Abstract
Recent studies indicate that the effect of thyrotropin-releasing hormone (TRH) on the regulation of food intake may be mediated by histaminergic neurons. To elucidate the anatomical basis for a functional relationship between TRH- and histamine-synthesizing neuronal systems, double-labeling immunocytochemistry was performed on the tuberomammillary nucleus (TMN) of rats, the exclusive location of histaminergic neurons. TRH-immunoreactive (IR) innervation of the histaminergic neurons were detected in all five subnuclei (E1-5) of the TMN, but was most prominent in the E4 and E5 subnuclei where 100% of the histamine-IR neurons were contacted. The number of TRH-IR varicosities in contact with histamine-IR neurons was also greatest in the E4 and E5 subnuclei, averaging 27.0±1.2 in E4 and 7.9±0.5 in E5. Somewhat fewer histamine-IR neurons were juxtaposed by TRH-IR varicosities in E2 and E3 and contacted by 6.3 ± 0.2 and 6.8 ± 0.2 varicosities/innervated cell, respectively. The number of juxtapositions of TRH-IR axon varicosities with histamine-IR neurons was the lowest in the E1 subnucleus (85.7±0.9%; 4.0±0.2 varicosities/innervated cell). Ultrastructural analysis demonstrated that TRH-IR axons established both asymmetric and symmetric type synapses on the perikaryon and dendrites of the histamine-IR neurons, although the majority of synapses were asymmetric type. These data demonstrate that TRH neurons heavily innervate histaminergic neurons in all subdivisions of the TMN, with the densest innervation in the E4 and E5 subdivisions, and are likely to exert activating effects.
Keywords: Histamine, TRH, tuberomammillary nucleus, innervations
Introduction
Thyrotropin-releasing hormone (TRH) is a tripeptide amide that is widely expressed throughout the central nervous system (Segerson et al., 1987). Its best known function is the central regulation of the hypothalamic-pituitary-thyroid axis, but TRH also has a fundamental role in the regulation of food intake (Lechan and Fekete, 2006). Recent studies have suggested that the anorexigenic effect of TRH is mediated by histaminergic neurons. In studies by Gotoh et al (Gotoh et al., 2007), central administration of TRH not only decreased food intake in a dose-dependent manner, but also increased the concentration of histamine and t-methylhistamine (a major metabolite of neuronal histamine) in the tuberomammillary nucleus (TMN) where histamine-synthesizing neurons reside in five, separate subnuclei (E1-E5) (Inagaki et al., 1990). In addition, the anorexic effects of TRH could be attenuated by pretreatment with the irreversible histidine decarboxylase inhibitor,α-fluoro-methyl histidine (α-FMH), suggesting that the central, anorexigenic effect of TRH is mediated by histaminergic neurons (Gotoh et al., 2007). More recent studies by Parmentier et al (Parmentier et al., 2009) have shown that histaminergic neurons in the TMN are excited by TRH and express both type 1 and type 2 TRH receptors, indicative of a direct action of TRH on these neurons. Anatomical evidence for direct synaptic associations between TRH neurons and histaminergic neurons, however, has not been previously demonstrated. In this study, therefore, we used double-labeling immunocytochemistry at light and electron microscopic levels to elucidate the interactions of the TRH-containing axons with histaminergic neurons, and determine the extent of the TRH-containing innervation in each of the five histaminergic neuronal populations in the TMN.
Results
Histamine-IR perikarya and dendrites were observed exclusively in the TMN, and organized in a pattern similar to that previously described (Inagaki et al., 1990; Panula et al., 1984). Large multipolar histamine-IR neurons were distributed in all five subnuclei (E1-5) of the tuberomammillary nucleus, forming the largest cell cluster in the E2 subnucleus close to the lateral surface of the hypothalamus (Fig. 1). Perikarya of the histamine-IR neurons in the E1 and E2 subnuclei were closely clustered, while the histamine-IR neurons in the E3-E5 subnuclei were more loosely organized (Fig. 1). TRH-IR neurons were also observed in the TMN, but almost exclusively in the E4 subnucleus (Fig. 1, 2, 3). Compared to the number of histamine-IR neurons in this subdivision, however, TRH neurons were far less abundant. Only scattered TRH-IR neurons were observed in the E1-E3 and E5 subnuclei.
Figure 1.
Distribution of the TRH-IR elements (black) and the histamine-IR neurons (brown) in the subnuclei of the tuberomammillary nucleus in for different coronal planes. The localization of the 5 subnuclei (E1-5) are labeled on the images. MR, mammillary recess; Scale bar = 500 μm.
Figure 2.
