Abstract
Communication between the serotonin system and the CRF system plays a pivotal role in the mediation of stress and stress reactivity. CRF appears to be inhibitory of serotonin neurotransmission through the CRF receptor type 1 (CRF-R1). Serotonin neurons also detect the urocortins, which are thought to be anxiolytic. Components of the CRF system in the serotonergic dorsal raphe region were examined in macaques that were ovary-intact or ovariectomized for 3 years living in a relatively natural environment. Female Japanese macaques (Macaca fuscata) were ovariectomized or tubal-ligated (n=5/group) and returned to their natal troop for 3 years. Quantitation of (1) CRF innervation of the serotonergic dorsal raphe, (2) CRF-Receptor type 1 (CRF-R1) in the dorsal raphe, (3) Urocortin 1 (UCN1) cells near the Edinger-Westfal nucleus and (4) UCN1 axons, was obtained with immunocytochemical staining and image analysis. There was no statistical difference in CRF axonal staining in the dorsal raphe, or in UCN1 axonal staining near the dorsal raphe. However, the average number of detectable UCN1 postive cells was significantly lower in the Ovx group than in the Intact group (p = 0.003). Average CRF-R1 positive pixel number and positive cell number were significantly higher in the Ovx group than in the Intact group (p=0.005 and 0.02, respectivly). The higher expression of CRF-R1 and lower expression of UCN1 in the Ovx group indicates they may be more vulnerable to stress. The greater expression of CRF-R1 could cause a greater inhibition of serotonin upon a stress-induced increase in CRF as well.
1. Introduction
Women experience ovarian failure and loss of ovarian steroid production around 50 years of age. Thus, with extended life spans, a woman may live 35–40 years without ovarian steroid secretion. After menopause, a significant number of women become more anxious, and less able to cope with stress, leading to new onset of depression (Conde et al., 2006; Heikkinen et al., 2006; Maki et al.; Tangen and Mykletun, 2008). The role of ovarian hormones in depression and stress sensitivity is of great interest for women transitioning through menopause. In addition, a significant number of young women lose ovarian function due to chemotherapy and require hormone therapy (Blumenfeld, 2012; Goswami and Conway, 2005).
The serotonin system plays a pivotal role in stress and affective disorders, including depression and anxiety. The administration of selective serotonin reuptake inhibitors (SSRIs) is to date, the most effective pharmacological intervention for depression and anxiety disorders (Cipriani et al., 2012; Gartlehner et al., 2011). The hypothalamic-pituitary adrenal axis has also been implicated in the etiology of depression (Morimoto et al., 1993; Nemeroff, 2004; Weiss et al., 1994), and an elevation in corticotropin releasing factor (CRF) in the hypothalamic paraventricular nucleus (PVN) is thought to underlie the hyperactivity of the HPA axis in depression (de Kloet et al., 2005; Holsboer, 1999; Keck and Holsboer, 2001). However, the CRF ‘system’ has multiple compnents including the CRF receptors 1 and 2, and the related peptide urocortins 1, 2 and 3 (UCN). (Clark and Kaiyala, 2003). The urocortins appear to have higher affinity for CRF-R2 than CRF; and they have been implicated in reducing the response to acute stress (Reul and Holsboer, 2002). On the other hand, CRF activation of CRF-R1 increases anxiety in mice and humans (Bailey et al., 2011; Smith et al., 1998).
This laboratory has shown that the ovarian steroids, estrogen (E) and progesterone (P) increase serotonin neural function (Bethea et al., 2002), protect serotonin neuronal health (Bethea et al., 2009) and increase gene and protein expression underlying dendritic spine proliferation on serotonin neurons (Bethea and Reddy, 2010; Bethea and Reddy, 2011; Rivera and Bethea, 2012). All of these actions could translate to improved mood and stress resilience in women. In addition, we found that administration of E or E+P to ovariectomized macaques decreased CRF gene and protein expression in the PVN of macaques (Bethea and Centeno, 2008), decreased CRF innervation of the dorsal and median raphe, decreased CRF-R1 receptors and increased CRF-R2 receptors in the dorsal raphe, increased detectable midbrain urocortin (UCN1) cell number and increased UCN1 fiber densitiy (Sanchez et al., 2010). Together these data suggest that ovarian hormone administration may improve stress resilience through the serotonin, CRF and UCN systems.
However, all of the former studies utilized a macaque model of hormone replacement therapy in which healthy adult monkeys were acquired that had been ovariectomized (Ovx) in other programs. The animals were rested for up to 8 months and then treated for 1 month with placebo, E or E+P via silastic capsules. This model was extremely cost effective and it demonstrated robust differences in serotonin-related, CRF and UCN1 gene and protein expression between treatment groups. However, it has certain limitations in comparison to natural menopause, although it resembles young women with chemotherapy-induced infertility. Of significance, the animals were Ovx for a relatively short period of time. In addition, the hormone therapy was continuous for one month rather than cycling for many months in the manner of intact females.
Therefore, we questioned the regulation of serotonin and CRF/UCN systems in intact monkeys compared to ovariectomized individuals, and whether the length of ovariectomy was an issue. To this end, we ovariectomized or ligated the fallopian tubes of young female Japanese macaques and maintained them for three years in an outdoor troop at the Oregon National Primate Research Center (ONPRC). These animals were not intended to fully model menopause, but rather to examine the effects of long-term ovariectomy, in a semi-natural, social environment. Other variables under current examination include diet and age.
We recently reported that expression of pivotal serotoin-related genes was reduced by long-term ovariectomy, which may partially reflect a decrease in serotonin cell number (Bethea et al., 2012). There were subtle differences between the regulation of serotonin-related genes by hormone replacement in the short-term Ovx monkeys compared to the differences in expression observed in the Intact versus long-term Ovx monkeys, again possibly related to the loss of serotonin neurons in the latter. We also reported that the same Ovx monkeys exhibited a decrease in positive social behavior in the troop and exhibited an increase in certain anxiety-related behaviors in temperament tests (Coleman et al., 2011).
In this study we extend our examination to the midbrain CRF and UCN1 systems in the long-term Ovx monkeys in comparison to their Intact troop mates.
2. Results
It was not possible to monitor the menstrual cycle in the individual tubal-ligated animals while they were in the outdoor corral or group housing; and they were in single cages for too brief a time to determine menstrual cycle stage from the presence of blood in the waste pans. Therefore, we measured estradiol (E), progesterone (P) and cortisol concentrations in serum obtained prior to euthanasia. Although previously published, this data is re-presented for convenience in Table 1. We found that serum E and P were very low in the ovariectomized animals. Low levels of E are produced by fat tissue and these concentrations are consistent with previous analyses of serum from ovariectomized monkeys (Bethea et al., 2009). Different concentrations of serum E and P were present among the Intact females, indicating that the Intact animals were at various stages of the menstrual cycle, as designated on Table 1. The cortisol concentrations were obtained prior to fenfluramine challenge, which has been published. There was no difference in basal cortisol between the groups. In addition, the cortisol response to fenfluramine did not differ between the groups (Bethea et al., 2012). We could not euthanize the animals at a particular stage of the cycle since this information was not available a priori. We hypothesize that the neurobiology results reflect the presence of cycling ovarian hormones in the Intact group; and reflect the near absence of these steroids in the Ovx group for a 3-year period. However, we cannot completely rule out that the possibility that the stage of the menstrual cycle at euthanasia increased the variance in the tubal-ligated (ovary) Intact group.
Table 1.
Steroid hormone concentrations in serum of female Japanese macaques prior to euthanasia and under propofol anesthesia.
| Treatment Group | Cortisol | Estradiol-17β | Progesterone | Stage |
|---|---|---|---|---|
| Animal ID | ng/ml | pg/ml | ng/ml | |
| Ovx | ||||
| 24018 | 319 | 14 | 0.30 | n/a |
| 24020 | 317 | 28 | 0.85 | n/a |
| 24028 | 194 | 20 | 0.69 | n/a |
| 24029 | 188 | 10 | 0.60 | n/a |
| Avg | 254±37 | 18±4 | 0.61±0.12 | |
| Intact | ||||
| 24032 | 250 | 19 | 0.23 | menstrual |
| 24037 | 175 | 64 | 0.12 | early-follicular |
| 24040 | 202 | 431 | 1.17 | early luteal |
| 24042 | 275 | 18 | 1.20 | late luteal |
| 24049 | 217 | 107 | 0.86 | mid-follicular |
| Avg | 223±18 | 127±77 | 0.72±0.23 | |
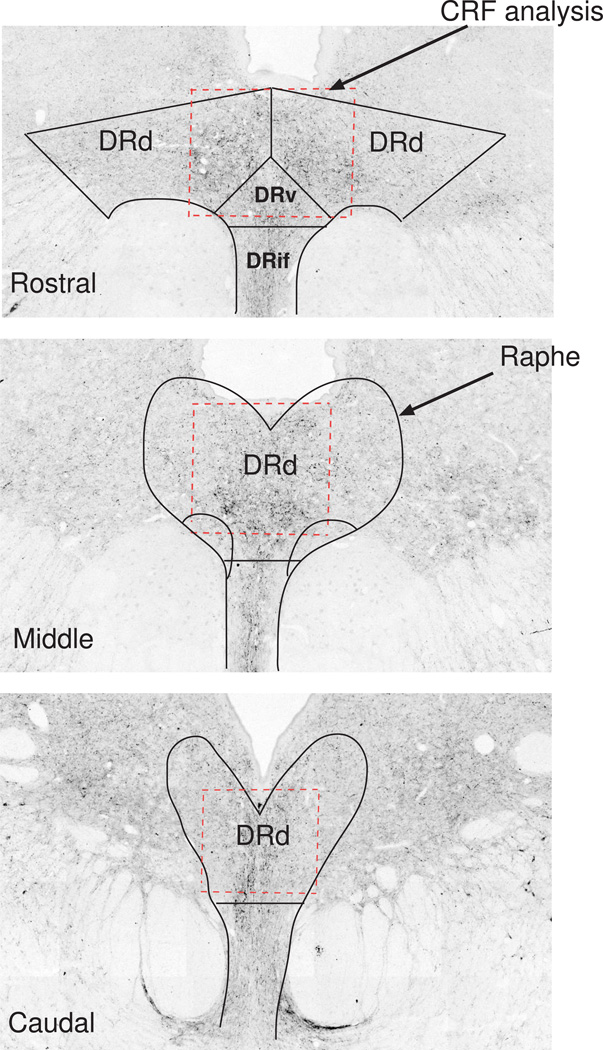
The location of the area analyzed for CRF fiber staining relative to the raphe subnuclei is illustrated in Figure 1 (dashed red box). Since the CRF fibers do not correspond to a particular subnucleus, the subnuclei were located based upon TPH2 gene expression as previously published (Sanchez et al., 2005). CRF staining in representative sections from the rostral, medial and caudal areas are shown. Routinely, representative sets of sections at 250 µm intervals contain 2 sections in the rostral area, 2 sections in the middle area and 3 sections in the caudal area. In our sections, the subnuclei at the rostral levels appear slightly different from reports with human brain (Bonkale et al., 2006). The Dorsal Raphe Ventrolateral (DRvl) subnucleus emerges in the middle levels rather than the rostral levels. This could be a species difference or it could be due to our plane of sectioning.
