dominated and thus we are essentially measuring only the first hydroxylation reaction. We also checked that product formation closely matched substrate depletion measured relative to an internal standard. The data shows that 1,25(OH)2D3 should be the preferred substrate for metabolism by CYP24A1 when substrate concentrations in the membrane are low (well below Km) where the velocity is determined by the term kcat/Km ([E][S]), where [E] is enzyme concentration and [S] is substrate concentration. This is consistent with the view of Jones and Tenenhouse [51] that 1,25(OH)2D3 is the preferred substrate for CYP24A1. The 3.8-fold higher kcat for 25(OH)D3 over that for 1,25(OH)2D3 which is in agreement with data reported by Taniguchi et al. (3.4-fold) [52], Annalora et al. (4.2-fold) [3] and Akiyoshi-Shibata et al. (2.4-fold) [2], indicates that rat CYP24A1 has a greater capacity to inactivate 25(OH)D3 when substrates are saturating. Other parameters in the living cell such as the presence of vitamin D binding protein, the concentrations of adrenodoxin and/or adrenodoxin reductase and the megalin transport system may also influence the relative rates of 1,25(OH)2D3 and 25(OH)D3 metabolism in vivo.
We compared the kinetics of 20(OH)D3 metabolism by rat CYP24A1 to those for 25(OH)D3 and 1,25(OH)2D3 metabolism in the phospholipid vesicle system and found that 20(OH)D3 showed a kcat only slightly lower than that for 1,25(OH)2D3 but a Km 19-fold higher. Its kcat was 5-fold lower than that for 25(OH)D3 and its Km was 3.5-fold higher. It is thus a considerably poorer substrate for CYP24A1 than 25(OH)D3 or 1,25(OH)2D3. However, its kcat/Km value of 386 min−1 (mol substrate/mol phospholipid)−1 is considerably higher than that for its metabolism to 20,23(OH)2D3 by CYP11A1 (24 min−1 (mol substrate/mol phospholipid)−1), an alternative pathway for 20(OH)D3 metabolism [17]. Like 25(OH)D3 and 1,25(OH)2D3, the favored site for initial hydroxylation of 20(OH)D3 by CYP24A1 is at C24. However, subsequent metabolism is clearly different as no subsequent oxidations were observed with 20(OH)D3, all products were dihydroxyvitamins D3 as shown by mass spectrometry. The presence of the 20-hydroxyl group prevents 23-hydroxylation as no 20,23(OH)2D3 or 20,23,24(OH)3D3 were seen among the products. Rather, the presence of the 20-hydroxyl group shifts the position of the side chain in the active site such that 20,25(OH)2D3 is the second major product of CYP24A1 action on 20(OH)D3. Other sites of hydroxylation in the minor products remain to be established.
Since the role of CYP24A1 is to inactivate 1,25(OH)2D3 [4], we tested the biological activity of the products of CYP24A1 action on 20(OH)D3. Inhibition of colony formation by SKMEL-188 melanoma cells in soft agar was used as the assay since the effect of the parent compound, 20(OH)D3, has been well documented in this system (see Section 1.) and the anchorage independent growth represents the best in vitro estimate of tumorigenicity. Both 20,24(OH)2D3 and 20,25(OH)2D3 caused significantly higher inhibition of colony formation in soft agar than the parent 20(OH)D3 and 1,25(OH)2D3 at concentrations of both 10−8 and 10−10 M. Since metabolism of 20(OH)D3 by CYP27A1 also produces 20,25(OH)2D3 as well as 20,26(OH)2D3 [38], we included the latter in our tests. It was also more potent than 20(OH)D3 and 1,25(OH)2D3in the inhibition of colony formation by melanoma cells.
In conclusion, the present study shows that CYP24A1 acts relatively slowly on 20(OH)D3 producing only dihydroxyvitamin D products, with the major ones being identified by NMR as 20,24(OH)2D3 and 20,25(OH)2D3. Both of these products inhibit anchorage-independent melanoma growth significantly more than the parent compound indicating that CYP24A1 plays an activating role on 20(OH)D3 rather than an inactivating role, which is in contrast to its well established inactivating activity on 1,25(OH)2D3. The anti-melanoma effects of 20,24(OH)2D3 and 20,25(OH)2D3, and the CYP27A1 product 20,26(OH)2D3, identify them as good candidates for further testing of their therapeutic utility in melanoma treatment.
