Abstract
Study Objective
We evaluated factors associated with physicians’ intentions to perform Pap smears in human papillomavirus-vaccinated women.
Design
Physicians were mailed a survey asking about intentions to change cervical cancer screening based on patients’ human papillomavirus vaccination status.
Participants
A national sample of 1,738 Family Physicians, Internal Medicine physicians, Pediatricians, and Obstetricians and Gynecologists was selected from the American Medical Association Physician Masterfile. Completed surveys were received from 1,118 physicians, of which 791 were included in the analyses.
Main Outcome Measures
Bivariate analyses compared physician, practice, and patient characteristics by intention change screening frequency. Significant variables were included in a multivariable logistic regression model.
Results
Overall, 81.8% (n = 647) physicians reported not planning to change Pap smear frequency for vaccinated women. Internal Medicine physicians were significantly more likely than Obstetrician/Gynecologists to report intentions to change frequency for vaccinated patients. Other factors significantly associated with the intention to change frequency were self-identification as a late adopter of new vaccines, a solo practice, and practicing primarily in a clinic or hospital-based setting.
Conclusions
Although it appears most clinicians understand that human papillomavirus vaccination should not alter current screening practices, there is a need to develop and evaluate interventions for physicians who are likely to change their screening pattern based on human papillomavirus vaccination receipt.
Keywords: human papillomavirus, HPV vaccines, Papanicolaou test, physicians
Introduction
In June 2006, a quadrivalent human papillomavirus (HPV) vaccine for 9-26 year-old females was approved and licensed by the Food and Drug Administration (FDA). In March 2007, the Centers for Disease Control and Prevention's Advisory Committee on Immunization Practices (ACIP) recommended routine vaccination of females aged 11-12 years as well as catch-up vaccination for females ages 13-26 years and vaccination of ages 9-10 years at the provider's discretion.1,2 By 2008, 37% of girls 13-17 years old had received at least one dose of HPV vaccine and 18% had completed the vaccination schedule of three doses.3 Current screening recommendations include cervical cancer screening beginning at age 21,4 which will soon encompass many young women who have been immunized against HPV. An understanding of the pathophysiology of HPV infection as a causative agent of cervical dysplasia would suggest that the need for screening for cervical cancer should be diminished in HPV immunized women. Results of cost effectiveness and epidemiologic studies have also indicated that a reduction in screening for HPV vaccine recipients may be forthcoming.5-8 However, it will likely take ~15-20 years to fully evaluate the effect of widespread HPV vaccination on cervical cancer incidence.9 Without population-based data to support the modification of current screening guidelines, the most recent cervical cancer screening guidelines in 2009 from the American College of Obstetricians and Gynecologists (ACOG) still recommend that women who have been immunized against HPV 16 and 18 should be screened with the same frequency as unvaccinated women.4,7
There is little information available on how physicians who provide cervical cancer screening may adapt their cervical cancer screening recommendations for their HPV immunized patients. While professional organizations may change guidelines based on the latest scientific and clinical advances, it is still providers who interpret and implement these recommendations at the patient level. Results from one study using data collected from August 2006 to May 2007 suggested almost 40% of physicians believed the HPV vaccine would impact cervical cancer screening frequency;10 however, more research is needed to assess intentions to change screening frequency among vaccinated females at three years post-vaccine licensure. The current study evaluates primary care providers’ knowledge and other practice-related factors associated with intentions to change Pap smear frequency among females who have received the HPV vaccine.
Materials and Methods
Between April 2009 and August 2009, a nationally representative sample of Family Physicians (FPs), Internal Medicine physicians (IM), and Obstetrician/Gynecologists (OBGYNs) was surveyed regarding their attitudes, knowledge, and recommendations for HPV vaccine for females. The Institutional Review Board determined the research met requirements for exemption and a waiver of informed consent was obtained. The current study represents a component of a larger study pertaining to physician recommendation of HPV vaccination. Of the previously published manuscripts using data from this larger study,11-13 none have included the primary outcome variable used in the current study (intention to change Pap frequency) nor have any of the papers used all items from the survey.