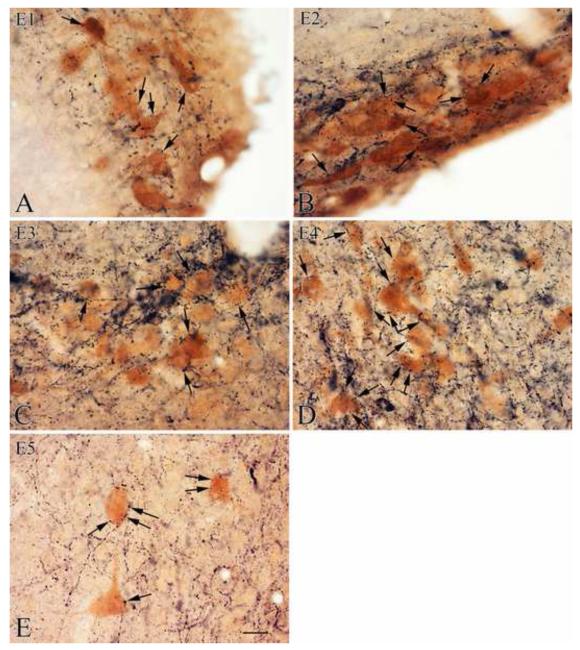
TRH-IR (black) innervation of histamine-IR neurons (brown) in the tuberomammillary nucleus. High power images illustrate the interaction of the TRH-IR axons and the histamine-IR neurons in the five subnuclei of the tuberomammillary nucleus (E1-5). Arrows point to TRH-IR axon varicosities in juxtaposition to histamine-IR neurons. Note, the especially dense TRH-IR innervation of the histamine-IR neurons in the E4 subnucleus (D). Scale bar = 20 μm.
Figure 3.
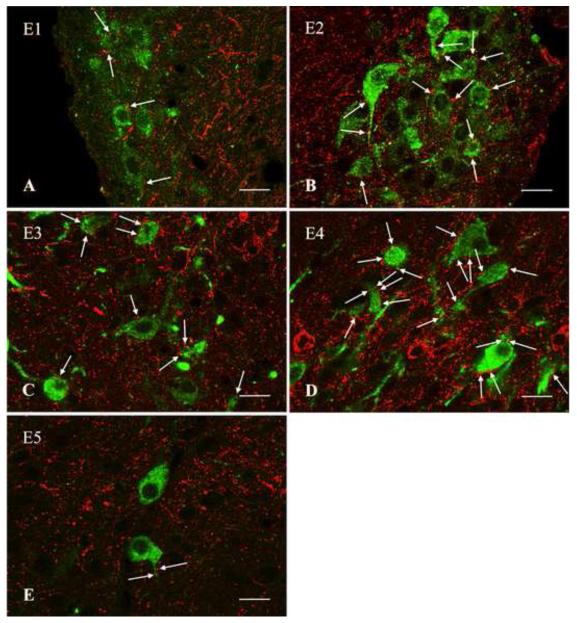
TRH-IR (red) innervation of histamine-IR neurons (green) in the tuberomammillary nucleus. High power, confocal microscopic images of immunofluorescent preparations illustrate the juxtaposition of the TRH-IR varicosities and the histamine-IR neurons in the five subnuclei of the tuberomammillary nucleus (E1-5). Arrows point to TRH-IR axon varicosities in juxtaposition to histamine-IR neurons. Images represent single optical sections with less than 0.8μm thickness. Scale bar = 20 μm.
TRH axons innervated all five subnuclei of the TMN, but the densest TRH-IR network was observed in the E4 subnucleus (Fig. 1,2,3). Results of the quantitative analyses of the morphological interaction of TRH-IR varicosities and histamine-IR neurons in the subnuclei of the TMN as determined by confocal microscopy are summarized in Table 1. TRH-IR axon varicosities were observed on the surface of all histamine-IR neurons in E4, averaging 27.0±1.2 varicosities per histamine-IR neuron (Fig. 2D, 3D). Similarly, all histamine-IR neurons were contacted by TRH-IR axon varicosities in the E5 subnucleus (Fig. 2E, 3E), but fewer TRH-IR varicosities (P<0.001) were observed on the surface of these cells (7.9±0.5). A dense TRH-IR innervation of E2 and E3 subnuclei (Fig. 2B,C, 3B,C) was observed, but somewhat fewer (P<0.001) histamine-IR neurons appeared to be contacted with 93.9±0.9% in E2 and 92.1±1.3% in E3, averaging 6.3±0.2 and 6.8±0.2 TRH-IR varicosities per histamine-IR neuron in each subnucleus, respectively. A less frequent interaction between TRH-containing axon terminals and histamine-IR neurons was observed in the E1 subnucleus (Fig. 2A, 3A), where only 85.7±0.9% of the histamine-IR neurons (significantly less than all other groups P<0.001) were contacted by an average of 4.0±0.2 TRH-IR axon varicosities/innervated cells (significantly less than all other groups P<0.001).
Table 1.
Quantitative analysis of the juxtaposition of TRH-IR axon varicosities and histamine-IR neurons in the 5 tuberomammillary subnuclei (E1-5).
