Abstract
Acetaminophen (APAP) overdose is currently the most frequent cause of drug-induced liver failure in the United States. Recently, it was shown that lysosomal iron translocates to mitochondria where it contributes to collapse of the mitochondrial membrane potential. Therefore, the purpose of this study was to investigate if cathepsin B, a lysosomal protease, is involved in APAP-induced hepatotoxicity. Cathepsin B activity was measured in subcellular liver fractions of C57Bl/6 mice 3 hr after 300 mg/kg APAP treatment. There was a significant increase in cytoplasmic cathepsin activity, concurrent with a decrease in microsomal activity, indicative of lysosomal cathepsin B release. To investigate the effect of cathepsin B on hepatotoxicity, the cathepsin inhibitor AC-LVK-CHO was given 1 hr prior to 300 mg/kg APAP treatment along with vehicle control. There was no difference between groups in serum ALT values, or by histological evaluation of necrosis, although cathepsin B activity was inhibited by 70–80% compared to controls. These findings were confirmed with a different inhibitor (z-FA-fmk) in vivo and in vitro. Hepatocytes were exposed to 5 mM acetaminophen. Lysotracker staining confirmed lysosomal instability, and cathepsin B release, but there was no reduction in cell death after treatment with cathepsin B inhibitors. Finally, cathepsin B release was measured in clinical samples from patients with APAP-induced liver injury. Low levels of cathepsin B were released into plasma from overdose patients.
Conclusion
APAP overdose causes lysosomal instability and release of cathepsin B into the cytosol but does not contribute to liver injury under these conditions.
Acetaminophen (APAP) is a safe drug when used as directed. However, an overdose can cause severe liver injury and even liver failure in rodents [1] and in human beings [2]. The toxicity of APAP strictly depends on the metabolic activation of APAP to a reactive metabolite, N-acetyl-p-benzoquinone imine (NAPQI), which depletes glutathione and reacts with protein sulfhydryl groups leading to protein adducts [3,4]. Experiments with the non-hepatotoxic regioisomer of APAP, 3′-hydroxyacetanilide, indicated that the toxicity does not correlate with the overall protein binding in the cell but with the capacity to bind to mitochondrial proteins [5,6]. This leads to inhibition of the mitochondrial respiration [7], a selective mitochondrial oxidant stress [8] and peroxynitrite formation [9], loss of mitochondrial DNA [9] and eventually formation of membrane permeability transition (MPT) pores, which trigger the collapse of the membrane potential, cessation of ATP synthesis and oncotic necrotic cell [10–13]. APAP induces similar mitochondrial dysfunction and necrotic cell death in human HepaRG cells [14] and in overdose patients [15].
In addition to the mitochondria, lysosomal components have been shown to be involved in APAP hepatotoxicity. Lysosomal iron can translocate to the mitochondria and promote the MPT during oxidant stress-induced liver cell injury [16] and APAP hepatotoxicity [17]. Lysosomes also contain a number of proteases, e.g. the cathepsin family. While cathepsins such as L and D, have been shown to be involved in numerous pathologies including multiple cancers (18–20) and neurological disorders (21), an important role for cathepsin B in liver injury was shown in various experimental models including TNF-mediated apoptosis [22–24], obstructive cholestasis [25], hepatic ischaemia-reperfusion injury [26] and lipotoxicity [27]. Both cathepsin B-deficient mice and pharmacological inhibitors were used in these investigations. Although the focus of these studies was on modulation of apoptotic cell death, certain models such as ischaemia-reperfusion injury [28] and obstructive cholestasis [29–31] involve predominantly necrotic cell death similar to APAP hepatotoxicity [32]. Furthermore, cathepsin B inhibition has been shown to attenuate mitochondrial cytochrome c release in other models [33], leading to mitochondrial and cellular protection. As cytochrome c release is a noted component of acetaminophen toxicity [34], this action may also lead to protection against APAP injury. Therefore, the objective of the current investigation was to evaluate if APAP can trigger lysosomal instability and release of cathepsin B and to assess the pathophysiological importance of cathepsin B in APAP-induced liver injury.
MATERIALS AND METHODS
Animals
Male C57Bl/6J mice (8–10 weeks old) were purchased from Jackson Laboratories (Bar Harbor, ME, USA). Animals received humane care according to the criteria outlined in the “Guide for the Care and Use of Laboratory Animals”. The experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Kansas Medical Center.
Patient selection and consent
Patients admitted to the medical intensive care unit at the University of Kansas Hospital were studied prospectively. The study was approved by the Institutional Review Board and all participants were required to sign a consent form. Diagnosis of APAP-induced liver injury was made by a physician at presentation. Evidence for APAP overdose included a patient-reported recent history of APAP ingestion in excess of 10 g/day, high serum APAP levels based on the Rumack-Matthews nomogram, and elevated serum transaminases. All patients fulfilled at least two of these criteria. Patients were excluded when other causes of liver injury (e.g. viral hepatitis, ischaemic injury, etc.) could not be ruled out. Blood samples were collected daily and alanine aminotransferase activity and prothrombin times (PT) were measured in the clinical laboratory. Plasma was obtained by centrifugation in our laboratory and stored at −80°C until analysis. Patients were divided into two groups: those with abnormal liver function test results (Abnormal LT; ALT ≥ 1,000 U/L and PT ≥ 20 sec.) and those with normal liver function test results (Normal LT; ALT < 100 U/L and PT < 20 sec.).
