Abstract
C3 deficiency is a rare disorder that leads to recurrent pyogenic infections. Here we describe a previously healthy 18 y/o Caucasian male with severe meningococcal disease. Total hemolytic activity was zero secondary to an undetectable C3. The C3 gene was normal by sequencing. Mixing the patient’s serum with normal human serum led to C3 consumption. An IgG autoantibody in the patient’s serum was identified that stabilized the classical pathway C3 and C5 convertases, thus preventing decay of these enzyme complexes. This autoantibody is an example of a C4 nephritic factor, with an additional feature of stabilizing the C5 convertase. Previous patients with C4 nephritic factor had membranoproliferative glomerulonephritis. Two years after presentation, this patient’s C3 remains undetectable with no evidence of renal disease. We revisit the role of autoantibodies to classical pathway convertases in disease, reviews the literature on C4-NeF and we comment on its detection in the clinical laboratory.
Keywords: C3 deficiency, Neisserial infection, C4 nephritic factor, autoimmunity
1. Introduction
The complement system is an ancient branch of the innate immune system. It serves as a first line of defense against invading pathogens, particularly bacteria accessing the intravascular space [1, 2]. Three recognition pathways have been described through which complement activation can be triggered: the classical, lectin and alternative. While each is initiated through a distinct mechanism, common to all three pathways is a series of proteolytic activation and amplification steps that converge to cleave C3 [3]. C3 is the central component of the complement cascades and the system’s most abundant protein [4]. It is a 190 kDa protein, synthesized predominantly in the liver, that is encoded by 41 exons on chromosome 19. It is translated as a single polypeptide chain, which is cleaved post-translationally to yield an α and a β chain held together by disulfide bonds.
C3 is activated via C3 convertases, enzyme complexes that cleave C3 to C3a and C3b. The C3 convertase shared by the classical and lectin pathways is composed of two subunits: C4b, which covalently binds the enzyme to a target, and C2a, which is the catalytic subunit. Similarly, the alternative pathway (AP) C3 convertase consists of a target binding subunit, C3b, and a catalytic subunit, Bb, the large subunit of the proenzyme Factor B. In both convertases the subunits are non-covalently linked to one another. These enzyme complexes are normally short-lived (a few minutes) due to spontaneous decay as well as to accelerated disassociation by complement inhibitors.
Regardless of the cause, total deficiency of C3 predisposes to recurrent pyogenic infections at an early age [5–7]. Point mutations or deletions in the C3 gene account for most cases of primary C3 deficiency [5]. In secondary C3 deficiency, C3 is synthesized normally but excessive consumption leads to a marked reduction or complete absence of serum C3 protein. For example, a total deficiency of the regulatory protein factor H (FH) or factor I (FI) produces C3 depletion because of inadequate inhibition of the AP C3 convertase. Another cause of secondary C3 deficiency is C3 nephritic factor (C3-NeF), an autoantibody that stabilizes the AP C3 convertase leading to excessive C3 turnover. Patients with secondary C3 deficiency may present with pyogenic infections as in primary deficiency but, in addition, also commonly develop glomerular disease. A few individuals with a C4 nephritic factor (C4-NeF) have also been described in the setting of lupus or glomerulonephritis [8–10]. C4-NeF stabilizes the classical pathway C3 convertase (C4b2a) and leads to C3 consumption in a fashion analogous to C3-NeF.
We report an 18-year-old patient with C3 deficiency who presented with a systemic Neisseria meningitidis infection. The basis for his C3 deficiency was an autoantibody stabilizing the classical pathway C3 convertase with an additional feature of also stabilizing the C5 convertase. In contrast to patients previously described with C4-NeF, this individual had normal renal function and no evidence of glomerulonephritis. We also review the literature on C4-NeF and discuss the strategy for its detection.
2. Case Report
An 18-year-old Caucasian male presented to a local emergency room with a 6 hr history of nausea, vomiting and periumbilical abdominal pain which woke him from sleep and progressed to include fever, rigors and headache. Physical examination revealed hypotension (blood pressure 87/48) and tachycardia (heart rate 125/min), but no photophobia or meningismus. On palpation he had diffuse abdominal pain without guarding or rebound. A complete blood count showed leukocytosis (18,500 leukocytes/µL) with 95% neutrophils and thrombocytopenia (107,000 platelets/µL). He was hydrated intravenously and admitted.
During the next eight hrs he developed an altered mental status, neck stiffness and a petechial rash. Following transfer to the intensive care unit, a lumbar puncture showed 23/µL white blood cells (normal 1–5/µL); CSF protein concentration of 20.6 mg/dL (normal 15–45 mg/dL), and a glucose concentration of 74 mg/dL. After blood and cerebrospinal fluid cultures had been obtained, ceftriaxone, acyclovir and corticosteroids were administered IV and a norepinephrine drip started.
Magnetic resonance imaging of the brain showed tiny foci of cerebral ischemia consistent with a vasculopathic process, but without evidence of cerebritis or encephalitis. Repeat laboratory studies demonstrated worsening leukocytosis (32,000 cells/µL) and thrombocytopenia (97,000 platelets/µL). Coagulation studies (PT, INR, PTT, and D-dimer) were consistent with disseminated intravascular coagulation (DIC) and led to the administration of three units of fresh frozen plasma. An EKG demonstrated ST-segment elevations suggestive of myocarditis. Acute cardiac injury was confirmed by 5- to 10-fold increase in the concentration of CK, CK-MB and troponin. Echocardiography showed global hypokinesia, decreased left ventricular ejection fraction (45–50%) and mild pulmonary hypertension.
Blood and cerebrospinal fluid cultures were positive for N. meningitidis, serogroup Y, and his antimicrobial coverage was narrowed to 2 g of ceftriaxone every 12 hrs. He improved rapidly and was discharged on hospital day #6 to complete a course of IV antibiotic therapy. At that time he was tolerating a general diet, ambulating without difficulty and stable from the cardiac, neurologic, hematologic and infectious standpoints.
The patient was a senior in high school. He drank alcohol socially and denied tobacco use. His medical history was notable for symptomatic gastroesophageal reflux with chronic H. pylori infection, seasonal rhinitis, and intermittent low back and knee pain without features of an inflammatory process. His medications included omeprazole and mometasone nasal spray. He had no prior history of serious infection nor was there a family history of recurrent infections, autoimmune diseases or immunodeficiency.