Figure 1.
Illustration of the CRF fiber area that was analyzed (red dashed box) relative to the subnuclei of the dorsal raphe nucleus at a rostral, middle and caudal level (solid black lines). The rostral levels contain the subnuclei called dorsal raphe dorsal (DRd), dorsal raphe ventral (DRv), and dorsal raphe infundibularis (DRif). The area identified as dorsal raphe ventral lateral (DRvl) makes an appearance in the middle region of our raphe sections in monkeys.
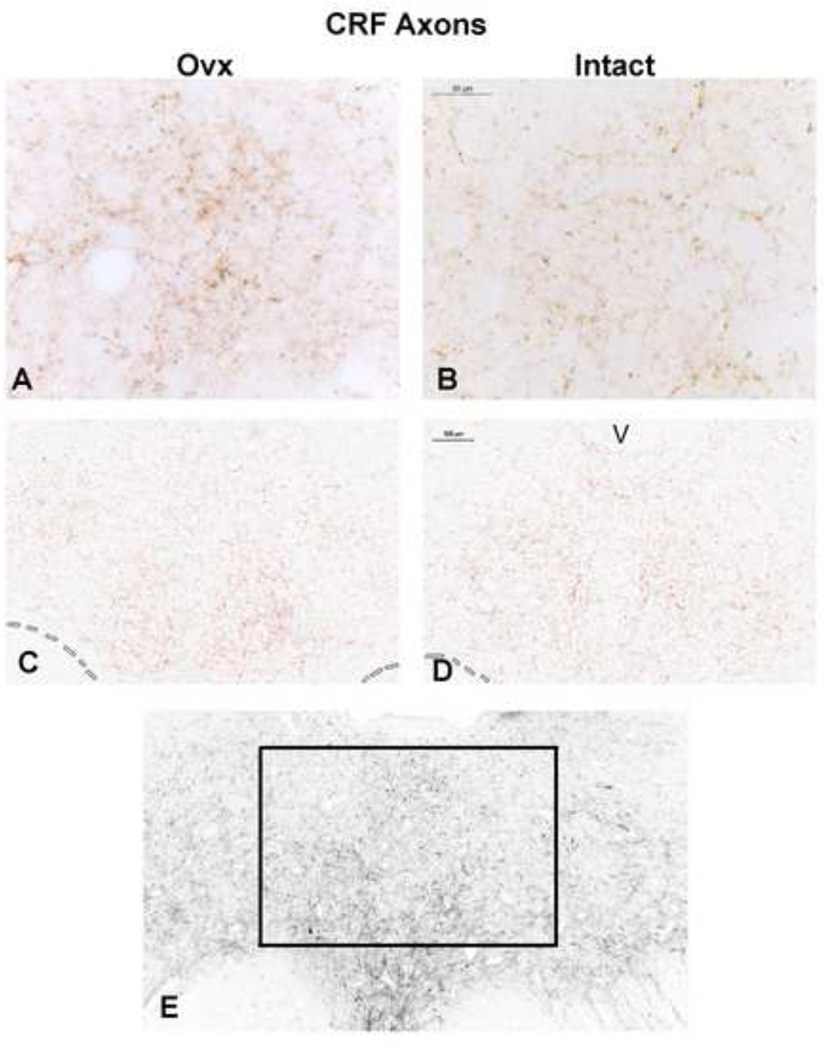
Representative CRF fiber staining in the dorsal raphe is shown in Figure 2. The CRF staining is largely in the boutons. An Ovx and an Intact animal are shown in panels A and B as observed with a Leica brightfield microscope. An Ovx and an Intact animal are shown in panels C and D following segmentation (red) as observed in Slidebook 4.2 and ImageJ. Panel E illustrates the rostral dorsal raphe as it appears in the montage generated by the Marianas and the area that was magnified and analyzed. The box contains the densest area of CRF fiber staining. This area corresponds roughly to the DRv and the middle of the DRd subnuclei as illustrated in Figure 1. Seven anatomical levels of the dorsal raphe from a rostral to a caudal level were subjected to image analysis.
Figure 2.
Representative photomicrographs of CRF axon staining in the dorsal raphe of an Ovx and Intact Japanese macaque. Panels A and B illustrate the appearance of the CRF axons with boutons under brightfield optics at a high magnification. Panels C and D illustrate images captured with Slidebook 4.2 on the Marianas stereology workstation that were segmented into positive (red) and negative pixels in ImageJ for quantitation. Panel E illustrates the entire montage of a rostral section and the area that was cropped and analyzed. The montage was generated with Slidebook 4.2 on the Marianas stereology workstation. Slidebook 4.2 builds a montgage from multiple pictures taken at low power over the entire field (the optics are not like a brightfield microscope).
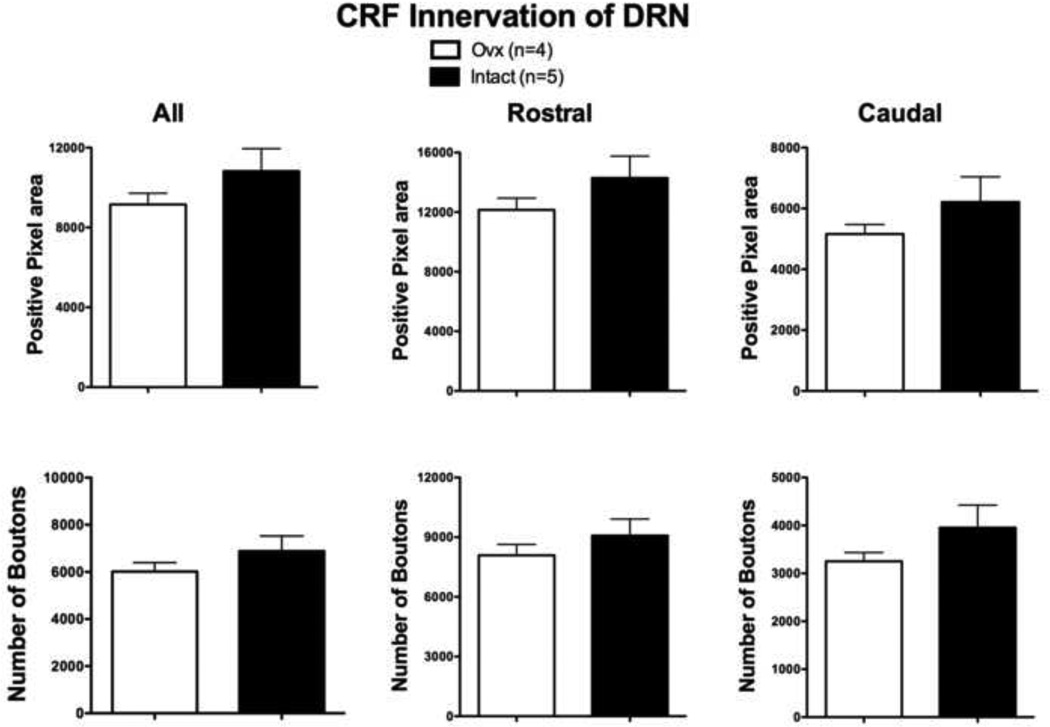
Figure 3 illustrates the quantitative analysis of CRF fiber density in the dorsal raphe. There was no significant difference in the CRF axonal bouton staining in the dorsal raphe. In the panel labeled ALL (7 sections), the overall average CRF positive pixel number and positive bouton number were not significantly different between the Intact group and the Ovx group (t (df 7) = 1.22; p= 0.26 and t (df 7)=1.088; p=0.31,respectively). Identical results were obtained with comparison of the total positive pixel number and total positive bouton number (t (df 7) = 1.22, p=0.26, and t (df 7)=1.088, p=0.31, respectively). Moreover, similar results were observed when the rostral (4 sections) and caudal (3 sections) areas were parsed. There did not appear to be a greater contribution from either region. The average of the areas analyzed for each animal equaled 817,394 pixels2 or 294,262 µm2.
Figure 3.
Histograms illustrating the quantitative analysis of CRF boutons in the dorsal raphe of long-term Ovx and Intact macaques. There was no difference in the overall positive pixel area or the number of boutons in the areas analyzed (labeled All). There was no difference in the rostral + middle positive pixel area or the number of boutons (labeled Rostral). There was no difference in the caudal positive pixel area or the number of boutons (labeled Caudal). The average of the areas analyzed for each animal equaled 817,394 pixels2 or 294,262 µm2.
