Highlights
► Methodological refinement improved survival from stroke in hypertensive rats. ► Post-stroke associated weight loss is reduced in animals with pre-stroke surgery. ► Surviving animals (±pre-stroke surgery) have equivalent infarct volumes. ► Surviving animals (±pre-stroke surgery) show same neurological deficit severity. ► Important implications for animal welfare/groups sizes required for stroke studies.
Keywords: Closed skull stroke model, Cranial burrhole, Hypertensive rat, Survival, Transient focal ischemia
Abstract
We describe a positive influence of pre-stroke surgery on recovery and survival in a commonly used experimental stroke model. Two groups of male, stroke-prone spontaneously hypertensive rats (SHRSPs) underwent transient middle cerebral artery occlusion (tMCAO). Group 1 underwent the procedure without any prior intervention whilst group 2 had an additional general anaesthetic 6 days prior to tMCAO for a cranial burrhole and durotomy. Post-stroke recovery was assessed using a 32 point neurological deficit score and tapered beam walk and infarct volume determined from haematoxylin–eosin stained sections. In group 2 survival was 92% (n = 12) versus 67% in group 1 (n = 18). In addition, post-tMCAO associated weight loss was significantly reduced in group 2. There was no significant difference between the two groups in experimental outcomes: infarct volume (Group 1 317 ± 18.6 mm3 versus Group 2 332 ± 20.4 mm3), and serial (day 0–14 post-tMCAO) neurological deficit scores and tapered-beam walk test. Drilling a cranial burrhole under general anaesthesia prior to tMCAO in SHRSP reduced mortality and gave rise to infarct volumes and neurological deficits similar to those recorded in surviving Group 1 animals. This methodological refinement has significant implications for animal welfare and group sizes required for intervention studies.
1. Introduction
The need for robust pre-clinical models of stroke is well recognised given the poor translation from bench to bedside for stroke interventions. Often, pre-clinical studies fail to consider co-morbidities associated with stroke clinical presentation. The most significant risk factors (hypertension, current smoking, abdominal obesity, diet and physical inactivity) account for 80% of the global risk for stroke with self-reported history of hypertension being the strongest of these risk factors (O’Donnell et al., 2010). The revised STAIR guidelines (Fisher et al., 2009) highlighted the need to include co-morbidities in pre-clinical stroke models to better model the clinical situation. A recent review cited that of 493 drugs tested in experimental stroke models only 84 were tested in hypertensive animals (generally the spontaneously hypertensive rat, SHR) (Howells et al., 2010). However, with the inclusion of risk factors such as ageing, hypertension and diabetes there is an increased risk of mortality, particularly in closed skull models using an intraluminal filament and beyond the 24 h recovery period commonly used in pre-clinical studies (Spratt et al., 2006; Rewell et al., 2010). Following tMCAO in rats, however, the infarct has been shown to evolve beyond 24 h out to 48–72 h (Li et al., 2000; Neumann-Haefelin et al., 2000), therefore, when assessing potential new therapeutic strategies it is important to set an experimental endpoint at or beyond the time when infarct is final to ensure efficacy is maintained and that infarct evolution is not simply delayed. Here, we describe a pre-stroke surgical procedure in the spontaneously hypertensive stroke prone rat (SHRSP) which has a positive impact on animal welfare and survival from a subsequent (6 days later) tMCAO. The resulting infarct and neurological deficit are reproducible and similar in size to outcome in surviving rats exposed to tMCAO without pre-surgery. Improved survival with this procedure was identified during a prior study involving pre-stroke central administration of a viral vector which involved a general anaesthetic, burrhole and durotomy to stereotaxically inject the virus 6 days prior to tMCAO (Ord et al., unpublished results).