Concerning his antibody status, his total Ig and IgG subclass levels were in the normal range. In 2007, he was immunized with Menactra (a polysaccharide-protein conjugate vaccine to prevent meningococcal disease) and laboratory studies in 2010 (after his acute illness) demonstrated a protective IgG titer to N. meningitidis serogroup W135; however, antibodies to serogroups Y, A and C were non-protective (Supplementary Table 1). Of note, he failed to mount an IgG response to the polysaccharide of the infecting organism, a Y serotype. He was reimmunized with Menactra in 2010. Follow up analysis two years later indicated that the antibody titer to W135 had decreased from 33 to 3 µg/ml. Immunization with the 14-valent pneumococcal polysaccharide vaccine (Pneumovax) led to antibody formation to two of the capsular serogroup. He did though have a protective level of antibodies against diptheria and tetanus. These results are consistent with an impaired IgG response to polysaccharide antigens.
Evaluation of the complement system was notable for a total hemolytic complement assay (THC or CH50) of <5 U/ml (normal 30–75 U/mL). Repeat testing in a second laboratory confirmed the absence of functional activity in both the classical and alternative pathways. Further studies revealed an undetectable C3 level by nephelometry. During follow-up studies over a year later, a C3-Nef assay was performed at a clinical laboratory (National Jewish, Denver, CO) in which the patient’s serum was mixed with NHS and C3 cleavage products were detected by 2D electrophoresis. The result of 0.55 (reference range is 0–0.3, which is the ratio of cleaved C3 to native C3) is considered positive, but not at the high levels usually observed with an autoantibody stabilizing the alternative pathway C3 convertase.
Two years later the patient is well and has had no additional infectious episodes. He carries antibiotics for use if he develops symptoms of illness. His kidney function and urinalysis remain normal.
3. Materials and Methods
3.1 Materials
Purified human complement proteins (C1, C2, C3, C3b, iC3b, C3c, C3d, C4, Factor B, C4 binding protein, and properdin), polyclonal antibodies to C3, Factor B and properdin and antibody sensitized sheep erythrocytes (EA cells) were purchased from Complement Technologies (Tyler, TX). A polyclonal antibody against C4 binding protein was obtained from AbCam (Cambridge, MA). Protein G agarose was purchased from Thermo Scientific (Rockford, IL). DGVB++ buffer (veronal buffered saline: 0.015% sodium 5′,5″-diethylbarbiturate (pH 7.35) and 71 mM NaCl supplemented with 2.5% dextrose, 0.1% gelatin, 1 mM MgCl2 and 0.15 mM CaCl2) was prepared as described previously [11]. EDTA-GVB buffer consisted of 0.1% gelatin, 0.015% sodium 5′, 5′-diethylbarbiturate (pH 7.35), 71 mM NaCl and 40 mM EDTA [12].
3.2 Sample collection
Informed consent was obtained from the patient under a protocol approved by the Institutional Review Board at the Children’s Hospital of Wisconsin. Blood was placed in standard EDTA vacutainer tubes for DNA isolation. For serum samples, blood was allowed to clot for 30 min at room temperature (RT), centrifuged (10 min, 1500 × g, RT) and the serum removed and frozen in aliquots.
3.3 Genomic sequencing
DNA was isolated from the peripheral blood cells using a QIAmp Blood Maxi Kit (Qiagen, Valencia, CA). Sequencing primers for genomic C3 were designed using the Primer 3 software (Supplement Table 2). Twenty-eight pairs of primers were designed to cover the 41 exons and intron/exon borders. A minimum of 50 base pairs into each intron was sequenced.
3.4 Western blotting
For C3 analyses, sera and purified complement proteins were electrophoresed on 10% Tris-Glycine polyacrylamide gels followed by transfer to a nitrocellulose membrane [13]. Membranes were blocked overnight with 5% non-fat dry milk in Phosphate Buffered Saline-Tween (PBS-T). Blots were probed with a 1: 5,000 dilution of goat anti-human C3 antibody (Complement Technologies, Tyler, Texas), followed by rabbit anti-goat IgG HRP secondary antibody (Sigma, St Louis, MO). The blots were developed with Super Signal West Pico Substrate (Thermo Scientific, Rockford, IL).
3.5 C3 ELISA
Native C3, C3b and iC3b were coated on ELISA wells (Nunc Immuno Modules, Rochester NY) at 2 µg/ml in PBS [14, 15]. After overnight incubation at 4°C, wells were blocked with TBS (10 mM Tris, pH 7.4, 150 mM NaCl) containing 1% BSA and 0.05% Tween-20. Serum samples were diluted in TBS containing 4% BSA and 0.05% Tween-20 and applied to the ELISA wells. Following incubation at 37 °C, samples were removed by washing with TBS-Tween-20. Secondary antibodies to IgM or IgG were applied to the wells (goat anti-human IgM, Sigma; or donkey anti-human IgG, Jackson Immunoresearch) using a 1:3000 dilution. Plates were incubated for 1 h at 37 °C, washed, developed using TMB (3, 3´, 5, 5´-tetramethylbenzidine) substrate (Thermo Scientific) and absorbance at 630 nm was determined.
3.6 IgG purification
The IgG fraction was purified from the patient’s serum and a control NHS using a Protein A column (Bio-Rad, Hercules, CA). The column was equilibrated in 50 mM borate (pH 8.5) containing 150 mM NaCl. In other experiments, a Protein G column (GE Healthcare, Piscataway, NJ), equilibrated in PBS, was used. In both cases, the serum was applied to the column followed by extensive washing in the equilibration buffer. Immunoglobulins were eluted with 200 mM glycine (pH 2.5) containing 500 mM NaCl. The eluate fractions were neutralized by the addition of 1 M Tris (pH 9.0). The IgG-containing fractions were pooled and dialyzed overnight against PBS. The IgG concentration was determined at an OD 280 nm using an absorbance coefficient of 1.35. Purity of the IgG was assessed by SDS-PAGE under reducing and non-reducing conditions employing Coomassie Brilliant Blue staining.
3.7 Preparation of the EAC14b2a cellular intermediates
The classical pathway C3 convertase was assembled on antibody-sensitized sheep erythrocytes (EA) as previously described [11] [10, 16]. Briefly, EA cells (5 × 108 cells/ml) were washed three times in DGVB++. Purified human C1 was added and the cells incubated at 30° C for 15 min, followed by washing in DGVB++. Human C4 was added and the mixture incubated for 15 min at 30° C. Following washing in DGVB++, purified human C2 was added in a limiting fashion (0.3 µg C2 to 5 × 108 cells). The cells were then incubated at RT for 4 min and washed. EAC14b2a cells were resuspended in DGVB++ and utilized immediately.
