Abstract
Background
Genetic and environmental factors likely influence susceptibility to nonsyndromic cryptorchidism, a common disease presenting at birth or in later childhood. We compared cases and controls to define differential risk factors for congenital vs. acquired cryptorchidism.
Methods
We compared questionnaire and clinical data from cases of congenital cryptorchidism (n=230), acquired cryptorchidism (n=182) and hernia/hydrocele (n=104) with a group of healthy male controls (n=358). Potential predictor variables (p<0.2 in univariable analysis) were included in stepwise multivariable logistic regression models.
Results
Temporary (odds ratio (OR) 0.5; 95% CI 0.4, 0.8) or exclusive (OR 0.6; 95% CI 0.4, 0.9) breastfeeding was reduced and soy formula feeding increased (OR 1.8; 95% CI 1.2, 2.9) in acquired but not congenital or hernia/hydrocele groups. Highest risk estimates were observed for primary soy formula feeding with limited or no breastfeeding (OR 2.5; 95% CI 1.4, 4.3; adjusted OR 2.7; 95% CI 1.4, 5.4) in the acquired group. Primary feeding risk estimates were equivalent or strengthened when multivariable models were limited to age >2 years, full term/non-SGA or Caucasian subjects. Pregnancy complications and increased maternal exposure to cosmetic or household chemicals were not consistently associated with either form of cryptorchidism in these models.
Conclusions
Our data support reduced breastfeeding and soy formula feeding as potential risk factors for acquired cryptorchidism. Although additional studies are needed, hormonally-active components of breast milk and soy formula could influence the establishment of normal testis position in the first months of life, leading to apparent ascent of testes in childhood.
Keywords: Cryptorchidism, Epidemiology, Infant feeding, Heredity, Environmental risk
Introduction
Cryptorchidism (undescended testis, UDT) is a common anomaly of newborn boys that may resolve spontaneously or present initially in later childhood, particularly in association with testicular retractility due to overactivity of the cremaster muscle. Longitudinal studies confirm that “acquired” cryptorchidism, or testes diagnosed as undescended after previous documentation of scrotal position at birth, is common (Villumsen and Zachau-Christiansen, 1966),(Hack and others, 2003). The etiology of acquired cryptorchidism remains unclear. A relative change in the position of incompletely descended testes with linear growth of the individual, physical ascent of testes and delayed referral are possible explanations for acquired cryptorchidism (Barthold and Gonzalez 2003).
Despite multiple epidemiologic, genetic and anatomic studies, the etiology of human cryptorchidism remains poorly understood (Bay and others, 2011). Mutations within coding regions of likely candidates such as the ligand-receptor pair insulin-like 3 (INSL3)-relaxin/insulin-like family peptide receptor 2 (RXFP2) occur in only 2-3% of cases (Ferlin and others, 2008) although epidemiologic studies suggest moderate genetic risk (Jensen and others, 2010; Schnack and others, 2008). Androgens appear to regulate testicular descent based on studies in experimental animals and in humans with mutations of the androgen receptor (Barthold and others, 2000; Foresta and others, 2008). Conversely, estrogens inhibit testicular descent in experimental animals by reducing androgen and/or INSL3 production by the testis (Nef and others, 2000) and/or by directly inhibiting development or function of the gubernaculum, the target organ that facilitates testicular descent and contains both estrogen and androgen receptors (Staub and others, 2005). Androgens and INSL3 contribute to postnatal development of the cremaster muscle (Kaftanovskaya and others, 2011). Accordingly, ubiquitous environmental chemicals with androgen antagonist and/or estrogen agonist activity may increase risk of cryptorchidism (Main and others, 2010). Unfortunately, the timing and degree of potential maternal exposures is difficult to validate and may not reflect fetal exposure. Similarly, previous studies of the perinatal risk factors for cryptorchidism are based on heterogeneous populations and methodology. In this ongoing study of the genetic basis of cryptorchidism, we have utilized strict inclusion criteria to identify nonsyndromic cases and to collect ancillary phenotypic and exposure data. The hypothesis we are testing is that maternal exposures and perinatal characteristics distinguish cryptorchid and non-cryptorchid boys, and that certain characteristics are unique to those presenting with cryptorchidism beyond the newborn period.
Methods
Clinical and phenotypic data were collected from families of boys recruited prospectively during 2004-2012 in IRB-approved studies of nonsyndromic cryptorchidism, with informed consent. Consecutive cases and controls meeting inclusion criteria are identified for recruitment from a single population using an electronic health record (EHR) database. All subjects are outpatients at a single hospital-based pediatric urology clinic with well-established patient referral patterns from a tri-state catchment area that includes primarily Delaware and to a lesser extent southern New Jersey, eastern Maryland and southeastern Pennsylvania. Cases are boys with cryptorchidism or inguinal hernia/hydrocele and controls are subjects referred to the same clinic for foreskin or voiding problems, mild antenatal urinary tract dilation or elective surgical circumcision. The hernia/hydrocele group is a comparably-aged case group with full testicular descent that overlaps the cryptorchid phenotype, as many cryptorchid boys also have an ipsilateral inguinal hernia.
To ensure accurate phenotypic classification, all participants are examined by an attending pediatric urologist co-investigator. Exclusion criteria include other genital anomalies such as hypospadias, chordee or other congenital penile anomaly and genetic or malformation syndromes. While retractile testis is usually a normal variant, a history of testis retractility, as documented by our own serial exam or those of referring pediatricians, is not uncommon in boys presenting at later ages and undergoing orchidopexy for cryptorchidism. Therefore, to minimize inclusion of boys at risk for acquired cryptorchidism, boys with retractile testes were excluded from the control and hernia/hydrocele groups. A standard EHR template used for new urology patients includes gestational age and weight at birth, timing of cryptorchidism diagnosis, testicular retractility and family history of cryptorchidism or inguinal hernia/hydrocele. Birth weights <2500 gm at ≥37 weeks’ gestation or <10th percentile for gestational age <37 weeks, based on a revised Babson and Benda nomogram, were coded as small for gestational age (SGA) (Fenton, 2003).
