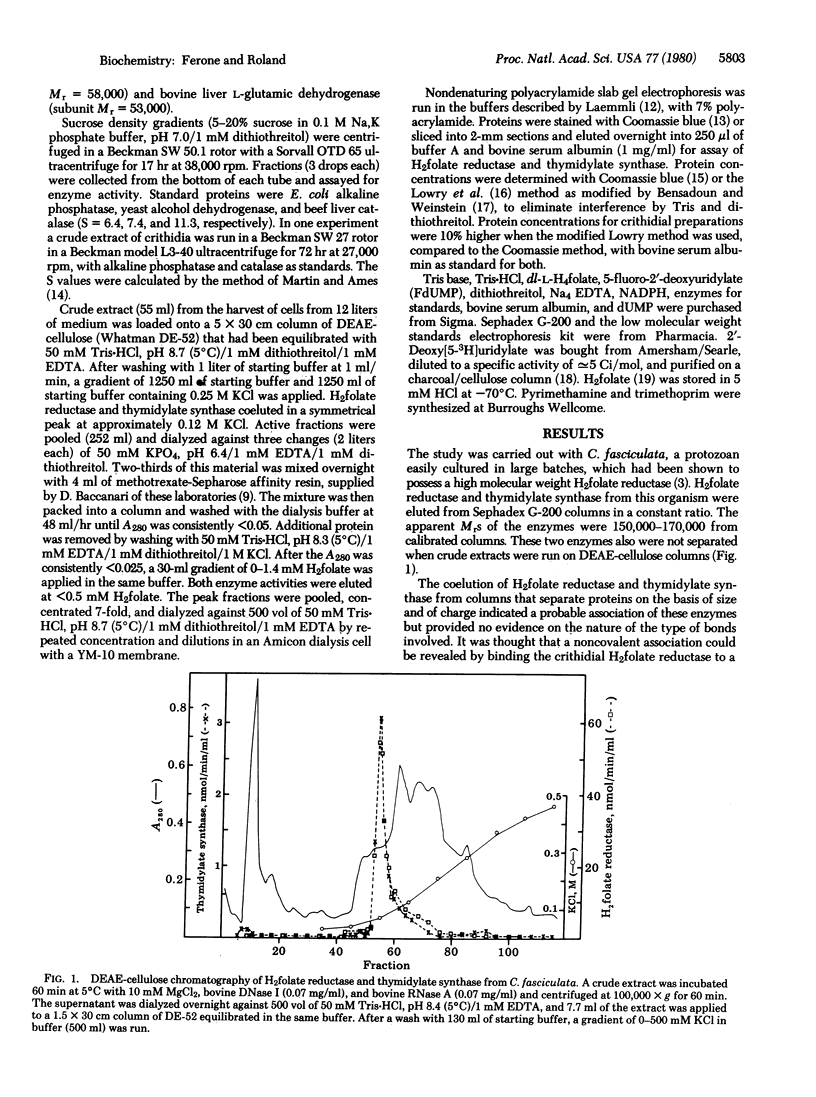
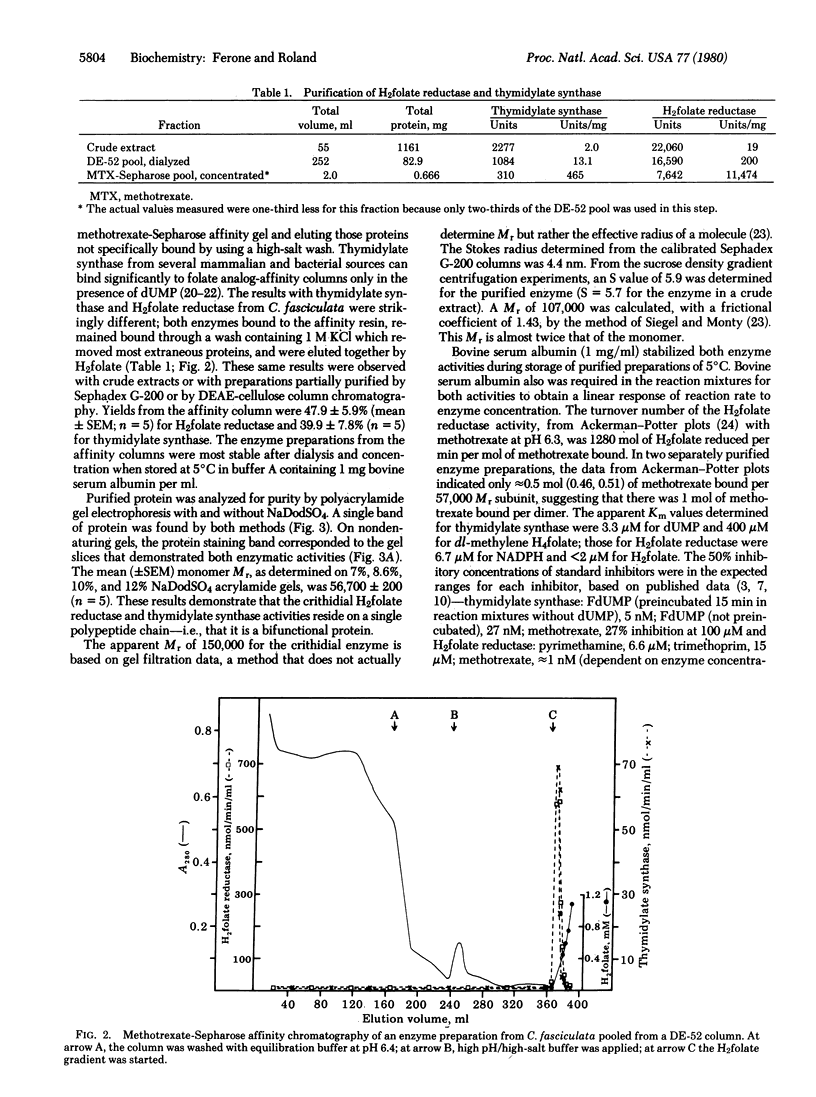
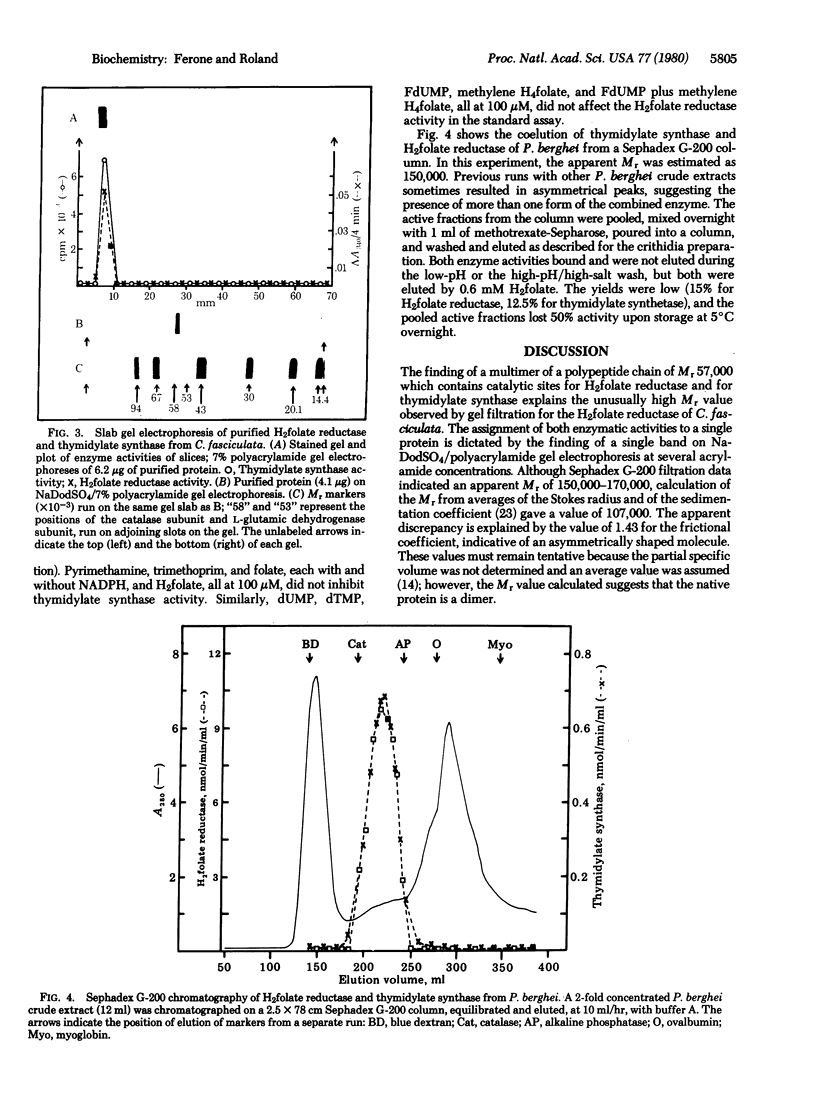
Abstract
The molecular weight of dihydrofolate reductase (5,6,7,8-tetrahydrofolate:NADP+ oxidoreductase, EC 1.5.1.3) from protozoa has been reported to be 5- to 10-fold larger than the isofunctional enzyme of most other organisms studied, based on gel filtration. This enzyme from the protozoal flagellate Crithidia fasciculata has been purified to homogeneity and found to be a bifunctional protein with thymidylate synthase (5,10-methylene tetrahydrofolate:dUMP C-methyltransferase, EC 2.1.1.45) activity. The purified protein, eluted from methotrexate-Sepharose columns by dihydrofolate, migrated as a single band on both nondenaturing and denaturing polyacrylamide gel electrophoresis. The monomer Mr is 56,700 +/- 200. The native Mr was calculated to be 107,000 from a sedimentation coefficient of 5.9 and Stokes radius of 4.4 nm. Dihydrofolate reductase and thymidylate synthase activities of the rodent malaria organism Plasmodium berghei also copurified on Sephadex G-200 and methotexate-Sepharose columns, suggesting that this unique bifunctional protein might occur throughout the Protozoa.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACKERMANN W. W., POTTER V. R. Enzyme inhibition in relation to chemotherapy. Proc Soc Exp Biol Med. 1949 Oct;72(1):1–9. doi: 10.3181/00379727-72-17313. [DOI] [PubMed] [Google Scholar]
- Baccanari D., Phillips A., Smith S., Sinski D., Burchall J. Purification and properties of Escherichia coli dihydrofolate reductase. Biochemistry. 1975 Dec 2;14(24):5267–5273. doi: 10.1021/bi00695a006. [DOI] [PubMed] [Google Scholar]