2. Materials and methods
2.1. Animals
Sixteen week old, male, SHRSP (270–310 g) were maintained “in-house” by brother–sister mating. The animals were kept in standard polycarbonate cages in small groups of two to three, with ad libitum access to rodent chow (RM1 expanded diet, SDS, England) and untreated tap water. All studies were carried out in accordance with the Animals Scientific Procedures Act 1986 and were approved by the University of Glasgow's Ethics Review Committee. In total, 30 animals were used with power calculations used to determine group sizes required to detect statistically significant differences.
2.2. Tail cuff plethysmography
Systolic blood pressure monitoring was carried out by non-invasive computerised tail-cuff, based on the plethysmographic method. Briefly, rats were preheated to ∼39 °C for ∼20 min and restrained by wrapping in a surgical drape, before a pneumatic pressure sensor was attached to the tail distal to a pneumatic pressure cuff, both under the control of a Programmed Electro-Sphygmomanometer. Systolic blood pressure values from each animal were determined from the mean of a minimum of six separate indirect pressure measurements. Animals had been previously habituated to the procedure before actual measurements were taken.
2.3. Surgical procedures
All surgical interventions were performed by the same surgeon and at the same time of day. Animals were randomly allocated to group 1 or 2. Rats were anaesthetised (2.5–3% isoflurane in oxygen) and artificially ventilated. Group 1 (n = 18) underwent tMCAO by advancing a silicone-coated monofilament (Doccol Corporation, USA) through the internal carotid artery to block the MCA for 45 min before reperfusion (total anaesthetic exposure <90 min). Group 2 animals (n = 12) were anaesthetised, positioned in a stereotactic frame and a 1 mm diameter burrhole made 6 days prior to tMCAO. A 24G Hamilton syringe needle was used to pierce the dura before sealing the burrhole with dental cement (Prontolute; Wright Cotterell, UK). The duration of anaesthesia for this additional procedure was typically 30 min, not exceeding 40 min on any occasion.
2.4. Infarct volume
Fourteen days following tMCAO, rats (n = 14) were killed by transcardiac perfusion fixation. Formalin-fixed, paraffin-embedded tissue sections (6 μm) were deparaffinised, rehydrated and stained with haematoxylin and eosin and infarct volume assessed over 8 coronal levels. Brains from remaining rats (n = 9) were used for biochemical analyses. Infarct measures were made blinded to group allocation.