Sample
Participants were randomly selected from the American Medical Association (AMA) Physician Masterfile, a database of all licensed US physicians irrespective of membership in the AMA or any other elective organization.14 FPs, Peds, and OBGYNs were sampled based on their proportional representation in the US primary care physician workforce. IM physicians were sampled as a pilot group and were not a representative sample. An external company responsible for maintaining the physician mailing list used a computer program to randomly select physicians according to study inclusion criteria. The sampling frame excluded physicians who: 1) were trainees, 2) were locum tenens, 3) primarily conducted non-patient care related professional activity (e.g., teaching, administration), 4) practiced only obstetrics, 5) were from the same practice, 6) were ≥ age 65 years (likely to be retired), and 7) listed a post office box for their address (precluding our ability to send the survey via Federal Express). A multiphase recruitment approach was used based on the Dillman15 method and is detailed elsewhere.12
Accounting for an estimated 65% response rate, the survey was mailed to 1,738 physicians: 818 FPs, 393 Peds, 200 IMs, and 327 OBGYNs. Of those surveys, 33 were undeliverable and 10 participants were identified as ineligible. Completed surveys were received from 1,118 physicians, including 500 FPs, 287 Peds, 105 IM, and 226 OBGYNs. After accounting for undeliverable surveys and ineligible participants, the overall response rate was 66.4% and specialty-specific response rates were 63.6% for FPs, 74.6% for Peds, 55.3% IM, and 69.8% for OBGYNs. Peds were not surveyed regarding the performance of Pap smears in their practice and therefore were not included in this substudy. Given that the physicians were randomly selected for participation by specialty and prior to data collection, the exclusion of Peds in the current substudy did not affect the random selection process for the other specialties. Providers who did not respond to the main outcome variable of interest were also excluded, leaving 791 respondents for the current analyses.
Instrument
The survey used for this study is described in greater detail elsewhere12 (the complete questionnaire can be obtained by e-mailing the corresponding author). In short, it consisted of 38 items and was developed based on existing questionnaires used to study HPV vaccination.16-19 The primary outcome measure was the response to the following question: “Do you plan to change the frequency with which you provide Pap test screening to females who have received the HPV vaccine?” Response options included “yes,” “no,” and “don't know.” These responses were collapsed to yes/don't know and no for the univariate and multivariable analyses because we intended to focus on factors that influence practitioners to change their current screening practices for vaccinated women, despite the current recommendations.4 This dichotomy serves to highlight differences between providers who intend to follow the current guidelines and those who might benefit from an educational intervention designed to prompt providers to follow the current guidelines. Demographic data collected about the primary care providers included age, gender, race, and ethnicity.
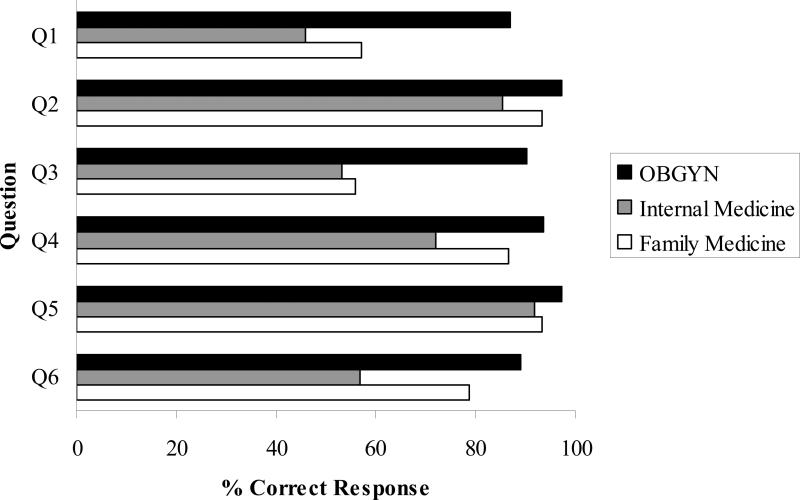
As shown in Figure 1, the survey also contained six items designed to ascertain participants’ knowledge regarding HPV infection and HPV vaccination; these items were reviewed for content validity by an expert panel. Response options included “true,” “false,” or “don't know.” Correct responses were summed to create a total knowledge score (range: 0-6), which was dichotomized into “high knowledge” (≥5 correct responses) and “low knowledge” (≤4 correct responses) based on a median split.
