Abstract
Ectopic expression of a neuronal receptor, Metabotropic Glutamate Receptor 1 (Grm1), in melanocytes has been implicated in melanoma development in mouse models. The human relevance of this receptor’s involvement in melanoma pathogenesis was demonstrated by detecting GRM1 expression in subsets of human melanomas, an observation lacking in benign nevi or normal melanocytes. Grm1-transformed mouse melanocytes and a conditional Grm1 transgenic mouse model confirmed a requirement for sustained expression of Grm1 for the maintenance of transformed phenotypes in vitro and tumorigenicity in vivo. Here, we investigate if continued GRM1 expression is also required in human melanoma cell lines by using two inducible, silencing RNA systems: the ecdysone/Ponasterone A and tetracycline on/off approaches to regulate GRM1 expression in the presence of each inducer. Various in vitro assays were performed to assess consequences of a reduction in GRM1 expression on cell proliferation, apoptosis, downstream targeted signaling pathways and in vivo tumorigenesis. We demonstrated that suppression of GRM1 expression in several human melanoma cell lines resulted in a reduction in the number of viable cells and a decrease in stimulated MAPK and PI3K/AKT and suppressed tumor progression in vivo. These results reinforce earlier observations where a reduction in cell growth in vitro and tumorigenesis in vivo were correlated with decreased GRM1 activities by pharmacological inhibitors of the receptor, supporting the notion that GRM1 plays a role in the maintenance of transformed phenotypes in human melanoma cells in vitro and in vivo and potentially be a therapeutic target for the treatment of melanoma.
Keywords: Melanoma, GRM1, siRNA, MAPK, PI3/AKT
Introduction
Malignant melanoma, the most deadly form of skin cancer which is also the fifth most common cancer in men and the sixth most common cancer in women poses a substantial clinical burden with over 76,000 Americans estimated to be diagnosed and 9,000 succumbing to the disease in 2012 (1). Using a transgenic mouse model (2), our group identified that the ectopic expression of metabotropic glutamate receptor 1 (Grm1), in melanocytes was sufficient to induce spontaneous melanoma development (3). Grm1 is a G-Protein coupled receptor whose expression is normally localized to the central and peripheral nervous system and has functional implications on learning and memory formation (4). Analysis of human melanoma cell lines and biopsy samples has detected GRM1 expression in 80% of cell lines and 65% of primary to metastatic biopsy samples but not in normal melanocytes or benign nevi (5, 6). In addition, we showed that stable mouse melanocytic clones with exogenously transfected Grm1 exhibited transformed phenotypes in vitro and were tumorigenic in vivo (7). Furthermore, a conditional Grm1 transgenic mouse model confirmed the requirement for continuous expression of Grm1 in murine melanocytes for initial tumor formation and progression in vivo (8).
The metabotropic glutamate receptor family encompasses 8 receptors which are divided into 3 groups according to agonist pharmacology, sequence homology and transduction mechanisms via coupling to second messenger systems (4, 9–11). Group I GRM1 and GRM5 receptors are coupled to the activation of phospholipase C through Gq proteins; group II GRM2 and GRM3 receptors and group III GRM4, GRM6, GRM7 and GRM8 receptors are negatively coupled to adenylyl cyclase through Gi/0 proteins in heterologous expression systems (4, 9–11). In addition to GRM1, two other GRMs have been shown to have roles in melanoma development. It was recently demonstrated that over-expression of Grm5 in mouse melanocytes can induce melanoma development in a transgenic mouse model (12). This over-expression of Grm5 was found to result in the activation of the MAPK pathway (12). Another group performed a large scale mutational analysis of GPCRs and identified mutations in GRM3 in ~17% of melanoma tumor samples (13). Some of these mutations were found to confer a growth advantage for the tumors through the action of MEK1/2, a kinase in the MAPK signaling pathway. Additionally, GRM3 mutant melanoma cells were found to be more responsive to MEK inhibition with AZD-6244 than wild-type cells especially when they also harbored BRAFV600E mutations (13). Taken together, these reports suggest that glutamate receptors and glutamatergic signaling may play greater roles than previously thought in melanoma biology. Given that large percentages of human melanomas examined showed GRM1 expression, we were interested to know if suppression of GRM1 expression may modulate the growth of human melanoma cells in vitro, stimulation of downstream signaling cascades as well as tumorigenic potentials in vivo. Here, we showed that siRNA targeted to GRM1 suppressed expression of the receptor led to decreased levels of activated mitogenic MAPK as well as anti-apoptotic PI3/AKT pathways resulting in reduced cell proliferation and increased apoptosis in vitro and in vivo.
Materials and Methods
Antibodies and Reagents
Antibodies against GRM1 (Novus Biologics, Littleton CO); Phospho-AKT (S473), total-AKT, phospho-ERK1/2, total-ERK1/2, Poly (ADP-ribose) polymerase PARP, Caspase-3 and cleaved Caspase-3 (Cell Signaling, Danvers MA); Phospho-AKT 2 (S474) (Genscript, Piscataway NJ); α-Tubulin (Sigma, St Louis MO); Anti-VgRXR-15C3 and 9B9 antibodies were a gift from the Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA; Zeocin, G418, blasticidin, hygromycin, Lipofectamine 2000, Plus Reagent, ViraPower Lentiviral Packaging mix (Invitrogen, Carlsbad, CA); doxycycline (Sigma, St Louis MO); Ponasterone A (Enzo Life Sciences, Plymouth Meeting, PA); Sucrose (Fisher Scientific, Pittsburg PA); DOTAP (Roche Applied Science, Indianapolis, IN); Polybrene (Chemicon, Temecula, CA).
Cell Culture
UACC903, 1205Lu and C8161 were provided by Dr. Jeffery Trent (The Translational Genomics Research Center, Phoenix, AZ), Dr. Meenhard Herlyn (Wistar Institute, Philadelphia, PA) and Dr. Mary Hendrix (Children’s Memorial Research Center, Chicago, IL). The cells were maintained in RPMI 1640 plus 10% fetal bovine serum (FBS). HEK293T cells and H1299 cells were provided by Dr. Renping Zhou and Dr. C. S. Yang (Rutgers State University, New Brunswick, NJ) and were maintained in DMEM plus 10% FBS.
Lentivirus Production
TetR cloned in lentiviral vector PLenti6L was a generous gift provided by Dr. Andrew Aplin (Kimmel Cancer Center, Philadelphia, PA). Lentivirus production was done using the ViraPower Packaging Mix according to manufacturer’s instruction.
