Summary
Highly active antiretroviral therapy (HAART) suppresses HIV-1 replication but cannot eliminate the virus because HIV-1 establishes latent infection. Interruption of HAART leads to a rapid rebound of viremia. Life-long treatment is therefore required. Efforts to purge the latent reservoir have focused on reactivating latent proviruses without inducing global T-cell activation. However, the killing of the infected cells after virus reactivation, which is essential for elimination of the reservoir, has not been assessed. Here we show that after reversal of latency in an in vitro model, infected resting CD4+ T cells survived despite viral cytopathic effects, even in the presence of autologous cytolytic T-lymphocytes (CTL) from most patients on HAART. Antigen-specific stimulation of patient CTLs led to efficient killing of infected cells. These results demonstrate that stimulating HIV-1-specific CTLs prior to reactivating latent HIV-1 may be essential for successful eradication efforts and should be considered in future clinical trials.
Introduction
The extremely stable latent reservoir for HIV-1 in resting memory CD4+ T cells (Chun et al., 1995; Chun et al., 1997a; Finzi et al., 1997; Wong et al., 1997 and Chun et al., 1997b) is a major barrier to viral eradication. In latently infected cells, the integrated provirus is transcriptionally silent (Hermankova et al., 2003 and Chun et al., 2003) but is able to produce replication-competent virus following cellular activation (Finzi et al., 1997; Wong et al., 1997 and Chun et al., 1997b). Because of the stability of the reservoir (Siliciano et al., 2003 and Strain et al., 2003), life-long antiretroviral therapy is required, raising concerns about adverse effects over decades of therapy, the evolution of resistance, and the financial burden of treatment. Strategies to eradicate HIV-1 from infected individuals are therefore urgently needed.
Efforts to eradicate HIV-1 have focused on reactivating latent proviruses. Early studies using IL-2 or IL-2 plus anti-CD3 antibodies to reactivate latent HIV-1 failed to eliminate the reservoir and caused significant toxicity due to global T cell activation (Chun et al., 1999; Prins et al., 1999; van Praag et al., 2001; Stellbrink et al., 2002 and Kulkosky et al., 2002). More recent studies have focused on identifying small molecules that reactivate latent virus without inducing host cell activation (Richman et al., 2009). Three FDA-approved drugs, valproic acid (Ylisastigui et al., 2004), suberoylanilide hydroxamic acid (SAHA) (Contreras et al., 2009; Archin et al., 2009 and Edelstein et al., 2009) and disulfiram (Xing et al., 2011) can reactivate latent virus in primary cell models and/or cells from infected individuals. Clinical studies of valproic acid, which has histone deacetylase (HDAC) inhibitor activity, have not shown a consistent decrease in the latent reservoir (Lehrman et al., 2005; Steel et al., 2006; Siliciano et al., 2007; Archin et al., 2008; Sagot-Lerolle et al., 2008 and Archin et al., 2010). These studies raise a critical issue: the fate of this reservoir after virus reactivation in resting CD4+ T cells. It is generally presumed that infected cells will die after reactivation of virus gene expression either as a result of viral cytopathic effects (CPE) or host immune responses, or both. Since newer approaches for reactivating latent HIV-1 utilize agents that do not induce global T cell activation, it is important to determine whether viral CPE or host responses can eliminate latently infected resting CD4+ T cells after virus reactivation.
