Abstract
Purpose
To investigate the effect of changes in expiratory intrathoracic pressure (ITP) on stroke volume (SV) at rest and during moderate exercise in patients with heart failure vs. healthy individuals.
Methods
SV was obtained by echocardiography during spontaneous breathing and during expiratory loads of 5 and 10 cm H2O produced by a ventilator in 11 patients with heart failure (61±9 years, EF: 32±4%, NYHA class I-II) and 11 age-matched healthy individuals at rest and during exercise at 60% of aerobic capacity on a semi-recumbent cycle ergometer.
Results
At rest, expiratory loading did not change heart rate, SV index (SVI) or cardiac index (CI) in either group. During moderate exercise, expiratory loading increased SVI and CI in patients with heart failure, but decreased SVI and CI in healthy individuals. There was a negative correlation between changes in gastric pressure and SVI (r=−0.51, p<0.05) in healthy individuals, while there was a positive correlation between changes in gastric pressure accompanying expiratory loading and CI (r=0.83, p<0.01) in patients with heart failure.
Conclusion
Expiratory loading during moderate exercise elicited increases in SVI and CI in patients with heart failure but decreased SVI and CI in healthy individuals. Improvements in cardiac function during submaximal exercise in patients with heart failure may be caused by a beneficial reduction in LV preload.
Keywords: preload, cardiorespiratory, exercise tolerance, stroke volume
Introduction
Patients with heart failure frequently experience expiratory flow limitation during submaximal exercise, possibly as a consequence of low end-expiratory lung volumes (11). Despite room to increase tidal volume by encroaching on the inspiratory reserve volume, end-expiratory lung volume remains near residual volume throughout exercise in many patients with heart failure (11). It may be hypothesized that heart failure patients maintain these low end-expiratory lung volumes, possibly resulting in expiratory flow limitation and increased expiratory intrathoracic pressure (ITP), in an attempt to optimize interactions between ITP and stroke volume (SV) which could improve the exercise response in this population (13).
Expiratory loading uses resistors to increase expiratory ITP, which, if maintained over successive respiratory cycles, elevates right atrial pressure and results in decreased venous return and smaller SV (5). Expiratory loading also reduces transmural pressure and left ventricular (LV) afterload (10, 23, 24) causing increased SV (29). In healthy individuals, reductions in LV preload are believed to outweigh the effect of reductions in LV afterload on SV, therefore expiratory loading results in a decreased SV (1, 21, 26). In contrast, patients with heart failure are more sensitive to changes in LV afterload due to a decreased LV compliance indicating that substantial increases in the elevated LV filling pressure cause small changes in LV volume, implying that the hearts of these patients operate on the upper flat part of the Starling curve (12, 19). Thus, expiratory loading is proposed to improve resting cardiac function in patients with heart failure by decreasing LV afterload and/or reducing effective filling to a level more acceptable for LV performance in these patients (9). However, the effect of expiratory loading on SV and cardiac output (CO) has never been determined during exercise in patients with heart failure.
Exercise influences cardiopulmonary interactions by augmenting the fluctuations in intrathoracic and gastric pressure, increasing tidal volume and increasing venous return following the contraction of peripheral skeletal muscles (18, 31), therefore the objective of this study was to determine the effect of expiratory loading on SV and CO during moderate exercise in patients with heart failure vs. healthy individuals. We hypothesized that increases in expiratory ITP would increase SV and CO during moderate exercise in patients with heart failure but would decrease SV and CO in age-matched healthy individuals.
Methods
Eleven patients with heart failure and 11 age-matched healthy individuals participated to the study. Patients with a history of ischemic or stable idiopathic heart failure of NYHA class I-II, with an ejection fraction ≤40%, with no history of dangerous arrhythmias and who were not pacemaker-dependent were included in the study. Ten patients with heart failure were taking angiotensin-converting enzyme inhibitors, 9 were on non-selective beta blockers/alpha-1 blockers, 2 on beta blockers, 7 were taking aspirin, 8 were on diuretics and 3 were using digoxin. Patients did not stop taking their medication during the study. Control participants consisted of age-matched healthy individuals with no history of cardiovascular abnormalities. Exclusion criteria included body mass index ≥34.9 kg/m2, a smoking history of >15 pack/year, an inability to perform exercise, allergies to lidocaine or latex and a deviated nasal septum. A blood sample was collected to ensure that hemoglobin levels were above 11 and 12 mg/dL for females and males, respectively. Ejection fraction, LV mass and brain natriuretic peptide (BNP) levels, measured within the year preceding the study, were obtained from medical records of patients. Patients had an ejection fraction of 32±4%, a LV mass of 269±67 g, and BNP levels of 248±193 pg/ml (a BNP cutoff value of 100 pg/ml differentiates heart failure from other causes of dyspnea (2)). This study conformed to the standards of the Declaration of Helsinki and was approved by the Mayo Clinic Institutional Review Board. All participants provided written informed consent.