Experimental protocols
All animals were fasted overnight. Animals were either treated with 300 mg/kg APAP or 600 mg/kg APAP diluted in warm saline via i.p. injection (Sigma-Aldrich Chemical Co., St. Louis, MO, USA). Animals treated with the cathepsin inhibitor benzyloxycarbonyl-phe-ala-fluoromethylketone (z-FA-FMK) received a single i.p. dose of 10 mg/kg z-FA-FMK (BD Pharmaceuticals, Franklin Lakes, NJ, USA) dissolved in pure DMSO, then diluted with phosphate-buffered saline. Vehicle control was the same dose and ratio of DMSO:saline. The animals receiving AC-LVK-CHO (EMD Chemicals, Gibbstown, NJ, USA) were given a single i.p. dose of 5 mg/kg AC-LVK-CHO dissolved in sterile water. Cathepsin B inhibitor and vehicle were injected 1 hr prior to 300 mg/kg or 2 hr after 600 mg/kg APAP. Groups of animals were killed by cervical dislocation under isoflurane anaesthesia 30 min., 3 hr or 12 hr after APAP injection. In addition to the APAP experiments, some animals were treated with 700 mg/kg galactosamine/100 μg/kg endotoxin (Salmonella abortus equi) 1 hr after either the DMSO/saline vehicle or 10 mg/kg z-FA-FMK. Blood was drawn from the vena cava into heparinized syringes and centrifuged for acquisition of plasma. The plasma was used for determination of alanine aminotransferase (ALT) activities. Immediately after collecting the blood, the livers were excised and rinsed in saline. A small section from each liver was placed in 10% phosphate-buffered formalin to be used for H&E staining and immunohistochemical analysis. The remaining liver was frozen in liquid nitrogen and stored at −80°C or immediately homogenized for subcellular fractionation.
Methods
Plasma ALT activities were determined with the Point Scientific ALT test (Point Scientific, Inc., Canton, MI, USA) and expressed as IU/litre. Total soluble GSH and GSSG were measured in the liver homogenate with a modified method of Tietze as described in detail [35].
Measurement of lysosomal integrity in primary cultured hepatocytes
Primary hepatocytes were isolated from mice as described in detail [36]. For live cell imaging, hepatocytes were allowed to adhere on glass bottom dishes for 3 hr after isolation, after which cells were treated with 5 mM APAP for 6 hr. After treatment, media was removed and cells were loaded with Lysotracker Red® (Invitrogen) (50 nM) in HBSS for 30 min. Cells were then washed and imaged on a Zeiss Axiovert 200 inverted fluorescence microscope using a Texas Red filter (Carl Zeiss GmbH, Goettingen, Germany). For experiments with cathepsin B inhibitors, cells were allowed to attach for 3 hr, cultures were washed with phosphate-buffered saline (PBS) and then plain culture medium (controls) or media containing 5 mM APAP were added. The cathepsin B inhibitors CA-074Me (Sigma) and Z-FA-fmk (BD) were dissolved in DMSO and diluted with culture medium to a final concentration of 50 μM. Cell were either pretreated for 30 min. before APAP or treated 2 hr after APAP addition. Cell viability was determined by lactate dehydrogenase (LDH) release as described [36] at 9 hr after APAP exposure.
Cathepsin B Activity
Cathepsin B activity was measured by Innozyme Fluorometric Cathepsin B Activity Kit (EMD Chemicals, Gibbstown, NJ) according to the manufacturer’s instructions. Protein was quantified before the start of the experiment and 50 μg of total protein was added into each well. Data are expressed as relative fluorescent units (RFU)/mg protein/min.
Histology and immunohistochemistry
Formalin-fixed tissue samples were embedded in paraffin and 5μm sections were cut. Replicate sections were stained with hematoxylin and eosin (H&E) for evaluation of necrosis[32].For the terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay, sections of liver were stained with the In Situ Cell Death Detection Kit, AP (Roche Diagnostics, Indianapolis, IN, USA) as described in the manufacturer’s instructions [32].
Isolation of subcellular fractions
Mitochondria and cytosolic fractions were isolated as described [9]. Briefly, the liver was homogenized in ice-cold isolation buffer (pH7.4) containing 220 mM mannitol, 70 mM sucrose, 2.5 mM HEPES, 10 mM EDTA, 1 mM EGTA and 0.1% bovine serum albumin. The nuclear fraction was excluded by 5-min. centrifugation at 1000 × g, followed by removal of the supernatant. Mitochondria were isolated by differential centrifugation (10,000×g) and washed with 2 ml of isolation buffer. The supernatant of the 10,000×g spin was centrifuged at 100,000 × g; the supernatant represented the cytosolic and the pellet the microsomal fraction, which contained the lysosomes. Protein was measured by the BCA assay and normalized for all relevant assays.
Statistics
Data are expressed as means ± S.E. Comparison between two groups were performed with Student’s t-test or one-way ANOVA followed by Bonferroni t-test for multiple groups. P<0.05 was considered significant.
RESULTS
Treatment with APAP induces release of cathepsin B from lysosomes in vivo
In order to investigate if the lysosomal enzyme cathepsin B is being released early during APAP hepatotoxicity, cathepsin B was measured at 3 hr after an overdose of 300 mg/kg APAP (fig. 1). At that time point, there is only a minor increase of plasma ALT activities reflecting the very early stages of liver injury (fig. 1A). However, the microsomal fraction of the liver homogenate, which contains the lysosomes [37], showed an 80% reduction in cathepsin B activity. In contrast, cytosolic cathepsin B activity more than doubled (fig. 1B). These data strongly suggest that cathepsin B leaks out of lysosomes during the early stages of cell death. Though an increase in cathepsin B activity could also be detected in plasma (fig. 1C), the absolute levels were very low compared to tissue. Similar data were obtained using plasma taken from APAP overdose patients at the time of peak ALT (table 1). There was a significant increase in plasma activity in the overdose patients with liver injury compared with healthy volunteers or overdose patients without liver injury. Even when much larger amounts of protein (1–2 mg) and full patient time courses were studied, cathepsin B activity in human plasma remained low (data not shown).