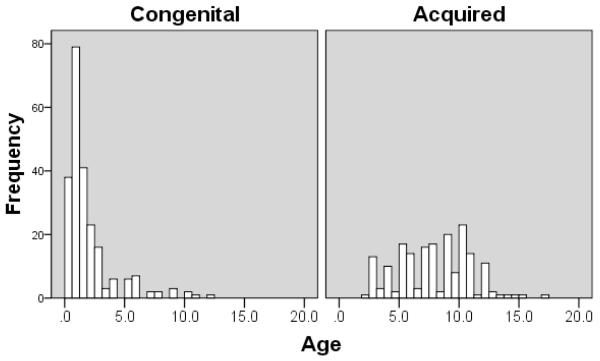
A preliminary analysis suggested differences in risk factors for cryptorchidism between boys diagnosed at birth and those in whom testes were reported as being descended during infancy but subsequently diagnosed as undescended. We are aware that parental history and/or pediatrician documentation are not always reliable in determining the timing of initial cryptorchidism diagnosis. Therefore, to study “congenital” and “acquired” forms of cryptorchidism as accurately as possible, we adopted a conservative approach to minimize inclusion of boys in the acquired group in whom cryptorchidism was present at birth but referral was delayed. We analyzed the age distribution of our cryptorchid patients and identified a major peak prior to age 2 followed by a lower but sustained frequency during childhood (Figure 1). This distribution was similar to that reported previously (Hack and others, 2003) although our postnatal peak is earlier and the secondary peak less pronounced, possibly because many congenital cases are referred to us for evaluation prior to 6 months of age. Based on our data and the recommendations of other investigators (Bruijnen and others, 2008) we categorized patients as having congenital cryptorchidism if surgery was performed at ≤24 months of age and in older boys whose families or pediatricians reported a history of undescended testes at birth and in whom referral was delayed. Boys >24 months with no prior history of undescended testis were categorized as having acquired cryptorchidism.
Figure 1.
The distribution of age at presentation for boys in congenital (defined as age ≤2 years or undescended testes reported at birth, n=230) and acquired (defined as no history of undescended testis at birth; n=182) cryptorchidism groups.
Surgeon co-investigators document testicular position and presence or absence of a hernia sac, epididymal anomaly and/or testicular appendage at the time of orchidopexy. The highest testicular location is used in bilateral cases and defined as “proximal” for testes above the external inguinal ring (canalicular and abdominal) and “distal” for those below the external inguinal ring (superficial inguinal pouch, external ring, prescrotal and perineal). Participants diagnosed intra-operatively with absent testes are excluded.
Additional data were collected by a retrospective maternal questionnaire completed at the time of surgery/recruitment and included parental age at child’s birth; family history of urogenital or inguinal disease; maternal fertility, pregnancy and birth history; infant feeding type and breastfeeding duration; maternal vegetarian or soy diet (regular use of tofu, soy milk or other soy products or supplements); and the occurrence/frequency per month and specific types of skin lotions, nail polish, perfume, perfumed soap, insecticides (defined as “chemicals that kill bugs”), herbicides (“chemicals that kill weeds”), pesticides (“chemicals that kill rodents”), paints or glazes, paint strippers or thinners, glues and adhesives, gas/diesel fuel (direct skin contact), petroleum fumes/exhaust and greases/oils used by the participant’s mother.
We summarized demographics and other study variables and analyzed their distribution in each of the 3 case groups (congenital and acquired cryptorchidism, and hernia/hydrocele) relative to the control group. Categorical data were summarized by frequencies and percentages. Numerical data were summarized using either mean and standard deviation or median and 1st/3rd quartiles. Two sample t-tests or Mann-Whitney U tests, whichever appropriate, were used to compare the mean/median of the control group with each of the case groups. Similarly, chi-square or Fisher exact tests were used to compare the distribution of categorical variables between the control and each of the case groups. A univariable logistic regression model was used to examine the potential association of study variables of each case group with the control group in this data set. Odds ratios with 95% confidence intervals were reported for this purpose. A stepwise backward method of multivariable logistic regression was used to select the variables that are significantly associated with each case group compared to the control group. Variables with low frequencies (≤5% of cases) and/or those with p-values >0.2 in univariable logistic regression models were excluded in the stepwise multivariable selection model. Probabilities for entry and removal in the stepwise analysis were set to 0.05 and 0.10, respectively. Adjusted odds ratios with 95% confidence intervals (CI) are reported for variables retained in each model. All tests were two-tailed with the level of significance <0.05. Analyses were performed based on the available data only. IBM® SPSS® version 19.0 was used for all statistical analyses.
Results
Of 1027 eligible families recruited, 874 (85%) completed questionnaires; including 79% of control (n=358), 72% of cryptorchid (n=230 congenital; n=182 acquired) and 86% of hernia/hydrocele (n=104) subjects. The majority of boys in the control group (83%) underwent circumcision or circumcision revision. EHR and/or questionnaire data were missing for <5% of subjects for all variables except birth weight (13%). Following an interim data analysis showing no association of maternal diet and household exposures with cryptorchidism, these questions were omitted from a subset (14%) of questionnaires.
Descriptive characteristics of participants
There were no significant differences in birth weight, maternal age or paternal age at the time of birth between the control, cryptorchid and hernia/hydrocele groups (Table 1). Likewise, there were no significant differences in the prevalence of prematurity although twin gestation was more common in the acquired group. The age of boys in the congenital (mean 2.0, median 1.2) and acquired (mean 7.9, median 8.0) cryptorchid groups differed significantly from the other groups and from each other, as expected. In addition, while Caucasian participants comprised the majority of all groups, the proportion of African-American subjects was higher in the control as compared to the cryptorchidism and hernia/hydrocele groups (p<0.05). These findings may reflect the referral patterns of the studied populations; potential confounding due to age and race were considered in our multivariable analyses. There were no significant differences in the proportion of boys classified as SGA in case groups as compared to controls.
Table 1.
Characteristics of control, cryptorchid and hernia/hydrocele subjects and parents
| Variable | Control | Congenital | Acquired | Hernia/hydrocele | ||||
|---|---|---|---|---|---|---|---|---|
| N | N | N | N | |||||
| Age# | 358 | 2.0 (0.9, 7.0) |
230 | 1.2** (0.8, 2.0) |
182 | 8.0** (5.4, 10.0) |
104 | 3.0* (1,8, 5.7) |
| Race | 358 | 230 | 182 | 104 | ||||
| • Caucasian | 230 (64%) | 175* (76%) | 154** (85%) | 86** (83%) | ||||
| • African American | 85 (24%) | 36 (16%) | 15 (9%) | 11 (11%) | ||||
| • Asian | 16 (4.5%) | 7 (3%) | 0 | 5 (5%) | ||||
| • Other/Mixed | 27 (7.5%) | 12 (5%) | 13 (7%) | 1 (1%) | ||||
| Birth weight (gm)## | 305 | 3398 ± 660 | 215 | 3385 ± 625 | 159 | 3381 ± 710 | 84 | 3439 ± 569 |
| SGA1 | 10 (3%) | 10 (4.6%) | 3 (2%) | 0 | ||||
| Prematurity | 358 | 29 (8%) | 230 | 18 (8%) | 182 | 21 (12%) | 104 | 3 (3%) |
| Twin gestation | 358 | 1 (0.3%) | 240 | 1 (0.4%) | 182 | 6* (3%) | 104 | 2 (2%) |
| Maternal age## | 353 | 29 ± 6 | 230 | 29 ± 6 | 179 | 29 ± 6 | 104 | 29 ± 7 |
| Paternal age## | 340 | 31 ± 7 | 224 | 31 ± 7 | 174 | 31 ± 7 | 102 | 31 ± 7 |
Median (1st, 3rd quartiles)
Mean ± standard deviation
p≤0.05
p<0.01 vs. control group
SGA (small for gestational age): <2500 gm at ≥37 weeks’ gestation or age-adjusted birth weight <10th percentile based on Babson and Benda nomogram
Cryptorchidism was unilateral in 74% of cases and did not differ significantly between acquired and congenital groups. However, phenotypic severity was increased in boys classified as congenitally cryptorchid. Testes were more frequently in a proximal location (40 vs. 21%; OR 2.5; 95% CI 1.6, 3.9) and associated with a hernia (82 vs. 46%; OR 5.5; 95% CI 3.5, 8.6) or an epididymal anomaly (60 vs. 31%; OR 3.4; 95% CI 2.2, 5.1) in boys with congenital as compared to acquired cryptorchidism. Prior spontaneous postnatal testicular descent occurred in 25 (11%) of boys initially diagnosed at birth.
