Abstract
Background
Abnormal hip mechanics are often implicated in female runners with patellofemoral pain. We sought to evaluate a simple gait retraining technique, using a full-length mirror, in female runners with patellofemoral pain and abnormal hip mechanics. Transfer of the new motor skill to the untrained tasks of single leg squat and step descent was also evaluated.
Methods
Ten female runners with patellofemoral pain completed 8 sessions of mirror and verbal feedback on their lower extremity alignment during treadmill running. During the last 4 sessions, mirror and verbal feedback were progressively removed. Hip mechanics were assessed during running gait, a single leg squat and a step descent, both pre- and post-retraining. Subjects returned to their normal running routines and analyses were repeated at 1-month and 3-month post-retraining. Data were analyzed via repeated measures analysis of variance.
Findings
Subjects reduced peaks of hip adduction, contralateral pelvic drop, and hip abduction moment during running (P<0.05, effect size=0.69–2.91). Skill transfer to single leg squatting and step descent was noted (P<0.05, effect size=0.91–1.35). At 1 and 3 months post retraining, most mechanics were maintained in the absence of continued feedback. Subjects reported improvements in pain and function (P<0.05, effect size=3.81–7.61) and maintained through 3 months post retraining.
Interpretation
Mirror gait retraining was effective in improving mechanics and measures of pain and function. Skill transfer to the untrained tasks of squatting and step descent indicated that a higher level of motor learning had occurred. Extended follow-up is needed to determine the long term efficacy of this treatment.
Keywords: patellofemoral pain, running, rehabilitation, hip, patella
Introduction
Running is one of the most popular forms of exercise with upwards of 16 million Americans participating in the sport today (National Sporting Goods Association, 2009). However, runners report an alarmingly high annual injury rate between 19.4 and 79.3% (van Gent et al. 2007). Patellofemoral pain syndrome (PFP) is the most prevalent type of knee pain among runners (Taunton et al. 2002). As with most knee injuries, there is a sex bias associated with PFP with 68% of sufferers of PFP being female (Taunton et al. 2002).
Abnormal hip mechanics in females with PFP have been reported during running, squatting, and during a step down maneuver (Willson and Davis, 2008, Dierks et al. 2008, Souza and Powers, 2009a, b, Noehren et al. 2012). These motions include excessive contralateral pelvic drop (Dierks et al., 2008, Willson et al., 2008), hip adduction (Willson et al. 2008, Dierks et al, 2008), and hip internal rotation (Dierks et al. 2008, Souza et al. 2009a,b). Together, these motions likely increase the dynamic Q-angle of the knee (Powers 2003, 2010) resulting in an increase in joint stress on the lateral aspect of the patellofemoral joint (Besier et al. 2008, Huberti and Hayes, 1984, Lee et al. 2001).
The gluteus medius and maximus are the primary stabilizers of the hip in the frontal and transverse planes. Because weakness of this musculature has been identified in individuals with active PFP, hip strengthening is often advocated for the treatment of PFP. Hip strengthening has clearly been shown to result in short term pain reduction in PFP cohorts (Dolak et al. 2011, Mascal et al. 2003, Ferber et al. 2011, Earl et al. 2011, Nakagawa et al. 2007, Boling et al. 2006). However, the ability of hip strengthening to change lower extremity mechanics is largely unproven. After a hip strengthening program in healthy females with normal running mechanics, Snyder and colleagues (Snyder et al. 2007) actually reported an increase of hip adduction excursion, the opposite of the desired direction. More recently, Willy and Davis (2011) suggested that a 6-week program that significantly increased hip strength had no effect on abnormal hip mechanics during running. In females with PFP, Earl et al. (Earl et al. 2011) failed to find any change in hip and knee kinematics after a hip strengthening and flexibility program. Therefore, it appears that hip strengthening alone is insufficient to change the proximal mechanics that have been associated with PFP in females. If the underlying mechanics of PFP are not addressed by a treatment modality, then symptoms will likely eventually return.
Treatments for PFP that directly address faulty proximal mechanics have shown promise. Gait retraining, using real-time kinematic feedback, has been suggested as an effective treatment of PFP in female runners (Noehren et al. 2010). Using a real time motion analysis system, the hip adduction angle during each stance phase of treadmill running was provided to the runner in real time. Subjects were encouraged match their hip angle to a normative target range provided on the monitor. Significant improvements in hip mechanics, knee pain and overall function were found at post-gait retraining. Interestingly, the reductions in abnormal hip mechanics during running transferred to the untrained task of single leg squatting. Importantly, these subjects were able to maintain their new movement patterns at the 1-month follow up. Improved pain and function scores also persisted, thus suggesting the potential for long-term changes in mechanics, pain, and function.
There are limitations to the work by Noehren and colleagues (Noehren et al. 2010). First, most rehabilitation settings do not have the resources to purchase and/or operate a real-time motion capture system. Thus, the clinical utility of real-time kinematic gait retraining is limited to research oriented treatment centers at best. In order to implement this gait retraining technique in clinical settings, it is possible that visual feedback could be provided by a mirror rather than a motion capture system. If successful, mirror feedback would provide a simple, cost-effective method of retraining. In addition, it might be more informative to assess the transfer of the new movement pattern during running to a more functional activity than the single leg squat, such as step descent. Finally, subjects were only followed for 1 month post-gait retraining. Most studies define chronic PFP as having a duration of at least 3 months (Crossley et al. 2006, Souza and Powers 2009a,b, Nakagawa et al., 2009). Thus, investigations of potential treatments of PFP should likely follow subjects for at least 3 months post-intervention to demonstrate stronger evidence of symptom resolution.
Therefore, the purpose of this study was to examine the effect of a clinically applicable, gait retraining program on hip mechanics, pain and function in runners with PFP. We hypothesized that mirror gait retraining would improve measures of abnormal hip mechanics during running. We further hypothesized that these changes would persist through 3 months post-intervention. We also hypothesized that subjects would successfully transfer the new movement pattern to the untrained tasks of SLS and step descent with similar persistence through 3 months. Finally, it was hypothesized that subjects would report a decrease in reported pain and an increase in function through 3 months post-intervention.
