Abstract
Objective
No evidence-based methods exist to identify prescription drug use disorder (PDUD) in primary care (PC) patients prescribed controlled substances. Aberrant drug-related behaviors (ADRBs) are suggested as a proxy. Our objective was to determine whether ADRBs documented in electronic medical records (EMRs) of patients prescribed opioids and benzodiazepines could serve as a proxy for identifying PDUD.
Design
A cross-sectional study of PC patients at an urban, academic medical center.
Subjects
264 English-speaking patients (ages 18–60) with chronic pain (≥3 months), receiving ≥1 opioid analgesic or benzodiazepine prescription in the past year, were recruited during outpatient PC visits.
Outcome Measures
Composite International Diagnostic Interview defined DSM-IV diagnoses of past-year PDUD and no disorder. EMRs were reviewed for 15 pre-specified ADRBs (e.g. early refill, stolen medications) in the year before and after study entry. Fisher’s exact test compared frequencies of each ADRB between participants with and without PDUD.
Results
61 participants (23%) met DSM-IV PDUD criteria and 203 (77%) had no disorder; 85% had one or more ADRB documented. Few differences in frequencies of individual behaviors were noted between groups, with only “appearing intoxicated or high” documented more frequently among participants with PDUD (n=10, 16%) vs. no disorder (n=8, 4%), p=0.002. The only common ADRB, “emergency visit for pain,” did not discriminate between those with and without the disorder (82% PDUD vs. 78% no disorder, p=0.6).
Conclusions
EMR documentation of ADRBs is common among PC patients prescribed opioids or benzodiazepines, but unsystematic clinician documentation does not identify PDUDs. Evidence-based approaches are needed.
Keywords: Prescription drug use disorder, diagnosis, aberrant drug-related behaviors, primary care, chronic pain
Introduction
Chronic pain is a common presenting problem in the primary care (PC) setting; approximately 22% of PC patients report chronic pain.(1) Over the past two decades, opioid prescribing for chronic non-cancer pain has dramatically increased, accompanied by rising rates of opioid misuse, unintentional overdose, and legal prosecution of physicians.(2–6) Benzodiazepine prescribing has similarly become a topic for debate, not only due to risks for medication abuse and diversion, but also because of the association of benzodiazepines to opioid-related deaths.(7) Given that primary care physicians (PCPs) provide the vast majority of psychoactive substances nationally,(8) they must take great care in the decision to prescribe these medications and monitor for side effects to avoid such negative outcomes. In addition, PCPs must assess for risk of prescription drug use disorder (PDUD), defined as abuse of or dependence on prescription opioids and prescription benzodiazepines and/or any illicit substance and/or alcohol dependence while receiving opioid and/or benzodiazepine pharmacotherapy.(9, 10) One suggested method of monitoring patients prescribed opioids and benzodiazepines is assessing for aberrant drug-related behaviors (ADRBs).(9)
Experts in pain medicine define ADRBs as behaviors suggesting out of control use of medications, one hallmark of addiction. Examples of such behaviors include insisting on a medication by name, claiming non-narcotic medications “don’t work”, buying medications off the street, making frequent emergency visits for pain medications, asking for an early refill, and spending extensive time discussing medications.(11) ADRBs may also include taking a medication in a manner not prescribed – e.g., taking someone else’s medication, unsanctioned dose escalations, multiple prescription locations or different providers writing prescriptions.(11) Finally, in addition to behaviors that are suggestive of a patient taking medication in an out of control manner, some behaviors suggest medication diversion, the transfer of legally obtained drugs into illegal channels –where patients exchange or sell their prescription drugs to someone else.(4, 12) It is worth noting that when ADRBs do occur, there can be multiple possible explanations for the behavior and hence consideration of a differential diagnosis of their etiology is appropriate, with PDUD being one of several plausible explanations.(13)
Currently, experts recommend monitoring patients prescribed chronic opioids for aberrant drug-related behaviors, based on the assumption that ADRBs indicate medication misuse, diversion, or addiction.(14–19) However, the evidence supporting this assumption is very limited: studies using ADRBs have relied on physician recall and/or documentation of ADRBs in a patient’s chart as a proxy for PDUD.(20–23) In order to establish an evidence base for the use of ADRBs as a proxy for prescription drug use disorder in patients with chronic pain prescribed opioids and benzodiazepines, we compared chart documentation of ADRBs to a systematically obtained patient diagnosis of prescription drug use disorder.
