Abstract
Aims
We hypothesized that pretreating urinary catheters with benign Escherichia coli HU2117 plus an anti-pseudomonal bacteriophage (ΦE2005-A) would prevent P. aeruginosa biofilm formation on catheters – a pivotal event in the pathogenesis of catheter-associated urinary tract infection (CAUTI).
Methods and Results
Silicone catheter segments were exposed to one of four pretreatments (sterile media; E. coli alone; phage alone; E. coli plus phage), inoculated with P. aeruginosa, then incubated up to 72 hours in human urine before rinsing and sonicating to recover adherent bacteria. P. aeruginosa adherence to catheters was almost 4 log10 units lower when pretreated with E. coli plus phage compared to no pretreatment (P < 0.001) in 24-hour experiments and more than 3 log10 units lower in 72-hour experiments (P < 0.05). Neither E. coli nor phage alone generated significant decreases.
Conclusions
The combination of phages with a pre-established biofilm of E. coli HU2117 was synergistic in preventing catheter colonization by P. aeruginosa.
Significance and Impact of Study
We describe a synergistic protection against colonization of urinary catheters by a common uropathogen. E. coli-coated catheters are in clinical trials; adding phage may offer additional benefit.
Keywords: Escherichia coli, Pseudomonads, Bacteriophages, Biofilms, Probiotics
INTRODUCTION
Catheter-associated urinary tract infection (CAUTI), one of the most common hospital-associated infections, is increasingly caused by highly resistant gram-negative organisms (Hidron et al., 2008). Pseudomonas aeruginosa is an important cause of CAUTI and is also one of the most problematic multidrug-resistant (MDR) gram-negative pathogens (Giamarellou and Poulakou, 2009, McGowan, 2006). Many strains of P. aeruginosa are resistant to all commonly used antimicrobial agents (McGowan, 2006), so there is a critical need for novel approaches to suppress the growth of this pathogen in the bladder. In the numerous patients who have a legitimate requirement for long-term urinary catheter use, no strategy is truly effective at preventing bladder invasion by urinary pathogens (Trautner et al., 2005).
CAUTI is also a biofilm-related infection, which contributes to the low efficacy of standard antimicrobial therapy (Donlan and Costerton, 2002, Maki and Tambyah, 2001). Organisms in biofilms are not consistently eradicated by the same therapeutically achievable levels of antimicrobial agents to which their planktonic counterparts are susceptible, and biofilms may provide a niche for antimicrobial-resistant organisms (Donlan and Costerton, 2002). Eradication of pathogenic organisms from a urinary tract with ongoing catheterization is nearly impossible; antibiotic use leads to temporary suppression followed by emergence of resistant strains (Maki and Tambyah, 2001).
Our bacterial interference approach to CAUTI treatment and prevention exploits the tenacity of biofilms by utilizing urinary catheters coated with a biofilm of Escherichia coli HU2117 (a derivative of E. coli 83972) in order to colonize the catheter and bladder with this benign, and potentially protective, strain (Prasad et al., 2009, Trautner et al., 2007). Our previous in vitro work with E. coli HU2117-coated catheters supports a potential role for this organism in preventing recurrent CAUTI against such diverse organisms as enterococci, Candida, Providencia, and pathogenic E. coli (Prasad et al., 2009, Trautner et al., 2008, Trautner et al., 2002, Trautner et al., 2003, Trautner et al., 2007). However, in clinical trials, some biofilm-forming organisms, notably P. aeruginosa, overgrew the E. coli HU2117 on urinary catheters (Prasad et al., 2009, Trautner et al., 2007). Here, we explore the potential of lytic bacteriophages (‘phages’) to improve the persistence of the benign E. coli on the catheter and in the bladder. The difficulty of eradicating biofilm infections from medical devices has prompted studies of the use of lytic phages for this application (Donlan, 2009). Phages are viruses that are highly specific to one or a few related target bacterial species; they are self-replicating in the presence of host cells and self-limiting in their absence; phages are frequently effective against multidrug-resistant organisms since they kill cells by different molecular mechanisms; they cannot infect eukaryotic cells and can be selected so as not to infect normal, beneficial microflora.
Many bacteriophages are known to exhibit narrow host range, and their host bacteria will develop resistance (Lenski and Levin, 1985, Mizoguchi et al., 2003), making total eradication of the target species unlikely. However, our goal is not to eradicate the pathogenic species (P. aeruginosa), but rather to shift the ecological balance of the urinary tract early on, such that growth of the benign biofilm is favored over growth of uropathogens. Emergence of phage-resistant populations in co-evolving host-phage systems takes some time, and can be slowed through the use of phage mixtures (Lenski and Levin, 1985, Maki and Tambyah, 2001, McGowan, 2006, Labrie et al., 2010). Additionally, in P. aeruginosa, phage resistance often arises through modification of bacterial surface structures whose loss reduces bacterial virulence, such as the type IV pili necessary for biofilm formation by P. aeruginosa (Labrie et al., 2010, Chibeu et al., 2009). Thus, development of phage resistance may come at the cost of biofilm-forming ability.
We explored the following hypotheses: (1) pretreating urinary catheters with benign E. coli HU2117 plus an anti-pseudomonal phage will prevent pathogen colonization of the catheter surface when challenged with P. aeruginosa in the host range of the phage, (2) phage-resistant variants of P. aeruginosa arising during these bacterial interference experiments will have diminished virulence either in terms of growth rate or catheter adherence. Our experimental design modeled an “at the bedside” treatment approach in which a cocktail of phages targeting pre-existing bladder populations of uropathogenic species would be added to the bladder at the time of insertion of a urinary catheter coated with benign E. coli HU2117.