Acknowledgments
This work was supported by NIH [Grant R01AR052190] to AS, by the University of Western Australia and by the College of Pharmacy at the University of Tennessee Health Science Center. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Abbreviations
- (20(OH)D3)
20S-hydroxyvitamin D3
- (25(OH)D3)
25-hydroxyvitamin D3
- (20,24(OH)2D3)
20S,24-dihydroxyvitamin D3
- (20,25(OH)2D3)
20S,25-dihydroxyvitamin D3
- (20,26(OH)2D3)
20S,26-dihydroxyvitamin D3
- (1,25(OH)2D3)
1α,25-dihydroxyvitamin D3
- ESI
electrospray ionization
- HSQC
heteronuclear single quantum correlation
- cyclodextrin
2-hydroxypropyl-β-cyclodextrin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ohyama Y, Okuda K. Isolation and characterization of a cytochrome P-450 from rat kidney mitochondria that catalyzes the 24-hydroxylation of 25-hydroxyvitamin D3. J Biol Chem. 1991;266:8690–5. [PubMed] [Google Scholar]
- 2.Akiyoshi-Shibata M, Sakaki T, Ohyama Y, Noshiro M, Okuda K, Yabusaki Y. Further oxidation of hydroxycalcidiol by calcidiol 24-hydroxylase. A study with the mature enzyme expressed in Escherichia coli. Eur J Biochem. 1994;224:335–43. doi: 10.1111/j.1432-1033.1994.00335.x. [DOI] [PubMed] [Google Scholar]
- 3.Annalora A, Bobrovnikova-Marjon E, Serda R, Lansing L, Chiu ML, Pastuszyn A, et al. Rat cytochrome P450C24 (CYP24A1) and the role of F249 in substrate binding and catalytic activity. Arch Biochem Biophys. 2004;425:133–46. doi: 10.1016/j.abb.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 4.Jones G, Prosser DE, Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch Biochem Biophys. 2012;523:9–18. doi: 10.1016/j.abb.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 5.Sakaki T, Sawada N, Nonaka Y, Ohyama Y, Inouye K. Metabolic studies using recombinant escherichia coli cells producing rat mitochondrial CYP24 CYP24 can convert 1alpha,25-dihydroxyvitamin D3 to calcitroic acid. Eur J Biochem. 1999;262:43–8. doi: 10.1046/j.1432-1327.1999.00375.x. [DOI] [PubMed] [Google Scholar]
- 6.Schuster I. Cytochromes P450 are essential players in the vitamin D signaling system. Biochim Biophys Acta. 2011;1814:186–99. doi: 10.1016/j.bbapap.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 7.Hamamoto H, Kusudo T, Urushino N, Masuno H, Yamamoto K, Yamada S, et al. Structure-Function Analysis of Vitamin D 24-Hydroxylase (CYP24A1) by Site-Directed Mutagenesis: Amino Acid Residues Responsible for Species-Based Difference of CYP24A1 between Humans and Rats. Molecular pharmacology. 2006;70:120–8. doi: 10.1124/mol.106.023275. [DOI] [PubMed] [Google Scholar]
- 8.Prosser DE, Kaufmann M, O’Leary B, Byford V, Jones G. Single A326G mutation converts human CYP24A1 from 25-OH-D3-24-hydroxylase into -23-hydroxylase, generating 1alpha,25-(OH)2D3-26,23-lactone. Proc Natl Acad Sci U S A. 2007;104:12673–8. doi: 10.1073/pnas.0702093104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakaki T, Sawada N, Komai K, Shiozawa S, Yamada S, Yamamoto K, et al. Dual metabolic pathway of 25-hydroxyvitamin D3 catalyzed by human CYP24. Eur J Biochem. 2000;267:6158–65. doi: 10.1046/j.1432-1327.2000.01680.x. [DOI] [PubMed] [Google Scholar]
- 10.Beckman MJ, Tadikonda P, Werner E, Prahl J, Yamada S, DeLuca HF. Human 25-hydroxyvitamin D3-24-hydroxylase, a multicatalytic enzyme. Biochemistry. 1996;35:8465–72. doi: 10.1021/bi960658i. [DOI] [PubMed] [Google Scholar]
- 11.Wagner D, Hanwell HE, Schnabl K, Yazdanpanah M, Kimball S, Fu L, et al. The ratio of serum 24,25-dihydroxyvitamin D3 to 25-hydroxyvitamin D3 is predictive of 25-hydroxyvitamin D3 response to vitamin D3 supplementation. J Steroid Biochem Mol Biol. 2011;126:72–7. doi: 10.1016/j.jsbmb.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 12.St-Arnaud R, Naja RP. Vitamin D metabolism, cartilage and bone fracture repair. Mol Cell Endocrinol. 2011;347:48–54. doi: 10.1016/j.mce.2011.05.018. [DOI] [PubMed] [Google Scholar]