3.8 C3 convertase stabilization assay
Normal human serum (NHS) and the patient’s serum were heat inactivated at 56° C for 30 min and then diluted 1:5 in DGVB++. EAC14b2a cells (50 µl) were mixed with 50 µl of DGVB++, NHS or patient’s serum [8, 10, 16] and incubated at 30° C to allow decay of the convertase. At specified time points, guinea pig serum, diluted 1:20 in 40 mM EDTA-GVB buffer, was added as a source of complement components and the samples were incubated for 1 h at 37° C. After centrifugation, absorbance at 414 nm was determined in the supernatant.
In other experiments, the sera were first pre-treated with Protein G agarose to remove IgG per the manufacturer’s instructions. Western blots were performed on the supernatants from the Protein G treatment to assess the efficiency of IgG removal. The blots were probed with a donkey anti-human IgG HRP labeled antibody (Jackson Immunoresearch).
3.9 C5 convertase stabilization hemolysis assays
Two types of assays were performed to assess stabilization of the classical pathway C5 convertase. The first assay began with assembly of the C3 convertase on EA cells followed by addition of the patient’s serum or IgG, NHS or normal IgG, or buffer alone (as described above). Following addition of the serum or IgG preparations, C3 was added to form the C5 convertase (C4b2a3b) and then allowed to decay at 30° C. At the indicated times, C3 depleted serum, diluted 1:60 in EDTA-GVB buffer, was added as a source of terminal pathway components. The samples were incubated for 1 h at 37° C, and absorbance determined in the supernatants at 414 nm. To account for decay of the C3 convertase that might occur while treating the samples with the patient and control sera and IgG, the data were analyzed as the ratio of C5 convertase remaining after 15 min, compared to the level of C5 convertase that was present in each sample at time zero.
In the second assay, the C5 convertase was assembled by adding C3 at the same time as the C2. The patient’s serum, IgG, NHS, normal IgG or buffer alone were then added and samples were incubated at 30° C to allow for decay. C3 depleted serum was added and hemolysis assessed as described above.
4. Results
4.1 Sequencing of the patient’s C3 gene
Genomic DNA was sequenced using twenty-seven pairs of primers that encompassed all 41 exons and intron/exon borders of the C3 gene. The sequences were aligned to the reference sequence available in GenBank with the accession number NG 009557. No abnormalities were detected. The patient was homozygous for the more common slow form of C3 (Arg80) and the more frequent Pro at position 292. The putatively normal gene suggested that the C3 protein was being synthesized.
4.2 Patient’s serum demonstrates C3 degradation products
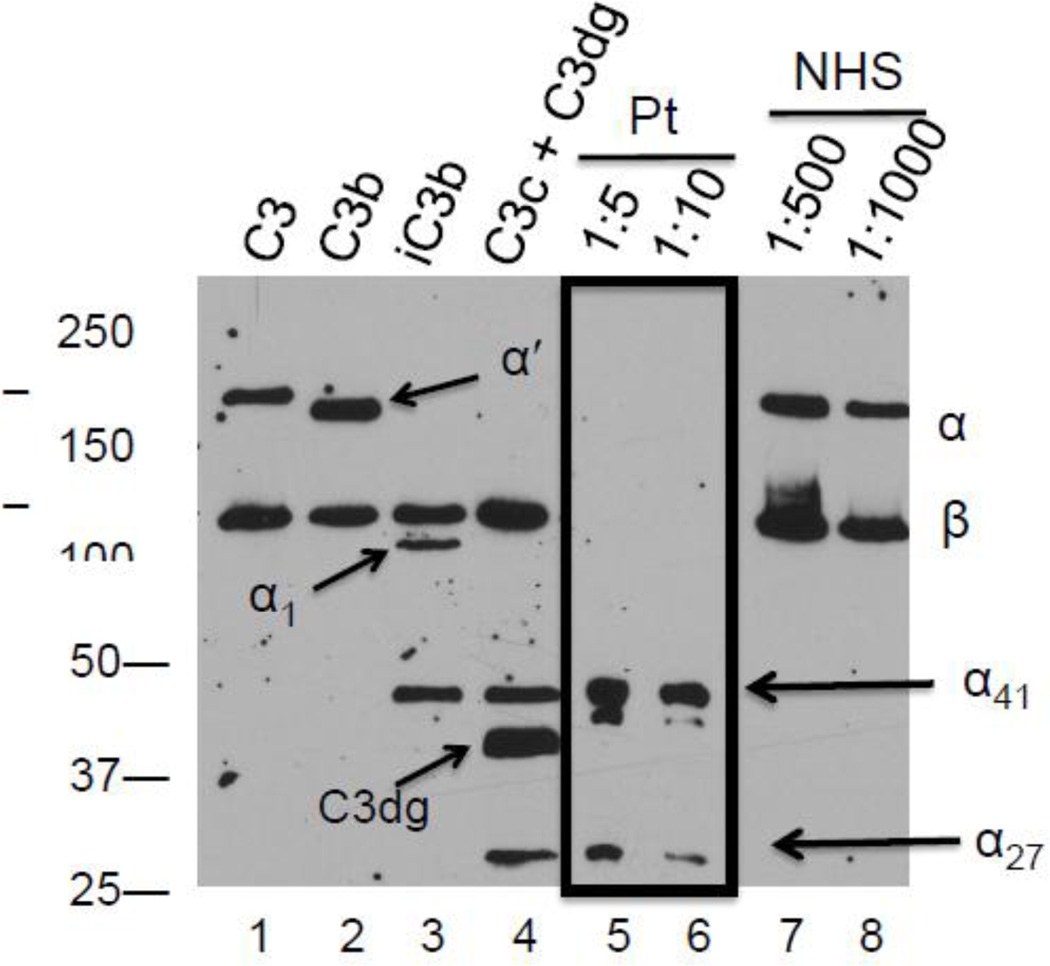
On multiple occasions, C3 antigen was below detectable limits by nephelometry. Therefore, his serum was analyzed for C3 by Western blotting at a 1:5 and a 1:10 dilution; in this assay, NHS is routinely diluted 1:500 to 1:1000. In NHS, as expected, under reducing conditions only the intact α (115 kDa) and β chains (75 kDa) of C3 were observed (Fig 1). However, in the patient’s serum, α chain fragments (α41 and α27; 41 kDa and 27 kDa, respectively) were observed. These bands correspond to those in the C3c fragment (Fig 2). This finding is consistent with C3 undergoing activation to C3b followed by its degradation to C3c and C3dg by cofactor activity. These fragments are rapidly cleared from the circulation. Thus, their presence points to continuous, excessive C3 turnover.
Figure 1.
