Abstract
Background
Patients with von Hippel-Lindau disease (VHL) commonly develop pancreatic cysts and neuroendocrine tumors (PNETs). Solid microcystic serous adenoma (SMSA), a rare tumor described in VHL patients, can be mistaken for PNET on imaging.
Methods
Clinical, pathologic and radiologic data were reviewed on VHL patients who underwent surgery for a pre-operative diagnosis of PNET since 1994 at one institution. Blinded to the pathological diagnoses, radiologists reassessed available imaging.
Results
For 55 patients, 79 pancreatectomies were performed for presumed PNETs. Ten (18.2%) patients underwent 12 (15.2%) resections for tumors diagnosed as SMSA on final pathology. The average size of a SMSA leading to surgery was 3.6 ±0.4 cm. Four out of 11 SMSAs were still mistaken for PNETs when imaging was reassessed. Mean FDG-PET SUV was higher for 17 PNETs (12.1 ±1.2) compared to 6 SMSAs (4.2 ±0.5; p=0.002). The mean doubling time of SMSAs and PNETs was similar. Seven (15.2%) patients with pathologically-proven PNETs had malignant disease.
Conclusions
SMSAs can mimic PNETs on non-functional imaging; FDG-PET may help differentiate them. A high index of suspicion is needed to minimize operations performed for SMSA and to counsel VHL patients of their risks of undergoing surgery for a lesion with no known malignant potential.
Introduction
With an incidence of approximately one in 36,000 newborns, von Hippel-Lindau (VHL) disease is an autosomal dominant multi-system cancer syndrome resulting from a germline mutation of the VHL tumor suppressor gene on the short arm of chromosome 3 (3p25-26).[1] With a penetrance of 90% by 65 years of age, patients with VHL develop various benign and malignant tumors, including hemangioblastomas of the brain and spinal cord (10–72%), retinal hemangioblastomas (25–60%), endolymphatic sac tumors (10%), renal cysts and renal cell carcinomas (RCC) (25–60%), pheochromocytomas (10–20%), and solid and cystic pancreatic tumors (35–70%).[1]
Pancreatic neuroendocrine tumors (PNETs) are found in up to 17% of VHL patients.[2, 3] Most VHL-associated PNETs are non-functional, and most are benign, whereas non-VHL associated PNETs have a higher rate (60–70%) of malignancy.[4, 5] The primary goal in the management of VHL patients is to prevent the development of metastatic disease while minimizing morbidity. Since the majority of VHL-associated PNETs do not become malignant, and given that these patients are at risk of developing recurrent pancreas tumors as well as other malignant (RCC) and benign but life-impacting tumors (e.g., spinal and brain hemangioblastomas), an individualized approach is used for VHL patients with PNETs to minimize morbidity and potentially unnecessary surgeries. We had previously recommended resecting VHL-associated PNETs if (1) the tumor is ≥ 3 cm, (2) if the tumor is ≥ 2 cm in the head of the pancreas to allow a chance at an enucleation rather than a pancreaticoduodenectomy, or (3) the patient is undergoing laparotomy for other elective procedures such as nephrectomy.[6] Besides a larger size, other factors that appear to be associated with an increased risk of malignancy in VHL patients with PNETs include a more rapidly growing tumor with a doubling time of less than 500 days and a germline VHL gene mutation in Exon 3.[2] Furthermore, since RCC is the major life-limiting malignancy in VHL patients, the status and management of a patient’s RCC should factor strongly in the management scheme of their PNET. Overall, a multidisciplinary approach must be used for VHL patients.
Generally asymptomatic, pancreatic cysts occur in the majority of VHL patients and rarely needs treatment. Referred to as “VHL-associated serous cystic neoplasm” (WHO classification[7], Box 1), these lesions are indistinguishable at the histological level from serous cystic tumors occurring sporadically. Salient histological features include a single layer of cuboidal epithelial cells with glycogen-rich clear cytoplasm organized in cysts filled with serous fluid.[7] In VHL patients, serous cystic neoplasms can involve the pancreas in either a diffuse or patchy fashion. The most frequent forms of pancreatic “serous adenoma” in patients with VHL are (1) the classical microcystic cystadenoma, a well-circumscribed, sponge-like lesion filled by microscopic cysts arranged around a central, dense fibronodular scar from which thin fibrous septa radiate to the periphery, and (2) the macrocystic variant, also referred to as oligocystic or ill-demarcated serous adenoma[8], composed of macroscopically visible large cysts lying within a fibrous stroma that lacks a central stellate scar. A microcystic adenoma may not have a central scar and can be formed by the smallest cysts, arranged in dense acini with minute or absent lumen, conferring the lesion a solid gross appearance. The WHO entity of “solid serous adenoma” refers to a lesion as such, reported to occur sporadically in non-VHL patients[9]. To emphasize the microcystic nature of some serous adenomas seen in VHL pancreata[10] and to convey the clinically-significant solid appearance of the densest lesions, we herein use the term “solid microcystic serous adenoma” (SMSA), a variant that could be included within the broader WHO classification of “VHL-associated serous cystic neoplasm”. Because these SMSAs are richly vascularized and have dense solid appearance, they can be mistaken for PNETs on imaging scans.[11] Over the years, we have occasionally observed a clinical conundrum in some VHL patients who have large growing tumors with radiologic workup consistent with PNETs which were then resected with final pathology showing that the targeted lesions were SMSAs.
