Abstract
Blood transfusion is a well-established risk factor for adverse outcomes during sepsis. The specific mechanisms responsible for this effect remain elusive, and few studies have investigated this phenomenon in a model that reflects not only the clinical circumstances in which blood is transfused, but also how packed red blood cells (PRBC) are created and stored. Using a cecal ligation and puncture model of polymicrobial sepsis as well as creating murine allogeneic and stored PRBC in a manner that replicates the clinical process, we have demonstrated that transfusion of PRBCs induces numerous effects on leukocyte subpopulations. In polymicrobial sepsis, these responses are profoundly dissimilar to the pro-inflammatory effects of PRBC transfusion observed in the healthy mouse. Transfused septic mice, as opposed to mice receiving crystalloid resuscitation, had a significant loss of blood, spleen and bone marrow lymphocytes, especially those with an activated phenotype. Myeloid cells behaved similarly, although they were able to produce more reactive oxygen species. Overall, transfusion in the septic mouse may contribute to the persistent immune dysfunction known to be associated with this process, rather than simply promote pro- or anti-inflammatory effects on the host. Thus, it is possible that blood transfusion contributes to the multiple known effects of sepsis on leukocyte populations that have been shown to result in increased morbidity and mortality.
Keywords: cytokine, leukocyte, mouse, cecal ligation and puncture
Introduction
Sepsis is the leading cause of death in the critically ill with 750,000 cases and 210,000 deaths annually (1); in fact, in the general surgery population, the incidence of sepsis is greater than the incidence of pulmonary embolism and myocardial infarction combined (2). Sepsis mortality has been attributed to derangements in the innate and adaptive immune systems (3). In addition, there are well known co-factors that are associated with an increased morbidity and mortality during sepsis, including blood transfusion (4). These associations have led to restrictive policies for transfusion in many hospitals in an attempt to improve outcomes (4).
Evidence increasingly demonstrates the adverse effects of blood transfusions in terms of subsequent patient infection and mortality. In trauma and critically ill patients, blood transfusions are associated with increased morbidity and mortality, especially with increased amounts of transfusion, as well as with the transfusion of blood that has been stored for longer periods of time (5, 6). Blood transfusion is an independent risk factor for ventilator-associated pneumonia, perioperative/post-injury infection, and multiple organ dysfunction syndrome; in fact, the effect of blood transfusions being associated with poor outcomes is present even after adjustment for confounding factors such as age and severity of injury (6, 7).
Although the cause of the increased morbidity and mortality associated with packed red blood cell (PRBC) transfusion is assuredly multifactorial, one of the major causes is believed to be the effect of PRBC transfusion on host protective immunity (8, 9). The mechanisms behind blood transfusion-induced alterations in protective immunity remain unclear. Although debated, allogeneic, stored PRBCs are presumed to induce T-cell anergy, leukocyte dysfunction and expansion of immunosuppressive cell populations (10, 11).
Analysis of transfusion in a clinically relevant animal model is lacking. This is of importance because the contribution of transfusion to poor outcomes is generally associated with critically ill patients or individuals who have sustained a major systemic inflammatory response, whether from surgery or trauma. In fact, the only randomized control trials that illustrate worsened outcomes with PRBC transfusion (in certain subsets) have been carried out in ICU populations (TRICC and TRACS trials) (12, 13).
There are several challenges that have hindered translational research regarding PRBCs’ impact on this patient population. Difficulties exist in creating murine allogeneic stored packed red blood cells similar to what would be given to a human (14), as well as limitations in murine models of sepsis and trauma (15). Finally, there is a degree of anemia, especially in regard to hemorrhagic or traumatic shock where the immediate benefits of blood transfusion outweigh any risk (16). Therefore, isolating the mechanisms behind this well-known phenomenon has remained problematic.
In this report, we have attempted created a murine model of blood transfusion that better recapitulates the treatment of typical human PRBCs, including storage and allogenicity, and then to determine the effect of transfusion on the immune system in both healthy animals, as well as in a clinically relevant model of murine sepsis. We propose that transfusion of PRBCs will result in the alteration of the phenotype and functionality of specific immune effector cell populations dependent upon whether the animal is healthy or septic. In the septic individual, these transfusions alter immune function, possibly leading to further risk for infection, end organ damage and mortality.
Materials and Methods
Mice
All experiments were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Florida. Specific pathogen-free male C57BL/6 and BALB/c mice were purchased from the Jackson Laboratory (Bar Harbor, ME) at 6–7 weeks of age and allowed to acclimatize for one week before being used for experimental procedures. Mice were maintained on standard rodent food and water ad libitum.
Allogeneic and stored PRBCs
BALB/c mice were placed in a CO2 chamber until they become unresponsive, and then blood was immediately collected by intracardiac puncture using heparinized syringe. Whole blood was placed in sterile 1.6 ml tubes containing citrate-phosphate-dextrose anticoagulant-preservative (CPDA) solution (1:6), mixed gently, and centrifuged at 4,000 rpm for 5 minutes at 4°C. The plasma and buffy coat layers were carefully aspirated and Adsol™ additive solution was added to the PRBCs (1:6) and stored at 4° C in the dark until use (14 days). Hematocrit was measured at different time points (60% at day 0) and no significant changes were identified up to 14 days (57%).
Cecal Ligation and Puncture (CLP)
For induction of polymicrobial sepsis in C57BL/6 (B6) mice, CLP was performed under isoflurane anesthesia as previously described (17). Briefly, cecum was exposed after a laparotomy and ligated with 2-0 silk suture, and punctured through and through with a 25-gauge needle. The cecum was returned in the abdomen and the incision was closed using surgical clips. After the procedure, the mice were administered 0.05–2 mg/kg buprenorphine in 1 ml 0.9 % saline, returned to their respective cages and closely monitored for any signs of distress. The IACUC requires euthanasia for moribund mice that are then considered as non-survivors.
Transfusion
B6 mice underwent cannulation of one of the femoral arteries under isoflurane anesthesia as previously described (18). Total blood volume for the mouse was estimated to be 70 ml/kg, or 1.75 mls of blood for a 25 gm mouse. Animals were transfused with sterile room temperature Ringer’s Lactate (LR, 1mL) or 600–700 µL of stored allogeneic PRBC (derived from BALB/C mice), which is equivalent to 3–4 units of human PRBCs. The volume difference between the crystalloid and PRBC delivered to the mouse was to compensate for the differences in intravascular volume between colloid and crystalloid. In addition, mice were not bled prior to transfusion or infusion of crystalloid as we did not wish to add shock to one of the variables to consider nor create significant anemia in the crystalloid group of mice as a confounder. Mice that underwent CLP were transfused on post-operative day two. Again, mice were not bled prior to transfusion or crystalloid infusion as septic mice require resuscitation (and they would be at risk for death if we remove further intravascular volume) as well as not wishing to create severe anemia in the crystalloid group. Mice were subsequently sacrificed one day post-transfusion and their blood, spleen, and bone marrow were harvested as previously described (19).
Flow Cytometry
Spleens, whole blood, and bone marrow were harvested and single cell suspensions were created by passing the cells through 70 µm pore sized cell strainers (BD Falcon, Durham, NC). Erythrocytes were then lysed using ammonium chloride lysis buffer and washed two times using phosphate-buffered saline (PBS) without calcium, phenol red, or magnesium. Cells were stained with the following antibodies for flow cytometric studies: FITC anti-CD8, FITC anti-CD11c, PE anti-CD69, PE anti-IA/IE, APC anti-CD4, PE Cy7 anti-CD25, PE Cy7 anti-CD11b, APC anti- Gr-1, and Pacific Blue anti-CD19 and anti-Ly6G (BD Pharmingen, Billerica, MA). Samples were acquired and analyzed using an LSRII flow cytometer (BD Biosciences) and FACSDiva™ (BD Biosciences) (19).
LPS stimulation
106 splenocytes were placed in 24-well culture plates (Corning Life Sciences, Lowell, MA) with 500 µL Dulbecco’s modified Eagle medium (DMEM, Cellgro, Manasa, VA) supplemented with 10% fetal calf serum, 10 mM HEPES, glutamine, and penicillin/streptomycin (complete DMEM). Cultured cells were stimulated with 1 µg/ml LPS (Escherichia coli O26:B6; Sigma-Aldrich; St Louis, MO). Culture supernatant was harvested 24 hours later and stored at −80°C until assayed.