Figure 1.
Plasma alanine aminotransferase (ALT) activity (A), intracellular cathepsin B activity (B) and plasma cathepsin B activity (C) were measured in untreated control animals and 3 hr after 300 mg/kg acetaminophen (APAP). Cathepsin B activities (100%) were 440±48 RFU/mg protein/min (microsomal fraction), 138±11 RFU/mg/min (cytosol) and 1.4±1.0 RFU/mg/min (plasma). Data represent means ± SE of n = 4 animals per group. *P<0.05 (compared to controls, C)
Table 1.
Plasma Cathepsin B Activities and Liver Injury in Acetaminophen Overdose Patients.
| Group | Cathepsin B (RFU/mg/min) | Age | ALT (U/L) | PT (s) |
|---|---|---|---|---|
| Healthy Volunteers | 1.0 ± 0.6 | 46 ± 4 | 21 ± 5 | ND |
| Normal LT | 0.8 ± 0.2 | 33 ± 7 | 61 ± 14 | 15 ± 1 |
| Abnormal LT | 3.0 ± 0.2* | 39 ± 6 | 4,524 ± 1,156* | 36 ± 7* |
ND = Not determined; PT = prothrombin time (reference range: 11–16 s [53]. Cathepsin B activity was measured in plasma from healthy volunteers, patients with normal liver function tests (Normal LT), and patients with abnormal liver function tests (Abnormal LT). Plasma samples were diluted in assay buffer. 50 μg of protein were loaded in each well of a 96-well plate and cleavage of fluorescent substrate was measured over time. Data are expressed as mean ± SE of n = 3–6 patients per group.
P < 0.05 (Compared to healthy volunteers or Normal LT)
Pathophysiological relevance of lysosomal cathepsin B release in vivo
In order to assess the potential role of cathepsin B in the pathophysiology of APAP hepatotoxicity, animals were treated with 5 mg/kg of the water-soluble cathepsin B inhibitor AC-LVK-CHO one hour before a 300 mg/kg APAP dose. Cell death and GSH values were evaluated 30 min. and 12 hr post APAP dose. Compared to vehicle controls, cathepsin B activities were inhibited by about 70% in the AC-LVK-CHO samples (fig. 2A). APAP caused severe liver injury as indicated by a massive increase in plasma ALT activity (fig. 2B); however, there was no significant difference in injury between the drug- and vehicle-treated groups. These results were confirmed by histology using both H&E staining and the TUNEL assay, which showed severe centrilobular necrosis and extensive DNA fragmentation, respectively (fig. 2C). GSH levels were measured to confirm metabolic activation of APAP. There was a characteristic rapid GSH depletion in both groups 30 min. after APAP treatment suggesting a similar formation of reactive metabolites (fig. 3A). The GSH content was recovered by 12 hr (fig. 3A). In addition, the significant increase in GSSG levels and the GSSG-to-GSH ratio at that time indicate a substantial oxidant stress (fig. 3B,C). However, there was no significant difference between the groups. These data suggest that the cathepsin B suicide inhibitor, despite its efficacy in reducing cathepsin activities, did not protect against APAP-induced liver injury.
Figure 2.
Hepatic cathepsin B activities (A) and plasma alanine aminotransferase (ALT) activities (B) were measured after 12-hr treatment with 300 mg/kg APAP. Mice were pre-treated for 1 hr with saline (vehicle) or with the cathepsin B inhibitor AC-LVK-CHO (5 mg/kg). All data represent means ± SE of n=4 animals per group. *P<0.05 (compared to controls). (C) Representative images of H&E and TUNEL staining in each group. All images: 50x
Figure 3.
(A) Total glutathione (GSH+GSSG) and (B) glutathione disulfide (GSSG) were measured 30 min. or 12 hr after treatment with 300 mg/kg APAP or in untreated controls. The GSSG-to-GSH ratio (C) for each animal was calculated. Mice were pre-treated for 1 hr with saline (vehicle) or with the cathepsin B inhibitor AC-LVK-CHO (5 mg/kg). Data represent means ± SE of n = 4 animals per group. *P<0.05 (compared to controls, Ctrl).