Study protocol
Participants reported to the Human Integrative and Environmental Physiology laboratory on 2 occasions. The first visit included an incremental ergometry test to determine maximal aerobic capacity. The second visit included spirometry measurements and the determination of the cardiopulmonary response to 2 min of spontaneous breathing followed by 2 min of a first level of expiratory loading at 5 cm H2O and 2 min of a second level of expiratory loading at 10 cm H2O at rest and during steady-state exercise at an intensity of 60% of maximal aerobic capacity. During this same visit, the resting and exercising cardiopulmonary response to 2 min of inspiratory loading and 2 min of inspiratory unloading was also determined and these results have been published separately (14).
Maximal aerobic capacity
Maximal aerobic capacity was determined during an incremental workload exercise test on a semi-recumbent ergometer (Ergoselect II 1200, Ergoline, Bitz, Germany) with a 12-lead electrocardiograph (Case®, GE Healthcare, Milwaukee, WI). Initial workload was set at 25 W and increased by 25 W with each 2 minute stage until perceived exertion as assessed using the Borg scale (3). Breath by breath data were collected and analyzed every 5 sec using a metabolic system (CPX, Medgraphics, St Paul, MN). Maximal aerobic capacity was considered to be achieved when 2 of the following criteria were met: a respiratory exchange ratio >1.1, an increase in oxygen consumption <100 ml/min with a further increase in workload, or achievement of age-predicted maximal heart rate.
Pulmonary function, lung mechanics and manipulation of expiratory ITP
Spirometry measurements included assessment of forced vital capacity (FVC) and forced expiratory flow in 1 sec (FEV1) (CPFS/D™ USB, Medgraphics, St Paul, MN). Airflow was assessed through a mouthpiece attached in series to apneumotachograph with a switching valve connected to a two-way non-rebreathing valve, and tidal volume was obtained from the digital integration of the linearized flow signal following correction for drift. For assessment of esophageal (ITP) and gastric pressures, small latex balloons were simultaneously inserted through the nose into the esophagus and stomach while mouth pressure was measured from a line inserted in the pneumotachograph. Expiratory loading was created using a proportional assist ventilator (Respironics, model 622175, Murrysville, PA) connected to the exhalation arm of the non-rebreathing valve.
Cardiovascular function
SV was assessed by echocardiography on a semi-recumbent ergometer during a 2 min period of spontaneous breathing, 2 min of expiratory loading at 5 cm H2O and 2 min of expiratory loading at 10 cm H2O (Biosound, Esaote, Genoa, Italy) at rest and during exercise. LV outflow tract diameter was determined from the parasternal long axis view at rest and was assumed to remain constant during exercise (6). The time-velocity integral of the LV outflow tract was obtained in the 5 chamber view of the apical window. SV was calculated as: (0.785 X (LV outflow tract diameter)2 X time-velocity integral of the LV outflow tract) (17). During each condition, an average of all SVs measured was obtained for each individual. Beat-by-beat heart rate, mean arterial pressure (MAP) and systemic vascular resistance (SVR) were simultaneously obtained by finger arterial pressure waveform analysis (Nexfin, BMEYE, Amsterdam, Netherlands). CO was calculated as the product of heart rate and SV. Because weight was different between groups, SV and CO were indexed to body surface area (BSA) as calculated from the Du Bois and Du Bois formula (8).
