Abstract
Previous studies have reported alterations in numbers or function of regulatory T cells (Tregs) in myasthenia gravis (MG) patients, but published results have been inconsistent, likely due to the isolation of heterogenous “Treg” populations. In this study, we used surface CD4, CD25high, and CD127low/− expression to isolate a relatively pure population of Tregs, and established that there was no alteration in the relative numbers of Tregs within the peripheral T cell pool in MG patients. In vitro proliferation assays, however, demonstrated that Treg-mediated suppression of responder T cells (Tresp) was impaired in MG patients and was associated with a reduced expression of FOXP3 in isolated Tregs. Suppression of both polyclonal and AChR-activated Tresp cells from MG patients could be restored using Tregs isolated from healthy controls, indicating that the defect in immune regulation in MG is primarily localized to isolated Treg cells, and revealing a potential novel therapeutic target.
Keywords: Myasthenia gravis, Regulatory T cells, Forkhead box protein P3 (FOXP3)
1. Introduction
Myasthenia gravis (MG) is an organ-specific autoimmune disease caused in most cases by highly specific autoantibodies directed against the acetylcholine receptor (AChR) on skeletal muscle [1]. Although anti-AChR antibodies directly contribute to the degradation of AChR at the neuromuscular junctions in MG, cognate interactions of autoreactive T cells with B cells are necessary for the synthesis of anti-AChR antibodies [2,3]. Immune tolerance to self antigens is initially achieved during thymic development by the clonal deletion of potentially autoreactive T cells. However, some of these pathogenic cells, including some with reactivity to skeletal muscle AChR, survive clonal deletion in normal individuals, and are kept in check by peripheral tolerance mechanisms, most notably by a specialized subset of CD4+ T cells called regulatory T cells (Tregs) [4,5]. The thymus gland is the primary source of Tregs which constitute approximately 5–10% of the peripheral CD4+ T cell population, and play a crucial role in the maintenance of immune homeostasis against self-antigens [6,7]. The lack or dysfunction of these cells contributes to the pathogenesis and development of a number of experimental autoimmune diseases [8,9]. Additionally, a quantitative or qualitative alteration in Tregs has also been noted in patients with MG [10–14], suggesting a role for Treg abnormalities in human MG pathogenesis.
Studies to date examining the relative frequencies and function of Tregs in the peripheral circulation of MG patients have reported conflicting results including reductions in Treg numbers [10–12], impaired regulatory function [13], or no defect [14]. Moreover, all of those studies have used a single step enrichment protocol in which high surface expression of CD25 identified Tregs. However, the level of expression of CD25 that identifies Tregs for isolation has been inconsistently defined in the literature, and CD25 is also expressed by recently activated T cells [15], resulting in the inclusion of T cells with effector or pro-inflammatory properties in isolated “Treg” cell populations. These issues may explain the discordant results reported in previous studies examining circulating Tregs in MG.
It has been recently demonstrated that low expression of the IL-7Rα chain (CD127) combined with high expression of CD25 enables better identification and isolation of pure Treg populations with high suppressive functionality [16,17]. Accordingly, we have used high expression of CD25 (CD25high) and negative or low expression of CD127 (CD127low/−) to more precisely characterize the regulatory properties of Tregs in MG patients. Contrary to previous reports, we have found no alteration in the relative numbers of Tregs (CD4+CD25highCD127low/− cells or FOXP3+ cells) within the peripheral CD4+ T cell pool. Instead, we demonstrated a reduced cellular expression of FOXP3 within Tregs (CD4+CD25highCD127low/− cells), associated with a significant defect in the ability of these cells to suppress polyclonal-activated and AChR-activated responder T (Tresp) cells in vitro. Notably, both polyclonal and AChR-activated Tresp cells from MG patients could be effectively suppressed using Tregs isolated from healthy controls, while polyclonal-activated Tresp cells from controls were not suppressed by Tregs isolated from MG patients, strongly suggesting a primary intrinsic defect in the function of the isolated Treg cells in MG.
2. Materials and methods
2.1 Subjects
Peripheral blood samples were obtained from a total of 24 patients (12 males, 12 females) with clinically definite autoimmune MG (mean age 46.6 years, range 22 to 77), as well as from 22 healthy controls (mean age 42.1 years, range 26 to 59). The clinical diagnosis of MG was confirmed in all patients by elevated serum levels of anti-AChR antibodies. Patients were recruited from the MG clinic at the University of Illinois Medical Center, and clinical information including age, sex, date of diagnosis, MGFA clinical classification [18], manual muscle testing scores (MMT) [19], thymic pathology, and anti-AChR antibody titers, was extracted from their medical records. The clinical characteristics of each patient are summarized in Table 1. The protocol was approved by the University of Illinois at Chicago Institutional Review Board and informed consent was obtained from all study subjects.
TABLE 1.
Clinical Profile of MG Patients
| Patient | Age/Sex | Max. MGFA Class | MMT score | Disease Duration (months) | Thymic histology | Anti-AChR (nmol/L) | Treatment |
|---|---|---|---|---|---|---|---|
| 1 | 63/F | 4 | 15 | 36 | ND | 66 | pyrido, pred, PEX |
| 2 | 31/M | 3 | 0 | 6 | Thymoma | 28 | pred, IVIg, PEX |
| 3 | 33/M | 3 | 7 | 36 | ND | 74 | pyrido, pred |
| 4 | 61/M | 3 | 0 | 72 | normal | 0.9 | pred |
| 5 | 39/M | 4 | 6 | 108 | thymoma | 69 | pyrido, pred |
| 6 | 63/M | 5 | 6 | 3 | ND | 50 | pred |
| 7 | 39/M | 3 | 0 | 6 | thymoma | 41 | pyrido, pred, MMF |
| 8 | 22/F | 3 | 6 | 6 | ND | 89 | pyrido |
| 9 | 77/M | 4 | 13 | 12 | ND | 7 | pyrido |
| 10 | 59/M | 4 | 5 | 12 | ND | 9 | pred |
| 11 | 60/M | 3 | 7 | 24 | ND | 1 | pyrido, pred |
| 12 | 57/F | 5 | 20 | 540 | hyperplasia | 178 | pyrido, pred, cyclo, IVIg |
| 13 | 31/F | 3 | 56 | 96 | ND | > 2000 | pyrido |
| 14 | 32/F | 3 | 2 | 132 | hyperplasia | 0.5 | pred, MMF |
| 15 | 60/M | 5 | 0 | 140 | normal | 7.45 | pred |
| 16 | 55/M | 5 | 2 | 216 | ND | 0.24 | pyrido |
| 17 | 64/F | 3 | 4 | 132 | ND | 1.13 | pred, MMF |
| 18 | 34/F | 3 | 6 | 71 | ND | 37 | pred,AZA |
| 19 | 72/M | 4 | 3 | 72 | ND | 14.71 | pred, AZA |
| 20 | 36/F | 2 | 7 | 4 | ND | 0.9 | pyrido,pred |
| 21 | 29/F | 4 | 10 | 174 | Normal | 2000 | pyrido,pred |
| 22 | 34/F | 2 | 2 | 60 | Encapsulated thymoma | 13.5 | pred, AZA |
| 23 | 37/F | 2 | 5 | 84 | ND | 5.6 | pyrido,pred,AZA |
| 24 | 30/F | 2 | 3 | 252 | hyperplasia | 25 | pyrido |
Max=maximum, F=female, M=male, pyrido=pyridostigmine, pred=prednisone, AZA = azathioprine, IVIg= intravenous immunoglobulin, PEX=plasma exchange, MMF=mycophenolate mofetil, cyclo=cyclosporine, ND=thymectomy not done
2.2 Collection of peripheral blood mononuclear cells (PBMCs)
Blood samples were drawn into heparinized tubes (BD Vacutainer), diluted 1:1 with sterile HBSS (Ca++/Mg++ free) at room temperature and centrifuged on Ficoll-Paque gradients (GE Healthcare bioscience) at 800×g for 30minutes. Mononucleated cells (PBMCs) from the interface were recovered, washed in HBSS, viability determined by trypan blue dye exclsuion and used for the experiments. PBMCs were cryopreserved for subsequent use.