Box 1. WHO classification of serous neoplasms of the pancreas.
| Serous adenoma |
| Microcystic serous cystadenoma |
| Macrocystic serous cystadenoma |
| Solid serous adenoma |
| VHL-associated serous cystic neoplasm |
| Mixed serous neuroendocrine neoplasm |
| Serous cystadenocarcinoma |
In order to clarify how often SMSAs could be mistaken for PNETs pre-operatively in patients with VHL disease, we reviewed our experience with patients who underwent pancreatic resections for solid pancreatic lesions thought to be PNETs over a period of nearly 17 years at our institution. By detailing and contrasting SMSA and PNET imaging characteristics, growth rates, and clinical and histopathological features, we aim to help clinicians involved in the care of VHL patients to differentiate between the two entities to minimize the frequency of operations performed for SMSAs which at this point have no known malignant potential. To our knowledge, this is the most comprehensive series with clinical, pathological and radiologic correlations on SMSAs published thus far from one institution.
Patients and methods
From September 1994 to May 2011, 55 patients with VHL underwent pancreatectomies at the National Institutes of Health Clinical Center for lesions with a presumptive diagnosis of PNET. A prior publication reported on 39 of these patients.[2] All patients were treated on natural history and tissue procurement protocols approved by the National Cancer Institute Institutional Review Board. Patients underwent comprehensive pre-operative screening and follow-up evaluations, which included a complete history and physical examination, computed tomography (CT) of the chest, abdomen and pelvis, abdominal and brain magnetic resonance imaging (MRI), 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET, as clinically indicated since 2005 and routinely since 2009), and laboratory studies (including those to screen for functional tumors such as pheochromocytomas). Each patient was evaluated by a multidisciplinary team which included genetic counselors, urologic surgeons, endocrine surgeons, otolaryngologists, neurosurgeons, and others as needed depending on the patient’s clinical status. VHL germline mutations were determined by quantitative Southern blotting to detect gene rearrangements and by complete gene sequencing as described previously.[12] Follow-up generally included yearly laboratory tests and CT scans of the chest, abdomen and pelvis and other imaging modalities as needed depending on the disease manifestation.
Pre-operative diagnosis of PNET was made based on CT and/or MRI characteristics. Decisions to perform resections for PNET were based on criteria outlined previously.[2, 6] In general, solid lesions in the body and tail of the pancreas were resected when they reached a diameter of ≥ 3 cm or when they reached ≥ 2 cm in the head of the pancreas. Intra-operative ultrasound was used routinely.
For the 55 patients in this series, all pathology reports and histology slides were reviewed for confirmation of diagnosis of SMSA or PNET (S.B. and M.Q.). In addition to chromogranin A and synaptophysin, additional staining for pan-cytokeratin (AE1/AE3), epithelial membrane antigen, Masson’s Trichrome, CD31, Ki67 and CD10 was performed to better characterize SMCA using clinical grade reagents and appropriate controls. Correlations of tumors found during surgery and pathology with radiological findings were established by reviewing operative notes, pathology reports and slides, and radiology reports and images concurrently (S.T. and G.Q.P.). Blinded to the pathological diagnoses but aware some lesions may be SMSAs and not PNETs, radiologists (B.T. and P.L.C) reviewed all available digitalized pre-operative CT and MRI scans (since 1998). For each solid lesion, the arterial enhancement on CT was qualified as homogeneous or heterogeneous and T2-weighted MRI characteristics as iso-intense, moderate or bright; a radiological diagnosis of PNET, SMSA, or uncertain was then made. The longest perpendicular diameters per lesion were recorded and compared to the oldest available corresponding scan for calculation of tumor doubling time, [Ti × log2]/[3 × log (Di/Df)], where Ti represented the time interval in days, Di the initial longest diameter, and Df the final longest diameter. For 18 patients who had pre-operative 18F-FDG-PET scans, maximum standardized uptake values (SUV) were calculated on targeted lesions (C.M.).
Statistical analyses were performed on GraphPad Prism software version 5.04 (La Jolla, CA) to calculate 2-sided p-values (P2) with Fisher’s exact test when comparing proportions and with Mann-Whitney for comparison between groups.
Results
For 55 patients with VHL, a total of 79 pancreatic resections were performed for lesions thought to be PNETs based on pre-operative imaging; one patient had two separate pancreatectomies within a four-year interval (Table 1). Overall, half of the resections were enucleations. Three total pancreatectomies were performed when the residual pancreas was entirely replaced by cysts and degenerative changes and not suitable for pancreatico-enteric anastomoses. SMSA was the final diagnosis for 12 pancreatectomies (15.2% of pancreatectomies) performed for tumors assumed to be PNETs in 10 patients (18.2% of patients). The mean radiological size of the SMSAs that led to surgery was 3.6 ± 0.4 cm (median 3.2 cm; range 2.0 to 5.4 cm) on imaging studies, compared to 2.6 cm (median 2.4 cm; range 1.4 to 7.0 cm) for patients with PNETs alone (P2 = 0.004). As seen in Table 2, in nine patients (16.4% of patients), SMSA was the dominant and the only clinically relevant pre-operatively identified solid lesion that led to surgery. In one patient (Table 2, Patient 1), in addition to the dominant and clinically relevant SMSA tumor, a concurrent PNET was also found (and also identified pre-operatively) that would have met the established criteria for resection in addition to the SMSA tumor. Of all 10 patients with SMSA on the final diagnosis of targeted lesions, six did not have a concurrent PNET in the surgical specimen after extensive re-sampling while three had small foci of PNETs that would not have justified a pancreatectomy (Table 2, Patients 3, 4 and 5). Available preoperative serum chromogranin A levels were not significantly different between eight patients with PNETs (median 138 ng/ml, range 76 to 1105 ng/ml) and five with SMSA (median 135 ng/ml, range 98 to 326 ng/ml).
Table 1.
