Abstract
While conjugated polymer nanoparticles (CPNs) have been widely touted as ultra-bright labels for biological imaging, no direct comparative measurements of their intracellular brightness have been reported. Simple in vitro comparisons are not definitive since fluorophore brightness in vitro may not correspond with intracellular brightness. We have compared the fluorescence brightness of J774A.1 cells loaded with 24 nm methoxy-capped 2000 Mr polyethylene glycol lipid PFBT nanoparticles (PEG lipid-PFBT CPNs) to cells loaded with carboxy-functionalized quantum dots (Qdots) or a dextran-linked small molecule organic dye, Alexa fluor 488-dextran (AF488-dex). Under conditions likely to be used for biological imaging or flow cytometry, these CPNs are 175X brighter than Qdots and 1400X brighter than AF488-dex in cells. Evaluation of the minimum incubation concentration required for detection of nanoparticle fluorescence with a commercial flow cytometer indicated that the limit of detection for PEG lipid-PFBT CPNs was 19 pM (86 ppb), substantially lower than values obtained for Qdots (980 pM) or AF488-dex (11.2 nM). Investigation of the mechanism of cellular uptake of the three fluid-phase labels indicates that these particles are passively taken into macrophage cells via macropinocytosis without interaction with cell surface receptors, and ultimately localize in lysosomes. In addition, no cytotoxicity could be observed at any of the CPN concentrations tested. Together, these data suggest that these CPNs are appropriate and attractive candidates as fluid phase markers with significantly greater fluorescence brightness than existing dyes or nanoparticles. We expect that these CPNs will find application in both imaging and flow cytometry.
Keywords: Alexa dextran, Quantum dots, Conjugated Polymer Nanoparticles, Semiconducting Polymer Nanoparticles, LAMP-1, Cellular toxicity
Introduction
Analyses of cellular functions routinely employ fluorescence-based techniques such as fluorescence microscopy and flow cytometry. For example, confocal and wide field imaging techniques are used to visualize cell and organelle structure, while flow cytometry takes advantage of fluorescently labeled cells to analyze, sort, and classify populations of cells for a variety of applications. However, the success of both fluorescence imaging and flow cytometry depends on the availability of bright photo-stable fluorescent probes. Small molecule dye labels such as fluorescein or Texas red have been widely used, particularly as labels for endocytic compartments in live cells[1]. However, these organic fluorophores tend to photo-bleach rapidly[2] and are removed from the cellular environment via efflux pathways such as organic anion transporters[3] unless tethered to larger polymer molecules such as dextrans. Further, these fluorophores do not have sufficient fluorescent signal for straight forward single molecule imaging[4].
Fluorescent nanoparticles are a better photon source for biological applications due to their improved brightness, photo-stability, and lower susceptibility to transport out of the cell compared to small molecule labels[2, 5–7]. In addition, the nanoparticle surface can be coated with specific external shell materials like polyethylene glycol (PEG) lipid[8–11], amphiphilic block copolymer[9], or silica[12] to increase nanoparticle solubility and stability in aqueous environments and provide functional groups for targeting to cell surface specific receptors and active delivery to specific cellular locations[11, 13, 14]. However, some nanoparticles result in cellular toxicity, which can arise from either core nanoparticle or the external shell composition[15–17]. Cytotoxicity is most noted for semiconductor quantum dots, due to the possibility of leaching heavy metal ions such as highly toxic Cd+2 from the nanoparticle core.
Highly fluorescent organic dye polymers, such as PFPV (poly[{9,9-dioctyl-2,7-divinylenefluorenylene}-alt-co-{2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylene}], MEH-PPV(poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylenevinylene], PDHF (poly(9,9-dihexylfluorenyl-2,7-diyl) and PFBT (poly[(9,9-dioctylfluorenyl-2,7-diyl)-co-(1,4-benzo-{2,1,3}-thiadazole)]) have been used to synthesize extremely bright conjugated polymer nanoparticles by a simple reprecipitation method[11, 18]. According to this method, when rapidly diluted from an organic solvent phase into aqueous solution, hydrophobic polymer molecules fold to exclude water from their surfaces, creating nanoparticles with concentrated intrinsic fluorescence, and extremely high absorption cross sections in vitro. For example, unmodified PFBT nanoparticles have a reported extinction coefficient of 5 × 107 M−1cm−1, a value ca. 100-fold greater than quantum dot nanoparticles of similar size and 1000-fold greater than typical small molecule dyes[12]. In addition, nanoparticle excitation and emission can be tailored by mixing two different polymers, or doping with specific dyes[19, 20]. These physical and photophysical properties make reprecipitated conjugated polymer nanoparticles ideal tools for imaging in biological systems, including single particle tracking [21], multicolor applications [22] and biological sensor development[23, 24]. Conjugated polymer nanoparticles can also be prepared via miniemulsion[25–27] although with somewhat lower yield and typically much larger observed nanoparticle diameters. As a part of efforts to tether conjugated polymer nanoparticles to recognition molecules for cellular targeting, we have prepared reprecipitated conjugated polymer nanoparticles in the presence of amphiphilic functionalized PEG lipid, to create conjugated polymer nanoparticles encapsulated with functionalized PEG[11]. These new functionalized PEG lipid coated conjugated polymer nanoparticles possess improved properties relative to uncoated conjugated polymer nanoparticles, including resistance to aggregation, greater solubility in aqueous solution, and increased quantum yield[11]. It has been demonstrated that uncoated CPNs contain potentially nonreproducible surface chemical defects resulting from surface polymer oxidation that occurs during preparation[28] and surface coating by PEG may ameliorate this effect.
Because of the extremely high fluorescent brightness of conjugated polymer nanoparticles in vitro, it has been suggested that these nanoparticles are attractive candidates for use as cellular labels in biological imaging. Indeed, a series of manuscripts have shown that conjugated polymer nanoparticles are efficiently taken up into cells[29–34]. We have reported that uncoated conjugated polymer nanoparticles can be successfully used as endocytic markers[29]. However, figures of merit typically used to describe conjugated polymer nanoparticles brightness, including extinction coefficient, quantum yield, and fluorescence cross-sectional area, are obtained outside the cell, and do not necessarily translate directly into brightness inside the cell. For example, fluorophore signal may change or show diminished brightness inside cells, as has been reported for several organic dyes[35, 36]. In addition, fluorophores may be taken up into cells with a range of uptake efficiencies, leading to variations in relative intracellular signal(s) that reflect differences in intracellular concentration rather than the brightness of individual fluorophores. As a result, direct intracellular comparison of different fluorophores under biological conditions is required to appropriately assess relative brightness for biological imaging. In this manuscript, we compare PEG lipid coated conjugated polymer nanoparticles to commercially available Qdots and small molecule organic dyes with respect to spectral properties, mode and rate of cellular uptake, final destination in the macrophage cell line J774A.1, and relative brightness inside the cell. The resulting data indicates that these PEG lipid-coated conjugated polymer nanoparticles are exceptional candidates for biological labeling applications, including both cellular imaging and flow cytometry.
Materials and Methods
Reagents
The conjugated polymer PFBT (Mr = 48,000; polydispersity = 2.7) was purchased from American Dye source (Quebec, Canada). Methoxy 2000 Mr polyethylene glycol lipid was purchased from Avanti Polar Lipids. The J774A.1 mouse macrophage cell line was purchased from American Type Culture Collections. Texas red dextran (TR-dex) (Mr = 10,000), AF488-dex (Mr = 10,000), and carboxy Qdots 525 were purchased from Invitrogen. Allophycocyanin (APC) labeled lysosome-Associated Membrane Protein 1 (LAMP-1) monoclonal antibody was purchased from Southern Biotech. All other chemicals used were purchased from Sigma-Aldrich, Fisher Scientific, or VWR.