Methods
The data collection procedures and informed consent document were approved by the University of Delaware Human Subjects Research Board. An a priori power analysis was conducted using data from pilot work examining the difference between females with PFP and healthy controls. Using the variable peak hip adduction (HADD), it was revealed that 9 subjects (effect size=1.43 α= 0.05, β=0.20) were required to adequately power this study. Therefore, 10 qualified subjects were recruited for this study.
Subjects were female, between 18 and 40 years of age, running at least 10 km/week, comfortable with treadmill running at 3.35 m/sec, and free of any cardiac risk factors. All subjects were required to have retropatellar or peripatellar pain that was insidious in nature and self-rated at least at a “3” (moderate) on a visual analog scale of “0” to “10” during running. These symptoms were required to be present during running and at least one of the activities of jumping, squatting, kneeling, prolonged sitting, or stair descent. All subjects with patellofemoral instability or other knee diagnoses, history of any lower extremity surgery, or who were otherwise unhealthy were excluded.
All qualified subjects were invited for the kinematic screening session. In the presence of bilateral knee pain, the knee with the highest self-rated pain was analyzed. When pain was equal bilaterally, the most dominant limb was analyzed, defined as the limb used to kick a soccer ball. To analyze overall function, subjects completed the Lower Extremity Functional Scale (LEFS). The LEFS is a 20-question clinical measure. Subjects ranked the amount a lower extremity injury affects various tasks and activities on a scale of 0–4 with a “0” signifying “extreme difficulty” and a “4” signifying “no difficulty” with a score of 80/80 corresponding to no limitations. The LEFS has previously been validated in PFP populations and a minimal clinically important difference of 9 points has been established (Brinkley et al. 1999).
Thirty retro-reflective markers were attached to the involved lower extremity to analyze running, SLS and step descent mechanics. To control for the effect of footwear on mechanics, subjects wore standardized neutral running shoes (Nike Pegasus, Beaverton, OR). The use of standardized running shoes was particularly important as qualified runners were enrolled in this study for 3 months. During this time period, the shoes utilized by the subjects during their everyday running likely experienced considerable wear or were even replaced. Thus, our use of standardized running shoes controlled for this likelihood and allowed us to control for this potential influence on running mechanics. Placement of anatomical markers was recorded with a marker placement device (MPD). This device was used to improve marker placement reliability when gait data are being measured over time. Intraclass correlation coefficient values of at least 0.9 were reported for all hip and knee variables when using the MPD (Noehren et al. 2010). The marker placement measurements were used for all subsequent data collections for each subject.
Three-dimensional marker coordinates were captured with an 8-camera, MX Vicon motion analysis system (VICON, Oxford, UK). A standing calibration trial was collected while the subject stood on a force plate (Bertec, Worthington, OH) mounted in the center of the capture volume. To assure standardized lower extremity position for standing calibration trials at follow-up data sessions, each subject’s foot position was recorded with a foot tracing for the baseline standing calibration trial. Next, a spherical hip trial was collected to calculate the functional hip joint center (Hicks and Richards, 2005).
All kinematic and kinetic data were sampled at 200 Hz and 1000 Hz, respectively. After completing approximately 10 warm-up trials, running data were collected as subjects traversed a 25-meter runway at 3.35 m/sec (8 min/mile). Next, single leg squat data were collected as subjects performed a squat to approximately 60 degrees knee flexion. Movement data were then collected as subjects descended an 8-inch instrumented step. Both single leg squat and step descent speed was standardized to a 1 Hz count. Approximately 8 trials of both the SLS and step descent were collected to obtain 5 trials with the correct speed of movement. Finally, we collected baseline video during treadmill running for future education purposes.
Kinematic and kinetic data from 5 trials were filtered with an 8-Hz and 50-Hz, low-pass, fourth order, zero-lab Butterworth filter, respectively. Internal joint moments were calculated utilizing segment inertial properties (Dempster et al. 1959) and normalized to body mass and height. Single leg squat mechanics were analyzed at 45 degrees knee flexion as this index represents typical peak knee flexion seen during running in our lab. Step descent mechanics were indexed at the point of peak knee extensor moment, which has been reported to correspond to peak quadriceps muscle force (Andriacchi et al. 1984). Customized software (LabVIEW 8.0, National Instruments, Austin, TX) was used to extract the discrete variables of interest from five individual curves for the motion files. Means and standard deviations of these values were calculated.
Those subjects who demonstrated abnormal hip alignment during running were invited to participate in the gait retraining phase. Abnormal hip alignment during running was operationally defined as peak HADD greater than 1 standard deviation above the mean of our lab’s normative database of running (peak HADD qualifying criterion= 20°). For qualified subjects, data collected during the kinematic screening served as their baseline data for the gait retraining protocol. Runners who did not meet this kinematic inclusion criterion were dismissed from further participation in the study.
Participants who met the kinematic qualification criteria attended a total of eight gait retraining sessions over the course of two weeks. During the first training session, subjects were first shown their baseline video so they could visualize the abnormal hip and knee alignment that they exhibited during running. During gait retraining, visual feedback was provided by a full length mirror that was placed directly in front of the treadmill. Participants received scripted verbal cueing at the beginning of each session, consisting of “run with your knees apart with your kneecaps pointing straight ahead” and “squeeze your buttocks.” Subjects received additional verbal feedback during each training session if they were not maintaining the desired gait modifications. During all training sessions, each subject’s response to the cueing was analyzed subjectively using a standard video camera and compared with their baseline video. Pilot work suggested that subjects may attempt to run with a widened stance or an increased toe out in a maladapted attempt to reduce HADD and HIR, respectively. If either of these maladaptations was noted on the video feed, subjects were immediately cued to correct them.
