Abstract
Introduction
Epithelial Na+ Channels (ENaC) play a crucial role in ion and fluid regulation in the lung. In cystic fibrosis (CF) Na+ hyperabsorption results from ENaC over activity, leading to airway dehydration. Previous work has demonstrated functional genetic variation of SCNN1A (the gene encoding the ENaC α-subunit), manifesting as an alanine (A) to threonine (T) substitution at amino acid 663, with the αT663 variant resulting in a more active channel.
Methods
We assessed the influence of genetic variation of SCNN1A on the diffusing capacity of the lungs for carbon monoxide (DLCO) and nitric oxide (DLNO), together with alveolar capillary membrane conductance (DM), pulmonary capillary blood volume (VC), and alveolar volume (VA) at rest and during peak exercise in 18 patients with CF [10 homozygous for αA663 (AA group) and 8 with at least one T663 allele (AT/TT group)]. Due to the more active channel we hypothesized that the AT/TT group would show a greater increase in DLCO, DLNO, and DM with exercise due to exercise-mediated ENaC inhibition and subsequent attenuation of Na+ hyperabsorption.
Results
The AT/TT group had significantly lower pulmonary function, weight and BMI than the AA group. Both groups had similar peak workloads, relative peak oxygen consumptions, and cardiopulmonary responses to exercise. The AT/TT group demonstrated a greater increase in DLNO, DLNO/VA, and DM in response to exercise (% increases: DLNO= 18±11vs.41±38; DLNO/VA= 14±21vs.40±37; DM= 15±11vs.41±38, AAvs.AT/TT, respectively). There were no differences between groups in absolute diffusing capacity measures at peak exercise.
Conclusion
These results suggest that genetic variation of the alpha-subunit of ENaC differentially affects the diffusing capacity response to exercise in patients with CF.
Keywords: exercise, cystic fibrosis, diffusing capacity, DLNO, DLCO, ENaC polymorphism
Introduction
Cystic fibrosis (CF) is an autosomal recessive disorder caused by mutations in the gene encoding the cystic fibrosis transmembrane conductance regulator (CFTR) which affects 1 in 3419 white individuals and 1 in 12,163 non-white individuals (21). Cystic fibrosis transmembrane conductance regulator helps to maintain airway surface fluid depth through secretion of Cl-; the impairment in CFTR results in a depletion of the airway surface fluid. There are several mutations which result in CF, with the ΔF508 mutation being the most common (13). The ΔF508 mutation is a deletion of phenylalanine at amino acid 508 which causes defective trafficking of CFTR and results in the inability of CFTR to be inserted into the apical membrane of the cell (25).
Extravascular lung water has been estimated to be 3.6mL/Kg in healthy individuals, and mantinence of extravascular lung water is important for proper gas diffusion to occur (37). The diffusing capacity of the lung is reduced in disease states like CF where the lungs are dry, whereas if extravascular lung water increases, like during rapid saline loading or in heart failure, the diffusing capacity of the lung is also reduced(28, 30, 39). Airway surface fluid depth is of vital importance in pulmonary defense and function by aiding in mucus clearance in the airways. In the alveoli, the maintenance of optimal airway surface fluid depth is also essential in gas diffusion across the respiratory membrane (12). Increases in surface fluid depth are mediated primarily by Cl- secretion by CFTR, but also occur through Cl- secretion by Ca2+-dependent Cl- channels (CaCC) (5, 8). Surface fluid depth is decreased primarily through Na+ reabsorption by the epithelial Na+ channel (ENaC) (9, 11). Sodium is brought into the epithelial cell by ENaC where it is subsequently moved across the basolateral membrane by Na+/K+ ATPase into the interstitial space. Fluid follows through tight junctions and aquaporin channels where it is then cleared by the lymphatic system. Not only is Cl- secretion impaired in CF, but Na+ absorption via ENaC is dramatically increased due to the loss of CFTR mediated ENaC inhibition, potentiating the decreased surface liquid volume seen in CF, as well as impairing mucocilliary clearance, leading to airway obstruction, inflammation, recurrent infections, and decreasing the diffusing capacity of gases across the respiratory membrane (39).
Previous work has shown that exercise increases the diffusing capacity of gases in the lungs of both healthy individuals and those with CF (39). Individuals with CF demonstrated lower diffusing capacity of the lungs at rest and during exercise as compared to healthy individuals (39). Exercise can activate ENaC through the adrenergic pathway but can also inhibit ENaC through the purinergic pathway, which is activated by turbulent air flow in the airways during exercise (1). Several functional polymorphisms in the gene encoding ENaC (SCNN1A) have been reported, including a common variant resulting in an alanine (A) to threonine (T) substitution at amino acid 663 in the C-terminus of the alpha subunit. The more common variant (αA663) demonstrated lower channel activity as compared to the αT663 variant due to decreased ENaC expression (27, 35). In humans, the αT663 variant has been associated with hypertension, suggesting its increased channel activity resulted in increased Na+ reabsorption in the kidneys (2). Genetic variation of SCNN1A has also previously been shown to influence the diffusing capacity of the lungs in response to β2-adrenergic stimulation (3). Healthy individuals with the αA663 ENaC variant demonstrated a greater increase in lung diffusing capacity in response to exercise as compared to those with at least one copy of the αT663 variant (3). Additionally, in response to albuterol, an exogenous β2-receptor agonist, individuals with the αA663 ENaC variant had less Na+ in their exhaled breath condensate 90 minutes after beta2-agonist administration as compared to those with the αT663 ENaC variant, suggesting a greater removal of Na+ from the airways following activation (14).