Cytokine Production
One day after transfusion, whole blood was harvested by intracardiac puncture. The plasma was collected and stored at −80°C until the time of analysis. Assessments of cytokine concentrations from the mouse plasma and culture supernatant were performed using a commercially-available multiplexed Luminex kit (MILLIPLEX MAP, Mouse cytokine/Chemokine Panel; Millipore, Bellirica, MA). Cytokines evaluated included interleukin (IL)-1β, IL-6, IL-12 (p70), Interferon-inducible protein (IP)-10, keratinocyte-derived chemokine (KC), monocyte chemoattractant protein (MCP)-1, macrophage inflammatory protein (MIP) -1α, and tumor necrosis factor (TNF) α. All assays were performed according to the manufacturer’s protocols. Cytokine concentrations were determined using BeadView™ software (Millipore).
Reactive oxygen species detection
Splenocytes were prepared using a Ficoll density gradient (1.104 specific gravity) and washed using PBS without calcium, phenol red, or magnesium. Cells were then labeled for surface markers as described, and washed twice with PBS. Reactive oxygen species (ROS) production was determined using dihydrorhodamine 123 (DHR123; Invitrogen, Carlsbad CA). Subsequently, cells were stimulated with PMA at 37°C and evaluated by flow cytometry analysis at various points over the subsequent 30 min period. A minimum of 1 × 104 live, non-debris cells were collected for analysis.
Statistics
Continuous variables were first tested for normality and equality of variances. Differences among groups in flow cytometric analyses were evaluated using Student’s t-test. All statistical analyses were performed using GraphPad Prism 5.0 (GraphPad software, La Jolla, CA).
Results
Transfusion of stored allogeneic PRBC in a naïve mouse acutely induces a pro-inflammatory response
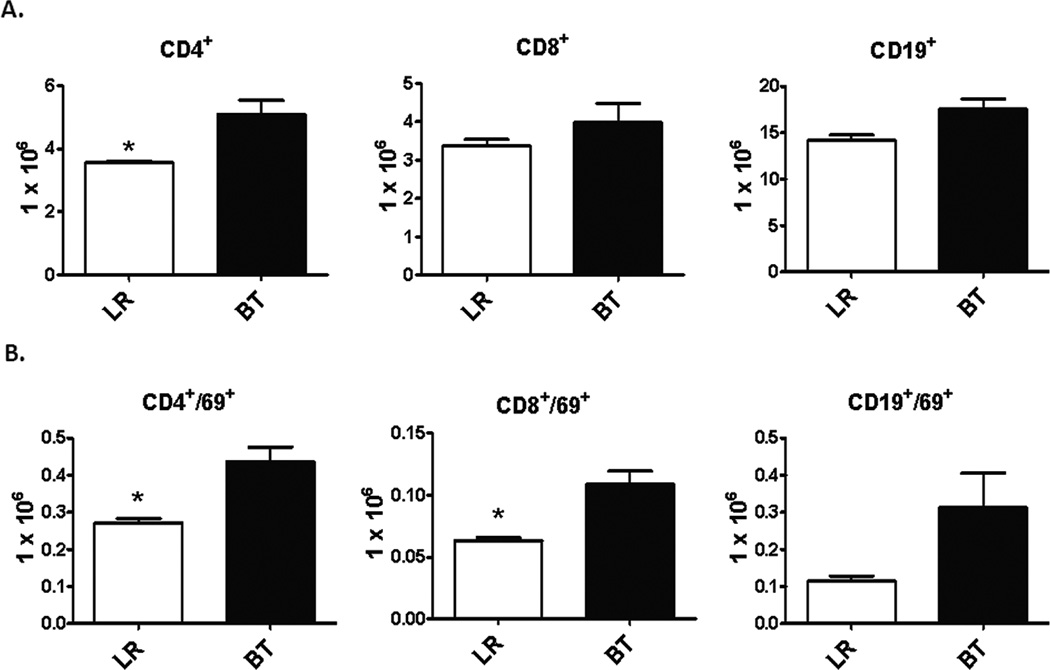
We initially sought to determine what the baseline immune response was to transfusion of aged, allogeneic PRBCs in a healthy animal. Twenty four hours after transfusion, analysis of the spleen demonstrated that there were significantly greater numbers of splenic CD4+ T cells, as well as a trend for increased CD8+ T cells and CD19+ B cells (Figure 1A). Almost all the lymphocytes had an increased activation status as determined by CD69 expression (Figure 1B). There was also a trend towards an increase in the number of CD11b+ myeloid cells (data not shown). There was no significant difference in the total number of leukocytes present in the spleens of animals that received Lactated Ringer’s solution (LR) or PRBC (data not shown).
Figure 1. Absolute numbers and activation status of splenocytes after PRBC transfusion in naïve mice.
Spleens from C57Bl/6 mice were harvested one day after receiving either allogeneic PRBC (n=3) or LR solution (n=3). Cellular phenotype were analyzed by flow cytometry using anti-CD4 (T-helper cells), anti-CD8 (cytotoxic T lymphocytes), and anti-CD19 (B cells). The activation status was determined by using anti-CD69. Data are shown in absolute numbers and are representative of three separate experiments, with an n=3 per experiment. (*p<0.05).
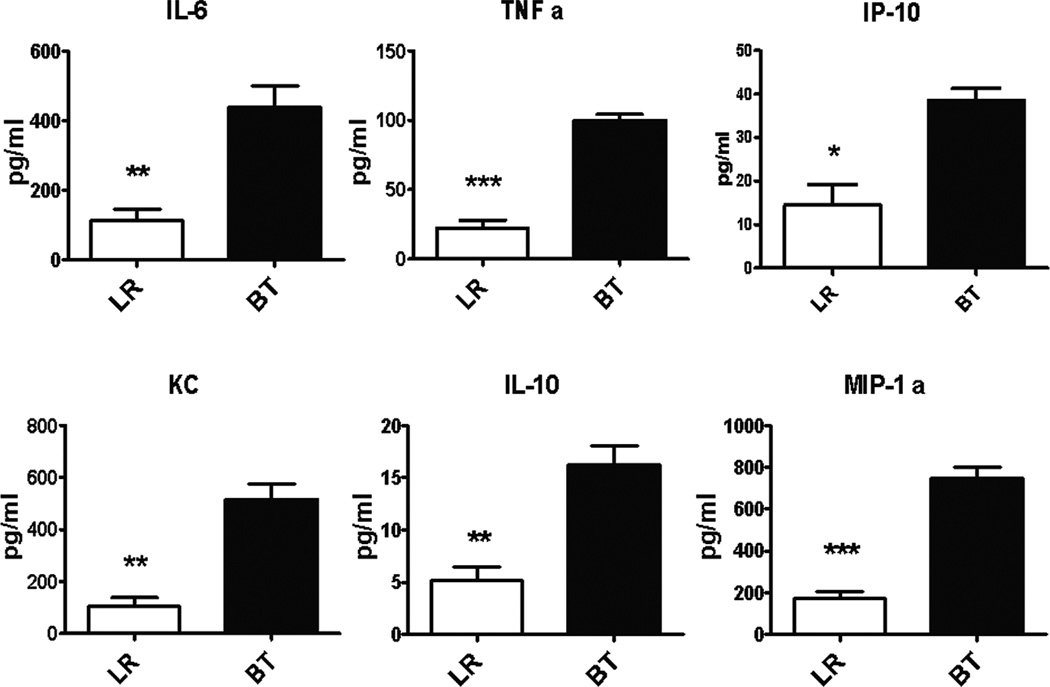
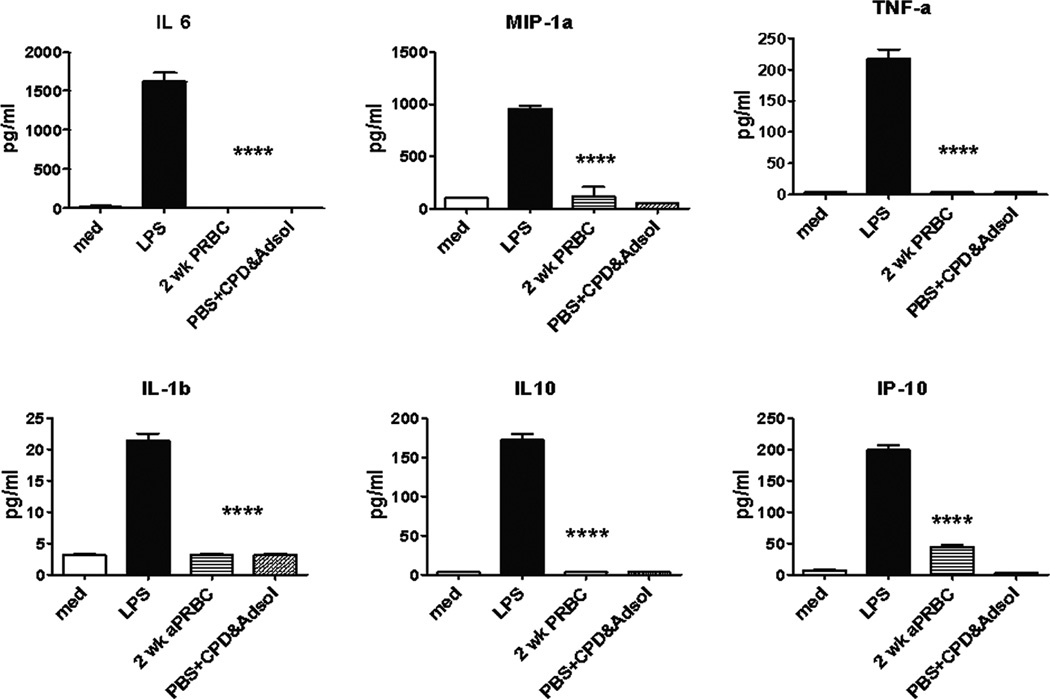
Analysis of these splenocytes further reinforced the concept that PRBC transfusion in a healthy animal has a similar effect to other inflammatory stimuli. Splenocytes from transfused mice produced significantly more proinflammatory cytokines and chemokines in response to ex vivo LPS stimulation (Figure 2). Exposure of splenocytes to 2 wk old PRBC or preservative solutions, CPDA and Adsol™, in vitro were not able to independently induce an exaggerated cytokine/chemokine response (Figure 3). Thus, although splenocytes from mice that received blood transfusion were more responsive to LPS stimulation, co-culturing of blood, blood products or preservatives with naïve spleen cells did not induce a similar increase in the production of cytokines/chemokines, indicating the absence of LPS contamination in the transfusate. Although not significant, pro-inflammatory cytokines in the plasma were detected at higher concentrations in PRBC-transfused healthy mice compared to mice that received LR solution (Figure 4).