- Bensadoun A., Weinstein D. Assay of proteins in the presence of interfering materials. Anal Biochem. 1976 Jan;70(1):241–250. doi: 10.1016/s0003-2697(76)80064-4. [DOI] [PubMed] [Google Scholar]
- Chalabi K. A., Gutteridge W. E. Presence and properties of thymidylate synthase in trypanosomatids. Biochim Biophys Acta. 1977 Mar 15;481(1):71–79. doi: 10.1016/0005-2744(77)90138-3. [DOI] [PubMed] [Google Scholar]
- Dolnick B. J., Cheng Y. c. Human thymidylate synthetase derived from blast cells of patients with acture myelocytic leukemia. Purification and chracterization. J Biol Chem. 1977 Nov 10;252(21):7697–7703. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Ferone R., Burchall J. J., Hitchings G. H. Plasmodium berghei dihydrofolate reductase. Isolation, properties, and inhibition by antifolates. Mol Pharmacol. 1969 Jan;5(1):49–59. [PubMed] [Google Scholar]
- Ferone R. Folate metabolism in malaria. Bull World Health Organ. 1977;55(2-3):291–298. [PMC free article] [PubMed] [Google Scholar]
- Gutteridge W. E., Jaffe J. J., McCormack J. J., Jr The gel-filtration behaviour of dihydrofolate reductases from culture forms of trypanosomatids. Biochim Biophys Acta. 1969;191(3):753–755. doi: 10.1016/0005-2744(69)90379-9. [DOI] [PubMed] [Google Scholar]
- Gutteridge W. E., McCormack J. J., Jr, Jaffe J. J. Presence and properties of dihydrofolate reductases within the genus Crithidia. Biochim Biophys Acta. 1969 May 27;178(3):453–458. doi: 10.1016/0005-2744(69)90214-9. [DOI] [PubMed] [Google Scholar]