2.5. Neurological outcome
Neurological outcome was determined before stroke surgery and at 1, 2, 3, 7, 10 and 14 days post-tMCAO from (a) number of footfalls recorded as rats traversed a 130 cm tapered beam and (b) using a battery of 10 tests to provide an overall neurological score (maximum score 32). Where possible, measures of neurological deficit were assessed blinded to group allocation.
2.6. Statistics
Data are presented as mean ± s.e.m. Groups were compared using a one-way ANOVA with Bonferroni's correction for multiple comparisons. Survival rates were compared using Fisher's exact test. A p value of <0.05 was deemed statistically significant.
3. Results
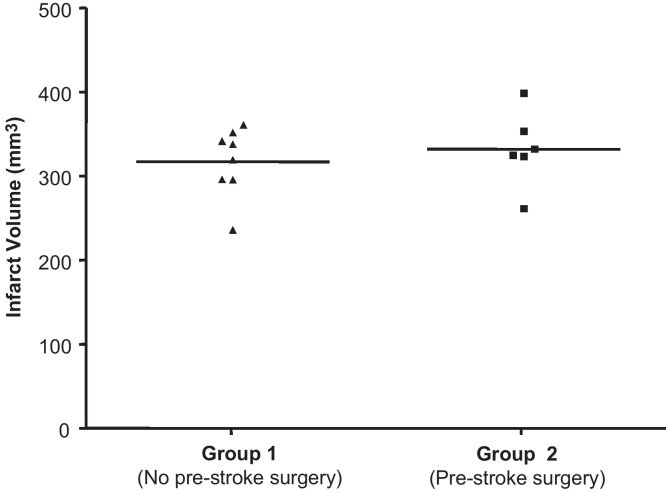
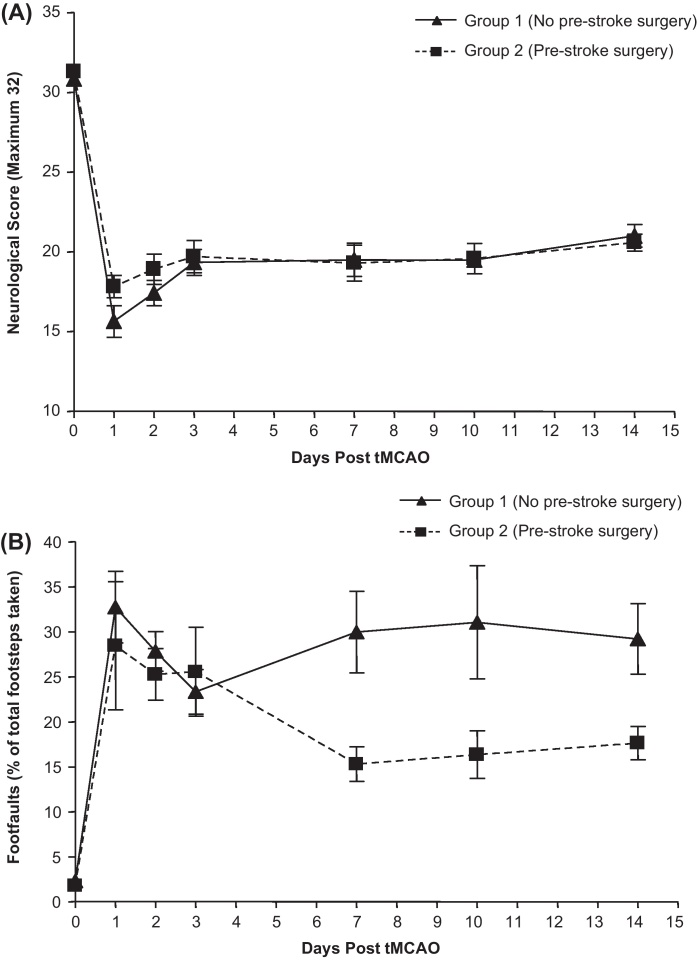
Survival was 67% in group 1 (12 out of 18 rats, no pre-stroke surgery) versus 92% in group 2 (11 out of 12 rats with pre-stroke surgery). Mortality occurred within 24–48 h of stroke onset and was attributed to the severity of the stroke, size of infarct and associated brain swelling and increased intracranial pressure resulting in depression of the cardiovascular and respiratory centres in the brainstem. Brain haemorrhage was not recorded in any instance. There was no significant difference in conscious systolic blood pressure [group 1 versus group 2 measured 3–4 days prior to tMCAO (baseline; mm Hg): 175.6 ± 5.4 versus 174.7 ± 5.0; at sacrifice: 172.7 ± 3.8 versus 177.9 ± 4.5] or pre-stroke body weight [group 1 versus group 2 (g): 282.2 ± 3.4 versus 272 ± 4.9] between groups. However, rats in group 2 had a lower weight loss associated with the stroke than group 1 rats indicative of a faster return to regular feeding patterns; weight loss from pre-stroke to sacrifice (+14 days) was group 1 31.1 ± 2.4 g versus group 2 18.3 ± 1.1 g; p = 0.0005. Final infarct volume was not significantly different between the groups [group 1 versus group 2 (mm3) 317 ± 18.6 versus 332 ± 20.4; Fig. 1]. Oedema was not measured as histological processing of the brains involved dehydration and, as such, assessment of brain swelling would be flawed. Furthermore, by day 14 post-tMCAO oedema or swelling associated with stroke would not persist. Longitudinal assessment of neurological score to day 14 post-tMCAO (Fig. 2A) and forelimb and hindlimb impairment were not significantly different between animals in groups 1 and 2 (Fig. 2B). Furthermore, the variation in the number of footfaults made on the tapered beam walk test was less in animals in group 2 versus group 1.