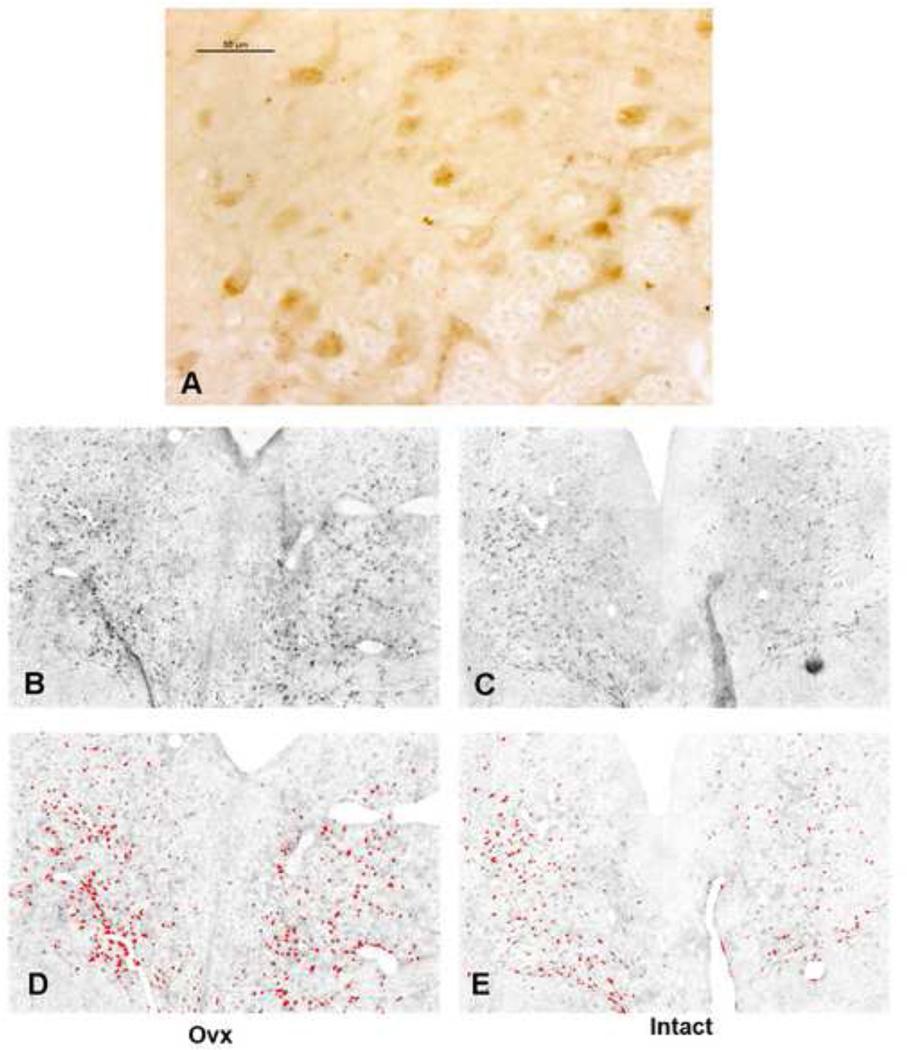
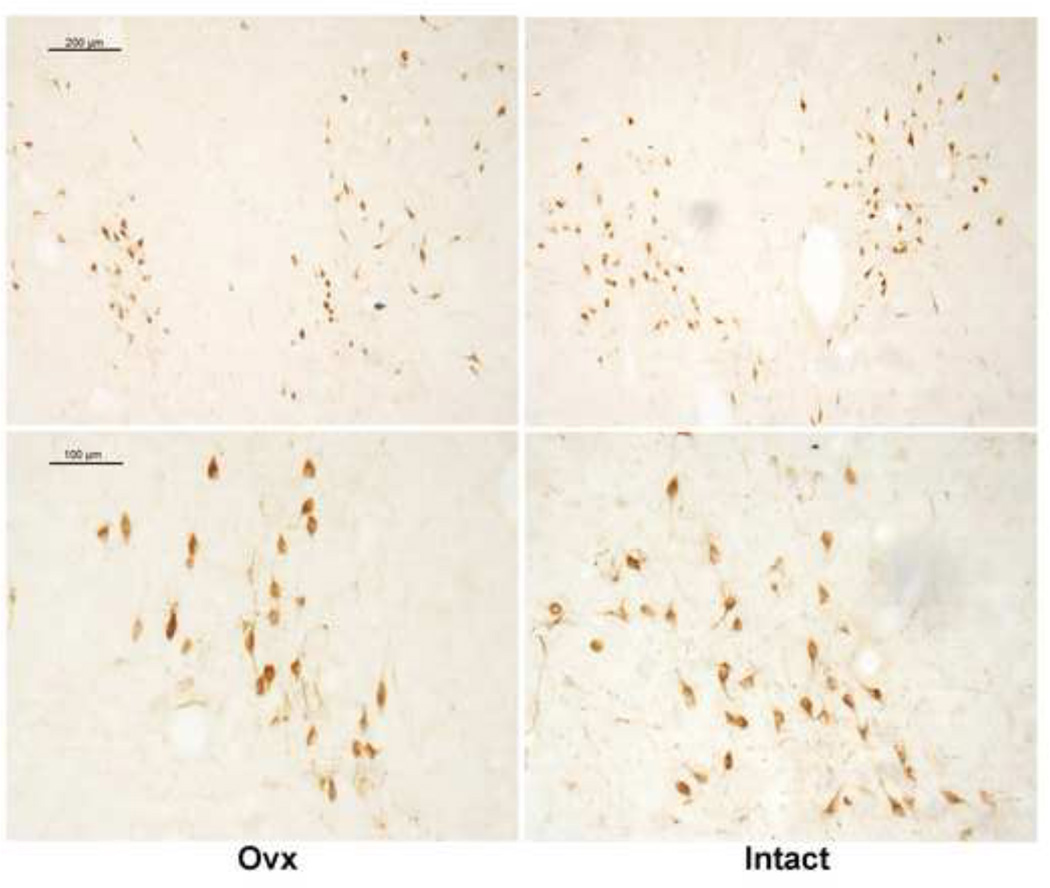
CRF-R1 immunostaining in the dorsal raphe is illustrated in Figure 4. CRF-R1 staining in the dorsal raphe of an Ovx animal is shown in panel A as observed with a Leica brightfield microscope. The staining is largely cellular and at higher power, it can be seen as patches on the cell surface depending on the plane of sectioning. Panels B and C illustrate representative CRF-R1 staining in an Ovx animal and in an Intact animal as captured with Slidebook 4.2 from the Marianas stereology workstation. Panels D and E illustrate the same sections following segmentation of positive signal with ImageJ. CRF-R1 immunostaining was not confined to any particular subnucleus. Three levels of the dorsal raphe, one each at a rostral, mid- and caudal level (250 – 500 µm apart) were immunostained from each animal and subjected to image analysis.
Figure 4.
Representative photomicrographs of CRF-R1 staining in the dorsal raphe of an Ovx and Intact Japanese macaque. Panel A illustrates the appearance of the CRF-R1 immunostaining in the dorsal raphe under brightfield optics at a high magnification. Panels B and C illustrate images of CRF-R1 immunostaining captured with Slidebook 4.2 on the Marianas stereology workstation. Slidebook 4.2 builds a montgage from multiple pictures taken at low power over the entire field. Panels D and E illustrate the same sections after background subtraction, erasure of folds or artifacts, and segmentation into positive (red) and negative pixels in ImageJ for quantitation.
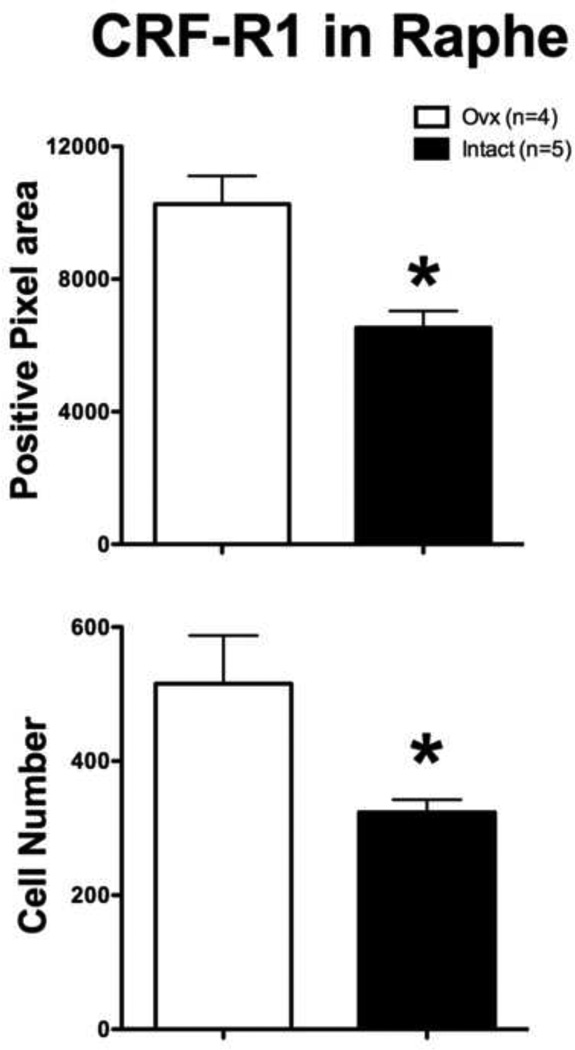
There was a higher expression of CRF-R1 in the Ovx group compared to the Intact group as illustrated in Figure 5. The overall average CRF-R1 positive pixel number (t (df 7)=3.983, p=0.005) and positive cell number (Mann-Whitney test U=0, p=0.016) were significantly higher in the Ovx group than in the Intact group. The standard t-test analysis of the positive cell number exhibited a significant difference in variance (F=11.44, p=0.04), which required the nonparametric Mann-Whitney test. Identical results were obtained with comparison of the total positive pixel number and total positive bouton number (t (df 7)=3.983, p=0.005 and U=0, p=0.016, respectively). The average of the areas analyzed for each animal equaled 556,711 pixels2 or 200,416 µm2.
Figure 5.
Histograms illustrating the quantitative analysis of CRF-R1 immunostaining in the dorsal raphe of long-term Ovx and Intact macaques. The Ovx group had significantly greater positive pixel area (p=0.005) and positive cell number (p = 0.02). The average of the areas analyzed for each animal equaled 556,711 pixels2 or 200,416 µm2.
Representative UCN1 cell staining in an Ovx animal and in an Intact animal as observed with a Leica brightfield microscope is illustrated in Figure 6. The staining was clearly cytoplasmic. These cells are located in the supraoptic area (SOA) adjacent to the Edinger-Westfal nucleus (May et al., 2008; Sanchez et al., 2010). Six levels of the rostral midbrain containing the SOA were subjected to image analysis.
Figure 6.
Representative photomicrographs of UCN1 immunostaining in the SOA at matching levels of an Ovx macaque and an Intact macaque under brightfield optics at a low (top) and higher (bottom) magnification. For analysis, the entire area shown at low magnification was captured on the Marianas stereology microscope with Slidebook 4.2. The images were segmented into positive and negative pixels with Image J for quantitative analysis. This process is not illustrated, but it is identical to that shown in Figure 4.