Methods
Study Design
This was a cross-sectional study of primary care patients with chronic pain who were recruited from the PC clinics of an urban, academic, safety-net medical center.(24, 24–26) Safety-net hospitals in the United States care for poor and vulnerable populations who may be uninsured or underinsured, and includes disproportionate numbers of underrepresented minorities.(26) We collected data from participants by an in-person interview with a trained member of the research team and by a subsequent electronic medical record (EMR) review for abstraction of prescription opioid and benzodiazepine data to assess entry criteria, as well as to identify any documentation of fifteen pre-specified behaviors considered by experts to be concerning for addictive disease.(10, 11) Further specific details of study methods have been published previously.(24, 25)
Setting
Research interviewers recruited patients waiting for scheduled PC visits. Interviewers were physicians, master-level professionals, college graduates, and college students who underwent 60+ hours of interview training. All participants were approached in the waiting rooms of the hospital-based primary care practice.(25)
Recruitment and Enrollment
Of the 833 (40.0%) patients eligible for the study based on explicit criteria, i.e. were 18 – 60 years of age, spoke English, endorsed pain of at least three months duration, reported use of any analgesic medication, including over-the-counter or prescription, in the prior month, and had a scheduled PC appointment, 589 patients (70.7%) agreed to participate. Informed consent was obtained from eligible patients, and participants were compensated $10 for the one time interview. The Boston University Medical Center Institutional Review Board approved the study.
All electronic medical record entries from 12 months prior to study entry were reviewed looking for documentation of any opioid or benzodiazepine prescription; a comprehensive list of all included medications has been previously published.(24, 25) Standardized chart abstraction forms were used. Electronic medical records were comprehensive including notes from all clinic visits, all emergency department records, all inpatient discharge summaries, phone calls, and an institutional prescription database. This data analysis was based on the 264 (44.8%) study participants who were prescribed an opioid pain reliever and/or a benzodiazepine in the 12 months prior to their interview.
Measures and Key Variables
Independent Variable: Prescription Drug Use Disorder (PDUD)
PDUD was defined as per DSM-IV diagnostic criteria for current (past year) abuse of or dependence on prescription opioids, prescription benzodiazepines. 26 Also included in this definition was any illicit substance and/or alcohol dependence while receiving opioid and/or benzodiazepine pharmacotherapy. During the interview portion, participants were assessed using the Composite International Diagnostic Interview (CIDI) v.2.1 module on Drug Disorders to diagnose drug use disorders.(28) Criteria for drug abuse included social, physical or legal consequences from use. The criteria for drug dependence included compulsive use, health consequences, and physical dependence (i.e., tolerance or withdrawal). Physical dependence alone did not suffice to meet the diagnosis. The CIDI-short-form (CIDI-SF) was used to measure current alcohol dependence; current alcohol abuse and past alcohol use disorders were not measured in order to reduce respondent time burden.(28) Thus participants with PDUD may have a lone prescription opioid and/or benzodiazepine disorder, a lone SUD (an illicit drug disorder and/or past year alcohol dependence) while receiving prescription opioids and/or benzodiazepines, or a comorbid prescription opioid and/or benzodiazepine disorder along with another SUD (i.e. comorbid illicit drug disorder and/or past year alcohol dependence).(28)
While it may be questioned whether a lone SUD while receiving prescription opioids and/or benzodiazepines truly constitutes PDUD, this is a definition that has been used clinically and also suggested by experts in pain and addiction.(10, 29, 30) However, this was taken into account and a sensitivity analysis was conducted, in which those with a lone other SUD while prescribed an opioid and/or benzodiazepine were analyzed as a unique group. As this analysis yielded similar results, for simplicity the data presented is from a two-group analysis comparing those with PDUD as defined above to those with no disorder. Nicotine dependence was not included in the variable SUD.