Figure 1. Questions Used to Determine Knowledge of HPV and HPV Vaccination.
Q1. Most HPV infections resolve without medical intervention. True
Q2. Treatment of cervical dysplasia/cancer permanently eliminates the causative infection. False
Q3. Genital warts are caused by the same HPV types that cause cervical cancer. False
Q4. Almost all cervical cancers are caused by HPV infection. True
Q5. The FDA approved the quadrivalent HPV vaccine for use in females ages 9-26. True
Q6. Females who have been diagnosed with HPV infection should not be given the HPV vaccine. False
Attitudinal factors regarding new vaccines and new technologies were measured in two separate questions that measured early vs. late adoption of new advancements:16 1) “Compared to my clinical peers, I am often the first to use a newly recommended vaccine,” and 2) “I tend to wait to adopt new medications, vaccines or procedures until I hear about them from several trusted colleagues” (α = .7220). For both questions, response options were presented on a five-point Likert-type scale (1=strongly disagree to 5=strongly agree). Given the relatively small number of responses for the strongly disagree and strongly agree options, the categories strongly disagree and somewhat disagree were collapsed into “disagree,” strongly agree and somewhat agree were collapsed into “agree,” and neutral remained an independent group.
Data Collection and Verification
Participants had the option of completing the survey via a paper-based scannable survey or a web-based survey; data for both survey formats were stored in a .CSV file. The accuracy of the scanned data was verified using a computer software program. The data were again verified by a study team member, who checked the accuracy of the data in the .CSV file against the actual survey for 10% of surveys randomly selected using a random numbers generator based on study ID number. The study coordinator met with the principal investigator to discuss and make decisions about instances of inaccuracy (e.g., participants selected more than one response when only one response was requested). Finally, the study data manager ran frequencies on all variables to ensure that no values fell outside the range of plausible values.
Data Analysis
Chi-square tests of homogeneity or Fisher's Exact tests were conducted to investigate differences between physicians’ plans to change the frequency of Pap smears and variables of interest. Multivariable logistic regression models using backward elimination were conducted to examine the association between each explanatory variable and the outcome measure, while controlling for all other variables in the model. The multivariable models were fitted using only the variables associated with primary outcomes (significance level of stay = < 0.1). Odds ratios (OR) and their 95% confidence interval (CI) were estimated from the logistic regression model. All analyses used two-tailed tests of significance with a significance level of p < 0.05.
Results
The majority of respondents were non-Hispanic white males, 50 years of age or older. There was no significant difference in plans to change Pap smear frequency based on any personal physician demographic factors (Table 1). Among physician practice characteristics, the majority practiced with two to fifteen physicians in their group, as part of a single specialty group, and were in private practice. The only practice characteristic associated with the likelihood of changing Pap smear frequency was a non-private practice setting (relative to a private practice setting; p < 0.001).
Table 1.