2.3 Antibodies and cell culture reagents
Allophycocyanin (APC)-conjugated anti-human CD4, Phycoerythrin (PE)-conjugated anti-human CD25, Phycoerythrin-Cy-7 (PE-Cy7)-conjugated anti-human CD127, Alexa Fluor 488 (AF488)-conjugated anti-human FoxP3, eFluor 450 conjugated anti-human CD31, and CD45RO, Biotin-conjugated anti-human CTLA4, APCeF780 conjugated anti-human HLA-DR, FITC-conjugated anti-human CD45RA, Pacific Blue (PB)-conjugated anti-human Helios, Streptavidin APCeF780 and respective isotype controls were purchased from eBioscience, CA, USA. RPMI 1640 media supplemented with 1% sodium pyruvate, 1% non-essential amino acids, 2mM L-glutamine, 20mM HEPES, 50 U/ml penicillin and 50 μg/ml streptomycin (all from GIBCO, CA, USA), 50 μM 2-ME, 10% heat inactivated human AB serum (Invitrogen, CA, USA) were used as culture medium. Anti-human CD3 (clone OKT3) and carboxyfluorescein succinimidyl ester (CFSE) were purchased from eBioscience and Invitrogen, respectively. Two different synthetic peptides representing two amino acid sequences: 1) aa195-212: DTPYLDITYHFVMQRLPL and 2) aa257-269: LLVIVELIPSTSS, of the human α-subunit of the nicotinic acetylcholine receptor (AChR) were synthesized and HPLC purified (96%) by the Protein Research Laboratory, University of Illinois at Chicago. Peptides were dissolved in DMSO at a concentration of 10mg/ml, aliquoted and stored at −80°C. The peptide sequences were selected based on previous studies [20,21].
2.4 Sorting /isolation of T cell subsets
PBMCs (1×108) were washed once and re-suspended in 500 μl flow cytometry buffer (PBS + 0.5% BSA + 2 mM EDTA) in a 5ml polystyrene round bottom tube. After addition of 5μl/107 cell volume APC-CD4, PE-CD25, and PECy7-CD127 antibodies, the cell suspension was mixed gently and incubated at 4°C for 30 min. Cells were then washed once in cold flow cytometry buffer. Labeled CD4+ T cells were sorted using a MoFlo (Becton Dickinson). The gates for the sorted T cell subsets were set to include only those events exhibiting the CD4-specific fluorescence. Based on the CD4 gate, cells were further sorted based on CD127 and/or CD25 expression [CD4+CD25highCD127low/− regulatory T cells (Treg cells) and CD4+CD25 responder T cells (Tresp)] (Fig.1). Cells were collected into 100% human AB serum and washed once with media (RPMI 1640 medium with 5% human AB serum) until they were ready to be plated in suppression assay. Sorted Treg and Tresp cells were > 90% pure. Isotype controls were used to determine the gating parameters.
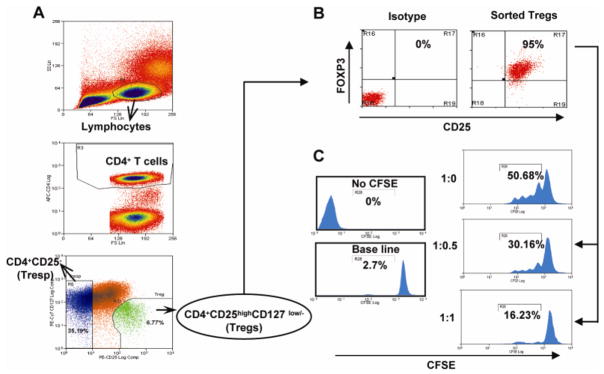
Figure 1.
Purification and in vitro suppressive function of CD4+CD25highCD127low/−FoxP3+ Treg. (A) Fluorescence-activated cell sorter (FACS) gating strategy used to isolate Treg and Tresp in PBMCs of healthy control. PBMCs were stained using CD4-APC, CD25-PE, and CD127-PECy7 antibodies and sorted by flow cytometry. First, a primary gate was set on live lymphocytes according to their forward/sideward scatter properties, and a secondary gate was set on the CD4+ population. Within the CD4+ T-cell population, Tregs were identified as CD4+CD25highCD127low/−, whereas Tresp were isolated as CD4+CD25− cells. (B) To verify the purity of sorted Tregs, the cells were fixed-permeabilized, stained with anti-Foxp3, and analyzed by flow cytometry. Scatter plots revealed that sorted Treg were > 90% pure. (C) To examine sorted Treg suppressive function in vitro, varying ratios of Tresp : Treg (1:0, 1:0.5, 1:1) were co-cultured (CFSE-labeled Tresp cells) in the presence of irradiated APCs and stimulated with anti-CD3 antibodies. Representative CFSE dilution plots illustrate sorted Treg cells were highly suppressive in a dose-dependent fashion in functional suppressor assays.
2.5 Flow cytometry
FACS sorted 6×104 Treg cells (CD4+CD25highCD127low) from each patient/healthy control were re-suspended in 100 μl flowcytometry buffer in a 5ml round bottom polystyrene tube. For these studies, we gated the top 1% of CD4+CD25highCD127low/− cells to ensure a pure Treg population. After addition of 5μl anti-human CD45RA, CD45RO, CD31, CTLA4, and HLA-DR the cell suspension was mixed gently and incubated at 4°C for 30 min. Cells were washed, pelleted and resuspended in 100 μl buffer.
Intracellular staining to determine FOXP3 and Helios expression was performed as per the manufacturer’s recommendations (eBioscience, CA, USA). Briefly, 6×104 sorted Treg cells (CD4+CD25highCD127low/−) per sample were washed once with flow cytometry buffer and fixed for 60 minutes. After washing with flow cytometry buffer, cell pellet was re-suspended in 100μl flow cytometry buffer. 10 μl AF488-conjugated anti-human FOXP3 and PB-conjugated anti-human Helios was added and cells were lightly vortexed and incubated at room temperature for 30 min. After incubation, flow cytometry buffer was added to each sample and cells were pelleted, resuspended in 200 μl buffer, and analyzed on a flow cytometer (CyAn ADP, DakoCytomation). Isotype controls were used to determine the gating parameters.
2.6 Reverse Transcription-Polymerase Chain Reaction
Total RNA was isolated from FACS-sorted CD4+CD25highCD127low/− Tregs cells using RNase Micro kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. The purity of RNA obtained was >1.75. For the synthesis of cDNAs, a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems) was used according to manufacturer’s instructions. Briefly, 2 μg of total RNA was reverse transcribed using MultiScribe™ Reverse Transcriptase (50 U/μl) in the presence of 2 μl Random primers, 0.8 μl 100 mM dNTP Mix, 1 μl of RNase Inhibitor and 10xRT Buffer in a final volume of 20 μl. The reaction was carried out in an iCycler (Biorad, Germany) thermocycler at 25°C 10 min, 37°C 120 min, 85°C 5 min. The following specific oligonucleotide primers were used in the multiplex PCR: i) human FOXP3 (PubMed Nucleotide Accession No. NM_001114377.1) sense primer is 5′-CAG CAC ATT CCC AGA GTT CCT C-3′, and the antisense primer is 5′-GCG TGT GAA CCA GTG GTA GAT C-3′. The predicted size of the amplified fragment by multiplex PCR is 153 base pairs (bp). ii) human β-actin (PubMed Nucleotide Accession No. NM_001101.3) sense primer is 5′-AGT CCT GTG GCA TCC ACG AAA CTA -3′, and the antisense primer is 5′-ACT CCT GCT TGC TGA TCC ACA TCT -3′. The predicted size of the amplified fragment by multiplex PCR is 276 bp. Reaction was performed triplicate in 50 μL using 200 nM of specific primers, 1μl cDNA, and 2x QIAGEN Multiplex PCR Master Mix (Qiagen, Valencia, CA) according to the manufacturer’s instructions. The thermal cycling protocol was as follows: initial PCR activation at 95 °C for 15 min, denaturation for 30 sec at 94 °C, annealing for 1.5 min at 58 °C, extension for 1.5 min at 72 °C. Thirty-five cycles were performed and final extension at 72 °C for 10 min. Finally, the reaction mixture containing PCR products were separated by 2% agarose gel electrophoresis along with 100 bp marker DNA. Gels were densitometrically (Bio-Rad) scanned and the cDNAs were normalized to that of the house keeping gene or internal control (β-actin), which was co-amplified along with the cDNA of interest.