Infant feeding characteristics
We observed unique and significant alterations in infant feeding patterns in acquired cryptorchidism (Table 2). Mothers of boys in the acquired group reported reduced breastfeeding (both supplemented and exclusive) and more common use of soy formula compared to those in the control group, while feeding patterns did not differ between congenital or hernia/hydrocele cases and controls and were similar to those reported for the states served by our hospital (http://www.cdc.gov/breastfeeding/data/reportcard.htm). Final descent of the testis to a dependent scrotal position may occur in response to the pituitary and testicular hormone secretion that occurs in the first 3 months of life. Consequently, we defined infant feeding as primary breastfeeding (reference; ≥3 months with or without non-soy or soy formula supplementation) and primary formula (non-soy or soy-based with <3 months of breastfeeding). Risk estimates increased for primary non-soy (OR 1.8, 95% CI 1.2, 2.7) and primary soy (OR 2.5; 95% CI 1.4, 4.3) formula use in acquired cryptorchidism (Table 2).
Table 2.
Infant feeding characteristics in boys with congenital or acquired cryptorchidism and hernia/hydrocele relative to unaffected control subjects
| Control | Cryptorchidism | Hernia/hydrocele | |||||
|---|---|---|---|---|---|---|---|
| Congenital | Acquired | ||||||
| N (%) | N (%) | Unadjusted OR (95% CI) |
N (%) | Unadjusted OR (95% CI) |
N (%) | Unadjusted OR (95% CI) |
|
| Any breast feeding |
241 (68%) |
155 (67%) |
1.0 (0.7, 1.4) |
96 (54%) |
0.5** (0.4, 0.8) |
66 (63%) |
0.8 (0.5, 1.3) |
| Breast, no formula |
111 (31%) |
62 (27%) |
0.8 (0.6, 1.2) |
37 (21%) |
0.6** (0.4, 0.9) |
39 (37%) |
1.3 (0. 8, 2.1) |
| Soy formula | 52 (15%) |
24 (10%) |
0.7 (0.4, 1.1) |
43 (24%) |
1.8** (1.2, 2.9) |
9 (9%) |
0.5 (0.3, 1.1) |
| Primary feeding # | |||||||
| Breast | 196 (55%) |
116 (50%) |
1.0 (reference) | 70 (39%) |
1.0 (reference) | 54 (52%) |
1.0 (reference) |
| Non-soy | 122 (35%) |
96 (42%) |
1.3 (0.9, 1.9) |
79 (44%) |
1.8** (1.2, 2.7) |
44 (42%) |
1.3 (0.8, 2.1) |
| Soy | 35 (10%) |
18 (8%) |
0.9 (0.5, 1.6) |
31 (17%) |
2.5** (1.4, 4.3) |
6 (6%) |
0.6 (0.2, 1.6) |
Breast: ≥3 months of breastfeeding with or without any type of formula feeding Non-soy/Soy: formula alone or with <3 months of breastfeeding
p≤0.01
Pregnancy, delivery and family history
In univariable analyses (Table 3), prior maternal pregnancy loss (miscarriage or stillbirth) was positively associated with congenital cryptorchidism and hernia/hydrocele, and marginally associated with acquired cryptorchidism. Persistent nausea and/or vomiting in the index pregnancy was associated with congenital cryptorchidism only. Caesarian section delivery was less common in all case groups relative to controls. No notable associations between diabetes or vaginal bleeding during pregnancy and cryptorchidism, potential risk factors in previous studies, were observed.
Table 3.
Frequency of occurrence and unadjusted odds ratios for pregnancy, delivery and family history characteristics of boys with congenital and acquired cryptorchidism or hernia/hydrocele relative to unaffected control subjects*
| Control | Cryptorchidism | Hernia/hydrocele | |||||
|---|---|---|---|---|---|---|---|
| Congenital | Acquired | ||||||
| N (%) |
N (%) |
Unadjusted OR (95% CI) |
N (%) |
Unadjusted OR (95% CI) |
N (%) |
Unadjusted OR (95% CI) |
|
| Pregnancy history | |||||||
| Prior miscarriage or stillbirth |
93 (26%) |
78 (34%) |
1.4* (1.0, 2.1) |
58 (33%) |
1.3# (0.9, 2.0) |
39 (38%) |
1.6* (1.0, 2.6) |
| Primiparity | 71 (20%) |
57 (25%) |
1.3# (0.9, 1.9) |
17 (9%) |
0.4** (0.2, 0.7) |
16 (15%) |
0.7 (0.4, 1.3) |
| Index pregnancy characteristics | |||||||
| Subfertility treatment | 8 (2%) |
7 (3%) |
1.3 (0.5, 3.7) |
10 (5%) |
2.5# (0.9, 6.5) |
5 (5%) |
2.2# (0.7, 6.8) |
| Persistent nausea and/or vomiting |
20 (6%) |
29 (13%) |
2.4** (1.3, 4.4) |
12 (7%) |
1.2 (0.6, 2.5) |
5 (5%) |
0.8 (0.3, 2.3) |
| Diabetes | 18 (5%) |
12 (5%) |
1.0 (0.5, 2.2) |
6 (3%) |
0.6 (0.3, 1.6) |
5 (5%) |
0.9 (0.3, 2.3) |
| Hypertension | 22 (6%) |
19 (8%) |
1.4 (0.7, 2.6) |
19 (10%) |
1.8# (0.9, 3.4) |
5 (5%) |
0.8 (0.3, 2.1) |
| Premature labor^ | 43 (12%) |
40 (17%) |
1.5# (0.9, 2.3) |
31 (17%) |
1.4# (0.9, 2.3) |
7 (7%) |
0.5# (0.2, 1.1) |
| Vaginal bleeding | 24 (7%) |
10 (4%) |
0.6 (0.3, 1.3) |
12 (7%) |
1.0 (0.5, 2.0) |
2 (2%) |
0.3# (0.1, 1.2) |
| Caesarian section | 57 (17%) |
25 (11%) |
0.6# (0.4, 1.0) |
22 (13%) |
0.7 (0.4, 1.2) |
1 (1%) |
0.1** (0.01, 0.4) |
| Family history | |||||||
| Cryptorchidism | |||||||
| • Any | 12 (3%) |
49 (22%) |
7.9** (4.1, 15.2) |
36 (20%) |
7.2** (3.6, 14.2) |
2 (2%) |
0.6 (0.1, 2.5) |
| • First-degree relative |
4 (1%) |
15 (7%) |
6.1** (2.0, 18.8) |
16 (9%) |
8.7** (2.9, 26,5) |
1 (1%) |
0.8 (0.1, 7.7) |
| Inguinal hernia | |||||||
| • Any | 3 (1%) |
22 (10%) |
12.5** (3.7, 42.3) |
19 (11%) |
13.9** (4.1, 47.8) |
13 (12%) |
16.6** (4.6, 59.4) |
| • First-degree relative |
1 (0.3%) |
4 (2%) |
6.3# (0.7, 56.5) |
6 (3%) |
12.3* (1.5, 102.8) |
0 | |
OR, odds ratio, CI, confidence interval
p<0.01
p<0.05
p<0.2.