Data analysis
Time aligned measurements of tidal volume, intrathoracic and gastric pressures, heart rate, MAP and SVR were acquired at a sampling frequency of 1000 Hz (PowerLab, ADInstruments, Colorado) and analyzed using commercially available software (LabChart 7.1, ADInstruments, Colorado). An observer blinded to both condition and group performed the analyses of the time-velocity integrals of the LV outflow tract. All measurements were performed twice, or until the calculated average SVs were within 5% of each other, and the average is reported. Briefly, a first measure of the average SV for each condition and for each individual was performed. A few weeks later, a second measure of the average SV was performed. The coefficient of variation for these measures during spontaneous breathing and both levels of expiratory loading were of 1.3 and 2.8% at rest and during exercise in healthy individuals, and of 1.7 and 1.9% at rest and during exercise in patients with heart failure. If both measures were not within 5% of each other, a third measure of SV was performed few weeks later. Two of the 3 measures that were within 5% of each other were averaged and reported. Comparisons of group characteristics were conducted using paired t tests. A mixed factorial analysis of variance was used to test for condition (spontaneous breathing (S), 5 cm H2O and 10 cm H2O) and group (healthy individuals vs. patients with heart failure) effects for all measurements. When main effects or a group and condition interaction were significant, post hoc analyses were performed using a Bonferroni correction. Pearson’s correlation coefficient was used for the analysis of associations between variables. Results are expressed as mean ± standard deviations and p values <0.05 were considered statistically significant.
Results
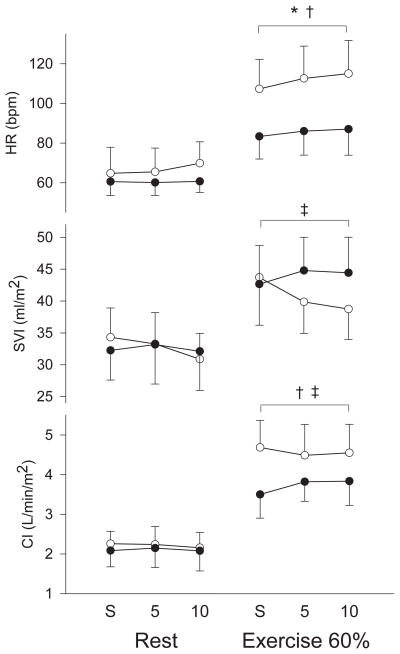
Age, height, body mass index, maximal respiratory exchange ratio, FVC, FEV1 and hemoglobin levels were not different between groups (Table 1). Weight and BSA were greater and maximal aerobic capacity, maximal workload, maximal heart rate, MAP and SVR were lower in patients with heart failure. At 60% of aerobic capacity, workload was lower in patients than healthy individuals (69±23 vs. 94±41 W, p=0.03) while the rates of perceived exertion were the same between groups (14±1 vs. 14±3, p=0.20). At rest, expiratory loading of 5 and 10 cm H2O resulted in a more positive peak expiratory ITP and a more negative peak inspiratory ITP in both groups of subjects while there were no changes in gastric pressure in either group (Table 2). Expiratory loading also resulted in increases in tidal volume and a slowing of the respiratory rate in both groups. Expiratory loading did not induce any changes in MAP or in SVR (Table 3). There were no changes in heart rate (p=0.16), stroke volume index (SVI) (p=0.14) or cardiac index (CI) (p=0.73) with expiratory loading in either group (Figure 1). Similarly, there were no changes in SV or CO with expiratory loading in either group (Table 3).
Table 1.
Participants’ characteristics
| Heart failure | Healthy | |
|---|---|---|
| Female/Male | 1/10 | 2/9 |
| Age (years) | 61 ± 9 | 61 ± 8 |
| Height (cm) | 178 ± 8 | 175 ± 8 |
| Weight (kg) | 96.8 ± 10.5 | 82.9 ± 10.9 * |
| BMI (kg/m2) | 30.5 ± 2.4 | 27.3 ± 4.1 |
| BSA (m2) | 2.15 ± 0.16 | 1.98 ± 0.14 * |
| MAP (mmHg) | 78 ± 11 | 100 ± 11 * |
| SVR (dyn·s/cm5) | 930 ± 350 | 1363 ± 359 * |
| VO2 max (ml/kg/min) | 19 ± 5.8 | 24.2 ± 4.7 * |
| Maximal workload (W) | 131 ± 42 | 159 ± 39 * |
| Maximal HR (bpm) | 118 ± 30 | 150 ± 24 * |
| Maximal RER | 1.14 ± 0.07 | 1.16 ± 0.03 |
| FVC (% predicted) | 93.6 ± 16.4 | 95.0 ± 16.1 |
| FEV1 (% predicted) | 91.5 ± 13.3 | 96.7 ± 14.4 |
| Hb (mg/dL) | 14.2 ± 1.0 | 13.4 ± 0.9 |
BMI: body mass index, BSA: body surface area, MAP: mean arterial pressure, SVR: systemic vascular resistance, VO2 max: maximal aerobic capacity, HR: heart rate, RER: respiratory exchange ratio, FVC: forced vital capacity, FEV1: forced expiratory flow in 1 second, Hb: haemoglobin.