Feedback exposure and treadmill runtime were tightly controlled. Runners attended 8 retraining visits over the course of the 2 weeks, during which treadmill runtime was gradually increased from 15 minutes to 30 minutes (Figure 1). This schedule is based on previous studies (Noehren et al. 2010, Barrios et al. 2010, Crowell and Davis, 2010). Feedback was gradually removed during the final 4 training sessions, in accordance with the feedback schedule, to shift dependence from external to internal cues and reinforce learning (Winstein and Schmidt, 1990). This was accomplished by decremental reductions in verbal cueing in addition to reductions in visual feedback by turning the mirror around so that subjects could not see themselves while running. During each period of feedback removal, running mechanics were monitored via a standard video stream that was only visible to the investigator. Once feedback was resumed, runners received retrospective verbal cueing on their running mechanics during the preceding feedback removal period. To ensure that feedback was consistent across subjects, runners were not permitted to run outside of the lab while participating in the gait retraining phase. Subjects were asked to verbally attest to the restrictions on running activity. Subjects were required to complete all 8 sessions.
Figure 1.
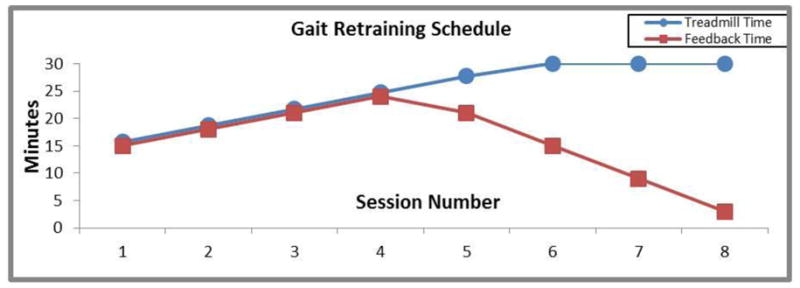
The gait retraining schedule: Over the first four visits, runtime and feedback time is increased from 15 minutes to 24 minutes. Over the last four visits, runtime is increased to 30 minutes while feedback is faded to 3 minutes by the eighth visit.
An instrumented gait analysis was repeated at the conclusion of the 2-week gait retraining program (POST). During this session, anatomical markers were placed according to the measurements obtained at the baseline visit using the marker placement device. Running, single leg squat and step descent data were collected in the same manner as during the baseline visit. Pain rating and LEFS data were recorded. After the post- retraining data collection, subjects were asked to return to their normal running routine. Further follow-up instrumented motion analyses, pain ratings, and LEFS were collected at 1MO and 3MO post-retraining. The variables of interest during running, squatting, and step descent were contralateral pelvic drop referenced to the lab (CPD), HADD, thigh adduction referenced to the lab (thigh ADD), hip abduction moment (HABDM), and hip internal rotation (HIR). Peak values were utilized for the analysis of running and the appropriately indexed values were utilized for analysis of the SLS and step descent.
Repeated measure, one way ANOVA’s were used for statistical analysis of running, SLS, and step descent. Sphericity of the data was assessed with Mauchly’s test with α<0.05. In the case of a positive Mauchly’s test, a Huhnh-Feldt correction was conducted during the analysis to generate accurate α scores (Field, 2008). Post hoc 2-tailed comparisons were conducted using a criterion α< 0.05, while a trend was defined as between α≤0.10 and α>0.05. Post hoc comparisons were conducted from PRE to POST. Comparisons were then made between POST and 1MO and again between POST and 3MO. Effect sizes were also calculated. A large effect size was defined as ≥ 0.80, moderate ≥ 0.40, and small <40 (Cohen, 1992).
Results
A total of 10 subjects completed the study (Table 1). An additional 3 subjects were lost to drop out. Two dropped out as they were unable to comply with the restriction of no running outside of the training sessions. The third subject dropped out due to health issues unrelated to the study. None of the 3 dropouts completed the 8-session training phase. Thus, an intention to treat analysis was not conducted.
Table 1.
Subjects demographics. Mean (SD).
| Group mean (sd) | |
|---|---|
| Age (years) | 22.4(5.0) |
| Running volume (km/week) | 23.7(11.3) |
| Body Mass Index (kg/m^2) | 22.0 (2.5) |
| Years running | 5.8 (2.9) |
| Years with knee pain | 4.3 (2.5) |
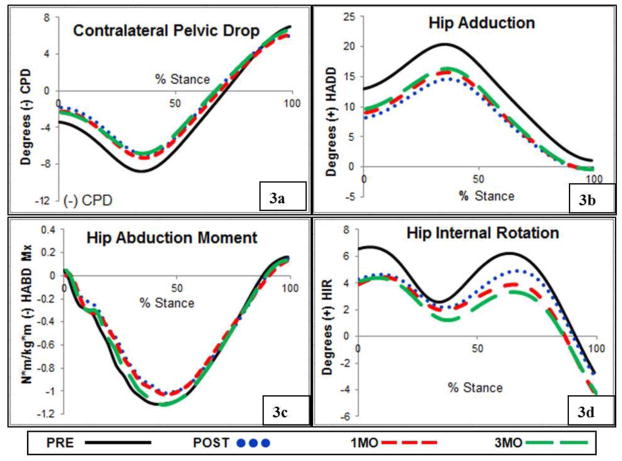
Subjects demonstrated a visible reduction in their peak HADD and CPD during running at POST (Figure 2). In addition, the ANOVA was significant for the variables of peak CPD, HADD, thigh ADD, and HABDM, leading to the examination of pair-wise comparisons (Table 2). At POST, subjects significantly reduced peak CPD (Figure 3a) and maintained these changes for peak CPD through 1MO and 3MO. At POST, subjects successfully reduced peak HADD by a mean of 5.9° (sd=1.5), (Figure 3b). However, at 1MO peak HADD increased by 1.1°, sd=1.2, and again by 0.6,° sd=2.1 at 3MO, moving closer to PRE levels. While significant, these changes were small and were associated with relatively small effect sizes (d=0.37 and d=0.23 at 1MO and 3MO, respectively). Peak thigh ADD was reduced at POST (d=1.32), suggesting that changes in both the thigh and the pelvis segment contributed to the overall reduction in peak HADD. Reductions in peak thigh ADD were maintained at 1MO and 3MO. At POST, peak HABDM was successfully reduced (Figure 3a). This reduction in peak HABDM was maintained through 1MO. However, peak HABDM increased significantly towards baseline levels at 3MO. The ANOVA was nonsignificant for peak HIR baseline values (Figure 3b).