2.7. CFSE-based polyclonal and AChR-stimulated suppression assays
Tresp cells were re-suspended in PBS (0.1% BSA) at 2×106 cells/ml and incubated with CFSE (2 μM final concentration) for 10 min at 37°C. Cells were washed and re-suspended in culture medium for 15 min. After a final wash step, cells were re-suspended in culture medium at the indicated cell concentrations. Suppression assays were performed as per the method of Venken et. al.[22]. Briefly, CFSE labeled Tresp cells were cultured in duplicates in 96-well round bottom plates (Falcon, NJ, USA) at 2×104 cells per well in 200 μl medium with 1×105 autologous antigen presenting cells (APCs) in the absence or presence equal numbers of Tregs (Tresp-Treg ratio 1:0 or 1:1). In parallel experiments, CFSE labeled Tresp cells were co-cultured with equal numbers of unlabeled Tresp cells to determine if the presence of unlabeled Tresp cells resulted in an apparent “suppression” of CFSE-labeled Tresps. The ratio of 1:1 was selected based on Treg suppressive capacity on Tresp cell proliferation using different ratios of Tresp-Treg in normal controls (Fig. 1C). APCs were separated from PBMCs by 1-h plastic adherence (to deplete T cells), and the adherent fraction was irradiated (2000 rads) immediately prior to use in the co-culture. Cell cultures were stimulated with 2 μg/ml anti-CD3 (non-antigen specific proliferation) or 5μg/ml AChR peptides (antigen specific proliferation). The AChR peptide concentration was selected based on an initial study of AChR peptide-stimulated Tresp cell proliferation using different concentrations (1, 3, 5, 10, 20 and 40 μg/mL) of peptides. Co-cultures of purified T cell populations and irradiated APCs, but in the absence of AChR peptide or anti-CD3, was used to assess background proliferation for each cell type/experiment. After 4 days (for anti CD3 stimulation), 7 days (for AChR stimulation) cells were harvested, stained for CD4 and the proliferation of cells based upon the dilution of CFSE in CD4+ lymphocytes was analyzed by flow cytometry. The suppressive capacity of Tregs towards Tresp cells in co-culture was expressed as the difference between the percentage of proliferation (CFSElow) of CD4+ Tresp cells cultured in the absence of Tregs and the percent proliferation of Tresp/Treg co-cultures. Dead cells were excluded by forward and side scatter. Signal from unlabeled Tregs alone [(Tresp:Tregs (0:1)] and/or unlabeled Tresp co-cultured with CFSE labeled Tresp [(Tresp:Tresp (1:1)] were used to set regions to exclude background levels that would interfere with CFSE diluted cells. The cell culture supernatant was stored at -80°C and used to assay for cytokines. In separate experiments, polyclonal-activated CD4+CD25− Tresp cells from healthy controls were mixed with either autologous (control) or allogeneic (from MG patients) CD4+CD25highCD127low/−Treg cells at a 1:1 ratio in the presence of allogeneic APCs. Similarly, both polyclonal and AChR-activated CD4+CD25− Tresp cells from MG patients cultured with autologous, irradiated APCs were mixed with autologous (MG) or allogeneic (from healthy controls) CD4+CD25highCD127low/−Treg cells. Suppression of Tresp proliferation by Tregs was assessed as described above.
2.8. Multi-plex analysis for cytokine production
IL-6, IL-10, IL-17 and IFN-γ were analyzed from culture supernatants using a Millipore custom made 4-plex kit (MILLIPLEX™ MAP, Millipore Corporation, USA). TGF-β1 was measured from acidified culture supernatants using TGFβ1 single-plex kit (MILLIPLEX™ MAP, Millipore Corporation, USA). Cytokine analysis was performed according to the manufacturer’s instructions. Briefly, following pre-wetting of the filter plates, 25μl of samples/ standards (in duplicate) were added to each well. 25μl of bead suspension was then added, the plates sealed and incubated with agitation on a plate shaker overnight at 4°C. The plates were washed twice and 25μl of detection antibody was then added; the plate was incubated for 60 min at room temperature with constant agitation. After incubation, 25μl of streptavidin-PE was added to each well and incubated with agitation on a plate shaker for 30 min at room temperature. The plate was again washed and cells were re-suspended in 150μl of sheath fluid and immediately read on a Bio-plex analyzer. For each analyte, a minimum of 50 bead events was collected. Standard curves were generated from lyophilized standard provided with each kit. The concentration for each analyte in cell supernatants was determined by interpolation from their corresponding standard curve. The sensitivity of four-plex (IL-6, IL-10, IL-17 and IFN-γ) assay was 3.2 pg/ml and single-plex TGFβ1 assay was 9.8 pg/ml.
2.9. Statistical Analysis
The data were subjected Student’s t-test to assess statistical significance between control subjects and MG patients, and to evaluate differences in percent proliferation of T cell co-cultures, with or without Tregs. Non-parametric measures of statistical dependence between two variables were also ascertained using Spearman’s rank correlation test for assessing the correlation between Treg suppressive ability vs. MMT using a computer based software package [Statistical Package for Social Sciences (SPSS 7.5) and GraphPad Prism 5]. Data are expressed as mean ± SEM. For all tests, P values of less than 0.05 were considered significant.
3. Results
3.1. Isolation of CD4+CD25highCD127low/− Tregs and in vitro suppressive assays
PBMCs were stained with fluorescent-labeled antibodies against CD4, CD25, and CD127 and analyzed by flow cytometry. Within the CD4+ T cell population, a small subset of cells with a high expression of CD25 and a low expression of CD127 could be visualized (Fig. 1A) comprising 3–7% of the total CD4+ T cell population. Phenotypic analysis of these CD4+CD25highCD127low/− cells revealed that they were largely comprised (> 90 %) of FOXP3-expressing cells, confirming their regulatory phenotype (Fig. 1B). We then used these CD4+CD25highCD127low/− cells in T cell proliferation/suppression assays as described in the Materials and methods section. The isolated Treg cells were tested for their ability to suppress the proliferation of Tresp in response to anti-CD3 activation in the presence of irradiated APCs over 4 days. Cellular proliferation was determined by CFSE dilution using flow cytometry for CFSE labeled Tresp cells [without Tregs], and after the addition of Tregs or unlabeled CD4+ CD25− T cells. No significant alteration in T cell proliferation was observed when CFSE-labeled Tresp cells were co-cultured with unlabeled CD4+CD25− T cells. As expected, we found that Tregs were able to significantly suppress the Tresp proliferation in a dose dependent manner. These assays confirmed the potent suppressive capability of these cells, with maximum suppression occurring at a Tresp:Treg ratio of 1:1 (Fig. 1C).
3.2. Treg-mediated suppression of polyclonal and AChR-activated T responder cells is impaired in individuals with MG
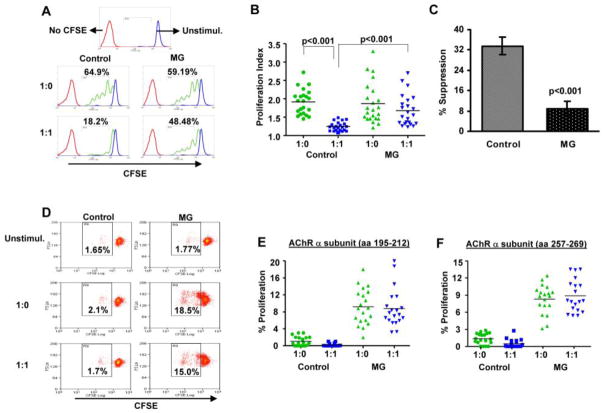
We sorted cells from the peripheral blood of MG patients (n = 23) and healthy controls (n = 22) and compared their suppressive properties using a suppression/proliferation assay (see Materials and Methods) based on the capacity of CD4+CD25highCD127low/− cells to inhibit proliferation of autologous Tresp cells (CD4+CD25−). Based on our findings in healthy controls (above), we used a Tresp/Treg ratio of 1:1 for these studies. Fig. 2A shows representative plots from an MG patient and a healthy control, demonstrating a robust Treg suppressive effect in the healthy control sample and an attenuation of this suppressive effect in the sample from the MG patient. In general, the mean proliferation index at baseline (before the addition of Tregs) was slightly lower (not statistically significant) in MG patients compared to controls (1.91 ± 0.07 vs. 1.872 ± 0.011). The mean proliferation index in the presence of Tregs was 1.24 ± 0.02 in healthy controls, compared to 1.67± 0.08 in MG patients (Fig. 2B); proliferation of Tresp cells was inhibited by a mean of 33.54% ± 2.32 in healthy controls compared to 9 % ± 2 in MG patients (Fig. 2C).
Figure 2.
MG Tregs fail to suppress polyclonal and antigen-specific T cell proliferation. CFSE-labeled Tresp cells were co-cultured with/without equal numbers of sorted CD4+CD25highCD127low/− cells, and stimulated with anti-CD3 antibodies or AChR-α peptides in the presence of irradiated APCs as described in Materials and methods. (A) Representative flow cytometry plots illustrate CFSE dilution profiles of anti-CD3 driven T cell proliferation in single healthy control and MG patient. Dot plot depiction of proliferation index (B) in T lymphocyte cultures (1:0 indicates Tresp cells cultured alone; 1:1 indicates Tresp/Treg co-cultured at 1:1 ratio), and percentage of Treg suppression (C) for MG patients (n =23) and healthy controls (n =22). (D) Flow cytometry dot plots illustrating CFSE dilution profiles for AChR-α subunit peptide [aa 195-212] stimulated T cell proliferation in a single healthy control and MG patient. (E) and (F) Percent proliferation in T lymphocyte cultures for stimulation with (E) AChR-α peptide [aa 195-212] (n = 20 MG patients and 20 healthy controls); and (F) AChR-α peptide [aa 257-269] (n = 19 MG patients and 19 controls) are shown.
