Abstract
Non-melanoma skin cancers (NMSC) are the most common type of cancer, occurring at a rate of over 1 million per year in the United States. Although their metastatic potential is generally low, they can and do metastasize, especially in the immune compromised host, and their surgical treatment is often quite disfiguring. Ultraviolet radiation (UVR) as occurs with sunlight exposure is generally regarded as causal for these malignancies, but UVR is also required for vitamin D synthesis in the skin. Based on our own data and that reported in the literature, we hypothesize that the vitamin D produced in the skin serves to suppress UVR epidermal tumor formation. In this review we will first discuss the evidence supporting the conclusion that the vitamin D receptor (VDR), with or without its ligand 1,25-dihydroxyvitamin D, limits the propensity for cancer formation following UVR. We will then explore three potential mechanisms for this protection: inhibition of proliferation and stimulation of differentiation, immune regulation, and stimulation of DNA damage repair (DDR).
Introduction
Over 1 million skin cancers occur annually in the United States, 80% of which are basal cell carcinomas (BCC), 16% squamous cell carcinomas (SCC), and 4% melanomas, making skin cancer by far the most common cancer afflicting humankind.1 Surgery is generally curative, but disfiguring and costly. Ultraviolet radiation (UVR) is the major etiologic agent for these cancers, but is also the principal means by which the body obtains vitamin D. Furthermore, the skin is capable of converting the vitamin D produced to its active metabolite 1,25(OH)2D, and this conversion is potentiated by UVR at least in part by cytokines such as TNF-α which are increased by UVR in the epidermis. This ability of the epidermis to make its own vitamin D and 1,25(OH)2D is likely to be of great importance for epidermal physiology and pathology. It is not at all clear, for example, whether the oral administration of vitamin D, various analogs, and/or circulating levels of 25OHD and 1,25(OH)2D has a major impact on processes within the skin—they may or they may not. Sun avoidance may reduce one’s risk of developing skin cancer, but this practice frequently results in suboptimal levels of vitamin D in the body, not to mention the epidermis. As pointed out in the analysis by Lucas et al.,2 the global disease burden due to UVR pales in comparison to the disease burden due to vitamin D deficiency, and although the latter can be prevented with vitamin D supplementation, the skin remains for most of the world’s population the major site of vitamin D availability. Most tissues have the vitamin D receptor (VDR), and several including the epidermis, are capable of producing their own 1,25(OH)2D. Vitamin D deficiency is associated with a number of diseases including, but not limited to, osteomalacia and rickets. Increased cancer risk ranks high among these diseases, including cancer of epithelial tissues such as breast, prostate, and colon. Perhaps because UVR increases the risk at least for non-melanoma skin cancers (NMSC), the potential for vitamin D and 1,25(OH)2D production in the epidermis to serve as protection against UVR induced epidermal carcinogenesis has received little attention. However, a low UVR dose may be protective against skin cancer via the vitamin D signaling mechanisms that will be reviewed in this article, and some epidemiologic evidence is consistent with a potential benefit of low dose UVR. For example, in the study by Armstrong and Kricker,3 a slight decrease in the incidence of SCC, BCC, and melanomas in 10 US populations was observed when the solar UV measurement was increased from 100 to 110, although higher levels increased the incidence. This same group,4 evaluating data from the Australian population, did not find a significant increase in SCC with time spent out of doors in the general population. Rosso et al.,5 in a multicentre European study, did not find a significant increase in SCC below a threshold of 70 000 accumulated hours of sunshine, although the development of BCC had a lower threshold. Before examining the animal data supporting this hypothesis, it is useful to review vitamin D production and metabolism in the skin as mediated by UVB.
Vitamin D metabolism in the skin
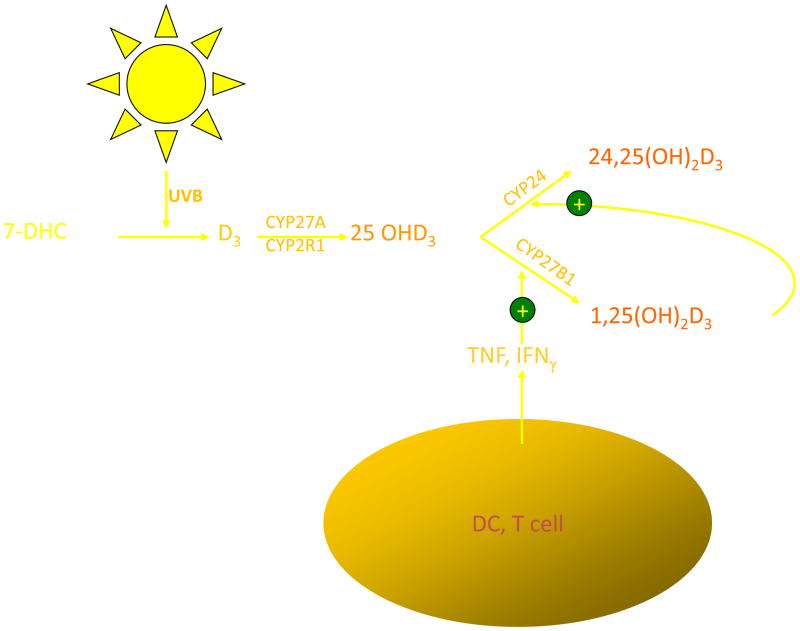
Vitamin D3 is produced from 7-dehydrocholesterol (7-DHC) (Fig. 1). Irradiation of 7-DHC with ultraviolet B (280–320 nm) (UVR) produces pre-D3, which subsequently undergoes a temperature-dependent rearrangement of the triene structure to form D3, lumisterol, and tachysterol. This process is relatively rapid and reaches a maximum within hours.6–8 Both the degree of epidermal pigmentation and the intensity of exposure correlate with the time required to achieve this maximal concentration of pre-D3 but do not alter the maximal level achieved. Although pre-D3 levels reach a maximum, the biologically inactive lumisterol and tachysterol accumulate with continued UV exposure. Thus, prolonged exposure to sunlight would not produce toxic amounts of D3 because of the photoconversion of pre-D3 to lumisterol and tachysterol. Melanin in the epidermis, by absorbing UVR, can also reduce the effectiveness of sunlight in producing D3 in the skin. Sunlight exposure increases melanin production, and so provides another mechanism by which excess D3 production can be prevented. The intensity of UVR is dependent on latitude and season. In Edmonton, Canada (52°N), very little D3 is produced in exposed skin from mid-October to mid-April, while in San Juan (18°N), the skin is able to produce D3 all year long9. Clothing and sunscreen effectively prevent D3 production in the covered areas. D3 produced in the skin can be secreted from the skin by a poorly understood process, carried to the liver and other tissues for further metabolism to 25-hydroxyvitamin D (25OHD), and then to the kidney to produce the biologically active metabolite 1,25(OH)2D by the enzyme CYP27B1. However, the keratinocyte contains the entire pathway for 1,25(OH)2D production from vitamin D.
Fig. 1.
Production of vitamin D and its metabolism to 1,25(OH)2D in the keratinocyte. UVB, via a photochemical reaction, breaks open the B ring of 7-dehydrocholesterol (7-DHC) to produce pre vitamin D3, which is subsequently converted first to 25OHD by the enzymes CYP27A1 and CYP2R1 and then to 1,25(OH)2D by CYP27B1. Regulation of CYP27B1 is primarily by cytokines such as tumor necrosis factor-α (TNF-α) and interferon-γ(IFN-γ).
The production of 1,25(OH)2D in the skin is under different regulation compared to its production by the kidney, where the parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF23) are the principal hormonal regulators (PTH stimulates, FGF23 inhibits). Keratinocytes respond to PTH with increased 1,25(OH)2D production, but these cells do not have the classic PTH receptor and do not respond to cyclic AMP.10 The mechanism by which PTH stimulates 1,25(OH)2D production in these cells remains unclear. The effect of FGF23 on keratinocyte CYP27B1 expression or function has not been reported. Furthermore, unlike the kidney, 1,25(OH)2D does not directly affect CYP27B1 expression in keratinocytes. Rather, 1,25(OH)2D regulates its own levels in the keratinocyte by inducing CYP24, the catabolic enzyme for 1,25(OH)2D3.11 Instead, cytokines such as tumor necrosis factor-α (TNF)12 and interferon-γ(IFN)13 are potent inducers of CYP27b1 activity in the keratinocyte. These cytokines are activated in the skin by UVB.
Vitamin D and skin cancer
1,25(OH)2D has been evaluated for its potential anticancer activity for approximately 25 years.14 Most cell types, including many cancer cells such as basal cell (BCC) and squamous cell (SCC) carcinomas15,16 as well as melanomas,17 contain the vitamin D receptor (VDR). Although epidemiologic evidence supporting the importance of adequate vitamin D nutrition (including sunlight exposure) for the prevention of at least some cancers, including those of the colon,18–22 is reasonably strong, such evidence is much weaker for skin cancers.23–25 One potential complication is that UVB radiation (UVR) has the dual effect of promoting vitamin D3 synthesis in the skin (which can be further converted to 1,25(OH)2D3) and increasing DNA damage leading to skin cancer. Thus, although UVR may be the most efficient means of providing the nutritional requirement for vitamin D, the advantage to the skin may be countered by the increased risk of mutagenesis if the UVR is excessive.