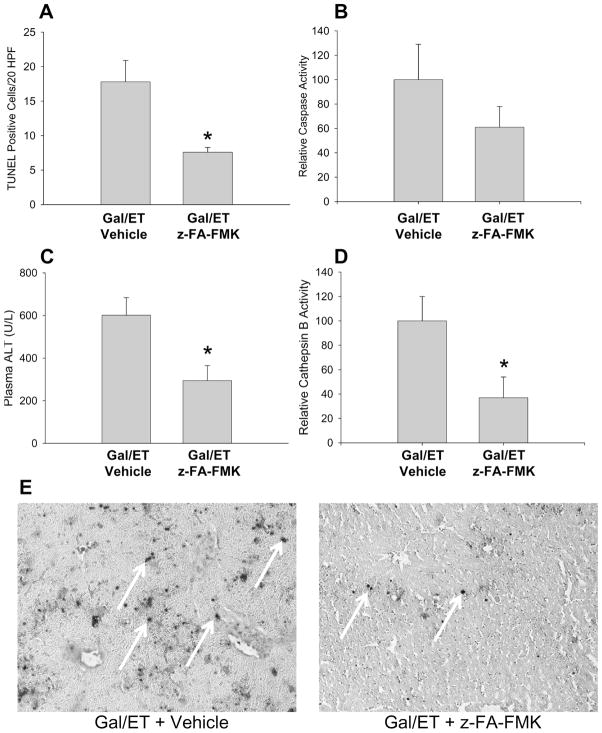
In order to confirm these findings with a different inhibitor, animals were pre-treated with 10 mg/kg of the cathepsin B inhibitor z-FA-fmk for 30 min. However, z-FA-fmk is only soluble in DMSO and even the saline-diluted inhibitor contained enough DMSO to eliminate any APAP toxicity at 6 hr when the animals were treated with a dose of 300 mg/kg APAP (data not shown). Therefore, a dose of 600 mg/kg APAP was used to potentially overcome the DMSO effect as previously shown for a JNK inhibitor [38,39] and the inhibitor z-FA-fmk or the DMSO-containing vehicle was injected 2 hr after APAP. Compared to vehicle controls, cathepsin B activities were inhibited by 80% in z-FA-fmk-treated animals (fig. 4A). APAP caused severe liver injury as indicated by the massive increase in plasma ALT activities (fig. 4B); however, there was no significant difference in injury between the drug- and vehicle-treated groups. These results were again confirmed by histology showing extensive centrilobular necrosis after APAP with no difference between the groups (fig. 4C). In addition, the TUNEL assay reflecting nuclear DNA damage supported the extensive centrilobular injury but no effect of the cathepsin B inhibitor fig. 4C). As there remained a possibility that this dose and route of administration of the drug was incapable at altering the pathophysiology, the inhibitor was also tested in a model previously shown to be mediated partially through cathepsin B [22–24,40]. Mice were treated with 10mg/kg z-FA-fmk or vehicle control one hour before 700mg/kg galactosamine and 100μg/kg endotoxin (Gal/ET) or saline control. TUNEL staining, ALT and cathepsin B levels were all found to be significantly decreased 6 hr after administration of the Gal/ET compared to vehicle controls (fig. 5A–E) consistent with a protective effect by the cathepsin B inhibitor. As the degree of inhibition of cathepsin B was consistent with the APAP experiments, and it has previously been shown that these types of suicide inhibitors are highly effective within an hour after treatment [41], it seemed likely this level of inhibition was sufficient to prevent or reduce injury in the APAP model if cathepsin B would be involved in the pathophysiology.
Figure 4.
Hepatic cathepsin B activities (A) and plasma alanine aminotransferase (ALT) activities (A) were measured after 12-hr treatment with 600mg/kg APAP. Mice were treated with saline, Saline/DMSO vehicle or 10 mg/kg z-FA-FMK, 2 hr after APAP administration. *P<0.05 (compared to control). #P<0.05 (compared to saline or DMSO). (C) Representative images of H&E and TUNEL staining in each group. All images: 50x
Figure 5.
Quantification of TUNEL staining (A) of galactosamine/endotoxin (Gal/ET) treated mice injected with either vehicle or 10mg/kg z-FA-FMK. Activities of hepatic caspase-3 (B), plasma ALT, (C) and hepatic cathepsin B (D) were also measured at 6 hr after Gal/ET treatment. Cathepsin B activities were 321±64 RFU/mg protein/min (vehicle) and 119±21 RFU/mg/min (z-FA-FMK). Representative images of TUNEL-stained slides are shown (E) (x100). TUNEL stain was only considered positive for specifically stained nuclei.
Lysosomal instability in cultured hepatocytes
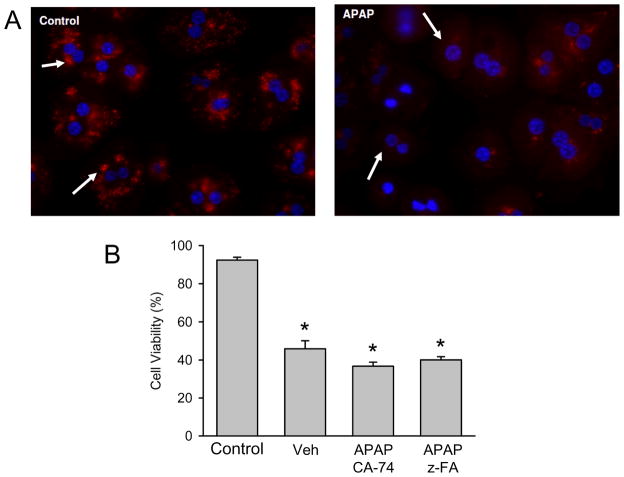
To confirm the in vivo results, lysosomal integrity was evaluated in primary cultured mouse hepatocytes. Cells were either untreated or exposed to 5 mM APAP for 6 hr. As was previously shown, this time point represents the early phase of cell necrosis at that concentration of APAP [36]. To investigate the potential lysosomal disruption, experiments were carried out with live cell imaging of isolated hepatocytes using Lysotracker Red, which localizes to acidic organelles [42]. Control hepatocytes showed punctate lysosomal staining characteristic of functional lysosomes (fig. 6A). Treatment with 5 mM APAP for 6 hr resulted in loss of the punctate staining and appearance of diffuse cytoplasmic signals indicating loss of lysosomal integrity (fig. 6A). In order to assess the potential effect of cathepsin B release on cell viability, cells were treated with 5 mM APAP 30 min. after exposure to either vehicle (0.1% DMSO in cell culture medium) or two different cathepsin B inhibitors (50 μM). In the vehicle-treated group, cell viability (measured as LDH release) declined from 92% to 48% at 9 hr after APAP (fig. 6B). Consistent with the in vivo findings, neither cathepsin B inhibitor affected the decline in cell viability caused by APAP (fig. 6B).
Figure 6.