Uterine contractions with or without delivery prior to 37 weeks’ gestation
A family history of cryptorchidism in first-degree or more distant (second- or third-degree) relatives was increased in cases of cryptorchidism but not hernia/hydrocele (Table 3). Cryptorchidism occurred in fathers and/or brothers of 7% and 9% of boys with congenital and acquired boys, respectively. Testes were reportedly retractile in 9 additional relatives (5 brothers, 3 fathers and 1 maternal uncle) of cryptorchid boys. Thirteen families (15%) with familial cryptorchidism reported 2 or more affected members. Maternal, paternal and bilateral transmission was noted for 40%, 45% and 5% of affected second-(uncle, half-brother or grandfather) or third-(cousin) degree relatives, respectively with similar distribution in the congenital and acquired groups, and was unknown in 10%. We failed to observe any significant phenotypic differences in cases with and without a reported positive family history. As the reported frequency of cryptorchidism in first-degree relatives is more reliable, we included this more stringent variable in our multivariable models.
Familial inguinal hernia was more commonly reported in hernia/hydrocele (12%), congenital (10%) and acquired (11%) cases than in controls (1%), suggesting the possibility that common genetic risk factors may exist for hernia and cryptorchidism. However, significant reporting bias may exist for this variable as we could not validate hernia site and/or type in other family members. Moreover, as the frequency in first-degree relatives was ≤5% for all groups, these data were not included in our multivariable models. A family history of inguinal hernia did not predict occurrence of an associated inguinal hernia in cryptorchid boys (OR 0.8; 95% CI 0.4, 1.7, adjusted for testicular position and age). Only 2 families of boys with cryptorchidism (1%) reported hypospadias in first-degree relatives, possibly reflecting exclusion of boys with hypospadias from the study. Parents of 4 cryptorchid boys (1%) and 1 boy (1%) in the hernia/hydrocele group reported a family history of testicular cancer.
Multivariable models
When predictor variables (defined as those with p<0.2 in univariable analysis) were included in stepwise regression models of congenital (Table 4) and acquired (Table 5) cryptorchidism, variables remaining as potential risk factors for both groups include cryptorchidism in first-degree relatives and real or threatened pregnancy loss (prior miscarriage or stillbirth, or premature labor in the index pregnancy). However, because prematurity and SGA are strongly associated with cryptorchidism and these risks vary with race/ethnicity (Hauck and others, 2011; Lauria and others, 2012), we performed restricted analyses of full term/non-SGA and Caucasian subjects in additional multivariable models. The only maternal variable consistently retained was nausea/vomiting during gestation in congenital cryptorchidism. In contrast, the strong associations between feeding choice and acquired cryptorchidism were maintained in all the restricted multivariable analyses, including an additional model in which controls ≤2 years of age were excluded to limit differential recall bias between groups (adjusted OR 2.1; 95% CI 1.0, 4.5 for primary soy and adjusted OR 1.9; 95% CI 1.1, 3.1 for primary non-soy feeding). The mean (7.8) and median (7.0) ages of this subset of controls were not significantly different from those of the acquired group (7.9 and 8.0, respectively).
Table 4.
Variables remaining in multivariable regression models of congenital cryptorchidism
| Variable |
All Adjusted OR# (95% CI) |
Full term, non-SGA Adjusted OR# (95% CI) |
Caucasian only Adjusted OR# (95% CI) |
|---|---|---|---|
| Age | 0.8 (0.7, 0.9) | 0.8 (0.7, 0.9) | 0.8 (0.7, 0.9) |
| Race | |||
| • Caucasian | 1.0 (reference) | 1.0 (reference) | - |
| • African American | 0.5 (0.3, 0.8) | 0.4 (0.2, 0.7) | - |
| • Asian | 1.2 (0.4, 3.7) | 1.1 (0.4, 3.4) | - |
| • Other/Mixed | 0.6 (0.3, 1.4) | 0.5 (0.2, 1.1) | - |
| Cryptorchidism in first- degree relative |
4.5 (1.4, 14.3) | 5.3 (1.5, 19.8) | 4.2 (1.1, 15.9) |
| Persistent nausea and/or vomiting |
2.5 (1.3, 4.7) | 3.0 (1.5, 6.2) | 2.3 (1.0, 5.1) |
| Prior miscarriage or stillbirth |
1.6 (0.3, 1.0) | - | 0.5 (0.3, 1.1) |
| C-section | 0.6 (0.3, 1.0) | - | 0.5 (0.3, 1.1) |
SGA: small for gestational age
OR, odds ratio; CI, confidence interval; Variables in each model: age, race (except Caucasian only model), primiparity, prior miscarriage or stillbirth, cryptorchidism in 1st-degree relative; premature labor or persistent nausea/vomiting in index pregnancy and delivery (vaginal or C-section).
Table 5.