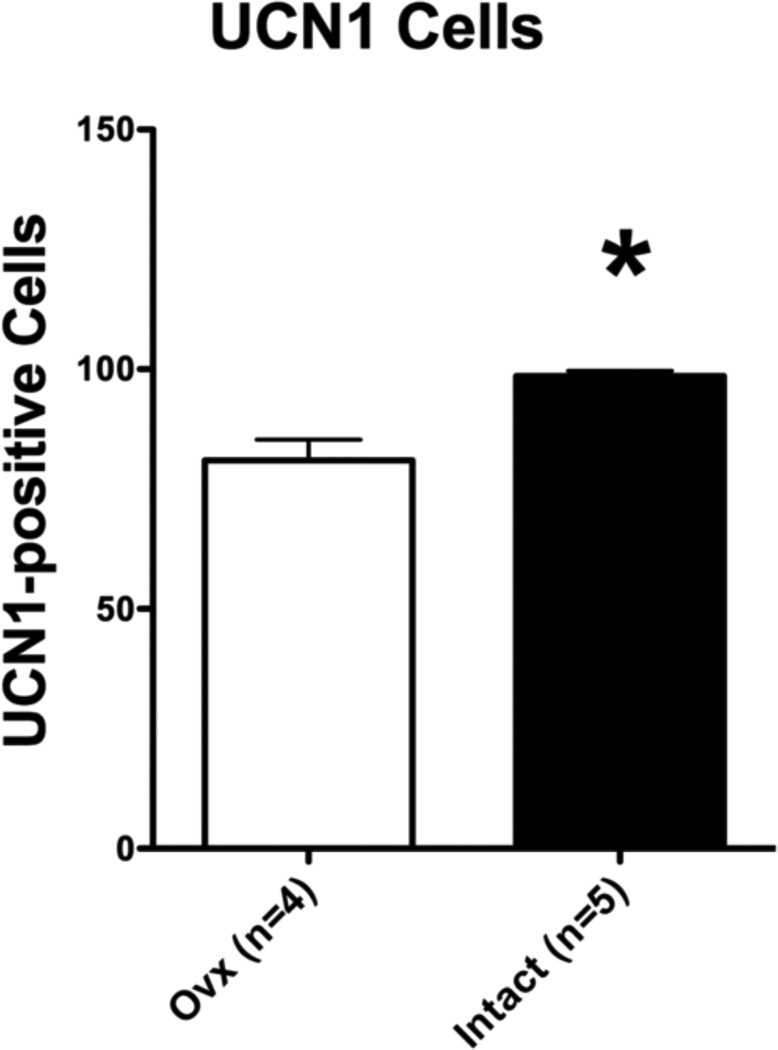
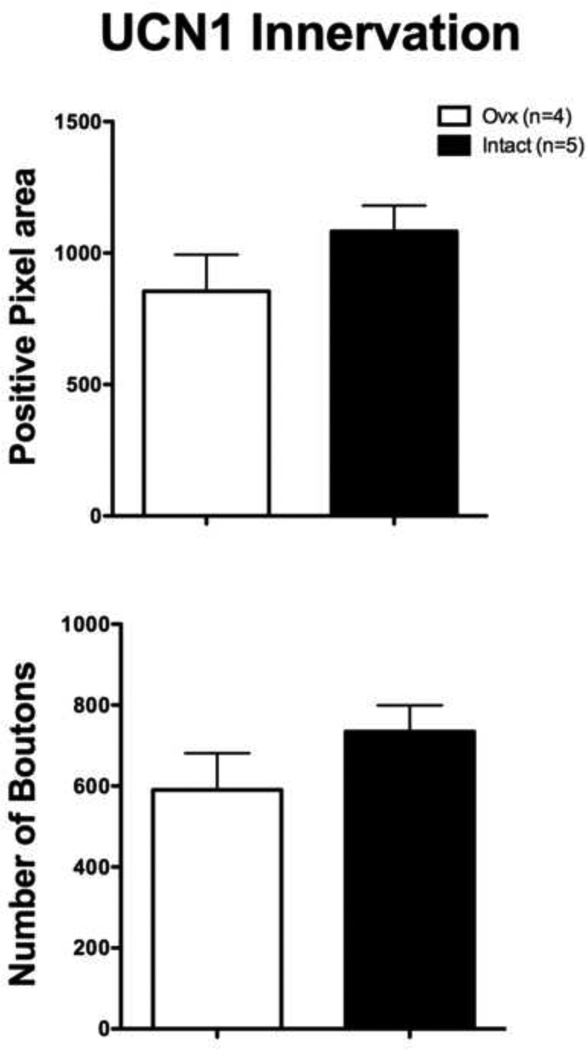
Figure 7 illustrates the quantitative analysis of UCN1 cell staining. The variance was significantly different between the groups (F=12.93, p=0.03), which necessitated the Mann-Whitney nonparametric test. The overall average number of detectable UCN1 postive cells was significantly lower in the Ovx group than in the Intact group (Mann-Whitney U=0, p=0.016). The average of the areas analyzed equaled 264,988 pixels2 or 95,396 µm2.
Figure 7.
Histograms illustrating the quantitative analysis of UCN1 immunostaining in the cell body area of long-term Ovx and Intact macaques. There were significantly fewer detectable UCN1 positive cells in the Ovx group than in the Intact group (p = 0.003). The average of the areas analyzed equaled 264,988 pixels2 or 95,396 µm2.
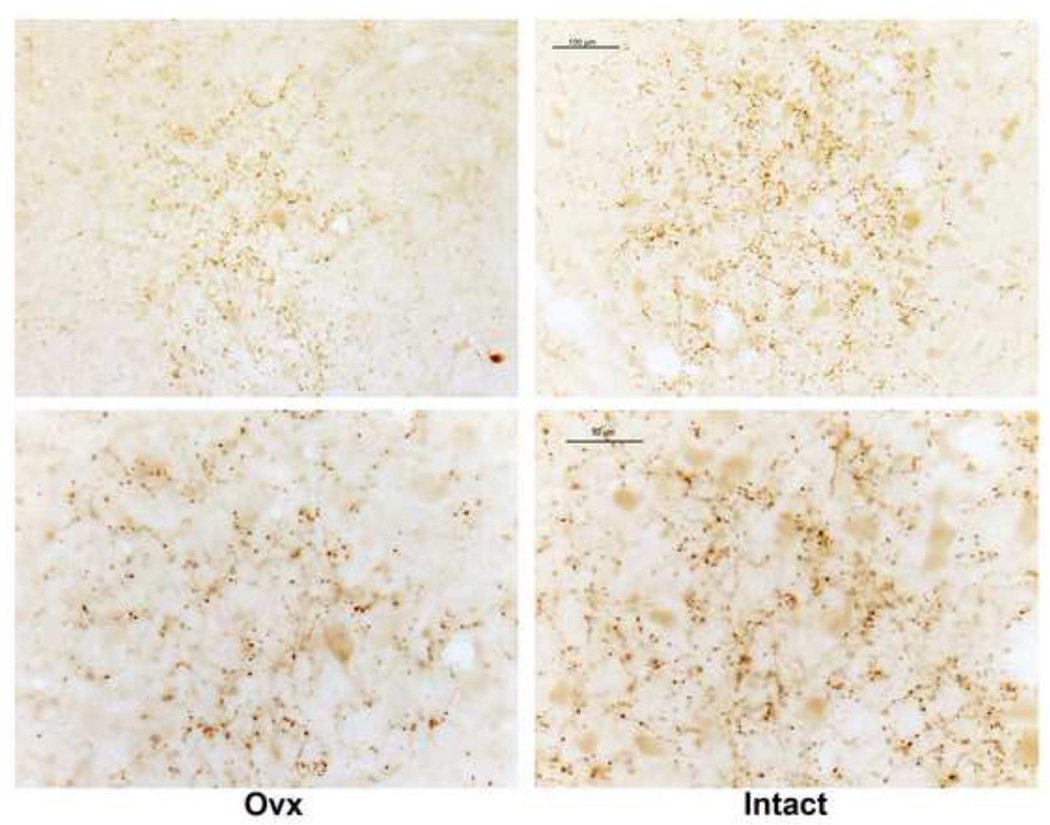
Figure 8 illustrates representative UCN1 fiber staining located rostral to the raphe of an Ovx and an Intact animal as observed with a Leica brightfield microscope. The staining was largely in the boutons. This little cluster of UCN1 fibers is located caudal to the Edinger-Westfall nucleus, but rostral to the dorsal raphe nucleus and it provides a reliable area for analysis. It is found in 3 levels of the midbrain at 250 µm intervals, which were subjected to image analysis.
Figure 8.
Representative photomicrographs of UCN1 axon immunostaining anatomically located between the SOA and dorsal raphe of a long-term Ovx- and an Intact-macaque under brightfield optics at a low and higher magnification. The cluster of axons appears larger in the Intact animal with more detectable butons. The images captured on the Marianas workstation contained the entire area. The images were segmented into positive and negative pixels with Image J for quantitative analysis. This process is not illustrated, but it is identical to that shown in Figure 2.
Figure 9 illustrates the quantitative analysis. There was no significant difference in the UCN1 axonal bouton staining in the midbrain although there was a trend toward higher values in the Intact versus the Ovx animals. The overall average UCN1 positive pixel number and positive bouton number were not significantly different between the Intact group and the Ovx group (t (df 7)=1.38, p= 0.21 and t (df 7)=1.33, p=0.22, respectively). Identical results were obtained with comparison of the total positive pixel number and total positive bouton number (t (df 7)=1.38, p= 0.21 and t (df 7)=1.33, p=0.22, respectively). The average of the areas analyzed equaled 180,037 pixels2 or 64,813 µm2.
Figure 9.
Histograms illustrating the quantitative analysis of UCN1 boutons in the small fiber plexus between the SOA and the dorsal raphe of long-term Ovx and Intact-macaques. There was no statistical difference in the positive pixel area or the number of boutons in the area analyzed. The average of the areas analyzed equaled 180,037 pixels2 or 64,813 µm2.
3. Discussion
During and after menopause a significant number of women report an increase in anxiety and vulnerability to stress (Conde et al., 2006; Heikkinen et al., 2006; Maki et al.; Tangen and Mykletun, 2008). Both the CRF and serotonin systems respond to stress and play roles in depression and anxiety disorders (Arango et al., 2002; Holsboer, 1999; Keck and Holsboer, 2001; Mann et al., 1996). Moreover, increasing evidence suggests that their function is inextricably linked. In humans, CRF terminals appose serotonin neurons in the raphe region (Ruggiero et al., 1999), and a postmortem study reported that patients with major depressive disorder had significantly more CRH-positive neurons in the PVN than normal controls (Raadsheer et al., 1994). Antidepressant SSRIs that increase available serotonin reduce the sensitivity of CRF neurons in the PVN (Stout et al., 2002), and cortisol levels return to normal in depressed patients treated with a variety of antidepressants (Himmerich et al., 2006; Schule et al., 2006). Moreover, repeated treatment with citalopram, an SSRI, decreased CRF and HPA axis activity in rodents (Moncek et al., 2003).