| E1 | E2 | E3 | E4 | E5 | |
|---|---|---|---|---|---|
| Percentage of histamine- IR neurons contacted by TRH-IR axons |
85.7±0.9a | 93.9±0.9b | 92.1±1.3c | 100.0±0.0d | 100.0±0.0d |
| Number of TRH aricosities/histamine- IR neuron |
4.0±0.2a | 6.3±0.2e | 6.8±0.2e | 27.0±1.8f | 7.9±0.5e |
significantly different from E2, E3, E4, E5
significantly different from E1, E3, E4, E5
significantly different from E1, E2, E4, E5
significantly different from E1, E2, E3
significantly different from E1, E4
ignificantly different from E1, E2, E3, E5
P<0.05
Ultrastructurally, TRH-IR nerve terminals labeled with electron dense DAB reaction product were observed to established close membrane appositions with histamine-IR perikarya and dendrites, recognized by the presence of highly electron dense deposits of immunogold-silver particles distributed throughout the labeled structures (Fig. 4). Tracing the juxtaposed TRH-IR terminals and histamine-IR neurons through a series of ultrathin sections, both asymmetric type and symmetric type synaptic specializations were observed on both the perikarya (Fig 4A) and dendrites (Fig. 4B) of histamine-synthesizing neurons. Analysis of 56 synapses between histamine neurons and TRH-IR terminals revealed 35 asymmetric and 14 symmetric synaptic associations. For 7 of the identified synapses, the specific type could not be unequivocally determined.
Figure 4.
Electron micrographs showing synaptic associations (arrows) between histamine-IR neurons and TRH-IR terminals in the tuberomammillary nucleus. The histamine-IR perikarya and dendrites are labeled with highly electron dense gold–silver granules, while the TRH-IR terminals are recognized by the presence of the electron dense DAB chromogen. Medium-power image illustrates an asymmetric type synapse established between a TRH-IR axon varicosity and a histamine-IR perikaryon (A) shown in greater detail in (B). A symmetric type axosomatic synapse is shown in (C and D). High-power magnification images show asymmetric (F, G) and symmetric (E, H) axodendritic synapses between TRH and histamine-IR structures. Arrows point to the synapses. HIS, histamine-IR structure; Nu, nucleus; TRH, TRH-IR structure; Scale bars=0,1 μm in (B, D-H); 1 μm in (A, C).
Discussion
Hypothalamic neuronal histamine is known to act as a central transmitter in the regulation of body weight, as an anorexic agent and to increase thermogenesis in brown adipose tissue (BAT) and lipolysis in white adipose tissue. Central administration of histamine reduces food intake in a number of experimental models (Itoh et al., 1991; Ookuma et al., 1989; Yasuda et al., 2005) that can be prevented by H1 receptor antagonists (Sakata et al., 1988a; Sakata et al., 1988b) and increases thermogenesis by increasing uncoupling protein-1 (UCP-1) in BAT (Jorgensen et al., 2006; Yasuda et al., 2004). In addition, histamine-deficient, histidine decarboxylase knockout mice develop obesity on a high fat diet (Jorgensen et al., 2006), and both histidine decarboxylase and H1R knockout mice develop late-onset obesity and hyperphagia (Hegyi et al., 2004; Masaki et al., 2004). Conversely, inhibition of histamine synthesis using the specific neuronal inhibitor of histidine decarboxylase, α-fluoromethylhistidine (FMH), increases feeding (Gray, 1959; Tuomisto et al., 1994). The mechanisms by which histaminergic neurons are integrated into the central mechanisms for weight regulation are not completely known, but the observation that the anorexic effects of TRH on food intake can be attenuated by preventing the synthesis of histamine raises the possibility that TRH-synthesizing neurons may comprise at least one of the feeding related inputs to histaminergic neurons.
In this study, we demonstrate that TRH-IR axons densely innervate all five subdivisions of the TMN and establish numerous, close appositions with histaminergic neurons in each subdivision. While the confocal analysis of the associations between TRH-containing axon terminals and histaminergic neurons cannot itself establish that they are all synaptic, axons in close justaposition to its targeted neuron have been reported to influence the neuron via non-synaptic transmission (Vizi et al., 2010). Nevertheless, by ultrastructural analysis, many of these associations were shown to be synaptic specializations with both the perikaryon and dendrites of histamine-IR neurons. While both symmetric and asymmetric type synaptic specializations were observed, the number of asymmetric type synapses was almost 3 times greater than the number of symmetric type specializations. Since the asymmetric type synaptic association is considered a characteristic feature of excitatory neuronal connections (Peters et al., 1991), the data suggest that TRH exerts primarily a stimulatory effect on the histaminergic neurons. This is in agreement with earlier findings showing that central administration of TRH increases histamine concentration in the TMN (Gotoh et al., 2007) and has a depolarizing effect on histaminergic neurons (Parmentier et al., 2009). Whether the activating effects of TRH are exerted via TRH-R1 or TRH-R2 receptors or both, remain controversial (Engel and Gershengorn, 2007). Although single cell PCR has demonstrated both types of receptors in some of the histaminergic neurons (Parmentier et al., 2009), by immuncytochemistry, only TRH-R2 has been visualized (Gotoh et al., 2007). How symmetric type TRH synapses contribute to the regulation of histaminergic neurons requires further study, but raises the possibility that the origin of TRH neurons that inervate TMN neurons may be diverse.