Characteristics of VHL patients with solid pancreatic tumors resected between September 1994 and May 2011
| All patients | Patients with SMSAs | Patients with PNETs | ||||||
|---|---|---|---|---|---|---|---|---|
| Patients with resection for solid tumors*; n, % | 55 | 100% | 10 | 18.2% | 46 | 83.6% | ||
| Mean age at surgery; years (range) | 41 | (18–71) | 46 | (32–56) | 40 | (18–71) | ||
| Resections performed*; n, % | 79 | 100% | 12 | 15.2% | 67 | 84.8% | ||
| Total pancreatectomy | 3 | 3.8% | 2 | 1 | ||||
| Pancreaticoduodenectomy | 16 | 20.3% | 1 | 15 | ||||
| Distal pancreatectomy | 20 | 25.3% | 7 | 13 | ||||
| Enucleation | 40 | 50.6% | 2 | 38 | ||||
| All Lesions (N = 55 pts.) | All SMSAs (N = 10 pts.) | Concurrent PNETs (N = 4 pts.) | PNETs only (N = 46 pts) | |||||
| Radiological findings | ||||||||
| Mean size†; cm (range) | 2.8 | (1.4–7.0) | 3.6 | (2.0–5.4) | 1.6 | (0.7–2.5) | 2.6 | (1.4–7.0) |
| Median; cm | 2.5 | 3.2 | 1.7 | 2.4 | ||||
| Pathological findings | ||||||||
| Total no. of solid tumors in specimens | 111 | 24 | 5 | 82 | ||||
| Mean no. of lesions per specimen (range) | 2 | (1.0–10.0) | 2.4 | (1.0–10.0) | 0.5 | (0.0–2.0) | 1.8 | (1.0–5.0) |
| Mean size; cm (range) | 2.1 | (0.3–7.5) | 2.2 | (0.3–6.0) | 1.3 | (0.3–2.2) | 2.1 | (0.5–7.5) |
| Median; cm | 1.8 | 2.0 | 1.3 | 1.8 | ||||
| PNET staging‡ | ||||||||
| T1, <2 cm; n, % | 2/4 | 50.0% | 15 | 32.6% | ||||
| T2, 2–4 cm; n, % | 2/4 | 50.0% | 25 | 54.3% | ||||
| T3, >4 cm, pancreas limited; n, % | 0 | 5 | 10.9% | |||||
| T4, beyond pancreas; n,% | 0 | 1 | 2.2% | |||||
| N1; n, % | 0 | 5 | 10.9% | |||||
| M1; n, % | 0 | 3 | 6.5% | |||||
| Grade 1, Ki67≤ 2% or < 2 mitoses/10 HPF; n, % | 2/4 | 50.0% | 29 | 80.6% | ||||
| Grade 2, Ki67 3–20% or 2–5 mitosis/10 HPF; n, % | 2/4 | 50.0% | 7 | 19.4% | ||||
One patient underwent 2 separate resections, once for PNET and once for SMSA, and some patients had >1 procedure performed at the same setting; thus the number resections > number of patients.
Radiological size of dominant lesion, except for visible PNET concurrent with SMSA.
European Neuroendocrine Tumor Society (ENETS) modified TNM staging and ENETS-World Health Organization Ki67 proliferative activity-based staging for the dominant lesion.[7, 20]
Abbreviations: HPF, high power field; PNET, pancreatic neuroendocrine tumor; SMCA, solid microcystic adenoma.
Table 2.
Characteristics of patients with clinically relevant SMSAs.
| Pts. | Gender | VHL mutation | Age at surgery | Surgery | Postop complications (Grade)* | Size of dominant SMCA† (cm) | No. of concurrent SMSA | Size of concurrent PNET† (cm) | Postop f/up (mos.) | New solid pancreas lesion(s) | No. of non-pancreatic VHL-related surgeries | Current status |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | Partial deletion | 45 | TP | Minor wound | 6 | - | 2.2 | 9 | - | 5 | Alive with RCC |
| 2 | M | Partial deletion | 48 | DP | None | 6 | - | none | 9 | - | 8 | Alive and well |
| 3 | M | Exon 1 | 55 | TP | None | 5.5 | 2 | 1.3 | 13 | - | 6 | Death from metastatic RCC |
| 4 | F | Exon 1 | 31 | DP + E | PF (Grade B) | 2 | - | 2.0, 0.7 | 14 | - | 1 | Alive and well |
| 5 | F | Exon 1 | 46 | DP | None | 2 | - | 0.3 | 16 | - | 0 | Alive and well |
| 6 | F | Exon 3 | 53 | DP§ | None | 3.2 | - | none | 58 | no | 8 | Alive (presumed) |
| 7 | M | Exon 3 | 58 | PD +E | PF (Grade B) | 3.5 | 3 | none | 68 | yes | 11 | Alive, metastatic RCC |
| 8 | F | Partial deletion | 55 | DP | None | 4 | - | none | 77 | yes | 1 | Alive and well |
| 9 | M | Deletion | 33 | DP | None | 1.1 | 10 | none | 160 | yes | 0 | Alive and well |
| 10 | F | Exon 1 | 45 | DP | None | 2 | 3 | none | 183 | yes | 0 | Alive and well |
PF grading as per Bassi C et al. 2005.[21]
Pathological gross measurement.
Concurrent with left partial nephrectomy for RCC.