Figure 2. Splenocyte cytokine and chemokine secretion of naïve mice after PRBC transfusion.
Spleens from C57Bl/6 mice were harvested one day after receiving either allogeneic PRBC (n=3) or LR solution (n=3). One million spleen cells were treated with LPS (1 µg/ml). Culture supernatant were collected 24 hours later and assessed for cytokine/chemokine production using multiplex Luminex kit. Data was confirmed by a second experiment (*p<0.05; **p<0.01, ***p<0.001).
Figure 3. Splenocyte cytokine and chemokine secretion after exposure to PRBC, preservative solution, or LPS.
One million spleen cells from naïve mice were treated with either LPS (1 µg/ml), PRBC (30% volume), or CPD and Adsol solution (30% volume). Cells in medium only served as untreated controls. Culture supernatants were collected 24 hours later and assessed for cytokine/chemokine production using multiplex Luminex kit. ****p<0.0001, one-way ANOVA.
Figure 4. Plasma cytokine and chemokine production of naïve mice after PRBC transfusion.
Blood was collected from mice one day after receiving either allogeneic PRBC (n=9) or LR (n=9) in heparinized syringe by intra-cardiac puncture. Plasma cytokine/chemokine levels were measured using multiplex Luminex kit. Data shown is the mean from three separate experiments.
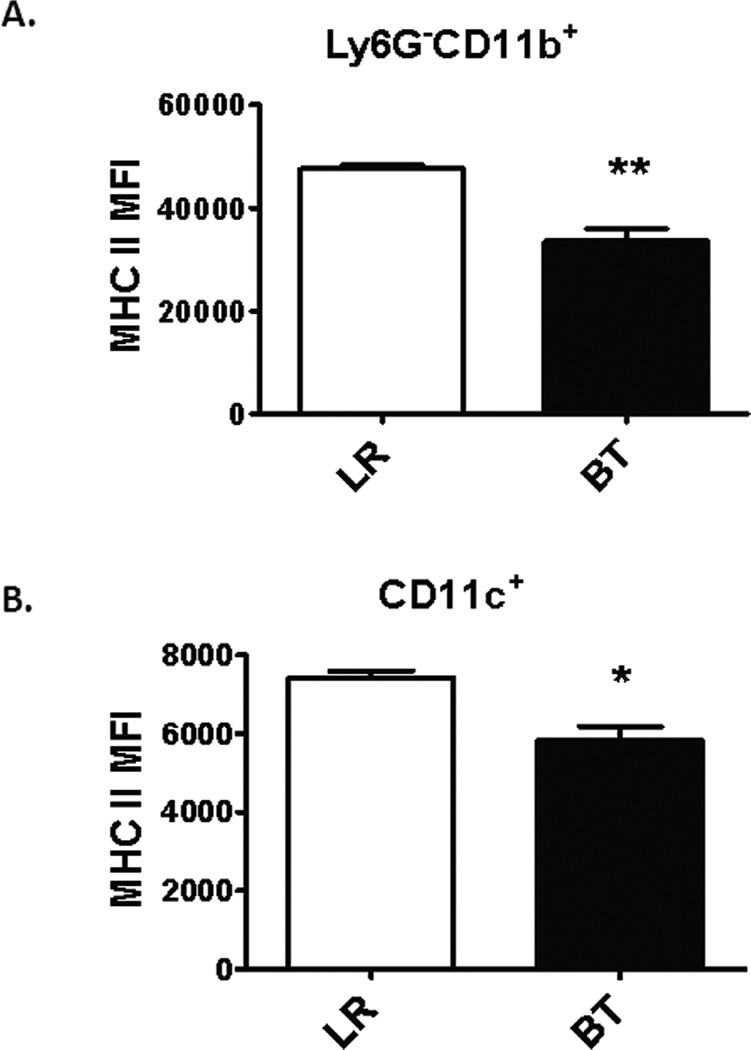
Pro-inflammatory events associated with exposure to microbial products or danger signals can also lead to the eventual down regulation of cell surface markers that are upregulated in the early acute inflammatory period. One prototypical response to an inflammatory event is the loss of MHCII+ expression on circulating monocytes as well as other myeloid-derived cells (20). Interestingly, one day after transfusion, there was a significant decrease in the mean fluorescence intensity (MFI) of circulating MHCII+ expression on both Ly6G−CD11b+ monocytic (Figure 5A) and CD11c+ dendritic cells (Figure 5B) consistent with reduced antigen presentation capacity.
Figure 5. MHCII+ status of circulating monocytes and dendritic cells in naïve mice after PRBC transfusion.
Blood was collected from C57Bl/6 mice one day after receiving either allogeneic PRBC (n=3) or LR (n=3) in heparinized syringe by intra-cardiac puncture. The MHCII expression (IA/IE+) of monocytes (Ly6G−CD11b+) and dendritic cells (CD11c+) were analyzed by flow cytometry. Data shown are representative of three separate experiments (*p<0.05; **p<0.01).