Preparation and characterization of CPNs
The method of preparation of PEG lipid-PFBT nanoparticles is described in detail elsewhere[11]. Briefly, one ml of PFBT (250 ppm) dissolved in HPLC grade tetrahydrofuran (THF) was diluted rapidly into 9 ml distilled-deionized H2O containing 50 ppm methoxy-capped 2000 Mr PEG lipid under mild sonication to facilitate fast mixing. THF was removed from the suspension under vacuum evaporation, and filtered through a 220 nm PVDF syringe filter. A very dilute solution (ca. 100 pM) of the resulting nanoparticles was spread on formvar copper grids by drop casting. The size of the nanoparticles was measured using a Hitachi H7600 transmission electron microscope (TEM) at 120 kV on a cryostage cooled with liquid nitrogen. The diameter of the CPNs was measured with Image J. The determined particle diameters were fit in to a Gaussian distribution using Sigma Plot (Systat). Hydrodynamic size of the nanoparticles was measured by dynamic light scattering (DLS) using a Malvern Zetasizer (ZS90) at 25°C as previously described[11]. Nanoparticle concentration was estimated from the mass of conjugated polymer diluted into aqueous solution and the TEM size, assuming complete polymer to nanoparticle conversion, as previously described[29].
Fluorescent intensity measurements of the fluorophores (AF488-dex, Qdots, and CPNs)
Fluorescence emission spectra of AF488-dex, Qdots, and CPNs were acquired using a photon counting spectrofluorometer (Photon Technology International; QM-4). In order to achieve measurable fluorescence intensity, a 500 μM stock solution of AF488-dex was diluted to 112 nM; a 8 μM Qdot stock was diluted to 22.4 nM, and 6 nM CPN stock was diluted to 0.6 nM in water. The emission spectra of the diluted solutions were measured from 495 nm – 650 nm using 488 nm excitation, (4 nm bandpass) for all of the three fluorophores. Fluorescence emission of each of the fluorophores was also recorded under similar conditions but using the corresponding excitation maximum for each fluorophore (494 nm for AF488-dex, 400 nm for Qdots and 460 nm for CPNs).
Cell culture
Mouse macrophage-like J774A.1 cells were grown in Dulbecco’s Modified Eagles Medium (DMEM; Mediatech) supplemented with 10% heat inactivated Fetal Bovine Serum (FBS; Hyclone), L –glutamine (2 mM), penicillin (100 units/mL) and streptomycin (100 g/mL), in a humidified incubator with 5% CO2 at 37°C. The viability of the cells was 97% or more with each passage, as determined by Trypan blue exclusion assay.
Fluorescence microscopy of fluorophore uptake
J774A.1 cells were grown in optical bottom culture plates until about 70% confluent, at which time the cells were incubated with either 0.6 nM (2.7 ppm) CPNs, 22.4 nM Qdots, and 112 nM AF488-dex overnight (16 h) in DMEM + 10% FBS at 37°C and 5% CO2. For co-localization experiments, 200 nM TR-dex was also added together with each fluorophore. Following incubation, cells were washed three times with Ringer’s Buffer (RB - 10 mM HEPES, 155 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 2 mM NaH2PO4, 10 mM glucose, pH 7.2–7.4), and incubated without extracellular fluorophore under culture conditions (chased) for ≥4 hours prior to imaging. Under these chase conditions, sufficient time has elapsed that all endocytic cargo is assumed to be delivered to terminal vacuoles[1], which for fluid phase uptake in macrophage cells is the lysosome[37].[1] Fluorescence imaging was performed on an inverted epifluorescence microscope (Olympus IX71) with xenon arc lamp excitation and a 60X/1.45NA objective. CPNs, Qdots and AF488-dex were viewed with 494 nm excitation (20 nm bandpass) and 531 nm emission (22 nm bandpass) while TR-dex was viewed with excitation wavelength 575 nm (25 nm bandpass) and emission at 624 nm (40 nm bandpass).
Flow cytometric studies of fluorophore uptake
J774A.1 cells grown in 35 mm culture dishes were incubated with Qdots, AF488-dex, and CPNs separately in DMEM containing 10% heat inactivated FBS. For time course studies, cells were incubated with fluorophore for each of 1, 2, 4 and 8 h, with 0.6 nM (2.7 ppm) CPNs, and with Qdots (22.4nM) and AF488-dex (112nM) concentrations that were 37-fold and 186-fold higher, respectively. At individual time points, cell plates were chilled on ice and the cells were detached from the surface by pipetting. Detached cells were pelleted, and washed 3X with RB before suspension in RB. Fluorescence from 10,000 cells was measured by flow cytometer (BD FACScan) at an excitation wavelength of 488 nm with the emission in the green channel. The data from FACScan were analyzed using FlowJo software (Treestar). In the dose dependent studies, cells were incubated with each fluorophore for 8 hours over the concentration range(s) of 22 to 600 pM (CPNs), 1.4 to 22.4 nM (Qdots), or 4.5 to 112 nM (AF488-dex). Cells were prepared for flow cytometer analysis as for the time course studies.
Immunocytochemical analysis
J774A.1 cells grown in glass bottom culture dishes were incubated with fluorophores at 37°C for 16 hours in a 5% CO2 incubator. Cells were washed 3X with RB, and then chased for 4 hours in DMEM culture medium. The cells were fixed with 4% paraformaldehyde in RB at 37° C for 10 minutes, and washed 3X again with RB before blocking with blocking buffer (RB containing 1.5% BSA, 0.3% Triton-X solution) for 2 hours at 4°C. The cells were next incubated with the LAMP-1 specific antibody labeled with Allophycocyanin (APC) (200-fold dilution of 0.1 mg/ml stock solution) for 16 hours in RB containing 1.0% BSA and 0.3 % Triton-X at 4°C. The cells were washed with blocking buffer 3× 15 minutes each at room temperature. Fluorescence imaging was performed by inverted epifluorescence microscope (Olympus IX71) with xenon arc lamp as excitation source and a 60X/1.45NA objective The fluorescence of each of CPNs, Qdots, and AF488-dex were observed with 494 nm excitation (20 nm bandpass) and emission of 531 nm (22 nm bandpass); Allophycocyanin fluorescence of the antibody was observed with 575 nm excitation (25 nm bandpass) and emission of 624 nm (40 nm bandpass).
Blocking nanoparticle uptake using inhibitors of macropinocytosis
Known inhibitors of macropinocytosis were used to help elucidate the mechanism of cellular uptake of CPNs, as described in Fernando et al[29]. Briefly, J774A.1 cells were plated in 35 mm tissue culture plates and grown to ~ 70% confluence. The cells were preincubated with methyl-β-cyclodextrin (2.5 mg/mL final concentration), wortmannin (100 ng/ml final concentration), and LY294002 (20 μg/ml final concentration) in culture medium for 30 min, followed by incubation of cells with 0.60 nM (2.7 ppm) CPNs for 1.5 – 2 hours, also in media. Methyl-β-cyclodextrin and wortmannin are insoluble in media and require dilution in DMSO; cells treated with these inhibitors were therefore exposed to small amounts of DMSO. An additional vehicle control was performed in which cells were exposed to equivalent amounts of DMSO in the absence of inhibitors prior to incubation with CPNs. Cell processing, flow cytometry measurements and data analysis were carried out as described above. Statistical analysis of the mean fluorescence for each treatment compared to the untreated and vehicle controls was done using ANOVA in SigmaPlot. We have previously demonstrated that under these conditions, there is less than 10% cytotoxicity from the inhibitor alone[29], indicating that observed decreases in CPN uptake in the presence of individual inhibitors do not reflect inhibitor cytotoxicity.