Juxtaposition of TRH-IR axons to histamine-IR neurons was observed in all subnuclei of the TMN, but the densest innervation pattern was observed in the dorsomedialy located E4 subnucleus. Here, approximately four times more TRH-IR axons were observed on the surface of the innervated histamine-IR neurons than any other TMN subnucleus. The physiological significance for this innervation pattern is currently unclear, as very little is known about the functional differences of the various TMN subnuclei. Early anatomical studies suggest that all TMN subnuclei have similar projection patterns. Namely, neurons retrogradely labeled from any single, specific projection area distribute equally in all TMN subnuclei, and individual TMN neurons have widespread projection fields (Ericson et al., 1987; Kohler et al., 1985), indicating that they may subserve similar functions. Restraint stress, insulin-induced hypoglycaemia and foot shock, however, induce neuronal activation preferentially in the E4 and E5 TMN subnuclei (Miklos and Kovacs, 2003), suggesting functional heterogenity. In addition, Mahia et al. (Mahia and Puerto, 2006) demonstrated that electrolytic lesions of the TMN subnuclei have differential effects on the food intake, such that lesions of the ventral E1 and E2 subnuclei result in hyperphagia, whereas similar lesions of E3 and E4 subnuclei have no effect. Electrolytic lesions not only damage neuronal perikarya, however, but also axons of passage. Since the caudal arcuate nucleus is closely juxtaposed to the E3 and E4 subnuclei, ablation of the axons emanating from the arcuate nucleus might have confounded interpretation of the data. Further studies using focal injections of TRH into the different subnuclei of the TMN, therefore, will be necessary before definitive conclusions can be rendered about the preferential role of any of the TMN subnuclei in the mediation of the anorexigenic effects of TRH.
In addition to the effects of TRH on food intake, TRH also participates in the central control of thermoregulation through effects independent of elevations in circulating thyroid hormone levels. Without affecting circulating T3 levels, intracerebroventricular administration of TRH increases rectal temperature and temperature of BAT that can be prevented by pretreatment with antibodies to the type 1 TRH receptor (Vizi et al., 2010). In addition, exposure of the total TRH KO mouse to cold results in hypothermia that cannot be fully rescued by restoring thyroid hormone levels (Yamada et al., 2003). Since bilateral denervation of the sympathetic nerves innervating BAT or the administration of β-adrenergic antagonists markedly attenuate the thermogenic response of centrally administered TRH (Vizi et al., 2010), the effects of TRH are presumably mediated through the sympathetic innervation of BAT. Activation of histaminergic neurons also increases thermogenesis through activation of the sympathetic innervation to BAT. Thus, it is possible that the TRH innervation of histaminergic neurons in the TMN may also mediate the thermogenic effect of this peptide on BAT.
Histaminergic neurons in the TMN may also mediate the effect of TRH on wakefulness. Both TRH and histamine are known to increase arousal (Haas and Panula, 2003; Nishino et al., 1997), and inhibition of histamine synthesis by α-FMH blocks the effect of the TRH analog, montirelin, to induce arousal, suggesting that the effect is mediated by histaminergic neurons. Since wakefulness results in c-Fos activation in all TMN subnuclei (Ko et al., 2003), it is possible that all subnuclei may be involved in the mediation of the effects of TRH on arousal.
In summary, we observe that TRH-IR axons densely innervate the histaminergic neurons in all subnuclei of the TMN, primarily establishing asymmetric synapses on these neurons indicative of an excitatory nature of the synaptic specializations. We hypothesize that this association mediates, at least in part, the well-described central effects of TRH on appetite, thermogenesis and arousal.
Experimental Procedure
Animals
The experiments were carried out on ten adult, male, Wistar rats, weighing 280–320 g, housed under standard environmental conditions (light between 06:00 and 18:00 h, temperature 22±1 °C, rat chow and water ad libitum). All experimental protocols were reviewed and approved by the Animal Welfare Committee at the Institute of Experimental Medicine of the Hungarian Academy of Sciences and Tufts Medical Center.
Animal preparation for double-labeling immunocytochemistry at light and electron microscopic levels
Animals were deeply anesthetized with ketamine/xylazine (ketamine 50 mg/kg, xylazine 10 mg/kg body weight, ip) and injected intracerebroventricularly with 40 μg of colchicine in 2 μl 0.9% saline under stereotaxic control. Twenty hours later, the animals were anaesthetized as described above and perfused transcardially with 20 ml 0.01 M phosphate-buffered saline (PBS), pH 7.4, followed sequentially by 100 ml of 2% paraformaldehyde/4% acrolein in 0.1 M phosphate buffer (PB), pH 7.4, and then by 50 ml of 2% paraformaldehyde in the same buffer. The brains were rapidly removed and stored in PBS, pH 7.4, for 24 h at 4 °C.