(A) Lysotracker fluorescence in control hepatocytes and in cells 6 hr after treatment with 5 mM acetaminophen (APAP). Primary mouse hepatocytes were isolated and treated with APAP; cells were loaded with Lysotracker after 6 hr and subjected to live cell imaging. (B) Primary mouse hepatocytes were either untreated or exposed to 5 mM APAP for 9 hr. Some cells were pre-treated for 30 min. with DMSO/cell culture medium (0.1% DMSO per well) or 50 μM of the cathepsin B inhibitors z-FA-FMK or CA-074Me. Cell viability was assessed by LDH release. Data represent means ± SE of n = 4 independent experiments. *P<0.05 (compared to untreated controls, C)
DISCUSSION
Lysosomal instability during APAP hepatotoxicity
The objective of the current investigation was to evaluate the potential release of cathepsin B from lysosomes during APAP hepatotoxicity. Our data clearly demonstrate that there is a shift of cathepsin B activity from the microsomal fraction of the liver cell homogenate to the cytosol early after APAP overdose in vivo. Similarly, the more punctate staining of Lysotracker Red in cultured control hepatocytes is lost after APAP exposure. Together, these data indicate lysosomal instability and release of lysosomal content including cathepsin B into the cytosol during the early phase of APAP hepatotoxicity. These findings are consistent with our recent reports showing release of lysosomal iron into the cytosol and uptake into mitochondria after APAP treatment or oxidant stress in cultured hepatocytes [16,17]. Although the mechanism of the lysosomal instability remains unclear, the fact that this is a very early event in the injury process increases the likelihood it is related to the initial GSH depletion and formation of protein adducts after APAP overdose. Secondary effects due to toll-like receptor-9 activation by DNA fragments may also contribute to this effect [43]. However, this mechanism depends on extensive DNA fragmentation during cell necrosis and release of these fragments into the plasma, which generally is a later event [15]. Furthermore, the lysosomal instability may also be related to the activation of autophagy, which involves the fusion of the primary autophagosome with lysosomes to form autophagolysosomes [44]. Further studies are necessary to evaluate the contribution of these different mechanisms to the observed release of cathepsin B from lysosomes.
Pathophysiological relevance of lysosomal cathepsin B release
The fundamental question of the study was to assess the pathophysiological relevance of cathepsin B release from lysosomes for APAP hepatotoxicity. Cathepsin B has been implicated as mediator of cell death in various liver disease models including TNF-mediated apoptosis [22–24], obstructive cholestasis [25], hepatic ischaemia-reperfusion injury [26] and lipotoxicity [27]. These studies used both the same cathepsin B inhibitors as in our study as well as cathepsin B-deficient mice. Using two different cathepsin inhibitors, which are suicide substrates and irreversibly reduced hepatic cathepsin B activity by >70–80%, no protective effect against APAP-induced cell death was observed both in vivo and in cultured hepatocytes. This suggests that despite the lysosomal release of cathepsin B into the cytosol and the plasma, this protease had no relevant impact on liver cell death. In previous studies, it was postulated that cathepsin B is involved in promoting mitochondrial dysfunction although the exact mechanism remained unclear [22,23]. However, in APAP hepatotoxicity the mitochondrial dysfunction may be initiated by protein binding [5], amplified by c-jun-N-terminal kinase (JNK) activation and translocation to the mitochondria [38], Bax translocation to the mitochondria [45], and most importantly a substantial mitochondrial oxidant stress and peroxynitrite formation [8,9], which is aggravated by additional mitochondrial iron uptake [17]. Together, these events provide very strong pathogenic signals that limit any impact cathepsin B might have had.
It was also demonstrated that cathepsin B is released into the plasma together with other cell contents. This could be a passive release due to cell necrosis or active exocytosis as demonstrated in a rat model [46]. Previous reports showed the cellular release of proteases such as calpains [47] and secretory phospholipase A2 [48], during APAP hepatotoxicity. It was hypothesized that the release of these mediators from dying cells is involved in the progression of liver injury [48,49]. However, because the >70–80% inhibition of cathepsin B activity had no effect on APAP-induced cell death, it is unlikely that this enzyme was involved in the amplification of APAP-mediated liver injury under these conditions, especially given that this 70–80% inhibition was sufficient to partially ablate liver injury in the galactosamine/endotoxin model. The fact that in overdose patients also very limited cathepsin B activity was detectable in plasma during the peak injury phase suggests that there was less lysosomal instability during APAP-induced hepatocellular cell death in human beings than was observed in mice. Thus, a role of cathepsin B in APAP-induced liver injury is even less likely than in mice.
Solvents and cathepsin B inhibitor administration
In our study, we used the cathepsin B inhibitor z-FA-FMK, which is only soluble in DMSO and tolerates only very limited dilution in saline before precipitation. This is similar to the previous experience with the JNK inhibitor SP600125 where administration of a significant amount of DMSO can not be avoided [38,39]. However, DMSO is a potent inhibitor of P450 and consequently protects against APAP toxicity [49,50], even at the low doses administered in these inhibitor studies [51]. To overcome this problem, the DMSO-soluble inhibitor was injected after APAP and the dose of APAP was increased to 600 mg/kg, which results in liver injury despite the presence of DMSO [38,39]. Under these conditions, the cathepsin B inhibitor was not protective in vivo or in vitro despite the fact that cathepsin B enzyme activity was inhibited by 80%. Bypassing the DMSO-based inhibition of cathepsin B using a water-soluble inhibitor still resulted in no protection, further implicating a limited role for cathepsin B in APAP toxicity.
In a previous paper, z-FA-FMK has been used as control for a caspase inhibitor and no protection against APAP hepatotoxicity was observed [52]. According to the authors, the cathepsin B inhibitor was dissolved in DMSO, diluted in saline and then injected 15 min. before APAP [52]. Attempts to reproduce these data failed due to the fact that the solvent itself effectively protected. These data are consistent with our previous findings that the estimated amount of DMSO that was used as pre-treatment (0.2–0.3 ml/kg) is more than sufficient to inhibit the metabolic activation of APAP and almost completely prevent liver injury [51]. Furthermore, as no cathepsin activities were measured by El-Hassan et al. [52], it remained unclear if the inhibitor actually worked. In contrast to these concerns, our data clearly document that both water-soluble and DMSO-soluble inhibitors were effective in reducing cathepsin B enzyme activity without affecting APAP-induced cell injury in vivo or in vitro. In addition, this degree of cathepsin B inhibition was sufficient to significantly attenuate liver injury in the galactosamine/endotoxin model.