The response of leukocytes to allogeneic stored PRBC during sepsis is different to that of healthy mice
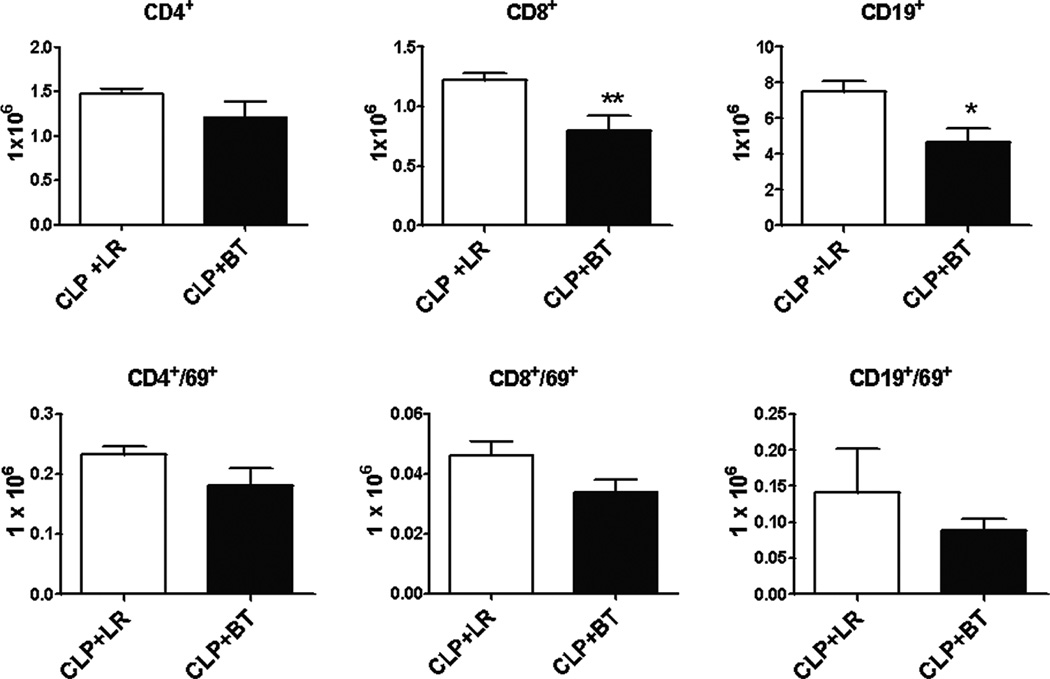
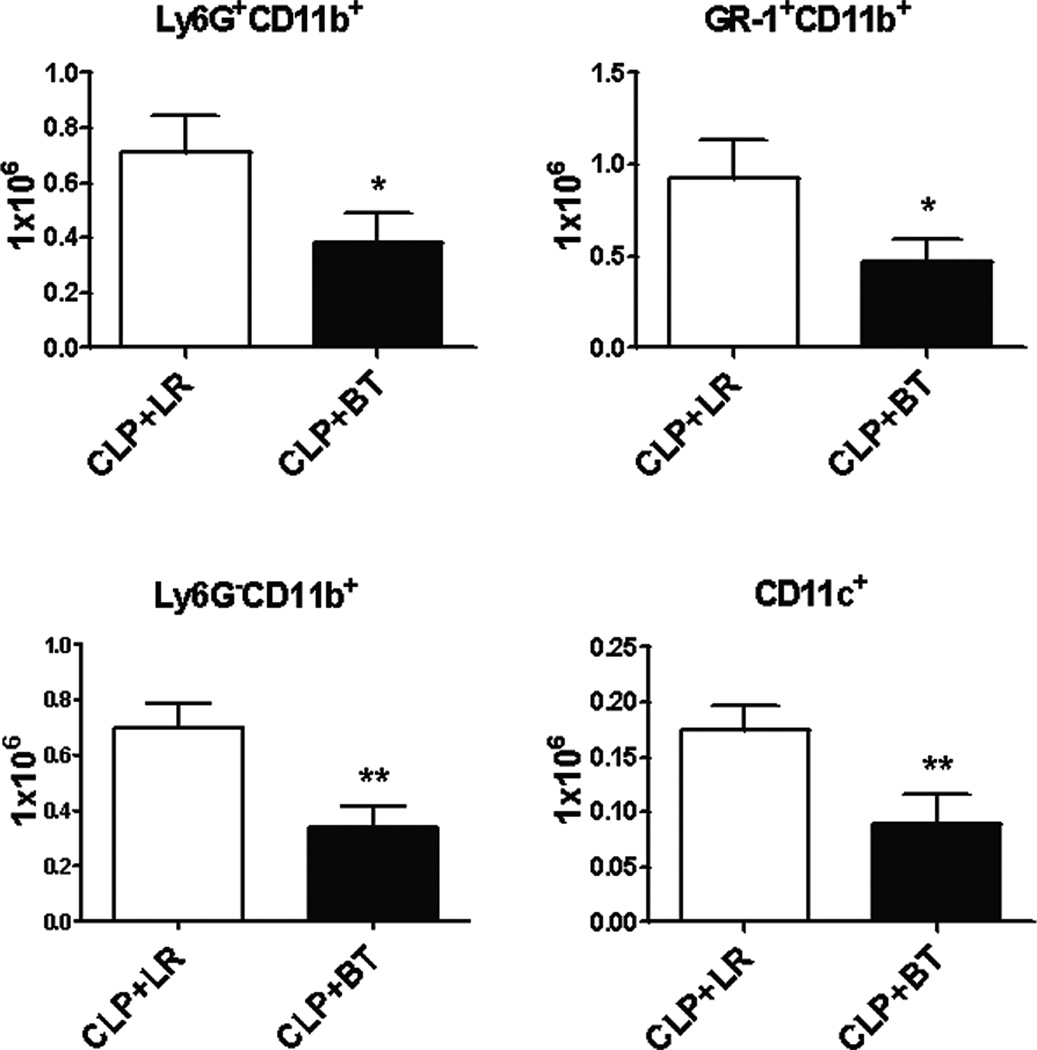
Surprisingly, PRBC transfusion following polymicrobial sepsis induced alterations that are dissimilar to the effects observed in a healthy mouse. Interestingly, PRBC administration was associated with a significant loss of CD8+ T cells and CD19+ B cells in the spleen, in contrast to an increase seen in naïve mice. In addition, the activation status of most lymphocytes, as determined by CD69 expression was decreased, rather than increased as compared to animals receiving crystalloid solution (Figure 6 and Table 1). In addition, there was also an absolute loss of splenic, myeloid-derived leukocytes, including Ly6G+CD11b+ neutrophils, Ly6G−CD11b+ monocytes, Gr-1+CD11b+ myeloid cells and CD11c+ dendritic cells (Figure 7). Associated with this was a further overall loss in the total number of leukocytes present in the spleen (data not shown).
Figure 6. Absolute numbers and activation status of splenocytes after PRBC transfusion in septic mice.
Male C57BL/6 mice received either allogeneic PRBC (n=11) or LR (n=9) two days after CLP. Spleens were harvested one day after transfusion and analyzed by flow cytometry using anti-CD4 (T-helper cells), anti-CD8 (cytotoxic T lymphocytes), and anti-CD19 (B cells). The activation status was determined by using anti-CD69. Data shown are the mean from three separate experiments (*p<0.05; **p<0.01).
Table 1.
Absolute cell counts in spleen one day after LR or PRBC transfusiona
| Absolute cell count (× 106) |
CD4 | CD4/69 | CD8 | CD8/69 | CD19 | CD19/69 |
|---|---|---|---|---|---|---|
| LR | 3.6 ± 0.04*# | 0.3 ± 0.01* | 3.4 ± 0.16# | 0.06 ± 0.01* | 14.2 ± 0.5# | 0.1 ± 0.01 |
| BT | 5.1 ± 0.44*§ | 0.4 ± 0.04*§ | 4.0 ± 0.49§ | 0.11± 0.01*§ | 17.5 ± 1.1§ | 0.3 ± 0.09 |
| CLP+LR | 1.5 ± 0.06# | 0.2 ± 0.01 | 1.2 ± 0.06#+ | 0.05 ± 0.01 | 7.5 ± 0.6#+ | 0.14± 0.06 |
| CLP+BT | 1.2 ± 0.18§ | 0.2 ± 0.03§ | 0.8± 0.12§+ | 0.03 ± 0.01§ | 4.6 ± 0.8§+ | 0.1 ± 0.02 |
Values represent the mean ± SE.
p<0.05, LR vs BT;
p<0.05, LR vs CLP+LR;
p<0.05, BT vs CLP+BT;
p<0.05, CLP+LR vs CLP+BT
Figure 7. Absolute numbers of splenic myeloid cells after PRBC transfusion in CLP mice.
Myeloid derived leukocytes from spleens of septic mice were analyzed by flow cytometry using anti-CD11b and anti-Ly6G (neutrophils), anti-Gr-1 and anti-CD11b (MDSCs), and anti-CD11c (dendritic cells). Monocytes were CD11b+ and Ly6G−. Data shown are the mean of three separate experiments (*p<0.05; **p<0.01).