Dependent Variable: Aberrant Drug-Related Behaviors (ADRBs)
Two years of electronic medical record data were reviewed for each participant in order to assess ADRBs, records from twelve months prior to and post study entry, looking for documentation of fifteen pre-specified behaviors. These behaviors were recognized in the published literature as signs of potential addiction or diversion in patients prescribed controlled substances.(11) While our list of behaviors was based on the work of Portenoy and meant to represent the concepts he first put forward, our nomenclature was slightly modified. Standardized chart abstraction forms were used.
Statistical Analysis
Using Fisher’s exact test, frequencies of each ADRB and cumulative numbers of ADRBs were compared between participants with PDUD and no disorder.
Results
The demographic characteristics of the 264 participants, stratified by presence or absence of PDUD, are presented in Table 1. Twenty-three percent (61/264) of study participants met criteria for current PDUD. We found few demographic differences between the two groups (i.e., age, gender, race, and education). Characteristics included mean age mid-40s, majority African American, and over two-thirds with 12 or more years of education. At least 50% of each group, with and without PDUD, were receiving disability payments and over 95% of each group had severely disabling pain.(31) However, participants with PDUD were more likely to have the following characteristics: post-traumatic stress disorder (PTSD), depression, smoker, past time in jail, and a family history of substance abuse (Table 2).
Table 1.
Demographic characteristics of PC participants with chronic pain and analgesic medication use stratified by prescription drug use disorder (PDUD) (n=264)
| Variable | PDUD n = 61 (23 %) | No Disorder n = 203 (77 %) | p-value | |
|---|---|---|---|---|
|
| ||||
| Mean Age, yr | 45.9 | 47.3 | 0.24 | |
|
| ||||
| Female Gender | 49% | 58% | 0.24 | |
|
| ||||
| Race | African American | 54% | 60% | 0.26 |
| Hispanic | 13% | 9% | ||
| White | 28% | 20% | ||
| Other | 5% | 11% | ||
|
| ||||
| Education | >12 yrs | 66% | 73% | 0.26 |
|
| ||||
| On Disability | 57% | 51% | 0.47 | |
|
| ||||
| PTSD | 49% | 33% | 0.02 | |
|
| ||||
| Depression | 56% | 33% | 0.002 | |
|
| ||||
| Current Smoker | 70% | 45% | <0.001 | |
|
| ||||
| Ever in Jail | 71% | 33% | <0.001 | |
|
| ||||
| Family Substance Abuse | 74% | 48% | <0.001 | |
Table 2.
Frequency of Aberrant drug-related behaviors stratified by prescription drug use disorder (PDUD) (n=264)
| Behavior | PDUD n = 61 n (%) | No Disorder n = 203 n (%) | p-value |
|---|---|---|---|
| Appears intoxicated/high | 10 (16%) | 8 (4%) | 0.002 |
| Use someone else’s medication | 3 (5%) | 8 (4%) | 0.8 |
| Bought medication off the street | 3 (5%) | 3 (2%) | 0.1 |
| Extensive time discussing medication | 3 (5%) | 9 (4%) | 1.0 |
| Involvement in an accident | 3 (5%) | 29 (14%) | 0.07 |
| Number of prescription locations | 3 (5%) | 4 (2%) | 0.2 |
| Insists non-narcotics don’t work | 1 (2%) | 8 (4%) | 0.7 |
| Try to get scripts from other MDs | 1 (2%) | 3 (2%) | 1.0 |
| Urgent visit for pain | 50 (82%) | 159 (78%) | 0.6 |
| Early refill | 9 (15%) | 29 (14%) | 1.0 |
| Urgent visit for narcotic pain meds | 9 (15%) | 14 (7%) | 0.06 |
| Insists on medication by name | 7 (12%) | 22 (11%) | 0.8 |
| Lost medication | 5 (8%) | 11 (5%) | 0.5 |
| Increase dose w/o authorization | 4 (7%) | 12 (6%) | 0.6 |
| Reports stolen medication | 3 (5%) | 3 (2%) | 0.1 |
Among patients receiving prescriptions for opioids in the prior 12 months, 15% received the equivalent of 20 tablets of 5 mg oxycodone in ≤2 fills, 12.6% received 21–60 tablets in ≤3 fills, 22.7% received 61–150 tablets in ≤3 fills, and 49.6% received >150 tablets or >3 fills of any amount (e.g. 4 prescriptions of 20 tablets each). The majority of those in the last category received >6 fills. Of those receiving benzodiazepines in the prior 12 months in one or more prescriptions (n=66), 17% received less than 30 pills, 15% received 30–100 pills, 15% received 101–200 pills, and 41% received >200 pills. Eight patients (12%) received benzodiazepine prescriptions from outside psychiatrists, for which specific pill counts and fills were unavailable.