MATERIALS AND METHODS
Organisms and culture conditions
E. coli HU2117 is a derivative of E. coli 83972, which was originally isolated in a 12-year old Swedish girl who carried it asymptomatically in her bladder (Andersson et al., 1991). E. coli HU2117 has a deletion in papG and thus cannot express functional P-fimbriae, associated with pyelonephritis (Hull et al., 2002). Our challenge pathogen was P. aeruginosa E2005-A, a clinical isolate from the CDC Clinical and Environmental Microbiology Branch culture collection. This organism was the host for phage ΦE2005-A, which was collected from the Snapfinger Creek Wastewater Treatment Plant in Dekalb County, Georgia, as previously described (Fu et al., 2010). Bacterial stocks were stored in Luria broth (LB) plus 10% glycerol at −80°C and propagated on Trypticase Soy Agar (TSA) plates (Becton, Dickinson and Company BBL™ Trypticase™ Soy Agar, BD211043) at 37°C, as needed. E. coli and P. aeruginosa were separately subcultured first on solid media (TSA), then in LB broth overnight (rocking, 37°C). This overnight liquid culture was diluted to an optical density at 600 nm (OD600) of 0.2–0.3 (approximately 108 organisms per mL) prior to use. Phage ΦE2005-A was propagated using the soft agar overlay method and stored at 4°C, shielded from light (Adams, 1959). Human urine was collected from one female donor, sterilized by filtration (0.22 μm), and stored in large-volume batches at 4°C until needed. We chose to work in real urine as our prior bacterial interference studies have all been done in human urine, both in vitro and in vivo.
Phage susceptibility of clinical P. aeruginosa isolates
We determined the susceptibility of 18 clinical isolates of urinary P. aeruginosa to the 6 phages in our library in order to choose a phage for the bacterial interference experiments. Lytic assays were performed using the spot test method (Kutter and Sulakvelidze, 2005). Briefly, bacterial lawns of each P. aeruginosa strain were seeded in soft agar that was allowed to solidify for at least 5 minutes; 15 μL droplets of phage suspensions (at approximately 108 PFU mL−1) were placed on the agar and allowed to air dry for 30 minutes. Plates were incubated overnight, and each phage zone of lysis was graded on a 0–4 scale of opaque (0, no lysis) to clear (4, total lysis).
Experimental set up and protocol
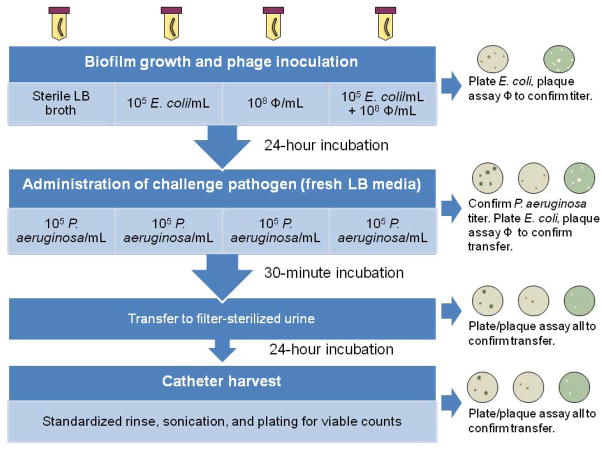
24-hour exposure experiments
All-silicone urinary catheters (BARDEX Uncoated Silicone Foley Catheters, Bard Medical Division, Covington, GA, Cat. No. 165812) were each sliced into four segments (approximately 6 cm each) using sterile technique. Each catheter segment underwent one of four pretreatment conditions in an individual tube of 50 mL LB broth (Figure 1) prior to exposure to the challenge pathogen. Specifically, in step 1, catheter segments were aseptically transferred to tubes containing one of the following: i) sterile media (control); ii) 105 E. coli colony-forming units (CFU) mL−1; iii) 108 PFU mL−1 phage ΦE2005-A; iv) 105 E. coli CFU mL−1 plus 108 PFU mL−1 phage ΦE2005-A. After a 24-hour incubation at 37°C with rocking, each catheter segment was transferred into a fresh tube of LB broth and inoculated with P. aeruginosa at a concentration of 105 CFU mL−1 for a 30-minute incubation at 37°C with rocking. Catheter segments were then moved to fresh tubes of filter-sterilized human urine for the final 24-hour incubation at 37°C with rocking. Catheters were harvested by immersing each 6-cm catheter segment in 3 different tubes of 50 mL phosphate-buffered saline (PBS), followed by flushing the inner catheter lumen with 30 mL PBS. Each segment was then aseptically cut into three 1-cm sub-segments; each sub-segment was placed into a tube containing 1 mL of PBS plus 0.01% sodium dodecyl sulfate (SDS) and was sonicated for 10 min. at 50 Hz. The sonicated fluid was plated onto selective media (TSA and LB agar plus 100 μg mL−1 ampicillin; the LB plus ampicillin media selected for P. aeruginosa, which is innately resistant to ampicillin while prohibiting growth of E. coli HU2117) and incubated overnight to quantify the numbers of adherent P. aeruginosa and E. coli (Trautner et al., 2002, Trautner et al., 2003). The detection limit for this method was 1 CFU per 1-cm catheter segment sample. Planktonic bacteria were quantified by plating dilutions of incubated urine on the same types of selective media (TSA and LB agar plus 100 μg mL−1 ampicillin) and incubating overnight. At each step in the protocol, plaque assays were performed using the soft agar overlay method to enumerate phage titers (Kutter and Sulakvelidze, 2005).
Figure 1. Experimental protocol.