Abbreviations: CNS, central nervous system; DP, distal pancreatectomy; f/up, follow-up; E, enucleation; PD, pancreaticoduodenectomy; PF, pancreatic fistula; PNET, pancreatic neuroendocrine tumor; RCC, renal cell carcinoma; SMSA, solid microcystic adenoma; TP, total pancreatectomy
None of the 10 SMSA patients developed a metastasis from a pancreas tumor, but with long-term follow-up, four patients who had SMSAs without concurrent PNETs have new solid pancreas tumors of undetermined pathological diagnosis. Three patients developed RCC, and one died due to RCC progression (Table 2). For the patients with pathologically-confirmed PNETs, the majority had tumors which were Grade 1 according to the WHO/European Neuroendocrine Tumor Society proliferation grading system) with < 2 mitoses per 10 HPF or Ki-67% index of ≤ 2% (Table 1). There were no Grade 3 tumors. Seven (15.2%) out of the 46 patients with resected PNETs had malignant disease: Five were found to have metastatic disease at time of surgery (two with nodal metastases only, two with nodal and liver metastases, and one with liver only metastases) while two patients developed metastases to liver during follow-up. For those patients, the mean radiological size of their primary tumor was 4.0 ± 0.7 cm (median 4.0 cm, range 1.8 to 7.0 cm). In line with criteria proposed for resection of PNET with increase malignant potential, the smallest tumor (1.8 cm) associated with nodal metastases had a doubling time of 411 days and presented in a patient bearing a germline mutation on Exon 3 of the VHL gene.[2]
Retrospective reassessment of pre-operative CT and MRI scans was done on 39 patients with available digitalized imaging (Table 3). Blinded to the final pathological diagnoses, radiologists described 60 solid lesions with histological correlates known only by S.T. and G.Q.P. The radiologists were aware that these patients had undergone surgery with final diagnoses being PNET, SMSA, or both. The radiologic diagnosis was accurate in 5 (45.5%) of 11 SMSAs and in 45 (91.8%) of 49 PNETs (P2 = 0.001). Four SMSAs were misdiagnosed as PNETs while two SMSAs had “uncertain” characteristics. No PNET was mistaken for SMSA while four PNETs (8.2%) had “uncertain” characteristics. The homogeneity of arterial enhancement on CT scan could not conclusively distinguish SMSAs from PNETs (P2 = 0.2) and neither did the T2-weighted MRI images (P2 = 1.0). Distinctions were more noticeable with functional 18F-FDG-PET imaging (Figure 1). The mean maximal standardized uptake value (SUV) was significantly higher in 17 PNETs (12.1 ± 1.2, range 3.9 to 18.9) compared to 6 SMSAs (4.2 ± 0.5, range 3.1 to 6.2; P2 = 0.002); however this distinction is not absolute as there is some overlap due to some PNETs with lower SUVs.
Table 3.
Blinded retrospective radiological reassessment of solid pancreatic lesions
| Patients with SMSA | Patients with PNET | p-value | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Pre-operative images available for review | 8 | 31 | |||
| CT and MRI | 7 | 87.5 | 23 | 74.2 | n.s. |
| CT only | 1 | 12.5 | 8 | 25.8 | |
| No. solid lesions with histological correlates | 11 | 49 | |||
| Correct diagnosis | 5 | 45.5 | 45 | 91.8 | 0.001* |
| Uncertain radiological diagnosis | 2 | 18.2 | 4 | 8.2 | |
| SMSA misdiagnosed as PNET | 4 | 36.4 | - | - | |
| Homogeneous arterial enhancement (CT) | 5/11 | 45.5 | 34/49 | 69.4 | n.s. |
| Moderate or high T2 enhancement (MRI) | 7/10 | 77.8 | 24/37 | 91.9 | n.s. |
Fisher exact test
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging; n.s., not significant; PNET, pancreatic neuroendocrine tumor; SMSA, solid microcystic adenoma.
Figure 1. Uptake of PNETs and SMSAs on pre-operative 18F-FDG-PET scans.
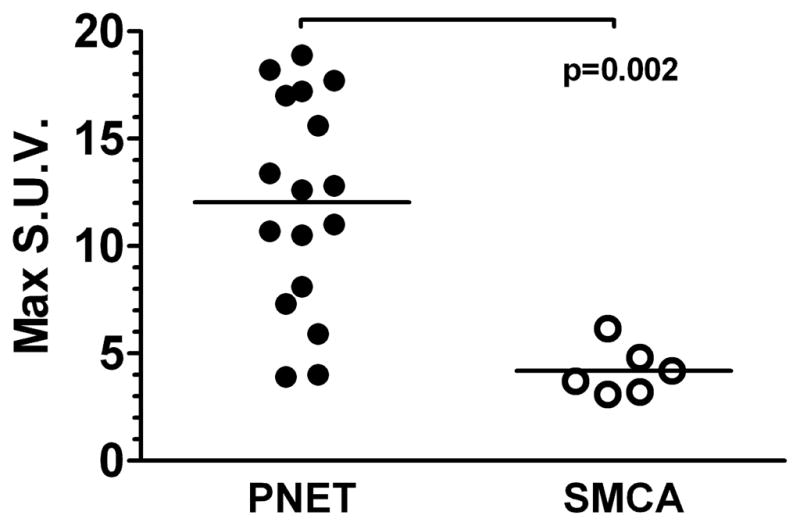
Maximum standardized uptake values (S.U.V) of 17 PNETs and 6 SMSAs in 18 patients with available pre-operative PET scans. Lines represent mean values.
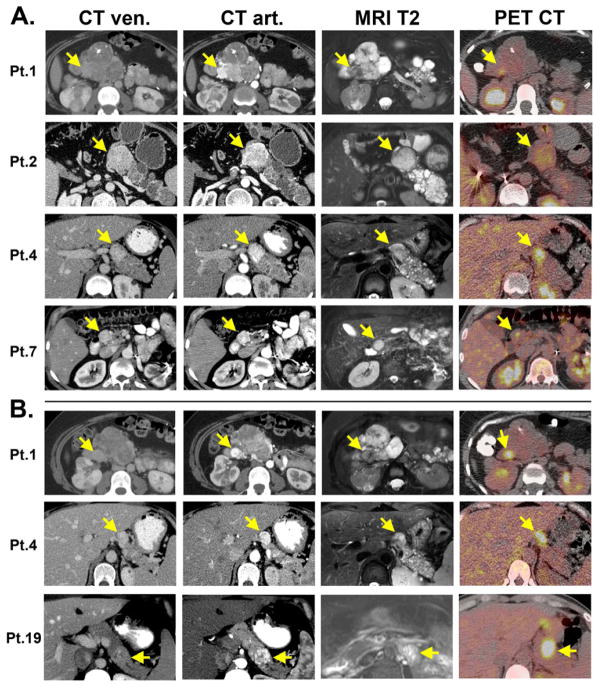
To illustrate the difficulty in distinguishing SMSA from PNET, we compiled representative images for four patients with SMSAs (Figure 2A) and three with PNETs (Figure 2B). In all cases, CT scans timed to capture early arterial enhancement is crucial to identify these pancreas lesions (whether SMSA or PNET) since the tumors become iso-attenuating on the venous phase. As seen in Figure 2, for both CT and MRI scans, the enhancement patterns for SMSAs resemble PNETs. SMSAs did not tend to uptake 18F-FDG with strong avidity; two SMSA tumors with higher signals (seen in Patients 1 and 4) had adjacent PNETs which probably caused in some signal “bleeding” onto the SMSAs.