Cytotoxicity studies
The Cell Titer Blue assay was used to assess cytotoxicity of CPNs. J774A.1 cells were plated at 10K/well in a black 96-well plates in DMEM + 10% FBS at 37°C and 5% CO2 and incubated with CPNs in culture medium for 16–18 hours at concentrations ranging from 0 ppm (control) to 67.5 ppm (15 nM). The background fluorescence from the CPNs at λex = 546 and λem = 585 nm was recorded using a Genios top reading fluorescence plate reader (Tecan). Cell Titer Blue reagent was then added to all according to manufacturer’s instructions, incubated with the cells for an additional 2 hours, and then the fluorescence in each well was remeasured. Individual well background fluorescence was subtracted from the total fluorescence to determine Cell Titer Blue fluorescence and then converted to % viability versus the untreated control wells. Statistical analysis of cell viability versus the untreated control was done using ANOVA in SigmaPlot followed by Bonferroni comparison; P-values less than 0.05 were used to conclude a significant difference between samples and controls.
Results
In this study we explore the suitability of methoxy-functionalized PEG lipid-coated conjugated polymer nanoparticles for application as intracellular probes for cellular imaging and flow cytometry. We compare the advantages these nanoparticles offer over commercially available fluorophores such as Qdots and Alexa fluor dextran for ex vivo cell labeling in J774A.1 cells. Our goal was to determine the relative brightness of the CPNs to Qdots and organic dyes when loaded into cells, their respective uptake efficiency and mechanism of cell entry, and their final intracellular localization. We also evaluate the cytotoxicity of PEG lipid-CPNs.
In these studies, we use CPNs as a representative PEG lipid conjugated polymer nanoparticle. These nanoparticles were synthesized from commercially available methoxy-functionalized PEG lipid and PFBT by reprecipitation, as previously described[11]. Based on spectral behavior and functional end group reactivity, the resulting nanoparticle structure is presumed to have a fluorescent PFBT-lipid core, surrounded by a corona of PEG molecules that results in high solution stability and increased quantum yield relative to bare PFBT particles[11]. Similar behavior was observed for PEG lipid coated conjugated polymer nanoparticles prepared with other functional end groups (e.g. carboxy, biotin) that allow for conjugation to biorecognition molecules[11]. Transmission Electron Microscope characterization of the CPNs used here indicates that the particles are approximately spherical in shape with a mean particle diameter of 24±5 nm, as shown in Figure 1a and 1b. Dynamic light scattering (DLS) analysis of this preparation gave a DLS diameter of 59 ± 2 nm, with a moderate polydispersity index of 0.14 ±0.03. The observed size difference between TEM and DLS measurements is consistent with 20 to 30 nm differences previously reported for PEG-coated conjugated polymer nanoparticles prepared by miniemulsion[34] and presumably reflects substantial hydration of the PEG surface. Some additional inflation of measured hydrodynamic radius by small amounts of high molecular weight particles is possible in this moderately polydisperse sample, although we have seen no evidence of the existence of aggregates. The measured size for this preparation is identical to our previous preparations[11], indicating that this method produces nanoparticles of highly reproducible size. The choice of methoxy as functional end group is arbitrary here, as we have seen no impact of the functional group in PEG lipid molecule on nanoparticle spectral behavior[11] or cellular uptake.
Figure 1.

TEM characterization and size distribution of CPNs. (a) Typical TEM image of CPNs. (b) Histogram of measured CPN diameter from TEM image analysis using Image J software. Histogram fit to Gaussian. Mean diameter = 24 ± 5 nm.
Nanoparticle uptake can change as a function of particle size and surface characteristics[15, 38–41], as alteration of nanoparticle surface or size can lead to differential interaction with cell surface receptors that can facilitate uptake. Hence, direct comparisons of nanoparticle uptake are only relevant for nanoparticles with similar size and surface characteristics. To directly compare the behavior and uptake of these CPNs, we chose a representative commercially available Qdot with an amphiphilic coating and approximately equivalent size. In this case, the semiconductor CdSe or CdTe core with a Zn sulfide shell was coated with a carboxy-functionalized (proprietary) amphiphilic polymer layer. The result was a ca. 20 nm quantum dot with a hydrophilic surface. A range of commercially available fluorescent dyes are available for comparison to the CPNs; we chose to use AF488-dex, since it is among the brightest of the available organic dyes at the wavelength typically used for biological imaging and flow cytometry. As a result, signal performance of AF488-dex relative to CPNs represents a “best case” behavior for organic dyes.
Biological imaging and analysis is typically carried out with 488 nm excitation, using a 488 nm argon-ion laser. In particular, commercially available flow cytometry instruments widely used to detect cell labeling are equipped with 488 nm excitation. We compared the spectral behavior of CPNs to Qdots and AF488 under these conditions. Figure 2A shows a comparison of the fluorescence intensities of the three fluorophores at an excitation wavelength of 488 nm. In this case, CPNs are so much brighter than either Qdots or organic dyes that they cannot be compared at equivalent concentrations; concentrations that allow adequate AF488 signal result in overloaded detection for CPNs. Spectra shown in Figure 2 were obtained with 0.6 nM (2.8 ppm) CPNs, while Qdots and AF488-dex concentrations were 37 and 186 fold higher, respectively. The spectra demonstrate that CPNs have a wide emission spectrum (λmax= 540 nm) compared to Qdots and AF488-dex. Based on the integrated fluorescence intensity under the spectra, we estimate that the measured signal intensity for CPNs would be 160 times brighter than Qdots and 600 times brighter than AF488 under these conditions when corrected for the differences in concentration of the different fluorophores. We note that these measurements were not obtained at the excitation maximum for either CPNs λmax = 460 nm) or Qdots (λmax = 400 nM). When the fluorescence intensity was compared using the respective absorption maxima of each fluorophore (Figure 2B), CPNs have still higher relative signal; CPNs are ca. 37-fold brighter than Qdots and 510-fold brighter than AF488-dextran under ideal excitation conditions. We have seen no impact of functional end group on PEG lipid coated conjugated polymer nanoparticle spectra or quantum yields; hence, data obtained for CPNs also reflect that for PEG lipid-coated PFBT nanoparticles with other PEG lipid end groups[11]. We note that single molecule brightness comparisons have been made between Qdot 565, IgG-AF488 and a polystyrene PEG PFBT Np prepared using an alternate protocol[42]; the relative brightness of this alternative type of PFBT CPN was reported to be somewhat lower than we observe here.
Figure 2.
In vitro comparison of the fluorescence intensity of CPNs, Qdots, and AF488-dex. Fluorescence emission spectra of 0.6 nM CPNs (solid), 22.4 nM Qdots (dashed) and 112 nM AF488-dex (dotted) obtained using (a) excitation wavelength of 488 nm and (b) at the optimal excitation wavelength of each fluorophore (494 nm for AF488-dex, 400 nm for Qdots and 460 nm for CPNs; excitation bandpass = 4 nm). All spectra were acquired using a steady state spectrofluorometer (emission bandpass = 4 nm).