Western blot of patient’s serum demonstrates C3 degradation fragments. C3 fragments in serum were separated by electrophoresis on a 10% Tris-glycine gel under reducing conditions and transferred to a nitrocellulose membrane. The blot was developed with a polyclonal goat anti-human C3 as described in Methods. Purified C3, C3b, iC3b and C3c and C3dg (10 ng each) served as controls (lanes 1–4). Antigenically-reactive C3 fragments in the patient’s serum were detectable only at low dilutions (1:5 and 1:10-lanes 5 and 6) whereas they were readily detectable at high dilutions of normal human serum (1:500 and 1:1000). The data shown are representative of 3 experiments.
Figure 2.
Schematic diagrams of C3 activation and degradation.
4.3 Normal C3 is activated by the patient’s serum
Mixing experiments were performed to assess whether the patient’s serum cleaved native C3 in NHS (Fig 3). C3 fragments consistent with iC3b were readily detectable (Fig 3A, lanes 2, 3 and 4) and increased as more of the patient’s serum was added (compare lane 2 to lanes 3–7). Mixing undiluted patient’s serum with undiluted NHS followed by overnight incubation led to complete cleavage of the C3 α chain (Fig 3B). Therefore, the patient’s serum contains a factor that promotes C3 cleavage and the cleavage pattern is consistent with cofactor-mediated degradation of C3b (Fig 2).
Figure 3.
Patient’s serum activates C3 in normal human serum (NHS). A. The patient’s serum and NHS were diluted 1:50 in PBS and then mixed together at the indicated ratios and processed as described in Methods. Lanes 1, 9, 10 and 11 are controls (10 ng/lane). At the 1:50 dilution, C3 fragments were not detectable in the patient’s serum (lane 8). Therefore, C3 bands detected by Western blot (lanes 2–7) are derived from C3 in the NHS. Samples (lanes 2–6) were incubated for one hr at 37°C. A representative experiment of 8 independent experiments is shown. B. Undiluted serum from the patient and NHS were mixed together at a 1:1 ratio and incubated overnight at 37°C. Lanes 1, 4, 5, and 6 are controls (10 ng/lane). The patient’s serum utilized in lanes 2 and 3 were obtained ~6 months apart. This experiment was performed one time.
4.4 The patient’s serum stabilizes the classical/lectin pathway C3 convertase
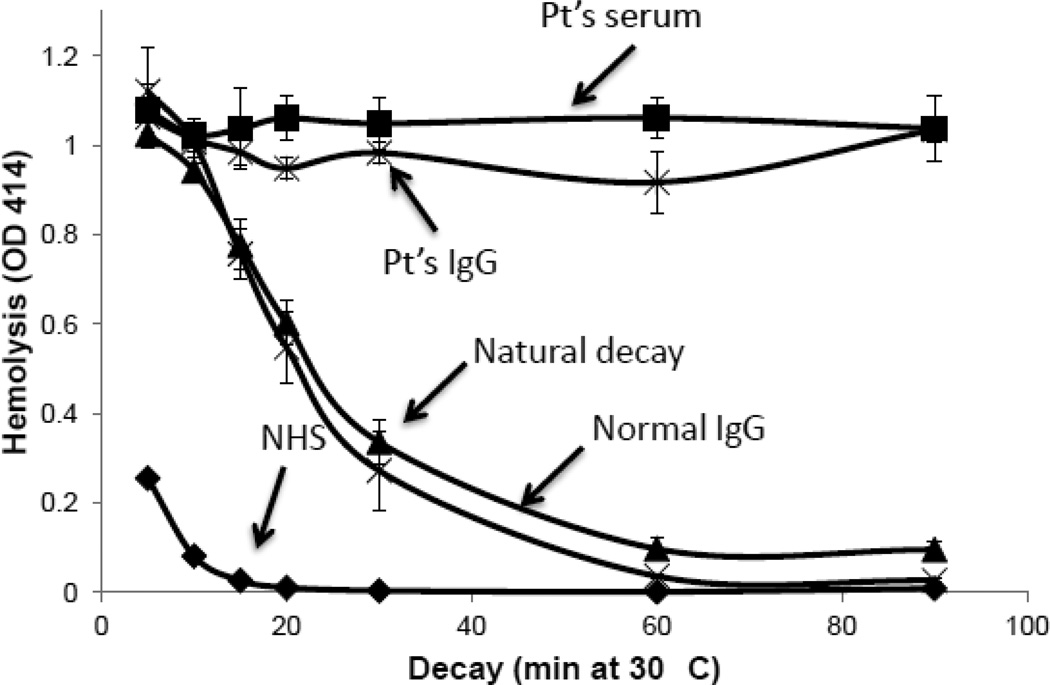
The normal FB levels made it unlikely that the AP was being excessively activated. To determine therefore whether the patient’s serum stabilized the classical/lectin pathway C3 convertase (CP-C3con), assays were performed using hemolysis of antibody sensitized sheep red blood cells (EA) as the readout for convertase activity. The CP-C3con was assembled on EA and then allowed to decay for various times before addition of terminal pathway complement components to produce hemolysis (Fig 4). The convertase spontaneously decays (natural decay), resulting in decreased hemolysis at later time points. If the convertase is stabilized, more hemolysis is observed. The samples to which buffer alone was added demonstrated the expected natural decay of the convertase. The accelerated decay of the convertase upon exposure to NHS is due to C4b binding protein (C4BP) in the NHS. In contrast, when the patient’s serum was added, there was no decay of the CP-C3con. If the assay time was extended to eight hrs, there was still no decay in the presence of the patient’s serum (not shown).
Figure 4.
The classical pathway C3 convertase is stabilized in the presence of the patient’s serum. The classical pathway C3 convertase (C4b2a) was established on antibody sensitized sheep erythrocytes (EA) as described in Methods. Patient’s serum, NHS or buffer (natural decay) was added and the convertase allowed to decay at 30° C for the times noted. EDTA-treated guinea pig serum was then added and hemolysis was quantitated following incubation at 37°C for 1 hr. Results are representative of 3 independent experiments. The half-life of the classical pathway convertase in buffer (~25 min as shown by the middle curve) is indefinitely extended in the presence of the patient’s serum (upper curve). The accelerated decay of the convertase upon exposure to NHS (compared to the buffer control) is due to C4b binding protein (C4BP) in the NHS (lower curve).
4.5 IgG stabilizes the classical pathway C3 convertase
We next depleted the patient’s serum and NHS of IgG using protein G beads and repeated the assays described above (Fig 5A). Untreated, the patient’s serum prevented decay of the convertase. Following a single absorption of the serum with protein G beads, hemolysis was reduced by 50%. A second exposure to protein G beads reduced hemolysis to 16%, similar to that observed with NHS. Thus, the stepwise loss of stabilizing capacity paralleled the removal of IgG (Fig 5B).
Figure 5.