The potential for vitamin D signaling as protection against epidermal tumor formation received an important boost when Zinser et al.26 demonstrated that 85% of VDR null mice developed skin tumors within two months following 7,12-dimethyl-benzanthracene (DMBA) administration. No tumors were found in the wildtype controls. The tumors were mostly sebaceous, squamous, and follicular papillomas; however, several BCC were also observed. No SCC were reported. These results have been confirmed using topical administration of DMBA/TPA, although only papillomas were seen in the VDR null mice unlike RXRα null mice which developed both BCC and SCC.27 More relevant to the concept of photoprotection is the study by Ellison et al. 28 demonstrating that VDR null mice were also more susceptible to tumor formation following UVB. These tumors included SCC. We have confirmed these results with our own studies.29 The appearance of BCC in at least some of these studies (including our own) is surprising since the typical malignancy induced in mouse skin by UVR, ionizing radiation, or chemical carcinogens is SCC not BCC.30 The appearance of BCC is characteristic of tumors formed when hedgehog (Hh) signaling is disrupted,31 although activation of Hh signaling (Ptch−/+ mice) also predisposes to UVR induced SCC formation.32 The question then becomes, through what mechanism (s) does vitamin D signaling protect against UVB induced tumor formation? We will examine three potential mechanisms and pathways within those mechanisms: regulation of proliferation and differentiation with particular attention to the hedgehog (Hh) and wnt/β-catenin pathways, immunoregulation, and DNA damage repair.
Vitamin D regulation of epidermal proliferation and differentiation
The epidermis is composed of four layers of keratinocytes at different stages of differentiation (reviewed in ref. 33). The basal layer (stratum basale, SB) rests on the basal lamina separating the dermis and epidermis. Within this layer are the stem cells. These cells proliferate, providing the cells for the upper differentiating layers. They contain an extensive keratin network comprised of keratins K5 and K14. As the cells migrate upward from this basal layer they acquire the characteristics of a fully differentiated corneocyte, which is eventually sloughed off. The layer above the basal cells is the spinous layer (stratum spinosum, SS). These cells initiate the production of the keratins K1 and K10, which are the keratins characteristic of the more differentiated layers of the epidermis. Cornified envelope precursors such as involucrin also appear in the spinous layer as does the enzyme transglutaminase K, responsible for the ε-(γ-glutamyl)lysine cross-linking of these substrates into the insoluble Cornified envelope. The granular layer, stratum granulosum (SG), lying above the spinous layer, is characterized by electron-dense keratohyalin granules containing profilaggrin and loricrin, which is a major component of the Cornified envelope. The granular layer also contains lamellar bodies—lipid-filled structures that fuse with the plasma membrane, divesting their contents into the extracellular space between the SG and stratum corneum (SC) where the lipid contributes to the permeability barrier of skin. As the cells pass from the granular layer to the Cornified layer (SC), they undergo destruction of their organelles with further maturation of the Cornified envelope into an insoluble, highly resistant structure surrounding the keratin–filaggrin complex and linked to the extracellular lipid milieu. The outer layer of the epidermis provides not only a barrier to water loss (permeability barrier) but a barrier to invasion by infectious organisms.
As noted above, the keratinocytes of the epidermis are unique in their ability to produce vitamin D3 from the precursor 7-dehydrocholesterol (7-DHC) and to convert the vitamin D produced to the active metabolite 1,25(OH)2D. 1,25(OH)2D increases involucrin, transglutaminase activity, loricrin, filaggrin, and Cornified envelope formation at subnanomolar concentrations,34–39 while inhibiting proliferation at concentrations above 1 nM. Much of these actions are in conjunction with or via changes in calcium responsiveness due to the ability of 1,25(OH)2D to induce the calcium receptor40,41 and the phospholipase C enzymes42–44 that regulate the intracellular calcium levels critical for the differentiation process. The antiproliferative effects are accompanied by a reduction in the expression of c-myc45 and cyclin D146 and an increase in the cell cycle inhibitors p21cip and p27kip. In addition, 1,25(OH)2D and its receptor regulate the processing of the long chain glycosylceramides that are critical for permeability barrier formation47 and induce the receptors—toll-like receptor 2 (TLR2) and its coreceptor CD14—that initiate the innate immune response in skin.48 Activation of these receptors leads to the induction of CYP27B1 (the enzyme that produces 1,25(OH)2D), which in turn induces cathelicidin, resulting in the killing of invasive organisms.48,49 Although the most striking feature of the VDR-null mouse is the development of alopecia50,51 (also found in many patients with mutations in the VDR), referred to as hereditary vitamin D resistance,52 these mice also exhibit a defect in epidermal differentiation as shown by reduced levels of involucrin and loricrin and loss of keratohyalin granules.53,54 Furthermore, these mice show a reduction in the lipid content of the lamellar bodies concomitant with a reduction in glucosylceramide production and transport into the lamellar bodies leading to a defective permeability barrier.47 The CYP27B1 null mouse also shows a reduction in levels of the epidermal differentiation markers and altered permeability barrier55 with a blunted innate immune response48 and poor resistance to infections,56 but these mice do not have a defect in hair follicle cycling.
Two pathways that appear to be important for vitamin D signaling in the epidermis, with respect to the proliferation and differentiation that we believe underlie the predisposition of the VDR null mouse to tumor formation, are the Hh and wnt/ β-catenin pathways.
The hedgehog (Hh) pathway (Fig. 2)
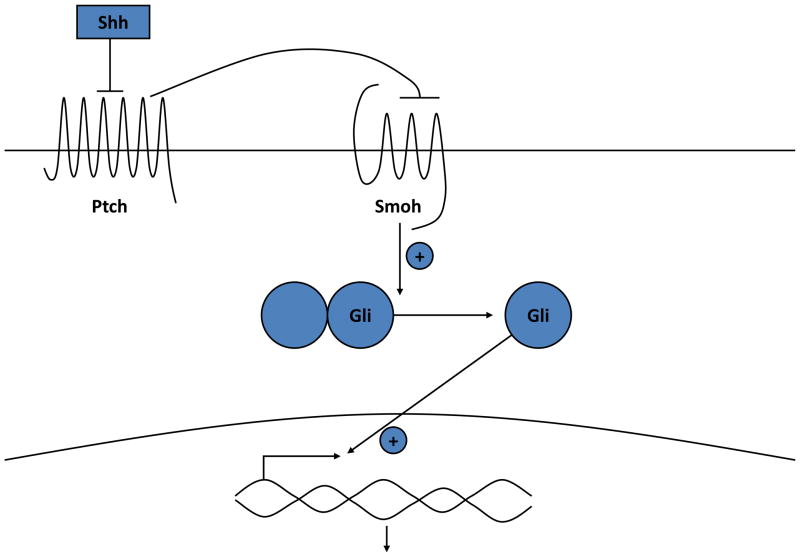
Fig. 2.
The hedgehog signaling pathway. In the absence of Shh, Ptch 1 suppresses signaling by smoothened (Smo). Binding of Shh to Ptch 1 relieves this inhibition. Activation of Smo leads to the activation and translocation of transcription factors of the Gli family into the nucleus, with subsequent changes in gene expression.
Ptch 1 is the membrane receptor for Shh. In the absence of Shh, Ptch 1 inhibits the function of another membrane protein smoothened (Smo). Shh reverses this inhibition, freeing Smo to enable the activation of a family of transcription factors Gli1, Gli2, and Gli3. Suppressor of fused (Sufu) may maintain these transcription factors in the cytoplasm and/or limit their activity in the nucleus such that the loss of Sufu leads to increased Hh signaling.57,58 Gli1 and 2 overexpression in keratinocytes can increase the expression of one another as well as Ptch 1, the anti-apoptotic factor bcl2, cyclins D1 and D2, E2F1, and cdc45 (all of which promote proliferation), while suppressing genes associated with keratinocyte differentiation such as K1, K10, involucrin, loricrin and the VDR.59–63
The appearance of BCC is characteristic of tumors formed when Hh signaling is disrupted,64 although activation of Hh signaling (Ptch−/+ mice) also predisposes to UVR induced SCC formation.32 We have found that the epidermis and epidermal portion (utricles) of the hair follicles of adult VDR null animals overexpress elements of the Hh signaling pathway.29 These results suggest that one of the causes of the increased susceptibility of the epidermis to malignant transformation is due to a loss of VDR regulation of Hh signaling in the epidermis. Furthermore, we29 found that 1,25(OH)2D suppressed all elements of the Hh pathway in a dose dependent fashion that required the VDR. These results have been confirmed at least for Gli1 by others65 in a study demonstrating that 1,25(OH)2D could reduce tumor growth in Ptch null mice. Furthermore, the promoters of Shh and Gli1 have been found to bind to VDR,66 suggesting a direct genomic action on these genes. However, vitamin D may regulate this pathway not only via the genomic actions of 1,25(OH)2D acting through its receptor, VDR, but also by direct inhibition by vitamin D independent of its receptor. The latter possibility stems from observations that vitamin D itself, as well as its precursor 7-dehydrocholesterol, can bind to and inhibit the actions of smoothened (Smo), a critical step in Hh signaling.67,68 1,25(OH)2D was less effective68 suggesting a direct effect of vitamin D itself, but the relative role of this non-genomic to the genomic actions of vitamin D is not clear.