Figure 2.
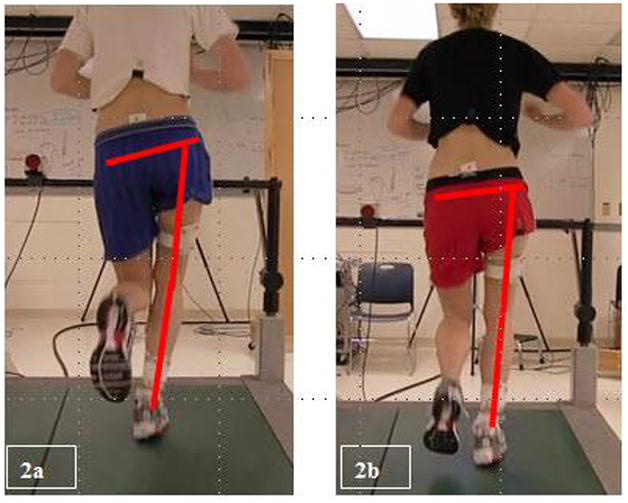
Representative subject during treadmill running a) PRE and b) POST. Note the reduction of contralateral pelvic drop and hip adduction at POST. This subject demonstrated a 3.8°, 8.1°, 4.5° reduction in contralateral pelvic drop, hip adduction, and thigh adduction respectively.
Table 2.
Group mean (SD) variables during for the variables of interest at the 4 time points.
| Variable | PRE | P | d | POST | P | d | 1MO | P | d | 3MO |
|---|---|---|---|---|---|---|---|---|---|---|
| Run | ||||||||||
| CPD | −9.0° (2.5) | 0.02* | 0.82 | −7.1° (2.2) | 0.171 | 0.19 | −7.5° (2.3) | 0.782 | 0.05 | −7.0° (2.2) |
| HADD | 20.7° (1.0) | 0.000* | 2.91 | 14.8° (3.1) | 0.02* | 0.37 | 15.9° (2.7) | 0.05* | 0.60 | 16.4° (2.5) |
| Thigh ADD | 9.8° (1.2) | 0.17* | 1.32 | 7.2° (2.7) | 0.92 | 0.04 | 7.3° (1.8) | 0.29 | 0.47 | 8.1° (1.4) |
| HABDM (N* m/kg* m) | −1.180 (0.185) | 0.042* | 0.69 | −1.054 (0.184) | 0.605 | 0.11 | −1.074 (0.173) | 0.05* | 0.61 | −1.153 (0.145) |
| HIR | 8.6° (5.4) | n/s | 0.21 | 7.1° (8.7) | n/s | 0.11 | 6.2° (7.9) | n/s | 0.19 | 5.7° (6.3) |
| Squat | ||||||||||
| CPD | 0.6° (2.0) | n/s | 0.11 | 2.3° (2.5) | n/s | 0.10 | 2.6° (2.6) | n/s | 0.04 | 2.2° (2.4) |
| HADD | 11.6° (3.4) | 0.002* | 1.35 | 7.6° (2.6) | 0.83 | 0.06 | 7.7° (2.6) | 0.10 | 0.69 | 9.2° (2.1) |
| Thigh ADD | 11.5° (2.0) | 0.04* | 0.68 | 10.1° (2.2) | 0.48 | 0.15 | 9.8° (2.2) | 0.92 | 0.03 | 10.1° (1.9) |
| HABDM (N* m/kg* m) | −0.470 (0.064) | 0.004* | 0.91 | −0.412 (0.070) | 0.06 | 0.28 | −0.431 (0.071) | 0.02* | 0.18 | −0.477 (0.039) |
| HIR | 3.0° (6.4) | n/s | 0.38 | 5.9° (8.5) | n/s | 0.24 | 3.9° (7.6) | n/s | 0.25 | 4.0° (6.4) |
| Step | ||||||||||
| CPD | −5.0° (3.5) | 0.72 | 0.07 | −5.2° (2.7) | 0.05* | 1.09 | −4.0° (3.0) | 0.001* | 1.28 | −1.8° (2.6) |
| HADD | 15.1° (6.8) | 0.03* | 0.69 | 11.6° (3.2) | 0.51 | 0.07 | 11.1° (4.2) | 0.88 | 0.04 | 11.8° (4.2) |
| Thigh ADD | 7.8° (2.7) | 0.10 | 0.42 | 6.8° (2.2) | 0.13 | 0.12 | 6.2° (2.2) | 0.55 | 0.16 | 6.4° (2.0) |
| HABDM (N* m/kg* m) | −0.556 (0.122) | n/s | 0.34 | −0.520 (0.085) | n/s | 0.10 | −0.498 (0.0813) | n/s | 0.18 | −0.506 (0.083) |
| HIR | 7.0° (5.7) | n/s | 0.03 | 7.3° (8.9) | n/s | 0.16 | 6.0° (8.8) | n/s | 0.10 | 8.0° (5.1) |
Variables for running were indexed to their peak values and were indexed to the discreet time points for the squat and step descent. Pairwise comparisons are indicated for PRE-POST, POST-1MO, and POST-3MO with respective effect sizes (d).
indicates P<0.05, n/s indicates repeated measures ANOVA was non-significant.