p < 0.05 between healthy and heart failure.
Table 2.
Ventilatory responses to expiratory loading at rest and during moderate exercise
|
|
||||||
|---|---|---|---|---|---|---|
| Healthy | Heart failure | |||||
| S | 5 | 10 | S | 5 | 10 | |
| Rest | ||||||
| Peak exp ITP (cm H2O) | 2.1 ± 3.6 * | 4.2 ± 4.4 | 6.2 ± 4.2 | 3.6 ± 2.0 * | 5.0 ± 1.8 | 7.4 ± 3.2 |
| Peak insp ITP (cm H2O) | −3.7 ± 1.6 * | −6.9 ± 2.9 | −8.7 ± 4.3 | −3.7 ± 2.0 * | −6.4 ± 2.2 | −7.8 ± 3.3 |
| Pg (cm H2O) | 1,4 ± 3,4 | 1,0 ± 3,0 | 0,8 ± 2,6 | 0,5 ± 3,6 | 0,5 ± 3,5 | 0,9 ± 4,1 |
| Tidal volume (L) | 1.33 ± 0.30 * | 1.65 ± 0.44 | 1.60 ± 0.44 | 1.29 ± 0.12 * | 1.67 ± 0.35 | 1.78 ± 0.68 |
| RR (breath/min) | 14.9 ± 3.3 * | 12.0 ± 4.7 | 10.9 ± 5.7 | 15.1 ± 4.1 * | 12.1 ± 4.1 | 10.1 ± 4.1 |
| Exercise | ||||||
| Peak exp ITP (cm H2O) | 3.8 ± 5.0 * | 6.3 ± 5.5 | 8.8 ± 3.9 | 4.4 ± 1.9 * | 7.0 ± 2.2 | 9.3 ± 2.1 |
| Peak insp ITP (cm H2O) | −10.2 ± 4.6 * | −10.9 ± 4.0 | −13.2 ± 3.8 | −7.8 ± 3.4 * | −10.1 ± 3.2 | −11.4 ± 3.4 |
| Pg (cm H2O) | −0,4 ± 0,6 * | −0,5 ± 0,8 | −0,1 ± 0,7 | 0,7 ± 4,5 * | 1,2 ± 4,6 | 1,7 ± 5,0 |
| Tidal volume (L) | 3.07 ± 0.90 * | 3.64 ± 1.28 | 3.45 ± 1.28 | 2.64 ± 0.52 * | 2.98 ± 0.80 | 3.07 ± 0.73 |
| RR (breath/min) | 24.9 ± 6.1 | 24.0 ± 7.8 | 23.9 ± 7.9 | 25.7 ± 5.8 | 22.9 ± 5.3 | 22.2 ± 6.1 |
S: spontaneous breathing, 5: expiratory load of 5 cmH2O, 10: expiratory load of 10 cmH2O, ITP: intrathoracic pressure, Pg: gastric pressure, RR: respiratory rate.
main effect for expiratory loading.
Table 3.