In this study, we sought to determine the influence of genetic variation at amino acid 663 of the alpha-subunit of ENaC on diffusing capacity of the lungs at rest and during exercise in patients with CF. Exercise was utilized as a means to endogenously alter ion and fluid regulation in the lung. We predicted that patients with the αT663 ENaC variant, which has shown greater basal activity in previous work (27, 35), would show a greater increase in diffusing capacity with exercise, due to a larger attenuation of Na+ reabsorption, and subsequent hydration of the airways.
Methods
Subjects
Eighteen subjects with mild to moderate CF, with a positive sweat test (≥60mmol/L) and at least one ΔF508 allele, were recruited for study through the Arizona Respiratory Center and its affiliated CF clinic at the University of Arizona Medical Center. To ensure subjects were clinically stable to participate in the study exclusion criteria included: FEV1 ≤ 40% predicted, experimental CF drugs, pulmonary exacerbation within the last six months resulting in ≥50cc of blood in the sputum, antibiotics for pulmonary exacerbation, pregnancy, smoking, inability to exercise, or cardiovascular abnormalities. The protocol was reviewed and approved by the University of Arizona Institutional Review Board, and all subjects provided written informed consent before participation in the study. All aspects of the study were performed according to the declaration of Helinski.
Protocol
Upon arrival to the laboratory in a two hour fasted state, subjects were consented and a buccal swab was performed for assessment of the amino acid at position 663 of αENaC and verification of ΔF508 mutation on CFTR. A venous blood sample was drawn for the assessment of hemoglobin concentration. Subjects were outfitted with a 12-lead electrocardiogram (Marquette electronics, Milwaukee, WI) to monitor heart rhythms, and pulmonary function tests were completed according to American Thoracic Society standards (23). Predicted values for pulmonary function tests were calculated according to equations from NHANES III (17). Baseline simultaneous measurements of the diffusing capacity of the lungs for carbon monoxide (DLCO) and the diffusing capacity of the lungs for nitric oxide (DLNO) were taken in triplicate. Baseline peripheral oxygen saturation (SaO2) was assessed via pulse oximetry with a finger sensor (Nellcor N-600 Pulse Oximeter, Boulder, CO). Subjects then completed a subject-specific maximal exercise capacity test on a cycle ergometer (Corival Lode B.V., The Netherlands) based on their body size, reported type, speed and intensity of exercise training, and predicted V̇O2 (18). DLCO and DLNO were taken during each stage of exercise and recovery. Subjects exercised at an initial workload that ranged from 15-40 watts (mean initial workload was 24±6 watts) and the workload was increased by this same amount every three minutes until exhaustion (ie. initial workload of 25 watts with a 25 watt increase in workload every three minutes). The exercise test ended with a three-minute recovery period at the initial workload. Exhaustion was determined by an inability to maintain a pedal rate of 60-80 revolutions per minute, a rating of perceived exertion (RPE) of at least 18 out of 20, or a respiratory exchange ratio (RER) greater than or equal to 1.15.
Oxygen consumption (V̇O2), carbon dioxide production (V̇)CO2), respiratory rate (RR), tidal volume (TV), minute ventilation (VE), heart rate (HR), and oxygen saturation (SaO2) were monitored continuously and averaged every three seconds throughout the test via a metabolic cart (Medical Graphics CPX/D; St. Paul, MN) interfaced with a Perkin Elmer MGA-1100 mass spectrometer (Wesley, MA) as described previously (30, 31). Accurate SaO2 values were ensured by instructing the subjects to maintain a relaxed grip and verifying that there were no discrepancies in HR between the pulse oximeter HR and that on the electrocardiogram.
Measurement of cardiac output, alveolar-capillary membrane conductance, and pulmonary capillary blood volume
Cardiac output, pulmonary capillary blood volume (VC) and alveolar-capillary membrane conductance (DM) were determined using a rebreathe technique by measuring the disappearance of acetylene, carbon monoxide (CO), and nitric oxide (NO) with respect to helium, as previously described (16, 30-33). Triplicate maneuvers of cardiac output (Q), the diffusing capacity of the lungs for carbon monoxide (DLCO), and nitric oxide (DLNO) were performed at baseline and during recovery. Single maneuvers of Q, DLCO, and DLNO were performed during each stage of exercise.
Measures of Q, DLCO, and DLNO were taken in an upright and seated position on the cycle ergometer using a rebreathing technique, with gases sampled by a Perkin Elmer MGA-1100 mass spectrometer (Wesley, MA) and Seivers Instruments NO analyzer (Boulder, CO) integrated with custom analysis software, as described previously (29, 31, 38, 39). Subjects breathed into a five-liter rebreathing bag containing 9% helium, 0.7% acetylene, 0.3% carbon monoxide (C18O), 40 PPM NO, and 35% O2 with a respiratory rate controlled at 32 breaths per minute via metronome. The 40 PPM NO was diluted in the anesthesia bag immediately prior to each maneuver from an 800 PPM NO tank. C18O was used instead of the more common C16O to enable the mass spectrometer to distinguish between C16O and N2, which have similar molecular weights. The total volume of gas used in the rebreathing maneuver was standardized at 1575mL at rest, and was based off of the tidal volume of the subject during exercise. Consistent bag volumes were ensured using a timed switching circuit which resulted in the desired volume, given a constant rate of flow from the tank. The switching circuit and tank were calibrated for accurate volumes before each test. At the end of a normal expiration (EELV, end-expiratory lung volume) subjects were switched into the rebreathing bag and breathed the test gas for eight to ten breaths. Following each maneuver, the rebreathing bag was completely emptied via vacuum and refilled immediately prior to the next maneuver.