In summary, our data demonstrate lysosomal instability after APAP overdose leading to release of cathepsin B into the cytosol and also into plasma. However, neither the cathepsin B inhibitor z-FA-FMK, which reduced the enzyme activity by 80%, nor the water-soluble cathepsin B inhibitor AC-LVK, which reduced the enzyme activity by >70%, had a significant impact on APAP-induced liver cell injury in vivo or in vitro. These data are in contrast to a number of other liver disease models where cathepsin B has been critically implicated in the mechanism of cell death. However, our data in the APAP model of acute liver injury may reflect the stronger insult on mitochondria in the APAP pathophysiology, which drowns out the potential impact of cathepsin B liberation. Interestingly, very little cathepsin B activity was observed in plasma of APAP overdose patients. These data suggest that cathepsin B may not be a relevant therapeutic target for treating patients with APAP-induced liver injury.
Acknowledgments
This work was supported in part by the National Institutes of Health [R01 DK070195 and R01 AA12916 to H.J.], and by grants from the National Center for Research Resources (5P20RR021940-07) and the National Institute of General Medical Sciences (8 P20 GM103549-07) from the National Institutes of Health. B.L. Woolbright and M.R. McGill were supported by the “Training Program in Environmental Toxicology” (T32 ES007079-26) from the National Institute of Environmental Health Sciences.
References
- 1.Mitchell JR, Jollow DJ, Potter WZ, Gillette JR, Brodie BB. Acetaminophen-induced hepatic necrosis. IV. Protective role of glutathione. J Pharmacol Exp Ther. 1973;187:211–7. [PubMed] [Google Scholar]
- 2.Larson AM, Polson J, Fontana RJ, Davern TJ, Lalani E, Hynan LS, et al. Acetaminophen-induced acute liver failure: results of a United States multicenter, prospective study. Hepatology. 2005;42:1364–72. doi: 10.1002/hep.20948. [DOI] [PubMed] [Google Scholar]
- 3.Hinson JA, Reid AB, McCullough SS, James LP. Acetaminophen- induced hepatotoxicity: role of metabolic activation, reactive oxygen nitrogen species, and mitochondrial permeability transition. Drug Metab Rev. 2004;36:805–22. doi: 10.1081/dmr-200033494. [DOI] [PubMed] [Google Scholar]
- 4.Cohen SD, Pumford NR, Khairallah EA, Boekelheide K, Pohl LR, Amouzadeh HR, et al. Selective protein covalent binding and target organ toxicity. Toxicol Appl Pharmacol. 1997;143:1–12. doi: 10.1006/taap.1996.8074. [DOI] [PubMed] [Google Scholar]
- 5.Tirmenstein MA, Nelson SD. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3′-hydroxyacetanilide, in mouse liver. J Biol Chem. 1989;264:9814–9. [PubMed] [Google Scholar]
- 6.Qiu Y, Benet LZ, Burlingame AL. Identification of hepatic protein targets of the reactive metabolites of the non-hepatotoxic regioisomer of acetaminophen, 3′-hydroxyacetanilide, in the mouse in vivo using two-dimensional gel electrophoresis and mass spectrometry. Adv Exp Med Biol. 2011;500:663–73. doi: 10.1007/978-1-4615-0667-6_99. [DOI] [PubMed] [Google Scholar]
- 7.Meyers LL, Beierschmitt WP, Khairallah EA, Cohen SD. Acetaminophen-induced inhibition of hepatic mitochondrial respiration in mice. Toxicol Appl Pharmacol. 1988;93:378–87. doi: 10.1016/0041-008x(88)90040-3. [DOI] [PubMed] [Google Scholar]
- 8.Jaeschke H. Glutathione disulfide formation and oxidant stress during acetaminophen-induced hepatotoxicity in mice in vivo: the protective effect of allopurinol. J Pharmacol Exp Ther. 1990;255:935–41. [PubMed] [Google Scholar]
- 9.Cover C, Mansouri A, Knight TR, Bajt ML, Lemasters JJ, Pessayre D, et al. Peroxynitrite-induced mitochondrial and endonuclease-mediated nuclear DNA damage in acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2005;315:879–87. doi: 10.1124/jpet.105.088898. [DOI] [PubMed] [Google Scholar]
- 10.Kon K, Kim JS, Jaeschke H, Lemasters JJ. Mitochondrial permeability transition in acetaminophen-induced necrosis and apoptosis of cultured mouse hepatocytes. Hepatology. 2004;40:1170–1179. doi: 10.1002/hep.20437. [DOI] [PubMed] [Google Scholar]
- 11.Loguidice A, Boelsterli UA. Acetaminophen overdose-induced liver injury in mice is mediated by peroxynitrite independently of the cyclophilin d-regulated permeability transition. Hepatology. 2011;54:969–78. doi: 10.1002/hep.24464. [DOI] [PubMed] [Google Scholar]