Light microscopic double-labeling immunocytochemistry for TRH and histamine in the tuberomammillary nucleus
For light microscopy, brains from 3 animals were cryoprotected in 30% sucrose in PBS at 4 °C overnight, then frozen on dry ice. Serial 25 μm thick coronal sections through the posterior hypothalamus were cut on a freezing microtome and collected in cryoprotectant solution (30% ethylene glycol; 25% glycerol; 0.05 M phosphate buffer) and stored at −20 °C until use. Series of sections from each brain were treated with 1% sodium borohydride in distilled water for 30 min and with 0.5% Triton X-100/0.5% H2O2 in PBS for 15 min. To reduce nonspecific antibody binding, the sections were treated with 2% normal horse serum in PBS for 20 min and then incubated in sheep TRH antiserum (Wittmann et al., 2009) at 1:50,000 dilution in PBS containing 2% normal horse serum and 0.2% sodium azide (antiserum diluent) for 2 days at 4 °C. After rinses in PBS, the sections were incubated in biotinylated donkey anti-sheep IgG for 2 h (1:500; Jackson Immunoresearch Lab, West Grove, PA) followed by the avidin–biotin–peroxidase complex (ABC Elite; 1:1000; Vector Laboratories, Burlingame, CA) in 0.05M Tris buffer for 1 h at room temperature. The immunoreaction was developed with 0.05% DAB, 0.15% nickel-ammonium-sulfate (Ni) and 0.005% H2O2 in 0.05M TB. The chromogen was then further intensified by a silver intensification technique to yield a black precipitate (Liposits et al., 1984). After visualization of TRH, the sections were incubated in sheep antiserum to histamine (Fekete and Liposits, 2003) at 1:1,000 dilution for 2 days at 4 °C., followed by donkey anti-sheep IgG (1:500; Jackson Lab) and in ABC (1:1,000). The immunolabeling was visualized by 0.025% DAB/0.0036% H2O2 in 0.05M TRIS buffer pH 7.6 to yield a brown reaction product. Using the silver intensified Ni-DAB and DAB fluorochromes sequentially, two antibodies raised the same species can be used for innervation studies without crossreaction (Fekete and Liposits, 2003; Liposits et al., 1988), because the use of low pH gold chloride solution during the silver intensification procedure elutes the antigens from the sections (Harlow and Lane, 1988), and the black silver precipitate completely fills the profiles and thereby obscures any potential, brown, DAB precipitate. Thus, the TRH-immunoreactive (IR) fibers were labeled black by silver-intensified Ni-DAB, and the histamine-IR neurons were labeled brown with DAB and could be easily distinguished in the same section. The sections were mounted on glass slides and coverslipped using DPX mounting medium (Sigma-Aldrich). Images were taken using a Zeiss AxioImager M1 microscope equipped with AxioCam MRc5 digital camera (Carl Zeis Inc., Göttingen, Germany)
Double-labeling immunofluorescence for TRH and histamine in the tuberomammillary nucleus
Since counting of the TRH-IR varicosities on the surface of the histamine-IR neurons is more precisely performed on 0.8 μm thick optical sections by laser scanning confocal microscopy than conventional light microscopy, double-labeling immunofluorescence for TRH and histamine was undertaken on TMN sections from 3 rats. Following the above described pretreatment, sections from each brain were incubated in a mixture of mouse anti-TRH serum at 1:4000 dilution and sheep antiserum against histamine (1:20.000) for 2 days at 4 °C. After washing in PBS, the sections were immersed in a mixture of Alexa 555-conjugated donkey anti-mouse IgG (1:500, Jackson Labs) and donkey biotinylated anti-sheep IgG (1:500) for 2h at room temperature. This was followed by treatment in ABC (1:1000) in 0.05M Tris buffer for 1 h at room temperature. The sections were then rinsed in PBS and the immunoreaction product amplified by the tyramide signal amplification (TSA) kit according to the manufacturer’s instructions (Life technologies Corporation). After further rinses, the sections were incubated in fluorescein-conjugated streptavidin (1:250, Vector Laboratories) for 1 h. The sections were mounted onto glass slides, coverslipped with Vectashield mounting medium (Vector Laboratories), and analyzed using a Radiance 2000 confocal microscope (Bio-Rad Laboratories, Hemel Hempstead, UK) using the following laser excitation lines: 488 nm for FITC, 543 nm for Alexa 555 and dichroic/emission filters, 560 nm/500–530 nm for FITC and 570–590 nm for Alexa 555. All images shown represent a single optical section (less than 0.8 μm thick) that were captured through 60x oil lens. For quantitative analyses, 9-11 sections were studied from each animal (n=3). Z-stack series of images were taken of all histamine-IR neurons contained in the studied sections using a 60X oil immersion lens. The juxtaposition of the TRH-IR varicosities and the histamine-containing neurons was traced through the series of optical sections using Image-Pro Plus software (Media Cybernetics, Inc., Bethesda, MD). A TRH varicosity was considered to be juxtaposed to the histamine-IR neuron if a visible gap was not seen by the observer between the two immunoreactive structures. The histamine-IR nuclei of the 5 subnuclei (E1-5) of the tuberomammillary nucleus were counted separately. The quantitative data was analysed by one way ANOVA analyses followed by Newman Kuels post hoc test using STATISTICA 9 software (StatSoft Inc. Tulsa, OK)