Figure 2. Radiological features of SMSAs (A) and PNETs (B).
A. Pathologically-confirmed SMSAs (arrows) seen in 4 patients using CT scans with intravenous contrast timed on venous (CT ven) and arterial (CT art) phases, T2-weighted magnetic resonance imaging (MRI T2) and 18F-FDG PET scans (PET CT). In addition to having a SMSA with intense enhancement on arterial phases of CT scans, Patients 1 also had a more typical macrocystic disease in the pancreas with partly calcified stellate central scars. Patient 2 had a pancreaticoduodenectomy performed 4 years earlier for a pathologically-confirmed PNET. B. Pathologically-confirmed PNETs (arrows) adjacent to the SMSAs concurrently seen in Patients 1 and 4 above. Bright 18F-FDG-PET uptake in both PNETs of Patients 1 and 4 showed some signal “bleeding” into the adjacent SMSAs in panel A. Patient 19 had some necrosis within the pathologically-confirmed PNET, giving rise to a cystic-like lesion within the tumor with high T2-weighted signal. In contrast to SMSAs, this PNET had high 18F-FDG-PET uptake (SUV = 18.2).
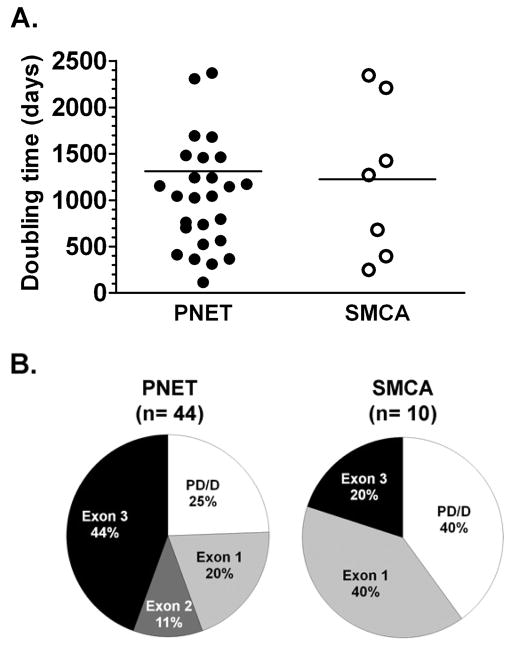
The mean doubling time was similar for both PNETs (1311 ± 288 days, range 114 to 8225) and SMSAs (1224 ± 316 days, range 248 to 2343) (Figure 3A). There was no clear genotype-phenotype association for the 10 patients with SMSAs and no clear pattern of association with a specific VHL gene mutation (Figure 3B).
Figure 3. A. Tumor growth rates.
The tumor doubling time for 24 patients (27 PNET tumors and 7 SMSA tumors) with available digitalized imaging were estimated using the longest diameter from the most recent pre-operative CT scan compared to the oldest available. Lines represent mean values; one value (8225 days) for a PNET was off-scale and not shown. B. Genotype-phenotype evaluation: VHL gene germline mutational status distribution seen in patients with PNETs and SMSAs. PD/D, partial deletion or deletion.
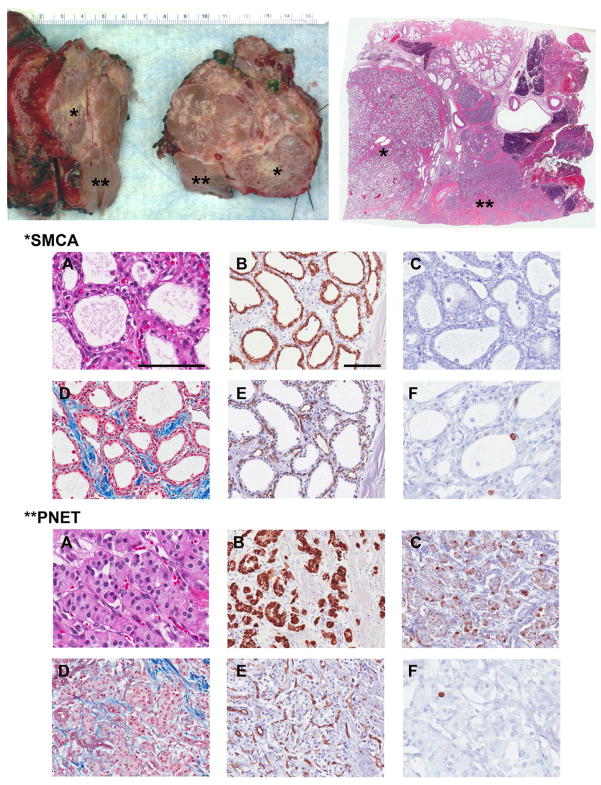
For illustrative purposes, a study of the different histological components of SMSA compared with PNET was carried out for Patient 1 who had both clinically relevant SMSA and PNET which were adjacent to each other (Figure 4). The epithelial component of both lesions stained strongly positive for pan-cytokeratins (Figure 4B). Epithelial membrane antigen (EMA) stained strongly in the SMSA, but not readily in the PNET (not shown). As expected, the SMSA did not stain for chromogranin A (Figure 4C) and synaptophysin (not shown) but the PNET did. Masson’s trichrome stain highlighted the dense fibrous components surrounding the SMSA with septa interdigitating into the lesion more obviously than for the PNET (Figure 4D). Although red blood cells could be seen readily in the stroma of both the SMSA and the PNET in the H&E slides (Figure 4A), the dense network of capillaries was made evident by CD31 staining (Figure 4E) which highlighted the endothelial cells in both tumors. Remarkably, Ki67-positive cells, providing evidence of active cell division, was documented in the epithelium of SMSA microcysts (Figure 4F) and in the PNET. Similar to the majority of the PNETs (Table 1), Ki67 was positive in < 2% of epithelial cells in the two SMSAs in which Ki67 was tested. CD10 stain used to rule out metastasis from RCC was negative (not shown).