DNA Transfection and Lentivirus transduction
Ponasterone inducible system
C8161 cells were transfected with 4 μg of pVgRXR plasmid DNA (A gift from Dr. Danny Rangasamy, The Australia National University, Canberra, Australia) with Lipofectamine 2000 reagent as per manufacturer’s protocol. Stable clones expressing pVgRXR receptor were selected using 5 μg/ml of Zeocin in RPMI plus 10% FBS. Receptor expression was confirmed by western blotting. C8161 pVgRXR cells were transfected with 4 μg inducible siGRM1 plasmid DNA with the siRNA sequence 5′ GATGTACATCATTATTGCC 3′ (7), cloned into the pIND vector containing 5 repeats of ecdysone response elements (14) using Lipofectamine 2000. Stable C8161 pVgRXR siGRM1 clones were generated by selection with 5 μg/ml Zeocin and 300 μg/ml G418. Induction of siGRM1 was achieved by treating the cells with 1–10 μM of Ponasterone A (PonA) for 7 days as described (15, 16).
Tetracycline/doxycycline inducible system
UACC903 cells were transduced overnight with TetR lentivirus particles and 7.5 μg/ml Polybrene. Stable UACC903 TetR cells were selected with 1 μg/ml blasticidin. Stable 1205 Lu TetR cells were a gift from Dr Andrew Aplin and were maintained in RPMI plus 10% FBS and 5 μg/ml blasticidin. UACC903 TetR and 1205 Lu TetR cells were transfected using DOTAP reagent with 4 μg of siGRM1 plasmid DNA with the sequence 5′ATGTACATCATTATTGCC3′ cloned into the pRNATin-H1.2/Hygro vector as described (7). Stable UACC903 TetR siGRM1 clones were generated by selection with 1 μg/ml blasticidin and 10 μg/ml hygromycin while 1205 Lu TetR siGRM1 clones were selected with 5 μg/ml blasticidin and 100 μg/ml hygromycin. Induction of siGRM1 was carried out by treating the cells with 10 ng/ml of doxycycline for 7 days.
Western blotting
Protein lysates were extracted from C8161 pVgRXR or C8161 pVgRXR-siGRM1 A10 or C8161 pVgRXR-siGRM1 A13 treated with plain media, vehicle control (DMSO) or 10 μM Ponasterone A (PonA) as described previously (6) and assayed for the expression of GRM1, phosphorylated and total ERK1/2, tubulin, Caspase-3 and cleaved Caspase-3 with the corresponding antibodies. Similarly, protein extracts from UACC903 TetR, UACC903 TetR siGRM1-8, UACC903 TetR siGRM1-11, 1205 Lu TetR, 1205LuTetR siGRM1-1 or 1205 Lu TetR siGRM1-9 treated with either plain media or 10 ng/ml doxycycline for 7 days and assayed for GRM1, phosphorylated and total ERK1/2, phosphorylated AKT (S473), phosphorylated AKT2 and α-Tubulin expression. The blots were scanned and the intensity of the protein bands analyzed with Optiquant Software (Perkin Elmer Life Sciences, Shelton, CT).
MTT assays
MTT assays performed as described (6) with 1× 103 cells per well of C8161 pVgRXR siGRM1 clones A10, and C8161 pVgRXR siGRM1 A13 or C8161 pVgRXR. The conditions were no treatment (NT), vehicle (DMSO) or 1 μM, 5 μM, and 10 μM of PonA for 7 days. Absorbance was determined on days 0, 3 and 7 using a Tecan Plate reader (Infinite 200 Tecan USA, Durham, NC).
In vivo Xenografts
Ponasterone inducible system
C8161 pVgRXR siGRM1 A13 cells and vector control C8161 pVgRXR cells were injected at 106 cells per site in the dorsal flanks of 5–6 weeks old athymic nude mice. When the tumor volumes reached ~10–20 mm3 as measured with a vernier caliper, mice were randomly divided into two groups with similar tumor volumes and 6 mice per group. One group was treated with vehicle - olive oil (Veh) and the other one with PonA (10mg/kg). Mice were treated twice a week with vehicle or PonA administered via intraperitoneal injection as described (16) and measured once a week with a vernier caliper. Tumor volumes were calculated as described (17). All tumor bearing mice were euthanized after 37–42 days due to tumor burden in the control groups.
Tetracycline/doxycycline inducible system
1205 Lu TetR siGRM1-9, 1205 Lu TetR siGRM1-1 or UACC903 TetR siGRM1-8 and UACC903 TetR siGRM1-11 cells were injected at 106 cells per site in the dorsal flanks of 5–6 weeks old athymic nude mice. When the tumor volumes reached ~10–20 mm3 as measured with a vernier caliper, mice were divided into no treatment (NT) or doxycycline (Dox) groups with similar tumor volumes in each group with 6 mice per group. A 0.1% doxycycline, 1% w/v sucrose solution was provided to the animals and replaced bi-weekly in the Dox treatment groups as previously described (7). The tumor volume was measured once a week and all tumor bearing mice euthanized when tumor burden in the control groups approached maximum permitted levels.
Immunohistochemistry
Immunohistochemical staining on excised tumor xenografts from C8161 pVgRXR and C8161 pVgRXR siGRM1 A13 PonA or vehicle treated controls, 1205 Lu TetR and 1205 Lu TetR siGRM1-9 doxycycline treated and not treated controls to detect changes in the number of apoptotic and proliferating cells (cleaved Caspase-3 and Ki-67, respectively) was performed by Tissue Analytical Services at the Cancer Institute of New Jersey. The number of stained cells were quantified with a digital Aperio ScanScopeGL system and ImageScope software (v 10.1.3.2028) (Aperio Technologies Inc., Vista, CA) according to the manufacturer’s protocol with modifications as described (18).
Statistics
Statistics analysis was calculated using Microsoft Excel® with two-sample t-tests with statistical significance set at p<0.05.
Results
Ponasterone A and doxycycline inducible suppression of GRM1 expression
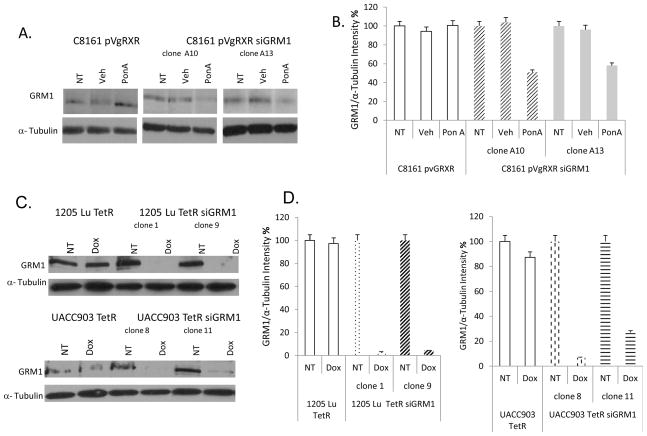
GRM1 positive C8161 human melanoma cells were transfected with a plasmid construct encoding a heterodimeric receptor consisting of the ecdysone receptor (EcR) and the mammalian homologue of the Ultraspiracle protein, the retinoid X receptor (RXR) (pVgRXR). Cells expressing the receptor were then transfected with a plasmid encoding GRM1 siRNA (siGRM1). This construct also contains 5 copies of the ecdysone response element (EcRE) which are upstream of a minimal promoter, which can drive expression of siGRM1 in the presence of the inducer, an analogue of ecdysone, Ponasterone A (PonA) (14, 15). We showed that in the presence of the inducer, 10 μM PonA, GRM1 expression was diminished in several clones with examples of two such clones shown (Figure 1A), while cells containing the RXR receptor but lacking the siGRM1-RNA, C8161 pVgRXR did not show any decrease in levels of GRM1 expression (Figure 1A). Quantitation of the intensity of the western blots indicate a ~40% decrease in C8161 pVgRXR siGRM1 A13 and a ~50% decrease in C8161 pVgRXR siGRM1 A10 (Figure 1B).