Electronmicroscopic double-labeling immunocytochemistry for TRH and histamine in the tuberomammillary nucleus
For electronmicroscopy, serial 25 μm thick coronal sections from 5 rats were cut on a Leica VT 1000S vibratome (Leica Microsystems, Wetzlar, Germany) through the rostro-caudal extent of the posterior hypothalamus and collected in PBS. The sections were treated with 1% sodium borohydride in 0.1 M PB for 30 min, followed by 0.5%H2O2 in PBS for 15 min. The sections were cryoprotected in 15% sucrose in PBS for 15 min at room temperature and in 30% sucrose in PBS overnight at 4 °C. The sections were placed in a tinfoil dish and quickly frozen over liquid nitrogen, then thawed at room temperature. This cycle was repeated three times to improve antibody penetration into the tissue. To reduce the nonspecific antibody binding, the sections were treated with 2% normal horse serum in PBS for 20 min. Sections were incubated in mouse antiserum against TRH (1:10,000) for 4 days at 4 °C, followed by biotinylated donkey anti-mouse IgG (1:500; Jackson Immunoresearch) for 20 h at 4 °C and ABC Elite Complex (1:1000) for 1 h at room temperature. Immunoreactivity was detected in 0.025% DAB/0.0036% H2O2 in 0.05 M Tris buffer, pH 7.6. The sections were then placed into sheep anti-histamine serum (1:250) for 2 days at 4 °C and after rinsing in PBS and 0.1% cold water fish gelatin/1% bovine serum albumin (BSA) in PBS, were incubated in donkey anti-sheep IgG conjugated with 0.8 nm colloidal gold (Electron Microscopy Sciences, Fort Washington, PA) diluted at 1:100 in PBS containing 0.1% cold water fish gelatin and 1% BSA. The sections were washed in the same diluent and PBS, followed by a 10 min treatment in 1.25% glutaraldehyde in PBS. After rinsing in 1X Aurion ECS buffer, the gold particles were silver intensified with the R-Gent SE-LM kit (Aurion, Wageningen, The Netherlands) (Branchereau et al., 1995). Sections were osmicated using 1% osmium tetroxide in 0.1M PB for 30 min, and then treated with 2% uranyl acetate in 70% ethanol for 30 min. Following dehydration in an ascending series of ethanol and propylene oxide, the sections were flat embedded in Durcupan ACM epoxy resin (Sigma-Aldrich Co) on liquid release agent (Electron Microscopy Sciences)-coated slides, and polymerized at 56 °C for 2 days. Ultrathin 50–60 nm sections taken from the E4 subnucleus of the TMN, which receives the densest TRH-IR innervation, were cut with Leica ultracut UCT ultramicrotome (Leica Microsystems, Wetzlar, Germany), collected onto Formvar-coated, single slot grids, and examined with a JEOL electron microscope. Ninety synaptic associations were detected between TRH-IR axons and histamine-IR neurons, Of these synapses, 56 were traced through serial ultrathin sections. These synaptic associations were characterized according Gray (Gray, 1959). The morphological characteristics were confirmed on neighboring ultrathin sections. Differentiation between synaptic contacts between TRH-containing axon terminals and histaminergic cell bodies vs dendrites was determined by demonstrating the presence or absence of a nucleus in serial sections. Perikarya and dendrites were only considered histamine-immunoreactive if the structure consistently contained silver grains through multiple neighboring ultrasections.
Specificity of antisera
The specificity of histamine antiserum and the sheep antiserum to TRH was reported previously (Fekete and Liposits, 2003; Wittmann et al., 2009). Mouse antiserum to TRH was generated similarly to sheep TRH antiserum (Fekete and Liposits, 2003; Wittmann et al., 2009). Briefly, the immunogenic complex was prepared by mixing 23 mg TRH (Bachem AG, Budendorf, Switzerland), 24 mg BSA (Sigma-Aldrich), and 15μL acrolein (Sigma-Aldrich) in 4 mL PBS. The mixture was kept at room temperature overnight. The reaction was stopped by the addition of 10 mg sodium borohydride. Finally, the conjugate was dialyzed against PBS. For initial immunization, 250 μg TRH-acrolein-BSA complex in 100μL PBS was emulsified with an equal volume of Freund’s complete adjuvant (Sigma) and injected subcutaneously. Subsequent boosts with Freund’s incomplete adjuvant were administered at 28 day intervals. The animals were decapitated 8 days after the third immunization and the serum was separated by centrifugation. The antiserum was affinity-purified on a column loaded with TRH-coupled CNBr-activated Sepharose 4 Fast Flow gel (Amersham Pharmacia Biotech UK, Buckinghamshire, UK). Specificity of the antiserum for immunocytochemistry was tested by preabsorption with TRH (Bachem) at 80 μg/mL concentration, which resulted in the complete loss of immunostaining. Specificity of the antiserum was further verified by double-labeling of sections with the sheep anti-TRH and the well characterized rabbit anti-TRH no. 31 antiserum (Lechan and Jackson, 1982), which resulted in a complete colocalization of the two signals in axons as well as in cell bodies in colchicine-treated animals.
Highlights.