Extended time course experiments: 24-, 48-, and 72-hour exposures
To investigate how the phage-enhanced interference effect would function over a longer duration of time, the interference experiments were extended to 72 hours, with daily movement of catheters to fresh filter sterilized urine. In these experiments, three (rather than one) catheter segments were placed in each 50-mL tube at the start of each experiment. At the 24-hour and 48-hour time points, one catheter segment was removed for processing as previously described, while the remaining catheter segment(s) were transferred to a fresh 50-mL tube of urine and incubated further.
Statistical analyses
Viable counts from catheter sub-segments and from spent media were log-transformed using the following algorithm: log10 [raw data + 1]. This transformation normalized the data and avoided the undefined function log10 0. For each catheter segment in each tube, the mean of the three 1-cm catheter subsections was taken as the representative value for that catheter in that particular experiment. All experiments were repeated at least 4 times. Analyses of variance (ANOVAs) were done to compare the mean numbers of P. aeruginosa adherent to catheters or recovered from the urine under the four pretreatment conditions, followed by the appropriate post-hoc comparison tests (Holm-Sidak method for comparisons to the “no pretreatment” control, or Dunn’s method for all pairwise comparisons). The correlation between adherent and planktonic P. aeruginosa was determined using the Pearson correlation test. Numbers of E. coli adherent to, or in the urine surrounding, phage-treated versus untreated catheters were compared by T tests. Statistical comparisons were performed using SigmaPlot version 11.0 software (Systat Software, Inc., San Jose, California).
Characterization of potentially phage-resistant variants of P. aeruginosa
Biochemical testing
P. aeruginosa isolates recovered from catheters or from urine after 24 or more hours of exposure to ΦE2005-A were subcultured onto TSA before storing at −80°C in LB with 10% glycerol. These variants were tested for oxidase activity, ability to grow on MacConkey agar and LB agar with 100 μg mL−1 of ampicillin, and for ability to produce distinct colony morphology and pigments on Cetrimide agar (EMD Chemicals, Gibbstown, NJ). Strains that were oxidase positive and grew on both MacConkey and LB agars, and produced colony morphology characteristic of P. aeruginosa on Cetrimide agar were identified using the Vitek II automated identification system (BioMérieux, Durham, North Carolina).
Phage susceptibility
We also characterized the susceptibility of potentially phage ΦE2005-A-resistant variants of P. aeruginosa to seven anti-pseudomonal phages (ΦE2005-A, ΦPaer4, ΦW2005-B, ΦE2005-C, ΦPaer14, and ΦM4) (Table 1). Lytic assays were performed using the spot test method as before (Kutter and Sulakvelidze, 2005). Each phage zone of lysis was graded on a 0–4 scale of opaque (0, no lysis) to clear (4, total lysis).
Table 1.
Characteristics of six anti-pseudomonal phages used in this study
| Phage | Bacterial host | Source |
|---|---|---|
| ΦE2005-A | P. aeruginosa EAMS2005-A* | CDC† |
| ΦPaer4 | P. aeruginosa Paer4 | CDC† |
| ΦW2005-B | P. aeruginosa EAMS2005-B* | CDC† |
| ΦE2005-C | P. aeruginosa EAMS2005-C* | CDC† |
| ΦPaer14 | P. aeruginosa Paer14 | CDC† |
| ΦM4 | P. aeruginosa M4 | HPA Colindale‡ |
NOTE. CDC = Centers for Disease Control and Prevention.
Clinical isolates.
Phage and bacterial host strains received from the CDC originated in the collections of the Biofilm Laboratory in the Clinical and Environmental Microbiology Branch, CDC, Atlanta, Georgia.
Health Protection Agency, Colindale, United Kingdom.
Growth curve assay
Standard growth curves of all potentially phage ΦE2005-A-resistant P. aeruginosa variants and the parent strain P. aeruginosa E2005-A were performed in filter-sterilized human urine: growth curve media was sampled for plate counts every hour for 8 hours and again at 24 hours. Doubling times of 4 repetitions per isolate were compared to the parent strain of P. aeruginosa using the Wilcoxon Rank Sum Test.
Catheter biofilm formation assay
Two of the slowest-growing and two of the fastest-growing experimental variants (as determined by length of doubling time) were tested alongside parent strain P. aeruginosa E2005-A for biofilm formation on non-pretreated catheters through overnight incubation in filter-sterilized human urine. Using the biofilm growth protocol already described, at least 3 repetitions were completed for each variant, with data analysis as per the interference experiments above.
RESULTS
Phage host range in clinical P. aeruginosa isolates
We chose phage ΦE2005-A for our bacterial interference protocol because ΦE2005-A lysed over 50% of the clinical P. aeruginosa isolates tested and made clear, well-demarcated plaques, while its host P. aeruginosa also had distinct colonies on agar plates.