- Jaffe J. J., McCormack J. J., Meymarian E. Comparative properties of schistosomal and filarial dihydrofolate reductases. Biochem Pharmacol. 1972 Mar 1;21(5):719–731. doi: 10.1016/0006-2952(72)90064-0. [DOI] [PubMed] [Google Scholar]
- Jaffe J. J., McCormack J. J., Meymarian E., Doremus H. M. Comparative activity and properties of lactate dehydrogenase, xanthine dehydrogenase, and dihydrofolate reductase in normal and Brugia pagangi-infected Aedes aegypti. J Parasitol. 1977 Jun;63(3):547–553. [PubMed] [Google Scholar]
- Kammen H. O. A rapid assay for thymidylate synthetase. Anal Biochem. 1966 Dec;17(3):553–556. doi: 10.1016/0003-2697(66)90192-8. [DOI] [PubMed] [Google Scholar]
- Kirschner K., Bisswanger H. Multifunctional proteins. Annu Rev Biochem. 1976;45:143–166. doi: 10.1146/annurev.bi.45.070176.001043. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- McCuen R. W., Sirotnak F. M. Thymidylate synthetase from Diplococcus pneumoniae, properties and inhibition by folate analogs. Biochim Biophys Acta. 1975 Apr 19;384(2):369–380. doi: 10.1016/0005-2744(75)90038-8. [DOI] [PubMed] [Google Scholar]
- Nagelschmidt M., Jaenicke L. Dihydrofolat-Reduktase aus bäckerhefe. Reinigung und Eigenschaften. Hoppe Seylers Z Physiol Chem. 1972 May;353(5):773–781. [PubMed] [Google Scholar]
- Reid V. E., Friedkin M. Thymidylate synthetase in mouse erythrocytes infected with Plasmodium berghei. Mol Pharmacol. 1973 Jan;9(1):74–80. [PubMed] [Google Scholar]