TRH-containing axons heavily innervate all subnuclei of the TMN and contact histamine-IR neurons
The greatest number of TRH-containing axonal contacts occur in the E4 subnucleus of the TMN
TRH axons establish asymmetric synaptic contacts with histaminergic perikarya and dendrites
TRH would appear to have direct activating effects on TMN histaminergic neurons
Acknowledgements
This work was supported by Grants from the Hungarian Science Foundation (OTKA K81845), Seventh EU Research Framework Programme (Health-F2-2010-259772), Lendület Award of the Hungarian Academy of Sciences and the National Institutes of Health DK-37021.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Branchereau P, Van Bockstaele EJ, Chan J, Pickel VM. Ultrastructural characterization of neurons recorded intracellularly in vivo and injected with lucifer yellow: advantages of immunogold-silver vs. immunoperoxidase labeling. Microsc Res Tech. 1995;30:427–36. doi: 10.1002/jemt.1070300509. [DOI] [PubMed] [Google Scholar]
- Engel S, Gershengorn MC. Thyrotropin-releasing hormone and its receptors --a hypothesis for binding and receptor activation. Pharmacology & therapeutics. 2007;113:410–9. doi: 10.1016/j.pharmthera.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Ericson H, Watanabe T, Kohler C. Morphological analysis of the tuberomammillary nucleus in the rat brain: delineation of subgroups with antibody against L -histidine decarboxylase as a marker. The Journal of comparative neurology. 1987;263:1–24. doi: 10.1002/cne.902630102. [DOI] [PubMed] [Google Scholar]
- Fekete C, Liposits Z. Histamine-immunoreactive neurons of the tuberomammillary nucleus are innervated by alpha-melanocyte stimulating hormone-containing axons. Generation of a new histamine antiserum for ultrastructural studies. Brain research. 2003;969:70–7. doi: 10.1016/s0006-8993(03)02279-0. [DOI] [PubMed] [Google Scholar]
- Gotoh K, Fukagawa K, Fukagawa T, Noguchi H, Kakuma T, Sakata T, Yoshimatsu H. Hypothalamic neuronal histamine mediates the thyrotropin-releasing hormone-induced suppression of food intake. Journal of neurochemistry. 2007;103:1102–10. doi: 10.1111/j.1471-4159.2007.04802.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray EG. Axo-somatic and axo-dendritic synapses of the cerebral cortex: an electron microscope study. Journal of anatomy. 1959;93:420–33. [PMC free article] [PubMed] [Google Scholar]
- Haas H, Panula P. The role of histamine and the tuberomamillary nucleus in the nervous system. Nature reviews. Neuroscience. 2003;4:121–30. doi: 10.1038/nrn1034. [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane D. Antibodies A laboratory manual. Cold Spring Harbor Laboratory; USA: 1988. [Google Scholar]
- Hegyi K, Fulop KA, Kovacs KJ, Falus A, Toth S. High leptin level is accompanied with decreased long leptin receptor transcript in histamine deficient transgenic mice. Immunology letters. 2004;92:193–7. doi: 10.1016/j.imlet.2003.11.029. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Toda K, Taniuchi I, Panula P, Yamatodani A, Tohyama M, Watanabe T, Wada H. An analysis of histaminergic efferents of the tuberomammillary nucleus to the medial preoptic area and inferior colliculus of the rat. Experimental brain research. Experimentelle Hirnforschung. Experimentation cerebrale. 1990;80:374–80. doi: 10.1007/BF00228164. [DOI] [PubMed] [Google Scholar]
- Itoh Y, Oishi R, Saeki K. Feeding-induced increase in the extracellular concentration of histamine in rat hypothalamus as measured by in vivo microdialysis. Neuroscience letters. 1991;125:235–7. doi: 10.1016/0304-3940(91)90037-t. [DOI] [PubMed] [Google Scholar]
- Jorgensen EA, Vogelsang TW, Knigge U, Watanabe T, Warberg J, Kjaer A. Increased susceptibility to diet-induced obesity in histamine-deficient mice. Neuroendocrinology. 2006;83:289–94. doi: 10.1159/000095339. [DOI] [PubMed] [Google Scholar]
- Ko EM, Estabrooke IV, McCarthy M, Scammell TE. Wake-related activity of tuberomammillary neurons in rats. Brain research. 2003;992:220–6. doi: 10.1016/j.brainres.2003.08.044. [DOI] [PubMed] [Google Scholar]
- Kohler C, Swanson LW, Haglund L, Wu JY. The cytoarchitecture, histochemistry and projections of the tuberomammillary nucleus in the rat. Neuroscience. 1985;16:85–110. doi: 10.1016/0306-4522(85)90049-1. [DOI] [PubMed] [Google Scholar]
- Lechan RM, Jackson IM. Immunohistochemical localization of thyrotropin-releasing hormone in the rat hypothalamus and pituitary. Endocrinology. 1982;111:55–65. doi: 10.1210/endo-111-1-55. [DOI] [PubMed] [Google Scholar]
- Lechan RM, Fekete C. The TRH neuron: a hypothalamic integrator of energy metabolism. Progress in brain research. 2006;153:209–35. doi: 10.1016/S0079-6123(06)53012-2. [DOI] [PubMed] [Google Scholar]
- Liposits Z, Setalo G, Flerko B. Application of the silver gold intensified 3,3′diaminobenzidine chromogen to the light and electron microscopic detection of the luteinizing hormone-releasing hormone system of the rat brain. Neuroscience. 1984;13:513–25. doi: 10.1016/0306-4522(84)90245-8. [DOI] [PubMed] [Google Scholar]