We next synthesized two peptides representing sequences of the human AChR-α subunit (aa195-212 and aa257-259), in which at least one of the two peptides have been determined to induce proliferation of PBMCs in the majority of patients with MG [21]. To assess Treg-mediated suppression of AChR-stimulated T cell proliferation, CD4+CD25highCD127low/− cells were added to the T cell culture in the presence of AChR peptide. Fig. 2D shows plots from an MG patient and a healthy control, demonstrating a scant proliferation in response to the AChR-α peptide in the age-matched healthy control and a modest proliferative response with an apparent lack of suppressive effect of Treg cells in the sample from the MG patient. In MG patients, a low frequency of proliferating cells was detected, ranging from 2 to 18 % for p195-212 (n = 20) and from 3.14 to 12.40 % for p257-269 (n=19). No significant suppression of AChR-induced proliferation was seen after the addition of Tregs to the culture (Fig. 2E and 2F). In no case was the proliferative response to either AChR peptide greater than 3% of CFSE-labeled cells in any of the healthy controls (n = 19).
3.3. Relative Treg numbers are not altered in MG patients (compared to controls), but isolated Tregs have reduced cellular expression levels of FOXP3
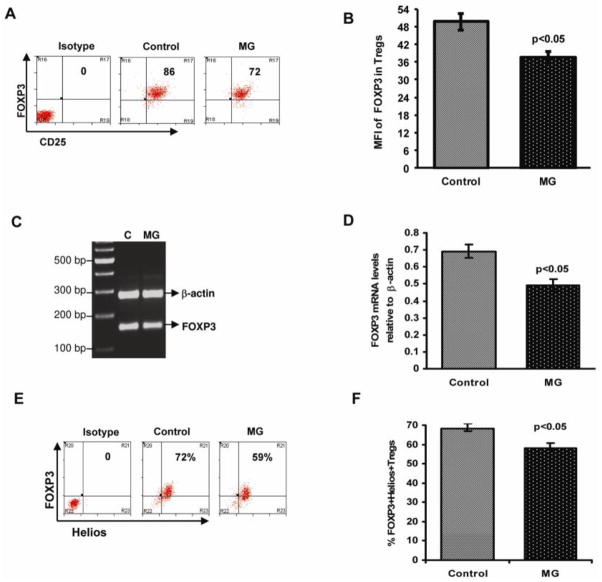
We found no deficiency in the relative circulating frequencies of FOXP3-expressing cells or isolated CD4+CD25highCD127low/− Tregs in MG patients compared to healthy controls, with a mean frequency of 4.957% ± 0.530 of the CD4+ T cells for MG patients and 4.66 ± 0.295 for healthy controls. Regulatory T cells are characterized by the expression of the forkhead lineage-specific transcription factor FOXP3, which endows these cells with their suppressive function[23]. Therefore, we analyzed intracellular FOXP3 expression within the population of isolated Treg cells (CD4+CD25highCD127low/−). Similar to the control subjects, > 90% of CD4+CD25highCD127lowcells from MG patients expressed FOXP3 protein. We next analyzed the level of cellular FOXP3 expression in the CD4+CD25highCD127low/− cells (Tregs), and found that mean cellular expression of FOXP3 (mean fluorescent intensity [MFI]) within isolated Tregs was significantly decreased (39.9± 2.9) for MG patients, compared to healthy controls (52.57± 3.57) (Figure 3A and 3B). We also determined messenger RNA (mRNA) expression levels of FOXP3 by RT-PCR, and found that a FOXP3 mRNA levels were also significantly decreased in CD4+CD25highCD127low/− Treg cells from MG patients (Figure 3C and 3D). The function of the Ikaros family transcription factor Helios in Tregs is relatively unknown. However, recent studies suggest that FOXP3+ Helios+ Tregs are functionally stable and do not produce inflammatory cytokines [24]. Therefore, we analyzed co-expression of Helios and FOXP3 in isolated Tregs (Fig.3E and F) from MG patients and healthy controls. A significantly reduced proportion of Tregs from MG patients were FOXP3+ and Helios+ [58.4 % ± 2.2] when compared to healthy controls [67.09 % ± 2.7].
Figure 3.
Attenuated FOXP3 expression in Treg cells from MG patients. Sorted CD4+CD25highCD127low/− Tregs were fixed and permeabilized, stained with anti-Foxp3/Helios and analyzed by flow cytometry. (A) Representative flow cytometry dot plot depicts the intensity of intracellular FOXP3 protein expression in Treg cells from an MG patient and an age-matched healthy control. (B) The bar diagram shows intensity of intracellular FOXP3 protein expression in Tregs in MG patients (n =24) and controls (n = 24). FOXP3 mRNA expression was determined by Multiplex PCR. Total RNA, extracted from sorted CD4+CD25highCD127low/− Treg cells from normal healthy controls (n=5) and MG patients (n=5), was reverse transcribed and a 158bp fragment corresponding to FOXP3 was amplified and separated on a 2% agarose gel. Gels were densitometrically scanned and cDNAs were normalized to that of β-actin, which was coamplified along with the cDNA of interest. (C) Representative gel from MG patient and control. (D) Bar diagram showing relative FOXP3 mRNA expression in isolated Tregs from MG patients compared to healthy controls. (E) Representative flow cytometry dot plot depicts Helios and FOXP3 protein co-expression in Treg cells from an MG patient and an age-matched healthy control. (F) Bar diagram showing intensity co-expression of Helios and FOXP3 protein in Tregs in MG patients (n =14) and controls (n = 14).
3.4. Tresp cells from MG patients are effectively suppressed by Tregs isolated from healthy control subjects
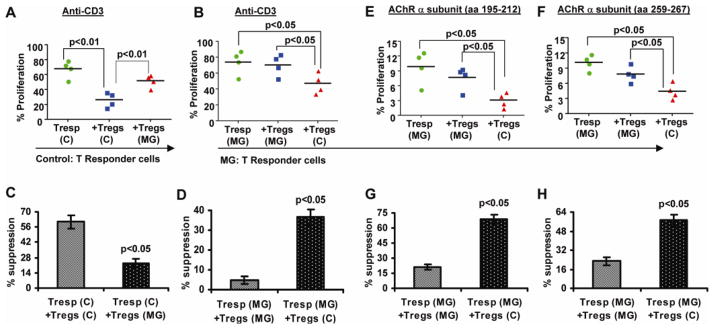
It is possible that a resistance of Tresp cells to suppression may contribute to the apparent functional impairment of Tregs observed in MG patients. To test this possibility, we conducted experiments in which polyclonal (anti-CD3) stimulated Tresp cells from MG patients were co-cultured with autologous (MG) Treg cells or with Treg cells isolated from a healthy donor, and vice versa. In all experiments, Tresp cells were co-cultured with autologous APCs. Tresp cells from healthy controls were effectively inhibited by autologous Tregs (60.81 % suppression), but were not inhibited by Tregs from MG patients (22.9 %) (Fig. 4A and 4C). In the case of MG Tresp cell proliferation, normal Tregs from healthy controls inhibited the proliferation of MG Tresp cells (mean 36.58% suppression) more potently, compared to autologus MG Tregs which afforded minimal suppression (4.66%]) (Fig. 4B and 4D). In separate experiments, Treg cells from healthy controls exerted a potent inhibition of AChR-α-stimulated Tresp (from MG patients) cellular proliferation compared to autologous (MG) Tregs, for both stimulation with AChR-α aa 195-212 (Fig 4E and 4G) and AChR-α aa 259-267 (Fig. 4F and 4H). In sum, both polyclonal and AChR-activated MG Tresp cells could be more effectively suppressed by Tregs from healthy subjects, while Tregs from MG patients failed to suppress autologous (MG) Tresp cells as well as control Tresp cells. These studies indicate that the observed defect in suppressive function of Tregs in MG is primarily a Treg-intrinsic defect rather than a resistance of MG Tresp cells to suppression
Figure 4.
Defect in suppression of T cell proliferation in MG is primarily intrinsic to isolated Tregs. Experiments in which T cell subsets from MG patients and healthy controls were “cross-cultured” as described in “Materials and methods” are shown. Treg cells from MG patients fail to suppress anti-CD3 induced proliferation in Tresp cells from healthy controls (A and C), while Tregs from healthy controls effectively suppress Tresp from MG patients (B and D). Tregs from healthy controls suppress AChR-α peptide induced proliferation of Tresp from MG patients, whereas autologous MG Tregs mediate only minimal suppression (E,F,G and H).