PRE= baseline measures, POST= post-gait retraining measures, 1MO= measures at 1 month post-gait retraining, 3MO= measures at 3 months post-gait retraining, CPD=contralateral pelvic drop, HADD= hip adduction, Thigh ADD= Thigh adduction, HABDM=Internal hip abduction moment, HIR= hip internal rotation.
Figure 3.
A) Subjects reduced CPD from PRE to POST and maintained these changes through 3MO. B) Subjects reduced HADD from PRE to POST with a small amount of drifting towards baseline at 1MO and 3MO. C) Subjects reduced HABDM from PRE to POST and maintained these levels at 1MO. However, values returned to baseline levels at 3MO. D) No reductions were noted for HIR.
The improved hip mechanics noted during running transferred to the untrained task of SLS, with the exception of CPD (Table 2). The ANOVA was significant for HADD (P=0.000), thigh ADD (P=0.01) and HABDM (P=0.008), but not for CPD (P= 0.08). Analysis of pairwise comparisons revealed that HADD and thigh ADD were significantly reduced at POST with reductions maintained at 1MO, However, a trend of drifting (by 1.1 deg) of HADD towards baseline levels (P=0.10, d=0.69) was noted at 3MO. Interesting, thigh adduction also drifted towards baseline levels at 3MO during the SLS (d=0.92). Similarly, HABDM was reduced at POST (d=0.91) and with a trend towards baseline levels at POST (P=0.058). However, HABDM drifted towards baseline at 3MO (P=0.017) and this drift was associated with a large effect size (d=1.19). As with running, no changes were noted for HIR during the SLS.
Similar to SLS, the improved hip mechanics during running transferred to the untrained task of step descent. During step descent, the ANOVA was significant for CPD (P=0.001), HADD (P=0.009) and thigh ADD (P=0.01), but not HIR (P=0.81) or HABDM (P=0.14). Examination of pairwise comparisons revealed that CPD was not reduced at POST, but interestingly was reduced from POST values at 1MO (d=1.09) and reduced even more at 3MO (d=2.98). HADD was reduced at POST, and maintained at 1MO and 3MO. There was a trend (P=0.10) of reduction in thigh ADD at POST with no significant changes between POST-1MO and POST-3MO, indicating retention of the kinematic change.
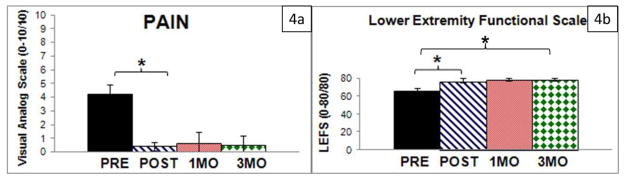
Both pain and LEFS scores were improved from PRE to POST (Figure 4a,b). These changes were associated with large effect sizes (d=7.61 and d= 3.81, respectively) and the score change of 9 points required to be a minimal clinically important difference (Brinkley et al., 1999). Subjects maintained these improvements through 1MO and 3MO. The 1.8 point (sd=2.2) increase in LEFS score between POST and 3MO was significant, yet did not exceed the threshold for a minimal clinical important difference.
Figure 4.
A) Subjects reported a significant reduction in visual analog score of pain from PRE to POST and maintained this reduction through 3MO. B) Lower extremity functional scale scores increased PRE to POST and maintained this reduction from POST to 1MO. A significant increase in this score resulted from POST to 3MO, albeit less than the minimally clinically important difference for this measure.
Discussion
Our primary goal was to determine if mirror gait retraining would reduce abnormal running mechanics in females with PFP and if these changes would persist through 3MO. We also investigated if the new movement skill during running would transfer and persist in the untrained tasks of single leg squat and step descent. Finally, we sought to determine if these changes in mechanics would be reflected in improvements in pain and function. Based on these data, it appears that mirror gait retraining is an effective treatment to reduce abnormal mechanics during running. These changes generally persisted through at least 3MO. Interestingly, this new movement skill transferred to the untrained tasks of single leg squat and step descent. As with running, alterations in mechanics during these untrained tasks were either maintained or continued to improve through 3MO. Reflecting these changes in dynamic alignment, measures of pain and function improved and remained at these levels through 3MO.
During running, the reductions in peak HADD were greater than any other variable tested. Interestingly, post hoc examination of subject characteristics (age, baseline running volume, baseline pain levels, and years of running experience) failed to yield any discernible factor that predicted a runner’s ability to decrease HADD after the retraining intervention. HADD was likely the easiest variable for subjects to visualize by focusing on the space between their knees. As the gluteus medius musculature is the primary stabilizer of the hip in the frontal plane, runners may have altered the neuromuscular control of this muscle to achieve reductions in HADD and CPD. Indeed, increased latency and decreased duration of activation of the gluteal musculature has been implicated in PFPS in females during running and stair negotiation (Willson et al. 2012, Cowan et al. 2009, Brindle et al. 2003). However, electromyography is necessary to investigate changes in neuromuscular control of the gluteal musculature. It is unknown if changes in gluteal strength accompanied changes in hip mechanics. However, the retraining period was brief (2 weeks) and thus, was likely insufficient to stimulate true strengthening. It is also possible that subjects may have shifted their trunk over the stance limb to decrease CPD, resulting in less HADD. Our marker set did not include the trunk and thus, we are unable to comment on the potential contribution of altered trunk mechanics. However, peak thigh adduction during running was also reduced by 2.6° indicating that alterations in CPD did not account for all of the reduction in peak HADD. The large reduction in HADD likely had a marked effect on the dynamic Q-angle (Powers 2003, 2010). Decreasing the dynamic Q-angle has been suggested to decrease lateral tracking of the patella, thus decreasing lateral joint stress of the patellofemoral joint (Huberti and Hayes 1984, Lee et al. 2003, Besier et al. 2008). The decrease in lateral patellofemoral joint stress would decrease forces on the subchondral bone, leading to a decrease in pain (Besier et al. 2008).