Hemodynamic responses to expiratory loading at rest and during moderate exercise
|
|
||||||
|---|---|---|---|---|---|---|
| Healthy | Heart failure | |||||
| S | 5 | 10 | S | 5 | 10 | |
| Rest | ||||||
| MAP (mmHg) | 100 ± 11 † | 100 ± 12 | 100 ± 12 | 78 ± 11 | 79 ± 13 | 79 ± 13 |
| SVR (dyn·s/cm5) | 1409 ± 342 † | 1414 ± 364 | 1422 ± 369 | 930 ± 350 | 932 ± 353 | 956 ± 346 |
| SV (ml) | 67 ± 10 † | 65 ± 11 | 61 ± 10 | 70 ± 9 | 72 ± 11 | 69 ± 11 |
| CO (L/min) | 4.4 ± 0.6 | 4.4 ± 0.9 | 4.2 ± 0.9 | 4.5 ± 0.9 | 4.6 ± 0.9 | 4.5 ± 0.9 |
| Exercise | ||||||
| MAP (mmHg) | 115 ± 13 * † ‡ | 107 ± 12 | 109 ± 15 | 90 ± 9 * | 91 ± 11 | 92 ± 10 |
| SVR (dyn·s/cm5) | 818 ± 149 * ‡ | 719 ± 146 | 720 ± 170 | 674 ± 323 * | 683 ± 336 | 693 ± 344 |
| SV (ml) | 87 ± 17 † | 73 ± 13 | 77 ± 12 | 92 ± 14 | 96 ± 11 | 95 ± 10 |
| CO (L/min) | 9.3 ± 1.8 | 8.9 ± 2.1 | 9.0 ± 1.9 | 7.7 ± 1.5 | 8.4 ± 1.2 | 8.4 ± 1.4 |
S: spontaneous breathing, 5: expiratory load of 5 cmH2O, 10: expiratory load of 10 cmH2O, MAP: mean arterial pressure, SVR: systemic vascular resistance, SV: stroke volume, CO: cardiac output.
main effect for expiratory loading,
main effect for group
group and condition interaction.
Figure 1.
Cardiovascular response to expiratory loading at rest and during moderate exercise in patients with heart failure (black circles) and healthy individuals (white circles). * main effect for expiratory loading, † main effect for group ‡ group and condition interaction. CI: cardiac index, HR: heart rate, S: spontaneous breathing, SVI: stroke volume index, 5: expiratory load of 5 cm H2O, 10: expiratory load of 10 cm H2O.
During moderate exercise, both levels of expiratory loading resulted in increases in peak expiratory ITP and gastric pressure and in an increased negativity of peak inspiratory ITP in both groups (Table 2). Expiratory loading also resulted in increases in tidal volume without significant changes in respiratory rate. While expiratory loading decreased MAP and SVR in healthy individuals, it did not change MAP and SVR in patients (Table 3). Expiratory loading increased SVI and CI in patients with heart failure and decreased SVI and CI in healthy individuals (SVI: p=0.01, CI: p=0.03) (Figure 1). Similarly, expiratory loading increased SV and CO in patients and decreased SV and CO in healthy individuals (Table 3). Heart rate increased with expiratory loading in both groups (p<0.01) (Figure 1). In healthy individuals, there was a negative correlation between changes in gastric pressure and SVI (r=−0.51, p=0.02) with expiratory loading. In patients with heart failure, there was a positive correlation between changes in gastric pressure accompanying expiratory loading and CI (r=0.83, p<0.001).
Discussion
In accordance with our hypothesis, these novel findings show that both levels of expiratory loading elicited increases in SVI, heart rate and CI during moderate exercise in patients with heart failure. As suggested under resting conditions (9), improvements in exercising cardiac function with expiratory loading in patients with heart failure may be caused by a decreased LV afterload combined with a beneficial reduction in LV preload following a pressure gradient change for systemic venous return. In healthy individuals, both levels of expiratory loading elicited reductions in SVI and CI but increases in heart rate during moderate exercise, supporting reports that reductions in venous return outweigh reductions in LV afterload in healthy individuals (1, 21, 26).
Our findings of decreases in exercising SVI and CI in healthy individuals further support that increasing expiratory ITP compromised venous return and LV filling in this population (7, 22), while an overriding influence of LV afterload in patients with heart failure (12) supports our observed increases in exercising SVI and CI. These important findings of improved cardiac function with expiratory loading during exercise in patients with heart failure are in accordance with the results of Grace et al. (9) who showed that expiratory loading improved resting CO in patients with a pulmonary arterial wedge pressure ≥ 19 mmHg. Conversely, patients with a pulmonary arterial wedge pressure of 12 mmHg, equivalent to the pressure observed in healthy individuals, had a decreased resting CO with expiratory loading (9). The authors suggested that the increase in resting CO induced by expiratory loading in patients with elevated filling pressures was due to a reduced LV preload as it decreases effective filling to a level more acceptable for LV performance in this population (9, 19). At rest, half of our patients with heart failure had a decrease in CO while the other half had an increase in CO, suggesting that the resting pulmonary arterial wedge pressures of our patients were both below and above 19 mmHg, which resulted in an average lack of change in CO with expiratory loading. Patients with heart failure have greater increases in pulmonary capillary wedge pressure during exercise (4, 12). With exercise, all but one of our patients with heart failure had an increase in CO with expiratory loading, supporting greater pulmonary arterial wedge pressures in this group. Therefore, it is suggested that improvements in exercising cardiac function with expiratory loading depended on an optimal LV filling and a decreased LV afterload (20).