Custom software was used to calculate the rate of disappearance of the gases with each breath calculated from the slope of the exponential disappearance for each gas with respect to helium (31). Membrane conductance and binding of carbon monoxide to hemoglobin contribute to the diffusion capacity of the lungs for carbon monoxide (33). Unlike DLCO, DLNO is based primarily on membrane conductance (DMNO) as nitric oxide is scavenged 280 times faster by hemoglobin than carbon monoxide, causing the uptake of NO to be instantaneous (DLNO≈DMNO). Therefore, DLNO is considered a direct measure of membrane conductance as the diffusion resistance of the blood is insignificant (19, 20, 26, 33). DMNO was then used to calculate the DM for carbon monoxide (DMCO) by correcting for their different diffusion constants based on molecular weight and solubility as described previously (19, 20, 26, 33). The correction factor based on molecular weight and solubility is 1.93 (16), however this correction factor is too low to produce values of VC that are physiologically plausible. Correction factors in used in previous work have been as high as 2.49 (24, 33, 36, 41). More recently, Ceridon et al. demonstrated that a correction factor of 2.11 is most appropriate during exercise by comparing the DLNO with the more traditional DLCO to calculate DM and Vc using DLCO at multiple oxygen tensions (6). Because this study utilized exercise, we have utilized 2.11 as the correction factor. VC was then calculated from the DLCO measured by subtracting the resistance to diffusion associated with alveolar-capillary barrier (DMCO). Finally, we corrected for individual differences in the rate of gas uptake and binding to hemoglobin due to each individual's hemoglobin concentration and alveolar partial pressure of oxygen.
Assessment of hemoglobin
Hemoglobin concentration was assessed at the University of Arizona Medical Center Pathology Laboratory using a cyanide-free hemoglobin method on an ADVIA 2120 Hemotology system. Subject's hemoglobin concentration (assessed from the baseline blood draw) was utilized in the calculation of VC, as described above.
SCNN1A genotyping and subject grouping
The inside of each cheek of the subject was swabbed, and the swabs were immediately placed in a stabilizing buffer for storage purposes. SCNN1A was genotyped and the αENaC amino acid at position 663 encoded by each SCNN1A allele was determined at the University of Arizona Genetics Core Laboratory using a Taqman SNP assay for rs#2228576. Briefly, initial DNA quantitation and quality control was performed using PicoGreen (Life Technologies). Pre-validated primers and probe sets for TaqMan Allelic Discrimination Assay were obtained from Life Technologies. Reactions were run at 10uL, containing TaqMan Universal PCR Master Mix, No AmpEraseR UNG (Life Technologies), 10ng total DNA, and 1X Assay Mix. All samples were processed and analyzed on 7900 Real-Time PCR System (Life Technologies) with cycling conditions (95°C for 10 minutes, 50 cycles of 92°C for 15 seconds and 60°C for 1 minute) and Genotyper software (SDS system, version 2.3). Subjects were then grouped according the amino acid at position 663 of αENaC. Individuals homozygous for SCNN1A alleles encoding alanine at amino acid 663 were grouped in the AA genotype, and individuals with at least one SCNN1A allele encoding threonine at amino acid 663 of αENaC were grouped into the AT/TT genotype, as only one subject was homozygous for threonine at amino acid 663.
Statistical analysis
The SPSS statistical software package (v.19; SPSS, Inc., Chicago, IL) was used for all statistical analyses. After confirming equality of variance with a Levene's Test, a one-sided independent samples t-test was used to determine significance between genotype groups at rest and peak exercise, to determine significance between genotype groups in percent change from rest to peak exercise and also to determine significance from rest to peak exercise within genotype groups. Using Bonferroni correction, an α level of 0.025 or lower was used for determining statistical significance. All data are presented as mean ± standard deviation unless stated otherwise.
Results
Eighteen individuals with CF participated in the study (Subject demographics, Table 1). Ten subjects were homozygous for alleles resulting in an alanine at amino acid 663 (AA genotype group), and eight subjects had at least one allele resulting in a threonine (AT/TT genotype group) at amino acid 663. Only one subject was homozygous for threonine at amino acid 663 and that subject was included in the AT/TT group. There were no differences in age, height, or forced expiratory volume in one second over forced vital capacity (FEV1/FVC) between genotype groups. Weight, body mass index (BMI), forced vital capacity (FVC) and forced expiratory volume in one second (FEV1) were significantly lower in the AT/TT genotype group than in the AA genotype group (Table 1).