Physician Factors, Practice Factors, Patient Characteristics, and Immunization Support by Intentions to Change Pap Test Frequency (N =791)
| Factor | Total (N =791) n (%)a | No (N =647) n (%)a | Yes/Don't Know (N =144) n (%)a | p b |
|---|---|---|---|---|
| Physician factors | ||||
| Demographic and personal | ||||
| Age (yr) (missing=17) | ||||
| 25-39 | 187 (24.2) | 158 (24.9) | 29 (20.7) | 0.356 |
| 40-49 | 262 (33.9) | 217 (34.2) | 45 (32.1) | |
| 50+ | 325 (42.0) | 259 (40.9) | 66 (47.1) | |
| Gender (missing=9) | ||||
| Male | 452 (57.8) | 361 (56.4) | 91 (64.1) | 0.094 |
| Female | 330 (42.2) | 279 (43.6) | 51 (35.9) | |
| Race (missing =13) | ||||
| White/Caucasian | 581 (74.7) | 477 (75.0) | 104 (73.2) | 0.663 |
| Other | 197 (25.3) | 159 (25.0) | 38 (26.8) | |
| Ethnicity (missing=19) | ||||
| Hispanic or Latino | 44 ( 5.7) | 37 ( 5.8) | 7 ( 5.1) | 0.726 |
| Not Hispanic or Latino | 728 (94.3) | 597 (94.2) | 131 (94.9) | |
| Knowledge (missing=3) | ||||
| Low (0-4 correct answers) | 270 (34.3) | 202 (31.3) | 68 (47.9) | <0.001 |
| High (5-6 correct answers) | 518 (65.7) | 444 (68.7) | 74 (52.1) | |
| Attitudinal Factors | ||||
| Early vs. late adopter | ||||
| First to use a new vaccine (missing=10) | ||||
| Agree | 227 (29.1) | 204 (32.0) | 23 (16.0) | 0.001 |
| Neutral | 324 (41.5) | 255 (40.0) | 69 (47.9) | |
| Disagree | 230 (29.4) | 178 (27.9) | 52 (36.1) | |
| Wait to adopt (missing=11) | ||||
| Agree | 345 (44.2) | 271 (42.6) | 74 (51.4) | 0.119 |
| Neutral | 223 (28.6) | 184 (28.9) | 39 (27.1) | |
| Disagree | 212 (27.2) | 181 (28.5) | 31 (21.5) | |
| Practice factors | ||||
| Practice characteristics | ||||
| No. of physicians (missing=7) | ||||
| 1 | 157 (20.0) | 119 (18.5) | 38 (26.8) | 0.063 |
| 2-15 | 512 (54.3) | 430 (67.0) | 82 (57.7) | |
| 16+ | 115 (14.7) | 93 (14.5) | 22 (15.5) | |
| No. of specialties (missing=8) | ||||
| Single | 547 (69.9) | 458 (71.5) | 89 (62.7) | 0.060 |
| Multiple | 210 (26.8) | 165 (25.7) | 45 (31.7) | |
| Other | 26 ( 3.3) | 18 ( 2.8) | 8 ( 5.6) | |
| Type (missing=6) | ||||
| Private practice | 566 (72.1) | 484 (75.3) | 82 (57.7) | <0.001 |
| Other | 219 (27.9) | 159 (24.7) | 60 (42.3) | |
| Patient characteristics | ||||
| Patient payment method | ||||
| Private insurance (missing=50) | ||||
| 0-50% of patients | 347 (46.8) | 277 (45.3) | 70 (53.8) | 0.077 |
| 51-100% of patients | 394 (53.2) | 334 (54.7) | 60 (46.2) | |
| Patient race (majority) | ||||
| Non-Hispanic White | 596 (76.3) | 497 (77.5) | 99 (70.7) 0.085 | |
| Other | 185 (23.7) | 144 (22.5) | 41 (28.3) |
Percentages may not add up to 100% due to missing data.
χ2 tests of homogeneity used to compare groups; Fisher's Exact test used for variables with ≤ 5 in each cell.
Most respondents scored in the high knowledge range (65.7%). Respondents with a low knowledge score were more likely to intend to change Pap smear frequency compared to those with a high knowledge score (p < 0.001). Specifically, among physicians who planned to continue the same Pap smear frequency, 68.7% had a high knowledge score compared to 52.1% of physicians who either planned to change the Pap smear frequency or who don't know.
With respect to attitudinal factors regarding new vaccines and new technologies, 29.1% agreed with the statement: “Compared to my clinical peers, I am often the first to use a newly recommended vaccine,” and were considered early adopters. On the other hand, 44.2% agreed with the question: “I tend to wait to adopt new medications, vaccines or procedures until I hear about them from several trusted colleagues,” and were deemed late adopters. Attitudinal factors related to vaccines only were significantly related to physicians’ intentions to change Pap smear frequency (p < 0.001). Early adopters of new vaccines were less likely to change their Pap smear frequency for vaccinated patients. Among respondents who plan to change Pap smear frequency or who don't know, 16% are among the first to use new vaccines compared to 32% of respondents who plan to continue their current Pap smear frequency. No statistically significant difference was noted among early vs. late adopters when medications and procedures were included in the question.