CRF producing neurons and projections are widespread and play a role in stress-related psychiatric disorders (Clark and Kaiyala, 2003). CRF innervation of the serotonergic dorsal raphe nucleus has been described and CRF receptors are found in the dorsal raphe of rodents (Linthorst, 2005; Lowry et al., 2000; Valentino et al., 2010) and primates (Sanchez et al., 2010). Within the CRF family, UCN1 plays a role in attenuation of the stress response (Pan and Kastin, 2008). Subsequently, the opposing effects of CRF and UCN1 on serotonin were established in rodents (Valentino et al., 2010). Administration of CRF directly into the dorsal raphe nucleus inhibits serotonergic activity, and CRF-R1 antagonists block this effect (Denihan et al., 2000). Conversely, the stimulatory effect of UCN and CRF-R2 is supported by increased 5-HT efflux in the basolateral amygdala (a projection region of the dorsal raphe) with intra-raphe administration of the CRF-R2 agonist, UCN2. This effect was completely blocked by antisauvagine-30 (ASV-30), a relatively selective CRF-R2 antagonist (Amat et al., 2004).
In the absence of hormone therapy, postmenopausal women exhibit higher release of ACTH and cortisol when administered a challenge of dexamethasone plus CRH (Kudielka et al., 1999) suggesting that their CRF system may be hyper-responsive. In animal models, the effects of ovarian steroids on the HPA axis are complex. Several studies initially indicated that treatment of ovariectomized female or male rats with estrogen increased CRF mRNA (Li et al., 2003; Lund et al., 2004). Subsequently, it was recognized that acute E treatment increases CRF expression, but low dose chronic E treatment decreased stress-induced ACTH in a rodent model (Dayas et al., 2000). In addition, 5 days of E treatment to ovariectomized macaques prevented the elevation in cortisol induced by icv administration of interleukin 1-alpha (Xia-Zhang et al., 1995).
We previously found that 1 month of hormone replacement in short-term (5–8 months) ovariectomized monkeys decreased CRF mRNA and protein in the PVN (Bethea and Centeno, 2008). In the same paradigm, we showed that E ± P supplementation decreased CRF innervation of the dorsal raphe and median raphe; decreased CRF-R1 in the dorsal raphe; increased CRF-R2 in the dorsal raphe; increased UNC1 immunostained neurons in the SOA near the Edinger-Westfal nucleus; and increased UNC1 axon density in a cluster of fibers rostral to the dorsal raphe (Sanchez et al., 2010). We also found that CRF axon density in the caudal portion of the dorsal raphe was increased in ovarian intact individuals who were stress-sensitive, whereas UCN1 axon density was decreased (Weissheimer, 2010). Hence, ovarian steroids, stress and stress sensitivity can all regulate CRF and UCN1. It is important to note that all of these studies were done indoors with animals in either single cages (Weissheimer, 2010) or paired housing (Bethea and Centeno, 2008; Sanchez et al., 2010).
In this study with Japanese macaques in an outdoor troop setting, long-term Ovx had no effect on CRF innervation of the dorsal raphe although the possibility of a type 2 error is present due to the small number of animals. Hormone replacement is not equivalent to having intact ovaries so comparisons to the earlier study are limited, and our current observation has several interpretations or caveats. It may suggest that in an outdoor social environment (that can only be described as very ‘laid-back’), the presence or absence of ovarian hormones did not significantly impact CRF production. The short-term Ovx in indoor caged housing had an elevation in CRF gene and protein expression that was ameliorated by hormone therapy (Sanchez et al., 2010). This may imply that indoor caged animals are under low, but chronic stress that is not present in the outdoor troop; a notion that is supported by other studies (Olsson, 2007; Refinement, 2009; Schapiro et al., 2000; Schapiro, 2002; Schapiro, 1996). Alternatively, we may not have the power to detect a difference. However, the trend toward Intact animals having higher CRF fibers in the dorsal raphe than the Ovx group is the opposite of earlier data. Although the ranks of the individuals did not change, the Intact individuals participated in sexual behavior to a significantly greater extent than the Ovx individuals (Coleman et al., 2011). There is an increase in fighting in the troop during mating season that would have involved the Intact animals to a much larger extent than the Ovx animals. Since the animals were euthanised in November (mating season), the Intact animals may have had more monkey-type stress at that time, which would contribute to higher CRF immunodetection in the Intact animals. It is possible that a different result would have been found in a different season. In addition, the animals in this study were ovariectomized at a younger age than in previous studies with indoor rhesus macaques.
CRF-R1 is considered the ‘anxiogenic’ receptor. We found that dorsal raphe CRF-R1 protein was significantly elevated in the long-term Ovx females. This could make the animals more sensitive to stress, and cause a greater inhibition of serotonin when stress is present. Although there was little effect of long-term Ovx on anxiety behaviors in the troop environment, certain parameters of anxiety behavior were increased in temperament tests that were conducted indoors with brief single caging.
In our previous study of hormone therapy in short-term Ovx macaques, we found that CRF-R1 immunoreactivity was elevated in Ovx and suppressed by ovarian steroid treatment (Sanchez et al., 2010), which is consistent with this study in which CRF-R1 immunoreactivity is suppressed in the Intact group compared to the Ovx group. Given that the protein expression and immunoreactivity are derived from a change in gene expression, then the mechanism by which ovarian steroids suppress CRF-R1 gene expression is of interest. In general, the ability of E to decrease gene expression appears to rely on indirect mechanisms. In one pathway, ligand activated steroid receptors sequester NFkB and thereby decrease gene transcription that is dependent upon binding of NFkB to its response element, NRE (Cerillo et al., 1998; McKay and Cidlowski, 1999). Hence, genes that are driven by NFkB can be deactivated with E treatment. We have shown that E treatment decreases NFkB translocation to the nucleus in serotonin neurons of the dorsal raphe (Bethea et al., 2006). An unexplored mechanism involves repressor element silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF). REST is a repressor of transcription of TPH2 (Patel et al., 2007) and CRF (Seth and Majzoub, 2001). Hence, it is present in serotonin neurons. It is attractive to speculate that E affects REST in some fashion, which may influence CRF-R1 expression. It is also important to note that CRF terminals are on serotonin neurons as well as GABA neurons (Waselus et al., 2005). Therefore, CRF via CRF-R1 may stimulate GABA neurons that in turn inhibit serotonin neurons.
We also observed that there were fewer UCN1 positive cells in the midbrain SOA. There was a trend toward lower axon density in the Ovx group, but due to the small number of monkeys there remains the possibility of a type 2 statistical error in which a real effect is not detected. Nonetheless the data suggest that there may be a reduction in UCN1 production and transport resulting in fewer detectable cells or axons. Or, the reduction in UCN1 neurons in the long-term Ovx may be a decrease in cell number rather than a decrease in UCN1 production. Nonetheless, the UCN1 reserve appears to be reduced in the long-term Ovx and upon stress, further changes might be observed. In the short-term Ovx animals housed indoors, there was a significant decrease in the number of detectable UNC1 positive neurons and also significantly decreased UNC1 axon density (Sanchez et al., 2010), which mirrors this study. Whether these observations are due to a decrease in UCN1 cell number or a decrease in UCN1 expression is unresolved.
UCN1 decreases feeding behavior, and also participates in the stress response (Oki and Sasano, 2004; Pan and Kastin, 2008; Spina et al., 1996). We previously showed that relative UCN1 gene expression was 1000x higher than UCN2, and 10,000x higher than UCN3 in the raphe region (Sanchez et al., 2010). UCN1 and 2 bind to CRF-R2 with greater affinity than CRF, suggesting that in the raphe, low extracellular concentrations of UCN1 would preferentially bind CRF-R2. Both UCN1 knockout mice and CRF-R2 knockout mice exhibit increased anxiety-like behavior (Pan and Kastin, 2008). Thus, it has been generalized that activation of CRF-R1 by CRF increases anxiety-like behavior whereas activation of CRF-R2 by UCN1 decreases anxiety-like behavior. The elevated anxiety behavior exhibited by the long-term Ovx animals when brought indoors for temperament tests is consistent with stress causing an increase in CRF release which binds to the increased CRF-R1 receptors. A decrease in UCN1 would not act to ameliorate the stress response, which may last longer in the Ovx group.
Altogether the increase in CRF-R1 and the decrease in UCN1 in the Ovx group may lead to lower serotonin availability in the Ovx group compared to the Intact group. This suggestion is supported by our previous observation that fenfluramine-induced serotonin release as indicated by subsequent prolactin release, was significantly lower in the Ovx group than in the Intact group (Bethea et al., 2012). In addition, Ovx animals have fewer serotonin neurons and decreased gene expression of Fev, TPH2, SERT and 5HT1A. However, as previously discussed, the age at which the animals were ovariectomized contributes developmental variables in addition to the presence or absence of ovarian steroids (Bethea et al., 2012). Altogether, the increase in anxiety behavior in the temperament tests exhibited by the Ovx group together with their lower response to fenfluramine, lower serotonin-related gene expression, higher CRF-R1 and lower UCN1 compared to intact animals encourages the hypothesis that removal of ovarian steroids in women may lead to decreased serotonin and consequent mood deterioration.
The usual computation of human years to monkey years is 3 to 1, so the Ovx group is somewhat comparable to women after 9 years of steroid deprivation, though significantly younger than menopausal women. Nonetheless, these studies promote further understanding of the effects of long-term ovarian steroid loss in females. For example, women with a relatively stress-free life or who are very stress-resilient may not notice changes in anxiety after menopause. Others may find that they are easily stressed or experience increases in anxiety and decreased coping mechanisms. In addition, monkey chow is significantly lower in fat and sugar than the typical western diet. In light of the inflammatory conditions induced by a western diet (Pistell et al., 2010; Posey et al., 2009), the endpoints examined in this study may be worsened with ovariectomy of older animals on a western diet.
In conclusion, we found (a) increased protein expression of CRF-R1 in the dorsal raphe, (b) decreased protein expression of UCN1 in the SOA cell body region and (c) a trend toward decreased UNC1 in midbrain axons, in long-term Ovx macaques living in an outdoor, stable troop. This was similar to results obtained with short-term Ovx rhesus macaques housed indoors. The pivotal difference between indoor short-term and outdoor long-term Ovx animals was in the CRF innervation of the dorsal raphe. CRF axon density was similar in outdoor long-term Ovx and Intact macaques, whereas it was significantly higher in caged short-term Ovx macaques compared to animals administered E±P therapy. We speculate that Ovx alone increases vulnerability to stress or anxiety by increasing CRF-R1 and decreasing UCN1, but many factors may impact CRF delivery to the dorsal raphe including age at ovariectomy, stress, stress sensitivity and season.