Variables remaining in multivariable regression models of acquired cryptorchidism
| Variable |
All Adjusted OR# (95% CI) |
Full term, non-SGA Adjusted OR# (95% CI) |
Caucasian only Adjusted OR# (95% CI) |
|---|---|---|---|
| Age | 1.3 (1.2, 1.4) | 1.3 (1.2, 1.4) | 1.3 (1.2, 1.4) |
| Race | |||
| • Caucasian | 1.0 (reference) | 1.0 (reference) | - |
| • African American | 0.2 (0.1, 0.5) | 0.2 (0.1, 0.6) | - |
| • Other/Mixed | 0.7 (0.3, 1.5) | 0.4 (0.2, 1.2) | - |
| Cryptorchidism in first- degree relative |
8.0 (2.3, 28.0) | 7.5 (1.7, 32.3) | 7.5 (1.9, 28.5) |
| Primary infant feeding* | |||
| • Breast | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| • Non-soy formula | 1.8 (1.1, 2.9) | 1.9 (1.1, 3.2) | 1.6 (0.9, 2.7) |
| • Soy formula | 2.7 (1.4, 5.4) | 3.0 (1.4, 6.4) | 3.5 (1.6, 7.6) |
| Maternal hypertension | 2.8 (1.3, 6.3) | - | - |
| Premature labor | - | - | 1.9 (0.9, 3.7) |
SGA: small for gestational age
OR, odds ratio; CI, confidence interval; Variables in each model: age, race (except Caucasian only model), primiparity, prior miscarriage or stillbirth, cryptorchidism in 1st-degree relative, premature labor or maternal hypertension in index pregnancy and primary infant feeding.
Breast: ≥3 months of breastfeeding ± any formula; Non-soy/Soy: <3 months of breastfeeding ± formula
Prior history of miscarriage or stillbirth was associated with hernia/hydrocele for the entire group (adjusted OR 1.8; 95% CI 1.1, 2.9) and for Caucasian participants (adjusted OR 1.8; 95% CI 1.0, 3.1), suggesting potentially overlapping reproductive risks for mothers of boys with cryptorchidism and inguinal hernia. Reduced frequency of Caesarian section was retained in the hernia/hydrocele model, possibly a chance finding related to the small sample size (data not shown).
Maternal exposures
We found no increased occurrence or frequency (reported use per month) of exposure to any household product in univariable analyses of cases, other than an association of insecticide exposure with hernia/hydrocele (Supplemental Table 1) that was not sustained in multivariable analysis. However, we did observe significantly reduced ORs for skin lotion and/or perfume in the cryptorchid groups and for a lotion*perfume interaction covariate in the acquired (OR 0.6; 0.4, 0.9) but not the congenital group. When included in the multivariable models in Table 5, our main observations were unchanged or strengthened for lotion*perfume (adjusted ORs 0.4-0.5), family history of cryptorchidism in first-degree relatives (adjusted ORs 6.0-6.5) and primary soy feeding (adjusted ORs 4.0-4.9).
Discussion
Our data suggest that familial and some pregnancy variables are correlated with occurrence of both congenital and acquired cryptorchidism. In contrast, infant feeding choice was associated with the risk of cryptorchidism presenting after infancy but not at birth, nor in boys with hernia/hydrocele, an overlapping phenotype without cryptorchidism. Breastfeeding was less common in acquired cryptorchidism, by choice or necessity, and risk estimates are highest in boys fed soy formula with limited or no exposure to breast milk. Other investigators reported increased formula use (Jones and others, 1998), reduced breastfeeding (Mori and others, 1992) or feeding difficulties (Davies and others, 1986) in cryptorchid boys, although specific feeding choices and outcomes were not well characterized. It is possible that common, undefined risk(s) predispose to both maternal lactational difficulties and acquired cryptorchidism; alternatively, infant feeding practices may influence postnatal testicular position in susceptible individuals whose testes have not descended completely to dependent scrotal position at birth.
Androgens or other reproductive hormones likely contribute to descent of testes, which may occur during neonatal (Acerini and others, 2009; Scorer, 1964) or pubertal (Hack and others, 2010) activation of the hypothalamic-pituitary-gonadal axis or in response to gonadotropin therapy (Ashley and others, 2010) and may correlate with serum testosterone levels (Gendrel and others, 1978). Moreover, cremaster muscle activity and testicular retractility are greatest during periods of low circulating testicular hormone levels (between 6 months of age and puberty), and among boys who develop acquired cryptorchidism (Barthold and Gonzalez, 2003). These data suggest a correlation between the early postnatal hormonal environment and subsequent testis position.
Breast milk from several species contains high levels of LHRH, and serum LH levels are significantly lower in female pups when suckling is temporarily prevented (Baram and others, 1977), but the hormonal response of newborn males to breast milk has not been reported. In contrast, soy formula exposes infants to high levels of phytoestrogens with potential estrogenic and/or anti-androgenic activity (Cao and others, 2009; Chen and Rogan, 2004; Lund and others, 2004) during a critical period of hormone production and completion of testicular descent (Scorer, 1964). Notably, effects of soy exposure in animal models include impaired Leydig cell function (Akingbemi and others, 2007) and decreased plasma testosterone levels during the postnatal hormone surge (Sharpe and others, 2002). A causal role for soy formula exposure in the acquired presentation of cryptorchidism is biologically plausible but can only be confirmed by prospective studies.
Our data also support a role for genetic susceptibility in risk for both congenital and acquired cryptorchidism. While recall bias may contribute to relative underreporting of cryptorchidism in family members of unaffected participants, the reported frequency of familial cryptorchidism in the control and hernia/hydrocele groups (1-3%) is similar to the population prevalence although lower than reported previously (Jensen and others, 2010). Although we could not independently confirm the accuracy of family history responses, our data mirror those reported in previous case-control studies (Brouwers and others, 2010; Elert and others, 2005) and removal of familial cryptorchidism variables from our regression models maintained or augmented risk estimates for feeding variables (data not shown). We did not find evidence for preferential maternal transmission as noted in some studies (Jensen and others, 2010; Schnack and others, 2008); ours and another case-control study (Elert and others, 2005) are likely underpowered to assess this risk.
SGA birth is correlated with cryptorchidism risk in many studies (Akre and others, 1999; Berkowitz and others, 1995; Biggs and others, 2002; Boyd and others, 2006; Brouwers and others, 2010; Damgaard and others, 2008; Hjertkvist and others, 1989; Jensen and others, 2012; Jones and others, 1998; Mayr and others, 1999; Virtanen and others, 2006) but prematurity was not necessarily an independent risk factor for persistent cryptorchidism (Hjertkvist and others, 1989; Jones and others, 1998), although effects of prematurity and SGA birth may be additive (Akre and others, 1999; Jensen and others, 2012). In the present series, SGA prevalence was similar in cryptorchid cases (3.5%) and controls (3.2%) and relatively low compared to series including only boys with persistent and/or treated cryptorchidism (11-15% incidence in cases vs. 4-10% in controls) although frequency of prematurity was similar (Berkowitz and others, 1995; Boyd and others, 2006; Brouwers and others, 2010; Jones and others, 1998). In a very large Danish birth cohort study that excluded boys with other congenital malformations, the SGA prevalence (defined as weight <20th percentile for gestational age) was 24.9% and 19% for surgically treated cases and controls, respectively (Jensen and others, 2012). The lower SGA prevalence in our series may relate in part to recruitment bias not present in population-based studies. Indeed, we may have selected against inclusion of SGA boys in both case and control groups by excluding those with any form of hypogonadism or with neuromuscular syndromes such as cerebral palsy that are associated with cryptorchidism (Depue, 1988) and intrauterine growth retardation (O’Callaghan and others, 2011) but were not excluded, for example, in the Jensen et al study (2012). Accordingly, while intrauterine growth restriction increases risk for cryptorchidism, fetal growth was normal in the vast majority of boys with nonsyndromic cryptorchidism in the present series, perhaps because we limited recruitment to otherwise healthy case-control populations.