Across the three specialties, 81.8% of physicians reported they did not plan to change the Pap smear frequency for women who received the HPV vaccine. OBGYNs were least likely to change Pap smear frequency (88.9%), followed by FPs (81.8%) and IM (67.7%) (data not shown).
Findings from the multivariable analyses of factors associated with intention to change Pap smear frequency for HPV vaccinated women are shown in Table 2. After adjusting for all other factors, IM physicians were more than twice as likely as OBGYNs to report intention to change the Pap smear frequency (OR, 2.21; 95% CI, 1.08-4.54), whereas FPs were not significantly different than OBGYNs. Physicians who were solo practitioners were more than twice as likely than those in medium (2-15) or large (16+) practices to report intention to change Pap smear frequency in vaccinated women (OR, 2.57; 95% CI, 1.16-4.84). The likelihood of intention to change Pap smear frequency was also more than twice as high for physicians practicing in a clinic or hospital setting when compared to those in private practice (OR, 2.57; 95% CI, 1.63-4.06). Both physicians who were late adopters of new vaccines (OR, 2.08; 95% CI, 1.15-3.75) and those who were neither late nor early adopters (OR, 2.33; 95% CI, 1.33-4.06) had more than a two-fold greater odds of intention to change their Pap smear practices in vaccinated women. Knowledge of HPV and HPV vaccination was not significantly associated with intention to change Pap smear screening frequency.
Table 2.
Logistic Regression Model of Predictors of Intentions to Change Pap Test Frequency (N =720)
| Factor | Adjusted Odds Ratio (95% Confidence Interval) |
|---|---|
| Knowledge | |
| High | 1.00 |
| Low | 1.47 (0.95 – 2.29) |
| First to use new vaccine | |
| Agree | 1.00 |
| Neutral | 2.33 (1.33 – 4.06) |
| Disagree | 2.08 (1.15 – 3.75) |
| No. of Physicians | |
| 16+ | 1.00 |
| 2-15 | 1.00 (0.55 – 1.83) |
| 1 | 2.37 (1.16 – 4.84) |
| Practice Type | |
| Private Practice | 1.00 |
| Other | 2.57 (1.63 – 4.06) |
| Primary Clinical Specialty | |
| OBGYN | 1.00 |
| Family Medicine | 1.43 (0.83 – 2.46) |
| Internal Medicine | 2.21 (1.08 – 4.54) |
Discussion
This study is among the first to examine factors related to physicians’ plans to change Pap smear frequency for women who have been vaccinated against HPV. Current cervical cancer screening recommendations do not vary based on HPV immunization status, reflecting a concern that cervical cancer rates will not diminish for decades, even if widespread HPV vaccination is accomplished. In general, cancer screening programs balance adequate testing to prevent cancer and avoidance of overtesting. However, the impact of HPV vaccination on this balance is unknown.
Even in the presence of established guidelines, it is providers who actually perform and recommend cervical cancer screening to their patients. Their knowledge and other practice-related factors could influence the implementation and success of any sufficient, yet cost effective methods of cancer screening. Understanding these factors and how they contribute to changing practice patterns should be paramount when changing screening recommendations. In our study we found that 18.5% of respondents who perform Pap smears for their patients intend to change Pap smear frequency based on HPV vaccination status. Our results using data from 2009 differ from those reported in a study conducted by Wong et al.,10 who surveyed primary care providers from August 2006 to May 2007 and found 38.2% were expecting to change Pap smear frequency for women who received HPV vaccination. In that study, other significant indicators of intention to change Pap smear frequency were belief in new screening technologies and current adherence to Pap smear recommendations. Physician knowledge of HPV or HPV vaccination was not assessed. Given that HPV vaccine does not confer immunity against all types of HPV that are associated with cervical cancer, the desired outcome of vaccination would be for providers to continue screening according to established guidelines and not change their practice solely on vaccination status.