3.5. Circulating Treg cells from MG patients contain reduced numbers of naïve and recent thymic emigrant (RTE) Tregs, and have an altered surface phenotype
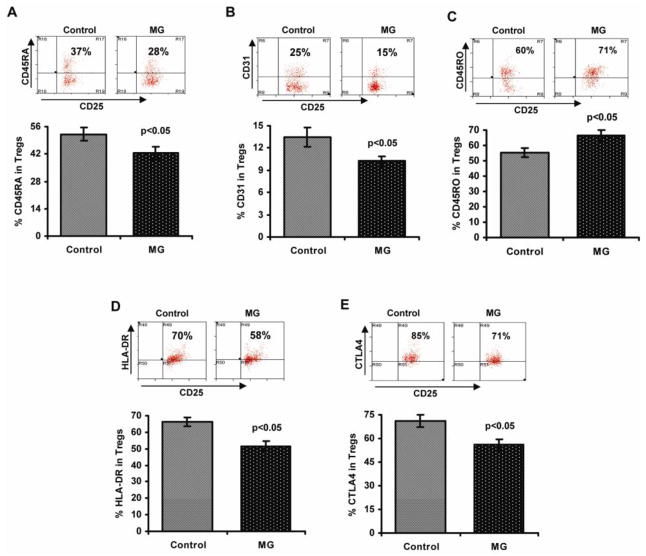
Most Tregs in the peripheral blood of healthy adults express CD45RO, identifying them as activated or memory cells [25]. These cells may derive from CD45RA+FOXP3+ naïve Tregs, but there is also evidence that a proportion are generated from highly differentiated memory CD4+ effector T cells (iTregs) [26,27]. CD45RO+ Tregs are highly suppressive, but they are short-lived and susceptible to apoptosis [28]. The proportion of CD45RO+ Tregs increases with age, while the relative numbers of CD45RA+ Tregs decreases [27]. An alteration in the homeostatic composition of the naïve vs memory Treg subsets may underlie some of the defects reported in Treg function in autoimmunity (including our findings in MG). Along these lines, we assessed the percentage of naïve (CD45RA+), memory (CD45RO+), and recent thymic emigrant (RTE) Treg cells (CD31+) within the isolated Treg populations from nine MG patients and nine age-matched healthy controls (Fig. 5), and observed a significant decrease in the percentage of CD45RA+ (Fig. 5A) and CD31+ (Fig. 5B) cells in Tregs isolated from MG patients; and a corresponding increase in the percentage of CD45RO+ cells (Fig. 5C).
Figure 5.
Phenotypic characterization of CD4+CD25highCD127low/− Tregs in MG patients vs healthy controls. Cell staining was performed as described in “Materials and methods.” Representative flow cytometry plots and bar diagrams showing the percentage of CD45RA+ (A), CD31+ (B), CD45RO+ (C) Treg cell subsets; percentage of cells expressing HLA-DR (D) and CTLA-4 (E) in Tregs isolated from MG patients (n=14) and control subjects (n=14).
HLA-DR, and CTLA-4 are surface markers that have been found to be important in the identification and functional characterization of CD4+ Tregs [29,30]. In particular, CTLA-4 is stably activated by FOXP3 and is critical for Treg suppressive function [30,31] Accordingly, the percentage of HLA-DR (Fig. 5D) and CTLA-4 (Fig. 5E) expressing Tregs was significantly decreased in MG patients compared to healthy controls.
3.6. Tregs from MG patients do not effectively suppress the secretion of pro-inflammatory cytokines and fail to up-regulate the production of IL-10
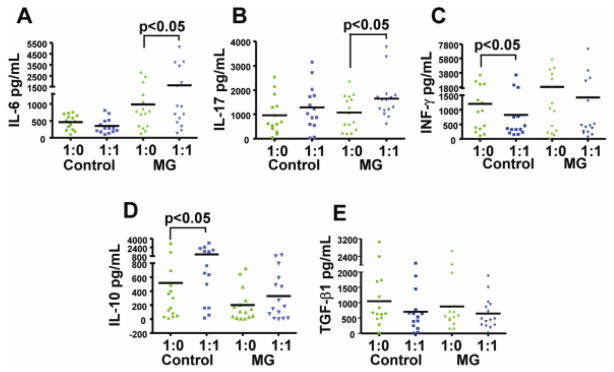
The cytokines IL-6, IL-17, IL-10, TGF-β, and IFN-γ were measured in the proliferation/suppression assays described above performed with anti-CD3 polyclonal activation in MG patients (n = 15) and healthy controls (n = 14). As shown in Figure 6, Tresp cells (cultured alone) from MG patients secreted significantly higher levels of IL-6 (Fig. 6A) and IFN-γ (Fig. 6C), and lower levels of IL-10 (Fig. 6D), consistent with an increased pro-inflammatory environment. In Tresp/Treg co-cultures, IL-6 (6A) and IL-17 (Fig. 6B) levels were observed to increase in MG patients. In contrast, no significant change in these cytokine levels was seen in controls compared to baseline assays (Tresp alone). In addition, a robust (expected) increase in IL-10 levels was seen in Tresp/Treg co-cultures compared to Tresp (cultured alone) cultures in the healthy controls. In MG patients, IL-10 levels did not change significantly with the addition of Tregs to the culture. Additionally, IFN-γ levels decreased significantly with the addition of Tregs in control subjects, but not MG patients. No significant difference was observed between patients and controls for secretion of TGF-β (Fig. 6E). Taken together, these results indicate that Tregs from MG patients did not effectively suppress the secretion of pro-inflammatory cytokines (IL-6, IL-17, and IFN-γ), and failed to up-regulate the production of IL-10.
Figure 6.
Defective Treg suppressive function in MG patients is associated with elevated levels pro-inflammatory cytokines and failure to up-regulate the production of IL-10. The cytokines IL-6 (A), IL-17 (B), INF-γ (C), IL-10 (D) and TGF-β (E) were measured in the supernatants of the proliferation assays from anti-CD3 stimulated cultures after 4 days of proliferation. Respective cytokine levels are plotted for Tresp (CD4+CD25−) cultures (1:0) and Tresp/Treg co-cultures (1:1) for MG patients (n = 15) and healthy controls (n = 14). Significant differences are indicated.
3.7. Clinical Correlations of FOXP3 expression and suppressive function of Tregs
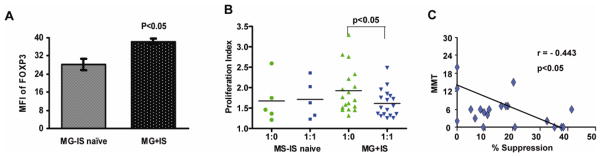
Most of the MG patients in our study were treated with prednisone or other immunosuppressive drugs, and it is conceivable that these drugs may have an effect on Treg function. Therefore, we examined Treg suppressive function and FOXP3 expression in subsets of patients on immunotherapy (n = 18) vs. patients who received no immunotherapy (n = 5). Firstly, there was no difference in the relative numbers of FOXP3-expressing cells or the numbers of CD4+CD25highCD127low/− cells within the CD4+ T cell pool in immunosuppressed vs. immunosuppression-naive patients (data not shown). Secondly, there was no significant reduction in the magnitude of Tresp cell proliferation (in the absence of Tregs) in the MG patients on immunotherapy (Fig. 7); rather there was a trend for higher levels of baseline proliferation, perhaps explained by enhanced disease severity in patients requiring immunosuppressive drugs. Importantly, isolated CD4+CD25highCD127low/− cells from immunosuppression-naïve MG patients demonstrated a significantly lower cellular expression of FOXP3 (Fig. 7A), and more effectively suppressed Tresp cell proliferation (Fig. 7B), compared to their immunosuppressed counterparts. While the numbers of patients not receiving immunosuppressive treatment is small, the data suggests that rather than impairing the function of Tregs, immunosuppressive therapy actually enhances Treg-mediated suppression and FOXP3 expression.
Figure 7.
Clinical Correlations of FOXP3 expression and suppressive function of Tregs. FOXP3 expression (MFI) in CD4+CD25highCD127low/− Tregs (A), and percentage suppression of Tresp proliferation by Tregs (B) was determined in 5 patients who were immunosuppression naïve (MG-IS naive) and 18 patients who were treated with immunosuppression (MG + IS). Immunosuppressive treatment was associated with increased FOXP3 expression and Treg suppression of Tresp cell proliferation. (C) An inverse correlation was found between manual muscle testing (MMT) scores and the percentage of Tresp suppression (n=20) with a Spearman’s correlation coefficient of r = −0.443 (p < 0.05).
Finally, we analyzed whether there was a correlation between clinical measurements and Treg function. We observed a significant (p< 0.05) inverse correlation between manual muscle testing scores (MMT) scores (higher score indicates more severe disease) and the suppressive capacity (% suppression) of Tregs (Fig. 7C). We observed no correlation between Treg suppressive function and serum anti-AChR antibody titers (data not shown).
