Abstract
Tenofovir (TFV) is a nucleotide reverse transcriptase inhibitor and IQP-0528 is a nonnucleoside reverse transcriptase inhibitor that also blocks virus entry. TFV and IQP-0528 alone have shown antiviral activity as microbicide gels. Because combination therapy will likely be more potent than mono-therapy, these drugs have been chosen to make a combination microbicide gel containing 2.5% TFV/1% IQP-0528. Safety and efficacy testing was done to evaluate five prototype combination gels. The gels retained TZM-bl cell and ectocervical and colorectal tissue viability. Further, the epithelium of the ectocervical and colorectal tissue remained intact after a 24 hour exposure. The ED50 calculated from the formulations for IQP-0528 was ~32 nM and for TFV was ~59 nM and their inhibitory activity was not affected by semen. The ED50 of TFV in the combination gels was ~100-fold lower than when calculated for the drug substance alone reflecting the activity of the more potent IQP-0528. When ectocervical and colorectal tissue were treated with the combination gels, HIV-1 p24 release was reduced by ≥1 log10 and ≥2 log10, respectively. Immunohistochemistry for the ectocervical tissues treated with combination gels showed no HIV-1 infected cells at study end. With the increased realization of receptive anal intercourse among heterosexual couples often in conjunction with vaginal intercourse, having a safe and effective microbicide for both mucosal sites is critical. The safety and efficacy profiles of the gels were similar for ectocervical and colorectal tissues suggesting these gels have the potential for dual compartment use.
Keywords: HIV prevention, combination microbicide, rectal microbicide, pyrimidinedione, topical gel
Global HIV-1 incidence is declining in part due to changes in sexual behavior. The recent success of male circumcision (Auvert et al., 2005; Bailey et al., 2007), treatment as prevention (Cohen et al., 2011), pre-exposure prophylaxis (PrEP) (Grant et al., 2010), and peri-coital use of topical microbicides (Abdool Karim et al., 2010) should make a more pronounced impact on the epidemic as they are implemented. Unfortunately, not all approaches have been successful in all populations (FHI, 2011; MTN 1% TFV gel, 2011; MTN Viread, 2011). Consequently, work is needed to optimize product choice to improve efficacy and ensure their use. The current microbicide paradigm is single drug agents for vaginal application. Combination therapy, which has been successful in treating HIV-1-infected patients as realized by reduced morbidity and mortality (Lucas, 2012), is now being considered for microbicides. Our goal is to provide a potent combination microbicide product that could be used rectally as well as vaginally. This will expand the populations that would benefit from not only a combination gel, but also a dual compartment product.
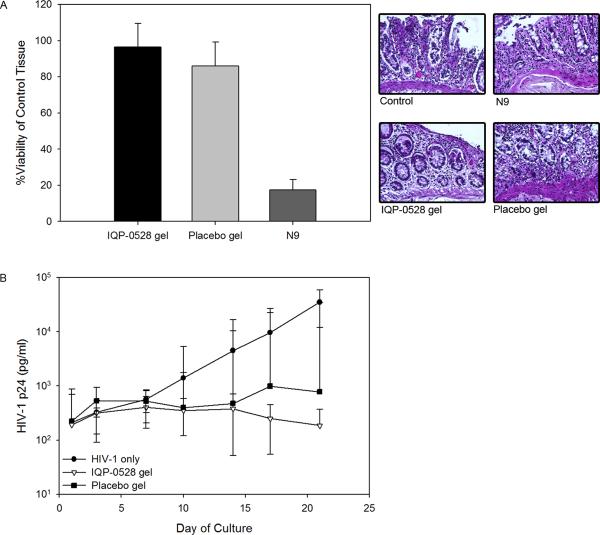
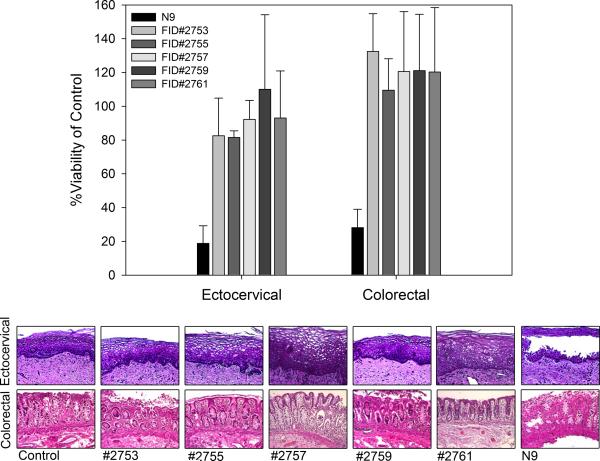
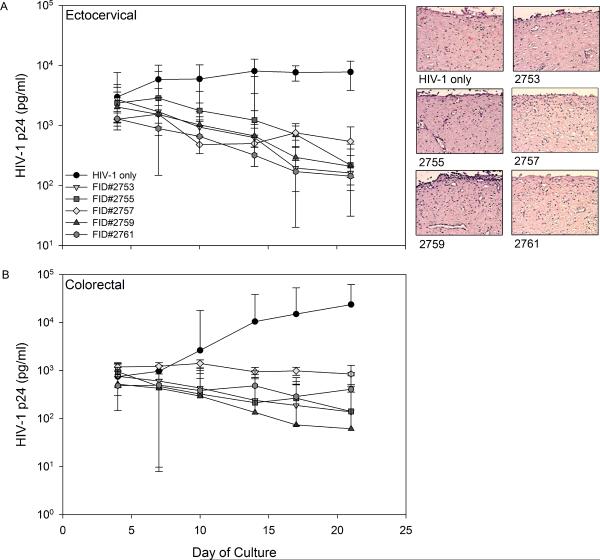