Although some physicians reported considering a change in Pap smear frequency, most OBGYNs were planning to continue their current practice. This intention to uphold current practices likely reflects attention and adherence to ACOG guidelines at the time the study was conducted, which recommended that screening frequency should not differ based on HPV vaccination status.4,7 Relative to FPs and IM physicians, OBGYNs may have more exposure to HPV literature, which may be supported by the higher frequency of correct responses to the knowledge items in this study. OBGYNS also may have a particularly vested interest in the elimination of cervical cancer. Taken together, OBGYNs may be more informed than the other specialties and therefore acting in accordance to guidelines. On the other hand, previous studies have shown the reluctance of OBGYNs to change screening intervals or extend the screening intervals despite ACOG recommendations,21 possibly motivated by legal concerns, patient history, missed opportunity to screen patients annually for other gynecological needs, and patient reluctance to extend the screening interval.22,23 Of interest, one recent study showed that OBGYNs were more likely to overscreen relative to established guidelines for Pap smear frequency compared to internists or family practitioners.24 While our study did not address the current Pap smear intervals used by each physician, available studies suggest that OBGYNs may be the slowest to adopt recommendations for a longer interval between Pap smears. In our study, IM physicians were most likely to report intention to change Pap smear frequency, which is consistent with the Wong et al. study.10 Previous research has also indicated that IMs are the most likely to follow established cervical cancer screening guidelines.10,24
Additionally, we found physicians in private practice were less likely to report intention to change Pap smear practices even when controlling for demographic criteria. Although this observation has not specifically been examined in previous studies, we can infer that the same factors noted in studies of OBGYNs are relevant: patient acceptance, legal issues, and a desire to see patients annually for other counseling and screening.22,23
The reverse is true of physicians in solo practice across practice specialties, who are more likely to report intentions to change Pap smear frequency in vaccinated women. In this study, a change in Pap smear frequency, presumably to a longer interval, represents a deviation from established guidelines. Solo practitioners may be less aware of current guidelines and practice patterns due to less frequent discussion with colleagues or lack of exposure to new health care innovations and guidelines that are readily disseminated in academic centers. If and when changes to screening guidelines are made, this group may need additional targeted interventions.
Finally, our survey contained two questions related to the early or late adoption of new vaccines, medications, and procedures by physicians. Physicians who were self-described as late adopters of new vaccines or who were neither late nor early adopters, were more likely to report intention to change their Pap smear frequency. Early adopters were less likely to report intention to change their Pap smear frequency, even after controlling for level of knowledge. This finding suggests that early adopters may be more likely to follow established guidelines and/or seek out a broader range of information about the practical implications of HPV vaccination for their patients. Practitioners who are early adopters of new technologies and guidelines are looked to as leaders and may influence the practice of other physicians.25,26 Therefore, they may be another group for whom interventions to disseminate new guidelines would be the most valuable and efficient in terms of reaching the most providers.
Although our study is among the first to evaluate intentions to change Pap smear frequency post vaccine licensure, findings should be considered in light of certain limitations. The IM sample was not selected in proportion to the general population of practitioners, but instead included as an exploratory group in a larger study focused on other primary care specialties. Thus the findings from this group may not be generalizable to the larger population of IM physicians. Second, physicians were asked if they intended to change Pap smear recommendations for their HPV immunized patients; however, there was no measure of whether the physicians were following current guidelines. Also, we did not ask if the frequency would increase or decrease, nor did we assess the degree of change in the frequency. Presumptively, the frequency would decrease given that the vaccine is intended to reduce the incidence of HPV infection and therefore logic would follow that providers would not increase screening frequency if they expect to find less disease. Regardless of the directionality of change, we expected to find no change in current cervical cancer screening practices at the time of the study because there was no change in guidelines for such screening. Additionally, the survey item linked to our main outcome of interest did not specify whether HPV vaccination referred to full vaccination or series initiation. Furthermore, we did not calculate item non-response frequency given the complexity of calculating this frequency due to skip patterns in the survey. It should also be noted that a power analysis was conducted to determine the sample size necessary for the larger study to determine physician and practice characteristics associated with our main study outcome (recommendation of HPV vaccination by age group).12 However, given the exploratory nature of the current study, we did not perform a power analysis prior to embarking on this sub analysis. Finally, it is unknown whether non-responders to the survey (approximately one-third) would have different views of HPV vaccination or would change the Pap smear frequency for their patients based on these views.