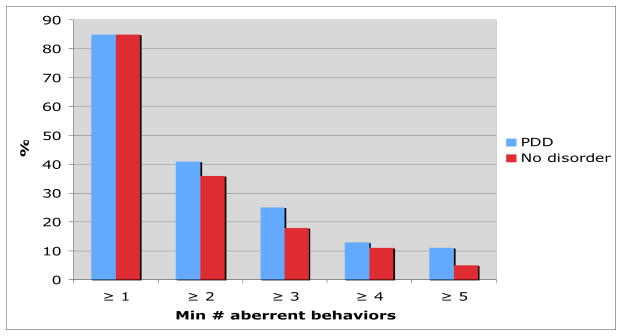
Of the fifteen behaviors assessed, only “appearing intoxicated or high“ was documented more frequently in the EMRs of participants with PDUD (n=10, 16%) vs. no disorder (n=8, 4%), p=0.002. However, the frequency was low and this behavior was present in the charts of those with no disorder (Table 2). Indeed most participants in both groups had at least one ADRB (85%, p=1.0). With respect to the remaining fourteen behaviors, the frequency of any one documented behavior was not significantly different among patients with PDUD and with no disorder. An urgent visit for pain was the most common aberrant behavior, but frequency did not differ between the two groups (PDUD vs. no PDUD - 82% vs. 78% p=0.6). Early refill was present in about 15% of both groups of patients. The remaining behaviors were present in 15% or less of participants. With respect to cumulative behaviors, patients with PDUD do not have significantly more documented ADRBs in their EMR (Figure). Rather, having one or two behaviors in the chart was common; over 80% of the patients with and without PDUD had at least one behavior in their chart and almost 40% in both groups had two ADRBs.
Figure 1.
Figure Cumulative aberrant drug-related behaviors among participants with prescription drug use disorder and no disorder.
No results were statistically significant.
Discussion
By using patients’ electronic medical records and research interviews, this study investigated whether aberrant drug-related behaviors (ADRBs) documented in patients’ charts reflected a diagnosis of prescription drug use disorder. If documented ADRBs were indeed viable indicators of PDUD, then the EMR abstractions would have revealed significant differences in patients’ charts; patients with a diagnosis of PDUD would have had more ADRBs documented in their medical charts than those with no diagnosis. The data reveal no differences; ADRBs as noted in routine primary care practice do not identify patients with PDUDs. Despite the thoughtful clinical insight that went into the development of these recommended “clinical pearls,” empirically they do not yield the desired outcome, identification of patients that should be of great concern to the clinician. However, the negative results of this study nevertheless do provide insight about physician recording in the EMR and suggest that relying on non-systematic documentation to identify addiction or diversion is not useful.