Table 1. Subject demographics.
| AA Genotype | AT/TT Genotype | |
|---|---|---|
| n | 10 | 8 |
| Age (years) | 24±8 | 21±7 |
| Height (cm) | 168±10 | 169±5 |
| Weight (kg) | 70±17 | 56±8* |
| BMI (kg/m2) | 25±4 | 20±2* |
| FVC (L) | 3.87±1.37 | 3.36±1.10 |
| FVC (%predicted) | 65±37 | 29±29* |
| FEV1 (L/sec) | 2.92±1.33 | 2.55±.99 |
| FEV1 (%predicted) | 60±39 | 25±25* |
| FEV1/FVC (%) | 71±15 | 72±8 |
| FEF50 (%predicted) | 50±35 | 27±14 |
| MVV (L/min) | 112±48 | 96±28 |
| MVV (%) | 72±19 | 102±67 |
Values are mean±SD; BMI=Body mass index; FVC=forced vital capacity; FEV1= Forced expiratory volume in 1 second; FEF50=Forced expiratory flow at 50% of forced vital capacity; MVV= Maximum voluntary ventilation; AA genotype= homozygous for SCNN1A resulting in alanine at amino acid 663; AT/TT genotype= at least one copy of SCNN1A resulting in threonine at amino acid 663;
p<0.05 between groups.
Table 2 depicts the cardiopulmonary measures taken at rest and during peak exercise. No differences were seen in cardiopulmonary parameters at rest or in response to peak exercise between the AA and AT/TT groups (Table 2). Both groups reached similar peak workloads and similar significant increases in heart rate, cardiac output, systolic blood pressure, oxygen consumption, respiratory rate, and minute ventilation in response to peak exercise were seen in both groups (Table 2).
Table 2. Cardiopulmonary response to exercise.
| AA Genotype | AT/TT Genotype | |||
|---|---|---|---|---|
|
|
|
|||
| Rest | Peak Exercise | Rest | Peak Exercise | |
| Workload (Watts) | 0 | 100±32 | 0 | 106±44 |
| Heart Rate | 94±20 | 140±51† | 92±14 | 137±20† |
| Cardiac Output (L/min) | 5±2 | 11±4† | 4±1 | 12±4† |
| SBP (mmHg) | 107±11 | 144±29† | 106±9 | 137±20† |
| DBP (mmHg) | 71±9 | 70±16 | 68±8 | 65±14 |
| MAP (mmHg) | 83±9 | 94±19 | 81±8 | 89±8 |
| VO2 (mL/kg/min) | 6±1 | 22±6† | 7±2 | 26±12† |
| RER | .92±.14 | 1.20±.13 | .89±.17 | 1.10±.05 |
| RR (breaths/min) | 20±5 | 38±8† | 24±7 | 43±11† |
| VT BTPS (mL) | 854±352 | 1751±692† | 753±275 | 1498±495† |
| VE BTPS (L/min) | 16±6 | 62±17† | 17±5 | 61±15† |
Values are mean±SD, SBP= systolic blood pressure, DBP= diastolic blood pressure, MAP= mean arterial pressure, VO2= oxygen consumption, RER=respiratory exchange ratio, RR= respiratory rate, VT BTPS = tidal volume standardized for temperature, pressure, and humidity, VE BTPS= minute ventilation standardized for temperature, pressure, and humidity. AA genotype= homozygous for SCNN1A resulting in alanine at amino acid 663; AT/TT genotype= at least one copy of SCNN1A resulting in threonine at amino acid 663. No significant differences were seen between genotypes in these measures.
=p<0.05 from rest to peak exercise within group.
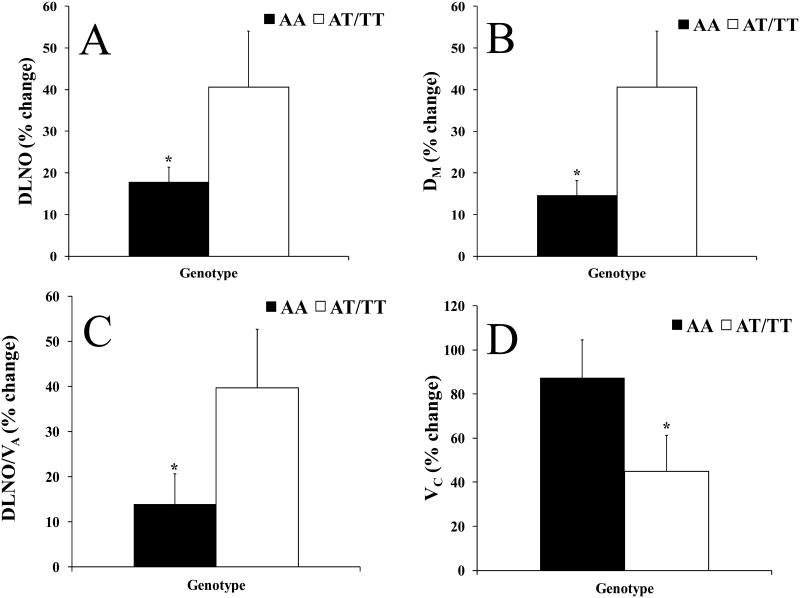
No significant differences were seen in absolute lung diffusion measures at rest or during peak exercise; although at rest the AA group tended to have higher DM and DLNO/VA than the AT/TT group (Table 3). The AA group demonstrated an attenuated percent increase in DLNO, DM, and DLNO/VA from rest to peak compared to the AT/TT group (% increase: DLNO= 17.8±3.5 vs. 40.6±13.4; DLNO/VA= 13.8±6.8 vs. 39.7±13.0; DM= 14.6±3.5 vs. 40.7±13.4, AA vs. AT/TT, p<0.025 for DM, Figure 1 panels A-C). Additionally, the AA group had a larger percent increase in VC with exercise than the AT/TT group (% increase: 87.2±7.2 vs 44.9±16.2, AA vs. AT/TT, Figure 1 panel D).