- 13.Guryev O, Carvalho RA, Usanov S, Gilep A, Estabrook RW. A pathway for the metabolism of vitamin D3: unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1) Proc Natl Acad Sci U S A. 2003;100:14754–9. doi: 10.1073/pnas.2336107100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Slominski A, Semak I, Zjawiony J, Wortsman J, Li W, Szczesniewski A, et al. The cytochrome P450scc system opens an alternate pathway of vitamin D3 metabolism. FEBS J. 2005;272:4080–90. doi: 10.1111/j.1742-4658.2005.04819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuckey RC, Li W, Shehabi HZ, Janjetovic Z, Nguyen MN, Kim TK, et al. Production of 22-hydroxy-metabolites of vitamin D3 by cytochrome P450scc (CYP11A1) and analysis of their biological activities on skin cells. Drug Metab Dispos. 2011;39:1577–88. doi: 10.1124/dmd.111.040071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tuckey RC, Li W, Zjawiony JK, Zmijewski MA, Nguyen MN, Sweatman T, et al. Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. FEBS J. 2008;275:2585–96. doi: 10.1111/j.1742-4658.2008.06406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tuckey RC, Nguyen MN, Slominski A. Kinetics of vitamin D3 metabolism by cytochrome P450scc (CYP11A1) in phospholipid vesicles and cyclodextrin. Int J Biochem Cell Biol. 2008;40:2619–26. doi: 10.1016/j.biocel.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Slominski AT, Kim TK, Shehabi HZ, Semak I, Tang EK, Nguyen MN, et al. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012;26:3901–15. doi: 10.1096/fj.12-208975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Slominski AT, Kim TK, Chen J, Nguyen MN, Li W, Yates CR, et al. Cytochrome P450scc-dependent metabolism of 7-dehydrocholesterol in placenta and epidermal keratinocytes. Int J Biochem Cell Biol. 2012;44:2003–18. doi: 10.1016/j.biocel.2012.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janjetovic Z, Brozyna AA, Tuckey RC, Kim TK, Nguyen MN, Jozwicki W, et al. High basal NF-kappaB activity in nonpigmented melanoma cells is associated with an enhanced sensitivity to vitamin D3 derivatives. Br J Cancer. 2011;105:1874–84. doi: 10.1038/bjc.2011.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li W, Chen J, Janjetovic Z, Kim TK, Sweatman T, Lu Y, et al. Chemical synthesis of 20S-hydroxyvitamin D3, which shows antiproliferative activity. Steroids. 2010;75:926–35. doi: 10.1016/j.steroids.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slominski AT, Janjetovic Z, Fuller BE, Zmijewski MA, Tuckey RC, Nguyen MN, et al. Products of vitamin D3 or 7-dehydrocholesterol metabolism by cytochrome P450scc show anti-leukemia effects, having low or absent calcemic activity. Plos One. 2010;5:e9907. doi: 10.1371/journal.pone.0009907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Slominski A, Tuckey RC, Janjetovic Z, Kulkarni A, Chen J, et al. 20-Hydroxyvitamin D3 inhibits proliferation of cancer cells with high efficacy while being non-toxic. Anticancer Res. 2012;32:739–46. [PMC free article] [PubMed] [Google Scholar]
- 24.Zbytek B, Janjetovic Z, Tuckey RC, Zmijewski MA, Sweatman TW, Jones E, et al. 20-Hydroxyvitamin D3, a product of vitamin D3 hydroxylation by cytochrome P450scc, stimulates keratinocyte differentiation. J Invest Dermatol. 2008;128:2271–80. doi: 10.1038/jid.2008.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slominski AT, Janjetovic Z, Kim TK, Wright AC, Grese LN, Riney SJ, et al. Novel Vitamin D Hydroxyderivatives Inhibit Melanoma Growth and Show Differential Effects on Normal Melanocytes. Anticancer Res. 2012;32:3733–42. [PMC free article] [PubMed] [Google Scholar]