The wnt/β-catenin pathway (Fig. 3)
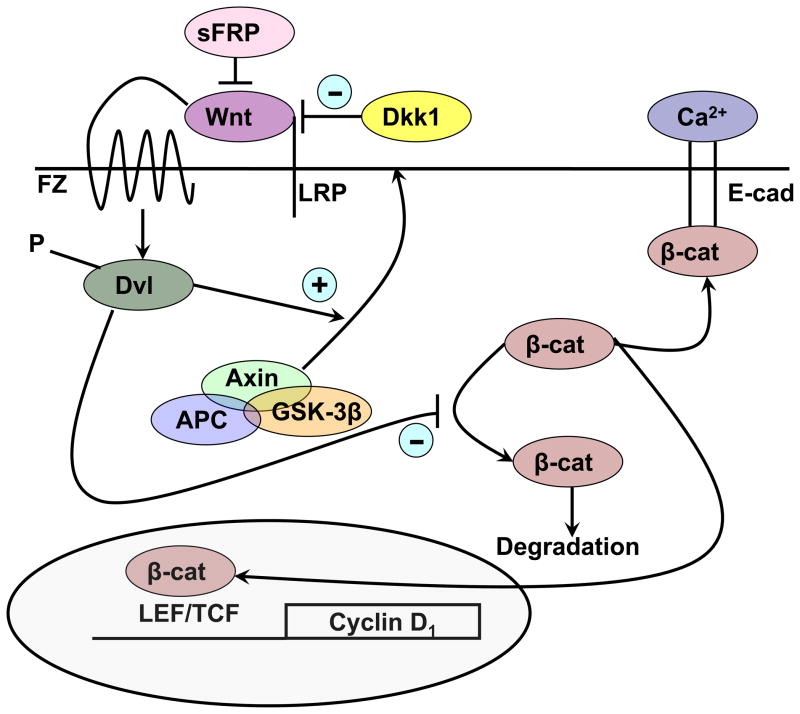
Fig. 3.
The wnt signaling pathway. Wnts bind to their frizzled receptors (FZ) and coreceptors LRP in the membrane. This binding can be blocked by dickkopf (Dkk) or soluble frizzled related proteins (sFRP). Activation of FZ by wnt results in phosphorylation of disheveled (Dvl) which induces the disruption of the axin/APC/GSK-3β complex and recruitment of axin to the membrane. When active, this complex phosphorylates β-catenin, leading to its proteosomal degradation. However, following wnt stimulation, β-catenin is no longer degraded and can enter the nucleus, where in combination with members of the LEF/TCF family, it can induce expression of its target genes such as cyclin D1. β-Catenin also binds to the E-cadherin complex in the plasma membrane, a complex stabilized by calcium, where it may play a role in differentiation.
Wnt signaling via activation of β-catenin has a complex role in VDR function. Wnt ligands bind to their seven-transmembrane frizzled receptors and an LRP5 or LRP6 co-receptor leading to phosphorylation of disheveled (Dvl) resulting in disruption of the axin/APC complex and inhibition of the kinase activity of glycogen synthase kinase 3β (GSK-3β). Phosphorylation by GSK-3β of the serine(s) within exon 3 of β-catenin results in its degradation by the E3 ubiquitin ligase. Thus, wnt signaling increases the availability of β-catenin in the nucleus, where it binds to transcription factors of the T-cell factor (TCF) and lymphoid enhancer factor (LEF) families to promote the expression of genes such as cyclin D1 and c-myc69 important for proliferation. β-Catenin also forms part of the adherens junction complex with E-cadherin where it may play an important role in keratinocyte differentiation.70 Tyrosine phosphorylation of E-cadherin, as occurs after calcium administration to keratinocytes, promotes the binding of β-catenin and other catenins to the adherens junction complex70,71 making it less available for transcriptional activity. Over expression and/or activating mutations in the β-catenin pathway lead to skin tumors, in this case pilomatricomas or trichofolliculomas (hair follicle tumors),72–74 indicative of the hyperproliferative response to β-catenin in these cells. In colon cancer cells, VDR has been shown to bind to β-catenin, and reduce its transcriptional activity in a ligand dependent fashion.75 Furthermore, in these cells, 1,25(OH)2D has been shown to increase E-cadherin expression, such that β-catenin is redistributed from the nucleus to the plasma membrane where it forms a complex with E-cadherin and other catenins at adherens junctions.76 We46 have obseved similar phenomena in keratinocytes. However, the suppression of β-catenin signaling by 1,25(OH)2D does not necessarily require E-cadherin.77 Rather, β-catenin binds to VDR in its AF-2 domain, binding that enhances the ability of 1,25(OH)2D to activate the transcriptional activity of the VDR,77 but blocks the transcriptional activity of β-catenin.77 Mutations in the AF-2 domain that block coactivator binding do not necessarily block β-catenin binding.77 Palmer et al.78 evaluated the interaction between VDR and β-catenin in transcriptional regulation in keratinocytes, and identified putative response elements for VDR and β-catenin/LEF in a number of genes. These interactions were either positive or negative, depending on the gene being evaluated. The hypothesis put forward is that genes in which the interaction was positive (i.e. stimulated transcription) benefited from β-catenin acting as a coactivator for VDR on VDREs, whereas in situations where the interaction was negative (i.e. suppression of transcription), VDR prevented β-catenin from binding to TCF/LEF required for transcription in those genes. We have found in keratinocytes that knockdown of VDR reduces E-cadherin expression and formation of the β-catenin/E-cadherin membrane complex, resulting in increased β-catenin transcriptional activity, whereas 1,25(OH)2D administration has the opposite effect.46 This was associated with increased (with VDR knockdown) or decreased (with 1,25(OH)2D administration) keratinocyte proliferation and cyclin D1 expression, respectively. These actions in the epidermis (and intestinal epithelium) appear to differ from those in the hair follicle in that Cianferotti et al.79 found a reduction in the proliferation of keratinocytes in the dermal portion of the hair follicle (below the bulge) in VDR null mice, and no stimulation of proliferation when β-catenin was overexpressed in these cells, in contrast to the stimulation of proliferation in control animals. Thus, VDR/β-catenin interactions can be positive or negative, depending on the gene/cell/function being evaluated; however, in the epidermis in the absence of VDR, the unchecked activity of wnt/β-catenin appears to be proliferative and inhibitory of differentiation.
Vitamin D regulation of epidermal immunity
The potential role for vitamin D and its active metabolite 1,25(OH)2D to modulate the immune response rests on the observation that VDR is found in activated dendritic cells, macrophages, and lymphocytes,80,81 that these cells produce 1,25(OH)2D (i.e. express CYP27B1),80 and that 1,25(OH)2D regulates their proliferation and function.82 Two forms of immunity exist, adaptive and innate, and each are regulated by 1,25(OH)2D.
Adaptive immunity
The adaptive immune response involves the ability of T and B lymphocytes to produce cytokines and immunoglobulins, respectively, to specifically combat the source of the antigen presented to them by cells such as macrophages and dendritic cells. Vitamin D exerts an inhibitory action on the adaptive immune system. In particular, 1,25(OH)2D suppresses proliferation and immunoglobulin production and retards the differentiation of B-cell precursors into plasma cells.81 In addition, 1,25(OH)2D inhibits T-cell proliferation,83 in particular the Th1 cells capable of producing IFN-γ and IL-2 and activating macrophages84 and Th17 cells capable of producing IL-17 and IL-22.85,86 In contrast IL-4, IL-5, and IL-10 production increase,87 shifting the balance to a Th2 cell phenotype. CD4+/ CD25+ regulatory T-cells (Treg) are also increased by 1,25 (OH)2D88 leading to increased IL-10 production. The IL-10 so produced is one means by which Treg can block Th1 and Th17 development. At least in part, these actions on T-cell proliferation and differentiation stem from actions of 1,25(OH)2D on dendritic cells to reduce their maturation and antigen presenting capability.89 The ability of 1,25(OH)2D to suppress the adaptive immune system is beneficial for conditions in which the immune system is directed at self—i.e. autoimmunity90 and following transplants91—but might not be good for tumor surveillance.