Relative fluorophore uptake and intracellular brightness
We compared the efficiency and mechanism of CPNs uptake into J774A.1 macrophage cells with that for commercial Qdots and AF488-dex dyes under equivalent conditions. Our goal was to determine the relative brightness of the CPNs versus Qdots and organic dyes in vivo, and to evaluate their respective mechanism of cell entry and final intracellular location(s).
First, to determine the time course of fluorophore uptake and to quantify the resulting intracellular signal, cells were incubated with each of CPNs, Qdots, and AF488-dex over time periods up to eight hours. The intracellular fluorescence of each loaded cell was determined by flow cytometry (Figure 3). As for in vitro experiments, cells were incubated in higher concentrations of Qdots and AF488-dex in order to place the signal from these fluorophores on the same scale as the CPN signal. As shown in Figure 3, we were able to observe good CPN signal with an hour-long incubation at a concentration of 0.6 nM (2.7 ppm) whereas 37-fold and 186-fold higher concentrations of Qdots and AF488-dex took ca. 2 and 4 hours respectively to get a discernable fluorescent intensity. This difference in time to achieve measureable intracellular fluorescence reflects differences in fluorophore brightness, and does not necessarily imply a difference in the uptake mechanism. After eight hour incubation, the observed intracellular CPNs fluorescence was ca. 175-fold and 1400-fold greater than Qdots and AF488-dex fluorescence intensities when corrected for the differences in fluorophore concentration bathing the cell. We note that this correction for differences in extracellular fluorophore concentration assumes equivalent uptake efficiency; apparent intracellular brightness could be inflated or diminished by differences in relative uptake efficiencies for the different fluorophores. Regardless of assumptions, these observations indicate that CPNs are efficiently taken up into this cell type, and have remarkably bright intracellular fluorescence.
Figure 3.
Time course of fluorophore uptake by cells. J774A.1 cells were pulsed with AF488-dex (black; 112 nM), Qdots (light grey; 22.4 nM), or CPNs (dark grey; 0.6 nM) for the indicated times, and cell-associated fluorescence was analyzed by flow cytometry. Error bars represent the standard deviation of the mean fluorescence at 488 nm excitation for at least 3 independent measurements of 10,000 cells.
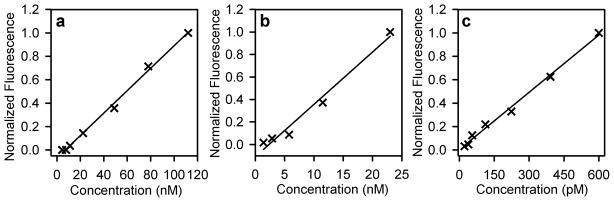
To examine the relationship between extracellular fluorophore concentration and intracellular fluorescence for CPNs, Qdots, and AF488-dex, J774A.1 cells were incubated for 8 hours with each of the three fluorophores over a range of concentrations; as for previous experiments, concentrations of Qdots and AF488-dex were 37-fold and 186-fold higher than that used for CPNs. The resulting cell-associated fluorescence under each condition was quantified by flow cytometry. As shown in Figure 4, cellular uptake of each fluorophore resulted in dose-dependent cellular fluorescence detectable by flow cytometry. These data indicate that extremely low concentrations of the CPN label can be detected in live cells using a standard flow cytometer equipped with a <15 mW argon ion laser. Depending on the sensitivity of the individual cytometer, even lower concentrations of CPNs could be detectable.
Figure 4.
Dose dependent uptake of CPNs, Qdots, and AF488-dex in J774A.1 macrophage-like cells. Individual graphs represent uptake of (a) AF488-dex; (b) Qdots; and (c) CPNs. Cells were pulsed with different concentrations of fluorophore for 8 hours, washed, and analyzed for cell-associated fluorescence by flow cytometry. Data reflects the mean fluorescence at 488 nm excitation for at least 3 independent measurements of 10,000 cells. Normalized fluorescence intensity at each dosage reflects the ratio of mean fluorescence intensity value at that dosage to mean fluorescent intensity value of the highest dosage.
The higher intracellular signal of CPNs compared to Qdots and AF488-dex is highlighted by their relative detection limits under these conditions. While the observed detection limit for CPNs was 19 pM (86 ppb), corresponding detection limits for Qdots and AF488-dex were 980 pM and 11.2 nM, respectively.
CPN concentrations used in this study are low. CPNs form in response to dilution from an organic phase to an aqueous phase, resulting a stock solution with low CPN concentrations. It should be noted that we have further diluted this low concentration into media for cell studies. However, given the extremely bright fluorescence of these particles, these very low loading concentrations are more than adequate, and we have investigated cell loading over the range of concentrations likely to be used. Also, we have previously demonstrated that the high solution stability of PEG lipid coated conjugated polymer nanoparticles allows these particles to be concentrated via ultra filtration[11]. Hence, if higher concentrations were required for specific experiments, such as toxicity studies, these low concentration stock solutions could be concentrated to allow treatment of cells with dramatically more concentrated CPN solutions.
Fluorophore uptake mechanisms
Application of different fluorophores as labels for cellular imaging or flow cytometry requires that their final intracellular location be established. For example, dextran-coupled dyes have been widely used as intracellular labels, an application that takes advantage of their known fluid-phase uptake and trafficking to lysosomes[1]. To investigate the uptake and cellular trafficking of CPNs, and to compare it to that for Qdots and AF488-dex, a series of experiment were performed in J774A.1 macrophage cells.
In the first set of experiments, cells were incubated in fluorophore solutions overnight in the presence of TR-dex, washed to remove any extracellular fluorophore, and then incubated under culture conditions (chased) for at least an additional four hours prior to imaging. Since dextran-labeled dyes can be present in a variety of endosome types (e.g. early endosomes, late endosomes, late-endosome-lysosomal fusion compartments, lysosomes) depending on the timescale of the experiment following initial uptake[37], a long chase was necessary to allow sufficient time for all endocytic cargo to be delivered to terminal vacuole(s), which for fluid-phase uptake in macrophage cells is the lysosome[37]. A 2 hour chase is generally assumed to be sufficient for complete lysosomal delivery [1], and macropinosomes have been shown to deliver fluid-phase cargo to lysosomes in less than 20 minutes[37]. Hence, a four hour chase, as chosen here, is more than sufficient to allow all fluorophore to be delivered to lysosomes. In addition, use of a long pulse and chase helped minimize cell-to-cell variation frequently observed when using shorter experimental time scales. Figure 5 demonstrates that under these conditions, each fluorophore was taken into the cell, resulting in a vessicular staining pattern of intracellular fluorescence consistent with fluorophore localization in intracellular organelles (green), rather than dispersed uniformly throughout the cytosol. A comparison of the cellular localization of each of the fluorophores (Figure 5 - green) demonstrates that the pattern of TR-dex fluorescence (red) mirrors (i.e. merges with) that for each of CPNs, Qdots, and AF488-dex in these experiments. Dextran coupled dyes are known to be taken up by host cells via fluid phase pinocytosis (usually macropinocytosis) and are trafficked along the cell’s endocytic pathway, finally accumulating in lysosomes[1]. Hence, co-localization of TR-dex and the respective fluorophores in these experiments indicates trafficking to the lysosome, and suggests simultaneous uptake via a single endocytic mechanism. These data suggest that each of these fluorophores enter the cell via a fluid-phase endocytic mechanism, and are ultimately located in membrane-bound organelles, presumably lysosomes, located in the perinuclear region of the cells. We point out that under the conditions of these experiments, cells could not phagocytose extracellular material (cells were grown in complement free media to preclude complement-mediated phagocytosis and nanoparticles were not opsonized, to prevent Fc-receptor mediated phagocytosis). Hence, fluorophores entering the cell through endocytic mechanism(s) in these experiments must do so through fluid-phase endocytosis, rather than phagocytosis.