Stabilizing activity for the classical pathway C3 convertase in the patient’s serum is lost by removal of IgG. Serum IgG was removed by one or two adsorption(s) with Protein G Sepharose. (A) The effect of IgG depleted serum on C3 convertase stability was assessed in an hemolysis assay monitoring convertase decay as described in Methods. Samples were allowed to decay for 30 min at 30°C. Shown is the mean +/−SEM of 3 experiments. Removal of IgG from the patient’s serum (bars 4 and 5) partially abrogated hemolysis mediated by the stabilized convertase whereas there was no effect on IgG removal from NHS (bars 7 and 8). Decay of the convertase in the presence of NHS and IgG depleted patient serum was accelerated compared to the buffer alone control due to the presence of C4BP in the serum. (B) Western blot assessment of the efficacy of IgG removal from sera by Protein G adsorption. The blots were performed as described in Methods and developed using donkey anti-human IgG (1:5,000).
To confirm and extend this finding, the IgG-containing fractions obtained from the patient’s serum and from NHS (assessed for purity by SDS-PAGE and Coomassie blue staining, see Supplement Fig 1) were employed. IgG from the patient’s serum stabilized the CP-C3con whereas IgG from NHS did not (Fig 6). Importantly, if either C4BP or soluble CR1 (sCR1) were added, decay also did not occur (Supplemental Fig 2). Taken together, these results indicate that an IgG autoantibody in the patient’s serum is capable not only of stabilizing the CP-C3con, but also of abrogating C4BP- and CR1-mediated convertase decay.
Figure 6.
Patient’s purified IgG stabilizes the classical pathway C3 convertase. IgG was isolated from NHS and the patient’s serum using a Protein A column and its effect on C3 convertase stability was assessed as described in Methods. Addition of patient IgG to convertase bearing cells recapitulated the effect produced by intact patient serum (top two curves), whereas IgG from NHS had no impact on the spontaneous decay of the convertase (middle two curves). The accelerated decay of the convertase upon exposure to NHS (compared to the buffer control is due to C4b binding protein (C4BP) in the NHS (lower curve). The data represent two independent experiments (+/− SEM).
4.6 IgG in the patient’s serum stabilizes the classical/lectin pathway C5 convertase
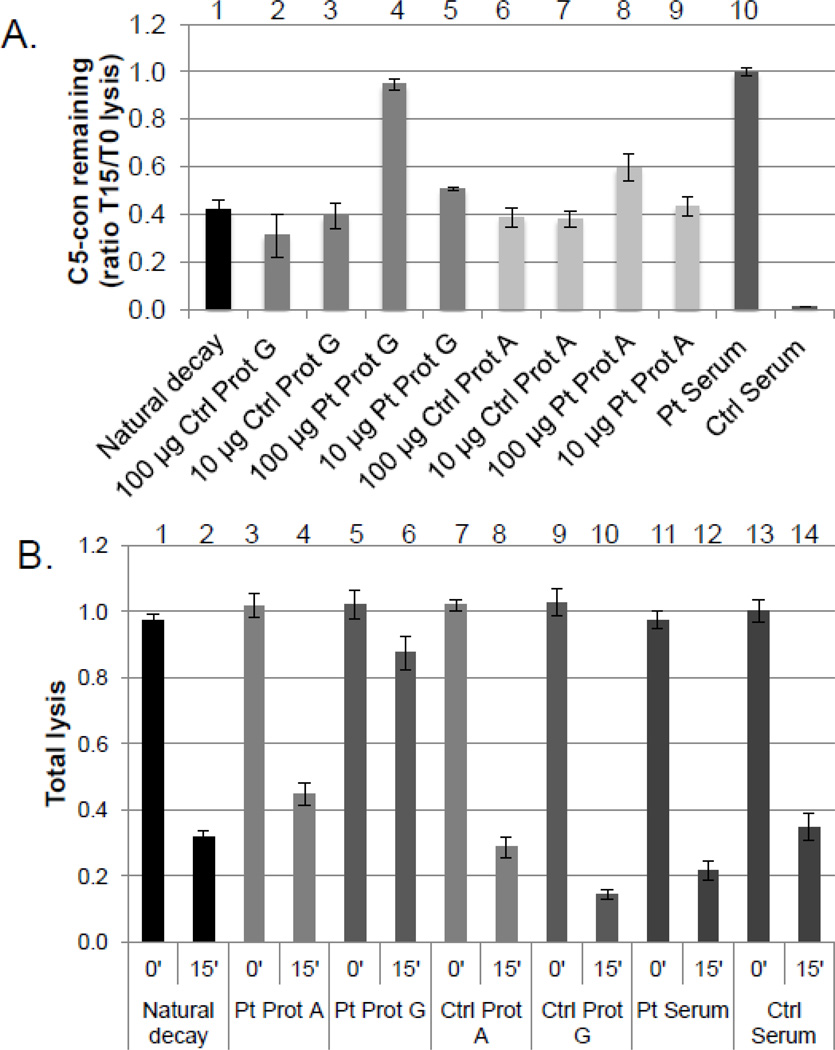
The cleavage of C3 by a C3 convertase may lead to the formation of a C5 convertase. This tri-molecular complex (C4b2a3b; CP-C5con) cleaves C5 and the newly generated C5b then initiates the terminal pathway. Since the C5 convertase is formed on the existing C3 convertase, the possibility of the patient’s IgG stabilizing the CP-C5con was investigated. The CP-C3con was assembled and then treated with the patient’s serum or IgG. The IgG was purified over a Protein A or a Protein G column. Protein A does not bind IgG3. Purified C3 was then added to form the CP-C3con. Following a decay period, samples were incubated with C3 depleted serum as a source of terminal pathway components. The patient’s IgG, purified by Protein G but not by Protein A, stabilized the C5 convertase, although the concentration of antibody required for stabilization was approximately 10- fold higher than that needed to stabilize the C3 convertase (Fig 7a). The fact that the stabilizing autoantibody was purified by Protein G but not by Protein A places it in the IgG3 subclass.
Figure 7.
IgG purified from the patient’s serum by Protein G stabilizes the classical pathway C5 convertase. Classical pathway C5 convertase sensitized cells were prepared as described in Methods. A. Effect of serum or IgG on the formation of the C5 convertase. C5 convertase formed in the presence of either the patient’s serum or 100 µg of the patient’s IgG was stabilized as evidenced by a T15/T0 ratio of 1.0 (compare bars 4 and 10 to bar 1). Substantial stabilization was observed only in the presence of IgG isolated from Protein G, not Protein A (bar 4 vs bar 8). No stabilization was observed with either NHS or normal IgG. The results shown are the mean +/− SEM of three experiments. B. Effect of whole serum or purified IgG on the stability of preformed C5 convertase. The C5 convertase was preformed on the EA cells as described in Methods. The patient’s IgG isolated by Protein G but not Protein A chromatography stabilized the preformed C5 convertase. Stabilization was not observed using IgG from NHS regardless of the isolation method employed. At the concentration of the patient’s serum used here, the C5 convertase was not stabilized. This is likely due to a concentration effect, as a higher dose of the purified IgG (100 µg) was required for C5 convertase stabilization. The results shown are the mean +/− SEM of three experiments.