Figure 1. Inducible suppression of GRM1.
(A.) Suppression of GRM1 protein expression in the ecdysone regulated system in C8161 human melanoma cells in the presence of the inducer, 10 μM Ponasterone A (PonA) in two independent clones. No suppression is observed in control C8161 pVgRXR cells in the presence of PonA. Samples were normalized to α-Tubulin. NT- No treatment, Veh - (Vehicle) DMSO. (B.) Quantitation of C8161 pVgRXR siGRM1 clones A10 and A13 western blots from A. using Optiquant software to determine % change in intensity. (C.) Suppression of GRM1 expression using the tetracycline/doxycycline regulated system in two independent clones from 1205 Lu and UACC903 human melanoma cell lines in the presence of 10 ng/ml doxycycline. No suppression is observed in control 1205 Lu TetR and UACC903 TetR in the presence of doxycycline. Equal loading was shown by levels of α-Tubulin. NT-No treatment, Dox- Doxycycline. (D.) Quantitation of western blots of 1205 Lu TetR siGRM1 and UACC903 TetR siGRM1 clones from panel C using Optiquant software to determine % change in intensity.
For the tetracycline inducible system, GRM1 positive UACC903 and 1205 human melanoma cells were infected with a lentiviral construct to ensure high expression levels of the Tet repressor (TetR). The TetR positive cells were then transfected with si-GRM1-RNA and treated with 10 ng/ml doxycycline to induce expression of siGRM1. Several independent clones were isolated and examples of some of the clones are shown. 1205 Lu TetR siGRM1 clones 1 and 9 and UACC903 TetR clones 8 and 11 exhibited decreased GRM1 expression compared to 1205 Lu TetR and UACC903 TetR cells that did not display any decrease in GRM1 expression after treatment with doxycycline (Figure 1C). Quantitation of the intensity of the no treatment and doxycycline treated samples showed that in the 1205 Lu TetR siGRM1 cells, clone 1 and clone 9 exhibited a ~90% decrease in GRM1 expression while (Figure 1D, left panel). UACC903 TetR siGRM1 clones 8 and 11 exhibited a ~90% and ~80% decrease in GRM1 expression respectively (Figure 1D, right panel).
Reduced GRM1 expression led to suppression in cell growth and enhanced apoptosis in vitro
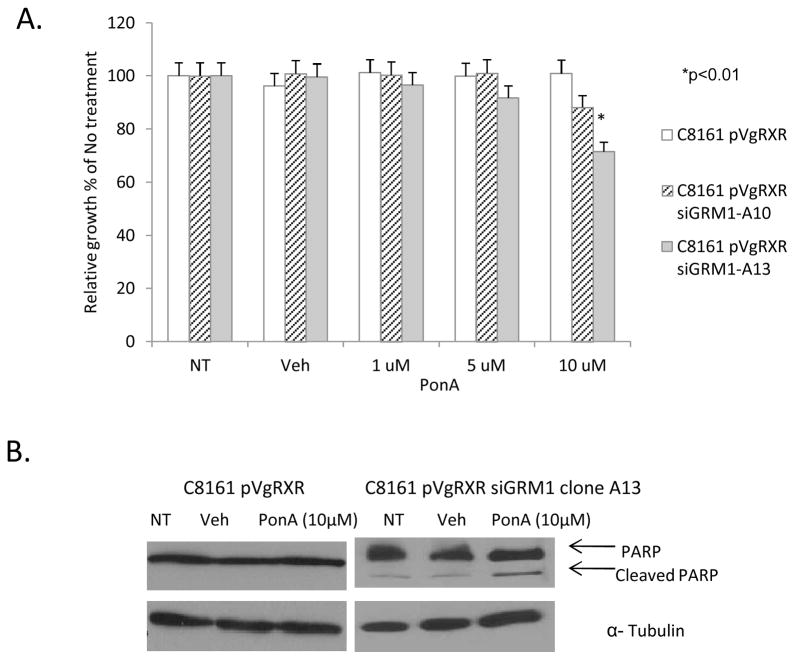
We have shown previously that when the GRM1 receptor is rendered non-functional by treatment with a competitive or non-competitive GRM1 antagonist, it results in suppression of proliferation in vitro in several GRM1-expressing human melanoma cell lines (6, 19). We performed MTT cell viability/proliferation assays on clones from the ecdysone regulated siRNA expression system; we showed that treatment with the inducer, PonA resulted in a 15–30% decrease in C8161 pVgRXR siGRM1 cells while control C8161 pVgRXR cells did not show any modulation in cell growth (Figure 2A). Furthermore, we also showed that a reduction in viable cell number at least in part could be attributed to an increase in apoptotic cell population as assessed by a rise in levels of an apoptotic marker, the cleaved form of Poly ADP-ribose Polymerase (PARP) (Figure 2B).
Figure 2. In vitro growth properties in C8161 siGRM1 human melanoma cells.
(A.) MTT cell viability/proliferation assays showing decreased viability and proliferation in C8161 human melanoma cells with suppressed GRM1 expression by the ecdysone regulated system in the presence of 10 μM PonA. (B.) C8161 pVgRXR siGRM1 cells treated with 10 μM PonA showing increased cleavage of PARP an indicator of apoptosis but not in control C8161 pVgRXR cells. Equal loading was shown by levels of α-Tubulin. NT - No treatment, Veh - Vehicle- DMSO, PonA - Ponasterone A.