Tenofovir (TFV) is a nucleotide reverse transcriptase inhibitor (NRTI) and IQP-0528 is a pyrimidinedione, non-nucleoside reverse transcriptase inhibitor (NNRTI). Both drugs have been formulated separately as microbicide gels (Rohan et al., 2010; Watson Buckheit et al., 2011). The 1% TFV gel was effective against HIV-1, but the hyperosmolar gel induced epithelial fracture/sloughing of ectocervical and colorectal explants (Rohan et al., 2010). The 0.25% IQP-0528 gel was effective against HIV-1 and safe toward ectocervical explant cultures (Mahalingam et al., 2011). To extend this finding, 0.25% IQP-0528 and hydroxyethylcellulose (HEC) placebo gels were evaluated in colorectal tissue (IRB-approved). For tissue viability, the gels were diluted 1:5 in medium for even spread and applied to the apical surface and remained there for 24 hours (Abner et al., 2005). Controls included no treatment and a 1:5 dilution of Gynol II (containing 2% nonoxynol-9 [N9]). After 24 hours, the explants were washed and viability was assessed using the MTT [1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan] assay on the first explant and epithelial integrity was assessed by histology on the second explant. In polarized colorectal tissue, overnight exposure to the 0.25% IQP-0528 and the placebo gels retained tissue epithelium as shown by histology (Fig. 1A) and viability as demonstrated by the MTT assay (Fig. 1A). Next, protection against HIV-1 infection was tested by diluting the gels 1:5 with 5×104 TCID50 of HIV-1BaL in medium (Abner et al., 2005). Controls included explants exposed to virus alone. After an overnight culture, the explants were washed and fresh medium was applied to the basolateral compartment. Basolateral medium was harvested and replenished every 3 to 4 days. The supernatant was tested by HIV-1 p24gag ELISA (Perkin-Elmer, Waltham, MA). The 0.25% IQP-0528 gel blocked infection of the colorectal tissue as shown by a 2.3 log10 reduction of HIV-1 p24 in the culture supernatant (p < .05; ANOVA with Bonferroni adjustments) (Fig. 1B). The placebo gel also reduced HIV-1 p24 release by 1.7 log10; however, three of seven explants had robust HIV-1 replication comparable to the control explants (p = ns). These data confirm that the single entity gel in an HEC base was safe and effective in colorectal tissue. The safety and efficacy of the single entity gel to ectocervical and colorectal tissues provided evidence that IQP-0528 should perform well when combined with TFV. To address combination prevention, five novel gel formulations containing 2.5% TFV/1% IQP-0528 (FID#2753, FID#2755, FID#2757, FID#2759, and FID#2761) (Ham et al., 2012) were compared for their safety and potency in polarized ectocervical and colorectal explants. Three of the combination gels were in an HEC base (FID#2753, 2755, 2761) similar to the single entity gel while the other two were in an hydroxypropylcellulose (HPC) base (FID#2755 and 2759) (Ham et al., 2012). The combination gels, irrespective of HEC or HPC, retained the viability of colorectal and ectocervical explants and preserved the epithelium (Fig. 2). Gynol II showed a >70% reduction in colorectal and ectocervical tissue viability along with loss of the epithelium. Evaluating tissue efficacy, the five gel combinations reduced HIV-1 p24 release by the ectocervical tissue (Fig. 3A) as well as the colorectal tissue (Fig. 3B) by ≥1 log10 and ≥2 log10, respectively (p < .05; ANOVA with Bonferroni adjustments). The reduction in released p24 corresponded to a lack of infected cells in ectocervical tissue at study end as shown by the lack of p24 positive cells (red cells) after immunohistochemistry staining (Fig. 3A).