Fig. 1.
Determination of final infarct volume. Infarct volume (mm3) in SHRSP was determined on day 14 following tMCAO from H&E stained sections over 8 coronal levels. Each data point represents an individual animal, horizontal bar represents mean.
Fig. 2.
Assessment of neurological deficit following tMCAO. (A) 32-point neurological score measured general condition, righting reflex, grip strength, paw placement, circling, horizontal bar, inclined platform, visual forepaw reaching, rotation and mobility. (B) Movement on tapered beam was tracked, footfalls onto an under-hanging ledge recorded for both ipsi- and contralateral side and expressed as a percentage of all foot-steps taken. Measures assessed at baseline and days 1, 2, 3; group 1 (n = 12), group 2 (n = 11) and 7, 10 and 14; group 1 (n = 5), group 2 (n = 5) post-tMCAO (not all animals assessed on for neurological deficit at later times). Group data presented are mean ± s.e.m.
4. Discussion
Pre-stroke general anaesthesia and cranial burrhole surgery improved survival in SHRSP exposed to 45 min tMCAO and reduced post-tMCAO associated weight loss. Systolic blood pressure, infarct volumes and neurological deficits were similar to surviving animals from the no pre-stroke surgery group. This experimental refinement improves the utility, animal welfare and validity of pre-clinical stroke models exhibiting stroke-associated co-morbidities, such as hypertension and has been supported by the regulatory authorities governing pre-clinical studies. The reduced mortality rate directly addresses the requirement to implement the 3R's (refinement, reduction, replacement) in stroke research whilst allowing the inclusion of co-morbidity models, in line with STAIR (Fisher et al., 2009).
Consideration of stroke risk factors is often overlooked in pre-clinical studies with most using healthy, young rats (Howells et al., 2010). In addition to the animal model used, the stroke models differ with both permanent and transient MCAO available. While the intraluminal filament model used here is the most commonly used stroke model (both permanent and transient MCAO) the main disadvantages are reproducibility and higher mortality in normotensive or hypertensive rats. Models requiring craniectomy and durotomy have low or absent mortality (reviewed in Macrae, 2011). Successful experimental refinements have been described for the intraluminal filament model using aged, hypertensive and diabetic animals through modification of the occluding filament used for a 2 h tMCAO resulting in a significant improvement in both successful stroke induction and a reduction in mortality (Spratt et al., 2006). Furthermore, modification of the dosing regime in aged animals for the induction of diabetes with streptozotocin improved survival thus allowing induction of tMCAO on this background (Rewell et al., 2010). In both cases, outcome (infarct volume) was assessed 24 h post-tMCAO. However, final infarct volume in rat tMCAO models can continue to evolve out to 48–72 h with a concomitant increase in brain oedema and higher mortality (Li et al., 2000; Neumann-Haefelin et al., 2000). The technical modification described here allowed outcome measures to be determined 14 days post-tMCAO, providing serial data on functional outcome and final infarct. Furthermore, the extent of post-tMCAO associated weight loss was significantly reduced in those animals that underwent pre-stroke surgery suggesting an overall improvement in animal welfare in this group with a quicker return to regular eating patterns. Together, these demonstrate both a refinement in the procedure with a concomitant reduction in mortality or reduction in the number of animals required for a study through an improved survival so addressing 2 of the 3Rs of animal experimentation. Given that the procedural associated mortality with normotensive and hypertensive animals involves the same mechanisms, we speculate that the experimental refinement described here will reduce mortality for normotensive animals also when closed skull stroke models are used.