4. Materials and Methods
The Institutional Animal Care and Use Committee of the Oregon National Primate Research Center (ONPRC) approved this study, which followed the guidelines of the Animal Welfare Act.
Animals
Ten young females ~3.5 years of age, born into a troop of Japanese macaques housed in a naturalistic setting (corral, see below) were chosen. At the time, there were 12 matriarchal lineages in the troop; and there were enough animals born in Spring 2003 to use 10 individuals with 2 alternates. It was not feasible to remove 10–12 adult females from our troop because of their contribution to social stability. The Japanese macaques go through puberty between 3 and 4 years of age. A recent paper examining the timing of puberty in rhesus macaques found that animals born in the spring ovulate in the fall at the age of approximately 30 months (Wilson et al., 2012). This suggests that the animals assigned to this study likely had their first ovulation in Fall, 2005. Other than that, we were unable to determine the stages of puberty, such as Tanner stage in girls (Lloyd et al., 1996; Oldehinkel et al., 2011; Travers et al., 1995).
Five pairs of young females were selected that represented different ranks in the dominance hierarchy. In late November 2006 (about 1 year after first ovulation), one individual of each pair was ovariectomized (Ovx; reproductive tract remains) and the other individual was sterilized by tubal ligation to prevent pregancy, but the ovaries remained intact. The surgeries were conducted during the annual round up of all of the animals in the corral. The animals were returned to the troop and allowed to mature for 3 years. Behavioral observations were conducted during their 3 years in the corral. One animal from the Ovx group died in the troop during year 1 and was replaced with an animal of similar age and lineage. Thus, 1 animal in the Ovx group was treated for slightly less than 3 years. Unfortunately, another Ovx animal died at the end of the 3 year period resulting in a final n=4 in the Ovx group. After 3 years, the animals were collected for temperament tests, pharmacological challenges and euthanasia. The results of the behavioral observations and pharmacological challenge are published (Bethea et al., 2012; Coleman et al., 2011).
Description of the troop
The monkeys used in this study were part of a troop of approximately 300 individuals housed in a two-acre outdoor corral at the Oregon National Primate Research Center (ONPRC). The corral is surrounded by steel walls, and contains several platforms and climbing structures for play and exploration. Monkeys are fed commercially available monkey chow twice daily, supplemented by daily fresh produce or grains. Water is available ad libitum. As part of general animal husbandry of ONPRC, all animals were given unique markings on their backs, allowing for individual identification. Animal care-staff walk through the corrals daily to look for sick or injured monkeys. Japanese macaques are seasonal breeders, and at the ONPRC, the birth season is typically between May and August, with the majority of births occurring in June and July. The mating season is typically November through February.
The Japanese macaque troop arrived at ONPRC in 1965. The troop composition is relatively stable and the age structure is comparable to that of a natural troop (Eaton, 1984; Maruhashi, 1982; Rostal et al., 1986). Like other macaque species, the hierarchical organization of the troop is along matriarchal lineages. The matriarchal lines and dominance hierarchies within the troop are well documented, and have remained stable for the past 45 years.
Annual round ups
At least once a year, the animals were “rounded up”, or brought from the corral into a capture run (“catch area”) adjacent to the corral. This round up allowed collection of the monkeys for procedures that could not be done in an open field, such as annual veterinary exams, weighing and dye marking. Typically, the monkeys were brought into this area one or two times per year, and so most were acclimated to this procedure. During the round-up, monkeys ran through tunnels into the catch area, where they jumped into transport boxes for transfer to single cages. Annual exams were done on all members of the corral in the fall, after the birthing season, and before the mating season. In addition to the physical examination, animals were also weighed and tested for tuberculosis, and new infants were identified. The animals were typically kept in cages in the catch area for approximately one week.
Surgeries
Subjects were either ovariectomized (Ovx; n=5) or tubal ligated (tubal-ligated or ovary intact; n=5) after puberty (at age 3.5 yrs), and returned to their semi-free ranging troop, for 3 years until approximately age 6.5 yrs. For ovariectomy or tubal ligation, each animal was sedated with ketamine (10 mg/kg) in its cage in the catch area and transported to a surgical suite. Ovariectomy and tubal ligation were performed by the surgical personnel of ONPRC using a laparoscopic approach.
Hormone assays
Cortisol, estradiol and progesterone concentrations in serum were performed utilizing a Roche Diagnostics Cobas e411 automated clinical platform instrument, which has been validated in the Endocrine Technology and Support Laboratory, ONPRC, for macaque serum by comparison with traditional radioimmunoassays. The sensitivity and range of the cortisol assay was 2 and 500 ng/ml, respectively. The sensitivity and range of the estradiol assay was 5 and 4250 pg/ml, respectively. The sensitivity and range of the progesterone assay was 0.035 and 59 ng/ml, respectively. Within-assay coefficients of variation were less than 5% and between-assay coefficients of variation were less than 10% for all assays.
Tissue Preparation
The monkeys were euthanized according to procedures recommended by the Panel on Euthanasia of the American Veterinary Association. Each animal was sedated with ketamine, transported to the necropsy suite, given an overdose of pentobarbital (25 mg/kg, i.v.), and exsanguinated by severance of the descending aorta.
Following euthanasia, the left ventricle of the heart was cannulated and the head of each animal was perfused with 1 liter of saline followed by 7 liters of 4% paraformaldehyde in 3.8% borate, pH 9.5 (both solutions made with DEPC-treated water [0.1% diethyl pyrocarbonate] to minimize RNase contamination). The brains were removed and dissected. Tissue blocks were post-fixed in 4% paraformaldehyde for 3 h, then transferred to 0.02M potassium phosphate-buffered saline (KPBS) containing 10%, followed by 20% glycerol and 2% dimethyl sulfoxide at 4°C for 3 days to cryoprotect the tissue. After infiltration, the block was frozen in isopentene cooled to −55°C, and stored at −80°C until sectioning. Sections (25 µm) were cut on a sliding microtome, and half were stored in cryoprotectant at −20°C until processing for immunocytochemistry (the other half were mounted on slides, dehydrated under vacuum and then frozen at −80°C until processing for ISH). Immunocytochemistry was employed in this study because all of the sections for ISH were used in a previous study and were depleted (Bethea et al., 2012).
Immunocytochemistry
Brain sections were removed from frozen storage and washed in KPBS for an hour. They were blocked in the appropriate normal serum for an hour, and then blocked in avidin and biotin for 20 minutes (Vector labs, Burlingame, CA). The primary antibody was diluted in 0.02 M KPBS/2% normal rabbit or goat serum/0.4% Triton X-100 and exposed to the experimental tissue for 48 hours at 4°C. After 48h, sections were washed in KPBS for an hour. The appropriate secondary antibody (Vector) was diluted 1:200 in KPBS/0.4% triton and incubated with the tissue for 60 minutes. After another KPBS wash, the tissue sections were incubated in VECTASTAIN Elite ABC solution (Vector) for one hour and washed in buffer again. Sections were then incubated in 0.02M KPBS with 0.05% DAB and 0.003% H2O2. Finally, the sections were washed, mounted on slides, dehydrated, dipped in xylene, and then coverslipped in DPX mountant for stereological analysis.
The CRF antibody was a gift from Dr. Wylie Vale (Salk Institute, La Jolla, CA) and was raised in rabbit against human CRF conjugated to human alpha globulin. Thus, in the CRF ICC, we used an additional blocking step with 1% human alpha globulin (Sigma G-2011, St. Louis, MO) for 20 minutes. Rabbit anti-human CRF (1/3,000) was diluted in 0.02 M KPBS/ 2% normal goat serum/ 0.4% triton/0.1% human alpha globulin. The secondary antibody was biotinylated goat anti-rabbit IgG (Vector). The antisera to CRF has been extensively characterized and previously applied to primate brain (Bassett and Foote, 1992).
The UCN1 antibody (Sigma-Aldrich, St. Louis, MO; Catalog No. U4757) was raised in rabbit against amino acids 25–40 of the human peptide. Prior to incubation with the primary antibody, an additional blocking step with 3% bovine serum albumin for an hour was included. Rabbit anti-UCN1 (1/15,000) was diluted in 0.02 M KPBS/2% normal goat serum/0.4% triton. The secondary antibody was biotinylated goat anti-rabbit IgG (Vector). The antisera to UCN1 has been extensively characterized and previously applied to rat and human brain (May et al.).
The CRF-R1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA; Catalog No. 12381) was an affinity purified goat polyclonal antibody raised against an internal region of CRF-R1 of human origin. Increasing dilutions of anti-CRF-R1 produced decreasing signal up to a titer of 1/4000, beyond which the signal disappeared. Anti-CRF-R1 was diluted 1/1000 in 0.02 M KPBS/2% normal rabbit serum/0.4% triton and incubated with the experimental tissue for 48 hours at 4°C. The secondary antibody was biotinylated rabbit anti-goat IgG (Vector). Absorption controls for this antibody were published for rhesus macaque (Sanchez et al., 2010). The antibody previously used for CRF-R2 was no longer available. Currently available CRF-R2 antibodies were not satifactory.
Densitometric Analysis
Each section was video-captured with the Marianas Stereology Workstation and Slidebook 4.2. A montage of the dorsal raphe was created by Slidebook, which was subjected to further analysis with Image J. The dorsal raphe changes shape from rostral to caudal levels. Unless otherwise specified, the representative sections were 250 µm apart and exhibited different anatomical levels of the dorsal raphe. The same size square was then used for all of the animals at that anatomical level. On each section, the operator outlined (boxed) the area of interest and the image was segmented into positive (stained) and negative (unstained) pixels. Three measurements were generated from the segmented image. The area covered by the positive pixels was obtained, called positive pixel area and the total area was obtained. The CRF and UCN fiber staining was confined to boutons. The number of CRF-R1 and UCN positive cells, or CRF and UCN boutons, was obtained using size cut-off values. The mean signal intensity is also obtained but it is not considered reliable with nonlinear assays such as ICC. The same procedure was applied at each anatomical level from the rostral to the caudal extent of the dorsal raphe nucleus. There is ~0.6 pixel/µm linear and 0.36 pixels2/µm2.
Statistical Analysis
The positive pixel area and the positive cell or bouton number were (a) averaged across levels and (b) totalled across levels, generating one overall average or total for each animal. The individual animal means and totals were subjected to statistical analysis with 2-tailed Student’s t–test. The variance in the groups reflects the difference between animals. F values were computed to assure the variances were equal and when not, the Mann-Whitney nonparametric test for comparison of 2 groups was applied.
All statistical analyses were conducted using the Prism Statistic Program 5.0 (GraphPad, San Diego, CA). A confidence level of p<0.05 was considered significant.