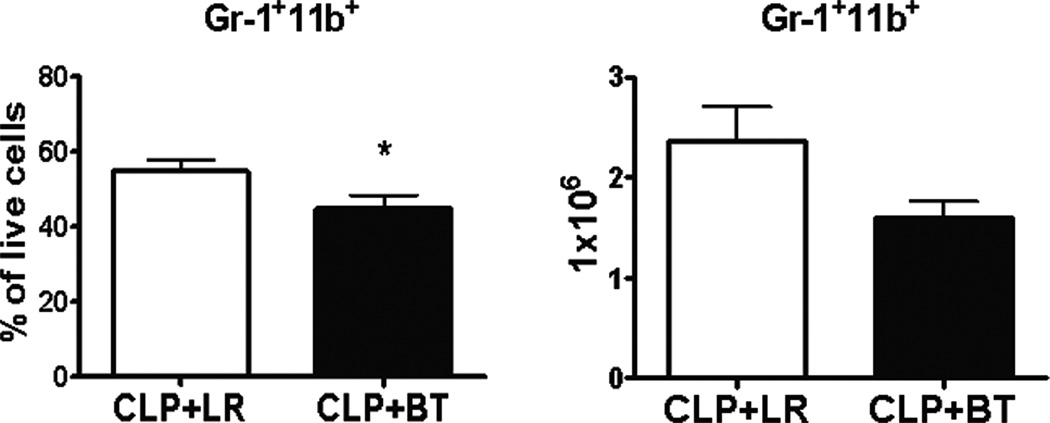
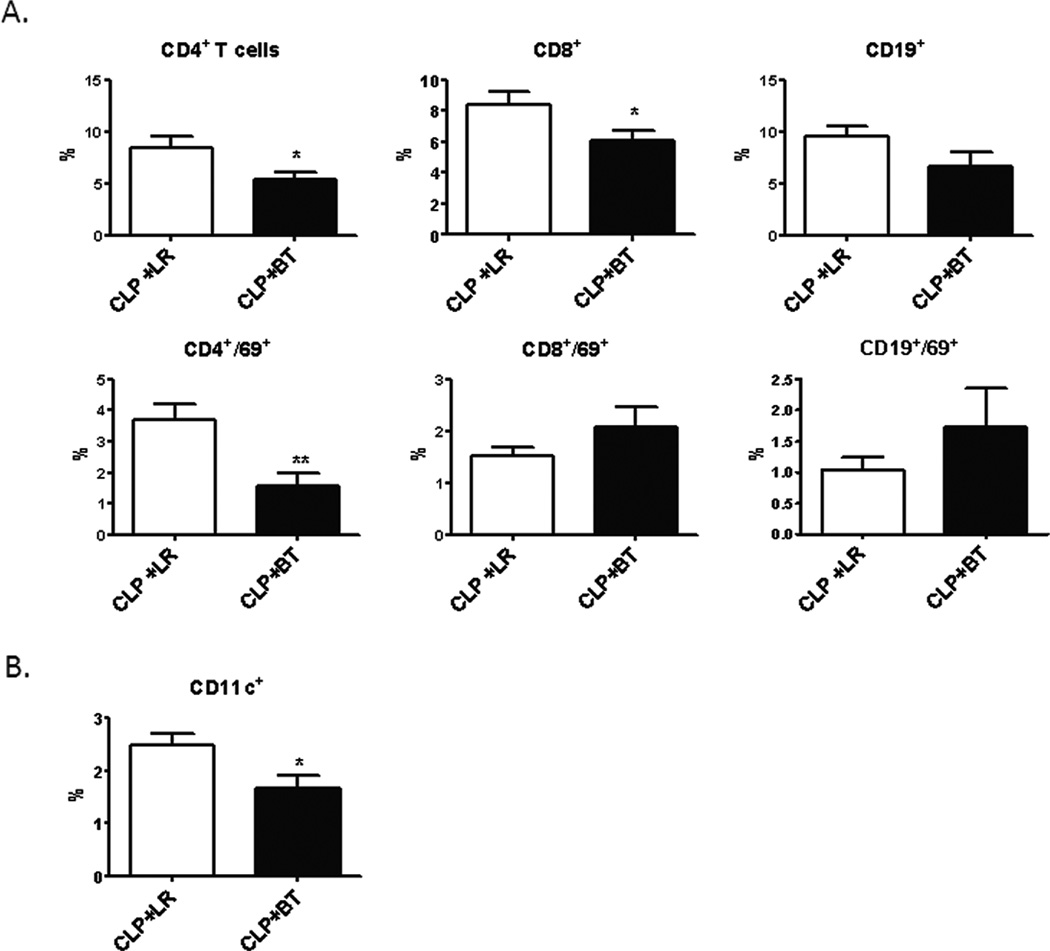
These effects associated with the transfusion of PRBCs during sepsis were not limited to the spleen. There was also a relative and absolute loss of myeloid precursors in the bone marrow of septic mice after PRBC transfusion, as compared to crystalloid resuscitation (Figure 8). The percentage of circulating lymphocytes also decreased, as well as the activation status of the circulating CD4+ T cells (Figure 9A). In addition, the percentage of circulating dendritic cells declined (Figure 9B).
Figure 8. Percent and absolute numbers of GR-1+11b+ cells in the bone marrow of mice after CLP and then resuscitated with PRBC or Ringer’s lactate.
The percent (left) and absolute number (right) of Gr-1+CD11c+ cells in the bone marrow of septic mice one day after receiving either PRBC (n=10) or LR (n=9) is shown as mean from three separate experiments (*p<0.05).
Figure 9. Percentage of circulating leukocytes in septic animals after resuscitation with PRBCs or Ringer’s lactate.
Blood was collected from septic mice one day after receiving either allogeneic PRBC (n=11) or LR (n=9) in heparinized syringe by intra-cardiac puncture. Leukocytes were analyzed by flow cytometry using anti-CD4 (T-helper cells), anti-CD8 (cytotoxic T lymphocytes), and anti-CD19 (B cells). The activation status was determined by using anti-CD69 (A). Dendritic cells were identified using anti-CD11c (B). Data shown is the mean from three separate experiments (*p<0.05; **p<0.01).
Allogeneic stored PRBC transfusion during sepsis is associated with a dysregulated inflammatory state
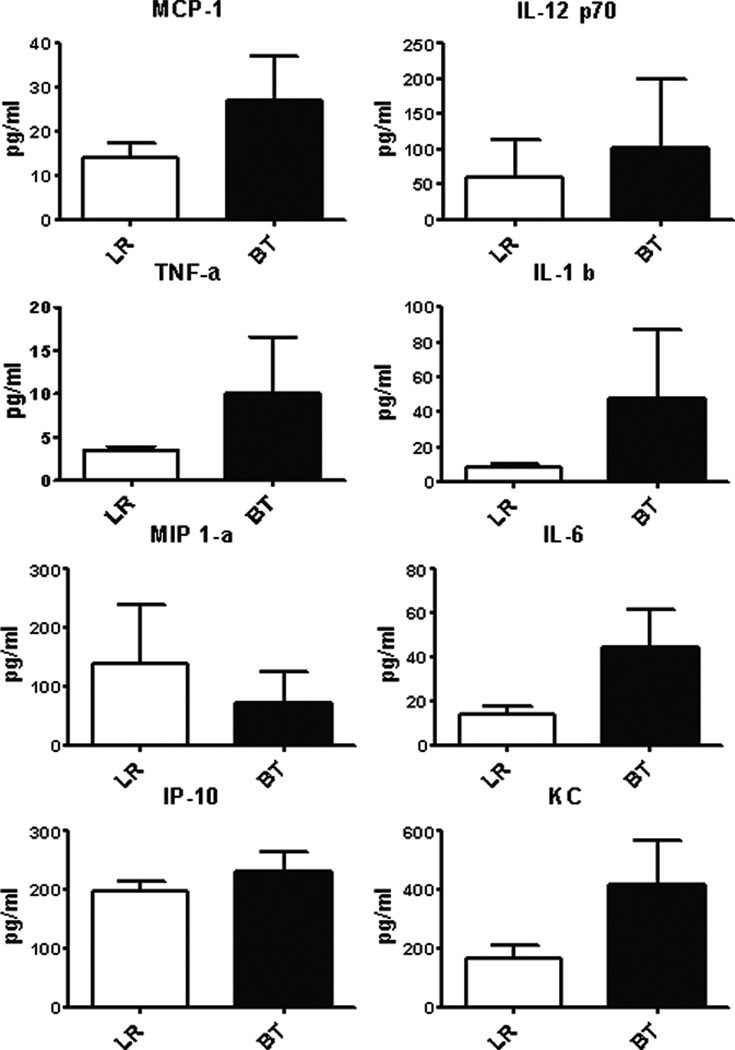
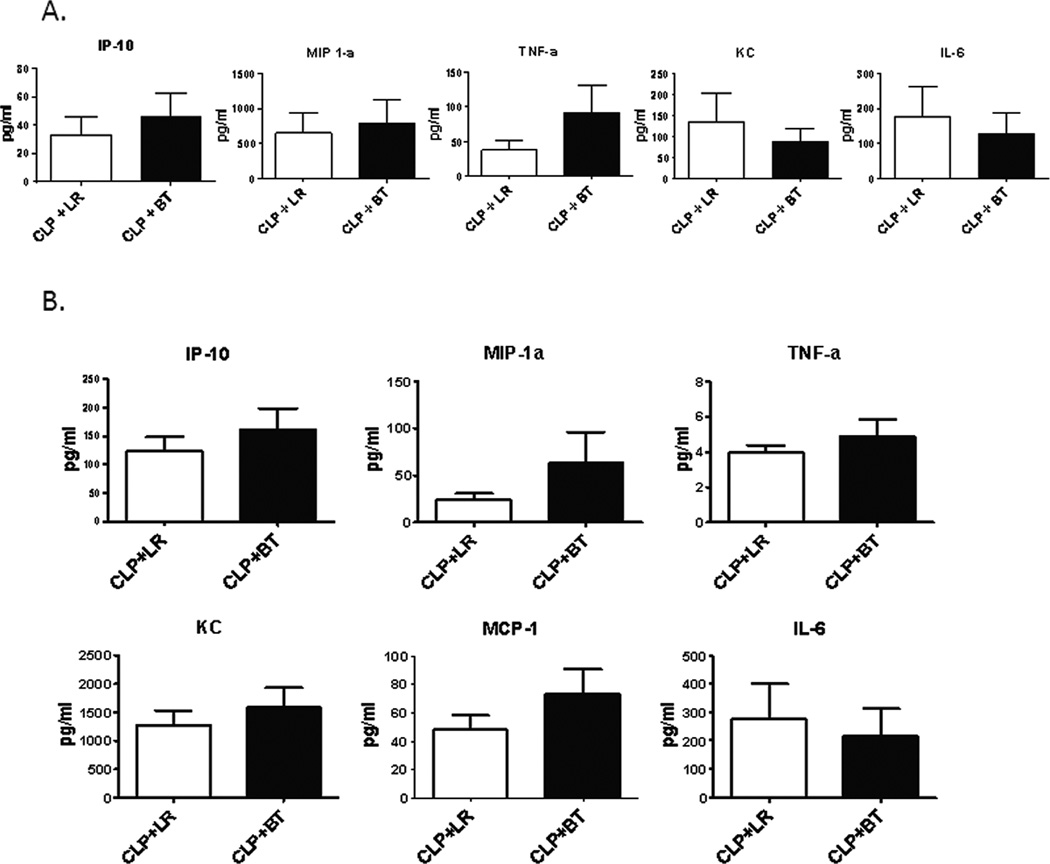
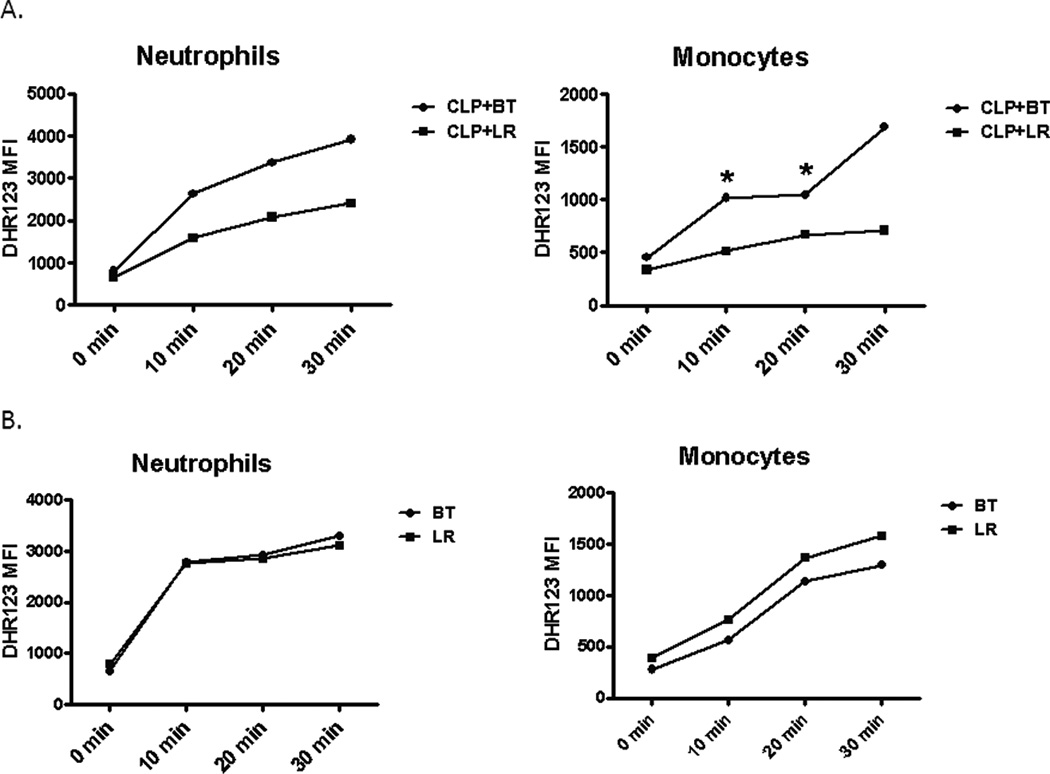
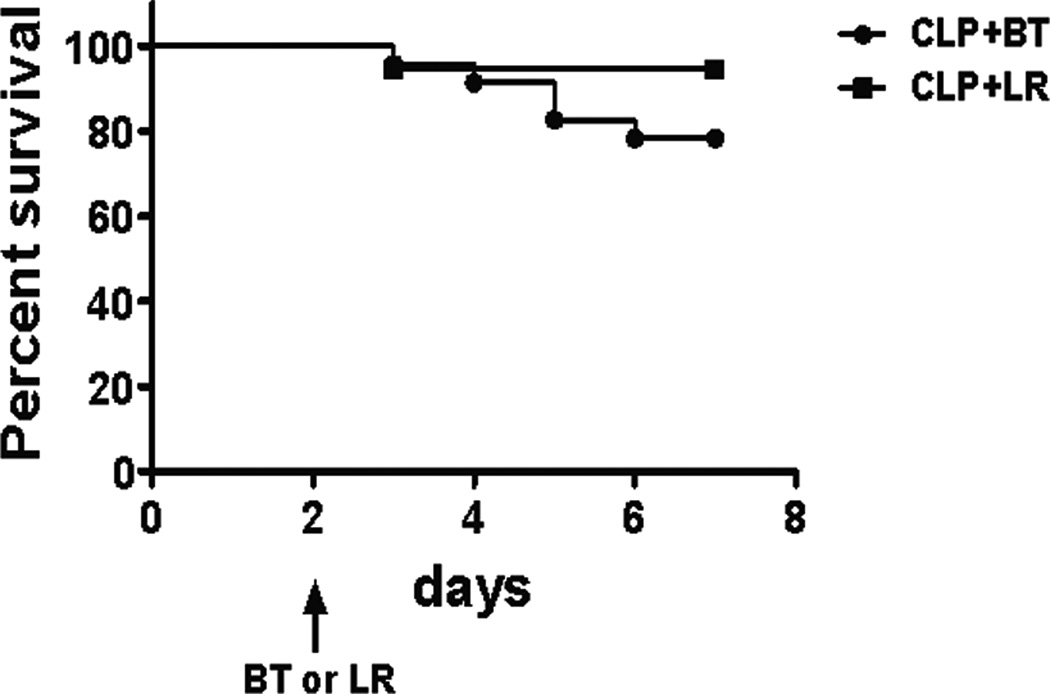