Table 3. DLCO, DLNO, DM, VC, VA, DLNO/VA, DLNO/DLCO, and SaO2 in response to exercise.
| AA | AT/TT | |||
|---|---|---|---|---|
|
|
|
|||
| Rest | Peak Exercise | Rest | Peak Exercise | |
| DLCO (mL/min/mmHg) | 21± 8 | 27±10 | 17±7 | 24±11 |
| DLNO (mL/min/mmHg) | 70±25 | 82±28 | 53±22 | 74±35 |
| DM (mL/min/mmHg) | 34±12 | 38±13 | 25±10 | 35±16 |
| Vc (mL) | 54±25 | 97±43 | 50±29 | 71±43 |
| VA (mL) | 2215±481 | 2364±679 | 2216±734 | 2127±682 |
| DLNO/VA | 31±7 | 35±7 | 25±8 | 33±7† |
| DLNO/DLCO | 3.3±.3 | 3.0±.2 | 3.1±.4 | 3.1±.2 |
| SaO2 (%) | 96±1 | 95±4 | 96±2 | 92±7 |
Values are mean±SD, DLCO= diffusing capacity of the lung for carbon monoxide, DLNO= diffusing capacity of the lung for nitric oxide, DM= alveolar-capillary membrane conductance. VC= pulmonary capillary blood volume, VA= alveolar volume, DLNO/VA= diffusing capacity of the lung for nitric oxide corrected for alveolar volume, SaO2= oxygen saturation. AA genotype= homozygous for SCNN1A resulting in alanine at amino acid 663; AT/TT genotype= at least one copy of SCNN1A resulting in threonine at amino acid 663. No significant differences were seen between genotypes.
=p<0.05 from rest to peak exercise within group.
Figure 1.
Panels depict the percent increase in response to peak exercise for A) the diffusing capacity of the lung for nitric oxide (DLNO), B) alveolar-capillary membrane conductance (DM), C) the diffusing capacity of the lung for nitric oxide corrected for alveolar volume (DLNO/VA), and D) pulmonary capillary blood volume (VC). The filled in bars represent the AA genotype (individuals homozygous for SCNN1A resulting in alanine at amino acid 663) and the open bars represent the AT/TT genotype (individuals with at least one allele resulting in threonine at amino acid 663). The error bars represent the SE of the mean. *p <0.05 between genotypes, **p<0.025 between genotypes.
Discussion
In the present study we demonstrate that genetic variation of SCNN1A manifested by changes at amino acid 663 is associated with differences in diffusing capacity of the lung in response to peak exercise in individuals with CF. Similar to previous studies, we have demonstrated an increase in both DLCO and DLNO with exercise in both groups (19, 33, 38, 39). Individuals with at least one allele resulting in a threonine at amino acid 663 (AT/TT group) demonstrated a significantly greater percent increase from rest to peak exercise in DLNO, DM, and DLNO/VA than individuals homozygous for alleles encoding the alanine variant. The AA group had a significantly greater percent increase from rest to peak exercise in VC. Interestingly, individuals in the AT/TT group had a significantly lower body weight, body mass index, and baseline pulmonary function (FVC, FEV1, and FEF50 %predicted). The lower resting pulmonary function in the AT/TT group may be due to the more functional ENaC resulting in a drier lung at rest which could reduce mucus clearance and result in the lower pulmonary function seen in the AT/TT group.
During exercise, the surface area for diffusion increases due to airway and capillary recruitment, and VC tends to drive the increase in DLCO and DLNO due to increases in cardiac output and ventilation. Individuals in the AT/TT group demonstrated a significantly greater percent increase from rest to peak exercise in DLNO, DM, and DLNO/VA, bringing the AT/TT group to similar levels of conductance as the AA group. The AT/TT group demonstrated this greater percent increase in these diffusing capacity parameters despite a significantly smaller percent increase in pulmonary capillary blood volume (VC). Given that the groups have similar maximal voluntary ventilation and alveolar volume, it is likely that these differences reflect alterations in the alveolar-capillary membrane. This suggests that the increase in conduction may be due to increased surface liquid volume in response to exercise. Improved airway hydration, through the use of hypertonic saline, has previously been shown to increase pulmonary function (FVC and FEV1) in individuals with CF, although no diffusing capacity data was available (12). Interestingly, recent studies have demonstrated an important relationship between DLNO and CT-derived structural abnormalities in patients with CF, suggesting that this technique strongly represents lung damage in this patient population(10).
There are various pathways that influence ion and, therefore, fluid regulation during exercise in the lungs. During exercise, epinephrine levels increase by up to 1000 fold causing stimulation of the β2-adrenergic receptor (β2-AR) (9). Activation of the β2-AR causes the release of Gαs subunit which activates adenylyl cyclase resulting in the conversion of ATP to cyclic-adenosine monophosphate (cAMP). Cyclic AMP subsequently activates protein kinase A, which phosphorylates CFTR, causing channel activation and movement of Cl- to the apical side of the airway epithelial cells and a CFTR mediated inhibition of ENaC activity (4, 7). Additionally, ENaC can be directly activated by protein kinase A during exercise. ENaC activity can also be upregulated during exercise through direct activation as a result of increases in shear stress in response to increased ventilation (11, 15, 34, 40).