Associations of prior pregnancy loss or premature labor, parity, persistent maternal nausea/vomiting and hypertension with congenital and/or acquired cryptorchidism or hernia/hydrocele were less robust, not sustained in models in which race or prematurity/SGA were excluded as potential confounders and/or not consistently associated with cryptorchidism in prior studies. However, our data suggest that common risk factors for inguinal hernia and cryptorchidism (maternal subfertility and/or fetal loss) may exist; similarly, low birth weight and gestational hormone use were associated with both phenotypes in a previous study (Depue, 1984). Few if any maternal factors such as tobacco, alcohol or analgesic use; gestational diabetes or vaginal bleeding; obesity, parity and mode of delivery are clearly or consistently correlated with persistent cryptorchidism in prior studies (Akre and others, 1999; Berkowitz and others, 1995; Boyd and others, 2006; Brouwers and others, 2010; Hjertkvist and others, 1989; Jensen and others, 2007; Jones and others, 1998; Kristensen and others, 2011; Mayr and others, 1999; Mongraw-Chaffin and others, 2008; Snijder and others, 2012; Swerdlow and others, 1983; Virtanen and others, 2006). Lack of reproducibility in published studies likely reflects differences in methodology and/or population heterogeneity. Birth registry databases are typically large but when documenting cryptorchidism at birth without complete or sufficiently long follow-up may exclude cases of acquired cryptorchidism or inappropriately include individuals with spontaneous testis descent after birth or with nonpalpable but absent testes. In some previous studies, high scrotal testes were considered cryptorchid (Brouwers and others, 2010; Damgaard and others, 2008; Kristensen and others, 2011), a classification that we do not adopt. Case-control studies may include only surgically-corrected cases of cryptorchidism, but may be underpowered or include syndromic cases. The present data suggest little overlap between potential maternal or perinatal risk factors for congenital and acquired cryptorchidism, phenotypes not modeled separately in prior studies. However, due to the retrospective phenotypic analysis, the present study may suffer from cryptorchid subgroup misclassification, maternal recall bias and/or be underpowered to detect potential associations. A prospective study design that involves expert exams and documentation beginning at birth is necessary to completely exclude phenotypic misclassification. Misclassification of control populations due to unrecognized testicular retractility or secondary ascent may also occur in either case-control or population-based studies but is less likely in the present series because of evaluation by experienced examiners. Other potential sources of bias include unmeasured risk variables and/or inaccurate data collection, although we expect documentation of feeding type to be potentially more reliable than other data. The omission of potentially strong correlates of cryptorchidism occurrence, such as genetic and feeding variables, may have contributed to undetected bias and partially account for inconsistent results in prior studies.
Reported associations between maternal exposure to environmental chemicals and human cryptorchidism do not show consistently significant trends (Virtanen and Adamsson, 2012). While differential recall bias is a potential concern and fetal exposure levels and/or timing remain undefined, we were unable to correlate maternal exposures with occurrence of cryptorchidism. The significance, if any, of reduced maternal use of lotion and/or perfume in association with acquired cryptorchidism is unclear; but this finding fails to support a role for phthalate exposure via maternal cosmetic use in cryptorchidism susceptibility.
Conclusions
With precise ascertainment of homogeneous case and unaffected control groups and separate analyses of congenital and acquired cryptorchidism, we observed novel relationships between soy formula exposure and reduced breastfeeding with occurrence of acquired cryptorchidism. Cases of congenital cryptorchidism did not show differences in feeding choice as would be expected for a phenotype that is established prenatally. The postnatal hormonal environment, potentially influenced by feeding choice in susceptible individuals, may facilitate or hinder migration of testes to a fully dependent position. Acquired cases comprise nearly half of those needing surgery and may account for an overall increase in cryptorchidism prevalence and/or orchidopexy. Long-term, prospective studies are needed to define factors associated with acquired cryptorchidism in otherwise healthy males and provide insight that will potentially reduce the need for medical intervention and improve reproductive health.
Supplementary Material
Acknowledgements
The authors wish to thank the Urology and OR nursing staff at A.I. duPont Hospital for Children, Coralee Karmazyn and Dawn Brown for their invaluable assistance, Dr. Robert Brent and Dr. Suzan Carmichael for their thoughtful reviews and our families for their gracious participation.
Supported by 1P20 RR20173-01 (NCRR), 1R01HD060769-01A1 (NICHD) and Nemours Biomedical Research
Abbreviations
- UDT
undescended testis
- EHR
electronic health record
- SGA
small for gestational age
- LHRH
luteinizing hormone-releasing hormone
- LH
luteinizing hormone
Footnotes
The authors have no financial disclosures or conflicts of interest to declare.