In the present study, there was a strong positive correlation between changes in gastric pressure and CI in patients with heart failure. Elevations in gastric pressure are shown to decrease venous return from the legs and to decrease blood flow through the inferior vena cava (28). Therefore, the correlation between gastric pressure and CI further suggests that a reduced venous return, due to the increased gastric pressure, reduces LV filling to a level more adequate for LV performance in patients with heart failure. On the other hand, we observed a negative relationship between gastric pressure and SVI during moderate exercise in healthy individuals, suggesting that the increased gastric pressure inhibits venous return and reduces CI during exercise in healthy individuals. These findings confirm previous results that increases in gastric pressure following abdominal muscles or diaphragmatic contraction induced by expiratory loading have been correlated to a fall in SV and CO in healthy individuals (27).
The breathing pattern in response to expiratory loading did not differ between healthy individuals and patients with heart failure. Expiratory loading during exercise has been reported to result in a slight hypoventilation through an unchanged or slightly increased tidal volume with a decreased respiratory rate in healthy individuals (25, 27). We observed an increase in tidal volume and a decreased respiratory rate at rest and an increased tidal volume without a significant decrease in respiratory rate during exercise in both groups. We also observed a more negative inspiratory ITP (increases of approximately 5 cm H2O) with expiratory loading both at rest and during exercise. A more negative inspiratory ITP would potentially increase LV afterload which could result in a reduced SV in patients with heart failure. However, our observations of increases in SVI and CI with expiratory loading in patients with heart failure suggest that the increase in negativity of inspiratory ITP had little impact on the exercising cardiac response to expiratory loading.
Expiratory loads of 5 and 10 cm H2O similarly increased SVI and CI during moderate exercise in relatively asymptomatic patients with stable heart failure, which supports our hypothesis that increased expiratory ITP helps to preserve exercising cardiac function in this population (13). The lack of effect between loads of 5 and 10 cm H2O on the cardiac response may be explained by different contributions from the reduction in venous return which may no longer be beneficial at 10 cm H2O, and from a decreased LV afterload. Many factors such as disease severity, observed through pulmonary capillary wedge pressure, heart size and/or volume status, could alter these cardiorespiratory interactions in patients with heart failure and therefore act as important influences in the SV response to increased expiratory ITP. For example, increases in expiratory ITP and gastric pressure in heart failure patients in a state of volume overload would reduce venous return and LV preload and improve the functional capacity of the over distended heart, possibly resulting in increased SVI and CI. LV afterload was solely defined as LV transmural pressure, however wall stress, representing afterload in the myocardial fibers during LV ejection, is also affected by the geometry of the left ventricle (30). We did not perform measurements of LV cavity size or wall thickness. We also did not acquire measurements of right ventricular function, which could lead to a better understanding of the role of changes in LV preload on cardiac function. Future studies should therefore include measurements of LV volumes and right ventricular function in order to gain a better understanding of the respective contribution of changes in LV preload and afterload on cardiac function.