MAPK signaling suppression by GRM1 siRNA
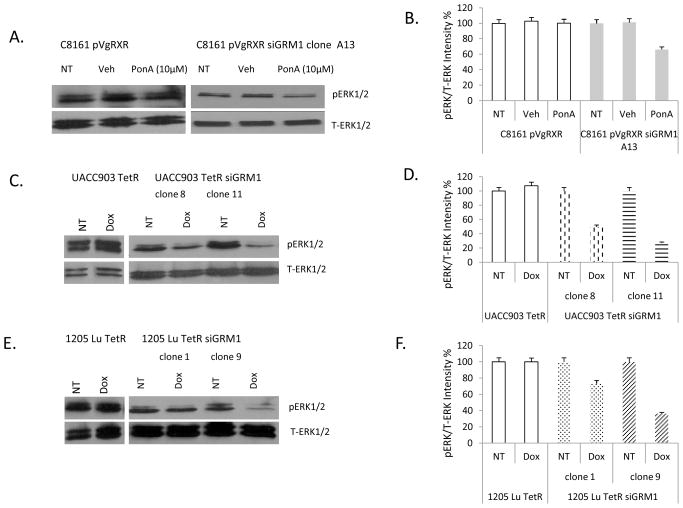
Possible alterations in MAPK signaling cascade by suppression of GRM1 in human melanoma cells were assessed. Treatment of C8161 pVgRXR siGRM1 A13 with the inducer, PonA, resulted in a decrease in the levels of activated/phosphorylated ERK1/2 in comparison to vector control C8161 pVgRXR (Figure 3A). Quantification of the intensities of phosphorylated form of ERK1/2 and normalized to the no treatment or vehicle controls, showed a 40% decrease in levels of activated ERK1/2 in C8161 pVgRXR siGRM1 A13 lysates (Figure 3B). Using the doxycycline inducible system, we also observed a reduction in ERK1/2 phosphorylation in UACC903 TetR siGRM1 clones induced with 10 ng/ml doxycycline, which was not observed in UACC903 TetR cells under similar conditions (Figure 3C). Quantitation of the western blots comparing phosphorylated ERK1/2 to total ERK1/2 showed a ~50% and ~75% reduction in intensity in UACC903 TetR siGRM1 clones 8 and 11 respectively (Figure 3D). In 1205 Lu TetR siGRM1 clones, we also observed a reduction in phosphorylated ERK1/2 with clone 9 showing greater suppression than clone 1(Figure 3E). Quantitation of the western blots comparing phosphorylated ERK1/2 to total ERK1/2 showed a ~27% and ~64% reduction in intensity in 1205 Lu TetR siGRM1 clones 1 and 9 respectively (Figure 3F).
Figure 3. Modulation of MAPK signaling with GRM1 siRNA.
(A.) Western immunoblots of protein lysates prepared from C8161 pVgRXR or C8161 pVgRXR siGRM1-A13 cells, not treated (NT) or treated with vehicle (DMSO) or with PonA and probed for phosphorylated (pERK1/2) and total ERK1/2 (T-ERK1/2). (B) Quantification of the intensities on the immunoblots from A. with Optiquant software with NT or Veh set as 100%. (C.) Suppression of ERK1/2 phosphorylation in UACC903 TetR siGRM1 clones but not in control UACC903 TetR after doxycycline (10 ng/ml) treatment. Equal loading was assessed with levels of total ERK1/2. (D.) Quantification of the intensities on the immunoblots from C. with Optiquant software. (E.) Suppression of ERK1/2 phosphorylation in 1205 Lu TetR siGRM1 clones and not in 1205 Lu TetR controls after doxycycline (10 ng/ml treatment). Equal loading was shown by levels of total ERK1/2. NT- No treatment, Dox - doxycycline. (F.) Quantification of the intensities on the immunoblots from E. with Optiquant software.
Inhibition of AKT phosphorylation by GRM1 siRNA
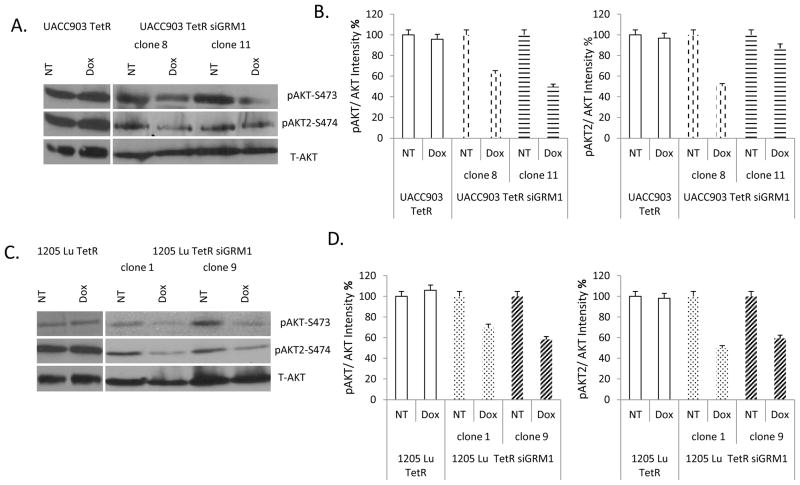
We have previously shown that the PI3K/AKT pathway functions downstream of Grm1 and also identified the Akt2 isoform to be the mediator of Akt phosphorylation in our system (20). Here, we investigated whether there are changes in AKT phosphorylation with siRNA-mediated suppression of GRM1 expression. Our results indicate that suppression of GRM1 expression by siRNA can inhibit the phosphorylation and activation of AKT in UACC903 human melanoma cells (Figure 4A). Similar results were observed in siGRM1-1205 Lu human melanoma cell lines (Figure 4C). Furthermore, we showed that AKT phosphorylation is mediated by the AKT2 isoform (Figures 4A and C). Quantitation of the western blots comparing phosphorylated AKT to total AKT showed a ~40% and ~50% reduction in intensity in UACC903 TetR siGRM1 clones 8 and 11 respectively (Figure 4B, left panel) while 1205 Lu TetR siGRM1 clones 1 and 9 showed a ~30% and ~40% decrease in clones 1 and 9 respectively (Figure 4D, left panel). Comparison of AKT2 phosphorylation normalized to total AKT showed a ~50 % and ~15% reduction in UACC903 TetR siGRM1 clones 8 and 11 respectively (Figure 4B, right panel) while 1205 Lu TetR siGRM1 clones 1 and 9 had a ~50% and ~40% decrease respectively (Figure 4D, right panel).
Figure 4. Modulation of PI3K/AKT signaling by GRM1 siRNA.
(A.) Suppression of total AKT (Serine 473) and AKT2 phosphorylation in UACC903 TetR siGRM1 clones 8 and 11, but not in UACC903 TetR after doxycycline (10 ng/ml) treatment. (B.) Quantification of the intensities on the immunoblots from A. with Optiquant software. (C.) Suppression of AKT (Serine 473) and AKT2 phosphorylation in 1205 Lu TetR siGRM1 clones and not in 1205 Lu TetR controls after doxycycline (10 ng/ml) treatment. (D.) Quantification of the intensities on the immunoblots from C. with Optiquant software. Total AKT was used to show equal loading. NT- No treatment, Dox -doxycycline.