Highlights.
CRF innervation of the dorsal raphe was similar in Ovx and Intact macaques in an outdoor troop.
CRF-R1 immunostaining was higher in long-term Ovx than Intact macaques in an outdoor troop.
UCN1 cell number was lower in long-term Ovx than Intact macaques in an outdoor troop.
Acknowledgements
We are deeply grateful to the technicians of the Division of Animal Resources, including surgery personnel, for their help with all aspects of this study. We especially thank Dr. Kris Coleman, Head of Behavioral Sciences, who provided expert knowledge and advice on the Japanese macaque troop, on Japanese macaque behavior and what it means to other monkeys. We also are especially grateful to Dr. Coleman’s assistant, Nicola Roberts, who was intimately involved in monitoring the behavior and well being of the animals in this study. This study was supported by NIH grants MH62677 to CLB and 8P51 OD-011092 for the operation of ONPRC.
Supported by: NIH grants MH62677 to CLB, P30-NS061800 to Dr. Sue Aicher, and 8P51-OD00011092 for the operation of ONPRC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, Foster AC, Watkins LR, Maier SF. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–519. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- Arango V, Underwood MD, Mann JJ. Serotonin brain circuits involved in major depression and suicide. Prog Brain Res. 2002;136:443–453. doi: 10.1016/s0079-6123(02)36037-0. [DOI] [PubMed] [Google Scholar]
- Bailey JE, Papadopoulos A, Diaper A, Phillips S, Schmidt ME, van der Ark P, Dourish CT, Dawson GR, Nutt DJ. Preliminary evidence of anxiolytic effects of the CRF1 receptor antagonist R317573 in the 7.5% CO2 proof-of-concept experimental model of human anxiety. J Psychopharmacol. 2011;25:1199–1206. doi: 10.1177/0269881111400650. [DOI] [PubMed] [Google Scholar]
- Bassett JL, Foote SL. Distribution of corticotropin-releasing factor-like immunoreactivity in squirrel monkey (Saimiri sciureus) amygdala. J Comp Neurol. 1992;323:91–102. doi: 10.1002/cne.903230108. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Frontiers in Neuroendocrinology. 2002;23:41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Reddy AP, Smith LJ. Nuclear factor kappa B in the dorsal raphe of macaques: an anatomical link for steroids, cytokines and serotonin. J Psychiatry Neurosci. 2006;31:105–114. doi: 10.1016/j.yfrne.2006.03.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Centeno ML. Ovarian steroid treatment decreases corticotropin-releasing hormone (CRH) mRNA and protein in the hypothalamic paraventricular nucleus of ovariectomized monkeys. Neuropsychopharmacology. 2008;33:546–556. doi: 10.1038/sj.npp.1301442. [DOI] [PubMed] [Google Scholar]
- Bethea CL, Reddy AP, Tokuyama Y, Henderson JA, Lima FB. Protective actions of ovarian hormones in the serotonin system of macaques. Front Neuroendocrinol. 2009;30:212–238. doi: 10.1016/j.yfrne.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Reddy AP. Effect of ovarian hormones on genes promoting dendritic spines in laser-captured serotonin neurons from macaques. Mol Psychiatry. 2010;15:1034–1044. doi: 10.1038/mp.2009.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Reddy AP. Ovarian steroids increase glutamatergic related gene expression in serotonin neurons of macaques. Mol Cell Neuroscience. 2011;49:251–262. doi: 10.1016/j.mcn.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethea CL, Smith AW, Centeno ML, Reddy AP. Long-term ovariectomy decreases serotonin neuron number and gene expression in free ranging macaques. Neuroscience. 2012;49:251–262. doi: 10.1016/j.neuroscience.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld Z. Chemotherapy and fertility. Best Pract Res Clin Obstet Gynaecol. 2012;26:379–390. doi: 10.1016/j.bpobgyn.2011.11.008. [DOI] [PubMed] [Google Scholar]
- Bonkale WL, Turecki G, Austin MC. Increased tryptophan hydroxylase immunoreactivity in the dorsal raphe nucleus of alcohol-dependent, depressed suicide subjects is restricted to the dorsal subnucleus. Synapse. 2006;60:81–85. doi: 10.1002/syn.20278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerillo G, Rees A, Manchanda N, Reilly C, Brogan I, White A, Needham M. The oestrogen receptor regulates NFkappaB and AP-1 activity in a cell-specific manner. J Steroid Biochem Mol Biol. 1998;67:79–88. doi: 10.1016/s0960-0760(98)00078-8. [DOI] [PubMed] [Google Scholar]
- Cipriani A, Purgato M, Furukawa TA, Trespidi C, Imperadore G, Signoretti A, Churchill R, Watanabe N, Barbui C. Citalopram versus other anti-depressive agents for depression. Cochrane Database Syst Rev. 2012;7:CD006534. doi: 10.1002/14651858.CD006534.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MS, Kaiyala KJ. Role of corticotropin-releasing factor family peptides and receptors in stress-related psychiatric disorders. Semin Clin Neuropsychiatry. 2003;8:119–136. doi: 10.1053/scnp.2003.50011. [DOI] [PubMed] [Google Scholar]
- Coleman K, Robertson ND, Bethea CL. Long-term ovariectomy alters social and anxious behaviors in semi-free ranging Japanese macaques. Behav Brain Res. 2011;225:317–327. doi: 10.1016/j.bbr.2011.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde DM, Pinto-Neto AM, Santos-Sa D, Costa-Paiva L, Martinez EZ. Factors associated with quality of life in a cohort of postmenopausal women. Gynecol Endocrinol. 2006;22:441–446. doi: 10.1080/09513590600890306. [DOI] [PubMed] [Google Scholar]
- Dayas CV, Xu Y, Buller KM, Day TA. Effects of chronic oestrogen replacement on stress- induced activation of hypothalamic-pituitary-adrenal axis control pathways. J Neuroendocrinol. 2000;12:784–794. doi: 10.1046/j.1365-2826.2000.00527.x. [DOI] [PubMed] [Google Scholar]
- de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- Denihan A, Kirby M, Bruce I, Cunningham C, Coakley D, Lawlor BA. Three-year prognosis of depression in the community-dwelling elderly. Brit J Psychiatry. 2000;176:453–457. doi: 10.1192/bjp.176.5.453. [DOI] [PubMed] [Google Scholar]
- Eaton GG. Aggression in adult male primates; a comparison of confined Japanese macaques and free-ranging olive baboons. Internat J Primatol. 1984;5:145–160. [Google Scholar]
- Gartlehner G, Hansen RA, Morgan LC, Thaler K, Lux LJ, Van Noord M, Mager U, Gaynes BN, Thieda P, Strobelberger M, Lloyd S, Reichenpfader U, Lohr KN. Second- Generation Antidepressants in the Pharmacologic Treatment of Adult Depression: An Update of the 2007 Comparative Effectiveness Review. 2011 [PubMed] [Google Scholar]
- Goswami D, Conway GS. Premature ovarian failure. Hum Reprod Update. 2005;11:391–410. doi: 10.1093/humupd/dmi012. [DOI] [PubMed] [Google Scholar]
- Heikkinen J, Vaheri R, Timonen U. A 10-year follow-up of postmenopausal women on long- term continuous combined hormone replacement therapy: Update of safety and quality-of-life findings. J Br Menopause Soc. 2006;12:115–125. doi: 10.1258/136218006778234093. [DOI] [PubMed] [Google Scholar]
- Himmerich H, Binder EB, Kunzel HE, Schuld A, Lucae S, Uhr M, Pollmacher T, Holsboer F, Ising M. Successful antidepressant therapy restores the disturbed interplay between TNF- alpha system and HPA axis. Biol Psychiatry. 2006;60:882–888. doi: 10.1016/j.biopsych.2006.03.075. [DOI] [PubMed] [Google Scholar]
- Holsboer F. The rationale for corticotropin-releasing hormone receptor (CRH-R) antagonists to treat depression and anxiety. J Psychiatr Res. 1999;33:181–214. doi: 10.1016/s0022-3956(98)90056-5. [DOI] [PubMed] [Google Scholar]
- Keck ME, Holsboer F. Hyperactivity of CRH neuronal circuits as a target for therapeutic interventions in affective disorders. Peptides. 2001;22:835–844. doi: 10.1016/s0196-9781(01)00398-9. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Schmidt-Reinwald AK, Hellhammer DH, Kirschbaum C. Psychological and endocrine responses to psychosocial stress and dexamethasone/corticotropin-releasing hormone in healthy postmenopausal women and young controls: the impact of age and a two-week estradiol treatment. Neuroendocrinology. 1999;70:422–430. doi: 10.1159/000054504. [DOI] [PubMed] [Google Scholar]
- Li XF, Mitchell JC, Wood S, Coen CW, Lightman SL, O'Byrne KT. The effect of oestradiol and progesterone on hypoglycaemic stress-induced suppression of pulsatile luteinizing hormone release and on corticotropin-releasing hormone mRNA expression in the rat. J Neuroendocrinol. 2003;15:468–476. doi: 10.1046/j.1365-2826.2003.01014.x. [DOI] [PubMed] [Google Scholar]
- Linthorst AC. Interactions between corticotropin-releasing hormone and serotonin: implications for the aetiology and treatment of anxiety disorders. Handb Exp Pharmacol. 2005:181–204. doi: 10.1007/3-540-28082-0_7. [DOI] [PubMed] [Google Scholar]
- Lloyd T, Martel JK, Rollings N, Andon MB, Kulin H, Demers LM, Eggli DF, Kieselhorst K, Chinchilli VM. The effect of calcium supplementation and Tanner stage on bone density, content and area in teenage women. Osteoporos Int. 1996;6:276–283. doi: 10.1007/BF01623385. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Rodda JE, Lightman SL, Ingram CD. Corticotropin-releasing factor increases in vitro firing rates of serotonergic neurons in the rat dorsal raphe nucleus: evidence for activation of a topographically organized mesolimbocortical serotonergic system. J Neurosci. 2000;20:7728–7736. doi: 10.1523/JNEUROSCI.20-20-07728.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TD, Munson DJ, Haldy ME, Handa RJ. Androgen inhibits, while oestrogen enhances, restraint-induced activation of neuropeptide neurones in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2004;16:272–278. doi: 10.1111/j.0953-8194.2004.01167.x. [DOI] [PubMed] [Google Scholar]