Figure 1. Pre-clinical testing of 0.25% IQP-0528 gel using polarized colorectal tissue.
Safety of the 0.25% IQP-0528 gel was evaluated by the 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT) assay and histology (A) (Abner et al., 2005). The data presented are the median ± standard deviation of three independent tissues. The histology is representative of one of those tissues. Efficacy of the 0.25% IQP-0528 gel was tested against HIV-1BaL exposure (B). Gel and virus were mixed and applied to the apical surface and cultured overnight before washing. Tissue supernatant was collected every three to four days for three weeks and stored at −80°C until tested. HIV-1 replication was measured by p24gag ELISA in the culture supernatant. Three independent tissues were evaluated. The data represent the median ± 95% confidence interval of the three tissues.
Figure 2. Safety of the tenofovir/IQP-0528 combination gels on polarized ectocervical and colorectal tissues.
Polarized tissue was set-up in duplicate and exposed to gel overnight. After washing, one of the duplicate tissues was processed for the 1-(4,5-dimethylthiazol-2-yl)-3,5-diphenylformazan (MTT) assay and the other duplicate was processed for histology (Abner et al., 2005; Cummins et al., 2007). The data presented are the median ± standard deviation of three independent tissues. The histology is representative of one of those tissues.
Figure 3. Efficacy of the tenofovir/IQP-0528 combination gels on polarized ectocervical and colorectal tissues.
Polarized tissue was set-up in duplicate and exposed to gel and HIV-1BaL overnight on the apical surface. After washing, fresh medium was replenished to the basolateral compartment. Medium was harvested and replenished every three to four days for three weeks. At study end, the ectocervical tissue was processed for immunohistochemistry for HIV-1 infected cells (p24 expressing; red color) (Cummins et al., 2007; Rohan et al., 2010). The data represent the median ± 95% confidence interval of three ectocervical (A) or colorectal (B) tissues. The immunohistochemistry is representative of one of the ectocervical tissues.
The preclinical testing of the 1% TFV and 0.25% IQP-0528 gels showed discrepant safety results (Mahalingam et al., 2011; Rohan et al., 2010). The IQP-0528 gel showed no alteration in ectocervical (Mahalingam et al., 2011) and colorectal (Fig. 1A) tissue viability or changes in the epithelium. However, the 1% TFV gel did induce epithelial changes (Rohan et al., 2010). This could be attributed to the elevated osmolality (>3,000 mOsm/kg). The TFV gel was reformulated (Dezzutti et al., 2012) to reduce the osmolality to ~800 mOsm/kg. This reduced glycerin (RG) 1% TFV gel showed improved retention of ectocervical and colorectal epithelium. When used rectally by persons who engage in receptive anal intercourse, the RG 1% TFV gel showed improved acceptability and no significant adverse events (AEs) (McGowan et al., 2012) as compared to the original 1% TFV gel (Anton et al., 2011) which had several AEs associated with rectal urgency and bloating. The osmolality of the single entity IQP-0528 and TFV/IQP-0528 gels ranges from 754.6 to 882 mOsm/kg (Ham et al., 2012; Mahalingam et al., 2011). While still hyperosmolar, they produced no changes to the ectocervical and colorectal tissue epithelium. The similar osmolality to the RG 1% TFV gel suggests the TFV/IQP-0528 gels should have no AEs when used rectally.