REFERENCES
- Acerini CL, Miles HL, Dunger DB, Ong KK, Hughes IA. The descriptive epidemiology of congenital and acquired cryptorchidism in a UK infant cohort. Archives of disease in childhood. 2009;94(11):868–872. doi: 10.1136/adc.2008.150219. [DOI] [PubMed] [Google Scholar]
- Akingbemi BT, Braden TD, Kemppainen BW, Hancock KD, Sherrill JD, Cook SJ, He X, Supko JG. Exposure to phytoestrogens in the perinatal period affects androgen secretion by testicular Leydig cells in the adult rat. Endocrinology. 2007;148(9):4475–4488. doi: 10.1210/en.2007-0327. [DOI] [PubMed] [Google Scholar]
- Akre O, Lipworth L, Cnattingius S, Sparen P, Ekbom A. Risk factor patterns for cryptorchidism and hypospadias. Epidemiology. 1999;10(4):364–369. [PubMed] [Google Scholar]
- Ashley RA, Barthold JS, Kolon TF. Cryptorchidism: pathogenesis, diagnosis, treatment and prognosis. Urol Clin North Am. 2010;37(2):183–193. doi: 10.1016/j.ucl.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Baram T, Koch Y, Hazum E, Fridkin M. Gonadotropin-releasing hormone in milk. Science. 1977;198(4314):300–302. doi: 10.1126/science.333582. [DOI] [PubMed] [Google Scholar]
- Barthold JS, Gonzalez R. The epidemiology of congenital cryptorchidism, testicular ascent and orchiopexy. The Journal of urology. 2003;170(6 Pt 1):2396–2401. doi: 10.1097/01.ju.0000095793.04232.d8. [DOI] [PubMed] [Google Scholar]
- Barthold JS, Kumasi-Rivers K, Upadhyay J, Shekarriz B, Imperato-Mcginley J. Testicular position in the androgen insensitivity syndrome: implications for the role of androgens in testicular descent. The Journal of urology. 2000;164(2):497–501. [PubMed] [Google Scholar]
- Bay K, Main KM, Toppari J, Skakkebaek NE. Testicular descent: INSL3, testosterone, genes and the intrauterine milieu. Nat Rev Urol. 2011 doi: 10.1038/nrurol.2011.23. [DOI] [PubMed] [Google Scholar]
- Berkowitz GS, Lapinski RH, Godbold JH, Dolgin SE, Holzman IR. Maternal and neonatal risk factors for cryptorchidism. Epidemiology. 1995;6(2):127–131. doi: 10.1097/00001648-199503000-00007. [DOI] [PubMed] [Google Scholar]
- Biggs ML, Baer A, Critchlow CW. Maternal, delivery, and perinatal characteristics associated with cryptorchidism: a population-based case-control study among births in Washington State. Epidemiology. 2002;13(2):197–204. doi: 10.1097/00001648-200203000-00015. [DOI] [PubMed] [Google Scholar]
- Boyd HA, Myrup C, Wohlfahrt J, Westergaard T, Norgaard-Pedersen B, Melbye M. Maternal serum alpha-fetoprotein level during pregnancy and isolated cryptorchidism in male offspring. Am J Epidemiol. 2006;164(5):478–486. doi: 10.1093/aje/kwj219. [DOI] [PubMed] [Google Scholar]
- Brouwers MM, de Bruijne LM, de Gier RPE, Zeielhuis GA, Feitz WFJ, Roeleveld N. Risk factors for undescended testis. J Pediatr Urol. 2010 doi: 10.1016/j.jpurol.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Bruijnen CJ, Vogels HD, Beasley SW. Review of the extent to which orchidopexy is performed at the optimal age: implications for health services. ANZ J Surg. 2008;78(11):1006–1009. doi: 10.1111/j.1445-2197.2008.04745.x. [DOI] [PubMed] [Google Scholar]
- Cao Y, Calafat AM, Doerge DR, Umbach DM, Bernbaum JC, Twaddle NC, Ye X, Rogan WJ. Isoflavones in urine, saliva, and blood of infants: data from a pilot study on the estrogenic activity of soy formula. J Expo Sci Environ Epidemiol. 2009;19(2):223–234. doi: 10.1038/jes.2008.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A, Rogan WJ. Isoflavones in soy infant formula: a review of evidence for endocrine and other activity in infants. Annu Rev Nutr. 2004;24:33–54. doi: 10.1146/annurev.nutr.24.101603.064950. [DOI] [PubMed] [Google Scholar]
- Damgaard IN, Jensen TK, Nordic Cryptorchidism Study G. Petersen JH, Skakkebaek NE, Toppari J, Main KM. Risk factors for congenital cryptorchidism in a prospective birth cohort study. PLoS ONE. 2008;3(8):e3051. doi: 10.1371/journal.pone.0003051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies TW, Williams DR, Whitaker RH. Risk factors for undescended testis. Int J Epidemiol. 1986;15(2):197–201. doi: 10.1093/ije/15.2.197. [DOI] [PubMed] [Google Scholar]
- Depue RH. Maternal and gestational factors affecting the risk of cryptorchidism and inguinal hernia. Int J Epidemiol. 1984;13(3):311–318. doi: 10.1093/ije/13.3.311. [DOI] [PubMed] [Google Scholar]
- Depue RH. Cryptorchidism, and epidemiologic study with emphasis on the relationship to central nervous system dysfunction. Teratology. 1988;37(4):301–305. doi: 10.1002/tera.1420370403. [DOI] [PubMed] [Google Scholar]
- Elert A, Jahn K, Heidenreich A, Hofmann R. Population-based investigation of familial undescended testis and its association with other urogenital anomalies. J Pediatr Urol. 2005;1:403–407. doi: 10.1016/j.jpurol.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Fenton TR. A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format. BMC Pediatr. 2003;3:13. doi: 10.1186/1471-2431-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferlin A, Zuccarello D, Zuccarello B, Chirico MR, Zanon GF, Foresta C. Genetic alterations associated with cryptorchidism. JAMA. 2008;300(19):2271–2276. doi: 10.1001/jama.2008.668. [DOI] [PubMed] [Google Scholar]
- Foresta C, Zuccarello D, Garolla A, Ferlin A. Role of hormones, genes, and environment in human cryptorchidism. Endocr Rev. 2008;29(5):560–580. doi: 10.1210/er.2007-0042. [DOI] [PubMed] [Google Scholar]
- Gendrel D, Job JC, Roger M. Reduced post-natal rise of testosterone in plasma of cryptorchid infants. Acta endocrinologica. 1978;89(2):372–378. doi: 10.1530/acta.0.0890372. [DOI] [PubMed] [Google Scholar]
- Hack WW, Meijer RW, Van Der Voort-Doedens LM, Bos SD, De Kok ME. Previous testicular position in boys referred for an undescended testis: further explanation of the late orchidopexy enigma? BJU international. 2003;92(3):293–296. doi: 10.1046/j.1464-410x.2003.04317.x. [DOI] [PubMed] [Google Scholar]
- Hack WW, van der Voort-Doedens LM, Goede J, van Dijk JM, Meijer RW, Sijstermans K. Natural history and long-term testicular growth of acquired undescended testis after spontaneous descent or pubertal orchidopexy. BJU international. 2010;106(7):1052–1059. doi: 10.1111/j.1464-410X.2010.09226.x. [DOI] [PubMed] [Google Scholar]
- Hauck FR, Tanabe KO, Moon RY. Racial and ethnic disparities in infant mortality. Semin Perinatol. 2011;35(4):209–220. doi: 10.1053/j.semperi.2011.02.018. [DOI] [PubMed] [Google Scholar]