Unlike the frontal plane measures of the hip during all tasks, we did not find any changes in the transverse plane at POST. This was somewhat surprising as HADD and HIR are coupled motions and participants also received specific cueing to reduce HIR during retraining sessions. The reason for this discrepancy may be attributed to the fact that our kinematic inclusion criterion was solely excessive peak HADD during running and not excessive peak HIR. Indeed, most subjects did not exhibit excessive peak HIR during running at baseline. As with excessive peak HADD, we operationally defined “excessive” as peak HIR greater than 1 standard deviation above our lab’s normative database (peak HIR during running: present study= 8.6°, sd=5.4 versus our lab’s normative database= 5.0, sd=6.7). Therefore, our participants may have had a high capacity to change HADD but a limited capacity to make changes to HIR.
Following the overall significant improvement in hip mechanics during running at POST, the majority of these changes persisted through 3 months. There was significant, albeit slight, drifting of HADD in the direction of baseline levels by 8/10 runners. Suggesting the importance of sufficient continued practice, the two runners with the greatest drifting of peak HADD during running from PRE to 3MO had the lowest reported running volume. However, the drift from POST to 1MO and to 3MO was only 1.1° and 1.6°, respectively. This drifting pattern may have been in response to an initial overcorrection by the subjects. Subjects, in fact, exhibited a very low value of 14.8°±3.1 for HADD following the retraining. This value was well below the peak HADD of our reference, normative database of runners (16.5°±3.5). At 3MO, peak HADD (16.4°±2.4) was nearly identical to that of our database. This suggests that the mechanics may have settled towards a more normal value at 3MO. Further evidence that a normalization of running occurred, peak CPD at 3MO was nearly identical to peak CPD of our normative database (−8.0°±2.8). Regardless, the general retention of changes in peak CPD, HADD, thigh ADD, and HABDM at 1MO and 3MO suggest that subjects learned a new motor skill (Sherwood and Lee, 2003, Salmoni et al. 1984).
Further evidence of skill acquisition was noted by the transfer of the new movement pattern learned during running to the untrained tasks of SLS and step descent (Salmoni et al. 1984, Schmidt, 1972, and Sherwood and Lee, 2003). HADD and thigh adduction (a trend) were reduced during SLS and step descent immediately following the retraining. Interestingly, CPD was not reduced at POST during step descent. Nevertheless, it was reduced at 1MO and then even further at 3MO. We speculate that subjects may have later reduced CPD at 1MO and 3MO due to continued practice of this new skill in the community setting. In contrast to the drifting of thigh ADD and the trend towards drifting noted in HADD during the SLS, these measures remained consistent during step descent at 3MO. While SLS is often used as a functional test, it is a movement that is rarely utilized during activities of daily living. Therefore, the SLS may not have been as well-reinforced during normal activities as the step descent. Future studies should examine changes in neuromuscular control of the gluteal musculature utilizing surface electromyography.
The focus of the retraining was to alter excessive HADD during running. However, the overarching goal of this intervention is to reduce pain and improve function in runners with PFP. The reductions in pain visual analog scores noted in this study were of greater magnitude (−90.5%, sd=7.8 greater than that reported in studies of other interventions of PFP (−43.1–87.5%) (Nakagawa et al. 2009, Crossley et al. 2002, Ferber et al. 2011, Earl et al. 2011, Boling et al. 2006). Furthermore, the duration of this intervention (2 weeks) was considerably shorter than that of previous intervention studies (3–8 weeks) of PFP that were focused on hip and knee strengthening. (Nakagawa et al. 2009, Crossley et al. 2002, Ferber et al. 2011, Earl et al. 2011, Boling et al. 2006). The greater reduction in pain and shorter duration of treatment makes gait retraining an appealing intervention to minimize financial and time investment for clinical settings. The 12.1 ±2.7 point increase in LEFS scores at POST exceeded the 9-point minimally clinically important difference associated with this measure (Brinkley et al. 1999). The increase in this score indicates improved function and suggests that the new movement pattern translated to functional movements that were previously painful. It is possible that participants experienced these improvements in pain and function due to the decrease in running volume while enrolled in the 2-week gait retraining phase. However, all runners reported returning to their pre-enrollment running volume at 1MO and 3MO. In fact, 6/10 of the runners reported higher running volume at 3MO, attributed to a decrease in their knee pain. Importantly, participants maintained POST levels of pain and LEFS through 3MO, suggesting potential for benefits lasting beyond the time interval examined in this study.
Overall, the changes in running and squatting mechanics, as well as pain and function in the current study compare favorably with real-time kinematic gait retraining (Noehren et al. 2010). The reductions seen in peak HADD and CPD during running are of the same magnitude, or greater, than Noehren et al. However, those authors also reported a trend in reduction in peak HIR, which was not seen in this study. The discrepancy is likely due to the fact that the subjects in Noehren’s study exhibited excessive HIR at baseline (Noehren: 11.0°±4.1 versus norm: 5.0°±7.1). Thus, the subjects in Noehren et al. had greater capacity for change for HIR than participants in the current study. Despite this difference, mirror retraining resulted in nearly identical improvements in pain and function compared with real-time kinematic feedback. Additionally, the reduction in HADD during the SLS was also similar to the changes in HADD during a SLS reported by Noehren et al. 2010 (Noehren et al. 2010 did not collect step data). Therefore, mirror gait retraining produces similar results compared with the more technical and costly method of real-time kinematic gait retraining, but with much greater clinical utility. Finally, the systematic changes in mechanics noted in both studies suggest that alterations in movement patterns were not random but were the result of the visual and scripted feedback.