We next investigated the impact semen may have on the activity of the drugs and the formulations. To address this issue, the ED50 of drug substances were determined to be 2000 nM for TFV and of 3 nM for IQP-0528 (Table 1) using the TZM-bl cell assay (NIH AIDS Research and Reference Reagent Program, DAIDS, NIAID) (Wei et al., 2002). IQP-0528 is approximately 1000-fold more potent than TFV with ED50's of ~3nM and ~2000 nM (Table 1). In the formulation, the ED50 of IQP-0528 was slightly increased (≤10-fold) and could be due to retention of the polar molecule in the formulation. Of interest, the ED50 of TFV decreased by ~100-fold suggesting increased potency. This was likely due to the activity of IQP-0528 in the formulation shifting the TFV response curve. The two molecules bind to the reverse transcriptase in different regions and have shown additive to synergistic activity when tested with primary HIV-1 isolates in vitro (Hartman et al., 2011). The data presented here support this finding. Because microbicides are often intended to be used coitally, the impact of semen needs to be taken into consideration. Several microbicide candidates have shown reduced efficacy in the presence of seminal plasma. The most affected class was the polyanions (Neurath et al., 2006; Patel et al., 2007). We and others have shown that NRTI and NNRTI activities are not affected by seminal plasma or whole semen (Kunjara Na Ayudhya et al., 2009; Neurath et al., 2006). In this study, TFV and IQP-0528 were not affected by the presence of whole semen (Table 1) suggesting that their potency should be retained during/after coitus.
Table 1.
Potency of tenofovir and IQP-0528 formulations.
| Tenofovir | IQP-0528 | ||||
|---|---|---|---|---|---|
|
|
|||||
| + semen | + semen | ||||
| APIa | CC50 | >300 μM | >300 μM | >50,000 nM | >50,000 nM |
| ED50 | 2 μM | 1.6 μM | 3 nM | 2 nM | |
| TI | >150 | >187 | >16,667 | >25,000 | |
| FID#2753 | CC50 | >4365 μM | >4365 μM | >1513 μM | >1513 μM |
| ED50 | 0.089 μM | 0.075 μM | 0.049 μM | 0.041 μM | |
| TI | >49000 | >58200 | >31000 | >36000 | |
| FID#2755 | CC50 | >4365 μM | >4365 μM | >1513 μM | >1513 μM |
| ED50 | 0.054 μM | 0.055 μM | 0.03 μM | 0.03 μM | |
| TI | >80800 | >79000 | >50000 | >50000 | |
| FID#2757 | CC50 | >4365 μM | >4365 μM | >1513 μM | >1513 μM |
| ED50 | 0.046 μM | 0.088 μM | 0.025 μM | 0.048 μM | |
| TI | >94000 | >49000 | >60000 | >31000 | |
| FID#2759 | CC50 | >4365 μM | >4365 μM | >1513 μM | >1513 μM |
| ED50 | 0.035 μM | 0.07 μM | 0.02 μM | 0.039 μM | |
| TI | >99000 | >62000 | >75000 | >38000 | |
| FID#2761 | CC50 | >4365 μM | >4365 μM | >1513 μM | >1513 μM |
| ED50 | 0.048 μM | 0.058 μM | 0.026 μM | 0.032 μM | |
| TI | >72000 | >75000 | >58000 | >47000 | |
Active pharmaceutical ingredient = API; 50% cytotoxic concentration = CC50; 50% effective dose = ED50; therapeutic index = TI.
Women who engage in receptive anal intercourse frequently report sequencing between the vagina and rectum during the same coital encounter (Gorbach et al., 2012). Providing these women the opportunity to have products to use at both mucosal sites should make a significant impact in the HIV-1 epidemic. Preclinical assessments here and elsewhere of TFV and IQP-0528 single entity gels (Mahalingam et al., 2011; Rohan et al., 2010) show they are effective at blocking HIV-1 infection in mucosal tissues. The 1% TFV gel showed modest protection against HIV-1 acquisition (39% reduction in HIV-1 incidence) when used peri-coitally (Abdool Karim et al., 2010), but not when used daily (MTN 1% TFV gel, 2011) suggesting TFV is marginally effective. The next advancement in product development is to combine TFV and IQP-0528 into a single gel (Ham et al., 2012). Our data show these gels to be safe for ectocervical and colorectal tissues and effective against HIV-1 infection. These combination gels should provide better protection than the single entity gels and expand the populations who could benefit from using a microbicide.
While single entity gels have shown at best modest prevention of incident HIV-1 infection, it is anticipated that combination gels will provide a greater benefit. Combinations of two and even three unformulated NRTI's and NNRTI's have shown improved protection against HIV-1 infection in colorectal tissue (Herrera et al., 2009). The combination of TFV and IQP-0528 described here were safe and effective at preventing HIV-1 for ectocervical and colorectal tissue. These data suggest the use of combination products designed for dual compartment use should provide better protection against HIV-1 infection and allow for more options for the persons wanting to incorporate these products into their sexual repertoire.