Clinicians prescribing opioids for patients with chronic pain are aware that some degree of aberrant drug-related behaviors may occur during the course of treatment. Accordingly, the provider should be aware that there is a differential diagnosis for ADRBs, of which PDUD is only one potential explanation.(27) Included in this differential is pain that is not adequately controlled, leading patients to increase their dose of medication without authorization and the subsequent need for an early refill.(27) For example, randomized controlled trials have demonstrated that opioid analgesia is not consistently effective; thus, patients with uncontrolled pain may take additional medication in an effort to find relief.(32, 33) In addition, some patients with adequately controlled pain may hoard medications, fearful of a time when the pain may suddenly worsen.(11, 34) This too can lead to early refills and even use of multiple prescription locations. Finally, a pain flare can send a patient to the emergency department in an effort to find relief. Thus, patients without PDUD can also plausibly have ADRBs documented in their charts. This study found that this is in fact the case, and these events do occur at a comparable frequency as ADRBs in patients with and without PDUD. The presence of these behaviors in those without PDUDs has been referred to as a pseudoaddiction, in which the aberrant behaviors will resolve once the pain is adequately treated.(13) It is clear that the patients in this study did not have adequately controlled pain, as over 95% of both groups of patients had severely disabling pain. Perhaps if the pain were better treated, then a true difference in documented ADRBs would exist between the groups. Alternatively, as suggested by Portenoy, Passik et al, perhaps many of the behaviors considered to be concerning are really more benign and not truly associated with addiction or diversion.(11, 27)
One documented behavior warrants a deeper investigation. From the fifteen ADRBs examined, only appearing intoxicated or high was documented more frequently in the EMRs of patients with PDUD. Any time a clinician suspects a patient of being intoxicated or high, further evaluation is warranted. Recently published guidelines offer clinicians a framework for safely initiating and continuing to prescribe opioids.(9) It is suggested that at every clinical visit, the patient be assessed for any risks associated with the opioids as well as any benefits received from the medication. Prescribing ought to continue when the benefits of a medication outweigh its potential risks, thus ensuring an ethically equitable distribution of benefits and burdens.(35) If a patient seems to be intoxicated or high, concern arises that the potential risks to the patient, as well as society, outweigh any benefits. In this situation, titration off of the opioid or benzodiazepine combined with the provision of adjunctive therapy for pain and/or anxiety, as well as referral to substance abuse treatment and/or psychiatry, is likely most appropriate. (36)
The other fourteen ADRBs were not significantly different between the groups. Since this study examined documented behaviors, the negative results suggest that relying on unsystematic physician documentation is not useful for identifying patients with prescription drug use disorder. Rather, in order to determine if ADRBs can be useful to monitor patients for PDUDs, a reasonable next step would be to assess implementation of standardized clinical assessment tools into clinical practice.
Although it may appear that ADRBs are not useful in detecting PDUD, the non-systematic collection of ADRBs may be the problem rather than the ADRBs themselves.(37) Validated tools have been studied to predict misuse of substances while being prescribed opioid analgesia for chronic non-cancer pain.(9) For example, the Screener and Opioid Assessment for Patients with Pain™ (SOAPP) is a tool to help risk-stratify patients at the initiation of opioid therapy.(20) The results provide an idea of how closely a patient ought to be monitored during treatment.(20) In addition, the Current Opioid Misuse Measure (COMM) provides a means for ongoing risk estimation via standardized assessment of aberrant behaviors.(38) The present authors have studied the COMM and caution that clinical assessment tools may perform differently in distinct patient populations, as the sensitivity and specificity of some tools may vary with the prevalence of disease.(24) As this study demonstrates, ADRBs as recorded in a patient’s EMR are not associated with PDUD. Thus, caution should always be utilized when monitoring any patient for the development of PDUD, even when standardized assessment tools are in place. As Butler et al stress, the purpose of these tools is not to serve as the basis for punitive measures.(38) Rather, they are to help clinicians have a means for consistent patient assessment and to limit the need to rely on haphazard documentation.
Finally, the results demonstrate that patients with PDUD were more likely to suffer from post-traumatic stress disorder and/or depression, to be current smokers, to have ever been in jail, and to have a family history of substance abuse. The present authors have previously explored these clinical risk factors for PDUD.(25) In addition, as previously reported, over 30% of patients without PDUD also suffered from comorbid mental illness, smoked, had been in jail, and had a family history of substance abuse.(25) Clearly there is a significant burden of comorbid mental illness in patients with chronic pain, offering further support of the need for an interdisciplinary approach to the management of chronic pain.(36)
This study has limitations. All participants with PDUD may not have been properly identified, through inadequacy of the study instruments or through inaccurate reporting on the part of study participants. This would lead to misclassification bias, in which patients with PDUD are inappropriately labeled as having no disorder, serving to bias the results towards the null hypothesis.(39) In addition, tolerance to, and withdrawal from, opioids and benzodiazepines can be naturally occurring phenomenon, but are nonetheless included in the diagnostic interview for PDUD.(10) This would serve to bias results away from the null hypothesis. Finally, the CIDI does not assess for drug diversion, a specific risk related to prescription opioids and benzodiazepines.(5, 12, 40) Even with its acknowledged limitations, the CIDI has been used widely and is considered to be a well-validated diagnostic instrument.(28) The sample size was small, and included only 61 patients with PDUD. Even if a larger sample size revealed statistically different findings, individual ADRBs would likely have little predictive value in diagnosing PDUD, as the majority of patients without a substance disorder also demonstrated each of the behaviors. Two years worth of chart entries were reviewed for each study participant, thus providing data to examine cumulative numbers of behaviors exhibited, which were not significant. However, when we looked at a combined number of behaviors, we did not stratify them and look at a hierarchy of more severe behaviors. Overall the results contribute to the recognition of the potential limitations of documented ADRBs and suggest a need to utilize a standardized approach to patient assessment as a potentially more informative way to gain clinical insight from ADRBs.