The CP-C5con convertase (C4b2a3b) was assembled on EA cells and allowed to decay (Fig 7b). The patient’s IgG purified via Protein G but not Protein A stabilized this pre-formed C5 convertase. These results are consistent with a polyclonal IgG antibody response to multiple neo-epitopes on the CP-C3 and C5 convertases.
5. Discussion
Herein we describe an 18-year-old male who presented with life-threatening sepsis and meningitis secondary to Neisseria meningitidis and whose serum contained no detectable total hemolytic complement or C3 in standard clinical laboratory tests. Genomic sequencing demonstrated an intact C3 gene. Western blotting of the patient’s serum established the presence of C3 degradation fragments, compatible with accelerated C3 consumption. This possibility was confirmed by mixing experiments in which a factor in the patient’s serum cleaved native C3 in NHS. The proteolytic activity was identified as a classical pathway C3 convertase (C4bC2a) stabilized by an autoantibody. Thus, the normally short-lived (min) enzyme complex was altered to one with a half-life of hrs. This autoantibody prevented spontaneous and regulatory protein mediated decay and, further, stabilized the C5 convertase.
C3-NeF, an autoantibody to the alternative pathway C3 convertase (C3bBb), was initially described in the 1970’s [17–19], and recently reviewed in depth by Paixão-Cavalcante et al [20]. It stabilized this enzyme complex and was associated with type II membranoproliferative glomerulonephritis (MPGNII; Dense Deposit Disease), systemic lupus erythematosus (SLE), and occasionally partial lipodystrophy [19]. There have also been four reports of an analogous C4-NeF, an autoantibody that stabilizes the CP C3-convertase [8–10], [21]. In one of these reports [9], 16 patients with lupus nephritis were screened and two were identified to have stabilizing activity for the CP C3 convertase. About the same time, another article identified a similar stabilizing activity in one patient with post-infectious glomerulonephritis [21]. In 1989, a study identified both C3-NeF and C4-NeF in two patients with hypocomplementemic MPGN [8]. The last report in the literature on C4-NeF was by Ohi and Yasugi in 1994 who screened sera from 100 patients with non-SLE hypocomplementemic (C3 < 40% of normal) membranoproliferative glomerulonephritis for C3- and C4-NeFs [10]. Thirty-one were positive for a nephritic factor, nine of them had only C4-NeF, and ten were positive for both C3- and C4-NeFs. The complement levels in the MPGN patients with only C4-NeF and the patient described herein are similar in that C3 was very low and C5 levels were about 50% of normal while C4 and Factor B were normal. However, in contrast to the previously reported cases [8–10, 21], our patient presented with sepsis and meningitis and had normal renal function that, despite ongoing C3 consumption, has remained so during a two-year follow up period.
Also notable in our patient is a lack of prior infections and absence over a two year follow up of additional infectious episodes. Given that C3 deficiency and the resultant lack of opsonic and bactericidal activity is a well-documented risk factor for pyogenic infections, it is likely that the development of the C4-NeF and the expansion of the corresponding B cell clones only recently predated the patient’s infection. Also, the patient has responded poorly to vaccination, presumably due to his C3 deficient state. The meningococcal vaccine was administered three years prior to his initial infection, but failed to provide protection against his serogroup Y infecting strain. Since that time, the patient has received yearly meningococcal (conjugated polysaccharide), pneumococcal (polysaccharide) and Haemophilus influenzae vaccines but continues to have undetectable or low antibody titers toward these antigens.
This patient’s C5 level was reduced by ~50%. Two explanations for this reduction have been considered. The first is that the stabilized CP C3 convertase cleaves C5 directly, a reaction reported to occur with about 10% the efficiency of C3 cleavage [22]. The second possibility is enhanced C5 cleavage by a stabilized C5 convertase (C4b2a3b). We provide evidence for the latter in in vitro experiments (Fig 7), but this may be less likely in vivo because the patient’s serum contains essentially no C3, which would be necessary to assemble the C5 convertase. These two explanations are not mutually exclusive.
The quantity of antibody required to stabilize the C5 convertase was considerably greater (10-fold) than what was needed to stabilize the C3 convertase, suggesting that either the binding of C3b to the C4b2a complex alters the neoepitopes or, alternatively, that only ~10% of the polyclonal antibody population can stabilize the C5 convertase. Additionally, stabilization of the C5 convertase by purified IgG was observed only if it was isolated using a Protein G column. IgG purified over a Protein A column did not stabilize the C5 convertase. In contrast, IgG purified from either Protein G or Protein A stabilized the C3 convertase to the same degree. Protein G binds to all isotypes of human IgG whereas Protein A binds poorly to IgG3. The discrepancy in the stabilization behavior of these two preparations supports the idea that this is a polyclonal response and that a subset of the autoantibodies containing IgG3 is specifically required for C5 convertase stabilization.
While there is a commercially available test for C3-NeF (National Jewish Center, Denver, CO), there is no such test for C4-NeF. However, the current test that is advertised for C3-NeF may give a low titer positive result if C4-NeF is present (Patricia Giclas, personal communication), as it did in our patient. This is because the basis of the test is the cleavage of native C3 by a factor in the unknown sample. Similar to the serum mixing experiments presented herein, the commercial test is performed by mixing NHS with the unknown sample and then monitoring for C3 cleavage products by electrophoresis. Thus, the presence of either C3-NeF or C4-NeF could result in cleavage of C3 in this assay. Therefore, if a patient has an undetectable, or very low, THC and undetectable C3, further testing should include C4 and Factor B (Table 2). In a patient with C4-NeF, Factor B and C4 levels will be normal. In contrast, a patient with C3-NeF will have reduced Factor B and normal C4 levels. Also, a patient does not necessarily need to have renal disease, as illustrated by our case. The proof that this is a C4-NeF then requires performing experiments as outlined in this report including mixing studies (Fig 3) and stabilization studies of the C4b2a enzyme (Fig 4). Thus, having defined the nature of the autoantibody in our patient’s serum, we could now reinterpret the positive result in the C3-NeF assay performed in the clinical laboratory as resulting from C4-NeF activity. In view of the lack of reports over the past nearly 20 years of a C4-NeF, we suspect the diagnosis has not been considered or, if so, not addressed with appropriate tests. This report serves as a reminder of this autoimmune disease and extends its clinical spectrum.