Highlights
Tenofovir/IQP-0528 microbicide combination gels were tested for safety and efficacy
Polarized ectocervical and colorectal tissues were used to evaluate these gels
Combination gels were safe toward mucosal tissue and blocked HIV infection
These gels provide the foundation of a dual compartment, combination product microbicide
ACKNOWLDEGMENTS
This work was supported by the U.S. National Institutes of Health (NIH) (U19 AI077289). The authors would like to thank the University of Pittsburgh Medical Center's Tissue Procurement program for their work in obtaining the ectocervical and colorectal tissues and the patients for their willingness to participate in research. The authors also thank Dr. Patrick Kiser for his mentorship and support of A.M. and S.R.U.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT G.G. is an employee of Particle Sciences which developed and manufactured the TFV/IQP-0528 gels used in this work. The remaining authors declare no conflict of interest.
REFERENCES
- Abdool Karim Q, Abdool Karim SS, Frohlich JA, Grobler AC, Baxter C, Mansoor LE, Kharsany AB, Sibeko S, Mlisana KP, Omar Z, Gengiah TN, Maarschalk S, Arulappan N, Mlotshwa M, Morris L, Taylor D. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abner SR, Guenthner PC, Guarner J, Hancock KA, Cummins JE, Jr., Fink A, Gilmore GT, Staley C, Ward A, Ali O, Binderow S, Cohen S, Grohskopf LA, Paxton L, Hart CE, Dezzutti CS. A Human Colorectal Explant Culture to Evaluate Topical Microbicides for the Prevention of HIV Infection. J Infect Dis. 2005;192:1545–1556. doi: 10.1086/462424. [DOI] [PubMed] [Google Scholar]
- Anton P, Cranston R, Carballo-Dieguez A, Kashuba A, Khanukhova E, Elliott J, Janocko L, Cumberland W, Mauck C, McGowan I. RMP-02/MTN-006: A phase 1 placebo-controlled trial of rectally applied 1% vaginal TFV gel with comparison to oral TDF. 18th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2011. #34LB. [Google Scholar]
- Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005;2:e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey RC, Moses S, Parker CB, Agot K, Maclean I, Krieger JN, Williams CF, Campbell RT, Ndinya-Achola JO. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369:643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JH, Godbole SV, Mehendale S, Chariyalertsak S, Santos BR, Mayer KH, Hoffman IF, Eshleman SH, Piwowar-Manning E, Wang L, Makhema J, Mills LA, de Bruyn G, Sanne I, Eron J, Gallant J, Havlir D, Swindells S, Ribaudo H, Elharrar V, Burns D, Taha TE, Nielsen-Saines K, Celentano D, Essex M, Fleming TR. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins JE, Jr., Guarner J, Flowers L, Guenthner PC, Bartlett J, Morken T, Grohskopf LA, Paxton L, Dezzutti CS. Preclinical testing of candidate topical microbicides for anti-human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture. Antimicrob Agents Chemother. 2007;51:1770–1779. doi: 10.1128/AAC.01129-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezzutti CS, Rohan LC, Wang L, Uranker K, Shetler C, Cost M, Lynam JD, Friend D. Reformulated tenofovir gel for use as a dual compartment microbicide. J Antimicrob Chemother. 2012 May 11; doi: 10.1093/jac/dks173. Epub ahead of print. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FHI FHI to Initiate Orderly Closure of FEM-PrEP. 2011 http://www.fhi360.org/NR/rdonlyres/erj6hevouwvddigjldvcca6tvtybfolh4nedyfeo4yz2i7la5 3jknim6ex42bme2rvyq6zfzovyrkb/FEMPrEPFactSheetJune2011.pdf.