Figure 4. Histopathological and immunohistochemistry characteristics of SMSA and PNET.
Pancreaticoduodenectomy gross specimen of Patient 1 and whole mount with SMSA (*) adjacent to PNET (**). Immunohistochemistry staining for both lesions: A, Hematoxylin and eosin (H&E); B, AE1/AE3 pan-cytokeratin; C, Chromogranin A; D, Masson’s Trichrome; E, CD31; F, Ki67. A: H&E images highlight the distinct morphologies for each lesion: For SMSA, dense coalescent small cysts are filled with serous fluid lined by a single layer of cuboidal epithelial cells with clear cytoplasm. For the adjacent well-differentiated PNET, epithelial cells with finely granular eosinophilic cytoplasm and characteristic “salt and pepper” chromatin in the nucleus are arranged in trabecular, nesting or glandular patterns. Clusters of red blood cells can readily be seen for both well-vascularized lesions. The main components of SMSA are highlighted: the epithelium (B), the smooth muscle fibers and collagen-rich stroma (D), and the dense capillary network (E). PNETs are positive for chromogranin A (C) and are also hypervascular (E). Both tumors show evidence of cell division with some Ki67 positivity (F). A and F photos were taken at 400x, all others at 200x; measure bars = 100 μm.
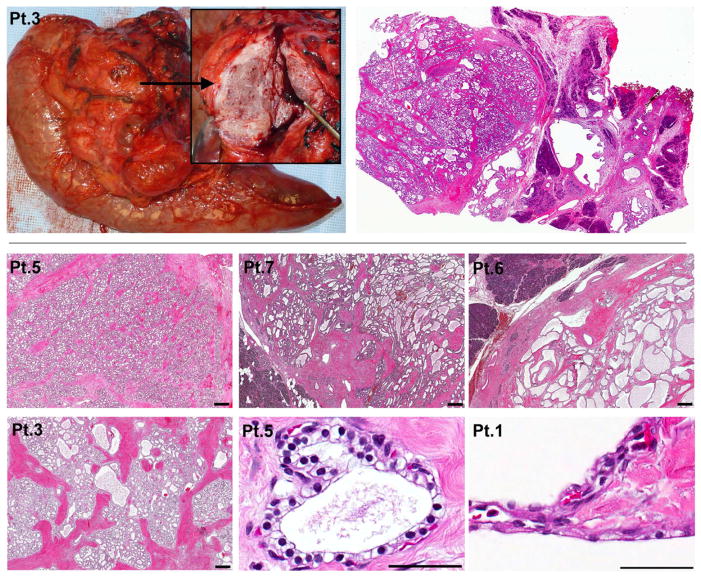
The spectrum of morphological features of SMSAs mistaken for PNETs by pre-operative imaging is presented in Figure 5. Tumors were characterized by microcysts organized in small acini of varying sizes, shapes and densities; however, all were surrounded and intersected by fibrous bands of varying thicknesses. All SMSA tumors lacked significant nuclear pleomorphism, cellular atypia, or invasive features that would have suggested a diagnosis of cystadenocarcinoma. This is consistent with long-term follow-up which showed that none of these 10 SMSA patients developed metastatic disease from a pancreas source (Table 2). These solid lesions were seen in association with more classical serous cystic changes of the pancreas (seen on CT images in Figure 2).
Figure 5. Spectrum of histopathological features of SMSAs.
Pancreaticoduodenectomy gross specimen and corresponding whole mount slide from Patient 3. H&E slides taken at 20x (measure bar = 400 μm) show microcysts organized in small acini of varying sizes in Patients 5, 7 and 6, clearly demarcated from the adjacent normal pancreas (darker purple staining) by a thick fibrotic layer. The interdigitating fibrous septa are of varying thicknesses, reaching at least 400 μm in some tumor areas for Patient 3. Typical cuboidal epithelial clear cells are shown at 400x (measure bar = 100 μm) for Patient 5, but some cells lining the cysts can also be flattened and intermixed with endothelial cells (Patient 1, 400x).
Discussion
Most patients with VHL disease require multiple surgical interventions throughout their lifetime to relieve symptoms related to benign tumor processes and to prevent and/or treat metastases from malignant tumors. Although solid pancreatic tumors are seen in < 20% of VHL patients, most of which are PNETs, these patients are also prone to develop benign pancreatic cystic disease of all variants, some of which are microcysts organized architecturally in such a dense fashion that they can be taken for PNETs on imaging. Accurate pre-operative imaging, along with a thoughtful multidisciplinary approach to their care, can help minimize the number of surgeries these patients undergo and potentially improve their quality of life.