Improving exercise capacity in patients with heart failure is of great importance as peak VO2 is the best predictor of survival in these patients (15). Many patients with heart failure perform exercise training through cardiac rehabilitation programs. Therefore, the use of expiratory loading while exercising could serve as a rationale strategy to improve cardiorespiratory interactions. Improved cardiac output during expiratory loading could lead to improved muscle perfusion and greater exercise duration and intensity, which could lead to improvements in peak VO2. The feasibility of expiratory loading while exercising remains to be determined, but could consist of a simple device with resistors. Inspiratory unloading has also been reported to reduce exertional leg discomfort and increase exercise endurance in patients with heart failure (16), and we recently demonstrated that inspiratory unloading improved SVI during moderate exercise in this population (14). Therefore, a combination of inspiratory unloading and expiratory loading, such as continuous positive airway pressure, could be ideal in improving exercise endurance in patients with heart failure. This breathing strategy, intended to reduce LV preload and afterload, may be particularly beneficial for patients with elevated pulmonary arterial wedge pressure and in a state of volume overload, however further studies including measurements of pulmonary arterial wedge pressure and blood volume will be needed to determine whether expiratory loading is beneficial for patients with more severe heart failure. In conclusion, both levels of expiratory loading elicited increases in SVI, heart rate and CI during moderate exercise in patients with heart failure, which may be caused by a decreased LV afterload combined with a beneficial reduction in LV preload following a pressure gradient change for systemic venous return.
Acknowledgments
Source of Funding: This study was supported by NIH Grant HL71478 and S. Lalande was supported by an American Heart Association Postdoctoral Fellowship (0920054G). The results of the present study do not constitute endorsement by ACSM.
Footnotes
Conflict of interest
None.
References
- 1.Aliverti A, Kayser B, Macklem PT. A human model of the pathophysiology of chronic obstructive pulmonary disease. Respirology. 2007;12(4):478–85. doi: 10.1111/j.1440-1843.2007.01106.x. [DOI] [PubMed] [Google Scholar]
- 2.Bhalla V, Willis S, Maisel AS. B-type natriuretic peptide: the level and the drug--partners in the diagnosis of congestive heart failure. Congest Heart Fail. 2004;10(1 Suppl 1):3–27. doi: 10.1111/j.1527-5299.2004.03310.x. [DOI] [PubMed] [Google Scholar]
- 3.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2(2):92–8. [PubMed] [Google Scholar]
- 4.Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail. 2010;3(5):588–95. doi: 10.1161/CIRCHEARTFAILURE.109.930701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cassidy SS, Mitchell JH. Effects of positive pressure breathing on right and left ventricular preload and afterload. Fed Proc. 1981;40(8):2178–81. [PubMed] [Google Scholar]
- 6.Christie J, Sheldahl LM, Tristani FE, Sagar KB, Ptacin MJ, Wann S. Determination of stroke volume and cardiac output during exercise: comparison of two-dimensional and Doppler echocardiography, Fick oximetry, and thermodilution. Circulation. 1987;76(3):539–47. doi: 10.1161/01.cir.76.3.539. [DOI] [PubMed] [Google Scholar]
- 7.Cournand A, Motley HL. Physiological studies of the effects of intermittent positive pressure breathing on cardiac output in man. Am J Physiol. 1948;152(1):162–74. doi: 10.1152/ajplegacy.1947.152.1.162. [DOI] [PubMed] [Google Scholar]
- 8.Du Bois D, Du Bois EF. A formula to estimate the approximate surface area if height and weight be known. 1916. Nutrition. 1989;5(5):303–11. discussion 312-3. [PubMed] [Google Scholar]
- 9.Grace MP, Greenbaum DM. Cardiac performance in response to PEEP in patients with cardiac dysfunction. Crit Care Med. 1982;10(6):358–60. doi: 10.1097/00003246-198206000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Innes JA, De Cort SC, Kox W, Guz A. Within-breath modulation of left ventricular function during normal breathing and positive-pressure ventilation in man. J Physiol. 1993;460:487–502. doi: 10.1113/jphysiol.1993.sp019483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson BD, Beck KC, Olson LJ, O’Malley KA, Allison TG, Squires RW, Gau GT. Ventilatory constraints during exercise in patients with chronic heart failure. Chest. 2000;117(2):321–32. doi: 10.1378/chest.117.2.321. [DOI] [PubMed] [Google Scholar]