Despite these limitations, this study has notable strengths. First, data were from a nationally representative sample of physician specialties most likely to be involved in HPV vaccination. The overall study response rate of ~66% is also higher than other provider studies specific to actual practices regarding HPV vaccination.27,28 Taken together, the sample and response rate enhance generalizability of the results to the larger population of US physicians practicing in the specialties studied. Additionally, this study is timely given that the clinical availability of HPV vaccine is relatively new and has important implications for cervical cancer prevention.
As time and empirical evidence begin to inform the most effective and affordable screening methodologies in light of HPV vaccination, the task of women's health care providers will be to ensure that women have adequate education about new guidelines and access to mutually informed providers. In addition to demonstrating that most clinicians understand that HPV vaccination should not change screening practices, our study provides some baseline information about factors likely to influence physicians’ plans for Pap smear frequency after HPV vaccination, regardless of current guidelines, which may be useful in planning appropriate interventions. Additional studies should be conducted as guidelines evolve. Along with patient education, these data may prove important in maximizing screening benefits and minimize screening harms for women who have received HPV vaccination.
Acknowledgments
The work contained within this publication was supported in part by the Survey Methods Core Facility at the H. Lee Moffitt Cancer Center & Research Institute.
Funding: This research was supported by a grant from the National Institutes of Health (R01AI076440-01).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest/Disclosure: Dr. Giuliano has received funding from Merck for consultancy and lectures.
References
- 1.Markowitz LE, Dunne EF, Saraiya M, et al. Quadrivalent human papillomavirus vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2007;56:1. [PubMed] [Google Scholar]
- 2.Landis SH, Muray T, Bolden S, et al. Cancer statistics, 1999. CA Cancer J Clin. 1999;49:8. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention National, state, and local area vaccination coverage among adolescents aged 13-17 years - United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:997. [PubMed] [Google Scholar]
- 4.Cervical Cytology Screening ACOG Practice Bulletin number 109, American College of Obstetricians and Gynecologists. Obstet Gynecol. 2009;114:1409. doi: 10.1097/AOG.0b013e3181c6f8a4. [DOI] [PubMed] [Google Scholar]
- 5.Myers E, Huh WK, Wright JD, et al. The current and future role of screening in the era of HPV vaccination. Gynecol Oncol. 2008;109:S31. doi: 10.1016/j.ygyno.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Franco EL, Cusick J, Hildesheim A, et al. Chapter 20: Issues in planning cervical cancer screening in the era of HPV vaccination. Vaccine. 2006;24:S3/171. doi: 10.1016/j.vaccine.2006.05.061. [DOI] [PubMed] [Google Scholar]
- 7.Massad LS, Einstein M, Myers E, et al. The impact of human papillomavirus vaccination on cervical cancer prevention efforts. Gynecol Oncol. 2009;58:997. doi: 10.1016/j.ygyno.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castle PE, Solomon D, Saslow D, et al. Predicting the effect of successful human papillomavirus vaccination on existing cervical cancer prevention programs in the United States. Cancer. 2008;113:3031. doi: 10.1002/cncr.23762. [DOI] [PubMed] [Google Scholar]
- 9.Wright TC, Van Damme P, Schmitt HJ, et al. Chapter 14: HPV vaccine introduction in industrialized countries. Vaccine. 2006;24:S3/122. doi: 10.1016/j.vaccine.2006.05.118. [DOI] [PubMed] [Google Scholar]
- 10.Wong C, Berkowitz Z, Saraiya M, et al. US physicians’ intentions regarding impact of human papillomavirus vaccine on cervical cancer screening. Sex Health. 2010;7:338. doi: 10.1071/SH09115. [DOI] [PubMed] [Google Scholar]