- Liposits Z, Sievers L, Paull WK. Neuropeptide-Y and ACTH-immunoreactive innervation of corticotropin releasing factor (CRF)-synthesizing neurons in the hypothalamus of the rat. An immunocytochemical analysis at the light and electron microscopic levels. Histochemistry. 1988;88:227–34. doi: 10.1007/BF00570278. [DOI] [PubMed] [Google Scholar]
- Mahia J, Puerto A. Lesions of tuberomammillary nuclei induce differential polydipsic and hyperphagic effects. The European journal of neuroscience. 2006;23:1321–31. doi: 10.1111/j.1460-9568.2006.04644.x. [DOI] [PubMed] [Google Scholar]
- Masaki T, Chiba S, Yasuda T, Noguchi H, Kakuma T, Watanabe T, Sakata T, Yoshimatsu H. Involvement of hypothalamic histamine H1 receptor in the regulation of feeding rhythm and obesity. Diabetes. 2004;53:2250–60. doi: 10.2337/diabetes.53.9.2250. [DOI] [PubMed] [Google Scholar]
- Miklos IH, Kovacs KJ. Functional heterogeneity of the responses of histaminergic neuron subpopulations to various stress challenges. The European journal of neuroscience. 2003;18:3069–79. doi: 10.1111/j.1460-9568.2003.03033.x. [DOI] [PubMed] [Google Scholar]
- Nishino S, Arrigoni J, Shelton J, Kanbayashi T, Dement WC, Mignot E. Effects of thyrotropin releasing hormone and its analogs on daytime sleepiness and cataplexy in canine narcolepsy. The Journal of neuroscience: the official journal of the Society for Neuroscience. 1997;17:6401–8. doi: 10.1523/JNEUROSCI.17-16-06401.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ookuma K, Yoshimatsu H, Sakata T, Fujimoto K, Fukagawa F. Hypothalamic sites of neuronal histamine action on food intake by rats. Brain research. 1989;490:268–75. doi: 10.1016/0006-8993(89)90244-8. [DOI] [PubMed] [Google Scholar]
- Panula P, Yang HY, Costa E. Histamine-containing neurons in the rat hypothalamus. Proceedings of the National Academy of Sciences of the United States of America. 1984;81:2572–6. doi: 10.1073/pnas.81.8.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmentier R, Kolbaev S, Klyuch BP, Vandael D, Lin JS, Selbach O, Haas HL, Sergeeva OA. Excitation of histaminergic tuberomamillary neurons by thyrotropin-releasing hormone. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:4471–83. doi: 10.1523/JNEUROSCI.2976-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A, Palay SL, deF Webster H. Neurons and their supporting cells. Oxford University Press; Oxford: 1991. The fine structure of the nervous system. [Google Scholar]
- Sakata T, Fukagawa K, Fujimoto K, Yoshimatsu H, Shiraishi T, Wada H. Feeding induced by blockade of histamine H1-receptor in rat brain. Experientia. 1988a;44:216–8. doi: 10.1007/BF01941710. [DOI] [PubMed] [Google Scholar]
- Sakata T, Ookuma K, Fukagawa K, Fujimoto K, Yoshimatsu H, Shiraishi T, Wada H. Blockade of the histamine H1-receptor in the rat ventromedial hypothalamus and feeding elicitation. Brain research. 1988b;441:403–7. doi: 10.1016/0006-8993(88)91423-0. [DOI] [PubMed] [Google Scholar]
- Segerson TP, Hoefler H, Childers H, Wolfe HJ, Wu P, Jackson IM, Lechan RM. Localization of thyrotropin-releasing hormone prohormone messenger ribonucleic acid in rat brain in situ hybridization. Endocrinology. 1987;121:98–107. doi: 10.1210/endo-121-1-98. [DOI] [PubMed] [Google Scholar]
- Tuomisto L, Yamatodani A, Jolkkonen J, Sainio EL, Airaksinen MM. Inhibition of brain histamine synthesis increases food intake and attenuates vasopressin response to salt loading in rats. Methods and findings in experimental and clinical pharmacology. 1994;16:355–9. [PubMed] [Google Scholar]
- Vizi ES, Fekete A, Karoly R, Mike A. Non-synaptic receptors and transporters involved in brain functions and targets of drug treatment. British journal of pharmacology. 2010;160:785–809. doi: 10.1111/j.1476-5381.2009.00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann G, Fuzesi T, Liposits Z, Lechan RM, Fekete C. Distribution and axonal projections of neurons coexpressing thyrotropin-releasing hormone and urocortin 3 in the rat brain. The Journal of comparative neurology. 2009;517:825–40. doi: 10.1002/cne.22180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M, Satoh T, Mori M. Mice lacking the thyrotropin-releasing hormone gene: what do they tell us? Thyroid: official journal of the American Thyroid Association. 2003;13:1111–21. doi: 10.1089/10507250360731505. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Masaki T, Sakata T, Yoshimatsu H. Hypothalamic neuronal histamine regulates sympathetic nerve activity and expression of uncoupling protein 1 mRNA in brown adipose tissue in rats. Neuroscience. 2004;125:535–40. doi: 10.1016/j.neuroscience.2003.11.039. [DOI] [PubMed] [Google Scholar]
- Yasuda T, Masaki T, Kakuma T, Hara M, Nawata T, Katsuragi I, Yoshimatsu H. Dual regulatory effects of orexins on sympathetic nerve activity innervating brown adipose tissue in rats. Endocrinology. 2005;146:2744–8. doi: 10.1210/en.2004-1226. [DOI] [PubMed] [Google Scholar]