- 26.Nguyen MN, Slominski A, Li W, Ng YR, Tuckey RC. Metabolism of vitamin D2 to 17,20,24-trihydroxyvitamin D2 by cytochrome P450scc (CYP11A1) Drug Metab Dispos. 2009;37:761–7. doi: 10.1124/dmd.108.025619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Slominski A, Semak I, Wortsman J, Zjawiony J, Li W, Zbytek B, et al. An alternative pathway of vitamin D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2. FEBS J. 2006;273:2891–901. doi: 10.1111/j.1742-4658.2006.05302.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slominski AT, Kim TK, Janjetovic Z, Tuckey RC, Bieniek R, Yue J, et al. 20-Hydroxyvitamin D2 is a noncalcemic analog of vitamin D with potent antiproliferative and prodifferentiation activities in normal and malignant cells. Am J Physiol Cell Physiol. 2011;300:C526–41. doi: 10.1152/ajpcell.00203.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Janjetovic Z, Tuckey RC, Nguyen MN, Thorpe EM, Jr, Slominski AT. 20,23-Dihydroxyvitamin D3, novel P450scc product, stimulates differentiation and inhibits proliferation and NF-kappaB activity in human keratinocytes. J Cell Physiol. 2010;223:36–48. doi: 10.1002/jcp.21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janjetovic Z, Zmijewski MA, Tuckey RC, DeLeon DA, Nguyen MN, Pfeffer LM, et al. 20-Hydroxycholecalciferol, Product of Vitamin D3 Hydroxylation by P450scc, Decreases NF-kappa B Activity by Increasing I kappa B alpha Levels in Human Keratinocytes. PloS one. 2009;4:e5988. doi: 10.1371/journal.pone.0005988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim TK, Wang J, Janjetovic Z, Chen J, Tuckey RC, Nguyen MN, et al. Correlation between secosteroid-induced vitamin D receptor activity in melanoma cells and computer-modeled receptor binding strength. Mol Cell Endocrinol. 2012;361:143–52. doi: 10.1016/j.mce.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang EK, Li W, Janjetovic Z, Nguyen MN, Wang Z, Slominski A, et al. Purified mouse CYP27B1 can hydroxylate 20,23-dihydroxyvitamin D3, producing 1alpha,20,23-trihydroxyvitamin D3, which has altered biological activity. Drug Metab Dispos. 2010;38:1553–9. doi: 10.1124/dmd.110.034389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhu J, Barycki R, Chiellini G, DeLuca HF. Screening of Selective Inhibitors of 1α,25-Dihydroxyvitamin D3 24-Hydroxylase Using Recombinant Human Enzyme Expressed in Escherichia coli. Biochemistry. 2010;49:10403–11. doi: 10.1021/bi101488p. [DOI] [PubMed] [Google Scholar]
- 34.Chen KS, Prahl JM, DeLuca HF. Isolation and expression of human 1,25-dihydroxyvitamin D3 24-hydroxylase cDNA. Proc Natl Acad Sci U S A. 1993;90:4543–7. doi: 10.1073/pnas.90.10.4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinczewski J, Slominski A. The potential role of vitamin D in the progression of benign and malignant melanocytic neoplasms. Exp Dermatol. 2010;19:860–4. doi: 10.1111/j.1600-0625.2010.01169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szyszka P, Zmijewski MA, Slominski AT. New vitamin D analogs as potential therapeutics in melanoma. Expert Rev Anticancer Ther. 2012;12:585–99. doi: 10.1586/era.12.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Denzer N, Vogt T, Reichrath J. Vitamin D receptor (VDR) polymorphisms and skin cancer: A systematic review. Dermatoendocrinol. 2011;3:205–10. doi: 10.4161/derm.3.3.16519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tieu EW, Li W, Chen J, Baldisseri DM, Slominski AT, Tuckey RC. Metabolism of cholesterol, vitamin D3 and 20-hydroxyvitamin D3 incorporated into phospholipid vesicles by human CYP27A1. J Steroid Biochem Mol Biol. 2012;129:163–71. doi: 10.1016/j.jsbmb.2011.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tuckey RC, Sadleir J. The concentration of adrenodoxin reductase limits cytochrome P450scc activity in the human placenta. Eur J Biochem. 1999;263:319–25. doi: 10.1046/j.1432-1327.1999.00483.x. [DOI] [PubMed] [Google Scholar]