Figure 5.
Fluorophore co-localization with TR-dex. J774A.1 cells in individual glass bottom culture dishes were pulsed overnight with fluorophores in the presence of TR-dex and chased for ≥4 h prior to imaging. Green images reflect CPN, Qdot, and AF488-dex fluorescence; red images reflect TR-dex fluorescence; in the merged image, yellow color indicates co-localization. Because of the differences in relative brightness, the different fluorophores were loaded into cells at different concentrations (CPNs = 0.6 nM; Qdots = 22.4 nM; AF488-dex = 112 nM). Scale bars = 10 μm.
Additional support for fluid-phase endocytosis as the mechanism of fluorophore entry under these conditions was provided by uptake experiments performed on ice. In these experiments, cells were bathed in the different fluorophores and incubated on ice. At low temperatures, cells cannot take in extracellular material by energy-dependent endocytic mechanisms, and any CPN fluorescence associated with cells under these conditions must result from either CPN binding to the cell surface or via energy independent uptake mechanisms such as diffusion through the cell membrane. After washing cells with ice-cold buffer, the relative intracellular fluorescence was evaluated by flow cytometry (Figure 3). For each of the fluorophores, very little cell-associated fluorescence can be observed under these conditions. This observation is consistent with uptake by an energy-dependent mechanism (i.e. endocytosis), and rules out simple diffusion as the mode of fluorophore uptake, since some diffusion could still occur at low temperature. In addition, the lack of interaction with the cell surface indicates that uptake does not occur through receptor-mediated endocytosis, which is initiated by interactions with receptors on the cell surface. However, while the fluorescent intensity of the CPN and AF488-dex samples incubated on ice were a very small percentage of the corresponding value at 37°C (0.2% and 2%, respectively), a somewhat higher value was observed with Qdots (13% of the value at 37°C). This observation suggests that there may be small amounts of either binding to the cell membrane or energy-independent cellular uptake, such as diffusion through cellular membranes, for these Qdots.
Flow cytometry experiments cannot distinguish between intracellular fluorescence and fluorescence resulting from fluorophore adsorption to the cell surface. Hence, the low measured CPN fluorescence observed by flow cytometry at low temperature indicates that there is very little physical adsorption (or receptor binding) of CPNs to the cell surface. A PEG coating is believed to reduce interaction with the cell surface for other nanoparticles, including Qdots[9, 43]. Consequently, the observation of little or no association of CPNs with the cells at low temperature is not unexpected. Similarly, dextran-coupled dyes do not typically interact with cell surfaces[44], consistent with the low AF488-dex fluorescence also associated with cells at low temperature. In contrast, the Qdots fluorescence associated with cells at low temperature may reflect adsorption of the proprietary amphiphilic coating to the cell surface, as well possible energy-independent uptake mechanism(s) such as diffusion through the cell membrane or receptor-mediated endocytosis.
A subsequent set of experiments were designed to further confirm that CPNs, like other fluid-phase markers, are ultimately delivered to lysosomes. LAMP-1 or CD107a is a protein that is trafficked to the membranes of late endosomes during endosomal maturation, and is retained in the lysosomal membrane[45]. Late endosomes mature to lysosomes or fuse with and/or deliver their contents to lysosomes[37], and the presence and contents of these vacuoles are closely linked[46]. Hence, while LAMP-1 cannot be used to identify lysosomes in the absence of late endosomes, it is commonly used to identify the presence of lysosome or lysosome-like late endosomes. To demonstrate that CPNs are finally localized to lysosomes in macrophage cells, immunocytochemical analysis was carried out using a Cy5 labeled anti-LAMP-1 antibody. In these experiments, a pulse-chase experiment was performed in which cells were incubated with fluorophore to allow uptake, washed, and incubated without extracellular fluorophore for ≥4 hours, more than sufficient time to allow delivery of endocytic cargo to lysosomes. Cells were then fixed and stained with anti-LAMP-1 antibody. As shown in Figure 6, CPN fluorescence (green) is present in large compartmentalized LAMP-1 immunostained organelles (red) near the nucleus; the fluorophore signal co-localizes with anti-LAMP-1 antibody (merge). Similar results were obtained for Qdots, and AF488-dex. Together, these data confirm that each fluorophore is taken up by these cells via solution phase endocytosis and is ultimately trafficked to LAMP-1-containing lysosomes.
Figure 6.
Co-localization of fluorophores with LAMP-1. J774A.1 cells were pulsed with CPNs (0.6 nM); Qdots (22.4 nM); or AF488-dex (112 nM), followed by a ≥4 hour chase. Cells were then paraformaldehyde fixed, detergent permeabilized, and stained with an anti-LAMP 1 allophycocyanin (APC) -conjugated antibody. Green images reflect CPN, Qdot, and AF488-dex fluorescence; red images reflect APC fluorescence; in the merged image, yellow color indicates co-localization of fluorophores and LAMP 1. Scale bars = 10 μm.
We have previously demonstrated that uptake of uncoated PFBT nanoparticles in J774A.1 macrophage cells takes place via macropinocytosis. For these bare particles, uptake was inhibited in the presence of chemical compounds that interfere with individual aspects of macropinocytosis (i.e wortmannin, cyclodextrin, and LY294002), while compounds known to inhibit other uptake mechanisms had no effect[29]. We performed a similar analysis with these PEG lipid PFBT particles. To establish that CPNs are taken up by macropinocytosis, we evaluated the sensitivity of uptake to wortmannin and LY294002, which block the action of phosphoinositide 3-kinase (PI3K)[47]. Since PI3K is required for spontaneous cell surface ruffling that is an integral part of macropinocytosis[47], inhibition of uptake in the presence of wortmannin and LY294002 indicates that nanoparticles enter the cell through macropinocytosis. As shown in Figure 7, both wortmannin and LY294002 result in significant inhibition of CPN uptake. An additional inhibitor, methyl-β-cyclodextrin, which inhibits cholesterol formation, also significantly reduces CPN uptake. Since cholesterol is involved in cell-surface ruffling, inhibition by cholesterol is also consistent with CPN uptake via macropinocytosis. Together, these data indicate that like uncoated CPNs, PEG-coated CPNs are taken into macrophage cells via macropinocytosis. Since macropinocytosis is a nonspecific uptake mechanism that does not require interaction with cell surface receptors[48], this characterization also indicates that interaction of CPNs with the cell surface is not required for uptake, consistent with the lack of CPN interaction with cell membrane observed at low temperature.
Figure 7.