During exercise, ENaC activity can be inhibited via activation of the purinergic (P2Y2) pathway by adenosine triphosphate and adenosine which are released from the epithelia in response to the mechanical stress of increased ventilation during exercise (8). The application of nucleotides to the airway lumen causes increased secretion and hydration of the airway surface liquid in both healthy and CF epithelia (5). ATP and adenosine interact with P2Y2 receptors on the apical side of the membrane causing a breakdown in phosphatidylinositol 4,5-bisphosphate (PIP2). Since PIP2 is required for protein kinase C-mediated ENaC activation, the breakdown of PIP2 results in the inhibition of ENaC. Purinergic stimulation during exercise can also increase calcium activated chloride channel activity through inositol trisphosphate (IP3) mediated release of Ca2+ from the endoplasmic reticulum Ca2+ stores (8, 22). This is important because even small changes in apical Cl- and, therefore, fluid can improve pulmonary function in patients with CF. The differences seen in DLCO and DLNO from rest to peak exercise between the genetic variants at amino acid 663 of ENaC may have resulted from the response to adrenergic stimulation of CFTR resulting in ENaC inhibition or more likely through purinergic inhibition of ENaC and CaCC activation.
In healthy individuals exercise resulted in opposite changes in diffusing capacity; AA individuals demonstrated a greater increase in diffusing capacity in response to peak exercise, whereas in CF AT/TT individuals demonstrated a greater increase in diffusing capacity (3). We hypothesize that in healthy individuals the AA group have a greater capacity to increase ENaC activity in response to adrenergic stimulation leading to fluid clearance and a greater increase in diffusing capacity. In individuals with CF, exercise may lead to inhibition of the more active T663 ENaC resulting in an increased surface liquid volume which may provide beneficial improvements in diffusing capacity.
Conclusion
This study suggests that genetic variation of the alpha-subunit of ENaC is associated with differences in the diffusing capacity response to exercise in CF. We hypothesize that the greater positive change in lung diffusion in the AT/TT group, which brought this group to overall levels similar to the AA group, could be due to exercise-induced inhibition of ENaC activity being more influential for improving surface liquid depth in the AT/TT group who have what was shown to be a more active ENaC in cell work.
Acknowledgments
Support for this work was provided by HL108962-01, the University of Arizona Clinical Scholars program and 5-T32-GM08400 Graduate Training Grant in Systems and Integrative Physiology.
We are sincerely grateful to the CF subjects who donated both their time and effort to be a part of this study. Support for this work was provided by HL108962-01, the University of Arizona Clinical Scholars program and 5-T32-GM08400 Graduate Training Grant in Systems and Integrative Physiology. The results of this study do not constitute endorsement by the American College of Sports Medicine.
Footnotes
Conflict of Interest: The authors have no conflict of interest to disclose.
Sarah E. Baker has no conflict of interest to disclose
Eric C. Wong has no conflict of interest to disclose
Courtney M. Wheatley has no conflict of interest to disclose
William T. Foxx-Lupo has no conflict of interest to disclose
Marina G. Martinez has no conflict of interest to disclose
Mary A. Morgan has no conflict of interest to disclose
Ryan Sprissler has no conflict of interest to disclose
Wayne J. Morgan has no conflict of interest to disclose
Eric M. Snyder has no conflict of interest to disclose
References
- 1.Alsuwaidan S, Li Wan Po A, Morrison G, Redmond A, Dodge JA, McElnay J, Stewart E, Stanford CF. Effect of exercise on the nasal transmucosal potential difference in patients with cystic fibrosis and normal subjects. Thorax. 1994;49(12):1249–50. doi: 10.1136/thx.49.12.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrosius WT, Bloem LJ, Zhou L, Rebhun JF, Snyder PM, Wagner MA, Guo C, Pratt JH. Genetic variants in the epithelial sodium channel in relation to aldosterone and potassium excretion and risk for hypertension. Hypertension. 1999;34(4 Pt 1):631–7. doi: 10.1161/01.hyp.34.4.631. [DOI] [PubMed] [Google Scholar]