Suppression of GRM1 expression by siRNA reduces in vivo tumorigenicity
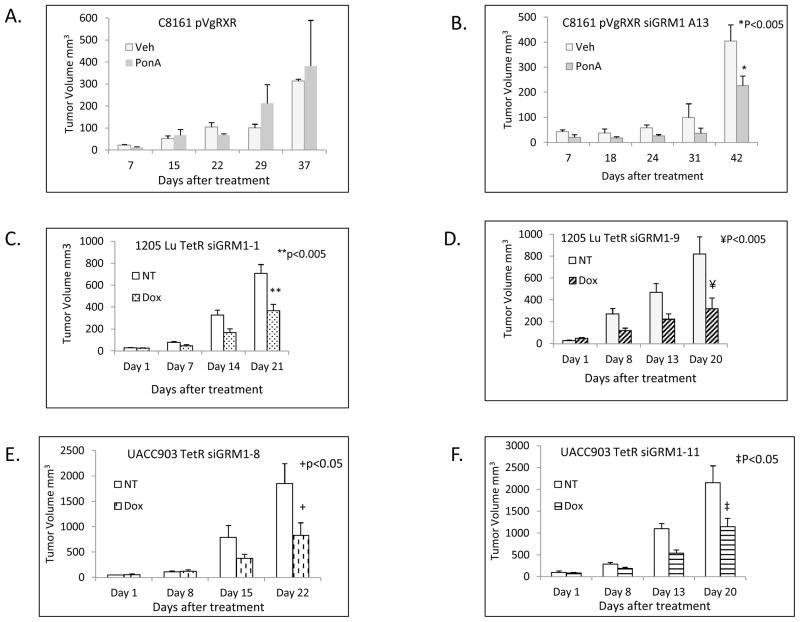
Next, we performed in vivo xenograft experiments with vector control C8161 pVgRXR and C8161 pVgRXR siGRM1 A13 cells (Figures 5A and B). The cells were inoculated into the dorsal flanks of nude mice and when the tumor volumes reached ~10–20 mm3, the mice were randomly divided into two groups, either treated with vehicle (olive oil) or 10 mg/kg of PonA in olive oil by intraperitoneal injections bi-weekly as described (16, 21). We showed that in the C8161 pVgRXR siGRM1 A13 xenografts treated with PonA, the tumors appeared to progress at a slower rate compared to those treated with the vehicle and resulted in ~45% (p<0.005) decrease in tumor volume. In contrast, in the control C8161 pVgRXR, there was no suppression of tumor growth when treated with PonA; in fact we observed slightly faster growth in comparison to vehicle treated ones (Figures 5A and B). We also performed xenograft studies with cells harboring tetracycline inducible siRNA. 1205 Lu TetR siGRM1 clones 1 and 9 or UACC903 TetR clones 8 and 11. Mice were randomly divided into no treatment and treatment groups when the tumor volumes reached ~10–20 mm3. A 0.1% doxycycline 1% sucrose w/v solution was administered in the drinking water in the Dox treatment groups. The data indicates that suppression of GRM1 in 1205 Lu cells siGRM1 cells treated with doxycycline resulted in ~50% (p<0.005) and ~60% (p<0.005) reduction in tumor volumes in clones 1 and 9 respectively compared to those in the no treatment control groups (Figures 5C and D). Similarly, in UACC903 TetR siGRM1 cells, inhibition of GRM1 expression resulted in ~56% (p<0.05) and ~47% (p<0.05) reduction in tumor growth in vivo in clones 8 and 11 respectively (Figures 5E and F).
Figure 5. In vivo xenograft tumorigenicity assays.
(A.) In vivo tumorigenicity assay of C8161 pVgRXR cells or (B.) C8161 pVgRXR siGRM1 A13 cells after treatment with vehicle (olive oil) or PonA (10 mg/kg) administered via intraperitoneal injection. All tumor bearing mice were euthanized after 37–42 days due to tumor burden in the control groups. *p<0.005 when PonA treated C8161 pVgRXR siGRM1 A13 tumor volumes were compared to vehicle treated ones. (C and D.) In vivo tumorigenicity assays of 1205 Lu TetR siGRM1 clone 1 (C) and clone 9 (D) No treatment (NT) or doxycycline treated (0.1% w/v) (Dox). * p<0.005 when 1205 Lu TetR siGRM1-9 Dox treated tumor volumes were compared to the not treated controls at day 21. ** p<0.005 when 1205 Lu TetR siGRM1-1 Dox treated tumor volumes were compared to the not treated controls at day 20. (E and F.) In vivo tumorigenicity assays of UACC903 TetR siGRM1 clone 8 (E) and clone 11 (F). No treatment (NT) and doxycycline treated (0.1% w/v) (Dox). * p<0.05 when UACC903 TetR siGRM1-8 Dox treated tumor volumes were compared to the not treated controls at day 22. ** p<0.05 when UACC903 TetR siGRM1-11 Dox treated tumor volumes were compared to the not treated controls at day 20.
Increased Caspase-3 cleavage and a decrease in Ki-67 with GRM1 suppression
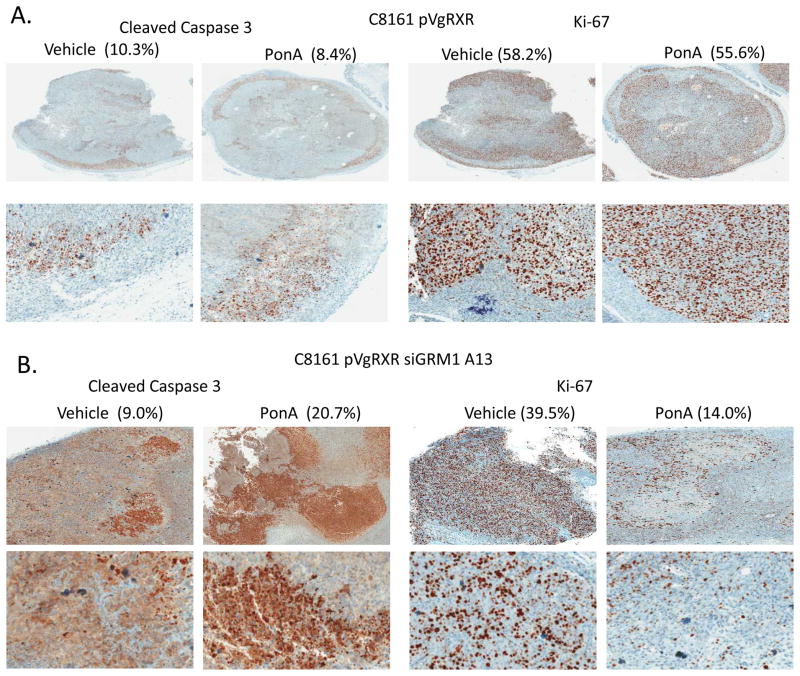
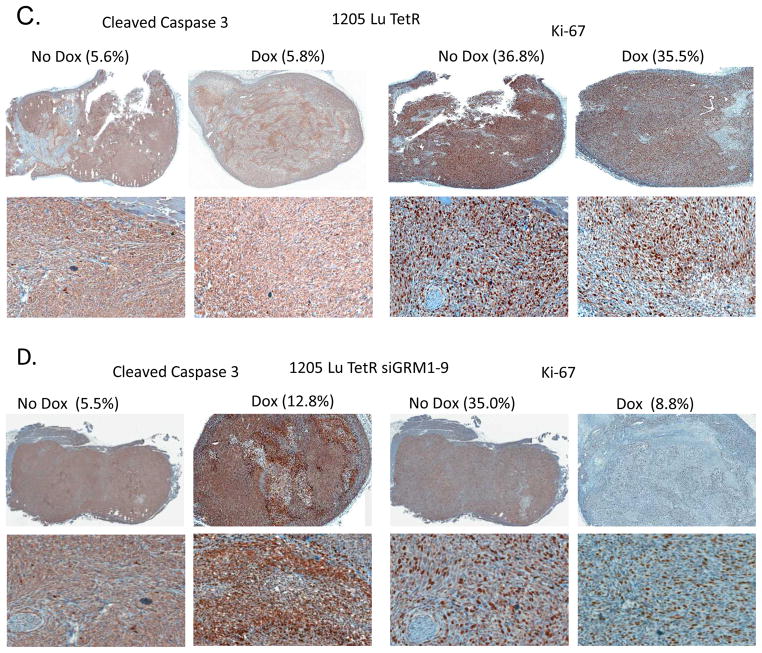
Tumors excised from C8161 pVgRXR, C8161 pVgRXR siGRM1 A13 PonA or vehicle treated samples and 1205 Lu TetR and 1205 Lu TetR siGRM1-9 doxycycline treated or no treatment controls were analyzed by immuno- histochemistry for the expression of the apoptosis marker, cleaved Caspase-3 and the proliferation marker, Ki-67. C8161 pVgRXR derived tumors did not show any significant increase in cleaved Caspace-3 or a decrease in Ki-67 (Figure 6A). C8161 pVgRXR siGRM1 A13 derived tumors treated with PonA exhibited a statistically significant increase in cells positive for cleaved Caspase-3 over vehicle treated samples, p<0.05 (Figure 6B, left panel). Analysis of the same samples showed a statistically significant decrease in Ki-67 expression in the PonA treated samples over vehicle treated samples, p<0.001 (Figure 6B, right panel). Using the doxycycline regulated siRNA system, no differences were observed in cleaved Caspase-3 and Ki-67 in the 1205 Lu TetR vector controls after doxycycline treatment (Figure 6C). In the 1205 Lu TetR siGRM1-9 derived tumors, treatment with doxycycline led to a statistically significant increase in cells positive for cleaved Caspase-3 over the no treated controls with p<0.05 (Figure 6D, left panel). Meanwhile, a statistically significant decrease in Ki-67 cells was detected in the doxycycline treated 1205 Lu TetR siGRM1-9 tumors than in the no treatment controls with p<0.001 (Figure 6D, right panel). These results showed that in vivo, suppression of GRM1 expression in these human melanoma cells has both anti-proliferative and pro-apoptotic effects.