The functional response of splenocytes in septic animals after transfusion reflects a different immune environment. PRBC transfused mice secreted more IP-10, MIP-1a, TNF-a, and IL-10 in response to LPS as compared to crystalloid-resuscitated, septic mice, similar to that seen in healthy naïve animals, but produced less KC and IL-6, which was opposite of what was seen in naïve mice (Figure 10A and Table 2). A similar trend was seen in plasma cytokine levels of these mice (Figure 10B). In addition, although there were fewer splenic neutrophils and monocytes, transfusion during sepsis increased the capacity of these cells to produce ROS (Figure 11A) compared to mice that received LR solution, a phenomenon not demonstrated in naive mice (Figure 11B). Finally, mortality was modestly increased in septic mice that received blood transfusion, although this did not reach statistical significance (Figure 12).
Figure 10. Splenocyte function of septic mice after PRBC transfusion.
(A) Spleens from septic mice were harvested one day after receiving either allogeneic PRBC (n=7) or LR solution (n=7). One million spleen cells were treated with LPS (1 µg/ml). Culture supernatant were collected 24 hours later and assessed for cytokine/chemokine production using multiplex Luminex kit. (B) Blood was collected from septic mice one day after receiving either allogeneic PRBC (n=13) or LR (n=11) in heparinized syringe by intra-cardiac puncture. Plasma cytokine/chemokine levels were measure using multiplex Luminex kit. Data shown are the mean from three separate experiments.
Table 2.
LPS stimulated spleen cytokine production a
| IP-10 (pg/ml) |
TNF a (pg/ml) |
MIP-1a (pg/ml) |
IL-6 (pg/ml) |
KC (pg/ml) |
IL-10 (pg/ml) |
|
|---|---|---|---|---|---|---|
| LR | 14.5 ± 4.7* | 22.2 ± 5.8* | 172.4 ± 34.6* | 115.1 ± 30.6* | 104.4 ± 33.1* | 5.2 ± 1.3 * |
| BT | 38.6 ± 2.7* | 100 ± 4.2* | 747.3 ± 51.4* | 439 ±60.5*§ | 515.2 ± 59.5*§ | 16.2 ± 1.8* |
| CLP+LR | 33.1 ± 12.8 | 35.8 ± 12.7 | 651.6 ± 297.8 | 176.7 ± 86.9 | 136.2 ± 67.8 | 99.8 ± 47.8 |
| CLP+BT | 45.6 ± 16.8 | 85.2 ± 41.4 | 797.3 ± 338.6 | 126.7 ± 162.8§ | 87.6 ± 31.5§ | 176 ± 99.4 |
Values represent the mean ± SE;
p<0.05, LR vs BT;
p<0.05, BT vs CLP+BT
Figure 11. Reactive oxygen species generation of splenic neutrophils and monocytes after PRBC transfusion during sepsis.