- Maki PM, Freeman EW, Greendale GA, Henderson VW, Newhouse PA, Schmidt PJ, Scott NF, Shively CA, Soares CN. Summary of the National Institute on Aging-sponsored conference on depressive symptoms and cognitive complaints in the menopausal transition. Menopause. 17:815–822. doi: 10.1097/gme.0b013e3181d763d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JJ, Malone KM, Diehl DJ, Perel J, Cooper TB, Mintun MA. Demonstration in vivo of reduced serotonin responsivity in the brain of untreated depressed patients. Am J Psychiatry. 1996;153:174–182. doi: 10.1176/ajp.153.2.174. [DOI] [PubMed] [Google Scholar]
- Maruhashi T. An ecological study of troop fissions of Japanese monkeys (Macaca fuscata yaku) on Yakushima Island, Japan. Primates. 1982;23:317–337. [Google Scholar]
- May PJ, Reiner AJ, Ryabinin AE. Comparison of the distributions of urocortin-containing and cholinergic neurons in the perioculomotor midbrain of the cat and macaque. J Comp Neurol. 2008;507:1300–1316. doi: 10.1002/cne.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay LI, Cidlowski JA. Molecular control of immune/inflammatory responses: interactions between nuclear factor-KB and steroid receptor-signaling pathways. Endocrine Reviews. 1999;20:435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- Moncek F, Duncko R, Jezova D. Repeated citalopram treatment but not stress exposure attenuates hypothalamic-pituitary-adrenocortical axis response to acute citalopram injection. Life Sci. 2003;72:1353–1365. doi: 10.1016/s0024-3205(02)02409-8. [DOI] [PubMed] [Google Scholar]
- Morimoto A, Nakamori T, Morimoto K, Tan N, Murakami N. The central role of corticotropin-releasing factor (CRF-41) in psychological stress in rats. J Physiology. 1993;460:221–229. doi: 10.1113/jphysiol.1993.sp019468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeroff CC. Early-Life Adversity, CRF Dysregulation, and Vulnerability to Mood and Anxiety Disorders. Psychopharmacol Bull. 2004;38:14–20. [PubMed] [Google Scholar]
- Oki Y, Sasano H. Localization and physiological roles of urocortin. Peptides. 2004;25:1745–1749. doi: 10.1016/j.peptides.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, Verhulst FC, Ormel J. Mental health problems during puberty: Tanner stage- related differences in specific symptoms. The TRAILS study. J Adolesc. 2011;34:73–85. doi: 10.1016/j.adolescence.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Olsson IA, Westlund K. More than numbers matter: The effect of social factors on behaviour and welfare of laboratory rodents and non-human primates. Appl Animal Behaviour Sci. 2007;103:229–254. [Google Scholar]
- Pan W, Kastin AJ. Urocortin and the brain. Prog Neurobiol. 2008;84:148–156. doi: 10.1016/j.pneurobio.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel PD, Bochar DA, Turner DL, Meng F, Mueller HM, Pontrello CG. Regulation of tryptophan hydroxylase-2 gene expression by a bipartite RE-1 silencer of transcription/neuron restrictive silencing factor (REST/NRSF) binding motif. J Biol Chem. 2007;282:26717–26724. doi: 10.1074/jbc.M705120200. [DOI] [PubMed] [Google Scholar]
- Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, Bruce-Keller AJ. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J Neuroimmunol. 2010;219:25–32. doi: 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posey KA, Clegg DJ, Printz RL, Byun J, Morton GJ, Vivekanandan-Giri A, Pennathur S, Baskin DG, Heinecke JW, Woods SC, Schwartz MW, Niswender KD. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am J Physiol Endocrinol Metab. 2009;296:E1003–E1012. doi: 10.1152/ajpendo.90377.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raadsheer FC, Hoogendijk WJ, Stam FC, Tilders FJ, Swaab DF. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology. 1994;60:436–444. doi: 10.1159/000126778. [DOI] [PubMed] [Google Scholar]
- Refinement JWGo. Refinements in husbandry, care and common procedures for non-human primates. Ninth report of the BVAAWF/FRAME/RSPCA/UFAW Joint Working Group on Refinement. Laboratory Animals. 2009;43:1–47. doi: 10.1258/la.2008.007143. [DOI] [PubMed] [Google Scholar]
- Reul JM, Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol. 2002;2:23–33. doi: 10.1016/s1471-4892(01)00117-5. [DOI] [PubMed] [Google Scholar]
- Rivera HM, Bethea CL. Ovarian steroids increase spinogenetic proteins in the macaque dorsal raphe. Neuroscience. 2012;208:27–40. doi: 10.1016/j.neuroscience.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostal DC, Glick BB, Eaton GG, Resko JA. Seasonality of adult male Japanese macaques (Macaca fuscata): androgens and behavior in a confined troop. Horm Behav. 1986;20:452–462. doi: 10.1016/0018-506x(86)90007-3. [DOI] [PubMed] [Google Scholar]
- Ruggiero DA, Underwood MD, Rice PM, Mann JJ, Arango V. Corticotropic-releasing hormone and serotonin interact in the human brainstem: behavioral implications. Neuroscience. 1999;91:1343–1354. doi: 10.1016/s0306-4522(98)00703-9. [DOI] [PubMed] [Google Scholar]
- Sanchez RL, Reddy AP, Centeno ML, Henderson JA, Bethea CL. A second tryptophan hydroxylase isoform, TPH-2 mRNA, is increased by ovarian steroids in the raphe region of macaques. Brain Res Mol Brain Res. 2005;135:194–203. doi: 10.1016/j.molbrainres.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Sanchez RL, Reddy AP, Bethea CL. Ovarian steroid regulation of the midbrain corticotropin releasing factor and urocortin systems in macaques. Neuroscience. 2010;171:893–909. doi: 10.1016/j.neuroscience.2010.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapiro SJ, Nehete PN, Perlman JE, Sastry KJ. A comparison of cell-mediated immune responses in rhesus macaques housed singly, in pairs, or in groups. Appl Anim Behav Sci. 2000;68:67–84. doi: 10.1016/s0168-1591(00)00090-3. [DOI] [PubMed] [Google Scholar]
- Schapiro SJ. Effects of social manipulations and environmental enrichment on behavior and cell- mediated immune responses in rhesus macaques. Pharmacol Biochem Behav. 2002;73:271–278. doi: 10.1016/s0091-3057(02)00779-7. [DOI] [PubMed] [Google Scholar]
- Schapiro SJ, Bloomsmith MA, Suarez SA, Porter LM. Effects of social and inanimate enrichment on the behavior of yearling rhesus monkeys. Am J Primatol. 1996;40:247–260. doi: 10.1002/(SICI)1098-2345(1996)40:3<247::AID-AJP3>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Schule C, Baghai TC, Eser D, Zwanzger P, Jordan M, Buechs R, Rupprecht R. Time course of hypothalamic-pituitary-adrenocortical axis activity during treatment with reboxetine and mirtazapine in depressed patients. Psychopharmacology (Berl) 2006;186:601–611. doi: 10.1007/s00213-006-0382-7. [DOI] [PubMed] [Google Scholar]
- Seth KA, Majzoub JA. Repressor element silencing transcription factor/neuron-restrictive silencing factor (REST/NRSF) can act as an enhancer as well as a repressor of corticotropin- releasing hormone gene transcription. J Biol Chem. 2001;276:13917–13923. doi: 10.1074/jbc.M007745200. [DOI] [PubMed] [Google Scholar]
- Smith GW, Aubry JM, Dellu F, Contarino A, Bilezikjian LM, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee KF. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- Spina M, Merlo-Pich E, Chan RK, Basso AM, Rivier J, Vale W, Koob GF. Appetite- suppressing effects of urocortin, a CRF-related neuropeptide. Science. 1996;273:1561–1564. doi: 10.1126/science.273.5281.1561. [DOI] [PubMed] [Google Scholar]
- Stout SC, Owens MJ, Nemeroff CB. Regulation of corticotropin-releasing factor neuronal systems and hypothalamic-pituitary-adrenal axis activity by stress and chronic antidepressant treatment. J Pharmacol Exper Therapeutics. 2002;300:1085–1092. doi: 10.1124/jpet.300.3.1085. [DOI] [PubMed] [Google Scholar]
- Tangen T, Mykletun A. Depression and anxiety through the climacteric period: an epidemiological study (HUNT-II) J Psychosom Obstet Gynaecol. 2008;29:125–131. doi: 10.1080/01674820701733945. [DOI] [PubMed] [Google Scholar]
- Travers SH, Jeffers BW, Bloch CA, Hill JO, Eckel RH. Gender and Tanner stage differences in body composition and insulin sensitivity in early pubertal children. J Clin Endocrinol Metab. 1995;80:172–178. doi: 10.1210/jcem.80.1.7829608. [DOI] [PubMed] [Google Scholar]
- Valentino RJ, Lucki I, Van Bockstaele E. Corticotropin-releasing factor in the dorsal raphe nucleus: Linking stress coping and addiction. Brain Res. 2010;1314:29–37. doi: 10.1016/j.brainres.2009.09.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waselus M, Valentino RJ, Van Bockstaele EJ. Ultrastructural evidence for a role of gamma- aminobutyric acid in mediating the effects of corticotropin-releasing factor on the rat dorsal raphe serotonin system. J Comp Neurol. 2005;482:155–165. doi: 10.1002/cne.20360. [DOI] [PubMed] [Google Scholar]
- Weiss JM, Stout JC, Aaron MF, Quan N, Owens MJ, Butler PD, Nemeroff CB. Depression and anxiety: role of the locus coeruleus and corticotropin releasing factor. Brain Res Bull. 1994;35:561–572. doi: 10.1016/0361-9230(94)90170-8. [DOI] [PubMed] [Google Scholar]
- Weissheimer KV, Herod SM, Cameron JL, Bethea CL. Interactions of corticotropin-releasing factor, urocortin and citalopram in a primate model of stress-induced amenorhea. Neuroendocrinology. 2010;92:224–234. doi: 10.1159/000319257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson ME, Bounar S, Godfrey J, Michopoulos V, Higgins M, Sanchez M. Social and emotional predictors of the tempo of puberty in female rhesus monkeys. Psychoneuroendocrinology. 2012 doi: 10.1016/j.psyneuen.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia-Zhang L, Xiao E, Ferin M. A 5-day estradiol therapy, in amounts reproducing concentrations of the early-mid follicular phase, prevents the activation of the hypothalamo- pituitary-adrenal axis by interleukin-1 alpha in the ovariectomized rhesus monkey. J Neuroendocrinol. 1995;7:387–392. doi: 10.1111/j.1365-2826.1995.tb00773.x. [DOI] [PubMed] [Google Scholar]