Innate immunity
The innate immune response is the critical first line of defense against invading pathogens. The response involves the activation of toll-like receptors (TLRs) in polymorphonuclear cells (PMNs), monocytes, and macrophages as well as in a number of epithelial cells including those of the epidermis, gingiva, intestine, vagina, bladder, and lungs.92 TLRs are transmembrane pathogen-recognition receptors that interact with specific membrane patterns (PAMP) shed by infectious agents that trigger the innate immune response in the host.93 Activation of TLRs leads to the induction of antimicrobial peptides and reactive oxygen species, which kill the organism. Among these antimicrobial peptides is cathelicidin. The expression of this anti-microbial peptide is induced by 1,25(OH)2D in both myeloid and epithelial cells,94,95 cells that also express CYP27B1 and so are capable of producing the 1,25(OH)2D needed for this induction. Stimulation of TLR2 by an antimicrobial peptide in macrophages96 or stimulation of TLR2 in keratinocytes by wounding the epidermis48 results in increased CYP27B1 expression, which in the presence of adequate substrate (25OHD) stimulates cathelicidin expression. Lack of substrate (25OHD), VDR, or CYP27B1 blunts the ability of these cells to respond to a challenge with respect to cathelicidin production.48,95,96
The major cells involved in adaptive immunity in the skin include the Langerhans cells, dendritic cells, and T-cells. The Langerhans cells are dendritic-like cells within the epidermis that when activated by invading organisms, migrate to the lymph nodes serving the skin where they present the antigens to the T-cells, initiating the adaptive immune response.97 Keratinocytes, on the other hand, are equipped with toll-like receptors that enable them to respond to invading organisms with elaboration of antimicrobial peptides such as cathelicidin49 but also a variety of cytokines that provoke an inflammatory response.98 UVB leads to a reduction in Langerhans cells and blunts their antigen presenting activity,99–101 but stimulates the innate immune function of keratinocytes perhaps as a consequence of UVB induced vitamin D/1,25(OH)2D production in the skin.102,103
The potential role of altered skin immunity by UVB with respect to skin carcinogenesis was suggested by Kripke and Fisher104 who found that skin tumors originally induced in mice by chronic UVR grew when transplanted into other UV-irradiated mice; however, it was found that the tumors did not grow further when transplanted into non-irradiated mice. What is less clear is whether 1,25(OH)2D would enhance or protect against UVR immunosuppression. One study demonstrated that topical 1,25(OH)2D at a high concentration (1.65 μM) protected against UVR induced suppression of contact hypersensitivity to oxazolone in mouse skin;105 however, a study in humans from the same group showed suppression of delayed hypersensitivity (Mantoux test) by topical 1,25(OH)2D at high doses with a trend toward additive suppression even at lower doses when combined with UVR.106 These data are limited, but raise some concerns about the balance between innate and adaptive immunity in tumor surveillance, and how that balance is affected by vitamin D.
Vitamin D regulation of the DNA damage response
UV wavelengths shorter than 280 nm (UVC) are absorbed by the ozone layer and do not reach the earth. UV wavelengths longer than 320 nm (UVA) are capable of penetrating into the dermis, and damage DNA (e.g. 8-oxoguanine production) primarily by oxidative processes. UVA does not stimulate vitamin D production. UVB, with a spectrum range of 280–320 nm, exerts its effects primarily in the epidermis, where it causes DNA cyclobutane pyrimidine dimers (CPD) and pyrimidine (6–4)pyrimidone photoproducts (6–4PP), which if not repaired result in C to T or CC to TT mutations, the UVB “signature” lesion.107,108 This type of mutation in p53 is common (50–90%) in both BCC and SCC30,109–111 as well as in actinic keratoses, the precursor lesions to SCC.112 Preventing UVR induced DNA damage from producing DNA mutations is the role of DNA damage repair (DDR), and p53 appears to have an important role in this process.113 DDR involves a cascade of damage recognition, and repair and signal transduction that coordinates the response of the cell cycle to DNA damage. DDR activates checkpoints that delay the cell cycle, provides time for repair, and directs damaged cells into senescent or apoptotic pathways. DDR involves a number of components, is well orchestrated, tightly controlled, and highly accurate in normal primary cells such that the spontaneous mutation rate is very low, and changes in copy number are negligible.114–116 During malignant transformation, DDR becomes less controlled, and mutation rates and copy number abnormalities increase by several orders of magnitude.114,115,117,118 Nucleotide excision repair (NER) is the principal means by which UVR damage is repaired. NER can remove DNA damage before DNA replication begins, and consequently plays a major role in reducing the amount of damage that becomes fixed as mutations during replication.119–121 The DNA damage is recognized, the DNA is unwound around the lesion, and 30 base pair portions of DNA containing the lesion are excised by endonucleases such as XPF and XPG followed by fill-in with DNA polymerases such as Polδ, Polε, and Polκ.
The NER process has two main branches distinguished by the mechanisms used for initial recognition of DNA damage:122 transcription coupled repair (TCR) during which DNA polymerases stop replication at the site of the lesion until it is repaired,123–127 and global genomic repair (GGR), during which non-transcribed regions of the genome are repaired.128 Keratinocytes in the epidermis of mice lacking VDR are deficient in DDR as demonstrated by a reduced rate of clearing CPDs and 6-4PPs following UVB.129 Moreover, 1,25(OH)2D increases CPD clearance and p53 in VDR intact mice130,131 as well as upregulation of two genes important for DDR: XPC (xeroderma pigmentosum complementation group C) and DDB2 (damage-specific DNA binding protein 2, also known as XPE).129,132 These actions of vitamin D signaling on DDR contribute to the reduced susceptibility of normal skin to UVB induced tumor formation.
Summary
UVB is critical for vitamin D production in the skin, but UVB is also the major cause of skin cancer. This article examines the question of whether the beneficial effects of vitamin D production can counter the harmful effects of carcinogenesis, and the predisposition of VDR null mice to UVB induced skin cancer suggests that it is a possibility. Three potential mechanisms for such protection were examined. The first mechanism focuses on the role of vitamin D signaling in keratinocyte proliferation and differentiation. In particular, two pathways affecting proliferation and differentiation, namely the hedgehog and wnt/β-catenin pathways, were evaluated. Mice lacking the VDR have increased expression of the hedgehog pathway and increased activation of the wnt/β-catenin pathway. 1,25(OH)2D suppresses both pathways in cells containing the VDR. Thus, in the absence of vitamin D signaling, overexpression of the hedgehog and wnt/β-catenin pathways leads to increased proliferation and decreased differentiation associated with tumor development. The second mechanism involves the role of vitamin D signaling in the immune system of the skin. 1,25(OH)2D/VDR promotes innate immunity but suppresses adaptive immunity. UVB likewise promotes innate immunity and suppresses adaptive immunity, perhaps in part by stimulating vitamin D/1,25-(OH)2D production. The net effect on tumor formation with respect to protection by vitamin D signaling is unclear. The third mechanism is DNA damage repair (DDR). The epidermis of VDR null mice show impaired DDR following UVR. 1,25-(OH)2D accelerates DDR. Thus, by these mechanisms one can conclude that the skin has evolved protective mechanisms against UVR induced carcinogenesis, and that these mechanisms involve vitamin D.
Acknowledgments
This work was supported by grants from the American Institute for Cancer Research, the VA Merit Review, the Department of Defense (CA110338), and the National Institute of Health (RO1 AR050023). Much of the data referred to was generated in collaboration with Drs. Arnaud Teichert, Yuko Oda, Dennis Oh, and JoEllen Welsh. Teresa Tong and Victoria Lee provided administrative support.
Footnotes
Contribution to the Vitamin D update collected papers.