4. Discussion
A prominent role for Tregs in the maintenance of immune tolerance has been emphasized recently, with evidence suggesting reduced numbers or function of these cells in autoimmune diseases [32–34], including MG [10–14]. Previous studies examining Tregs in human MG have reported conflicting results, but have only investigated the relative numbers and function of cells expressing the IL-2 receptor α-chain (IL-2Rα chain or CD25) or CD4+CD25high cells, in which the findings were likely obscured by the presence of contaminating non-Treg cells expressing CD25. Along these lines, it has recently been shown that activated/memory cells expressing high CD127 are also present within the CD4+CD25high T cell population, potentially confounding the classical functional assays for measuring CD4+CD25high Treg suppressive function [16,17]. Therefore, the present study utilized CD127 expression (CD127 low/−) to identify and eliminate these cells. To our knowledge, this is the only study to utilize this strategy to investigate immune regulatory function in MG patients, thereby isolating a purer population of Tregs (CD4+CD25highCD127low/−).
The number of Treg cells found in the peripheral blood of patients with autoimmune disease is influenced by Treg development, persistence, and proliferation in the periphery, which in turn are likely affected by disease status and the use of immunotherapy. Contrary to previous reports (10–12), we found that the relative numbers of Tregs (based on FOXP3 expression or CD4+CD25highCD127low/− phenotype) within the circulating CD4+ T cell population was not significantly different in MG patients compared to healthy controls. Furthermore, the frequency of circulating CD4+CD25highCD127low/− Tregs did not appear to differ in treated vs. untreated MG patients. Our results are in agreement with a more recent study showing no significant alterations in the numbers of Treg cells in the peripheral blood of patients with MG [35], and similar studies showing no difference in the frequency of CD4+CD25high cells in patients with multiple sclerosis [36] and type 1 diabetes mellitus [37].
In our studies, circulating CD4+CD25highCD127low/− (Tregs) cells from healthy controls effectively suppressed the proliferation of Tresp cells in a dose-dependent fashion (Fig. 1C). In contrast, Tregs from patients with MG showed an impaired ability to suppress the polyclonal proliferation of co-cultured autologous Tresp cells. We did not observe any significant enhancement of the intensity of anti-CD3-induced proliferation in MG patients compared to control subjects. We further investigated the source (Treg intrinsic?) of the suppressive defect by co-culturing Tregs from MG patients with allogeneic Tresp cells from control subjects, and Tregs from controls with Tresp from MG patients. We found that Tregs from MG patients were poor suppressors of Tresp proliferation whether co-cultured with autologous or normal control Tresp cells. In contrast, Tregs isolated from healthy controls were potent suppressors of both polyclonal and AChR-activated Tresp cells from MG patients, which were minimally suppressed by autologous MG Tregs. While control Tregs mediated a somewhat less potent suppression of MG Tresp cells (compared to their effects on autologous Tresp cells), the suppressive effect was significantly more robust compared to MG Tregs (Fig. 4). In sum, these experiments indicate that the observed impaired suppressive function of MG Tregs is predominantly due to an intrinsic Treg cell defect, although further studies are required to definitively determine the potential additional contribution of resistance of MG Tresp cells to suppression.
The efficiency of the Treg system is at least partially a consequence of Treg expression of a T cell receptor (TCR) repertoire that is biased toward self-recognition [39], so that the frequency of Treg precursors that target self-proteins is very high. These same attributes, however, may backfire when FOXP3 expression is attenuated in a subset of Tregs perhaps causing these cells to become autoreactive upon activation. Therefore, investigation of Treg suppression of autoantigen-specific T cell responses is of particular interest in the investigation of the role of Tregs in autoimmunity. We therefore specifically investigated whether Tregs from MG patients have a reduced ability to suppress AChR-reactive T cells. While the number of circulating AChR-specific T cells is obviously low, previous investigators have identified “myasthenogenic” AChR peptides that induce T cell proliferation in many patients with MG [20,21]. Using these peptides in Tresp:Treg co-culture experiments, we found consistent AChR-α-induced T cell proliferation in most MG patients (albeit to a limited extent), while observing no significant proliferation in our control samples. Significantly, CD4+CD25highCD127low/− Tregs showed virtually no activity in suppressing AChR-α-induced T cell proliferation in MG patients. The level of T cell proliferation induced by AChR-α peptide was low in MG patients, as might be expected due to the low frequency of circulating AChR-specific T cells. The absent or very limited proliferative response to AChR-α peptides in healthy control subjects precluded a relevant set of comparison data for these experiments. However, our findings in which Tregs from control subjects clearly suppress AChR-α-stimulated Tresp from MG patients (in which MG Tregs were not suppressive) provide a relevant ‘control’ for these studies, and suggest that Tregs from healthy controls effectively suppress even very low frequencies of antigen (AChR)-specific T cell proliferation. These results indicate that suppression of AChR-specific T cell responses is impaired in MG patients, and, as is the case for polyclonal activation, the defect appears to be primarily Treg intrinsic.
The transcription factor forkhead box protein P3 (Foxp3) is predominantly expressed in CD4+CD25+ Treg cells and is the master regulator for the development and function of these cells [23]. Mutations of the FOXP3 gene in humans results in an X-linked clinical syndrome characterized by immune dysregulation, polyendocrinopathy, and enteropathy (IPEX) [39]. It has been generally thought that FOXP3 expression serves as an “on-off” switch endowing T lymphocytes with suppressive ability. However, emerging evidence [40], including our own work showing an association between attenuated FOXP3 expression in Tregs and human autoimmune disease, suggests a paradigm in which alterations in its expression in lymphocytes may lead to loss of immune tolerance in a dose-dependent manner. Recent studies have shown that reduced or aberrant FOXP3 expression is correlated with Treg dysfunction [32,41]. We demonstrate herein that FOXP3 expression is significantly reduced in peripheral Tregs (CD4+CD25highCD127low/− cells) in MG patients at both the protein and mRNA levels. Interestingly, Balandina et. al.[42] have reported reduced FOXP3 expression in myasthenic thymocytes also associated with impaired function of thymic Treg cells. Genetic alterations in the FOXP3 gene as observed in IPEX patients are associated with reduced numbers of FOXP3+ Tregs or impaired Treg function, depending on the type of mutation[43]. It is not yet known if FOXP3 polymorphisms or alterations in genes that indirectly influence FOXP3 expression are associated with susceptibility to MG. However, it may be hypothesized that such genetic alterations could potentially affect Tregs in the periphery or Treg development in the thymus. An alteration in Treg generation in the thymus is suggested by our findings of a reduced percentage of circulating naïve and RTE Tregs, which is particularly intriguing given the high incidence of thymic pathology (hyperplasia and neoplasia) in MG. Future studies in which the expression levels of FOXP3 in Treg subsets (thymic derived vs. peripherally induced, naïve vs memory) are specifically analyzed and correlated with thymic pathology will help to more definitively localize the potential source of the defect in FOXP3 expression in MG patients. Two important features of FOXP3+ Tregs are that they constitutively express cytotoxic T lymphocyte antigen 4 (CTLA-4), which is only expressed after activation in other T cell subsets [44], and that FOXP3 controls the expression of CTLA-4 in Tregs [45,46]. In our study, a significantly lower proportion of isolated Tregs from MG patients expressed CTLA-4, presumably as a consequence of the attenuation of FOXP3 expression with resultant impaired suppressive function. We also found a reduced number of Tregs expressing HLA-DR in MG patients. MHC II expression on human Tregs has been reported to identify a distinct population of Tregs with high FOXP3 expression and with the capability to mediate strong contact-dependent suppression [29]. HLA-DR expressing Tregs have thus been hypothesized to be involved in homeostatic maintenance of Treg cells in vivo via presentation of self-antigens [29], so that a deficiency in this subset of Tregs may impact on tolerance to self-antigens like the AChR.
We further investigated the possible mechanisms of immune regulatory dysfunction in MG by examining cytokine profiles from cell-free culture supernatants obtained from our T cell proliferation/suppression assays described above. As expected, cultured Tresp cells from MG patients produced increased levels of IL-6 and IFN-γ, and reduced levels of IL-10 compared to controls. Interestingly, when Tregs were added to the cultures, IL-6 and IL-17 levels actually increased in MG patients (and IL-10 levels did not increase), confirming a functional inability to suppress the production of pro-inflammatory cytokines by MG Tregs. Not only is IL-6 a critical cytokine in the pathogenesis of MG (46), but it also directly inhibits Treg function [48]. Binding of IL-6 and IL-23 to their respective receptors leads to the activation of the STAT3 transcription factor, which in turn induces expression of RORγt, a key molecule in determining a Th17 fate [48]. It has recently been shown that human FOXP3+ Treg cells can differentiate into IL-17 producing cells upon TCR engagement in the appropriate cytokine milieu [48]. Therefore, it is possible that Treg cell plasticity (conversion to pathogenic Th17 cells) may underlie the functional disturbance in MG Tregs. Further investigation including examining cytokine profiles from isolated Tregs alone, additional cytokine assessments (IL-1β, IL-2, IL-21, IL-23), and assessing the expression of the orphan nuclear receptor RORγt in isolated Tregs may help clarify this issue.