In our series of 55 patients with VHL who underwent 79 pancreatectomies for solid pancreatic lesions thought to be PNETs on pre-operative evaluation, 12 pancreatectomies (15.2 % of pancreatectomies) in 10 patients (18.2% of patients) were done for lesions found to be microcystic adenomas with prominent solid components (SMSAs) on final pathology. Blinded reassessment of pre-operative CT and MRI scans confirmed the difficulty in distinguishing SMSAs from PNETs, even by experienced radiologists who were aware of the purpose of the study. PNETs are known to display bright and often homogeneous early arterial contrast enhancement on CT scans.[13] In our study, the SMSAs were also found to exhibit similar radiologic characteristics. Histological and immunohistochemistry studies confirmed the hypervascularity of SMSAs (and PNETs), which likely explains why SMSAs also display hyper-intense arterial contrast enhancement on CT and MRI scans. Although solid in nature, necrotic degeneration, hemorrhage, or abundant blood supply in PNETs could explain why the majority of PNETs had a higher than background T2 signal intensity by MRI, whereas the microcystic nature of SMSAs did not necessarily result in strikingly increased T2 signal intensity as would a more macrocystic tumor. Moreover, neither the rate of tumor growth, estimated by the doubling time, nor the specific VHL mutation could distinguish SMSAs from PNETs. The only modality we investigated that could potentially help distinguish those two entities was 18F-FDG-PET imaging. PNETs generally had higher SUV uptake than SMSAs, although several PNETs had low SUVs which overlapped with the SMSAs. A threshold SUV ≥ 5.0 had a sensitivity of 88% and a specificity of 83% for diagnosing a PNET from a SMSA. A threshold SUV ≥ 7.0 gave 100% specificity in distinguishing a PNET from a SMSA, albeit with a lower sensitivity of 82%.
Ten cases of clinically relevant non-VHL SMSAs were reported thus far in the literature since 1996.[9, 11] These reports in non-VHL patients (mean age 60.3 years, range 39 to 74) were of isolated lesions found with no clear gender preference, with size ranging from 1.9 to 4.5 cm, and presenting incidentally or in association with abdominal discomfort. Although the frequency of those reports have increased over the years, it is likely that SMSA is a rare solid pancreatic lesion in non-VHL patients. Our findings in VHL patients suggest a higher frequency in this particular group of patients who are younger (mean age 46.9 years, range 31 to 58) and who often (40%) have multiple SMSA lesions. Consistent with what we observed for VHL patients, other reports in non-VHL patients state the difficulty of distinguishing SMSAs from PNETs based on early contrast-phased CT scan imaging. Some suggested that heavily T2-weighted magnetic resonance cholangiopancreatography could better reveal the microcystic nature of SMSAs than conventional T2-weighted MRI.[14, 15]
Endoscopic ultrasound (EUS) and needle biopsy were not employed in our study since all lesions for which surgery was proposed were growing tumors with classic radiologic characteristics of PNETs in a population of patients with a predisposition to form PNETs. The frequency of SMSA “masquerading” as PNETs had not been established heretofore. Furthermore, it has been our experience that intra-operative ultrasound, used in all cases, was not sensitive enough to reveal the microcystic nature of SMSAs (with cyst sizes in the range of 100 to 200 μm). Although the addition of EUS-guided biopsy for cytology and immunohistochemistry could potentially confirm a PNET diagnosis in some cases, up to 22% of PNETs in VHL patients may be negative for chromogranin A, while approximately 17% are focally positive, with synaptophysin being more consistently detected.[16] Thus, the value of EUS to distinguish SMSA and PNET should be prospectively studied before definitive recommendations can be given.
The rarity of SMSAs precludes complete understanding of its natural history. However, no clear case of malignant transformation or metastatic spread has been reported or seen in our patients. Nonetheless, approximately 25 cases of serous cystadenocarcinoma are reported in the literature, all in non-VHL patients, and are defined by local invasive features or distant metastases.[17] In our series, no nuclear atypia or invasive features were found in any SMSA tumors. Two unusual cases of ectopic microcystic adenoma have been reported in VHL patients being followed at the NIH--one with a rapidly growing tumor arising in the ethmoid sinus and one within the lung parenchyma.[18, 19] Although both patients had pancreatic cysts, neither had solid pancreatic tumors. In VHL patients, benign microcystic cystadenomas have also been described in the epididymis and the broad ligament.[1] In fact, loss of heterozygosity at the VHL locus has been documented in all variants of serous cystic lesions in VHL patients, linking the pathophysiology of serous adenoma to VHL disease at the genetic and molecular level.[10]
Since PNETs in VHL patients do carry a risk of malignancy, as evidenced by the seven patients (15.2%) with PNETs who were found to have or to develop metastatic disease after pancreatectomy (Table 1), we still support prior resection guidelines for large or growing solid tumors that appear to be PNETs.[2, 6] However, for thoroughness during the counseling of patients for a pancreatectomy for a suspected PNET, it is important to disclose that due to limitations with pre-operative imaging, a small but not insignificant percent (15.2%) of pancreatectomies may be performed for a SMSA which at this time does not appear to have a malignant potential. Perhaps 18F-FDG-PET may help decrease this false positivity rate, but the data is early and limited at this time. We are continuing to use routine 18F-FDG-PET imaging on a clinical protocol to evaluate future patients with solid pancreas tumors.
Acknowledgments
Funding: National Institutes of Health.