References
- 1.Greenlee RT, Hill-Harmon MB, Murray T, Thun M. Cancer statistics. Ca-Cancer J Clin. 2001;51:15–36. doi: 10.3322/canjclin.51.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Lucas RM, McMichael AJ, Armstrong BK, Smith WT. Estimating the global disease burden due to ultraviolet radiation exposure. Int J Epidemiol. 2008;37:654–667. doi: 10.1093/ije/dyn017. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol, B. 2001;63:8–18. doi: 10.1016/s1011-1344(01)00198-1. [DOI] [PubMed] [Google Scholar]
- 4.English DR, Armstrong BK, Kricker A, Winter MG, Heenan PJ, Randell PL. Case-control study of sun exposure and squamous cell carcinoma of the skin. Int J Cancer. 1998;77:347–353. doi: 10.1002/(sici)1097-0215(19980729)77:3<347::aid-ijc7>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 5.Rosso S, Zanetti R, Martinez C, Tormo MJ, Schraub S, Sancho-Garnier H, Franceschi S, Gafa L, Perea E, Navarro C, Laurent R, Schrameck C, Talamini R, Tumino R, Wechsler J. The multicentre south European study ‘Helios’. II: different sun exposure patterns in the aetiology of basal cell and squamous cell carcinomas of the skin. Br J Cancer. 1996;73:1447–1454. doi: 10.1038/bjc.1996.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holick MF, McLaughlin JA, Clark MB, Doppelt SH. Factors that influence the cutaneous photosynthesis of previtamin D3. Science. 1981;211:590–593. doi: 10.1126/science.6256855. [DOI] [PubMed] [Google Scholar]
- 7.Holick MF, McLaughlin JA, Clark MB, Holick SA, Potts JT, Jr, Anderson RR, Blank IH, Parrish JA. Photosynthesis of previtamin D3 in human and the physiologic consequences. Science. 1980;210:203–205. doi: 10.1126/science.6251551. [DOI] [PubMed] [Google Scholar]
- 8.Holick MF, Richtand NM, McNeill SC, Holick SA, Henley JW, Potts JT., Jr Isolation and identification of previtamin D3 from the skin of exposed to ultraviolet irradiation. Biochemistry. 1979;18:1003–1008. doi: 10.1021/bi00573a011. [DOI] [PubMed] [Google Scholar]
- 9.Webb AR, Kline L, Holick MF. Influence of season and latitude on the cutaneous synthesis of vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 synthesis in human skin. J Clin Endocrinol Metab. 1988;67:373–378. doi: 10.1210/jcem-67-2-373. [DOI] [PubMed] [Google Scholar]
- 10.Bikle DD, Nemanic MK, Gee E, Elias P. 1,25-Dihydroxyvitamin D3 production by human keratinocytes. Kinetics and regulation. J Clin Invest. 1986;78:557–566. doi: 10.1172/JCI112609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Z, Munson SJ, Huang N, Portale AA, Miller WL, Bikle DD. The mechanism of 1,25-dihydroxyvitamin D(3) auto-regulation in keratinocytes. J Biol Chem. 2002;277:36987–36990. doi: 10.1074/jbc.M201404200. [DOI] [PubMed] [Google Scholar]
- 12.Bikle DD, Pillai S, Gee E, Hincenbergs M. Tumor necrosis factor-alpha regulation of 1,25-dihydroxyvitamin D production by human keratinocytes. Endocrinology. 1991;129:33–38. doi: 10.1210/endo-129-1-33. [DOI] [PubMed] [Google Scholar]
- 13.Bikle DD, Pillai S, Gee E, Hincenbergs M. Regulation of 1,25-dihydroxyvitamin D production in human keratinocytes by interferon-gamma. Endocrinology. 1989;124:655–660. doi: 10.1210/endo-124-2-655. [DOI] [PubMed] [Google Scholar]
- 14.Eisman JA, Martin TJ, MacIntyre I, Moseley JM. 1,25-dihydroxyvitamin-D-receptor in breast cancer cells. Lancet. 1979;2:1335–1336. doi: 10.1016/s0140-6736(79)92816-2. [DOI] [PubMed] [Google Scholar]
- 15.Ratnam AV, Bikle DD, Su MJ, Pillai S. Squamous carcinoma cell lines fail to respond to 1,25-dihydroxyvitamin D despite normal levels of the vitamin D receptor. J Invest Dermatol. 1996;106:522–525. doi: 10.1111/1523-1747.ep12343898. [DOI] [PubMed] [Google Scholar]
- 16.Kamradt J, Rafi L, Mitschele T, Meineke V, Gartner BC, Wolfgang T, Holick MF, Reichrath J. Analysis of the vitamin D system in cutaneous malignancies. Recent Results Cancer Res. 2003;164:259–269. doi: 10.1007/978-3-642-55580-0_19. [DOI] [PubMed] [Google Scholar]
- 17.Colston K, Colston MJ, Feldman D. 1,25-dihydroxyvitamin D3 and malignant melanoma: the presence of receptors and inhibition of cell growth in culture. Endocrinology. 1981;108:1083–1086. doi: 10.1210/endo-108-3-1083. [DOI] [PubMed] [Google Scholar]
- 18.Garland C, Shekelle RB, Barrett-Connor E, Criqui MH, Rossof AH, Paul O. Dietary vitamin D and calcium and risk of colorectal cancer: a 19-year prospective study in men. Lancet. 1985;1:307–309. doi: 10.1016/s0140-6736(85)91082-7. [DOI] [PubMed] [Google Scholar]
- 19.Bostick RM, Potter JD, Sellers TA, McKenzie DR, Kushi LH, Folsom AR. Relation of calcium, vitamin D, and dairy food intake to incidence of colon cancer among older women. The Iowa Women’s Health Study. Am J Epidemiol. 1993;137:1302–1317. doi: 10.1093/oxfordjournals.aje.a116640. [DOI] [PubMed] [Google Scholar]
- 20.Kearney J, Giovannucci E, Rimm EB, Ascherio A, Stampfer MJ, Colditz GA, Wing A, Kampman E, Willett WC. Calcium, vitamin D, and dairy foods and the occurrence of colon cancer in men. Am J Epidemiol. 1996;143:907–917. doi: 10.1093/oxfordjournals.aje.a008834. [DOI] [PubMed] [Google Scholar]
- 21.Garland FC, Garland CF, Gorham ED, Young JF. Geographic variation in breast cancer mortality in the United States: a hypothesis involving exposure to solar radiation. Prev Med. 1990;19:614–622. doi: 10.1016/0091-7435(90)90058-r. [DOI] [PubMed] [Google Scholar]
- 22.Hanchette CL, Schwartz GG. Geographic patterns of prostate cancer mortality. Evidence for a protective effect of ultraviolet radiation. Cancer. 1992;70:2861–2869. doi: 10.1002/1097-0142(19921215)70:12<2861::aid-cncr2820701224>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 23.van Dam RM, Huang Z, Giovannucci E, Rimm EB, Hunter DJ, Colditz GA, Stampfer MJ, Willett WC. Diet and basal cell carcinoma of the skin in a prospective cohort of men. Am J Clin Nutr. 2000;71:135–141. doi: 10.1093/ajcn/71.1.135. [DOI] [PubMed] [Google Scholar]
- 24.Hunter DJ, Colditz GA, Stampfer MJ, Rosner B, Willett WC, Speizer FE. Diet and risk of basal cell carcinoma of the skin in a prospective cohort of women. Ann Epidemiol. 1992;2:231–239. doi: 10.1016/1047-2797(92)90055-u. [DOI] [PubMed] [Google Scholar]
- 25.Weinstock MA, Stampfer MJ, Lew RA, Willett WC, Sober AJ. Case-control study of melanoma and dietary vitamin D: implications for advocacy of sun protection and sunscreen use. J Invest Dermatol. 1992;98:809–811. doi: 10.1111/1523-1747.ep12499962. [DOI] [PubMed] [Google Scholar]
- 26.Zinser GM, Sundberg JP, Welsh J. Vitamin D(3) receptor ablation sensitizes skin to chemically induced tumorigenesis. Carcinogenesis. 2002;23:2103–2109. doi: 10.1093/carcin/23.12.2103. [DOI] [PubMed] [Google Scholar]
- 27.Indra AK, Castaneda E, Antal MC, Jiang M, Messaddeq N, Meng X, Loehr CV, Gariglio P, Kato S, Wahli W, Desvergne B, Metzger D, Chambon P. Malignant transformation of DMBA/TPA-induced papillomas and nevi in the skin of mice selectively lacking retinoid-X-receptor alpha in epidermal keratinocytes. J Invest Dermatol. 2007;127:1250–1260. doi: 10.1038/sj.jid.5700672. [DOI] [PubMed] [Google Scholar]
- 28.Ellison TI, Smith MK, Gilliam AC, Macdonald PN. Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis. J Invest Dermatol. 2008;128:2508–2517. doi: 10.1038/jid.2008.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Teichert AE, Elalieh H, Elias PM, Welsh J, Bikle DD. Over-expression of hedgehog signaling is associated with epidermal tumor formation in vitamin D receptor-null mice. J Invest Dermatol. 2011;131:2289–2297. doi: 10.1038/jid.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Daya-Grosjean L, Sarasin A. The role of UV induced lesions in skin carcinogenesis: an overview of oncogene and tumor suppressor gene modifications in xeroderma pigmentosum skin tumors. Mutat Res, Fundam Mol Mech Mutagen. 2005;571:43–56. doi: 10.1016/j.mrfmmm.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 31.Aszterbaum M, Rothman A, Johnson RL, Fisher M, Xie J, Bonifas JM, Zhang X, Scott MP, Epstein EH., Jr Identification of mutations in the human PATCHED gene in sporadic basal cell carcinomas and in patients with the basal cell nevus syndrome. J Invest Dermatol. 1998;110:885–888. doi: 10.1046/j.1523-1747.1998.00222.x. [DOI] [PubMed] [Google Scholar]