Conclusion
Among primary care patients with chronic pain receiving prescription opioids and/or benzodiazepines, having at least one ADRB documented in the EMR is almost universal. In addition, frequencies of most individual behaviors are similar among those with and without PDUD. Reliance on non-systematic documentation of ADRBs to identify PDUD in primary care patients with chronic pain may not be useful. Based on these findings, physicians and researchers should be cautious before using documented ADRBs in the EMR as a proxy for PDUD. Prospective studies, in which ADRBs are systematically assessed, are needed. Only then can we determine the true significance of these behaviors.
Footnotes
All work was performed at Boston University Medical Center.
Conflict of Interest
The authors all report no conflicts of interest. R. Saitz is a consultant for Medical Directions Inc. and BMJ Publishing Group
Disclosures
The study was funded by K23 DA016665 from the National Institute of Drug Abuse. Dr. Jane Liebschutz had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. This work was previously presented at the Society of General Internal Medicine 32nd Annual Meeting in Miami, Florida on May 15, 2009, where it was a finalist for the Mack Lipkin Sr. Associate Member Awards.
References
- 1.Gureje O, Von Korff M, Simon GE, Gater R. Persistent pain and well-being: A world health organization study in primary care.[erratum appears in JAMA 1998 oct 7;280(13):1142] JAMA. 1998;280(2):147–51. doi: 10.1001/jama.280.2.147. [DOI] [PubMed] [Google Scholar]
- 2.Marks RM, Sachar EJ. Undertreatment of medical inpatients with narcotic analgesics. Annals of Internal Medicine. 1978;78:173–81. doi: 10.7326/0003-4819-78-2-173. [DOI] [PubMed] [Google Scholar]
- 3.Cicero TJ, Inciardi JA, Munoz A. Trends in abuse of oxycontin and other opioid analgesics in the united states: 2002–2004. J Pain. 2005;6(10):662–72. doi: 10.1016/j.jpain.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Bell J. The global diversion of pharmaceutical drugs: Opiate treatment and the diversion of pharmaceutical opiates: A clinician’s perspective. Addiction. 2010;105(9):1531–7. doi: 10.1111/j.1360-0443.2010.03014.x. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Emergency department visits involving nonmedical use of selected prescription drugs - united states, 2004–2008. MMWR Morb Mortal Wkly Rep. 2010;59(23):705–9. [PubMed] [Google Scholar]
- 6.Reidenberg MM, Willis O. Prosecution of physicians for prescribing opioids to patients. Clin Pharmacol Ther. 2007;81(6):903–6. doi: 10.1038/sj.clpt.6100127. [DOI] [PubMed] [Google Scholar]
- 7.Webster LR, Cochella S, Dasgupta N, et al. An analysis of the root causes for opioid-related overdose deaths in the united states. Pain Med. 2011;12 (Suppl 2):S26–35. doi: 10.1111/j.1526-4637.2011.01134.x. [DOI] [PubMed] [Google Scholar]
- 8.Smith DE. Prescribing practices and the prescription drug epidemic: Physician intervention strategies. J Psychoactive Drugs. 2012 Jan-Mar;44(1):68–71. doi: 10.1080/02791072.2012.662094. [DOI] [PubMed] [Google Scholar]
- 9.Chou R, Fanciullo GJ, Fine PG, et al. Clinical guidelines for the use of chronic opioid therapy in chronic noncancer pain. J Pain. 2009;10(2):113–30. doi: 10.1016/j.jpain.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.American Psychiatric Association, Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders (DSM-IV-TR) 4. Washington, DC: American Psychiatric Association; 2000. p. xxxvii.p. 943. [Google Scholar]