Factors which could potentially explain the reduced mortality include pre-stroke general anaesthesia (pre-conditioning), the burrhole itself and durotomy. Isoflurane preconditioning has been described to improve neurological outcome following tMCAO in rats (Kapinya et al., 2002; Li and Zuo, 2009) however, this effect was only described when experimental stroke was induced up to 48 h after the preconditioning stimulus [30 min (Li and Zuo, 2009) or 3 h (Kapinya et al., 2002) exposure to isoflurane anaesthesia]. In the current study, animals in group 2 were exposed to 30 min isoflurane 6 days before tMCAO, a sustained preconditioning effect for such a prolonged period between the initial isoflurane exposure and focal cerebral ischemia has, to our knowledge, not been described (reviewed in Kitano et al., 2007). Sustained protection has been reported up to 8 weeks after repeated hypoxic preconditioning in mice (Stowe et al., 2011) although the mechanism for this sustained protection involves mechanisms distinct and different to that implicated for inhalation anaesthetic preconditioning. Hence, it seems unlikely that an isoflurane preconditioning effect could be responsible for the improved survival in group 2, all the more because no improvement in neurological recovery or reduction in infarct volume was detected in surviving animals, which is in contrast to previous studies (Kapinya et al., 2002; Li and Zuo, 2009).
In humans with severe MCA ischemic strokes there is often a life-threatening, space-occupying brain oedema and this has been associated with mortality rates of up to 80% (Bardutzky and Schwab, 2007). In clinical studies, reducing intracranial pressure through procedures such as decompressive craniectomy reduced stroke mortality by 50% at 1 year versus best medical intervention (Vahedi et al., 2007) and a more recent review has concluded that the practice of opening the cranial vault and the dura for surgical decompression of cerebral oedema in acute ischemic stroke is still the treatment of choice in some patients (Cruz-Flores et al., 2012). By comparison, the cranial burrhole in the present study was significantly smaller than any surgical hemicraniectomy and was immediately sealed with dental cement to reduce the risk of infection. However, in humans, it has been shown that a craniotomy with mere durotomy via dural incisions can have the same beneficial effect of intracranial decompression following traumatic brain injuries as does the more invasive practice of craniectomy and duroplasty (Burger et al., 2008). Opening of the dura is crucial for effective reduction of intracranial pressure. Moreover, drainage of even small volumes of CSF can dramatically reduce increased ICP caused by oedema (Singhi and Tiwari, 2009). Hence, it is possible that the beneficial effect on survival shown in our study was a direct result of the durotomy which might have enabled excess cerebrospinal fluid to drain away as oedema and brain swelling evolved in the early hours to days after tMCAO. Further studies could be performed to elucidate the exact mechanism behind the improved survival and recovery shown in our report but, for ethical reasons, we do not think they are warranted. We have been able to ascertain, by using the described technique in subsequent studies, that the beneficial effects of the pre-stroke surgery are reduced if it is performed more than 7 days before tMCAO surgery.
In conclusion, we describe a methodological refinement to the tMCAO procedure which reduced mortality and post-tMCAO associated weight loss in SHRSP, an animal model exhibiting many of the co-morbidities associated with stroke clinically (hypertension, altered glucose handling and increased basal inflammatory status). Further, infarct size and neurological deficit were not different to values recorded in group 1 animals which survived the stroke surgery. As such, we believe this refinement can easily be employed by pre-clinical stroke researchers to improve survival when closed skull experimental stroke models are used, particularly when using hypertensive rats, thus reducing the total number of animals required to complete a study.
Acknowledgements
These studies were supported by a British Heart Foundation project grant (PG/07/226/24223) and a Capacity Building Award in Integrative Mammalian Biology (Biotechnology & Biological Science Research Council, British Pharmacological Society, Knowledge Transfer Network, Medical Research Council and Scottish Funding Council).