Interference experiments: after a 24-hour exposure
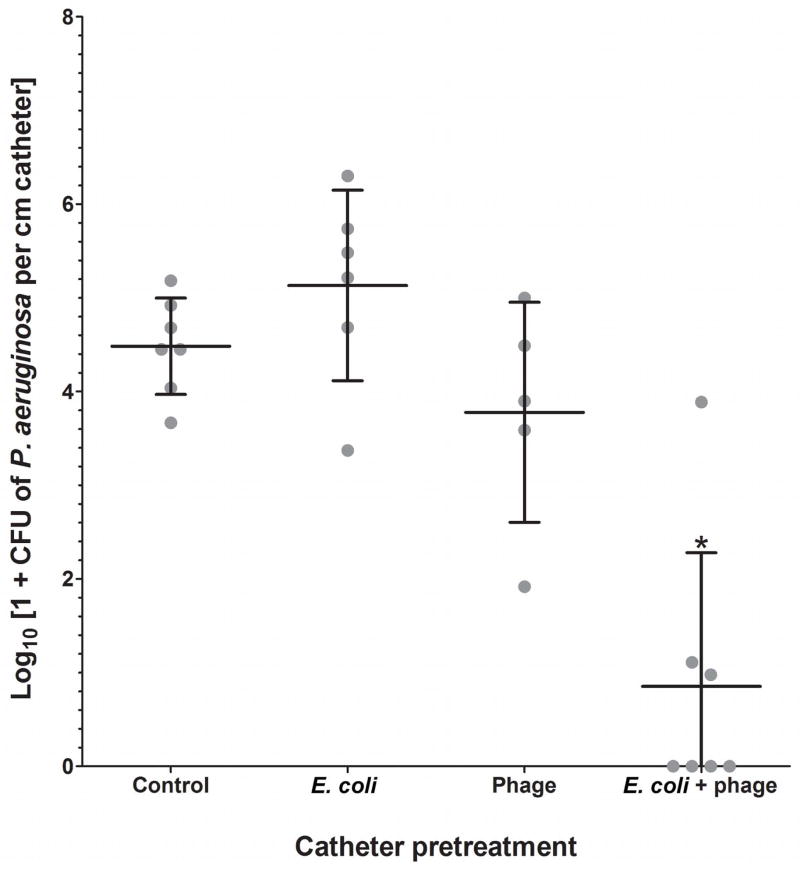
The E. coli plus phage combination was the only pretreatment condition that had a significant effect on the adherence of P. aeruginosa (P < 0.001, ANOVA followed by Holm-Sidak) (Figure 2). P. aeruginosa adherence to catheters was almost 4 log10 units lower following pretreatment with E. coli plus phage (0.90 log10 CFU cm−1), in comparison to no pretreatment (4.73 log10 CFU cm−1). In several experiments, no P. aeruginosa was recovered from catheters that had been pretreated with E. coli plus phage. The numbers of E. coli on catheters were not significantly affected by the presence of phage in the media (P = 0.4, T test).
Figure 2. Effect of catheter pretreatment on biofilm formation by P. aeruginosa after a 24-hour exposure.
Each dot represents the mean P. aeruginosa recovered from three catheter sub-segments in one experimental repetition, while the longer horizontal lines indicate averages of all repetitions. Error bars indicate standard deviation of the mean (n = 5 for phage-only treatment; 6 for E. coli-only treatment; 7 for untreated control and E. coli + phage treatment) (*P < 0.001, ANOVA followed by Holm-Sidak).
Extended time course experiments: after exposure for 24, 48, and 72 hours
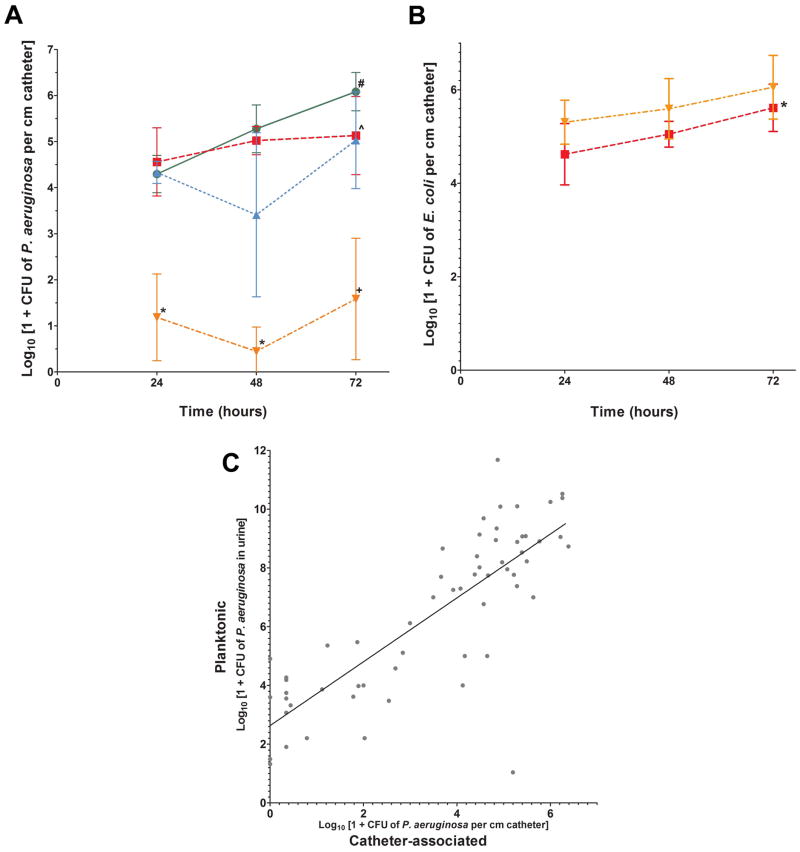
In extended time course experiments, the mean number of P. aeruginosa isolated from E. coli plus phage-pretreated catheters was more than 3 log10 units lower than that collected from untreated catheters at 24 hours (1.2 log10 vs. 4.3 log10 CFU cm−1), 48 hours (0.5 log10 vs. 5.3 log10 CFU cm−1), and 72 hours (1.6 log10 vs. 6.1 log10 CFU cm−1) (P < 0.05 for all, ANOVA, followed by Dunn’s Test) (Figure 3A). The reduction ranged from 3.6 fold to 11.7 fold. Averages of adherent P. aeruginosa per pretreatment condition differed significantly at 24-, 48-, and 72-hour time points (ANOVA, P = 0.004, P < 0.001, P < 0.001, respectively), with the significance arising from the comparison of the E. coli plus phage pretreatment to the control condition (no pretreatment). Of note, in the control condition, the numbers of catheter-adherent P. aeruginosa increased 1.8 log10 units over time from 24 to 72 hours (4.3 log10 CFU cm−1 vs. 6.1 log10 CFU cm−1, P = 0.03, Mann-Whitney U Statistic). No such increase was seen for P. aeruginosa on E. coli alone-pretreated catheters (4.6 log10 vs. 5.1 log10 CFU cm−1, P = 0.29, T test). However, at 72 hours, the differences between catheter-adherent P. aeruginosa in the control and E. coli pretreatment groups did not differ significantly (6.1 log10 vs. 5.2 log10 CFU cm−1 respectively, P = 0.08, T test). Overall, neither the E. coli-alone nor the phage-alone pretreatment produced any significant reductions in P. aeruginosa recovery from catheters when compared to control catheters at any time point.