- 12.Masubuchi Y, Suda C, Horie T. Involvement of mitochondrial permeability transition in acetaminophen-induced liver injury in mice. J Hepatol. 2005;42:110–116. doi: 10.1016/j.jhep.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Ramachandran A, Lebofsky M, Baines CP, Lemasters JJ, Jaeschke H. Cyclophilin D deficiency protects against acetaminophen-induced oxidant stress and liver injury. Free Radic Res. 2011;45:156–164. doi: 10.3109/10715762.2010.520319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H. HepaRG cells: A human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology. 2011;53:974–82. doi: 10.1002/hep.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGill MR, Sharpe MR, Williams CD, Taha M, Curry SC, Jaeschke H. The mechanism underlying acetaminophen-induced hepatotoxicity in humans and mice involves mitochondrial damage and nuclear DNA fragmentation. J Clin Invest. 2012;122:1574–83. doi: 10.1172/JCI59755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uchiyama A, Kim JS, Kon K, Jaeschke H, Ikejima K, Watanabe S, et al. Translocation of iron from lysosomes into mitochondria is a key event during oxidative stress-induced hepatocellular injury. Hepatology. 2008;48:1644–1654. doi: 10.1002/hep.22498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kon K, Kim JS, Uchiyama A, Jaeschke H, Lemasters JJ. Lysosomal iron mobilization and induction of the mitochondrial permeability transition in acetaminophen-induced toxicity to mouse hepatocytes. Toxicol Sci. 2010;117:101–108. doi: 10.1093/toxsci/kfq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masson O, Prébois C, Derocq D, Meulle A, Dray C, Daviaud D, et al. Cathepsin-D, a key protease in breast cancer, is up-regulated in obese mouse and human adipose tissue, and controls adipogenesis. PLoS One. 2011;6:e16452. doi: 10.1371/journal.pone.0016452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liaudet-Coopman E, Beaujouin M, Derocq D, Garcia M, Glondu-Lassis M, Laurent-Matha V, et al. Cathepsin D: newly discovered functions of a longstanding aspartic protease in cancer and apoptosis. Cancer Lett. 2006;237:167–79. doi: 10.1016/j.canlet.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Lankelma JM, Voorend DM, Barwari T, Koetsveld J, Van der Spek AH, De Porto AP, et al. Cathepsin L, target in cancer treatment? Life Sci. 2010;86:225–33. doi: 10.1016/j.lfs.2009.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Schechter I, Ziv E. Cathepsins S, B and L with aminopeptidases display β-secretase activity associated with the pathogenesis of Alzheimer’s disease. Biol Chem. 2011;392:555–69. doi: 10.1515/BC.2011.054. [DOI] [PubMed] [Google Scholar]
- 22.Guicciardi ME, Deussing J, Miyoshi H, Bronk SF, Svingen PA, Peters C, et al. Cathepsin B contributes to TNF-alpha-mediated hepatocyte apoptosis by promoting mitochondrial release of cytochrome c. J Clin Invest. 2000;106:1127–37. doi: 10.1172/JCI9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guicciardi ME, Miyoshi H, Bronk SF, Gores GJ. Cathepsin B knockout mice are resistant to tumor necrosis factor-alpha-mediated hepatocyte apoptosis and liver injury: implications for therapeutic applications. Am J Pathol. 2001;159:2045–54. doi: 10.1016/s0002-9440(10)63056-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gezginci S, Bolkent S. The effect of Z-FA-FMK on D-galactosamine/TNF-alpha-induced liver injury in mice. Cell Biochem Funct. 2007;25:277–286. doi: 10.1002/cbf.1352. [DOI] [PubMed] [Google Scholar]
- 25.Canbay A, Guicciardi ME, Higuchi H, Feldstein A, Bronk SF, Rydzewski R, et al. Cathepsin B inactivation attenuates hepatic injury and fibrosis during cholestasis. J Clin Invest. 2003;112:152–9. doi: 10.1172/JCI17740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baskin-Bey ES, Canbay A, Bronk SF, Werneburg N, Guicciardi ME, Nyberg SL, et al. Cathepsin B inactivation attenuates hepatocyte apoptosis and liver damage in steatotic livers after cold ischemia-warm reperfusion injury. Am J Physiol Gastrointest Liver Physiol. 2005;288:G396–402. doi: 10.1152/ajpgi.00316.2004. [DOI] [PubMed] [Google Scholar]
- 27.Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, et al. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–94. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- 28.Gujral JS, Bucci TJ, Farhood A, Jaeschke H. Mechanism of cell death during warm hepatic ischemia-reperfusion in rats: apoptosis or necrosis? Hepatology. 2001;33:397–405. doi: 10.1053/jhep.2001.22002. [DOI] [PubMed] [Google Scholar]
- 29.Gujral JS, Liu J, Farhood A, Jaeschke H. Reduced oncotic necrosis in Fas receptor-deficient C57BL/6J-lpr mice after bile duct ligation. Hepatology. 2004;40:998–1007. doi: 10.1002/hep.20380. [DOI] [PubMed] [Google Scholar]
- 30.Fickert P, Trauner M, Fuchsbichler A, Zollner G, Wagner M, Marschall HU, et al. Oncosis represents the main type of cell death in mouse models of cholestasis. J Hepatol. 2005;42:378–85. doi: 10.1016/j.jhep.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 31.Schoemaker MH, Gommans WM, Conde de la Rosa L, Homan M, Klok P, Trautwein C, et al. Resistance of rat hepatocytes against bile acid-induced apoptosis in cholestatic liver injury is due to nuclear factor-kappa B activation. J Hepatol. 2003;39:153–61. doi: 10.1016/s0168-8278(03)00214-9. [DOI] [PubMed] [Google Scholar]