Spleens from septic (A) and naïve (B) mice were harvested one day after receiving either PRBC or LR were stimulated ex vivo with PMA in the presence of DHR 123 at 37°C for up to 30 minutes. Mean fluorescence intensity (MFI) of DHR 123 was measured from neutrophil and monocyte gated cells (*p<0.05).
Figure 12. Survival of septic mice with blood versus crystalloid resuscitation.
C57Bl/6 mice underwent CLP with a 25-gauge needle. Survival of septic mice receiving either PRBC (n=23) or LR (n=18) on day 2 were followed up to 7 days after CLP. The figure is the combination of two separate survival studies performed.
Discussion
During sepsis, blood transfusion appears to modify, and in places, amplify the known immunological effects of sepsis, including further exaggerating inflammatory cytokine production, while simultaneously inducing immune-suppressive phenotypes known to affect mortality to primary and secondary infections. By using a relatively more clinically relevant model of murine PRBC creation and storage as well as transfusion during murine sepsis, our data indicate that the effects of blood on the recipient depend on the inflammatory status of the individual (4). Using the cecal ligation and puncture model of polymicrobial sepsis to reflect a human condition known to have increased morbidity and mortality with blood transfusion (17), we have demonstrated that the response by the recipient’s leukocytes do not reflect those of a healthy mouse that has been transfused PRBCs, nor a septic mouse that has received crystalloid resuscitation, which is known to improve outcomes in septic mice. Thus, the illustrated dissimilarities in leukocyte numbers, phenotype and function in transfused septic mice reflect an immune environment that may place the host at an increased risk for poor outcomes.
Previous data from our laboratory and others have demonstrated that ‘emergency myelopoiesis’ and an appropriate innate immune response are required for early survival during sepsis (21, 22). In addition, Hotchkiss, et al have demonstrated that a loss of lymphocytes, and defects in adaptive immunity, during the septic response results in increased mortality (23). Follow up studies have revealed that functioning activated B cells are vital to the immune system and to survival early in sepsis (24). Our work demonstrates that PRBC transfusion exacerbates the loss of both T and B cell populations after cecal ligation and puncture, as well as reducing their activation status both in the circulation and in secondary lymphoid tissue.
In addition, these transfusion-associated alterations are not limited to lymphoid populations. Our laboratory has demonstrated the importance of functioning myeloid derived cells to outcome during both primary and secondary infections, especially in the early septic period (<3 days) (19, 21, 22, 25). Mice transfused in the early phase of sepsis have an absolute loss of almost all their splenic myeloid cell populations. Transfusion of stored allogeneic PRBC appears to interfere with ‘emergency myelopoiesis’, as reflected by the relative and absolute deficiency in myeloid precursors present in the bone marrow of septic recipients.
Our ex vivo data demonstrates the increased sensitivity of splenocytes of healthy transfused mice exposed to LPS concurs with the in vivo data of Hod, et al. (9). In both cases, transfusion resulted in a significant increase in cytokine/chemokine production after LPS stimulation. The response of septic mice to similar transfusions and stimulation is not as robust.
It is clear that blood transfusion is associated with increased infections, morbidity and mortality in the surgical and critically ill populations (4). A recent meta-analysis that reviewed the efficacy of human blood transfusion in the critically ill revealed that almost all studies (42 of 45) indicate that the risks of PRBC transfusion outweigh the benefits; two of the remaining studies were neutral and the final remaining study demonstrated a benefit to transfusion in a subgroup of a single retrospective study (elderly patients with an acute myocardial infarction and a hematocrit <30%) (26). The same report also determined that 17 of the 18 studies that appropriately reviewed mortality demonstrated that PRBC transfusion was an independent predictor of death. Finally, all of the 22 studies that analyzed PRBCs association with nosocomial infection concluded that blood transfusion was an independent risk factor for infection (26). Recently, Juffermans et al determined that there was an association between the development of nosocomial bacterial infection and the transfusion of PRBCs in the initial phase of sepsis (27). We wished to use a mouse model that better reflected this human data to determine how transfusion of murine PRBCs affected specific systemic and local leukocytes known to affect outcome and infection during the septic response.
Similar to other animals models, rodent blood banking is not without some difficulty in accurately recapitulating the human process. Previous research has demonstrated that rat PRBCs, as compared to human blood, undergo premature degeneration, and in fact may be equivalent to human blood that had been stored for much longer periods of time (28). However, subsequent studies illustrated that if processed properly, murine PRBCs can be stored for up to a month and be used for transfusion research (29). Regardless, there are still other confounding factors in regard to murine PRBC storage, including but not limited to the pH, potassium and lactate level of the stored blood that would make studying PRBC stored beyond 14 days impractical (14). In addition, although using fresh syngeneic blood can be useful as a rodent model for massive transfusion and resuscitation research (30), our initial preliminary research has illustrated differences in the host response when transfused fresh syngeneic PRBCs are compared to stored allogeneic PRBCs (data not shown). In fact, fresh blood can reverse some of the adverse effects of stored PRBCs (31). Virtually no blood transfused in the critically ill is autologous, and although debated, much data would indicate that allogeneic blood is more likely to be associated with poor outcomes than autologous blood (32). In addition, on average, most PRBCs that are transfused in the United States are greater than fourteen days old (26), the storage age at which most analyses demonstrate an increased risk for morbidity and mortality after PRBC transfusion (as compared to fresh blood) (5, 33). Other studies verify the immunologic differences between fresh and stored murine PRBCs (9, 31). Therefore, to best recapitulate the human condition, we elected to utilize allogeneic murine PRBC that had been stored for 14 days, which is more similar to human blood that has been stored for 4 weeks (14, 29).
Although we have attempted to reasonably recreate an animal model of blood transfusion, this study does have specific weaknesses. It cannot be excluded that hyperviscosity and initial hypervolumemia may have contributed to the some of the immune changes displayed. Unfortunately, limitations in murine models do not allow an exact replication of human transfusions in anemic patients. In addition, the type of crystalloid used for resuscitation, specifically Lactated Ringers in our experiment, can contribute to immunologic alterations (34). Although researches should continually strive to be recapitulate the human condition, this model of transfusion a non-anemia mouse and use lactated ringers as one of the controls is typical for the current literature (9, 30, 31).
In conclusion, PRBC transfusion alters the immune response during sepsis. These effects appear to be specific to the mammalian response to infection/inflammation and are not necessarily present in naïve recipients of blood. The downstream effects of stored allogeneic PRBCs on immunity are complex and not specific to simple pro-inflammatory responses or immune suppression, but rather a combination of different alterations of murine leukocyte responses. Increasingly, data demonstrate that the mammalian response to severe trauma or infection places the host in a persistent, inflammatory/immunosuppressed, catabolic syndrome (PICS) that can lead to poor outcomes (19, 35). The displayed systemic effects of blood transfusion on immunity and myelopoesis in the setting of infection may play a role in exacerbating PICS which, in part, might explain some the worsened outcomes displayed by critically ill transfused patients.
Acknowledgments
We would like to thank LifeSouth Community Blood Centers for supplying/donating the CPD and Adsol™ solutions for blood preservation.