Figure 3. A) Effect of catheter pretreatment on biofilm formation by P. aeruginosa after exposure for 24, 48, and 72 hours.
Each point represents the averages of means of P. aeruginosa adherent to three catheter sub-segments from at least four repetitions per pretreatment condition (P. aeruginosa only (green ●); E. coli only (red ■); Phage only (blue ▲); E. coli + phage (orange ▼)). Error bars indicate standard deviation of the mean. #P < 0.03 for the Mann-Whitney rank sum test of P. aeruginosa retrieved from non-pretreated 24-hour catheters compared to non-pretreated 72-hour catheters; ^P = 0.3 for the T test of P. aeruginosa recovered from E. coli-pretreated 24-hour catheters compared to 72-hour catheters; *P < 0.05, Dunn’s, and +P < 0.001, Holm-Sidak post-hoc comparison of E. coli plus phage pretreatment to control. B) Recovery of E. coli from phage-treated and untreated catheters Data shown are averages of mean log E. coli CFU cm−1 (error bars are standard deviations of the mean, n = 5) (No phage (red ■); Phage (orange ▼)). *P = 0.04 for the T test of E. coli retrieved from non-phage-pretreated 24-hour catheters compared to 72-hour catheters. C) Relationship between the number of planktonic and biofilm P. aeruginosa. A positive correlation was identified by Pearson Product Moment Correlation (correlation coefficient = 0.810, P = 4.776 × 10−15, R2 = 0.6557).
For several replicates, catheter-associated P. aeruginosa were not detected at all for the E. coli plus phage-treated catheters. Pooling results from all tubes that contained phage in all experimental replicates, we found that P. aeruginosa was completely eradicated from catheters in 8 of 27 (30%) experiments in which catheters had been pretreated with E. coli plus phage, in contrast to 0 of 14 (0%) experiments in which catheters had not been pretreated with E. coli plus phage (P = 0.02, Fisher’s Exact Test). Again, the number of E. coli collected from E. coli only-pretreated catheters did not differ significantly from that recovered from E. coli plus phage-pretreated catheters at 24, 48, or 72 hours (T test, P = 0.074, P = 0.268, P = 0.252, respectively) (Figure 3B). E. coli biofilm on E. coli only-pretreated catheters increased by approximately 1 log10 over time, from 4.6 log10 at 24 hours to 5.6 log10 at 72 hours (P = 0.04, T test). The number of biofilm-associated P. aeruginosa correlated positively with the number of planktonic P. aeruginosa for all three time points (Pearson Correlation, P ≪ 0.001) (Figure 3C).
Characterization of phage-resistant experimental variants
Thirteen potentially phage-resistant isolates of P. aeruginosa E2005-A (recovered from phage-treated catheters) were subcultured and stored for further characterization. Many of these variants resembled their parent strain in colony morphology but were collected because they had grown in a tube that contained the phage and thus could have become resistant to that phage. Other isolates demonstrated considerable variety in terms of colony shape, size, and color. All variants were identified as P. aeruginosa.
Phage susceptibility of experimental P. aeruginosa isolates
The average lytic score for the thirteen potentially phage-resistant variants of P. aeruginosa E2005-A when tested with ΦE2005-A was 1.4, in contrast to the lytic score of 4 for the parent strain (completely clear plaques). Two variants were fully resistant to ΦE2005-A, three showed barely any clearing in plaques, and one remained fully susceptible to ΦE2005-A. The other seven variants displayed diminished susceptibility to ΦE2005-A in the form of semi-opaque plaques.
Growth rates
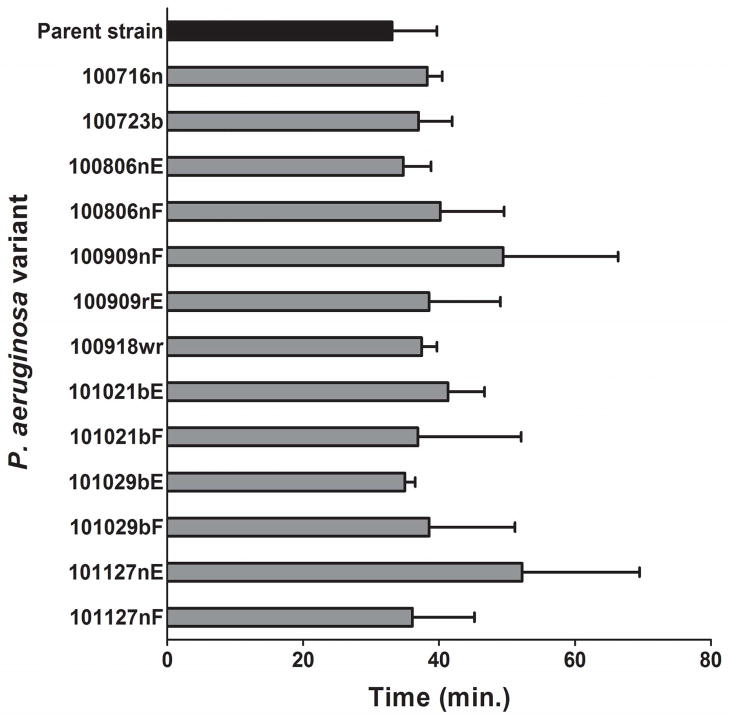
The doubling time of the parent strain P. aeruginosa E2005-A was 33 +/− 7 minutes, while the average doubling time of experimental variants was 40 +/− 10 minutes (P = 0.27, Wilcoxon Rank Sum Test) (Figure 4).