Table 2.
Laboratory Results Suggesting the Presence of a Nephritic Factora
| C3-NeF | C4-NeF | |
|---|---|---|
| TCHb | very lowc | very lowc |
| C3 | very lowc | very lowc |
| C4 | normal | normal |
| FB | very lowc | normal |
| C5 | reduced | reduced |
If one of these patterns is detected, follow up studies should be performed, such as serum mixing and hemolysis assays.
AP-CH50 would also be very low or undetectable.
Commonly undetectable
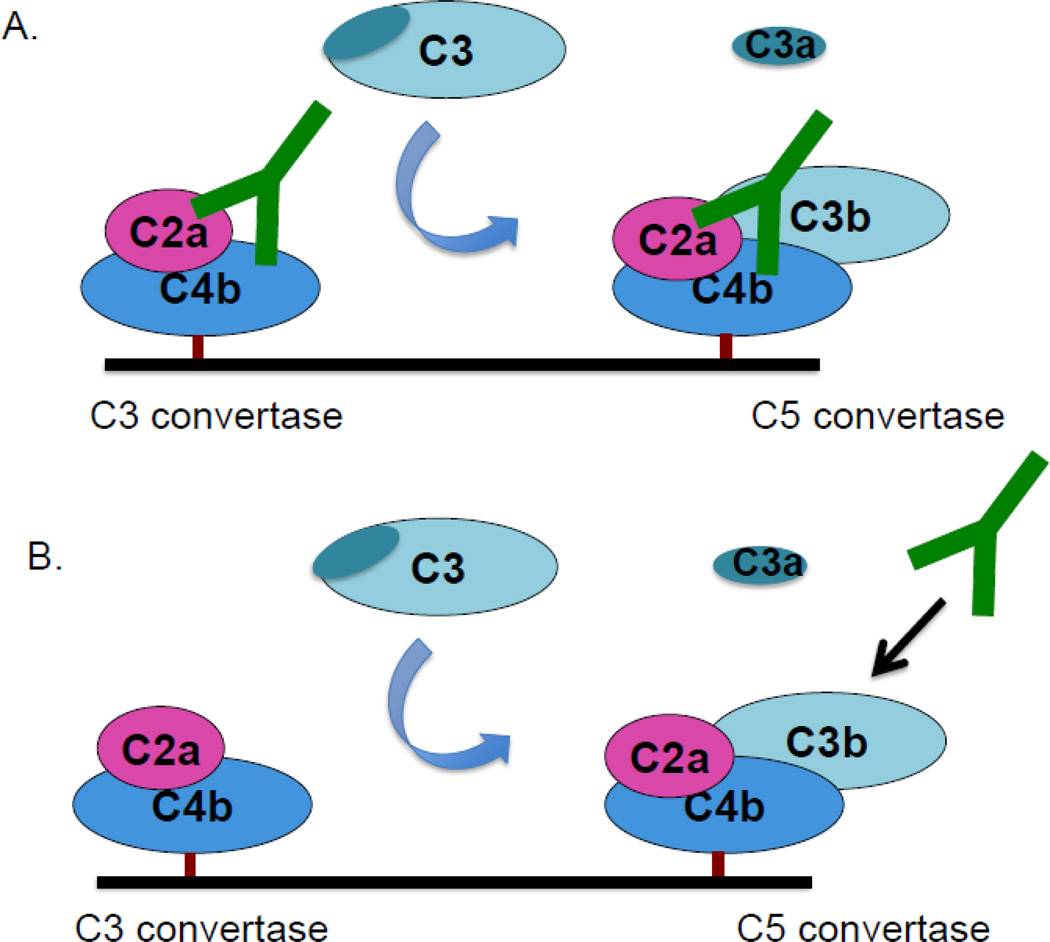
Structural and functional analyses of the classical pathway C3 and C5 convertases have been hampered by the highly transient nature of these bi- and tri-molecular enzymatic complexes. This patient’s autoantibody indefinitely stabilized the classical pathway C3 convertase. It also stabilized the C5 convertase, especially if added before C3b associates with C4b2a (Fig 8); additionally, a subset of the IgG autoantibody stabilizes the convertase even if added to the pre-formed C5 convertase. Further studies of the convertases utilizing this patient’s IgG may lend additional insight into the assembly and stabilizing forces for these complexes.
Figure 8.
Two models of convertase stabilization by the patient’s autoantibody. A. The patient’s IgG binds to and stabilizes the classical pathway C3 convertase. This convertase cleaves C3 to C3b and a fraction of this C3b then forms the C5 convertase. The C5 convertase is also stabilized by the IgG. B. After the C5 convertase is formed, it can still be stabilized by a subset of the autoantibodies.
In summary, we describe a unique case of C4-NeF presenting with a meningococcal infection and C3 deficiency but without renal disease. We outline an approach to making a definitive laboratory diagnosis of this autoantibody-induced syndrome.
Supplementary Material
Table 1.
Patient’s serum complement levelsa
| Patient | Reference range | |
|---|---|---|
| CH50 | <10 | 31–66 (units/ml) |
| AH50 | 0 | 77–159 (units/ml) |
| C2 | 2.0 | 1.6–3.5 (mg/dl) |
| C3 (antigen)** | <11 | 82–185 (mg/dl) |
| C3 (function) | 0 | 11249–42887 (units/ml) |
| C4 | 35 | 15–53 (mg/dl) |
| C5 | 3.4 | 5.5–11.3 (mg/dl) |
| Factor B | 24 | 13–28 (mg/dl) |
Concentrations of Factor H, C4 binding protein and Factor I were comparable to those in normal serum as assessed by Western blotting.
C3 antigen concentration in the sera from family members was normal (father:89; mother: 95; siblings: 85, 120, 101, and 93 (mg/dl).
Highlights.
We report a previously healthy 18 y/o with Neisserial sepsis and C3 deficiency.
The deficiency was identified as being due to accelerated consumption of C3.
The mechanism was classical pathway C3 convertase stabilization by an autoantibody.
Known as C4 nephritic factor, the presentation and lack of nephrosis are unique.
We review the C4 nephritic factor literature and outline a strategy for diagnosis.