- Rode W., Scanlon K. J., Hynes J., Bertino J. R. Purification of mammalian tumor (L1210) thymidylate synthetase by affinity chromatography on stable biospecific adsorbent. Stabilization of the enzyme with neutral detergents. J Biol Chem. 1979 Nov 25;254(22):11538–11543. [PubMed] [Google Scholar]
- Siegel L. M., Monty K. J. Determination of molecular weights and frictional ratios of proteins in impure systems by use of gel filtration and density gradient centrifugation. Application to crude preparations of sulfite and hydroxylamine reductases. Biochim Biophys Acta. 1966 Feb 7;112(2):346–362. doi: 10.1016/0926-6585(66)90333-5. [DOI] [PubMed] [Google Scholar]
- Slavík K., Rode W., Slavíková V. Purification of thymidylate synthetase from enzyme-poor sources by affinity chromatography. Biochemistry. 1976 Sep 21;15(19):4222–4227. doi: 10.1021/bi00664a014. [DOI] [PubMed] [Google Scholar]
- Spector T. Refinement of the coomassie blue method of protein quantitation. A simple and linear spectrophotometric assay for less than or equal to 0.5 to 50 microgram of protein. Anal Biochem. 1978 May;86(1):142–146. doi: 10.1016/0003-2697(78)90327-5. [DOI] [PubMed] [Google Scholar]
- Walter R. D., Königk E. Synthese der Desoxythymidylat-Synthetase und der Dihydrofolat-Reduktase bei synchroner Schizogonie von Plasmodium chabaudi. Z Tropenmed Parasitol. 1971 Sep;22(3):250–255. [PubMed] [Google Scholar]
- Wang C. C., Stotish R. L., Poe M. Dihydrofolate reductase from Eimeria tenella: rationalization of chemotherapeutic efficacy of pyrimethamine. J Protozool. 1975 Nov;22(4):564–568. doi: 10.1111/j.1550-7408.1975.tb05234.x. [DOI] [PubMed] [Google Scholar]