Along these lines, it has been reported that Foxp3+Helios+ Tregs are functionally stable and do not produce inflammatory cytokines upon stimulation or expansion [24]. Our finding of a enhanced proportion of Foxp3+Helios− Tregs amongst the CD4+CD25highCD127low/− cells isolated from MG patients compared to controls points to a potential mechanism whereby enhanced plasticity of these cells results in their production of pro-inflammatory cytokines. This hypothesis remains to be definitively demonstrated, but is partially substantiated by our observation of an increase in IL-17 and IL-6 levels in T cell culture supernatants after the addition of MG Tregs, suggesting that the added Tregs either produced IL-6 and IL-17 themselves, or enhanced production of these cytokines by Tresp cells. Again, future experiments are planned in which cytokine production in isolated Treg cells alone will be assessed.
We found no difference in TGF-β levels in culture supernatants from the T cell proliferation experiments, suggesting that TGF-β was not involved in the observed Treg dysfunction in MG patients. Similarly, we have found that IL-10, and not TGF-β, is required for in vitro Treg suppression of AChR-stimulated T cell responses in experimental MG [50–52].
The majority of the patients in this study were treated with prednisone or other immunosuppressant drugs, and it is conceivable that these agents may affect Treg function. While our subset analyses were limited by small numbers of immunosuppression-naïve patients, we found that Treg function and FOXP3 expression were actually less impaired in patients treated with immunosuppressive drugs compared to patients who were immunosuppression-naïve. While this is in agreement with another report [53], definitive determination of the effects of immunomodulatory treatments on Treg numbers and function in MG will require longitudinal studies in larger numbers of patients. Nonetheless, our findings and previous reports argue that the demonstrated Treg alterations are not an artifact of immunosuppressive treatment. In addition, we found that Treg suppressive capacity was also correlated with disease severity as measured by manual muscle testing. These findings suggest that the observed defects are clinically relevant and that tracking Treg function and phenotype may become a useful measure of efficacy for some MG treatments, possibly providing a much-needed therapeutic biomarker.
5. Conclusion
Taken together, our studies indicate that impaired immune regulation is present in patients with MG, characterized by defects in suppressive function and expression of FOXP3 in CD4+CD25highCD127low/− cells, without a significant deficiency in circulating numbers of FOXP3-expressing Tregs. Treg-mediated suppression of polyclonal and autoantigen (AChR)-specific T cell responses is impaired in MG, associated with altered levels of IL-6, IL-17, IFN-γ, and IL-10. This demonstrated immune regulatory defect appears to correlate with disease severity and is notably less severe in patients treated with immunosuppressive drugs. These findings reveal immune regulation defects that likely contribute to the loss of immune tolerance to the AChR in MG. Our results suggest that this defect primarily resides within the isolated population of CD4+CD25highCD127low/− cells, and may potentially be due to instability of FOXP3 expression in these cells and conversion of Treg cells to Th1, Th2 or Th17 effectors, a hypothesis that will require further study. While it is not yet clear whether Treg dysfunction plays a direct causal role in MG, it may provide a general explanation for why tolerance against auto-antigens becomes imbalanced, leading to enhancement of an individual’s susceptibility to autoimmunity. A general dysfunction of immune regulation may also explain the co-occurrence of different autoimmune diseases and the simultaneous presence of distinct autoantibodies in MG patients [54]. Further studies are needed to further localize and define the nature of the Treg dysfunction in MG, with correlations to relevant clinical disease parameters, most notably, autoantibody profiles and thymic pathology.
Highlights.
CD4+CD25highCD127low Tregs from MG patients have an impaired function.
Isolated Tregs from MG patients express reduced levels of FOXP3.
CD4+CD25highCD127low Tregs from controls restore normal suppression of MG T cells.
The Treg defect is more prominent in immunosuppression-naïve MG patients.
Footnotes
This work was funded by the NIH (National Institute of Neurologic Disorders and Stroke, K08NS058800, MNM; and National Institute of Allergy and Infectious Diseases, RO1 AI 058190, BSP); and the Muscular Dystrophy Association (MDA 185924, MNM and MDA 157286, JRS); and by the University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS), Award Number UL1RR029879 from the National Center For Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health.
Conflict of Interest Statement
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Meriggioli MN, Sanders DB. Myasthenia gravis: emerging clinical and biological heterogeneity. Lancet Neurol. 2009;8:475–490. doi: 10.1016/S1474-4422(09)70063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Protti MP, Manfredi AA, Straub C, et al. Immunodominant regions for T helper-cell sensitization on the human nicotinic receptor alpha subunit in myasthenia gravis. Proc Natl Acad Sci USA. 1990;87:7792–7796. doi: 10.1073/pnas.87.19.7792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang ZY, Okita DK, Howard J, Jr, et al. T-cell recognition of muscle acetylcholine receptor subunits in generalized and ocular myasthenia gravis. Neurology. 1998;50:1045–1054. doi: 10.1212/wnl.50.4.1045. [DOI] [PubMed] [Google Scholar]
- 4.Bouneaud C, Kourilsky P, Buosso P. Impact of negative selection on the T cell repertoire reactive to a self peptide: a large fraction of T cell clones escapes clonal deletion. Immunity. 2000;13:829–840. doi: 10.1016/s1074-7613(00)00080-7. [DOI] [PubMed] [Google Scholar]
- 5.Jiang H, Chess L. Regulation of immune responses by T cells. N Engl J Med. 2006;354:1166–1176. doi: 10.1056/NEJMra055446. [DOI] [PubMed] [Google Scholar]
- 6.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu Rev Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 7.Jonuleit H, Schmitt E, Stassen M, et al. Identification and functional characterization of human CD4[+] CD25[+] T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–1294. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baecher-Allan C, Hafler DA. Human regulatory T cells and their role in autoimmune disease. Immunol Rev. 2006;212:203–216. doi: 10.1111/j.0105-2896.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 9.Torgerson TR. Regulatory T cells in human autoimmune diseases. Springer Semin Immunopathol. 2006;28:63–76. doi: 10.1007/s00281-006-0041-4. [DOI] [PubMed] [Google Scholar]
- 10.Fattorossi A, Battaglia A, Buzzonetti A, et al. Circulating and thymic CD4+ CD25+ T regulatory cells in myasthenia gravis: effect of immunosuppressive treatment. Immunology. 2005;116:134–141. doi: 10.1111/j.1365-2567.2005.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li X, Xiao BG, Xi JY, et al. Decrease of Foxp3+ regulatory T cells and elevation of CD19+ BAFF-R+ B cells and soluble ICAM-1 in myasthenia gravis. Clin Immunol. 2008;126:180–188. doi: 10.1016/j.clim.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 12.Masuda M, Matsumoto M, Tanaka S, et al. Clinical implication of peripheral CD4+ CD25+ regulatory T cells and Th17 cells in myasthenia gravis patients. J Neuroimmunol. 2010;225:123–131. doi: 10.1016/j.jneuroim.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Wang H, Chi L, et al. The role of FoxP3+CD4+CD25hi Tregs in the pathogenesis of myasthenia gravis. Immunol Lett. 2009;122:52–57. doi: 10.1016/j.imlet.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 14.Huang YM, Pirskanen R, Giscombe R, et al. Circulating CD4+CD25- and CD4+CD25+ Tcells in myasthenia gravis and in relation to thymectomy. Scand J Immunol. 2004;59:408–414. doi: 10.1111/j.0300-9475.2004.01410.x. [DOI] [PubMed] [Google Scholar]
- 15.Baecher-Allan C, Brown JA, Freeman GJ, et al. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 16.Hartigan-O’Connor DJ, Poon C, Sinclair E, et al. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor α chain [CD127], allowing consistent identification and sorting of live cells. J Immunol Methods. 2007;319:41–52. doi: 10.1016/j.jim.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin [IL]-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaretski A, 3rd, Barohn RJ, Ernstoff RM, et al. Myasthenia gravis: recommendations for clinical research standards. Task force of the Medical and Scientific Advisory Board of the Myasthenia Gravis Foundation of America. Neurology. 2000;55:16–23. doi: 10.1212/wnl.55.1.16. [DOI] [PubMed] [Google Scholar]