Disruption of CPN uptake by macropinocytosis inhibitors. J774A.1 cells treated with β-methyl cyclodextrin, wortmannin, and LY 294002 were incubated with identical concentrations of CPNs, and cellular uptake was evaluated by flow cytometry. Data is presented as a percentage uptake relative to cells not treated with inhibitors; CPN uptake into cells in media in the absence of inhibitors (positive control) represents 100% uptake, and cellular fluorescence in the absence of both inhibitors and CPNs (negative control) represents 0% uptake. β-methyl cyclodextrin and LY 294002 are insoluble in media and were delivered to the cells in DMSO. The vehicle control reflects experiments in which equivalent volumes of DMSO were delivered to cells in the absence of inhibitors prior to incubation with CPNs in media. Data reflects the mean cell-associated fluorescence at 488 nm excitation for at least 3 independent measurements of 10,000 cells. Error bars are standard deviations.
Evaluation of nanoparticle toxicity
The utility of a given probe for biological imaging or analysis is compromised if the probe causes cell death or other deleterious effects. Hence, we evaluated the cytotoxicity of CPNs in cells over the concentration range likely to be used in cell imaging. First, cells were incubated with increasing amounts of CPNs for 16–18 h and the percentage of live cells was determined using the Cell Titer Blue Assay (Figure 8). Notably, the percentage of live J774.A1 cells in CPN treated wells were similar to the controls (i.e. cells that had not been exposed to CPNs) at every CPN concentration tested (P>0.05). These data indicate that CPNs have no discernable impact on cell viability over this concentration range. In addition, CPN loaded cells were examined under DIC illumination (x 600) using an epifluorescence microscope; we observed no detached cells or membrane blebbing that would reflect cell damage, even at the highest concentrations evaluated (67.5 ppm). Hence, these data indicate that these CPNs are not toxic to the macrophage cell line at the highest tested concentration. We note that the highest concentration evaluated for toxicity is ca. 25 fold higher than the working concentration needed for cell labeling.
Figure 8.
Cytotoxicity of CPNs. Cells were incubated with the indicated concentrations of nanoparticles for 16–18 hours, and viability analyzed using the Cell Titer Blue Assay. Percent viability is relative to cells incubated without CPNs. The standard deviation is shown based on the average of the wells used for each concentration. All nanoparticle concentrations resulted in P-values > 0.05 when compared with untreated control samples.
DISCUSSION
We have prepared small (24 nm) conjugated polymer nanoparticles via reprecipitation in the presence of methoxy PEG lipid. This method results in highly stable nanoparticles with extremely bright fluorescence, and different preparations consistently yield particles of equivalent size[11]; Nanoparticles of similar composition but larger size can also be prepared by miniemulsion[10, 34]. Reported outstanding figures of merit for CPNs suggest their application as labels for cellular imaging and analysis[31, 32, 42]. However, intracellular conjugated polymer nanoparticles brightness had not been evaluated with respect to competing labels. In this study, we compared the performance of CPNs with widely used commercially available fluorophores to analyze suitability for biological applications, including brightness under spectral condition likely to be used for both biological imaging and flow cytometry. These comparisons use 488 nm or 494 nm excitation, rather than the relative absorption maxima of either CPNs or Qdots, since these excitation wavelengths are commonly used for flow cytometry and imaging applications using argon ion lasers. In vitro comparison of fluorophore intensity in a steady state spectrofluorometer at an excitation wavelength of 488 nm indicates that when corrected for concentration differences; measured signal intensity for CPNs is ca. 160 times brighter than the Qdots and 600 times brighter than AF488 at this wavelength. However, the relative brightness of CPNs is much higher inside cells. We performed experiments in which cells were loaded with fluorophore by incubation with defined concentrations of fluorophore, and the fluorescence associated with those cells was evaluated by flow cytometry. Assuming equivalent cellular uptake and after correction for differences in fluorophore concentration, we estimate that the intracellular fluorescence signal of CPNs is 175 times brighter than the corresponding Qdots signal and 1400 times brighter than AF488-dex under these conditions. We note that neither CPNs nor Qdots are excited on resonance for these experiments, leading to somewhat reduced brightness for each of these fluorophores. However, the wavelengths chosen reflect conditions typically used for biological imaging and flow cytometry, and measured brightness obtained under these conditions is the appropriate comparison for these cell-based applications.
Flow cytometry experiments, as used here to assess intracellular signal, cannot distinguish between intracellular signal and signal resulting from fluorophore adsorption to the cell surface, and our reports of relative intracellular brightness assume that fluorophore signal measured by flow cytometry reflects intracellular fluorescence. This assumption is supported by experiments in which the different fluorophores were incubated with cells on ice, and the resulting cell-associated fluorescence was measured by flow cytometry. Very little resulting fluorescence was associated with cells under these conditions. Since low temperatures would necessarily inhibit active uptake but not interaction with the cell surface, the observed negligible fluorescence under these conditions suggests that any contribution from fluorophore interaction with the cell surface is small. We point out that Qdots show the largest amount of uptake and/or cell surface adsorption when incubated on ice and it is therefore probable that some quantity of Qdots are associated with the cell surface and/or taken into the cell by mechanism(s) not available to CPNs or dextran-coupled dyes.
Analysis of the mechanism of CPN uptake indicates that these particles are taken up by J774A.1 macrophage cells via fluid phase endocytosis. Co-localization of intracellular CPNs with TR-dex and inhibition of uptake in the presence of wortmannin, LY294002, and β methyl cyclodextrin give evidence that the mode of endocytosis is macropinocytosis, similar to our previous observations of uncoated PFBT nanoparticles uptake in macrophage cells[29]. In contrast to the majority of endocytic uptake mechanisms, macropinocytosis does not require interaction with the cell surface to initiate uptake[48]; instead, extracellular material is taken into the cell via spontaneous actin-mediated ruffles that nonspecifically enclose extracellular solution. Since it has been observed that PEG coating of nanoparticles can inhibit interaction with cell surface receptors responsible for other fluid-phase uptake mechanisms[9, 43], entry of CPNs via this nonspecific fluid phase uptake mechanism is perhaps not surprising. While macrophage cells have particularly high levels of constitutive macropinocytosis, many cell types can take in extracellular material by this mechanism[49], and would therefore be amenable to labeling with CPNs, with the modification of somewhat longer incubation time. For example, we have observed efficient conjugated polymer nanoparticle uptake into non-macrophage chinese hamster ovary (CHO) cells (unpublished data).
Co-localization of CPN fluorescence with TR-dex and labeled anti-LAMP-1 antibodies indicates that following macropinocytic uptake, these nanoparticles are trafficked from endosomes to lysosomes. In this respect, these nanoparticles behave similarly to the range of dextran-conjugated organic dyes such as Lucifer Yellow dextran, TR-dex, and FITC-dextran that are commonly used as fluid phase markers. Such fluorophores have been widely utilized as specific labels of endocytic compartments for analysis of cell function and endocytosis[1]. We suggest that the high brightness of these PEG-lipid CPNs, combined with their easy synthesis, resistance to aggregation, and photo-stability, makes them highly attractive substitutes for historically used fluid phase markers. For example, we have shown here that these characteristics allow them to be used as intracellular markers for flow cytometry.
We have previously demonstrated that functionalized PEG lipid-coated CPNs can be conjugated to biorecognition molecules and targeted to specific cell-surface receptors[11]. Howes et al have demonstrated coupling of larger PEG lipid coated conjugated polymer nanoparticles to BSA, as proof of principle that the PEG lipid functional group can be used to couple nanoparticles to proteins for targeted delivery[34]. Depending on the biorecognition molecule used and the choice of receptor to be targeted, such conjugation to specific biologically relevant molecules could initiate cellular uptake via receptor-mediated endocytic mechanisms, with subsequent delivery to individual cellular locations that could include the nucleus, cytosol, or individual organelles[50, 51]. In this case, targeted delivery with minimal competition from macropinocytic delivery would be made possible simply by using a cell line with low levels of constitutive macropinocytosis. In cell line with higher rates of macropinocytosis, targeted delivery could be facilitated by experimental conditions that minimize nonspecific endocytosis; targeted CPNs could be incubated with cells on ice and washed to remove CPNs not bound to cell surface receptors prior to incubation at physiological temperatures to allow endocytic uptake.