- 11.Portenoy RK. Opioid therapy for chronic nonmalignant pain: A review of the critical issues. J Pain Symptom Manage. 1996;11(4):203–17. doi: 10.1016/0885-3924(95)00187-5. [DOI] [PubMed] [Google Scholar]
- 12.Inciardi JA, Surratt HL, Cicero TJ, Kurtz SP, Martin SS, Parrino MW. The “black box” of prescription drug diversion. J Addict Dis. 2009;28(4):332–47. doi: 10.1080/10550880903182986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weissman DE, Haddox JD. Opioid pseudoaddiction--an iatrogenic syndrome. Pain. 1989;36(3):363–6. doi: 10.1016/0304-3959(89)90097-3. [DOI] [PubMed] [Google Scholar]
- 14.Dunbar SA, Katz NP. Chronic opioid therapy for nonmalignant pain in patients with a history of substance abuse: Report of 20 cases. J Pain Symptom Manage. 1996;11(3):163–71. doi: 10.1016/0885-3924(95)00165-4. [DOI] [PubMed] [Google Scholar]
- 15.Passik SD, Kirsh KL, McDonald MV, et al. A pilot survey of aberrant drug-taking attitudes and behaviors in samples of cancer and AIDS patients. J Pain Symptom Manage. 2000;19(4):274–86. doi: 10.1016/s0885-3924(00)00119-6. [DOI] [PubMed] [Google Scholar]
- 16.Michna E, Ross EL, Hynes WL, et al. Predicting aberrant drug behavior in patients treated for chronic pain: Importance of abuse history. J Pain Symptom Manage. 2004;28(3):250–8. doi: 10.1016/j.jpainsymman.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 17.Ives TJ, Chelminski PR, Hammett-Stabler CA, et al. Predictors of opioid misuse in patients with chronic pain: A prospective cohort study. BMC Health Serv Res. 2006;6:46. doi: 10.1186/1472-6963-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fleming MF, Balousek SL, Klessig CL, Mundt MP, Brown DD. Substance use disorders in a primary care sample receiving daily opioid therapy. J Pain. 2007;8(7):573–82. doi: 10.1016/j.jpain.2007.02.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirsh KL, Whitcomb LA, Donaghy K, Passik SD. Abuse and addiction issues in medically ill patients with pain: Attempts at clarification of terms and empirical study. Clin J Pain. 2002;18(4 Suppl):S52–60. doi: 10.1097/00002508-200207001-00006. [DOI] [PubMed] [Google Scholar]
- 20.Wasan AD, Butler SF, Budman SH, et al. Does report of craving opioid medication predict aberrant drug behavior among chronic pain patients? Clin J Pain. 2009;25(3):193–8. doi: 10.1097/AJP.0b013e318193a6c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butler SF, Budman SH, Fernandez K, Jamison RN. Validation of a screener and opioid assessment measure for patients with chronic pain. Pain. 2004;112(1–2):65–75. doi: 10.1016/j.pain.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 22.Weaver MF, Bond DS, Arnold BL, Waterhouse E, Towne A. Aberrant drug-taking behaviors and headache: Patient versus physician report. Am J Health Behav. 2006;30(5):475–82. doi: 10.5555/ajhb.2006.30.5.475. [DOI] [PubMed] [Google Scholar]
- 23.Compton P, Darakjian J, Miotto K. Screening for addiction in patients with chronic pain and “problematic” substance use: Evaluation of a pilot assessment tool. J Pain Symptom Manage. 1998;16(6):355–63. doi: 10.1016/s0885-3924(98)00110-9. [DOI] [PubMed] [Google Scholar]