Figure 6. Immunohistochemical (IHC) analysis.
IHC performed on excised tumor xenografts from (A) C8161 pVgRXR, (B) C8161 pVgRXR siGRM1 A13 PonA and vehicle treated controls (C) 1205 Lu TetR and (D) 1205 Lu TetR siGRM1-9 no treatment or Dox treated for cleaved Caspase-3 and Ki-67. Top panels, 10X magnification, bottom panels 100X magnifications of a section of the stained tumors. The numbers of stained cells were quantified with a digital Aperio ScanScopeGL system and ImageScope software and are expressed as percentages of the total number of cells counted. When vehicle treated samples are compared with PonA treated samples; cleaved Caspase-3, p<0.05 and Ki-67, p<0.001. When no Dox samples are compared with Dox treated samples; cleaved Caspase-3, p<0.05 and Ki-67, p<0.001.
Discussion
We have previously reported that targeted Grm1 expression to melanocytes was sufficient to induce spontaneous melanoma development in vivo; subsequently we also detected GRM1 expression in a subset of human melanoma cell lines and biopsies suggesting GRM1 may be involved in melanomagenesis (2, 3, 6). Additionally, two other metabotropic glutamate receptors have recently been documented to be involved in melanoma pathogenesis. Over-expression of Grm5 (12) and mutations in GRM3 (13) make this particular group of receptors and glutamate signaling attractive candidates for understanding the onset and progression of this deadly disease.
Gene silencing by short-interfering RNAs (siRNAs) (22) has been used to silence the expression of specific genes to study their roles in different cell types and in various organisms. Inducible gene expression systems have also been developed to regulate expression in a temporal and quantitative manner to aid in the study of gene function (21). These regulatable expression systems rely on small molecules that serve as inducers to modify synthetic transcription factors which regulate the expression of a target gene (21). The tetracycline operon based tetracycline inducible system (7, 23, 24), and the non-mammalian steroid based ecdysone inducible system (15, 16, 25), have been shown to be both reversible and efficient in regulating the expression of various mammalian genes. These inducible systems are especially critically important when siRNAs are utilized in inhibiting expression of genes crucial for cell or organism survival as silencing occurs only in the presence of the inducer (14, 26). We and others have shown that the sustained expression of Grm1 in mouse melanocytes is required for the maintenance of transformed phenotypes in vitro and tumor progression in vivo (7, 8). In our attempts to inhibit GRM1 expression in human melanoma cells, we have previously employed constitutively expressed GRM1 specific siRNAs with unfavorable consequence as the GRM1 knock-out cells exhibited a dormancy- like state before dying (unpublished observations). We selected the ecdysone/Ponasterone A and the tetracycline inducible gene expression systems to induce siRNA expression targeted to GRM1 in human melanoma cell lines.
It has been shown that GRM1 activation is coupled to the Extracellular-Signal-Regulated Kinase (ERK1/2), a component of the classical MAPK pathway through G-protein and kinase dependent mechanisms in neuronal cells (27). Activation of ERK1/2 by phosphorylation on tyrosine and threonine residues has a pivotal role in intracellular signaling and can mediate cellular processes such as cell proliferation, invasion, metastasis, survival angiogenesis and apoptosis (28, 29). Our group has demonstrated that similar to the neuronal system, stimulation of GRM1 by its agonist, L-Quisqualate leads to the activation of ERK1/2 in murine or human melanoma cells (6, 7, 30). The specificity of GRM1-mediated activation of ERK1/2 was demonstrated by pre-incubation of the cells with GRM1-antagonists; Bay 36-7620 or LY-367385 which abolished ERK1/2 phosphorylation (6, 7, 30). Activated MAPK pathway has also been detected with other metabotropic glutamate receptors in melanoma. The over-expression of Grm5 in mouse melanocytes was found to result in ERK1/2 activation (12) while mutations in GRM3 were found to activate MEK1/2 (13) indicating that the MAPK pathway has a fundamental role in melanomas linked to glutamate receptors. Stimulation of the MAPK pathway is indeed a classical event in melanoma due to mutations in BRAF and RAS (31, 32). In this report, our results indicate that suppression of GRM1 expression in human melanoma cells results in reduced activation of the MAPK pathway. These results suggest that in melanoma cells, MAPK activation can also result from upstream receptor mediated activation events such as the activation of G-protein coupled receptors including GRM1.