Several limitations should be noted in this study. First, this study is preliminary in nature. Because it lacked a control group, we cannot suggest that this intervention is an improvement over hip and knee strengthening, the current standard of care for patients with PFP. However, it is unlikely that the biomechanical changes that were noted would have occurred in the absence of the retraining. A randomized clinical trial is warranted comparing gait retraining with an intervention focused on hip and knee strengthening. Second, this study only followed the subjects up to 3MO. Consequently, the long term effects of this retraining intervention are unknown. Finally, it is unknown if the new running pattern noted post-gait retraining increases the risk of sustaining other injuries. Future study should include at least a 1-year follow-up to determine the long term effects of gait retraining for the treatment of PFP.
Conclusion
Gait retraining in female runners with PFP, using a full-length mirror, resulted in significant improvements in pain, function, and abnormal mechanics from their baseline measures. The new movement skill transferred to the untrained tasks of single leg squat and step descent, thus indicating acquisition of a new motor skill. Reductions in pain, function, and mechanics were generally maintained through 3 months, suggesting potential for long term changes. The results of this study are promising, as this technique requires only a treadmill and a full-length mirror. Further study is necessary to determine the long term efficacy of this treatment technique.
Acknowledgments
This work was supported by a Promotional of Doctoral Studies scholarship from the Foundation for Physical Therapy (Doctoral studies support for co-author RW), Drayer Physical Therapy Institute (Doctoral studies support for co-author RW and subject reimbursement), and NIH 1 S10 RR022396 (Shared instrumentation grant).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andriacchi TP, Andersson GB, Ortengren R, Mikosz RP. A study of factors influencing muscle activity about the knee joint. J Orthop Res. 1984;12:266–75. doi: 10.1002/jor.1100010306. [DOI] [PubMed] [Google Scholar]
- Barrios J, Crossley K, Davis I. Gait retraining to reduce the knee adduction moment through real-time visual feedback of dynamic knee alignment. Journal of Biomech. 2010;91:2208–13. doi: 10.1016/j.jbiomech.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besier TF, Gold GE, Delp SL, Fredericson M, Beaupre GS. The influence of femoral internal and external rotation on cartilage stresses within the patellofemoral joint. J Orthop Res. 2008;26:1627–35. doi: 10.1002/jor.20663. [DOI] [PubMed] [Google Scholar]
- Boling M, Bolgla L, Mattacola C, Uhl T, Hose R. Outcomes of weightbearing rehabilitation program for patients diagnosed with patellofemoral pain syndrome. Arch Of Phys Med And Rehab. 2006;87:1428–35. doi: 10.1016/j.apmr.2006.07.264. [DOI] [PubMed] [Google Scholar]
- Brindle TJ, Mattacola C, McCrory J. Electromyographic changes in the gluteus medius during stair ascent and descent in subjects with anterior knee pain. Knee Surg Sports Traumatol Arthrosc. 2003;11:244–251. doi: 10.1007/s00167-003-0353-z. [DOI] [PubMed] [Google Scholar]
- Brinkley J, Stratford P, Lott S, Riddle D. The lower extremity functional scale (LEFS): scale development, measurement properties and clinical application. Phys Ther. 1999;79:371–83. [PubMed] [Google Scholar]
- Cohen J. A power primer. Psych Bull. 1992;112:155–159. doi: 10.1037//0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Cowan SM, Crossley KM, Bennell KL. Altered hip and trunk muscle function in individuals with patellofemoral pain. Br J Sports Med. 2009;43:584–588. doi: 10.1136/bjsm.2008.053553. [DOI] [PubMed] [Google Scholar]
- Crossley K, Bennell K, Breen S, Cowan S, McConnell J. Physical therapy for patellofemoral pain: A randomized, double-blinded, placebo-controlled trial. Am J of Sp Med. 2002;30:857–67. doi: 10.1177/03635465020300061701. [DOI] [PubMed] [Google Scholar]
- Crowell HP, Davis IS. Gait retraining to reduce lower extremity loading in runners. Clin Biomech. 2011;26:78–83. doi: 10.1016/j.clinbiomech.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempster WT, Gabel WC, Felts WJ. The anthropometry of the manual work space for the seated subject. Am J Phys Anthropol. 1959;17:289–317. doi: 10.1002/ajpa.1330170405. [DOI] [PubMed] [Google Scholar]
- Dierks TA, Manal KT, Hamill J, Davis IS. Proximal and distal influences on hip and knee kinematics in runners with patellofemoral pain during and prolonged run. J Orthop Sports Phys Ther. 2008;38:448–456. doi: 10.2519/jospt.2008.2490. [DOI] [PubMed] [Google Scholar]
- Dolak KL, Silkman C, Medina McKeon J, Hosey RG, Latterman C, Uhl TL. Hip strengthening prior to functional exercises reduces pain sooner than quadriceps strengthening in females with patellofemoral pain syndrome: a randomized clinical trial. J Orthop Sports Phys Ther. 2011;41:560–570. doi: 10.2519/jospt.2011.3499. [DOI] [PubMed] [Google Scholar]
- Earl J, Hoch A. A proximal strengthening program improves pain, function, and biomechanics in women with patellofemoral pain syndrome. Am J Sports Med. 2010;59:110–2. doi: 10.1177/0363546510379967. [DOI] [PubMed] [Google Scholar]
- Field A. Discovering Statistics using SPSS. 2. Sage Publishing, London, Eng; 2005. p. 451. [Google Scholar]