This work was supported by grants 3 R01 GM040586-21S1 awarded by the National Institute of General Medical Sciences as well as the Shock Society Fellowship for Early Career Investigators. AB is supported by NIH NIGMS Grant K23 GM087709. AGC, MJD, and LFG were all supported by a training grant in burn and trauma research (T32 GM-08431).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicting financial interests.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Moore LJ, Moore FA, Todd SR, Jones SL, Turner KL, Bass BL. Sepsis in general surgery: the 2005–2007 national surgical quality improvement program perspective. Arch Surg. 2010;145:695–700. doi: 10.1001/archsurg.2010.107. [DOI] [PubMed] [Google Scholar]
- 3.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 4.Napolitano LM, Kurek S, Luchette FA, Corwin HL, Barie PS, Tisherman SA, Hebert PC, Anderson GL, Bard MR, Bromberg W, Chiu WC, Cipolle MD, Clancy KD, Diebel L, Hoff WS, Hughes KM, Munshi I, Nayduch D, Sandhu R, Yelon JA. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med. 2009;37:3124–3157. doi: 10.1097/CCM.0b013e3181b39f1b. [DOI] [PubMed] [Google Scholar]
- 5.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, Blackstone EH. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358:1229–1239. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 6.Robinson WP, 3rd, Ahn J, Stiffler A, Rutherford EJ, Hurd H, Zarzaur BL, Baker CC, Meyer AA, Rich PB. Blood transfusion is an independent predictor of increased mortality in nonoperatively managed blunt hepatic and splenic injuries. J Trauma. 2005;58:437–444. doi: 10.1097/01.ta.0000153935.18997.14. discussion 444-435. [DOI] [PubMed] [Google Scholar]
- 7.Bochicchio GV, Napolitano L, Joshi M, Bochicchio K, Shih D, Meyer W, Scalea TM. Blood product transfusion and ventilator-associated pneumonia in trauma patients. Surg Infect (Larchmt) 2008;9:415–422. doi: 10.1089/sur.2006.069. [DOI] [PubMed] [Google Scholar]
- 8.Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): an update. Blood Rev. 2007;21:327–348. doi: 10.1016/j.blre.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, Francis KP, Della-Latta P, Whittier S, Sheth S, Hendrickson JE, Zimring JC, Brittenham GM, Spitalnik SL. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115:4284–4292. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nathens AB, Nester TA, Rubenfeld GD, Nirula R, Gernsheimer TB. The effects of leukoreduced blood transfusion on infection risk following injury: a randomized controlled trial. Shock. 2006;26:342–347. doi: 10.1097/01.shk.0000228171.32587.a1. [DOI] [PubMed] [Google Scholar]
- 11.Baumgartner JM, Silliman CC, Moore EE, Banerjee A, McCarter MD. Stored red blood cell transfusion induces regulatory T cells. J Am Coll Surg. 2009;208:110–119. doi: 10.1016/j.jamcollsurg.2008.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, Tweeddale M, Schweitzer I, Yetisir E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 13.Hajjar LA, Vincent JL, Galas FR, Nakamura RE, Silva CM, Santos MH, Fukushima J, Kalil Filho R, Sierra DB, Lopes NH, Mauad T, Roquim AC, Sundin MR, Leao WC, Almeida JP, Pomerantzeff PM, Dallan LO, Jatene FB, Stolf NA, Auler JO., Jr Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. Jama. 2010;304:1559–1567. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- 14.Makley AT, Goodman MD, Friend LA, Johannigman JA, Dorlac WC, Lentsch AB, Pritts TA. Murine blood banking: characterization and comparisons to human blood. Shock. 2010;34:40–45. doi: 10.1097/SHK.0b013e3181d494fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deitch EA. Animal models of sepsis and shock: a review and lessons learned. Shock. 1998;9:1–11. doi: 10.1097/00024382-199801000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Corwin HL, Carson JL. Blood transfusion--when is more really less? N Engl J Med. 2007;356:1667–1669. doi: 10.1056/NEJMe078019. [DOI] [PubMed] [Google Scholar]
- 17.Cuenca AG, Delano MJ, Kelly-Scumpia KM, Moldawer LL, Efron PA. Cecal ligation and puncture. Curr Protoc Immunol. 2010;Chapter 19(Unit 19):13. doi: 10.1002/0471142735.im1913s91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Perl M, Chung CS, Perl U, Thakkar R, Lomas-Neira J, Ayala A. Therapeutic accessibility of caspase-mediated cell death as a key pathomechanism in indirect acute lung injury. Crit Care Med. 2010;38:1179–1186. doi: 10.1097/CCM.0b013e3181d4563f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O'Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, Swan R, Chung CS, Atkinson MA, Ramphal R, Gabrilovich DI, Reeves WH, Ayala A, Phillips J, Laface D, Heyworth PG, Clare-Salzler M, Moldawer LL. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–1474. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meisel C, Schefold JC, Pschowski R, Baumann T, Hetzger K, Gregor J, Weber-Carstens S, Hasper D, Keh D, Zuckermann H, Reinke P, Volk HD. Granulocyte-macrophage colony-stimulating factor to reverse sepsis-associated immunosuppression: a double-blind, randomized, placebo-controlled multicenter trial. Am J Respir Crit Care Med. 2009;180:640–648. doi: 10.1164/rccm.200903-0363OC. [DOI] [PubMed] [Google Scholar]
- 21.Delano MJ, Thayer T, Gabrilovich S, Kelly-Scumpia KM, Winfield RD, Scumpia PO, Cuenca AG, Warner E, Wallet SM, Wallet MA, O'Malley KA, Ramphal R, Clare-Salzer M, Efron PA, Mathews CE, Moldawer LL. Sepsis induces early alterations in innate immunity that impact mortality to secondary infection. J Immunol. 2011;186:195–202. doi: 10.4049/jimmunol.1002104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly-Scumpia KM, Scumpia PO, Delano MJ, Weinstein JS, Cuenca AG, Wynn JL, Moldawer LL. Type I interferon signaling in hematopoietic cells is required for survival in mouse polymicrobial sepsis by regulating CXCL10. J Exp Med. 2010 doi: 10.1084/jem.20091959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, Aspiotis R, Han Y, Nicholson DW, Karl IE. Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol. 2000;1:496–501. doi: 10.1038/82741. [DOI] [PubMed] [Google Scholar]
- 24.Kelly-Scumpia KM, Scumpia PO, Weinstein JS, Delano MJ, Cuenca AG, Nacionales DC, Wynn JL, Lee PY, Kumagai Y, Efron PA, Akira S, Wasserfall C, Atkinson MA, Moldawer LL. B cells enhance early innate immune responses during bacterial sepsis. J Exp Med. 208:1673–1682. doi: 10.1084/jem.20101715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuenca AG, Wynn JL, Kelly-Scumpia KM, Scumpia PO, Vila L, Delano MJ, Mathews CE, Wallet SM, Reeves WH, Behrns KE, Nacionales DC, Efron PA, Kunkel SL, Moldawer LL. Critical role for CXC ligand 10/CXC receptor 3 signaling in the murine neonatal response to sepsis. Infect Immun. 2011;79:2746–2754. doi: 10.1128/IAI.01291-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36:2667–2674. doi: 10.1097/CCM.0b013e3181844677. [DOI] [PubMed] [Google Scholar]
- 27.Juffermans NP, Prins DJ, Vlaar AP, Nieuwland R, Binnekade JM. Transfusion-related risk of secondary bacterial infections in sepsis patients: a retrospective cohort study. Shock. 2011;35:355–359. doi: 10.1097/SHK.0b013e3182086094. [DOI] [PubMed] [Google Scholar]
- 28.d'Almeida MS, Jagger J, Duggan M, White M, Ellis C, Chin-Yee IH. A comparison of biochemical and functional alterations of rat and human erythrocytes stored in CPDA-1 for 29 days: implications for animal models of transfusion. Transfus Med. 2000;10:291–303. doi: 10.1046/j.1365-3148.2000.00267.x. [DOI] [PubMed] [Google Scholar]
- 29.Gilson CR, Kraus TS, Hod EA, Hendrickson JE, Spitalnik SL, Hillyer CD, Shaz BH, Zimring JC. A novel mouse model of red blood cell storage and posttransfusion in vivo survival. Transfusion. 2009;49:1546–1553. doi: 10.1111/j.1537-2995.2009.02173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makley AT, Goodman MD, Friend LA, Deters JS, Johannigman JA, Dorlac WC, Lentsch AB, Pritts TA. Resuscitation with fresh whole blood ameliorates the inflammatory response after hemorrhagic shock. J Trauma. 2010;68:305–311. doi: 10.1097/TA.0b013e3181cb4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hendrickson JE, Hod EA, Hudson KE, Spitalnik SL, Zimring JC. Transfusion of fresh murine red blood cells reverses adverse effects of older stored red blood cells. Transfusion. 2011;51:2695–2702. doi: 10.1111/j.1537-2995.2011.03197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heiss MM, Mempel W, Jauch KW, Delanoff C, Mayer G, Mempel M, Eissner HJ, Schildberg FW. Beneficial effect of autologous blood transfusion on infectious complications after colorectal cancer surgery. Lancet. 1993;342:1328–1333. doi: 10.1016/0140-6736(93)92247-q. [DOI] [PubMed] [Google Scholar]
- 33.Weinberg JA, McGwin G, Jr, Vandromme MJ, Marques MB, Melton SM, Reiff DA, Kerby JD, Rue LW., 3rd Duration of red cell storage influences mortality after trauma. J Trauma. 69:1427–1431. doi: 10.1097/TA.0b013e3181fa0019. discussion 1431-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koustova E, Stanton K, Gushchin V, Alam HB, Stegalkina S, Rhee PM. Effects of lactated Ringer's solutions on human leukocytes. J Trauma. 2002;52:872–878. doi: 10.1097/00005373-200205000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Gentile LF, Cuenca AG, Efron PA, Ang D, Bihorac A, McKinley BA, Moldawer LL, Moore FA. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72:1491–1501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]