Acknowledgements
We thank Michael Triebwasser for donating the C3 sequencing primers and the Rheumatic Diseases Core Center for experimental support (NIH P30AR048335). This work was supported by NIH RO1 AI041592 and T32 HL007317.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Walport MJ. Complement. First of two parts. N. Engl. J. Med. 2001;344:1058–1066. doi: 10.1056/NEJM200104053441406. [DOI] [PubMed] [Google Scholar]
- 2.Walport MJ. Complement. Second of two parts. N. Engl. J. Med. 2001;344:1140–1144. doi: 10.1056/NEJM200104123441506. [DOI] [PubMed] [Google Scholar]
- 3.Gros P, Milder FJ, Janssen BJ. Complement driven by conformational changes. Nat Rev Immunol. 2008;8:48–58. doi: 10.1038/nri2231. [DOI] [PubMed] [Google Scholar]
- 4.Janssen BJ, Huizinga EG, Raaijmakers HC, Roos A, Daha MR, Nilsson-Ekdahl K, Nilsson B, Gros P. Structures of complement component C3 provide insights into the function and evolution of immunity. Nature. 2005;437:505–511. doi: 10.1038/nature04005. [DOI] [PubMed] [Google Scholar]
- 5.Reis E, Falcao DA, Isaac L. Clinical aspects and molecular basis of primary deficiencies of complement component C3 and its regulatory proteins factor I and factor H. Scand. J. Immunol. 2006;63:155–168. doi: 10.1111/j.1365-3083.2006.01729.x. [DOI] [PubMed] [Google Scholar]
- 6.Botto M, Kirschfink M, Macor P, Pickering MC, Wurzner R, Tedesco F. Complement in human diseases: Lessons from complement deficiencies. Mol. Immunol. 2009;46:2774–2783. doi: 10.1016/j.molimm.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 7.Figueroa J, Andreoni J, Densen P. Complement deficiency states and meningococcal disease. Immunol. Res. 1993;12:295–311. doi: 10.1007/BF02918259. [DOI] [PubMed] [Google Scholar]
- 8.Tanuma Y, Ohi H, Watanabe S, Seki M, Hatano M. C3 nephritic factor and C4 nephritic factor in the serum of two patients with hypocomplementaemic membranoproliferative glomerulonephritis. Clin. Exp. Immunol. 1989;76:82–85. [PMC free article] [PubMed] [Google Scholar]
- 9.Daha MR, Hazevoet HM, Vanes LA, Cats A. Stabilization of the classical pathway C3 convertase C42, by a factor F-42, isolated from serum of patients with systemic lupus erythematosus. Immunology. 1980;40:417–424. [PMC free article] [PubMed] [Google Scholar]
- 10.Ohi H, Yasugi T. Occurrence of C3 nephritic factor and C4 nephritic factor in membranoproliferative glomerulonephritis (MPGN) Clin. Exp. Immunol. 1994;95:316–321. doi: 10.1111/j.1365-2249.1994.tb06530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Medof ME, Kinoshita T, Nussenzweig V. Inhibition of complement activation on the surface of cells after incorporation of decay-accelerating factor (DAF) into their membranes. Journal of Experiment Medicine. 1984;160:1558–1578. doi: 10.1084/jem.160.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krych-Goldberg M, Hauhart RE, Subramanian VB, Yurcisin BM, II, Crimmins DL, Hourcade DE, Atkinson JP. Decay accelerating activity of complement receptor type 1 (CD35). Two active sites are required for dissociating C5 convertases. J. Biol. Chem. 1999;274:31160–31168. doi: 10.1074/jbc.274.44.31160. [DOI] [PubMed] [Google Scholar]
- 13.Fremeaux-Bacchi V, Miller EC, Liszewski MK, Strain L, Blouin J, Brown AL, Moghal N, Kaplan BS, Weiss RA, Lhotta K, Kapur G, Mattoo T, Nivet H, Wong W, Gie S, de Ligny BH, fischbach M, Gupta R, Hauhart R, Meunier V, Loirat C, Dragon-Durey MA, Fridman WH, Janssen BJC, Goodship THJ, Atkinson JP. Mutations in complement C3 predispose to development of atypical hemolytic uremic syndrome. Blood. 2008;112:4948–4952. doi: 10.1182/blood-2008-01-133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liszewski MK, Leung MK, Hauhart R, Fang CJ, Bertram P, Atkinson JP. Smallpox inhibitor of complement enzymes (SPICE): Dissecting functional sites and abrogating activity. J. Immunol. 2009;183:3150–3159. doi: 10.4049/jimmunol.0901366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dragon-Durey MA, Blanc C, Garnier A, Hofer J, Sethi SK, Zimmerhackl LB. Anti-factor H autoantibody-associated hemolytic uremic syndrome: review of literature of the autoimmune form of HUS. Semin. Thromb. Hemost. 2010;36:633–640. doi: 10.1055/s-0030-1262885. [DOI] [PubMed] [Google Scholar]
- 16.Kuttner-Kondo L, Hourcade DE, Anderson VE, Muqim N, Mitchell L, Soares DC, Barlow PN, Medof ME. Structure-based mapping of DAF active site residues that accelerate the decay of C3 convertases. J. Biol. Chem. 2007;282:18552–18562. doi: 10.1074/jbc.M611650200. [DOI] [PubMed] [Google Scholar]
- 17.Daha MR, Austen KF, Fearon DT. The incorporation of C3 nephritic factor (C3NeF) into a stabilized C3 convertase, C3b, Bb (C3NeF), and its release after decay of convertase function. J. Immunol. 1977;119:812–817. [PubMed] [Google Scholar]
- 18.Lyzoguvov VV, Tytarenko RG, Jha P, Wu X, Kolar G, Atkinson JP, Bora PS, Bora NS. Role of CD46 in laser induced choroidal neovascularization. Investigational Opthalmology and Visual Science. 2011;52 E-Abstract 2326. [Google Scholar]
- 19.Walport MJ, Davies KA, Botto M, Naughton MA, Isenberg DA, Biasi D, Powell RJ, Cheung NT, Struthers GR. C3 nephritic factor and SLE: report of four cases and review of the literature. QJM. 1994;87:609–615. [PubMed] [Google Scholar]
- 20.Paixao-Cavalcante D, Lopez-Trascasa M, Skattum L, Giclas PC, Goodship TH, de Cordoba SR, Truedsson L, Morgan BP, Harris CL. Sensitive and specific assays for C3 nephritic factors clarify mechanisms underlying complement dysregulation. Kidney Int. 2012 doi: 10.1038/ki.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halbwachs L, Leveille M, Lesavre PH, Wattel S, Leibowitch J. Nephritic factor of the classical pathway of complement. Immunoglobulin G autoantibody directed against the classical pathway C3 convertase enzyme. J. Clin. Invest. 1980;65:1249. doi: 10.1172/JCI109787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rawal N, Pangburn MK. Formation of high-affinity C5 convertase of the classical pathway of complement. J. Biol. Chem. 2003;278:38476–38483. doi: 10.1074/jbc.M307017200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.