- 3.Baker SE, Wheatley CM, Cassuto NA, Foxx-Lupo WT, Sprissler R, Snyder EM. Genetic variation of alphaENaC influences lung diffusion during exercise in humans. Respir Physiol Neurobiol. 2011;179(2-3):212–8. doi: 10.1016/j.resp.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Briel M, Greger R, Kunzelmann K. Cl- transport by cystic fibrosis transmembrane conductance regulator (CFTR) contributes to the inhibition of epithelial Na+ channels (ENaCs) in Xenopus oocytes co-expressing CFTR and ENaC. J Physiol. 1998;508(Pt 3):825–36. doi: 10.1111/j.1469-7793.1998.825bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Button B, Boucher RC. Role of mechanical stress in regulating airway surface hydration and mucus clearance rates. Respir Physiol Neurobiol. 2008;163(1-3):189–201. doi: 10.1016/j.resp.2008.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ceridon ML, Beck KC, Olson TP, Bilezikian JA, Johnson BD. Calculating alveolar capillary conductance and pulmonary capillary blood volume: comparing the multiple- and single-inspired oxygen tension methods. J Appl Physiol. 2010;109(3):643–53. doi: 10.1152/japplphysiol.01411.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chabot H, Vives MF, Dagenais A, Grygorczyk C, Berthiaume Y, Grygorczyk R. Downregulation of epithelial sodium channel (ENaC) by CFTR co-expressed in Xenopus oocytes is independent of Cl- conductance. J Membr Biol. 1999;169(3):175–88. doi: 10.1007/s002329900529. [DOI] [PubMed] [Google Scholar]
- 8.Chambers LA, Rollins BM, Tarran R. Liquid movement across the surface epithelium of large airways. Respir Physiol Neurobiol. 2007;159(3):256–70. doi: 10.1016/j.resp.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X, Eaton DC, Jain L. Alveolar epithelial ion and fluid transport B-adrenergic regulation of amiloride-sensitive lung sodium channels. American Journal of Physiology - Lung Cellular & Molecular Physiology. 2001;282:L609–L20. doi: 10.1152/ajplung.00356.2001. [DOI] [PubMed] [Google Scholar]
- 10.Dressel H, Filser L, Fischer R, Marten K, Muller-Lisse U, de la Motte D, Nowak D, Huber RM, Jorres RA. Lung diffusing capacity for nitric oxide and carbon monoxide in relation to morphological changes as assessed by computed tomography in patients with cystic fibrosis. BMC Pulm Med. 2009;9:30. doi: 10.1186/1471-2466-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eaton DC, Chen J, Ramosevac S, Matalon S, Jain L. Regulation of Na+ Channels in Lung Alveolar Type II Epithelial Cells. Proc Am Thorac Soc. 2004;1(1):10–6. doi: 10.1513/pats.2306008. [DOI] [PubMed] [Google Scholar]
- 12.Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, Belousova EG, Xuan W, Bye PT. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354(3):229–40. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- 13.Estivill X, Bancells C, Ramos C. Geographic distribution and regional origin of 272 cystic fibrosis mutations in European populations. The Biomed CF Mutation Analysis Consortium. Hum Mutat. 1997;10(2):135–54. doi: 10.1002/(SICI)1098-1004(1997)10:2<135::AID-HUMU6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 14.Foxx-Lupo WT, Wheatley CM, Baker SE, Cassuto NA, Delamere NA, Snyder EM. Genetic variation of the alpha subunit of the epithelial Na+ channel influences exhaled Na+ in healthy humans. Respir Physiol Neurobiol. 2011;179(2-3):205–11. doi: 10.1016/j.resp.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fronius M, Bogdan R, Althaus M, Morty RE, Clauss WG. Epithelial Na+ channels derived from human lung are activated by shear force. Respir Physiol Neurobiol. 170(1):113–9. doi: 10.1016/j.resp.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Guenard H, Varene N, Vaida P. Determination of lung capillary blood volume and membrane diffusing capacity in man by the measurements of NO and CO transfer. Respir Physiol. 1987;70(1):113–20. doi: 10.1016/s0034-5687(87)80036-1. [DOI] [PubMed] [Google Scholar]
- 17.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179–87. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 18.Hansen JE, Sue DY, Wasserman K. Predicted values for clinical exercise testing. Am Rev Respir Dis. 1984;129(2 Pt 2):S49–55. doi: 10.1164/arrd.1984.129.2P2.S49. [DOI] [PubMed] [Google Scholar]
- 19.Hsia CC. Recruitment of lung diffusing capacity: update of concept and application. Chest. 2002;122(5):1774–83. doi: 10.1378/chest.122.5.1774. [DOI] [PubMed] [Google Scholar]
- 20.Hsia CC, Raskin P. The diabetic lung: relevance of alveolar microangiopathy for the use of inhaled insulin. Am J Med. 2005;118(3):205–11. doi: 10.1016/j.amjmed.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 21.Kosorok MR, Wei WH, Farrell PM. The incidence of cystic fibrosis. Stat Med. 1996;15(5):449–62. doi: 10.1002/(SICI)1097-0258(19960315)15:5<449::AID-SIM173>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 22.Martins JR, Kongsuphol P, Sammels E, Dahimene S, Aldehni F, Clarke LA, Schreiber R, de Smedt H, Amaral MD, Kunzelmann K. F508del-CFTR increases intracellular Ca(2+) signaling that causes enhanced calcium-dependent Cl(-) conductance in cystic fibrosis. Biochim Biophys Acta. 2011;1812(11):1385–92. doi: 10.1016/j.bbadis.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 24.Phansalkar AR, Hanson CM, Shakir AR, Johnson RL, Jr, Hsia CC. Nitric oxide diffusing capacity and alveolar microvascular recruitment in sarcoidosis. Am J Respir Crit Care Med. 2004;169(9):1034–40. doi: 10.1164/rccm.200309-1287OC. [DOI] [PubMed] [Google Scholar]