Notably, evaluations of possible CPN cytotoxicity show no impact of nanoparticles on cell viability or structure at all tested concentrations. Similar low cytotoxicity was observed for unmodified PFBT nanoparticles[29] and other conjugated polymer nanoparticles[31], indicating that the benign behavior of these nanoparticles may reflect the characteristics of the conjugated polymer core, rather than simple shielding by PEG. While loading concentrations tested for cell damage were low, they are well above concentrations likely to be used for cell studies, given the extreme fluorescent brightness of this label.
A dogma of fluorescence labeling in cellular biology is that the amount of fluorophore probe added to the cells is sufficiently small so as to not perturb cell function. Depending on the concentration, even routinely used probes can impact the system being measured (e.g. FITC-dextran is used to measure intralysosomal pH, but is also a weak acid that could potentially act as a buffer). Hence, the lower probe concentration required for a given application, the less likely the probe is to alter the system under observation, and extremely fluorescent probes suitable for use at low concentrations are very desirable. Data presented here clearly indicates that the extreme brightness of the CPNs allows use of very low labeling concentrations inside the cell. When combined with their low cytotoxicity, these evaluations clearly demonstrate the high utility of conjugated polymer nanoparticles as labels for both biological imaging and flow cytometry.
Acknowledgments
The authors are thankful to the National Institutes of Health (1 R01 GM081040) for financial support.
References
- 1.Swanson J. In: Methods in Cell Biology. Wang Y, Taylor DL, editors. Vol. 29. Academic Press; San Diego: 1989. [Google Scholar]
- 2.Alivisatos AP, Gu W, Larabell C. Quantum dots as cellular probes. Annu Rev Biomed Eng. 2005;7:55. doi: 10.1146/annurev.bioeng.7.060804.100432. [DOI] [PubMed] [Google Scholar]
- 3.Abrahamse SL, Rechkemmer G. Identification of an organic anion transport system in the human colon carcinoma cell line HT29 clone 19A. Pflugers Arch. 2001;441:529. doi: 10.1007/s004240000437. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez-Suarez M, Ting AY. Fluorescent probes for super-resolution imaging in living cells. Nat Rev Mol Cell Biol. 2008;9:929. doi: 10.1038/nrm2531. [DOI] [PubMed] [Google Scholar]
- 5.Biju V, Itoh T, Anas A, Sujith A, Ishikawa M. Semiconductor quantum dots and metal nanoparticles: syntheses, optical properties, and biological applications. Anal Bioanal Chem. 2008;391:2469. doi: 10.1007/s00216-008-2185-7. [DOI] [PubMed] [Google Scholar]
- 6.Zheng J, Nicovich PR, Dickson RM. Highly fluorescent noble-metal quantum dots. Annu Rev Phys Chem. 2007;58:409. doi: 10.1146/annurev.physchem.58.032806.104546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baykal B, Ibrahimova V, Er G, Bengü E, Tuncel D. Dispersion of multi-walled carbon nanotubes in an aqueous medium by water-dispersible conjugated polymer nanoparticles. Chem Commun (Camb) 2010;46:6762. doi: 10.1039/c0cc00510j. [DOI] [PubMed] [Google Scholar]
- 8.Yu WW, Chang E, Falkner JC, Zhang J, Al-Somali A, Sayes CM, Johns J, Drezek R, Colvin VL. Forming biocompatible and nonaggregated nanocrystals in water using amphiphilic polymers. J Am Chem Soc. 2007;129:2871. doi: 10.1021/ja067184n. [DOI] [PubMed] [Google Scholar]
- 9.Smith AM, Duan H, Rhyner MN, Ruan G, Nie S. A systematic examination of surface coatings on the optical and chemical properties of semiconductor quantum dots. Phys Chem Chem Phys. 2006;8:3895. doi: 10.1039/b606572b. [DOI] [PubMed] [Google Scholar]
- 10.Howes P, Green M. Colloidal and optical stability of PEG-capped and phospholipid-encapsulated semiconducting polymer nanospheres in different aqueous media. Photochem Photobiol Sci. 2010;9:1159. doi: 10.1039/c0pp00106f. [DOI] [PubMed] [Google Scholar]
- 11.Kandel PK, Fernando LP, Ackroyd PC, Christensen KA. Incorporating functionalized polyethylene glycol lipids into reprecipitated conjugated polymer nanoparticles for bioconjugation and targeted labeling of cells. Nanoscale. 2011;3:1037. doi: 10.1039/c0nr00746c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu C, Szymanski C, McNeill J. Preparation and encapsulation of highly fluorescent conjugated polymer nanoparticles. Langmuir. 2006;22:2956. doi: 10.1021/la060188l. [DOI] [PubMed] [Google Scholar]
- 13.Delehanty JB, Mattoussi H, Medintz IL. Delivering quantum dots into cells: strategies, progress and remaining issues. Anal Bioanal Chem. 2009;393:1091. doi: 10.1007/s00216-008-2410-4. [DOI] [PubMed] [Google Scholar]
- 14.Walling MA, Novak JA, Shepard JRE. Quantum Dots for Live Cell and In Vivo Imaging. Int J Mol Sci. 2009;10:441. doi: 10.3390/ijms10020441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clift MJD, Rothen-Rutishauser B, Brown DM, Duffin R, Donaldson K, Proudfoot L, Guy K, Stone V. The impact of different nanoparticle surface chemistry and size on uptake and toxicity in a murine macrophage cell line. Toxicol Appl Pharmacol. 2008;232:418. doi: 10.1016/j.taap.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Ryman-Rasmussen JP, Riviere JE, Monteiro-Riviere NA. Surface coatings determine cytotoxicity and irritation potential of quantum dot nanoparticles in epidermal keratinocytes. J Invest Dermatol. 2007;127:143. doi: 10.1038/sj.jid.5700508. [DOI] [PubMed] [Google Scholar]
- 17.Fadeel B, Garcia-Bennett A. Better safe than sorry: Understanding the toxicological properties of inorganic nanoparticles manufactured for biomedical applications. Adv Drug Deliv Rev. 2010;62:362. doi: 10.1016/j.addr.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Tian Z, Yu J, Wu C, Szymanski C, McNeill J. Amplified energy transfer in conjugated polymer nanoparticle tags and sensors. Nanoscale. 2010;2:1999. doi: 10.1039/c0nr00322k. [DOI] [PubMed] [Google Scholar]
- 19.Wu CF, Zheng YL, Szymanski C, McNeill J. Energy transfer in a nanoscale multichromophoric system: Fluorescent dye-doped conjugated polymer nanoparticles. J Phys Chem C. 2008;112:1772. doi: 10.1021/jp074149+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu CF, Peng HS, Jiang YF, McNeill J. Energy transfer mediated fluorescence from blended conjugated polymer nanoparticles. J Phys Chem B. 2006;110:14148. doi: 10.1021/jp0618126. [DOI] [PubMed] [Google Scholar]
- 21.Yu J, Wu C, Sahu SP, Fernando LP, Szymanski C, McNeill J. Nanoscale 3D tracking with conjugated polymer nanoparticles. J Am Chem Soc. 2009;131:18410. doi: 10.1021/ja907228q. [DOI] [PubMed] [Google Scholar]