We would like to thank Ms. Silke A. Williams and Dr. Avi Z. Rosenberg for assistance on pathology archiving and imaging software. We also greatly appreciate Dr. Irina A. Lubensky and Dr. Ping Zhuang for thoughtful discussions on the pathology and molecular genetics of VHL tumors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Lonser RR, Glenn GM, Walther M, Chew EY, Libutti SK, Linehan WM, et al. von Hippel-Lindau disease. Lancet. 2003;361:2059–67. doi: 10.1016/S0140-6736(03)13643-4. [DOI] [PubMed] [Google Scholar]
- 2.Blansfield JA, Choyke L, Morita SY, Choyke PL, Pingpank JF, Alexander HR, et al. Clinical, genetic and radiographic analysis of 108 patients with von Hippel-Lindau disease (VHL) manifested by pancreatic neuroendocrine neoplasms (PNETs) Surgery. 2007;142:814–8. doi: 10.1016/j.surg.2007.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammel PR, Vilgrain V, Terris B, Penfornis A, Sauvanet A, Correas JM, et al. Pancreatic involvement in von Hippel-Lindau disease. The Groupe Francophone d’Etude de la Maladie de von Hippel-Lindau. Gastroenterology. 2000;119:1087–95. doi: 10.1053/gast.2000.18143. [DOI] [PubMed] [Google Scholar]
- 4.Phan GQ, Yeo CJ, Hruban RH, Lillemoe KD, Pitt HA, Cameron JL. Surgical experience with pancreatic and peripancreatic neuroendocrine tumors: review of 125 patients. J Gastrointest Surg. 1998;2:472–82. [PubMed] [Google Scholar]
- 5.de Wilde RF, Edil BH, Hruban RH, Maitra A. Well-differentiated pancreatic neuroendocrine tumors: from genetics to therapy. Nat Rev Gastroenterol Hepatol. 2012 doi: 10.1038/nrgastro.2012.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libutti SK, Choyke PL, Bartlett DL, Vargas H, Walther M, Lubensky I, et al. Pancreatic neuroendocrine tumors associated with von Hippel Lindau disease: diagnostic and management recommendations. Surgery. 1998;124:1153–9. doi: 10.1067/msy.1998.91823. [DOI] [PubMed] [Google Scholar]
- 7.Bosman FT, Carneiro F, Hruban RH, Theise ND. WHO Classification of Tumours of the Digestive System. 4. Lyon, France: International Agency for Research on Cancer; 2010. [Google Scholar]
- 8.Kosmahl M, Pauser U, Peters K, Sipos B, Luttges J, Kremer B, et al. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows Arch. 2004;445:168–78. doi: 10.1007/s00428-004-1043-z. [DOI] [PubMed] [Google Scholar]
- 9.Perez-Ordonez B, Naseem A, Lieberman PH, Klimstra DS. Solid serous adenoma of the pancreas. The solid variant of serous cystadenoma? Am J Surg Pathol. 1996;20:1401–5. doi: 10.1097/00000478-199611000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Mohr VH, Vortmeyer AO, Zhuang Z, Libutti SK, Walther MM, Choyke PL, et al. Histopathology and molecular genetics of multiple cysts and microcystic (serous) adenomas of the pancreas in von Hippel-Lindau patients. Am J Pathol. 2000;157:1615–21. doi: 10.1016/S0002-9440(10)64799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Machado MC, Machado MA. Solid serous adenoma of the pancreas: an uncommon but important entity. Eur J Surg Oncol. 2008;34:730–3. doi: 10.1016/j.ejso.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Stolle C, Glenn G, Zbar B, Humphrey JS, Choyke P, Walther M, et al. Improved detection of germline mutations in the von Hippel-Lindau disease tumor suppressor gene. Hum Mutat. 1998;12:417–23. doi: 10.1002/(SICI)1098-1004(1998)12:6<417::AID-HUMU8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 13.Marcos HB, Libutti SK, Alexander HR, Lubensky IA, Bartlett DL, Walther MM, et al. Neuroendocrine tumors of the pancreas in von Hippel-Lindau disease: spectrum of appearances at CT and MR imaging with histopathologic comparison. Radiology. 2002;225:751–8. doi: 10.1148/radiol.2253011297. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto T, Takahashi N, Yamaguchi T, Imamura Y. A case of solid variant type of pancreatic serous cystadenoma mimicking islet cell tumor. Clin Imaging. 2004;28:49–51. doi: 10.1016/S0899-7071(03)00102-5. [DOI] [PubMed] [Google Scholar]
- 15.Gabata T, Terayama N, Yamashiro M, Takamatsu S, Yoshida K, Matsui O, et al. Solid serous cystadenoma of the pancreas: MR imaging with pathologic correlation. Abdom Imaging. 2005;30:605–9. doi: 10.1007/s00261-004-0286-0. [DOI] [PubMed] [Google Scholar]
- 16.Lubensky IA, Pack S, Ault D, Vortmeyer AO, Libutti SK, Choyke PL, et al. Multiple neuroendocrine tumors of the pancreas in von Hippel-Lindau disease patients: histopathological and molecular genetic analysis. Am J Pathol. 1998;153:223–31. doi: 10.1016/S0002-9440(10)65563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King JC, Ng TT, White SC, Cortina G, Reber HA, Hines OJ. Pancreatic serous cystadenocarcinoma: a case report and review of the literature. J Gastrointest Surg. 2009;13:1864–8. doi: 10.1007/s11605-009-0926-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klein J, Zhuang Z, Lubensky I, Colby TV, Martinez F, Jr, Leslie KO. Multifocal microcysts and papillary cystadenoma of the lung in von Hippel-Lindau disease. Am J Surg Pathol. 2007;31:1292–6. doi: 10.1097/PAS.0b013e3180377aaf. [DOI] [PubMed] [Google Scholar]
- 19.Xu DS, Dirks MS, Quezado MM, Lubensky IA, Zhuang Z, Lonser RR, et al. A von Hippel-Lindau disease-associated microcystic adenoma of the ethmoid sinus: case report. Neurosurgery. 2011;69:E1017–E1021. doi: 10.1227/NEU.0b013e318223b7a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarpa A, Mantovani W, Capelli P, Beghelli S, Boninsegna L, Bettini R, et al. Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol. 2010;23:824–33. doi: 10.1038/modpathol.2010.58. [DOI] [PubMed] [Google Scholar]
- 21.Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 2005;138:8–13. doi: 10.1016/j.surg.2005.05.001. [DOI] [PubMed] [Google Scholar]