- 32.Gujral JS, Knight TR, Farhood A, Bajt ML, Jaeschke H. Mode of cell death after acetaminophen overdose in mice: apoptosis or oncotic necrosis? Toxicol Sci. 2002;67:322–8. doi: 10.1093/toxsci/67.2.322. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Berk M, McIntyre TM, Gores GJ, Feldstein AE. The lysosomal-mitochondrial axis in free fatty acid-induced hepatic lipotoxicity. Hepatology. 2008;47:1495–503. doi: 10.1002/hep.22183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Knight TR, Jaeschke H. Acetaminophen-induced inhibition of Fas receptor-mediated liver cell apoptosis: mitochondrial dysfunction versus glutathione depletion. Toxicol Appl Pharmacol. 2002;181:133–41. doi: 10.1006/taap.2002.9407. [DOI] [PubMed] [Google Scholar]
- 35.Jaeschke H, Mitchell JR. Use of isolated perfused organs in hypoxia and ischemia/reperfusion oxidant stress. Methods Enzymol. 1990;186:752–9. doi: 10.1016/0076-6879(90)86175-u. [DOI] [PubMed] [Google Scholar]
- 36.Bajt ML, Knight TR, Lemasters JJ, Jaeschke H. Acetaminophen-induced oxidant stress and cell injury in cultured mouse hepatocytes: protection by N-acetyl cysteine. Toxicol Sci. 2004;80:343–9. doi: 10.1093/toxsci/kfh151. [DOI] [PubMed] [Google Scholar]
- 37.Burtelow MA, Kaufmann SH, Karnitz LM. Retention of the human Rad9 checkpoint complex in extraction-resistant nuclear complexes after DNA damage. J Biol Chem. 2000;275:26343–8. doi: 10.1074/jbc.M001244200. [DOI] [PubMed] [Google Scholar]
- 38.Hanawa N, Shinohara M, Saberi B, Gaarde WA, Han D, Kaplowitz N. Role of JNK translocation to mitochondria leading to inhibition of mitochondria bioenergetics in acetaminophen-induced liver injury. J Biol Chem. 2008;283:13565–77. doi: 10.1074/jbc.M708916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saito C, Lemasters JJ, Jaeschke H. c-Jun N-terminal kinase modulates oxidant stress and peroxynitrite formation independent of inducible nitric oxide synthase in acetaminophen hepatotoxicity. Toxicol Appl Pharmacol. 2010;246:8–17. doi: 10.1016/j.taap.2010.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yan BZ, Wang W, Chen LY, Bi MR, Lu YJ, Li BX, et al. Role of cathepsin B-mediated apoptosis in fulminant hepatic failure in mice. World J Gastroenterol. 2009;15:1231–6. doi: 10.3748/wjg.15.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA. Activation of caspase 3 (CPP32)-like proteases is essential for TNF-alpha-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J Immunol. 1998;160:3480–6. [PubMed] [Google Scholar]
- 42.Chazotte B. Labeling lysosomes in live cells with LysoTracker. Cold Spring Harb Protoc. 2011:990–2. doi: 10.1101/pdb.prot5571. [DOI] [PubMed] [Google Scholar]
- 43.Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, et al. Acetaminophen-induced hepatotoxicity in mice is dependent on Tlr9 and the Nalp3 inflammasome. J Clin Invest. 2009;119:305–14. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ni HM, Bockus A, Boggess N, Jaeschke H, Ding WX. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology. 2012;51:222–32. doi: 10.1002/hep.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bajt ML, Farhood A, Lemasters JJ, Jaeschke H. Mitochondrial bax translocation accelerates DNA fragmentation and cell necrosis in a murine model of acetaminophen hepatotoxicity. J Pharmacol Exp Ther. 2008;324:8–14. doi: 10.1124/jpet.107.129445. [DOI] [PubMed] [Google Scholar]
- 46.Gupta S, Rogers LK, Smith CV. Biliary excretion of lysosomal enzymes, iron, and oxidized protein in Fischer-344 and Sprague-Dawley rats and the effects of diquat and acetaminophen. Toxicol Appl Pharmacol. 1994;125:42–50. doi: 10.1006/taap.1994.1047. [DOI] [PubMed] [Google Scholar]
- 47.Limaye PB, Apte UM, Shankar K, Bucci TJ, Warbritton A, Mehendale HM. Calpain released from dying hepatocytes mediates progression of acute liver injury induced by model hepatotoxicants. Toxicol Appl Pharmacol. 2003;191:211–26. doi: 10.1016/s0041-008x(03)00250-3. [DOI] [PubMed] [Google Scholar]
- 48.Bhave VS, Donthamsetty S, Latendresse JR, Cunningham ML, Mehendale HM. Secretory phospholipase A2-mediated progression of hepatotoxicity initiated by acetaminophen is exacerbated in the absence of hepatic COX-2. Toxicol Appl Pharmacol. 2011;251:173–80. doi: 10.1016/j.taap.2011.01.013. [DOI] [PubMed] [Google Scholar]
- 49.Park Y, Smith RD, Combs AB, Kehrer JP. Prevention of acetaminophen-induced hepatotoxicity by dimethyl sulfoxide. Toxicology. 1998;52:165–75. doi: 10.1016/0300-483x(88)90202-8. [DOI] [PubMed] [Google Scholar]
- 50.Yoon MY, Kim SJ, Lee BH, Chung JH, Kim YC. Effects of dimethylsulfoxide on metabolism and toxicity of acetaminophen in mice. Biol Pharm Bull. 2006;29:1618–24. doi: 10.1248/bpb.29.1618. [DOI] [PubMed] [Google Scholar]
- 51.Jaeschke H, Cover C, Bajt ML. Role of caspases in acetaminophen-induced liver injury. Life Sci. 2006;78:1670–6. doi: 10.1016/j.lfs.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 52.El-Hassan H, Anwar K, Macanas-Pirard P, Crabtree M, Chow SC, Johnson VL, et al. Involvement of mitochondria in acetaminophen-induced apoptosis and hepatic injury: roles of cytochrome c, Bax, Bid, and caspases. Toxicol Appl Pharmacol. 2003;191:118–29. doi: 10.1016/s0041-008x(03)00240-0. [DOI] [PubMed] [Google Scholar]
- 53.Wallach JB. Interpretation of Diagnostic Tests. 7. Vol. 4 Lippincott Williams & Wilkins; Philadelphia, PA: 2000. [Google Scholar]