- 19.Sanders DB, Tucker-Lipscomb B, Massey JM. A simple manual muscle test for myasthenia gravis: validation and comparison with the QMG score. Ann N Y Acad Sci. 2003;998:440–444. doi: 10.1196/annals.1254.057. [DOI] [PubMed] [Google Scholar]
- 20.Harcourt GC, Sommer N, Rothbard J, et al. A juxta-membrane epitope on the human acetylcholine receptor recognized by T cells in myasthenia gravis. J Clin Invest. 1988;82:1295–1300. doi: 10.1172/JCI113729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brocke S, Brautbar C, Steinman L, et al. In vitro proliferative responses and antibody titers specific to human acetylcholine receptor synthetic peptides in patients with myasthenia gravis and relation to HLA class II genes. J Clin Invest. 1988;82:1894–1900. doi: 10.1172/JCI113807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venken K, Thewissen M, Hellings N, et al. A CFSE based assay for measuring CD4+ CD25+ regulatory T cell suppression of auto-antigen specific and polyclonal T cell responses. J Immunol Meth. 2007;322:1–11. doi: 10.1016/j.jim.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 23.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 24.Kim YC, Bhairavabhotla R, Yoon J, et al. Oligodeoxynucleotides stabilize Helios-expressing Foxp3+ human T regulatory cells during in vitro expansion. Blood. 2012;119:2810–2818. doi: 10.1182/blood-2011-09-377895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Booth NJ, McQuaid AJ, Sobande T, et al. Different proliferative potential and migratory characteristics of human CD4+ regulatory T cells that express either CD45RA or CD45RO. J Immunol. 2010;184:4317–4326. doi: 10.4049/jimmunol.0903781. [DOI] [PubMed] [Google Scholar]
- 26.Valmori D, Merlo A, Souleimanian NE, et al. A peripheral circulating compartment of natural naïve CD4 Tregs. J Clin Invest. 2005;115:1953–1962. doi: 10.1172/JCI23963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seddiki N, Santner-Nanan B, Tangye SG, et al. Persistence of naïve CD45RA+ regulatory T cells in adult life. Blood. 2006;107:2830–2838. doi: 10.1182/blood-2005-06-2403. [DOI] [PubMed] [Google Scholar]
- 28.Vukmanovic-Stejic M, Zhang Y, Cook JE, et al. Human CD4+ CD25hiFoxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J Clin Invest. 2006;116:2423–2433. doi: 10.1172/JCI28941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baecher-Allan C, Wolf E, Hafler DA. MHC class II expression identifies functionally distinct human regulatory T cells. J Immunol. 2006;176:4622–4631. doi: 10.4049/jimmunol.176.8.4622. [DOI] [PubMed] [Google Scholar]
- 30.Sansom DM, Walker LS. The role of CD28 and cytotoxic Tlymphocyte antigen-4 [CTLA-4] in regulatory T-cell biology. Immunol Rev. 2006;212:131–148. doi: 10.1111/j.0105-2896.2006.00419.x. [DOI] [PubMed] [Google Scholar]
- 31.Jian N, Nguyen H, Chambers C, et al. Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc Natl Acad Sci USA. 2010;107:1524–1528. doi: 10.1073/pnas.0910341107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moes N, Rieux-Laucat F, Begue B, et al. Reduced expression of FOXP3 and regulatory T-cell function in severe forms of early-onset autoimmune enteropathy. Gastroenterology. 2010;139:770–778. doi: 10.1053/j.gastro.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Valencia XC, Yarboro G, Illei P, et al. Deficient CD4+CD25high T regulatory cell function in patients with active systemic lupus erythematosus. J Immunol. 2007;178:2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 34.Venken K, Hellings N, Thewissen M, et al. Compromised CD4+CD25high regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology. 2008;123:79–89. doi: 10.1111/j.1365-2567.2007.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsui N, Nakane S, Saito F, et al. Undiminished regulatory T cells in the thymus of patients with myasthenia gravis. Neurology. 2010;74:816–820. doi: 10.1212/WNL.0b013e3181d31e47. [DOI] [PubMed] [Google Scholar]
- 36.Michel L, Berthelot L, Pettré S, et al. Patients with relapsing-remitting multiple sclerosis have normal Treg function when cells expressing IL-7 receptor alpha-chain are excluded from the analysis. J Clin Invest. 2008;118:3411–3419. doi: 10.1172/JCI35365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brusko T, Wasserfall C, McGrail K, et al. No alterations in the frequency of FOXP3+ regulatory T-cells in type 1 diabetes. Diabetes. 2007;56:604–612. doi: 10.2337/db06-1248. [DOI] [PubMed] [Google Scholar]
- 38.Killebrew JR, Perdue N, Kwan A, et al. A self-reactive TCR drives the development of Foxp3+ regulatory T cells that prevent autoimmune disease. J Immunol. 2011;187:861–69. doi: 10.4049/jimmunol.1004009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gambineri E, Torgerson TR, Ochs HD. Immune dysregulation, polyendocrinopathy, enteropathy, and X-linked inheritance, a syndrome of systemic autoimmunity caused by mutations of FOXP3, a critical regulator of T cell homeostasis. Curr Opin Rheumatol. 2003;15:430–435. doi: 10.1097/00002281-200307000-00010. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Zhao Y. The regulation of Foxp3 expression in regulatory CD4[+]CD25[+]T cells: multiple pathways on the road. J Cell Physiol. 2007;211:590–597. doi: 10.1002/jcp.21001. [DOI] [PubMed] [Google Scholar]
- 41.Chauhan SK, Saban DR, Lee HK, et al. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J Immunol. 2009;182:148–153. doi: 10.4049/jimmunol.182.1.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Balandina A, Lécart S, Dartevelle P, et al. Functional defect of regulatory CD4+CD25+ T cells in the thymus of patients with autoimmune myasthenia gravis. Blood. 2005;105:735–741. doi: 10.1182/blood-2003-11-3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bacchetta R. Defective regulatory and effector T cell functions in patients with FOXP3 mutations. J Clin Invest. 2006;116:1713–1722. doi: 10.1172/JCI25112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25[+]CD4[+] regulatory T cells constitutively expressing cytotoxic T lymphocyte associated antigen 4. J Exp Med. 2000;192:303–310. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marson A, Kretschmer K, Frampton GM, et al. Foxp3 occupancy and regulation of key target genes during T-cell stimulation. Nature. 2007;445:931–935. doi: 10.1038/nature05478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng Y, Josefowicz SZ, Kas A, et al. Genome-wide analysis of Foxp3 target genes in developing and mature regulatory T cells. Nature. 2007;445:936–940. doi: 10.1038/nature05563. [DOI] [PubMed] [Google Scholar]
- 47.Aricha R, Mizrachi K, Fuchset S, et al. Blocking of IL-6 suppresses experimental autoimmune myasthenia gravis. J Autoimmun. 2011;36:135–141. doi: 10.1016/j.jaut.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Fujimoto M, Nakano M, Terabe F, et al. The influence of excessive Il-6 production in vivo on the development and function of Foxp3+ regulatory T cells. J Immunol. 2011;186:32–40. doi: 10.4049/jimmunol.0903314. [DOI] [PubMed] [Google Scholar]
- 49.Voo KS, Wang YH, Santori FR, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc Natl Acad Sci U S A. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sheng JR, Li LC, Ganesh BB, et al. Suppression of experimental autoimmune myasthenia gravis [EAMG] by Granulocyte-Macrophage Colony-Stimulating Factor [GM-CSF] is associated with an expansion of FoxP3+ regulatory T cells. J Immunol. 2006;177:5296–5306. doi: 10.4049/jimmunol.177.8.5296. [DOI] [PubMed] [Google Scholar]
- 51.Sheng JR, Li LC, Ganesh BB, et al. Regulatory T cells induced by GM-CSF suppress ongoing experimental myasthenia. Clin Immunol. 2008;128:172–180. doi: 10.1016/j.clim.2008.03.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sheng JR, Muthusamy T, Prabhakar BS, et al. GM-CSF-induced regulatory T cells inhibit anti-acetylcholine receptor-specific immune responses in experimental myasthenia gravis. J Neuroimmunol. 2011;240–241:65–73. doi: 10.1016/j.jneuroim.2011.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Luther C, Adamopoulou E, Stoeckle C, et al. Prednisolone treatment induces tolerogenic dendritic cells and a regulatory milieu in myasthenia gravis patients. J Immunol. 2009;83:841–848. doi: 10.4049/jimmunol.0802046. [DOI] [PubMed] [Google Scholar]
- 54.Vrolix K, Fraussen J, Molenaar PC, et al. The auto-antigen repertoire in myasthenia gravis. Autoimmunity. 2010;43:380–400. doi: 10.3109/08916930903518073. [DOI] [PubMed] [Google Scholar]