In addition to the suppressed MAPK pathway stimulation observed, we showed that suppressed GRM1 expression by siRNA leads to inhibition of another major signaling cascade, PI3/AKT. The PI3K/AKT pathway plays critical roles in cell survival, proliferation and anti-apoptosis through PTEN loss or aberrant AKT activation (20, 33, 34). Our earlier work in mouse melanocytes showed that Grm1 activation by its agonist L-Quisqualate could result in AKT activation and phosphorylation. The specificity of this activation was confirmed with the Grm1 specific antagonist Bay-36-7620 which blocked the activation and phosphorylation of AKT (20). Moreover, we showed that the AKT2 isoform mediated this activation (20). Here, we showed that a decrease in GRM1 expression by siRNA resulted in suppression of AKT phosphorylation and distinctively, the phosphorylation of AKT2. This suppression of AKT2 phosphorylation has significant implications as it has been shown to have pro-invasive properties in melanoma cells (35).
Despite the lack of specific GRM1 antagonists with clinical applications, our group has used Rilutek® (riluzole), an FDA approved drug for the treatment of Amyotropic Lateral Sclerosis (ALS) (36, 37) in clinical trials for melanoma patients (38, 39). Riluzole inhibits the release of glutamate from intracellular to extracellular environments (40, 41). GPCRs with oncogenic activity are know to create autocrine/paracrine loops to maintain receptor activation (42–44). Inhibition of glutamate release by riluzole in GRM1-expressing cells disrupts glutamate receptor signaling and thus functions as an antagonist to the receptor (6). Results from our Phase 0 and II trials strongly suggest that riluzole monotherapy has modest antitumor activity (38, 39), however, combining it with other anti-cancer agents may lead to additive or synergistic responses as shown in our pre-clinical studies (45). Based on these results perhaps it is not surprising that modulation of GRM1 expression results in concurrent suppression of activities of two major signaling pathways known to be critical in melanomagenesis.
Acknowledgments
Grant support: NIH GM66338 (JWT), R01CA124975-02S1 (JWT), New Jersey Commission for Cancer Research 09-1143-CCR-E0 (SC), NIH R01CA74077 (SC), NIH R01CA124975 (JSG).
Footnotes
Conflict of interest: None disclosed
References
- 1.American Cancer Society. Cancer facts & figures. Atlanta: American Cancer Society; 2012. [Google Scholar]
- 2.Zhu H, Reuhl K, Botha R, Ryan K, Wei J, Chen S. Development of early melanocytic lesions in transgenic mice predisposed to melanoma. Pigment Cell Res. 2000;13:158–64. doi: 10.1034/j.1600-0749.2000.130307.x. [DOI] [PubMed] [Google Scholar]
- 3.Pollock PM, Cohen-Solal K, Sood R, et al. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat Genet. 2003;34:108–12. doi: 10.1038/ng1148. [DOI] [PubMed] [Google Scholar]
- 4.Conn PJ, Pin J-P. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–37. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 5.Funusaka Y, Harada T, Aiba A, Nishigori C. Expression of metabotropic glutamate receptor 1 and phosphorylated extracellular signal-regulated kinase 1/2 proteins in human melanocytic lesions. Pigm Cell Res. 2006;19:256. [Google Scholar]
- 6.Namkoong J, Shin SS, Lee HJ, et al. Metabotropic glutamate receptor 1 and glutamate signaling in human melanoma. Cancer Res. 2007;67:2298–305. doi: 10.1158/0008-5472.CAN-06-3665. [DOI] [PubMed] [Google Scholar]
- 7.Shin SS, Namkoong J, Wall BA, Gleason R, Lee HJ, Chen S. Oncogenic activities of metabotropic glutamate receptor 1 (Grm1) in melanocyte transformation. Pigment Cell Melanoma Res. 2008;21:368–78. doi: 10.1111/j.1755-148X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohtani Y, Harada T, Funasaka Y, et al. Metabotropic glutamate receptor subtype-1 is essential for in vivo growth of melanoma. Oncogene. 2008;27:7162–70. doi: 10.1038/onc.2008.329. [DOI] [PubMed] [Google Scholar]
- 9.Tanabe Y, Masu M, Ishii T, Shigemoto R, Nakanishi S. A family of metabotropic glutamate receptors. Neuron. 1992;8:169–79. doi: 10.1016/0896-6273(92)90118-w. [DOI] [PubMed] [Google Scholar]
- 10.Houamed KM, Kuijper JL, Gilbert TL, et al. Cloning, expression, and gene structure of a G protein-coupled glutamate receptor from rat brain. Science. 1991;252:1318–21. doi: 10.1126/science.1656524. [DOI] [PubMed] [Google Scholar]
- 11.Masu M, Tanabe Y, Tsuchida K, Shigemoto R, Nakanishi S. Sequence and expression of a metabotropic glutamate receptor. Nature. 1991;349:760–5. doi: 10.1038/349760a0. [DOI] [PubMed] [Google Scholar]
- 12.Choi KY, Chang K, Pickel JM, Badger JD, 2nd, Roche KW. Expression of the metabotropic glutamate receptor 5 (mGluR5) induces melanoma in transgenic mice. Proc Natl Acad Sci. 2011;108:15219–24. doi: 10.1073/pnas.1107304108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prickett TD, Wei X, Cardenas-Navia I, et al. Exon capture analysis of G protein-coupled receptors identifies activating mutations in GRM3 in melanoma. Nat Genet. 2011;43:1119–26. doi: 10.1038/ng.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rangasamy D, Tremethick DJ, Greaves IK. Gene knockdown by ecdysone-based inducible RNAi in stable mammalian cell lines. Nat Protoc. 2008;3:79–88. doi: 10.1038/nprot.2007.456. [DOI] [PubMed] [Google Scholar]
- 15.No D, Yao TP, Evans RM. Ecdysone-inducible gene expression in mammalian cells and transgenic mice. Proc Natl Acad Sci U S A. 1996;93:3346–51. doi: 10.1073/pnas.93.8.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dang DT, Chen X, Feng J, Torbenson M, Dang LH, Yang VW. Overexpression of Kruppel-like factor 4 in the human colon cancer cell line RKO leads to reduced tumorigenecity. Oncogene. 2003;22:3424–30. doi: 10.1038/sj.onc.1206413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stepulak A, Sifringer M, Rzeski W, et al. NMDA antagonist inhibits the extracellular signal-regulated kinase pathway and suppresses cancer growth. Proc Natl Acad Sci U S A. 2005;102:15605–10. doi: 10.1073/pnas.0507679102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu TY, Saw CL, Khor TO, Pung D, Boyanapalli SS, Kong AN. In vivo pharmacodynamics of indole-3-carbinol in the inhibition of prostate cancer in transgenic adenocarcinoma of mouse prostate (TRAMP) mice: Involvement of Nrf2 and cell cycle/apoptosis signaling pathways. Mol Carcinog. 2011 doi: 10.1002/mc.20841. [DOI] [PubMed] [Google Scholar]