- 25.Puchelle E, Gaillard D, Ploton D, Hinnrasky J, Fuchey C, Boutterin MC, Jacquot J, Dreyer D, Pavirani A, Dalemans W. Differential localization of the cystic fibrosis transmembrane conductance regulator in normal and cystic fibrosis airway epithelium. Am J Respir Cell Mol Biol. 1992;7(5):485–91. doi: 10.1165/ajrcmb/7.5.485. [DOI] [PubMed] [Google Scholar]
- 26.Roughton FJ, Forster RE. Relative importance of diffusion and chemical reaction rates in determining rate of exchange of gases in the human lung, with special reference to true diffusing capacity of pulmonary membrane and volume of blood in the lung capillaries. J Appl Physiol. 1957;11(2):290–302. doi: 10.1152/jappl.1957.11.2.290. [DOI] [PubMed] [Google Scholar]
- 27.Samaha FF, Rubenstein RC, Yan W, Ramkumar M, Levy DI, Ahn YJ, Sheng S, Kleyman TR. Functional polymorphism in the carboxyl terminus of the alpha-subunit of the human epithelial sodium channel. J Biol Chem. 2004;279(23):23900–7. doi: 10.1074/jbc.M401941200. [DOI] [PubMed] [Google Scholar]
- 28.Smith AA, Cowburn PJ, Parker ME, Denvir M, Puri S, Patel KR, Cleland JG. Impaired pulmonary diffusion during exercise in patients with chronic heart failure. Circulation. 1999;100(13):1406–10. doi: 10.1161/01.cir.100.13.1406. [DOI] [PubMed] [Google Scholar]
- 29.Snyder EM, Beck KC, Hulsebus ML, Breen JF, Hoffman EA, Johnson BD. Short-term hypoxic exposure at rest and during exercise reduces lung water in healthy humans. J Appl Physiol. 2006;101(6):1623–32. doi: 10.1152/japplphysiol.00481.2006. [DOI] [PubMed] [Google Scholar]
- 30.Snyder EM, Beck KC, Turner ST, Hoffman EA, Joyner MJ, Johnson BD. Genetic variation of the beta2-adrenergic receptor is associated with differences in lung fluid accumulation in humans. J Appl Physiol. 2007;102(6):2172–8. doi: 10.1152/japplphysiol.01300.2006. [DOI] [PubMed] [Google Scholar]
- 31.Snyder EM, Johnson BD, Beck KC. An open-circuit method for determining lung diffusing capacity during exercise: comparison to rebreathe. J Appl Physiol. 2005;99(5):1985–91. doi: 10.1152/japplphysiol.00348.2005. [DOI] [PubMed] [Google Scholar]
- 32.Snyder EM, Olson TP, Johnson BD, Frantz RP. Influence of sildenafil on lung diffusion during exposure to acute hypoxia at rest and during exercise in healthy humans. Eur J Appl Physiol. 2008;103(4):421–30. doi: 10.1007/S00421-008-0735-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tamhane RM, Johnson RL, Jr, Hsia CC. Pulmonary membrane diffusing capacity and capillary blood volume measured during exercise from nitric oxide uptake. Chest. 2001;120(6):1850–6. doi: 10.1378/chest.120.6.1850. [DOI] [PubMed] [Google Scholar]
- 34.Tarran R, Button B, Boucher RC. Regulation of normal and cystic fibrosis airway surface liquid volume by phasic shear stress. Annu Rev Physiol. 2006;68:543–61. doi: 10.1146/annurev.physiol.68.072304.112754. [DOI] [PubMed] [Google Scholar]
- 35.Tong Q, Menon AG, Stockand JD. Functional polymorphisms in the alpha-subunit of the human epithelial Na+ channel increase activity. Am J Physiol Renal Physiol. 2006;290(4):F821–7. doi: 10.1152/ajprenal.00312.2005. [DOI] [PubMed] [Google Scholar]
- 36.van der Lee I, Zanen P. Diffusion capacity for nitric oxide and carbon monoxide. Chest. 2004;126(5):1708–9. doi: 10.1378/chest.126.5.1708-b. author reply 9-10. [DOI] [PubMed] [Google Scholar]
- 37.Wallin CJ, Leksell LG. Estimation of extravascular lung water in humans with use of 2H2O: effect of blood flow and central blood volume. J Appl Physiol. 1994;76(5):1868–75. doi: 10.1152/jappl.1994.76.5.1868. [DOI] [PubMed] [Google Scholar]
- 38.Wheatley CM, Baldi JC, Cassuto NA, Foxx-Lupo WT, Snyder EM. Glycemic control influences lung membrane diffusion and oxygen saturation in exercise-trained subjects with type 1 diabetes: alveolar-capillary membrane conductance in type 1 diabetes. European journal of applied physiology. 2011;111(3):567–78. doi: 10.1007/s00421-010-1663-8. [DOI] [PubMed] [Google Scholar]
- 39.Wheatley CM, Foxx-Lupo WT, Cassuto NA, Wong EC, Daines CL, Morgan WJ, Snyder EM. Impaired lung diffusing capacity for nitric oxide and alveolar-capillary membrane conductance results in oxygen desaturation during exercise in patients with cystic fibrosis. Journal of cystic fibrosis: official journal of the European Cystic Fibrosis Society. 2011;10(1):45–53. doi: 10.1016/j.jcf.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 40.Wirtz HR, Dobbs LG. The effects of mechanical forces on lung functions. Respir Physiol. 2000;119(1):1–17. doi: 10.1016/s0034-5687(99)00092-4. [DOI] [PubMed] [Google Scholar]
- 41.Zavorsky GS, Lands LC. Lung diffusion capacity for nitric oxide and carbon monoxide is impaired similarly following short-term graded exercise. Nitric Oxide. 2005;12(1):31–8. doi: 10.1016/j.niox.2004.11.002. [DOI] [PubMed] [Google Scholar]