- Hjertkvist M, Damber JE, Bergh A. Cryptorchidism: a registry based study in Sweden on some factors of possible aetiological importance. J Epidemiol Community Health. 1989;43(4):324–329. doi: 10.1136/jech.43.4.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MS, Bonde JP, Olsen J. Prenatal alcohol exposure and cryptorchidism. Acta Paediatr. 2007;96(11):1681–1685. doi: 10.1111/j.1651-2227.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- Jensen MS, Toft G, Thulstrup AM, Henriksen TB, Olsen J, Christensen K, Bonde JP. Cryptorchidism concordance in monozygotic and dizygotic twin brothers, full brothers, and half-brothers. Fertil Steril. 2010;93(1):124–129. doi: 10.1016/j.fertnstert.2008.09.041. [DOI] [PubMed] [Google Scholar]
- Jensen MS, Wilcox AJ, Olsen J, Bonde JP, Thulstrup AM, Ramlau-Hansen CH, Henriksen TB. Cryptorchidism and hypospadias in a cohort of 934,538 Danish boys: the role of birth weight, gestational age, body dimensions, and fetal growth. Am J Epidemiol. 2012;175(9):917–925. doi: 10.1093/aje/kwr421. [DOI] [PubMed] [Google Scholar]
- Jones ME, Swerdlow AJ, Griffith M, Goldacre MJ. Prenatal risk factors for cryptorchidism: a record linkage study. Paediatr Perinat Epidemiol. 1998;12(4):383–396. doi: 10.1046/j.1365-3016.1998.00144.x. [DOI] [PubMed] [Google Scholar]
- Kaftanovskaya EM, Feng S, Huang Z, Tan Y, Barbara AM, Kaur S, Truong A, Gorlov IP, Agoulnik AI. Suppression of insulin-like 3 receptor reveals the role of βετα-catenin and Notch signaling in gubernaculum development. Mol Endocrinol. 2011;25 doi: 10.1210/me.2010-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen DM, Hass U, Lesne L, Lottrup G, Jacobsen PR, Desdoits-Lethimonier C, Boberg J, Petersen JH, Toppari J, Jensen TK, Brunak S, Skakkebaek NE, Nellemann C, Main KM, Jegou B, Leffers H. Intrauterine exposure to mild analgesics is a risk factor for development of male reproductive disorders in human and rat. Hum Reprod. 2011;26(1):235–244. doi: 10.1093/humrep/deq323. [DOI] [PubMed] [Google Scholar]
- Lauria L, Lamberti A, Grandolfo M. Smoking Behaviour before, during, and after Pregnancy: The Effect of Breastfeeding. Scientific World Journal. 2012;2012:154910. doi: 10.1100/2012/154910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund TD, Munson DJ, Haldy ME, Setchell KD, Lephart ED, Handa RJ. Equol is a novel anti-androgen that inhibits prostate growth and hormone feedback. Biology of reproduction. 2004;70(4):1188–1195. doi: 10.1095/biolreprod.103.023713. [DOI] [PubMed] [Google Scholar]
- Main KM, Skakkebaek NE, Virtanen HE, Toppari J. Genital anomalies in boys and the environment. Baillieres Best Pract Res Clin Endocrinol Metab. 2010;24(2):279–289. doi: 10.1016/j.beem.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Mayr JM, Lawrenz K, Berghold A. Undescended testicles: an epidemiological review. Acta Paediatr. 1999;88(10):1089–1093. doi: 10.1080/08035259950168144. [DOI] [PubMed] [Google Scholar]
- Mongraw-Chaffin ML, Cohn BA, Cohen RD, Christianson RE. Maternal smoking, alcohol consumption, and caffeine consumption during pregnancy in relation to a son’s risk of persistent cryptorchidism: a prospective study in the Child Health and Development Studies cohort, 1959-1967. Am J Epidemiol. 2008;167(3):257–261. doi: 10.1093/aje/kwm311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Davies TW, Tsukamoto T, Kumamoto Y, Fukuda K. Maternal and other factors of cryptorchidism--a case-control study in Japan. Kurume Med J. 1992;39(2):53–60. doi: 10.2739/kurumemedj.39.53. [DOI] [PubMed] [Google Scholar]
- Nef S, Shipman T, Parada LF. A molecular basis for estrogen-induced cryptorchidism. Developmental biology. 2000;224(2):354–361. doi: 10.1006/dbio.2000.9785. [DOI] [PubMed] [Google Scholar]
- O’Callaghan ME, MacLennan AH, Gibson CS, McMichael GL, Haan EA, Broadbent JL, Goldwater PN, Dekker GA, Australian Collaborative Cerebral Palsy Research G. Epidemiologic associations with cerebral palsy. Obstet Gynecol. 2011;118(3):576–582. doi: 10.1097/AOG.0b013e31822ad2dc. [DOI] [PubMed] [Google Scholar]
- Schnack TH, Zdravkovic S, Myrup C, Westergaard T, Wohlfahrt J, Melbye M. Familial aggregation of cryptorchidism--a nationwide cohort study. Am J Epidemiol. 2008;167(12):1453–1457. doi: 10.1093/aje/kwn081. [DOI] [PubMed] [Google Scholar]
- Scorer CG. The Descent of the Testis. Archives of disease in childhood. 1964;39:605–609. doi: 10.1136/adc.39.208.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe RM, Martin B, Morris K, Greig I, McKinnell C, McNeilly AS, Walker M. Infant feeding with soy formula milk: effects on the testis and on blood testosterone levels in marmoset monkeys during the period of neonatal testicular activity. Hum Reprod. 2002;17(7):1692–1703. doi: 10.1093/humrep/17.7.1692. [DOI] [PubMed] [Google Scholar]
- Snijder CA, Kortenkamp A, Steegers EA, Jaddoe VW, Hofman A, Hass U, Burdorf A. Intrauterine exposure to mild analgesics during pregnancy and the occurrence of cryptorchidism and hypospadia in the offspring: the Generation R Study. Hum Reprod. 2012;27(4):1191–1201. doi: 10.1093/humrep/der474. [DOI] [PubMed] [Google Scholar]
- Staub C, Rauch M, Ferriere F, Trepos M, Dorval-Coiffec I, Saunders PT, Cobellis G, Flouriot G, Saligaut C, Jegou B. Expression of estrogen receptor ESR1 and its 46-kDa variant in the gubernaculum testis. Biology of reproduction. 2005;73(4):703–712. doi: 10.1095/biolreprod.105.042796. [DOI] [PubMed] [Google Scholar]
- Swerdlow AJ, Wood KH, Smith PG. A case-control study of the aetiology of cryptorchidism. J Epidemiol Community Health. 1983;37(3):238–244. doi: 10.1136/jech.37.3.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villumsen AL, Zachau-Christiansen B. Spontaneous alterations in position of the testes. Archives of disease in childhood. 1966;41(216):198–200. doi: 10.1136/adc.41.216.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen HE, Adamsson A. Cryptorchidism and endocrine disrupting chemicals. Mol Cell Endocrinol. 2012;355:208–220. doi: 10.1016/j.mce.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Virtanen HE, Tapanainen AE, Kaleva MM, Suomi AM, Main KM, Skakkebaek NE, Toppari J. Mild gestational diabetes as a risk factor for congenital cryptorchidism. J Clin Endocrinol Metab. 2006;91(12):4862–4865. doi: 10.1210/jc.2006-1420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.