- 32.Ping XL, Ratner D, Zhang H, Wu XL, Zhang MJ, Chen FF, Silvers DN, Peacocke M, Tsou HC. PTCH mutations in squamous cell carcinoma of the skin. J Invest Dermatol. 2011;116:614–616. doi: 10.1046/j.1523-1747.2001.01301.x. [DOI] [PubMed] [Google Scholar]
- 33.Bikle DD. Vitamin D and the skin: physiology and pathophysiology. Rev Endocr Metab Disord. 2012;13:3–19. doi: 10.1007/s11154-011-9194-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pillai S, Bikle DD. Role of intracellular-free calcium in the Cornified envelope formation of keratinocytes: differences in the mode of action of extracellular calcium and 1,25 dihydroxyvitamin D3. J Cell Physiol. 1991;146:94–100. doi: 10.1002/jcp.1041460113. [DOI] [PubMed] [Google Scholar]
- 35.Bikle DD, Pillai S, Gee E. Squamous carcinoma cell lines produce 1,25 dihydroxyvitamin D, but fail to respond to its prodifferentiating effect. J Invest Dermatol. 1991;97:435–441. doi: 10.1111/1523-1747.ep12481267. [DOI] [PubMed] [Google Scholar]
- 36.Hosomi J, Hosoi J, Abe E, Suda T, Kuroki T. Regulation of terminal differentiation of cultured mouse epidermal cells by 1-alpha,25-dihydroxyvitamin D3. Endocrinology. 1983;113:1950–1957. doi: 10.1210/endo-113-6-1950. [DOI] [PubMed] [Google Scholar]
- 37.Smith EL, Walworth NC, Holick MF. Effect of 1-alpha,25-dihydroxyvitamin D3 on the morphologic and biochemical differentiation of cultured human epidermal keratinocytes grown in serum-free conditions. J Invest Dermatol. 1986;86:709–714. doi: 10.1111/1523-1747.ep12276343. [DOI] [PubMed] [Google Scholar]
- 38.McLane JA, Katz M, Abdelkader N. Effect of 1,25-dihydroxy-vitamin D3 on human keratinocytes grown under different culture conditions. In Vitro Cell Dev Biol. 1990;26:379–387. doi: 10.1007/BF02623829. [DOI] [PubMed] [Google Scholar]
- 39.Hawker NP, Pennypacker SD, Chang SM, Bikle DD. Regulation of human epidermal keratinocyte differentiation by the vitamin D receptor and its coactivators DRIP205, SRC2, and SRC3. J Invest Dermatol. 2007;127:874. doi: 10.1038/sj.jid.5700624. [DOI] [PubMed] [Google Scholar]
- 40.Tu CL, Chang W, Xie Z, Bikle DD. Inactivation of the calcium sensing receptor inhibits E-cadherin-mediated cell-cell adhesion and calcium-induced differentiation in human epidermal keratinocytes. J Biol Chem. 2008;283:3519–3528. doi: 10.1074/jbc.M708318200. [DOI] [PubMed] [Google Scholar]
- 41.Tu CL, Oda Y, Komuves L, Bikle DD. The role of the calcium-sensing receptor in epidermal differentiation. Cell Calcium. 2004;35:265–273. doi: 10.1016/j.ceca.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 42.Xie Z, Bikle DD. Cloning of the human phospholipase C-gamma1 promoter and identification of a DR6-type vitamin D-responsive element. J Biol Chem. 1997;272:6573–6577. doi: 10.1074/jbc.272.10.6573. [DOI] [PubMed] [Google Scholar]
- 43.Xie Z, Bikle DD. Phospholipase C-gamma1 is required for calcium-induced keratinocyte differentiation. J Biol Chem. 1999;274:20421–20424. doi: 10.1074/jbc.274.29.20421. [DOI] [PubMed] [Google Scholar]
- 44.Xie Z, Bikle DD. Inhibition of 1,25-dihydroxyvitamin-D-induced keratinocyte differentiation by blocking the expression of phospholipase C-gamma1. J Invest Dermatol. 2001;117:1250–1254. doi: 10.1046/j.0022-202x.2001.01526.x. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto K, Hashimoto K, Nishida Y, Hashiro M, Yoshikawa K. Growth-inhibitory effects of 1,25-dihydroxyvitamin D3 on normal human keratinocytes cultured in serum-free medium. Biochem Biophys Res Commun. 1990;166:916–923. doi: 10.1016/0006-291x(90)90898-w. [DOI] [PubMed] [Google Scholar]
- 46.Bikle DD. The vitamin D receptor: a tumor suppressor in skin. Discov Med. 2011;11:7–17. [PMC free article] [PubMed] [Google Scholar]
- 47.Oda Y, Uchida Y, Moradian S, Crumrine D, Elias P, Bikle D. Vitamin D receptor and coactivators SRC 2 and 3 regulate epidermis-specific sphingolipid production and permeability barrier formation. J Invest Dermatol. 2009;129:1367–1378. doi: 10.1038/jid.2008.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zugel U, Bikle DD, Modlin RL, Gallo RL. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schauber J, Dorschner RA, Yamasaki K, Brouha B, Gallo RL. Control of the innate epithelial antimicrobial response is cell-type specific and dependent on relevant microenvironmental stimuli. Immunology. 2006;118:509–519. doi: 10.1111/j.1365-2567.2006.02399.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li YC, Pirro AE, Amling M, Delling G, Baron R, Bronson R, Demay MB. Targeted ablation of the vitamin D receptor: an animal model of vitamin D-dependent rickets type II with alopecia. Proc Natl Acad Sci U S A. 1997;94:9831–9835. doi: 10.1073/pnas.94.18.9831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, Masushige S, Fukamizu A, Matsumoto T, Kato S. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997;16:391–396. doi: 10.1038/ng0897-391. [DOI] [PubMed] [Google Scholar]
- 52.Malloy PJ, Pike JW, Feldman D. The vitamin D receptor and the syndrome of hereditary 1,25-dihydroxyvitamin D-resistant rickets. Endocr Rev. 1999;20:156–188. doi: 10.1210/edrv.20.2.0359. [DOI] [PubMed] [Google Scholar]
- 53.Xie Z, Komuves L, Yu QC, Elalieh H, Ng DC, Leary C, Chang S, Crumrine D, Yoshizawa T, Kato S, Bikle DD. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002;118:11–16. doi: 10.1046/j.1523-1747.2002.01644.x. [DOI] [PubMed] [Google Scholar]
- 54.Bikle DD, Elalieh H, Chang S, Xie Z, Sundberg JP. Development and progression of alopecia in the vitamin D receptor null mouse. J Cell Physiol. 2006;207:340–353. doi: 10.1002/jcp.20578. [DOI] [PubMed] [Google Scholar]
- 55.Bikle DD, Chang S, Crumrine D, Elalieh H, Man MQ, Choi EH, Dardenne O, Xie Z, Arnaud RS, Feingold K, Elias PM. 25 Hydroxyvitamin D 1 alpha-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J Invest Dermatol. 2004;122:984–992. doi: 10.1111/j.0022-202X.2004.22424.x. [DOI] [PubMed] [Google Scholar]
- 56.Muehleisen B, Bikle DD, Aguilera C, Burton DW, Sen GL, Deftos LJ, Gallo RL. PTH/PTHrP and vitamin D control antimicrobial peptide expression and susceptibility to bacterial skin infection. Sci Transl Med. 2012;4:135ra166. doi: 10.1126/scitranslmed.3003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barnfield PC, Zhang X, Thanabalasingham V, Yoshida M, Hui CC. Negative regulation of Gli1 and Gli2 activator function by suppressor of fused through multiple mechanisms. Differentiation. 2005;73:397–405. doi: 10.1111/j.1432-0436.2005.00042.x. [DOI] [PubMed] [Google Scholar]
- 58.Svard J, Heby-Henricson K, Persson-Lek M, Rozell B, Lauth M, Bergstrom A, Ericson J, Toftgard R, Teglund S. Genetic elimination of Suppressor of fused reveals an essential repressor function in the mammalian Hedgehog signaling pathway. Dev Cell. 2006;10:187–197. doi: 10.1016/j.devcel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 59.Regl G, Kasper M, Schnidar H, Eichberger T, Neill GW, Ikram MS, Quinn AG, Philpott MP, Frischauf AM, Aberger F. The zinc-finger transcription factor GLI2 antagonizes contact inhibition and differentiation of human epidermal cells. Oncogene. 2004;23:1263–1274. doi: 10.1038/sj.onc.1207240. [DOI] [PubMed] [Google Scholar]
- 60.Regl G, Kasper M, Schnidar H, Eichberger T, Neill GW, Philpott MP, Esterbauer H, Hauser-Kronberger C, Frischauf AM, Aberger F. Activation of the BCL2 promoter in response to Hedgehog/GLI signal transduction is predominantly mediated by GLI2. Cancer Res. 2004;64:7724–7731. doi: 10.1158/0008-5472.CAN-04-1085. [DOI] [PubMed] [Google Scholar]
- 61.Regl G, Neill GW, Eichberger T, Kasper M, Ikram MS, Koller J, Hintner H, Quinn AG, Frischauf AM, Aberger F. Human GLI2 and GLI1 are part of a positive feedback mechanism in basal cell carcinoma. Oncogene. 2002;21:5529–5539. doi: 10.1038/sj.onc.1205748. [DOI] [PubMed] [Google Scholar]
- 62.Grachtchouk M, Mo R, Yu S, Zhang X, Sasaki H, Hui CC, Dlugosz AA. Basal cell carcinomas in mice overexpressing Gli2 in skin. Nat Genet. 2000;24:216–217. doi: 10.1038/73417. [DOI] [PubMed] [Google Scholar]