- 24.Meltzer EC, Rybin D, Saitz R, et al. Identifying prescription opioid use disorder in primary care: Diagnostic characteristics of the current opioid misuse measure (COMM) Pain. 2011;152(2):397–402. doi: 10.1016/j.pain.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liebschutz JM, Saitz R, Weiss RD, et al. Clinical factors associated with prescription drug use disorder in urban primary care patients with chronic pain. J Pain. 2010;11(11):1047–55. doi: 10.1016/j.jpain.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baxter RJ, Mechanic RE. The status of local health care safety nets. Health Aff (Millwood) 1997;16(4):7–23. doi: 10.1377/hlthaff.16.4.7. [DOI] [PubMed] [Google Scholar]
- 27.McCarberg B, Passik SD. Expert guide to pain management. Philadelphia, PA: American College of Physicians; 2005. [Google Scholar]
- 28.Kessler RC, Andrews G, Mroczek D, Ustun B, Wittchen HU. The world health organization composite international diagnostic interview short-form (CIDI-SF) International Journal of Methods in Psychiatric Research. 1998;7(4):171–85. [Google Scholar]
- 29.Zacny J, Bigelow G, Compton P, Foley K, Iguchi M, Sannerud C. College on problems of drug dependence taskforce on prescription opioid non-medical use and abuse: Position statement. Drug Alcohol Depend. 2003;69(3):215–32. doi: 10.1016/s0376-8716(03)00003-6. [DOI] [PubMed] [Google Scholar]
- 30.Katz NP, Adams EH, Benneyan JC, et al. Foundations of opioid risk management. Clin J Pain. 2007;23(2):103–18. doi: 10.1097/01.ajp.0000210953.86255.8f. [DOI] [PubMed] [Google Scholar]
- 31.Von Korff M, Ormel J, Keefe FJ, Dworkin SF. Grading the severity of chronic pain. Pain. 1992;50(2):133–49. doi: 10.1016/0304-3959(92)90154-4. [DOI] [PubMed] [Google Scholar]
- 32.Martell BA, O’Connor PG, Kerns RD, et al. Systematic review: Opioid treatment for chronic back pain: Prevalence, efficacy, and association with addiction. Ann Intern Med. 2007;146(2):116–27. doi: 10.7326/0003-4819-146-2-200701160-00006. [DOI] [PubMed] [Google Scholar]
- 33.Ossipov MH, Lai J, King T, Vanderah TW, Porreca F. Underlying mechanisms of pronociceptive consequences of prolonged morphine exposure. Biopolymers. 2005;80(2–3):319–24. doi: 10.1002/bip.20254. [DOI] [PubMed] [Google Scholar]
- 34.Portenoy RK. Opioid therapy for chronic nonmalignant pain: Clinician’s perspective. J Law Med Ethics. 1996;24(4):296–309. doi: 10.1111/j.1748-720x.1996.tb01871.x. [DOI] [PubMed] [Google Scholar]
- 35.Jonsen AR, Siegler M, Winslade WJ. Clinical ethics: A practical approach to ethical decisions in clinical medicine. 7. New York: McGraw-Hill Medical; 2010. [Google Scholar]
- 36.Liebschutz JM, Alford DP. Safe opioid prescribing: A long way to go. J Gen Intern Med. 2011;26(9):951–2. doi: 10.1007/s11606-011-1797-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Krebs EE, Ramsey DC, Miloshoff JM, Bair MJ. Primary care monitoring of long-term opioid therapy among veterans with chronic pain. Pain Med. 2011;12(5):740–6. doi: 10.1111/j.1526-4637.2011.01099.x. [DOI] [PubMed] [Google Scholar]
- 38.Butler SF, Budman SH, Fernandez KC, et al. Development and validation of the current opioid misuse measure. Pain. 2007;130(1–2):144–56. doi: 10.1016/j.pain.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fletcher RH, Fletcher SW. Clinical epidemiology the essentials. 4. Baltimore: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 40.McCabe SE, Teter CJ, Boyd CJ. Medical use, illicit use, and diversion of abusable prescription drugs. J Am Coll Health. 2006;54(5):269–78. doi: 10.3200/JACH.54.5.269-278. [DOI] [PMC free article] [PubMed] [Google Scholar]