- 22.Wu C, Szymanski C, Cain Z, McNeill J. Conjugated polymer dots for multiphoton fluorescence imaging. J Am Chem Soc. 2007;129:12904. doi: 10.1021/ja074590d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu C, Bull B, Christensen K, McNeill J. Ratiometric single-nanoparticle oxygen sensors for biological imaging. Angew Chem Int Ed Engl. 2009;48:2741. doi: 10.1002/anie.200805894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan YH, Wu C, Ye F, Jin Y, Smith PB, Chiu DT. Development of ultrabright semiconducting polymer dots for ratiometric pH sensing. Anal Chem. 2011;83:1448. doi: 10.1021/ac103140x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tuncel D, Demir HV. Conjugated polymer nanoparticles. Nanoscale. 2010;2:484. doi: 10.1039/b9nr00374f. [DOI] [PubMed] [Google Scholar]
- 26.Landfester K. Miniemulsion Polymerization and the Structure of Polymer and Hybrid Nanoparticles. Angew Chem Int Ed. 2009;48:4488. doi: 10.1002/anie.200900723. [DOI] [PubMed] [Google Scholar]
- 27.Landfester K, Montenegro R, Scherf U, Guntner R, Asawapirom U, Patil S, Neher D, Kietzke T. Semiconducting polymer nanospheres in aqueous dispersion prepared by a miniemulsion process. Adv Mat. 2002;14:651. [Google Scholar]
- 28.Clafton SN, Beattie DA, Mierczynska-Vasilev A, Acres RG, Morgan AC, Kee TW. Chemical defects in the highly fluorescent conjugated polymer dots. Langmuir. 2010;26:17785. doi: 10.1021/la103063p. [DOI] [PubMed] [Google Scholar]
- 29.Fernando LP, Kandel PK, Yu J, McNeill J, Ackroyd PC, Christensen KA. Mechanism of cellular uptake of highly fluorescent conjugated polymer nanoparticles. Biomacromolecules. 2010;11:2675. doi: 10.1021/bm1007103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiss CK, Lorenz MR, Landfester K, Mailänder V. Cellular uptake behavior of unfunctionalized and functionalized PBCA particles prepared in a miniemulsion. Macromol Biosci. 2007;7:883. doi: 10.1002/mabi.200700046. [DOI] [PubMed] [Google Scholar]
- 31.Moon JH, McDaniel W, MacLean P, Hancock LE. Live-cell-permeable poly (p-phenylene ethynylene) Angew Chem Int Ed. 2007;19:8223. doi: 10.1002/anie.200701991. [DOI] [PubMed] [Google Scholar]
- 32.Wu C, Bull B, Szymanski C, Christensen K, McNeill J. Multicolor Conjugated Polymer Dots for Biological Fluorescence Imaging. ACS Nano. 2008;2:2415. doi: 10.1021/nn800590n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howes P, Thorogate R, Green M, Jickells S, Daniel B. Synthesis, characterisation and intracellular imaging of PEG capped BEHP-PPV nanospheres. Chem Commun (Camb) 2009:2490. doi: 10.1039/b903405f. [DOI] [PubMed] [Google Scholar]
- 34.Howes P, Green M, Levitt J, Suhling K, Hughes M. Phospholipid encapsulated semiconducting polymer nanoparticles: their use in cell imaging and protein attachment. J Am Chem Soc. 2010;132:3989. doi: 10.1021/ja1002179. [DOI] [PubMed] [Google Scholar]
- 35.Hama Y, Urano Y, Koyama Y, Bernardo M, Choyke PL, Kobayashi H. A comparison of the emission efficiency of four common green fluorescence dyes after internalization into cancer cells. Bioconjug Chem. 2006;17:1426. doi: 10.1021/bc0601626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Longmire MR, Ogawa M, Hama Y, Kosaka N, Regino CA, Choyke PL, Kobayashi H. Determination of optimal rhodamine fluorophore for in vivo optical imaging. Bioconjug Chem. 2008;19:1735. doi: 10.1021/bc800140c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Racoosin EL, Swanson JA. Macropinosome maturation and fusion with tubular lysosomes in macrophages. J Cell Biol. 1993;121:1011. doi: 10.1083/jcb.121.5.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang LW, Monteiro-Riviere NA. Mechanisms of Quantum Dot nanoparticle cellular uptake. Toxicol Sci. 2009;110:138. doi: 10.1093/toxsci/kfp087. [DOI] [PubMed] [Google Scholar]
- 39.Foged C, Brodin B, Frokjaer S, Sundblad A. Particle size and surface charge affect particle uptake by human dendritic cells in an in vitro model. Int J Pharm. 2005;298:315. doi: 10.1016/j.ijpharm.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 40.Dausend J, Musyanovych A, Dass M, Walther P, Schrezenmeier H, Landfester K, Mailander V. Uptake mechanism of oppositely charged fluorescent nanoparticles in HeLa cells. Macromol Biosci. 2008;8:1135. doi: 10.1002/mabi.200800123. [DOI] [PubMed] [Google Scholar]
- 41.Lai SK, Hida K, Chen C, Hanes J. Characterization of the intracellular dynamics of a non-degradative pathway accessed by polymer nanoparticles. J Control Release. 2008;125:107. doi: 10.1016/j.jconrel.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wu C, Schneider T, Zeigler M, Yu J, Schiro PG, Burnham DR, McNeill JD, Chiu DT. Bioconjugation of ultrabright semiconducting polymer dots for specific cellular targeting. J Am Chem Soc. 2010;132:15410. doi: 10.1021/ja107196s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang E, Thekkek N, Yu WW, Colvin VL, Drezek R. Evaluation of quantum dot cytotoxicity based on intracellular uptake. Small. 2006;2:1412. doi: 10.1002/smll.200600218. [DOI] [PubMed] [Google Scholar]
- 44.Luby-Phelps K. In: Methods in Cell Biology. Wang Y, editor. Vol. 29. Academic Press; San Diego: 1989. [Google Scholar]
- 45.Woodman PG, Futter CE. Multivesicular bodies: co-ordinated progression to maturity. Curr Opin Cell Biol. 2008;20:408. doi: 10.1016/j.ceb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Humphries WH, 4th, Szymanski CJ, Payne CK. Endo-lysosomal vesicles positive for Rab7 and LAMP1 are terminal vesicles for the transport of dextran. PLoS One. 2011 doi: 10.1371/journal.pone.0026626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Bio. 1996;135:1249. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009;10:364. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- 49.Jones AT. Macropinocytosis: searching for an endocytic identity and role in the uptake of cell penetrating peptides. J Cell Mol Med. 2007;11:670. doi: 10.1111/j.1582-4934.2007.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nahar M, Dutta T, Murugesan S, Asthana A, Mishra D, Rajkumar V, Tare M, Saraf S, Jain NK. Functional polymeric nanoparticles: an efficient and promising tool for active delivery of bioactives. Crit Rev Ther Drug Carrier Syst. 2006;23:259. doi: 10.1615/critrevtherdrugcarriersyst.v23.i4.10. [DOI] [PubMed] [Google Scholar]
- 51.Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: Peptoid molecular transporters. Proc Natl Acad Sci U S A. 2000;97:13003. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]