- 12.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17(5):1065–72. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 13.Lalande S, Johnson BD. Breathing strategy to preserve exercising cardiac function in patients with heart failure. Medical hypotheses. 2010;74(3):416–21. doi: 10.1016/j.mehy.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lalande S, Luoma CE, Miller AD, Johnson BD. Effect of changes in intrathoracic pressure on cardiac function at rest and during moderate exercise in health and heart failure. Experimental physiology. 2012;97(2):248–56. doi: 10.1113/expphysiol.2011.061945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mancini DM, Eisen H, Kussmaul W, Mull R, Edmunds LH, Jr, Wilson JR. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83(3):778–86. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 16.O’Donnell DE, D’Arsigny C, Raj S, Abdollah H, Webb KA. Ventilatory assistance improves exercise endurance in stable congestive heart failure. Am J Respir Crit Care Med. 1999;160(6):1804–11. doi: 10.1164/ajrccm.160.6.9808134. [DOI] [PubMed] [Google Scholar]
- 17.Oh JK, Seward JB, Tajik AJ. The echo manual. Lippincott Williams & Wilkins; 2006. p. 191. [Google Scholar]
- 18.Olafsson S, Hyatt RE. Ventilatory mechanics and expiratory flow limitation during exercise in normal subjects. J Clin Invest. 1969;48(3):564–73. doi: 10.1172/JCI106015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patterson SW, Starling EH. On the mechanical factors which determine the output of the ventricles. Journal of Physiology. 1914;48:357–379. doi: 10.1113/jphysiol.1914.sp001669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pinsky MR, Matuschak GM, Klain M. Determinants of cardiac augmentation by elevations in intrathoracic pressure. J Appl Physiol. 1985;58(4):1189–98. doi: 10.1152/jappl.1985.58.4.1189. [DOI] [PubMed] [Google Scholar]
- 21.Pouleur H, Covell JW, Ross J., Jr Effects of nitroprusside on venous return and central blood volume in the absence and presence of acute heart failure. Circulation. 1980;61(2):328–37. doi: 10.1161/01.cir.61.2.328. [DOI] [PubMed] [Google Scholar]
- 22.Powers SR, Jr, Dutton RE. Correlation of positive end-expiratory pressure with cardiovascular performance. Crit Care Med. 1975;3(2):64–8. doi: 10.1097/00003246-197503000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Robotham JL, Lixfeld W, Holland L, MacGregor D, Bryan AC, Rabson J. Effects of respiration on cardiac performance. J Appl Physiol. 1978;44(5):703–9. doi: 10.1152/jappl.1978.44.5.703. [DOI] [PubMed] [Google Scholar]
- 24.Ruskin J, Bache RJ, Rembert JC, Greenfield JC., Jr Pressure-flow studies in man: effect of respiration on left ventricular stroke volume. Circulation. 1973;48(1):79–85. doi: 10.1161/01.cir.48.1.79. [DOI] [PubMed] [Google Scholar]
- 25.Savourey G, Besnard Y, Launay JC, Guinet A, Hanniquet AM, Sendowski I, Caterini R, Bittel J. Positive end expiratory pressure (PEEP) slightly modifies ventilatory response during incremental exercise. Aviat Space Environ Med. 2001;72(1):21–4. [PubMed] [Google Scholar]
- 26.Scharf SM. Cardiovascular effects of airways obstruction. Lung. 1991;169(1):1–23. doi: 10.1007/BF02714137. [DOI] [PubMed] [Google Scholar]
- 27.Stark-Leyva KN, Beck KC, Johnson BD. Influence of expiratory loading and hyperinflation on cardiac output during exercise. J Appl Physiol. 2004;96(5):1920–7. doi: 10.1152/japplphysiol.00756.2003. [DOI] [PubMed] [Google Scholar]
- 28.Takata M, Wise RA, Robotham JL. Effects of abdominal pressure on venous return: abdominal vascular zone conditions. J Appl Physiol. 1990;69(6):1961–72. doi: 10.1152/jappl.1990.69.6.1961. [DOI] [PubMed] [Google Scholar]
- 29.Weber KT, Janicki JS, Hunter WC, Shroff S, Pearlman ES, Fishman AP. The contractile behavior of the heart and its functional coupling to the circulation. Prog Cardiovasc Dis. 1982;24(5):375–400. doi: 10.1016/0033-0620(82)90020-2. [DOI] [PubMed] [Google Scholar]
- 30.West JB. Best & Taylor’s Physiological Basis of Medical Practice. Williams & Wilkins; 1991. p. 220. [Google Scholar]
- 31.Willeput R, Rondeux C, De Troyer A. Breathing affects venous return from legs in humans. J Appl Physiol. 1984;57(4):971–6. doi: 10.1152/jappl.1984.57.4.971. [DOI] [PubMed] [Google Scholar]