Figure 4. Doubling times of experimental P. aeruginosa variants.
Hourly viable plate counts from each individual repetition were plotted on a logarithmic scale plot to select those data points within the exponential portion of each variant’s growth. A best-fit exponential curve was fitted to those selected exponential growth data points; the equation for the curve (in the general form y = kect, where y is number of organisms per mL, k and c are constants, e is Euler’s number (approximately 2.718), and t is time as the independent variable) was solved for doubling time. Four repetitions were performed for each variant. No statistically significant difference between parent strain and variants’ growth rates was found (P = 0.27, Wilcoxon Rank Sum Test).
Biofilm formation
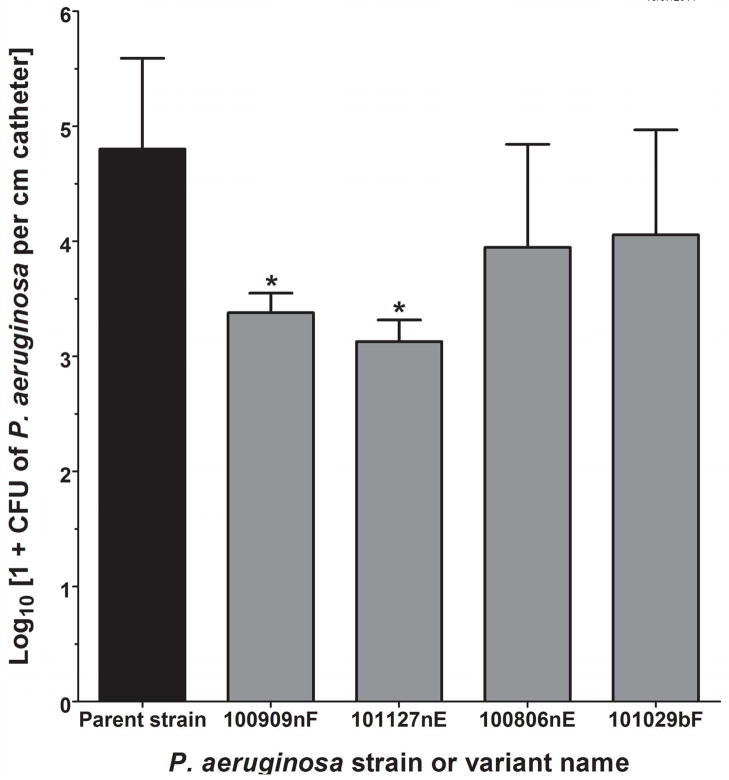
The two slowest-growing (100909nF and 101127nE) and two of the faster-growing experimental variants (100806nE and 101029bF) were compared with the parent strain for ability to form biofilms on urinary catheters after overnight incubation in filter-sterilized human urine. Mean doubling times of these 4 variants were as follows (4 repetitions): 49 minutes for 100909nF, 52 minutes for 101127nE, 35 minutes for 100806nE, and 39 minutes for 1010029bF. Significantly fewer catheter-adherent organisms were recovered from slow growers 100909nF and 101127nE than from the parent P. aeruginosa (3.38 log10 and 3.13 log10 respectively, versus 4.8 log10 CFU cm−1 for the parent strain), (P < 0.05 for both comparisons to the parent strain using ANOVA followed by Holm-Sidak); fast growers 100806nE and 101029bF did not have significantly reduced biofilm formation compared to the parent strain (Figure 5).
Figure 5. Catheter adherence ability of selected experimental variants.
The two slowest-growing (100909nF and 101127nE) and two of the faster-growing experimental variants (100806nE and 101029bF) were tested in 3–8 repetitions for adherence to urinary catheters during overnight incubation in urine. Significantly fewer numbers of 100909nF and 101127nE) were recovered from catheter surfaces than the parent strain (*P = 0.026, ANOVA).
DISCUSSION
In our previous in vitro bacterial interference studies, we found that E. coli 83972 (the parent strain for HU2117) itself was able to significantly impede catheter colonization by the following pathogens: uropathogenic E. coli, Candida albicans, Enterococcus faecalis, and Providencia stuartii (Trautner et al., 2002, Trautner et al., 2003). However, we did not challenge the E. coli-coated catheters in vitro with P. aeruginosa in those studies, and in vivo we found that this aggressive biofilm-forming organism could overgrow the E. coli HU2117 (Trautner and Darouiche, 2002, Prasad et al., 2009). The results of our current study imply that the combination of E. coli HU2117 and anti-pseudomonal phage could be effective at preventing catheter colonization by P. aeruginosa.
Our results demonstrate that E. coli HU2117 and phage ΦE2005-A had a strongly synergistic effect on preventing urinary catheter colonization by P. aeruginosa, both in overnight experiments and when the pathogen exposure time was lengthened to 72 hours. P. aeruginosa colonization of catheters was completely prevented in 30% of experiments in which the phage plus E. coli combination was used. In those experiments where colonization was not completely prevented, P. aeruginosa populations were several orders of magnitude lower than in all other treatments. Only the combination treatment significantly reduced P. aeruginosa biofilm formation. This is the first report of a synergistic effect of bacteriophage and a probiotic bacterium on prevention of pathogenic biofilm formation.