- 11.Bynum SA, Malo TL, Lee JH, et al. HPV vaccine information-seeking behaviors among US physicians: Government, media, or colleagues? Vaccine. 2011;29:5090. doi: 10.1016/j.vaccine.2011.04.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vadaparampil ST, Kahn JA, Salmon D, et al. Missed clinical opportunities: Provider recommendations for HPV vaccination for 11-12 year old girls are limited. Vaccine. 2011;29:8634. doi: 10.1016/j.vaccine.2011.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quinn GP, Murphy D, Malo TL, et al. A national survey about human papillomavirus vaccination: What we didn't ask, but physicians wanted us to know. J Pediatr Adolesc Gynecol. 2012 doi: 10.1016/j.jpag.2012.02.007. Doi:10.1016/j.jpag.2012.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Freed GL, Nahra TA, Wheeler JR. Counting physicians: Inconsistencies in a commonly used source for workforce analysis. Acad Med. 2006;81:847. doi: 10.1097/00001888-200609000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Dillman DA. Mail and Internet Surveys: The Tailored Design Method. Wiley; New York: 2000. [Google Scholar]
- 16.Riedesel JM, Rosenthal SL, Zimet GD, et al. Attitudes about human papillomavirus vaccine among family physicians. J Pediatr Adolesc Gynecol. 2005;18:391. doi: 10.1016/j.jpag.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Daley MF, Liddon N, Crane LA, et al. A national survey of pediatrician knowledge and attitudes regarding human papillomavirus vaccination. Pediatrics. 2006;118:2280. doi: 10.1542/peds.2006-1946. [DOI] [PubMed] [Google Scholar]
- 18.Kahn JA, Zimet GD, Bernstein DI, et al. Pediatricians’ intention to administer human papillomavirus vaccine: The role of practice characteristics, knowledge, and attitudes. J Adolesc Health. 2005;37:502. doi: 10.1016/j.jadohealth.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 19.Kahn JA, Rosenthal SL, Tissot AM, et al. Factors influencing pediatricians’ intention to recommend human papillomavirus vaccines. Ambul Pediatr. 2007;7:367. doi: 10.1016/j.ambp.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 20.Feemster KA, Winters SE, Fiks AG, et al. Pediatricians’ intention to recommend human papillomavirus (HPV) vaccines to 11- to 12-year-old girls postlicensing. J Adolesc Health. 2008;43:408. doi: 10.1016/j.jadohealth.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Saint M, Gildengorin G, Sawaya GF. Current cervical neoplasia screening practices of obstetrician/gynecologists in the US. Am J Obstet Gynecol. 2005;192:414. doi: 10.1016/j.ajog.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 22.Noller KL, Bettes B, Zinberg S, et al. Cervical cytology screening practices among obstetrician-gynecologists. Obstet Gynecol. 2003;102:259. doi: 10.1016/s0029-7844(03)00565-9. [DOI] [PubMed] [Google Scholar]
- 23.Sirovich BE, Woloshin S, Schwartz LM. Screening for cervical cancer: Will women accept less? Am J Med. 2005;118:151. doi: 10.1016/j.amjmed.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 24.Yabroff KR, Saraiya M, Meissner HI, et al. Specialty differences in primary care physician reports of papanicolaou test screening practices: A national survey, 2006-2007. Ann Intern Med. 2009;151:602. doi: 10.7326/0003-4819-151-9-200911030-00005. [DOI] [PubMed] [Google Scholar]
- 25.Lomas J, Enkin M, Anderson GM, et al. Opinion leaders vs audit and feedback to implement practice guidelines: Delivery after previous cesarean section. JAMA. 1991;265:2202–7. [PubMed] [Google Scholar]
- 26.Greco PJ, Eisenberg JM. Changing physicians’ practices. N Engl J Med. 1993;329:1271. doi: 10.1056/NEJM199310213291714. [DOI] [PubMed] [Google Scholar]
- 27.Kahn JA, Cooper HP, Vadaparampil ST, et al. Human papillomavirus vaccine recommendations and agreement with mandated human papillomavirus vaccination for 11-to-12-year-old girls: A statewide survey of Texas physicians. Cancer Epidemiol Biomarkers Prev. 2009;18:2325. doi: 10.1158/1055-9965.EPI-09-0184. [DOI] [PubMed] [Google Scholar]
- 28.McCave EL. Influential factors in HPV vaccination uptake among providers in four states. J Community Health. 2010;35:645. doi: 10.1007/s10900-010-9255-4. [DOI] [PubMed] [Google Scholar]