- 19.Le MN, Chan JL, Rosenberg SA, et al. The glutamate release inhibitor Riluzole decreases migration, invasion, and proliferation of melanoma cells. J Invest Dermatol. 2010;130:2240–9. doi: 10.1038/jid.2010.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shin SS, Wall BA, Goydos JS, Chen S. AKT2 is a downstream target of metabotropic glutamate receptor 1 (Grm1) Pigment Cell Melanoma Res. 2009;23:103–11. doi: 10.1111/j.1755-148X.2009.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saez E, Nelson MC, Eshelman B, et al. Identification of ligands and coligands for the ecdysone-regulated gene switch. Proc Natl Acad Sci. 2000;97:14512–7. doi: 10.1073/pnas.260499497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–11. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 23.Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–9. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- 24.Ohkawa J, Taira K. Control of the functional activity of an antisense RNA by a tetracycline-responsive derivative of the human U6 snRNA promoter. Hum Gene Ther. 2000;11:577–85. doi: 10.1089/10430340050015761. [DOI] [PubMed] [Google Scholar]
- 25.Downey PM, Lozza G, Petro R, et al. Ecdysone-based system for controlled inducible expression of metabotropic glutamate receptor subtypes 2, 5, and 8. J Biomol Screen. 2005;10:841–8. doi: 10.1177/1087057105280285. [DOI] [PubMed] [Google Scholar]
- 26.Wiznerowicz M, Szulc J, Trono D. Tuning silence: conditional systems for RNA interference. Nat Methods. 2006;3:682–8. doi: 10.1038/nmeth914. [DOI] [PubMed] [Google Scholar]
- 27.Thandi S, Blank JL, Challiss RA. Group-I metabotropic glutamate receptors, mGlu1a and mGlu5a, couple to extracellular signal-regulated kinase (ERK) activation via distinct, but overlapping, signalling pathways. Journal of Neurochemistry. 2002;83:1139–53. doi: 10.1046/j.1471-4159.2002.01217.x. [DOI] [PubMed] [Google Scholar]
- 28.Inamdar GS, Madhunapantula SV, Robertson GP. Targeting the MAPK pathway in melanoma: why some approaches succeed and other fail. Biochem Pharmacol. 2010;80:624–37. doi: 10.1016/j.bcp.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pearson G, Robinson F, Beers Gibson T, et al. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–83. doi: 10.1210/edrv.22.2.0428. [DOI] [PubMed] [Google Scholar]
- 30.Marin YE, Namkoong J, Cohen-Solal K, et al. Stimulation of oncogenic metabotropic glutamate receptor 1 in melanoma cells activates ERK1/2 via PKCepsilon. Cell Signal. 2006;18:1279–86. doi: 10.1016/j.cellsig.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 31.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54. doi: 10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 32.Marquette A, Bagot M, Bensussan A, Dumaz N. Recent discoveries in the genetics of melanoma and their therapeutic implications. Arch Immunol Ther Exp (Warsz) 2007;55:363–72. doi: 10.1007/s00005-007-0043-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parmiter AH, Nowell PC. The cytogenetics of human malignant melanoma and premalignant lesions. Cancer Treat Res. 1988;43:47–61. doi: 10.1007/978-1-4613-1751-7_3. [DOI] [PubMed] [Google Scholar]
- 34.Tsao H, Goel V, Wu H, Yang G, Haluska FG. Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. J Invest Dermatol. 2004;122:337–41. doi: 10.1046/j.0022-202X.2004.22243.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nogueira C, Kim KH, Sung H, et al. Cooperative interactions of PTEN deficiency and RAS activation in melanoma metastasis. Oncogene. 2010;29:6222–32. doi: 10.1038/onc.2010.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bensimon G, Lacomblez L, Delumeau JC, et al. A study of riluzole in the treatment of advanced stage or elderly patients with amyotrophic lateral sclerosis. Journal of Neurology. 2002;249:609–15. doi: 10.1007/s004150200071. [DOI] [PubMed] [Google Scholar]
- 37.Lacomblez L, Bensimon G, Leigh PN, et al. Long-term safety of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis & Other Motor Neuron Disorders. 2002;3:23–9. doi: 10.1080/146608202317576507. [DOI] [PubMed] [Google Scholar]
- 38.Yip D, Le MN, Chan JL, et al. A phase 0 trial of riluzole in patients with resectable stage III and IV melanoma. Clin Cancer Res. 2009;15:3896–902. doi: 10.1158/1078-0432.CCR-08-3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehnert J, Wen Y, Lee J, et al. A phase II trial of riluzole, an antagonist of metabotropic glutamate receptor (GRM1) signaling, in advanced melanoma. J Clin Oncol. 2010;28:15. doi: 10.1111/pcmr.12694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kretschmer BD, Kratzer U, Schmidt WJ. Riluzole, a glutamate release inhibitor, and motor behavior. Naunyn Schmiedebergs Arch Pharmacol. 1998;358:181–90. doi: 10.1007/pl00005241. [DOI] [PubMed] [Google Scholar]
- 41.Doble A. The pharmacology and mechanism of action of riluzole. Neurology. 1996;47:S233–41. doi: 10.1212/wnl.47.6_suppl_4.233s. [DOI] [PubMed] [Google Scholar]
- 42.Heasley LE. Autocrine and paracrine signaling through neuropeptide receptors in human cancer. Oncogene. 2001;20:1563–9. doi: 10.1038/sj.onc.1204183. [DOI] [PubMed] [Google Scholar]
- 43.Cuttitta F, Carney DN, Mulshine J, et al. Bombesin-like peptides can function as autocrine growth factors in human small-cell lung cancer. Nature. 1985;316:823–6. doi: 10.1038/316823a0. [DOI] [PubMed] [Google Scholar]
- 44.Dorsam RT, Gutkind JS. G-protein-coupled receptors and cancer. Nat Rev Cancer. 2007;7:79–94. doi: 10.1038/nrc2069. [DOI] [PubMed] [Google Scholar]
- 45.Lee HJ, Wall BA, Wangari-Talbot J, et al. Glutamatergic Pathway Targeting in Melanoma: Single-Agent and Combinatorial Therapies. Clin Cancer Res. 2011;17:7080–92. doi: 10.1158/1078-0432.CCR-11-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]