- 63.Nilsson M, Unden AB, Krause D, Malmqwist U, Raza K, Zaphiropoulos PG, Toftgard R. Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc Natl Acad Sci U S A. 2000;97:3438–3443. doi: 10.1073/pnas.050467397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, Vorechovsky I, Holmberg E, Unden AB, Gillies S, Negus K, Smyth I, Pressman C, Leffell DJ, Gerrard B, Goldstein AM, Dean M, Toftgard R, Chenevix-Trench G, Wainwright B, Bale AE. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 65.Uhmann A, Niemann H, Lammering B, Henkel C, Hess I, Nitzki F, Fritsch A, Prufer N, Rosenberger A, Dullin C, Schraepler A, Reifenberger J, Schweyer S, Pietsch T, Strutz F, Schulz-Schaeffer W, Hahn H. Antitumoral effects of calcitriol in basal cell carcinomas involve inhibition of hedgehog signaling and induction of vitamin D receptor signaling and differentiation. Mol Cancer Ther. 2012;10:2179–2188. doi: 10.1158/1535-7163.MCT-11-0422. [DOI] [PubMed] [Google Scholar]
- 66.Luderer HF, Gori F, Demay MB. Lymphoid enhancer-binding factor-1 (LEF1) interacts with the DNA-binding domain of the vitamin D receptor. J Biol Chem. 2011;286:18444–18451. doi: 10.1074/jbc.M110.188219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bijlsma MF, Spek CA, Zivkovic D, van de Water S, Rezaee F, Peppelenbosch MP. Repression of smoothened by patched-dependent (pro-)vitamin D3 secretion. PLoS Biol. 2006;4:e232. doi: 10.1371/journal.pbio.0040232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tang JY, Xiao TZ, Oda Y, Chang KS, Shpall E, Wu A, So PL, Hebert J, Bikle D, Epstein EH., Jr Vitamin D3 inhibits hedgehog signaling and proliferation in murine basal cell carcinomas. Cancer Prev Res. 2011;4:744–751. doi: 10.1158/1940-6207.CAPR-10-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 70.Xie Z, Bikle DD. The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-gamma1 activation and human keratinocyte differentiation. J Biol Chem. 2007;282:8695–8703. doi: 10.1074/jbc.M609135200. [DOI] [PubMed] [Google Scholar]
- 71.Bienz M. Beta-Catenin: a pivot between cell adhesion and Wnt signalling. Curr Biol. 2005;15:R64–R67. doi: 10.1016/j.cub.2004.12.058. [DOI] [PubMed] [Google Scholar]
- 72.Chan EF, Gat U, McNiff JM, Fuchs E. A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet. 1999;21:410–413. doi: 10.1038/7747. [DOI] [PubMed] [Google Scholar]
- 73.Gat U, DasGupta R, Degenstein L, Fuchs E. De Novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell. 1998;95:605–614. doi: 10.1016/s0092-8674(00)81631-1. [DOI] [PubMed] [Google Scholar]
- 74.Xia J, Urabe K, Moroi Y, Koga T, Duan H, Li Y, Furue M. Beta-Catenin mutation and its nuclear localization are confirmed to be frequent causes of Wnt signaling pathway activation in pilomatricomas. J Dermatol Sci. 2006;41:67–75. doi: 10.1016/j.jdermsci.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 75.Palmer HG, Gonzalez-Sancho JM, Espada J, Berciano MT, Puig I, Baulida J, Quintanilla M, Cano A, de Herreros AG, Lafarga M, Munoz A. Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of beta-catenin signaling. J Cell Biol. 2001;154:369–387. doi: 10.1083/jcb.200102028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shah S, Hecht A, Pestell R, Byers SW. Trans-repression of beta-catenin activity by nuclear receptors. J Biol Chem. 2003;278:48137–48145. doi: 10.1074/jbc.M307154200. [DOI] [PubMed] [Google Scholar]
- 77.Shah S, Islam MN, Dakshanamurthy S, Rizvi I, Rao M, Herrell R, Zinser G, Valrance M, Aranda A, Moras D, Norman A, Welsh J, Byers SW. The molecular basis of vitamin D receptor and beta-catenin crossregulation. Mol Cell. 2006;21:799–809. doi: 10.1016/j.molcel.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 78.Palmer HG, Anjos-Afonso F, Carmeliet G, Takeda H, Watt FM. The vitamin D receptor is a wnt effector that controls hair follicle differentiation and specifies tumor type in adult epidermis. PLoS One. 2008;3:e1483. doi: 10.1371/journal.pone.0001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cianferotti L, Cox M, Skorija K, Demay MB. Vitamin D receptor is essential for normal keratinocyte stem cell function. Proc Natl Acad Sci U S A. 2007;104:9428–9433. doi: 10.1073/pnas.0702884104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, Butcher EC. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 81.Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. 2007;179:1634–1647. doi: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 82.Bikle D. Vitamin D and immune function: understanding common pathways. Curr Osteoporosis Rep. 2009;7:58–63. doi: 10.1007/s11914-009-0011-6. [DOI] [PubMed] [Google Scholar]
- 83.Rigby WF, Stacy T, Fanger MW. Inhibition of T lymphocyte mitogenesis by 1,25-dihydroxyvitamin D3 (calcitriol) J Clin Invest. 1984;74:1451–1455. doi: 10.1172/JCI111557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lemire JM, Archer DC, Beck L, Spiegelberg HL. Immunosuppressive actions of 1,25-dihydroxyvitamin D3: preferential inhibition of Th1 functions. J Nutr. 1995;125:1704S–1708S. doi: 10.1093/jn/125.suppl_6.1704S. [DOI] [PubMed] [Google Scholar]
- 85.Daniel C, Sartory NA, Zahn N, Radeke HH, Stein JM. Immune modulatory treatment of trinitrobenzene sulfonic acid colitis with calcitriol is associated with a change of a T helper (Th) 1/Th17 to a Th2 and regulatory T cell profile. J Pharmacol Exp Ther. 2008;324:23–33. doi: 10.1124/jpet.107.127209. [DOI] [PubMed] [Google Scholar]
- 86.Bettelli E, Korn T, Oukka M, Kuchroo VK. Induction and effector functions of T(H)17 cells. Nature. 2008;453:1051–1057. doi: 10.1038/nature07036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Boonstra A, Barrat FJ, Crain C, Heath VL, Savelkoul HF, O’Garra A. 1 alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J Immunol. 2001;167:4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- 88.Penna G, Adorini L. 1 alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 89.Griffin MD, Xing N, Kumar R. Vitamin D and its analogs as regulators of immune activation and antigen presentation. Annu Rev Nutr. 2003;23:117–145. doi: 10.1146/annurev.nutr.23.011702.073114. [DOI] [PubMed] [Google Scholar]
- 90.Adorini L. Intervention in autoimmunity: the potential of vitamin D receptor agonists. Cell Immunol. 2005;233:115–124. doi: 10.1016/j.cellimm.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 91.Adorini L, Amuchastegui S, Daniel KC. Prevention of chronic allograft rejection by Vitamin D receptor agonists. Immunol Lett. 2005;100:34–41. doi: 10.1016/j.imlet.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 92.Liu PT, Krutzik SR, Modlin RL. Therapeutic implications of the TLR and VDR partnership. Trends Mol Med. 2007;13:117–124. doi: 10.1016/j.molmed.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 93.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 94.Gombart AF, Borregaard N, Koeffier HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxy-vitamin D3. FASEB J. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 95.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 96.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 97.Kripke ML, Munn CG, Jeevan A, Tang JM, Bucana C. Evidence that cutaneous antigen-presenting cells migrate to regional lymph nodes during contact sensitization. J Immunol. 1990;145:2833–2838. [PubMed] [Google Scholar]
- 98.Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol. 2004;195:298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 99.Toews GB, Bergstresser PR, Streilein JW. Epidermal Langerhans cell density determines whether contact hypersensitivity or unresponsiveness follows skin painting with DNFB. J Immunol. 1980;124:445–453. [PubMed] [Google Scholar]
- 100.Simon JC, Cruz PD, Jr, Tigelaar RE, Sontheimer RD, Bergstresser PR. Adhesion molecules CD11a, CD18, and ICAM-1 on human epidermal Langerhans cells serve a functional role in the activation of alloreactive T cells. J Invest Dermatol. 1991;96:148–151. doi: 10.1111/1523-1747.ep12515946. [DOI] [PubMed] [Google Scholar]