References
- Bardutzky J., Schwab S. Antiedema therapy in ischemic stroke. Stroke. 2007;38:3084–3094. doi: 10.1161/STROKEAHA.107.490193. [DOI] [PubMed] [Google Scholar]
- Burger R., Duncker D., Uzma N., Rohde V. Decompressive craniotomy: durotomy instead of duroplasty to reduce prolonged ICP elevation. Acta Neurochir Suppl. 2008;102:93–97. doi: 10.1007/978-3-211-85578-2_19. [DOI] [PubMed] [Google Scholar]
- Cruz-Flores S., Berge E., Whittle I.R. Surgical decompression for cerebral oedema in acute ischaemic stroke. John Wiley & Sons Ltd.; 2012. Cochrane Database of Systematic Reviews. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M., Feuerstein G.Z., Howells D.W., Hurn P.D., Kent T.A., Savitz S.I. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howells D.W., Porritt M.J., Rewell S.S.J., O’Collins V., Sena E.S., van der Worp H.B. Different strokes for different folks: the rich diversity of animal models of focal cerebral ischemia. J Cereb Blood Flow Metab. 2010;30:1412–1431. doi: 10.1038/jcbfm.2010.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapinya K.J., Löwl D., Fütterer C., Maurer M., Waschke K.F., Isaev N.K. Tolerance against ischemic neuronal injury can be induced by volatile anesthetics and is inducible NO synthase dependent. Stroke. 2002;33:1889–1898. doi: 10.1161/01.str.0000020092.41820.58. [DOI] [PubMed] [Google Scholar]
- Kitano H., Kirsch J.R., Hurn P.D., Murphy S.J. Inhalational anesthetics as neuroprotectants or chemical preconditioning agents in ischemic brain. J Cereb Blood Flow Metab. 2007;27:1108–1128. doi: 10.1038/sj.jcbfm.9600410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F., Silva M.D., Sotak C.H., Fisher M. Temporal evolution of ischemic injury evaluated with diffusion-, perfusion-, and T2-weighted MRI. Neurology. 2000;54:689–696. doi: 10.1212/wnl.54.3.689. [DOI] [PubMed] [Google Scholar]
- Li L., Zuo Z. Isoflurane preconditioning improves short-term and long-term neurological outcome after focal brain ischemia in adult rats. Neuroscience. 2009;164:497–506. doi: 10.1016/j.neuroscience.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae I.M. Preclinical stroke research—advantages and disadvantages of the most common rodent models of focal ischaemia. Br J Pharmacol. 2011;164:1062–1078. doi: 10.1111/j.1476-5381.2011.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann-Haefelin T., Kastrup A., de Crespigny A., Yenari M.A., Ringer T., Sun G.H. Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood–brain barrier damage, and edema formation. Stroke. 2000;31:1965–1973. doi: 10.1161/01.str.31.8.1965. [DOI] [PubMed] [Google Scholar]
- O’Donnell M.J., Xavier D., Liu L., Zhang H., Chin S.L., Rao-Melacini P. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): a case–control study. The Lancet. 2010;376:112–123. doi: 10.1016/S0140-6736(10)60834-3. [DOI] [PubMed] [Google Scholar]
- Rewell S.S.J., Fernandez J.A., Cox S.F., Spratt N.J., Hogan L., Aleksoska E. Inducing stroke in aged, hypertensive, diabetic rats. J Cereb Blood Flow Metab. 2010;30:729–733. doi: 10.1038/jcbfm.2009.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singhi S., Tiwari L. Management of intracranial hypertension. Indian J Pediatr. 2009;76:519–529. doi: 10.1007/s12098-009-0137-7. [DOI] [PubMed] [Google Scholar]
- Spratt N.J., Fernandez J., Chen M., Rewell S., Cox S., van Raay L. Modification of the method of thread manufacture improves stroke induction rate and reduces mortality after thread-occlusion of the middle cerebral artery in young or aged rats. J Neurosci Methods. 2006;155:285–290. doi: 10.1016/j.jneumeth.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Stowe A.M., Altay T., Freie A.B., Gidday J.M. Repetitive hypoxia extends endogenous neurovascular protection for stroke. Ann Neurol. 2011;69:975–985. doi: 10.1002/ana.22367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahedi K., Hofmeijer J., Juettler E., Vicaut E., George B., Algra A. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol. 2007;6:215–222. doi: 10.1016/S1474-4422(07)70036-4. [DOI] [PubMed] [Google Scholar]