Consistent with the observations that motivated this study, E. coli HU2117 alone did not prevent catheter colonization by P. aeruginosa. However, E. coli HU2117 alone did have some interference effect on P. aeruginosa, preventing the biofilm of this organism from increasing between 24 and 72 hours (in contrast to the untreated catheters). The inability of the phage-only treatment to prevent P. aeruginosa growth on the catheter was unexpected though, since Fu et al. found that phage alone could significantly decrease catheter adherence by P. aeruginosa by over 2 logs in a flowing model using diluted tryptic soy broth (Fu et al., 2010). The difference may be related to the presence of a hydrogel coating which enabled adsorption of the phages, or the use of a phage cocktail in the Fu et al. studies. Fu et al. hypothesized that phages were associating with the hydrogel coating on the catheters used in that study, whereas the current work used uncoated silicone catheters. In several instances, the E. coli HU2117 biofilm increased by more than 1 log over 72 hours of incubation, as we observed in human studies (Prasad et al., 2009). Unsurprisingly, the presence of anti-pseudomonal phage did not substantially affect E. coli HU2117 population sizes.
The results of the present study support our original hypothesis that the combination of phage and bacterial interference will reduce biofilm formation by P. aeruginosa on urinary catheters to a significantly greater extent than either phage treatment or treatment by bacterial interference alone. Phage alone was clearly insufficient to kill all P. aeruginosa cells prior to mixed biofilm formation on the catheter, and similarly, E. coli was unable to out-compete P. aeruginosa during biofilm formation. It is likely that in the combination E. coli plus phage pretreatment, the phage reduced the initial P. aeruginosa population sufficiently to allow the E. coli biofilm to become further established. As a consequence, a mixed biofilm does not appear to have formed, or when it did the only P. aeruginosa cells recovered were phage-resistant mutants, and those in low numbers. This suggests that if the initial P. aeruginosa population is held in check, the E. coli biofilm can develop and mitigate biofilm formation by P. aeruginosa, possibly due to reduced competitive ability of the phage-resistant mutants. The observed correlation between numbers of P. aeruginosa on catheters and in urine supports this numbers-based theory, as the correlation probably results from a cyclical seeding process from catheter to urine and back. Harcombe and Bull found that addition of a phage specific for E. coli in an E. coli/Salmonella enterica planktonic community resulted in a significant reduction in its host strain even though E. coli densities with phage present reached high levels in the absence of S. enterica (Harcombe and Bull, 2005). Kay et al. also observed a similar effect on P. aeruginosa in a P. aeruginosa/E. coli culture in the presence of a P. aeruginosa-specific phage (Kay et al., 2011). Both of these studies suggest that the combined effect of lytic phage infection and microbial competition can result in the predominance of one species in the biofilm, as observed in the present study.
Fu et al. addressed the issue of the rapid emergence of phage-resistant variants of P. aeruginosa by pretreating urinary catheters with a phage cocktail (Fu et al., 2010). We found that adding E. coli HU2117 to the catheter pretreatment is a highly effective alternative approach, since in 30% of the experiments in which both E. coli and phage had been applied, no P. aeruginosa was found on the catheter. A promising future direction for our interference studies will be to combine a cocktail of multiple phages with the E. coli-coated catheters, taking advantage of two approaches to maximize the pathogen suppression.
Despite the large reduction in P. aeruginosa growth following E. coli plus phage pretreatment, we did still observe frequent emergence of phage-resistant variants of P. aeruginosa in that context. Although the absolute doubling times of phage-resistant isolates of P. aeruginosa were all longer than for the parent strain, the wide standard deviations precluded finding a significant difference in growth rates. Nevertheless, the finding that slower-growing phage-resistant variants of P. aeruginosa had reduced virulence in terms of catheter adherence is a finding with potential clinical relevance, and is in accord with several other recent studies of the effect of phage exposure upon bacterial virulence. Phage resistance often arises through modification of bacterial surface structures (Labrie et al., 2010). Type IV pili and their associated twitching motility are essential for biofilm formation by P. aeruginosa (O’Toole and Kolter, 1998). In Chibeu et al.’s studies of another P. aeruginosa-phage pair, P. aeruginosa PAO1 and ΦKMV, the loss of type IV pili conferred resistance to phage ΦKMV but also resulted in diminished twitching motility (Chibeu et al., 2009). A mouse study of phage therapy for Salmonella enterica found a similar association of the acquisition of phage resistance with the loss of virulence (Capparelli et al., 2010). In our interference experiments, our goal is not to eradicate the pathogenic species, but rather to shift the ecological balance so that the benign E. coli can persist and protect the catheter and the bladder. Virulence modification in terms of biofilm formation suits this purpose very well.
In summary, pretreating urinary catheters with a benign E. coli biofilm in addition to anti-pseudomonal phages inhibits colonization by P. aeruginosa more effectively than either the E. coli biofilm or the phage pretreatment alone. This synergistic effect is promising when considering the application of this system in a clinical setting, where the phage may reinforce the ability of our benign E. coli biofilm to prevent uropathogen adherence and colonization.
Acknowledgments
BWT’s work was supported by the National Institutes of Health HD058985 and a VA Career Development Award from Rehabilitation Research & Development B4623; also with some use of resources and facilities at the Houston VA HSR& D Center of Excellence (HFP90-020) at the Michael E. DeBakey VA. SML was partially supported by the American Society for Microbiology/Center for Disease Control and Prevention International Postdoctoral Fellowship.
We appreciate the assistance of Megan Burger and Lisa Atkins in the interference experiments and in testing the variant strains of P. aeruginosa, respectively. We acknowledge Elizabeth Perez and Bette Jensen of CDC for assistance. This work was performed through a Research Collaboration Agreement between Baylor College of Medicine and The Centers for Disease Control and Prevention.
Footnotes
CONFLICT OF INTERESTS
The authors do not have any financial or other associations that might pose a conflict of interest.