- 40.Woods ST, Sadleir J, Downs T, Triantopoulos T, Headlam MJ, Tuckey RC. Expression of catalytically active human cytochrome P450scc in Escherichia coli and mutagenesis of isoleucine-462. Arch Biochem Biophys. 1998;353:109–15. doi: 10.1006/abbi.1998.0621. [DOI] [PubMed] [Google Scholar]
- 41.Ohyama Y, Noshiro M, Okuda K. Cloning and expression of cDNA encoding 25-hydroxyvitamin D3 24-hydroxylase. FEBS Lett. 1991;278:195–8. doi: 10.1016/0014-5793(91)80115-j. [DOI] [PubMed] [Google Scholar]
- 42.Tang EKY, Voo KJQ, Nguyen MN, Tuckey RC. Metabolism of substrates incorporated into phospholipid vesicles by mouse 25-hydroxyvitamin D3 1[alpha]-hydroxylase (CYP27B1) J Steroid Biochem Mol Biol. 2010;119:171–9. doi: 10.1016/j.jsbmb.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 43.Lambeth JD, Kitchen SE, Farooqui AA, Tuckey R, Kamin H. Cytochrome P-450scc-substrate interactions. Studies of binding and catalytic activity using hydrocholesterols. J Biol Chem. 1982;257:1876–84. [PubMed] [Google Scholar]
- 44.Hiwatashi A, Nishii Y, Ichikawa Y. Purification of cytochrome P-450D1 alpha (25-hydroxyvitamin D3-1 alpha-hydroxylase) of bovine kidney mitochondria. Biochem Biophys Res Commun. 1982;105:320–7. doi: 10.1016/s0006-291x(82)80047-8. [DOI] [PubMed] [Google Scholar]
- 45.Slominski A, Zbytek B, Slominski R. Inhibitors of melanogenesis increase toxicity of cyclophosphamide and lymphocytes against melanoma cells. Int J Cancer. 2009;124:1470–7. doi: 10.1002/ijc.24005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Annalora AJ, Goodin DB, Hong WX, Zhang Q, Johnson EF, Stout CD. Crystal structure of CYP24A1, a mitochondrial cytochrome P450 involved in vitamin D metabolism. J Mol Biol. 2010;396:441–51. doi: 10.1016/j.jmb.2009.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Headlam MJ, Wilce MC, Tuckey RC. The F-G loop region of cytochrome P450scc (CYP11A1) interacts with the phospholipid membrane. Biochim Biophys Acta. 2003;1617:96–108. doi: 10.1016/j.bbamem.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 48.Tuckey RC, Stevenson PM. Purification and analysis of phospholipids in the inner mitochondrial membrane fraction of bovine corpus luteum, and properties of cytochrome P-450scc incorporated into vesicles prepared from these phospholipids. Eur J Biochem. 1985;148:379–84. doi: 10.1111/j.1432-1033.1985.tb08849.x. [DOI] [PubMed] [Google Scholar]
- 49.Tuckey RC, Janjetovic Z, Li W, Nguyen MN, Zmijewski MA, Zjawiony J, et al. Metabolism of 1alpha-hydroxyvitamin D3 by cytochrome P450scc to biologically active 1alpha,20-dihydroxyvitamin D3. J Steroid Biochem Mol Biol. 2008;112:213–9. doi: 10.1016/j.jsbmb.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tang EK, Tieu EW, Tuckey RC. Expression of human CYP27B1 in Escherichia coli and characterization in phospholipid vesicles. FEBS J. 2012;279:3749–61. doi: 10.1111/j.1742-4658.2012.08736.x. [DOI] [PubMed] [Google Scholar]
- 51.Jones G, Tenenhouse HS. 1,25(OH)2D, the preferred substrate for CYP24. J Bone Miner Res. 2002;17:179–81. doi: 10.1359/jbmr.2002.17.1.179. [DOI] [PubMed] [Google Scholar]
- 52.Taniguchi T, Eto TA, Shiotsuki H, Sueta H, Higashi S, Iwamura T, et al. Newly established assay method for 25-hydroxyvitamin D3 24-hydroxylase revealed much lower Km for 25-hydroxyvitamin D3 than for 1alpha,25-dihydroxyvitamin D3. J Bone Miner Res. 2001;16:57–62. doi: 10.1359/jbmr.2001.16.1.57. [DOI] [PubMed] [Google Scholar]