- Gorbach PM, Jeffries R, Weiss RE, Cranston RD, Fuchs EJ, Javanbakht M, Hezerah M, Brown S, Voskanian A, Pines HA, Anton P. Order of Orifices: Sequence of Insertion and Ejaculation Locations during Anal Intercourse for Women, Implications for HIV Transmission Risks, Microbicides 2012. Sydney Australia: 2012. [Google Scholar]
- Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, Goicochea P, Casapia M, Guanira-Carranza JV, Ramirez-Cardich ME, Montoya-Herrera O, Fernandez T, Veloso VG, Buchbinder SP, Chariyalertsak S, Schechter M, Bekker LG, Mayer KH, Kallas EG, Amico KR, Mulligan K, Bushman LR, Hance RJ, Ganoza C, Defechereux P, Postle B, Wang F, McConnell JJ, Zheng JH, Lee J, Rooney JF, Jaffe HS, Martinez AI, Burns DN, Glidden DV. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med. 2010;363:2587–2599. doi: 10.1056/NEJMoa1011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ham AS, Ugaonkar SR, Shi L, Buckheit KW, Lakougna H, Nagaraja U, Gwozdz G, Goldman L, Kiser PF, Buckheit RW., Jr. Development of a combination microbicide gel formulation containing IQP-0528 and tenofovir for the prevention of HIV infection. J Pharm Sci. 2012;101:1423–1435. doi: 10.1002/jps.23026. [DOI] [PubMed] [Google Scholar]
- Hartman TL, Yang L, Buckheit RW., Jr. Antiviral interactions of combinations of highly potent 2,4(1H,3H)-pyrimidinedione congeners and other anti-HIV agents. Antiviral Res. 2011;92:505–508. doi: 10.1016/j.antiviral.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera C, Cranage M, McGowan I, Anton P, Shattock RJ. Reverse transcriptase inhibitors as potential colorectal microbicides. Antimicrob Agents Chemother. 2009;53:1797–1807. doi: 10.1128/AAC.01096-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunjara Na Ayudhya RP, Billitto N, Rooney J, Dezzutti C. Influence of Semen on Efficacy of Tenofovir 1% Gel. Keystone Symposia on Molecular and Cellular Biology: Prevention of HIV/AIDS; Keystone, Colorado. 2009. #233. [Google Scholar]
- Lucas S. Causes of death in the HAART era. Curr Opin Infect Dis. 2012;25:36–41. doi: 10.1097/QCO.0b013e32834ef5c4. [DOI] [PubMed] [Google Scholar]
- Mahalingam A, Simmons AP, Ugaonkar SR, Watson KM, Dezzutti CS, Rohan LC, Buckheit RW, Jr., Kiser PF. Vaginal microbicide gel for delivery of IQP-0528, a pyrimidinedione analog with a dual mechanism of action against HIV-1. Antimicrob Agents Chemother. 2011;55:1650–1660. doi: 10.1128/AAC.01368-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan I, Hoesley C, Andrew P, Janocko L, Dai J, Carballo-Dieguez A, Kunjara Na Ayudhya R, Piper J, Cranston C, Mayer K, Team M-P. MTN-007: A Phase 1 Randomized, Double-blind, Placebo-controlled Rectal Safety and Acceptability Study of Tenofovir 1% Gel. Conference on Retroviruses and Opportunistic Infections; Seattle, WA. 2012. #34LB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MTN 1% TFV gel MTN Statement on Decision to Discontinue Use of Tenofovir Gel in VOICE, a Major HIV Prevention Study in Women. 2011 http://www.mtnstopshiv.org/node/3909.
- MTN Viread MTN Statement on Decision to Discontinue Use of Oral Tenofovir Tablets in VOICE, a Major HIV Prevention Study in Women. 2011 http://www.mtnstopshiv.org/node/3619.
- Neurath AR, Strick N, Li YY. Role of seminal plasma in the anti-HIV-1 activity of candidate microbicides. BMC Infect Dis. 2006;6:150. doi: 10.1186/1471-2334-6-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Hazrati E, Cheshenko N, Galen B, Yang H, Guzman E, Wang R, Herold BC, Keller MJ. Seminal plasma reduces the effectiveness of topical polyanionic microbicides. J Infect Dis. 2007;196:1394–1402. doi: 10.1086/522606. [DOI] [PubMed] [Google Scholar]
- Rohan LC, Moncla BJ, Kunjara Na Ayudhya RP, Cost M, Huang Y, Gai F, Billitto N, Lynam JD, Pryke K, Graebing P, Hopkins N, Rooney JF, Friend D, Dezzutti CS. In vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide. PLoS One. 2010;5:e9310. doi: 10.1371/journal.pone.0009310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson Buckheit K, Yang L, Buckheit RW., Jr. Development of Dual-Acting Pyrimidinediones as Novel and Highly Potent Topical Anti-HIV Microbicides. Antimicrob Agents Chemother. 2011;55:5243–5254. doi: 10.1128/AAC.05237-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, Saag MS, Wu X, Shaw GM, Kappes JC. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46:1896–1905. doi: 10.1128/AAC.46.6.1896-1905.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]