References
- Adams M. Bacteriophages. New York: Interscience Publishers; 1959. [Google Scholar]
- Andersson P, Engberg I, Lidin-Janson G, et al. Persistence of Escherichia coli bacteriuria is not determined by bacterial adherence. Infect Immun. 1991;59:2915–21. doi: 10.1128/iai.59.9.2915-2921.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capparelli R, Nocerino N, Iannaccone M, et al. Bacteriophage therapy of Salmonella enterica: a fresh appraisal of bacteriophage therapy. J Infect Dis. 2010;201:52–61. doi: 10.1086/648478. [DOI] [PubMed] [Google Scholar]
- Chibeu A, Ceyssens PJ, Hertveldt K, et al. The adsorption of Pseudomonas aeruginosa bacteriophage phiKMV is dependent on expression regulation of type IV pili genes. FEMS Microbiol Lett. 2009;296:210–8. doi: 10.1111/j.1574-6968.2009.01640.x. [DOI] [PubMed] [Google Scholar]
- Donlan RM. Preventing biofilms of clinically relevant organisms using bacteriophage. Trends Microbiol. 2009;17:66–72. doi: 10.1016/j.tim.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Donlan RM, Costerton JW. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev. 2002;15:167–93. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu W, Forster T, Mayer O, et al. Bacteriophage cocktail for the prevention of biofilm formation by Pseudomonas aeruginosa on catheters in an in vitro model system. Antimicrob Agents Chemother. 2010;54:397–404. doi: 10.1128/AAC.00669-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giamarellou H, Poulakou G. Multidrug-resistant Gram-negative infections: what are the treatment options? Drugs. 2009;69:1879–901. doi: 10.2165/11315690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Harcombe WR, Bull JJ. Impact of phages on two-species bacterial communities. Applied and Environmental Microbiology. 2005;71:5254–9. doi: 10.1128/AEM.71.9.5254-5259.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidron AI, Edwards JR, Patel J, et al. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006–2007. Infect Control Hosp Epidemiol. 2008;29:996–1011. doi: 10.1086/591861. [DOI] [PubMed] [Google Scholar]
- Hull RA, Donovan WH, Del Terzo M, et al. Role of type 1 fimbria- and P fimbria-specific adherence in colonization of the neurogenic human bladder by Escherichia coli. Infect Immun. 2002;70:6481–4. doi: 10.1128/IAI.70.11.6481-6484.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay MK, Erwin TC, Mclean RJ, et al. Bacteriophage ecology in Escherichia coli and Pseudomonas aeruginosa mixed-biofilm communities. Appl Environ Microbiol. 2011;77:821–9. doi: 10.1128/AEM.01797-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutter E, Sulakvelidze A, editors. Bacteriophages: biology and applications. Boca Raton, Florida: CRC Press; 2005. [Google Scholar]
- Labrie SJ, Samson JE, Moineau S. Bacteriophage resistance mechanisms. Nat Rev Microbiol. 2010;8:317–27. doi: 10.1038/nrmicro2315. [DOI] [PubMed] [Google Scholar]
- Lenski R, Levin B. Constaints on the coevolution of bacteria and virulent phage--a model, some experiments, and predictions for natural communities. Am Nat. 1985;125:585–602. [Google Scholar]
- Maki D, Tambyah P. Engineering out the risk of infection with urinary catheters. Emerging Infectious Diseases. 2001;7:1–13. doi: 10.3201/eid0702.010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan JE., Jr Resistance in nonfermenting gram-negative bacteria: multidrug resistance to the maximum. Am J Med. 2006;119:S29–36. doi: 10.1016/j.amjmed.2006.03.014. discussion S62–70. [DOI] [PubMed] [Google Scholar]
- Mizoguchi K, Morita M, Fischer CR, et al. Coevolution of bacteriophage PP01 and Escherichia coli O157:H7 in continuous culture. Appl Environ Microbiol. 2003;69:170–6. doi: 10.1128/AEM.69.1.170-176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’toole GA, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- Prasad A, Cevallos ME, Riosa S, et al. A bacterial interference strategy for prevention of UTI in persons practicing intermittent catheterization. Spinal Cord. 2009;47:565–9. doi: 10.1038/sc.2008.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautner BW, Cevallos ME, Li H, et al. Increased expression of type-1 fimbriae by nonpathogenic Escherichia coli 83972 results in an increased capacity for catheter adherence and bacterial interference. J Infect Dis. 2008;198:899–906. doi: 10.1086/591093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautner BW, Darouiche RO. Prevention of urinary tract infection in patients with spinal cord injury. J Spinal Cord Med. 2002;25:277–83. doi: 10.1080/10790268.2002.11753628. [DOI] [PubMed] [Google Scholar]
- Trautner BW, Darouiche RO, Hull RA, et al. Pre-inoculation of urinary catheters with Escherichia coli 83972 inhibits catheter colonization by Enterococcus faecalis. J Urol. 2002;167:375–9. [PMC free article] [PubMed] [Google Scholar]
- Trautner BW, Hull RA, Darouiche RO. Escherichia coli 83972 inhibits catheter adherence by a broad spectrum of uropathogens. Urology. 2003;61:1059–62. doi: 10.1016/s0090-4295(02)02555-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautner BW, Hull RA, Darouiche RO. Prevention of catheter-associated urinary tract infection. Curr Opin Infect Dis. 2005;18:37–41. doi: 10.1097/00001432-200502000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trautner BW, Hull RA, Thornby JI, et al. Coating urinary catheters with an avirulent strain of Escherichia coli as a means to establish asymptomatic colonization. Infect Control Hosp Epidemiol. 2007;28:92–4. doi: 10.1086/510872. [DOI] [PMC free article] [PubMed] [Google Scholar]