- 101.Tang A, Udey MC. Inhibition of epidermal Langerhans cell function by low dose ultraviolet B radiation. Ultraviolet B radiation selectively modulates ICAM-1 (CD54) expression by murine Langerhans cells. J Immunol. 1991;146:3347–3355. [PubMed] [Google Scholar]
- 102.Glaser R, Navid F, Schuller W, Jantschitsch C, Harder J, Schroder JM, Schwarz A, Schwarz T. UV-B radiation induces the expression of antimicrobial peptides in human keratinocytes in vitro and in vivo. J Allergy Clin Immunol. 2009;123:1117–1123. doi: 10.1016/j.jaci.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 103.Mallbris L, Edstrom DW, Sundblad L, Granath F, Stahle M. UVB upregulates the antimicrobial protein hCAP18 mRNA in human skin. J Invest Dermatol. 2005;125:1072–1074. doi: 10.1111/j.0022-202X.2005.23872.x. [DOI] [PubMed] [Google Scholar]
- 104.Kripke ML, Fisher MS. Immunologic parameters of ultraviolet carcinogenesis. J Natl Cancer Inst. 1976;57:211–215. doi: 10.1093/jnci/57.1.211. [DOI] [PubMed] [Google Scholar]
- 105.Dixon KM, Deo SS, Norman AW, Bishop JE, Halliday GM, Reeve VE, Mason RS. In vivo relevance for photoprotection by the vitamin D rapid response pathway. J Steroid Biochem Mol Biol. 2007;103:451–456. doi: 10.1016/j.jsbmb.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 106.Damian DL, Kim YJ, Dixon KM, Halliday GM, Javeri A, Mason RS. Topical calcitriol protects from UV-induced genetic damage but suppresses cutaneous immunity in humans. Exp Dermatol. 2010;19:e23–e30. doi: 10.1111/j.1600-0625.2009.00955.x. [DOI] [PubMed] [Google Scholar]
- 107.Freeman SE, Hacham H, Gange RW, Maytum DJ, Sutherland JC, Sutherland BM. Wavelength dependence of pyrimidine dimer formation in DNA of human skin irradiated in situ with ultraviolet light. Proc Natl Acad Sci U S A. 1989;86:5605–5609. doi: 10.1073/pnas.86.14.5605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hussein MR. Ultraviolet radiation and skin cancer: molecular mechanisms. J Cutaneous Pathol. 2005;32:191–205. doi: 10.1111/j.0303-6987.2005.00281.x. [DOI] [PubMed] [Google Scholar]
- 109.Ziegler A, Leffell DJ, Kunala S, Sharma HW, Gailani M, Simon JA, Halperin AJ, Baden HP, Shapiro PE, Bale AE, et al. Mutation hotspots due to sunlight in the p53 gene of nonmelanoma skin cancers. Proc Natl Acad Sci U S A. 1993;90:4216–4220. doi: 10.1073/pnas.90.9.4216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ziegler A, Jonason AS, Leffell DJ, Simon JA, Sharma HW, Kimmelman J, Remington L, Jacks T, Brash DE. Sunburn and p53 in the onset of skin cancer. Nature. 1994;372:773–776. doi: 10.1038/372773a0. [DOI] [PubMed] [Google Scholar]
- 111.Brash DE, Rudolph JA, Simon JA, Lin A, McKenna GJ, Baden HP, Halperin AJ, Ponten J. A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma. Proc Natl Acad Sci U S A. 1991;88:10124–10128. doi: 10.1073/pnas.88.22.10124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bito T, Ueda M, Ahmed NU, Nagano T, Ichihashi M. Cyclin D and retinoblastoma gene product expression in actinic keratosis and cutaneous squamous cell carcinoma in relation to p53 expression. J Cutaneous Pathol. 1995;22:427–434. doi: 10.1111/j.1600-0560.1995.tb00758.x. [DOI] [PubMed] [Google Scholar]
- 113.Li G, Mitchell DL, Ho VC, Reed JC, Tron VA. Decreased DNA repair but normal apoptosis in ultraviolet-irradiated skin of p53-transgenic mice. Am J Pathol. 1996;148:1113–1123. [PMC free article] [PubMed] [Google Scholar]
- 114.Tlsty TD, Margolin BH, Lum K. Differences in the rates of gene amplification in nontumorigenic and tumorigenic cell lines as measured by Luria–Delbruck fluctuation analysis. Proc Natl Acad Sci U S A. 1989;86:9441–9445. doi: 10.1073/pnas.86.23.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tlsty TD. Normal diploid human and rodent cells lack a detectable frequency of gene amplification. Proc Natl Acad Sci U S A. 1990;87:3132–3136. doi: 10.1073/pnas.87.8.3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Maher VM, Dorney DJ, Mendrala AL, Konze-Thomas B, McCormick JJ. DNA excision-repair processes in human cells can eliminate the cytotoxic and mutagenic consequences of ultraviolet irradiation. Mutat Res, Fundam Mol Mech Mutagen. 1979;62:311–323. doi: 10.1016/0027-5107(79)90087-3. [DOI] [PubMed] [Google Scholar]
- 117.Bartkova J, Rezaei N, Liontos M, Karakaidos P, Kletsas D, Issaeva N, Vassiliou LV, Kolettas E, Niforou K, Zoumpourlis VC, Takaoka M, Nakagawa H, Tort F, Fugger K, Johansson F, Sehested M, Andersen CL, Dyrskjot L, Orntoft T, Lukas J, Kittas C, Helleday T, Halazonetis TD, Bartek J, Gorgoulis VG. Oncogene-induced senescence is part of the tumorigenesis barrier imposed by DNA damage checkpoints. Nature. 2006;444:633–637. doi: 10.1038/nature05268. [DOI] [PubMed] [Google Scholar]
- 118.Bielas JH, Loeb KR, Rubin BP, True LD, Loeb LA. Human cancers express a mutator phenotype. Proc Natl Acad Sci U S A. 2006;103:18238–18242. doi: 10.1073/pnas.0607057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen RH, Maher VM, McCormick JJ. Effect of excision repair by diploid human fibroblasts on the kinds and locations of mutations induced by (+/−)-7-beta,8-alpha-dihydroxy-9 alpha,10 alpha-epoxy-7,8,9,10- tetrahydrobenzo[a]pyrene in the coding region of the HPRT gene. Proc Natl Acad Sci U S A. 1990;87:8680–8684. doi: 10.1073/pnas.87.21.8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wood RD. DNA damage recognition during nucleotide excision repair in mammalian cells. Biochimie. 1999;81:39–44. doi: 10.1016/s0300-9084(99)80036-4. [DOI] [PubMed] [Google Scholar]
- 121.Sertic S, Pizzi S, Lazzaro F, Plevani P, Muzi-Falconi M. NER and DDR: classical music with new instruments. Cell Cycle. 2012;11:668–674. doi: 10.4161/cc.11.4.19117. [DOI] [PubMed] [Google Scholar]
- 122.Mellon I, Bohr VA, Smith CA, Hanawalt PC. Preferential DNA repair of an active gene in human cells. Proc Natl Acad Sci U S A. 1986;83:8878–8882. doi: 10.1073/pnas.83.23.8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mellon I, Spivak G, Hanawalt PC. Selective removal of transcription-blocking DNA damage from the transcribed strand of the mammalian DHFR gene. Cell. 1987;51:241–249. doi: 10.1016/0092-8674(87)90151-6. [DOI] [PubMed] [Google Scholar]
- 124.Mellon I, Rajpal DK, Koi M, Boland CR, Champe GN. Transcription-coupled repair deficiency and mutations in human mismatch repair genes. Science. 1996;272:557–560. doi: 10.1126/science.272.5261.557. [DOI] [PubMed] [Google Scholar]
- 125.Bohr VA. Gene specific DNA repair. Carcinogenesis. 1991;12:1983–1992. doi: 10.1093/carcin/12.11.1983. [DOI] [PubMed] [Google Scholar]
- 126.Hanawalt PC. Transcription-coupled repair and human disease. Science. 1994;266:1957–1958. doi: 10.1126/science.7801121. [DOI] [PubMed] [Google Scholar]
- 127.Hoeijmakers JH. Genome maintenance mechanisms for preventing cancer. Nature. 2001;411:366–374. doi: 10.1038/35077232. [DOI] [PubMed] [Google Scholar]
- 128.Wood RD, Mitchell M, Sgouros J, Lindahl T. Human DNA repair genes. Science. 2001;291:1284–1289. doi: 10.1126/science.1056154. [DOI] [PubMed] [Google Scholar]
- 129.Demetriou SK, Ona-Vu K, Teichert AE, Cleaver JE, Bikle DD, Oh DH. Vitamin D receptor mediates DNA repair and is UV inducible in intact epidermis but not in cultured keratinocytes. J Invest Dermatol. 2012;132:2097–2100. doi: 10.1038/jid.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dixon KM, Deo SS, Wong G, Slater M, Norman AW, Bishop JE, Posner GH, Ishizuka S, Halliday GM, Reeve VE, Mason RS. Skin cancer prevention: a possible role of 1,25dihydroxy-vitamin D3 and its analogs. J Steroid Biochem Mol Biol. 2005;97:137–143. doi: 10.1016/j.jsbmb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 131.Gupta R, Dixon KM, Deo SS, Holliday CJ, Slater M, Halliday GM, Reeve VE, Mason RS. Photoprotection by 1,25 dihydroxyvitamin D3 is associated with an increase in p53 and a decrease in nitric oxide products. J Invest Dermatol. 2007;127:707–715. doi: 10.1038/sj.jid.5700597. [DOI] [PubMed] [Google Scholar]
- 132.Moll PR, Sander V, Frischauf AM, Richter K. Expression profiling of vitamin D treated primary human keratinocytes. J Cell Biochem. 2007;100:574–592. doi: 10.1002/jcb.21061. [DOI] [PubMed] [Google Scholar]