- Ferber R, Kendall KD, Farr L. Changes in knee biomechanics after a hip-abductor strengthening protocol for runners with patellofemoral pain syndrome. J Athl Train. 2011;46:142–9. doi: 10.4085/1062-6050-46.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks J, Richards J. Clinical applicability of using spherical fitting to find hip joint centers. Gait & Posture. 2005;22:138–45. doi: 10.1016/j.gaitpost.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Huberti HH, Hayes WC. Patellofemoral Contact Pressures- The influence of Q-angle and tendofemoralcontact. J of Bone and Joint Surg Am. 1984;66A:715–24. [PubMed] [Google Scholar]
- Lee TQ, Yang BY, Sandusky MD, McMahon PJ. The effects of tibial rotation on the patellofemoral joint: assessment of the changes in in situ strain in the peripatellar retinaculum and the patellofemoral contact pressures and areas. J Rehabil Res Dev. 2001;38:463–69. [PubMed] [Google Scholar]
- Mascal CL, Landel R, Powers C. Management of patellofemoral pain targeting hip, pelvis, and trunk muscle function: 2 case reports. J Orthop Phys Ther. 2003;33:647–60. doi: 10.2519/jospt.2003.33.11.647. [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Muniz T, Baldon R, Maciel C, Reiff B, Serrao V. The effect of additional strengthening of hip abductor and lateral rotator muscles in patellofemoral pain syndrome: a randomized controlled pilot study. Clin Rehabil. 2008;22:1051–60. doi: 10.1177/0269215508095357. [DOI] [PubMed] [Google Scholar]
- Noehren B, Manal K, Davis I. Improving day to day reliability using a marker placement device. J of Biomech. 2010;28:1405–10. doi: 10.1002/jor.21172. [DOI] [PubMed] [Google Scholar]
- Noehren B, Pohl MD, Sanchez Z, Cunningham T, Latterman C. Proximal and distal kinematics in female runners with patellofemoral pain. Clin Biomech. 2012;27:366–371. doi: 10.1016/j.clinbiomech.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noehren B, Scholz J, Davis I. The effect of real-time gait retraining on hip kinematics, pain and function in subjects with patellofemoral pain syndrome. Br J Sports Med. 2010;34:691–696. doi: 10.1136/bjsm.2009.069112. [DOI] [PubMed] [Google Scholar]
- National Sporting Goods Association Web site [internet] Mount Prospect, (Ill) [cited 2009 Nov. 30 11/30/09]. Available from: http://www.nsga.org/files/public/2008RankedByTotal_4Web_080423.pdf.
- Powers C. The influence of altered lower extremity kinematics on patellofemoral joint dysfunction: a theoretical perspective. J Orthop Sports Phys Ther. 2003;33:639–46. doi: 10.2519/jospt.2003.33.11.639. [DOI] [PubMed] [Google Scholar]
- Powers C. The influence of abnormal hip mechanics on knee injury: a biomechanical perspective. J Orthop Sports Phys Ther. 2010;40:42–1. doi: 10.2519/jospt.2010.3337. [DOI] [PubMed] [Google Scholar]
- Salmoni AW, Schmidt RA, Walter CB. Knowledge of results and motor learning: a review and critical reappraisal. Psychological Bulletin. 1984;95:355–86. [PubMed] [Google Scholar]
- Schmidt RA. The case against learning and forgetting scores. Journal of Motor Behavior. 1972;4:79–8. doi: 10.1080/00222895.1972.10734922. [DOI] [PubMed] [Google Scholar]
- Selkowitz DM, Souza RB, Powers CM. Effect of femoral strapping on lower extremity kinematics and pain response in females with patellofemoral pain. Proceedings of the International Retreat: Patellofemoral pain syndrome: proximal, distal, and local factors. April 30 – May 2, 2009. Baltimore, MD. J Orthop Sports Phys Ther. 2009;3:A39–40. doi: 10.2519/jospt.2010.0302. [DOI] [PubMed] [Google Scholar]
- Sherwood D, Lee T. Schema theory: critical review and implications for the role of cognition in a new theory of motor learning. Res Quart Exer Sport. 2003;74:376–391. doi: 10.1080/02701367.2003.10609107. [DOI] [PubMed] [Google Scholar]
- Souza RB, Powers CM. Differences in hip kinematics, muscle strength, and muscle activation between subjects with and without patellofemoral pain. J Orthop Sp Phys Ther. 2009;39:12–9. doi: 10.2519/jospt.2009.2885. [DOI] [PubMed] [Google Scholar]
- Souza RB, Powers CM. Predictors of hip internal rotation during running: an evaluation of hip strength and femoral structure in women with and without patellofemoral pain. Am J Sport Med. 2009;37:579–587. doi: 10.1177/0363546508326711. [DOI] [PubMed] [Google Scholar]
- Stathopulu E, Baildam E. Anterior knee pain: a long term follow-up. Rheumatology. 2003;42:380–2. doi: 10.1093/rheumatology/keg093. [DOI] [PubMed] [Google Scholar]
- Taunton J, Ryan M, Clement D, McKenzie C, Lloyd-Smith R, Zumbo B. A retrospective case control analysis of 2002 running injuries. Br J Sports Med. 2002;36:95–101. doi: 10.1136/bjsm.36.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gent B, Siem GD, Middelkoop M, van Os T, Bierma-Zeinstra S, Koes B. Incidence and determinants of lower extremity running injuries in long distance runners: A systematic review. Br J Sports Med. 2007;41:469–80. doi: 10.1136/bjsm.2006.033548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willson JD, Davis IS. Lower extremity mechanics of females with and without patellofemoral pain across activities with progressively greater task demands. Clin Biomech. 2008;23:203–11. doi: 10.1016/j.clinbiomech.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Willson J, Kernozek T, Arndt Reznichek D, Straker S. Gluteal muscle activation during running in females with and without patellofemoral pain syndrome. Clin Biomech. 2011;26:735–740. doi: 10.1016/j.clinbiomech.2011.02.012. [DOI] [PubMed] [Google Scholar]
- Willy RW, Davis IS. The Effect of a Hip Strengthening Program on Mechanics during Running and during a Single Leg Squatting. J Orthop Phys Ther. 2011;41:625–32. doi: 10.2519/jospt.2011.3470. [DOI] [PubMed] [Google Scholar]
- Winstein C, Schmidt R. Reduced frequency of knowledge of results enhances motor skill learning. J Exp Psyc. 1990;16:677–91. [Google Scholar]