Direct killing of infected cells by HIV-1 through caspase-dependent or independent mechanisms has been observed in activated CD4+ T cells (Roshal et al., 2001; Bolton et al., 2002; Sakai et al., 2006 and Shedlock et al., 2008). Other studies showed that early events in abortive HIV-1 infection induced cell death in resting CD4+ T cells (Zhou et al., 2008 and Doitsh et al., 2010). However, whether the reversal of viral latency causes cell death in resting CD4+ T cells or not has not been assessed. Besides viral CPE, host immunity is also presumed to eliminate the latently infected CD4+ T cells after virus reactivation. Cytolytic T-lymphocytes (CTL) are a major component of the host response to HIV-1. CTL partially limit viral replication (Walker et al., 1987; Koup et al., 1994; Borrow et al., 1997; Schmitz et al., 1999; Gandhi and walker, 2002 and Hersperger et al., 2011) but show functional defects in patients with progressive disease that are not restored with HAART (Kalams et al., 1999; Saez-Cirion et al., 2007; Migueles et al., 2008; Migueles et al., 2009 and Hersperger et al., 2010). It is unknown whether CTL can kill resting CD4+ T cells in which latent infection has been reversed. In this study, we generated latently infected cells from primary CD4+ T cells as previously reported (Yang et al., 2009). SAHA was used to reactivate latent HIV-1 in resting CD4+ T cells. We found that virus reactivation did not cause death of infected cells. CTLs from patients on HAART failed to kill autologous latently infected CD4+ T cells after latent viruses were reactivated. Antigen-specific stimulation of patient CTL prior to virus reactivation led to rapid and effective killing of infected cells. Our results suggest that reactivation of latent HIV-1 will not purge the viral latent reservoir. Stimulation of HIV-1-specific CTL responses prior to virus reactivation may be essential for the viral eradication.
Results
Reactivation of latent HIV-1 in vitro without T cell activation does not affect the latent reservoir
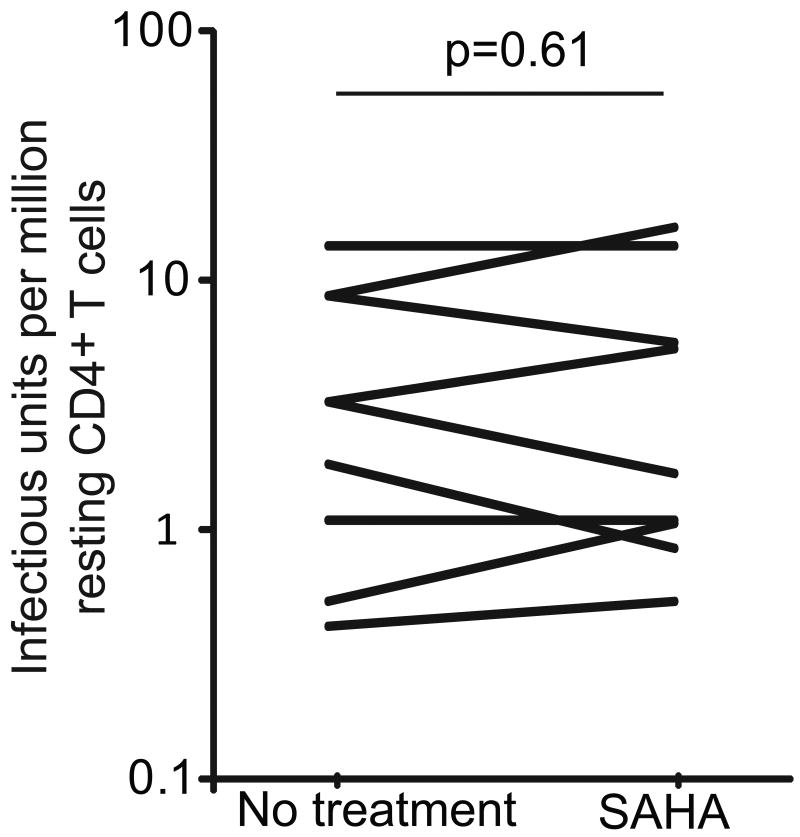
We first examined the stability of HIV-1 latent reservoir in resting CD4+ T cells after treatment with agents that reactivate latent HIV-1 without causing T cell activation. If viral CPE or CTL responses cause the death of infected resting CD4+ T cells, reactivating latent HIV-1 should result in reduction or elimination of latent reservoir. We used the histone deacetylase inhibitor SAHA, an FDA-approved drug used to treat cutaneous T-cell lymphoma. SAHA has been shown to reactivate latent HIV-1 in peripheral blood mononuclear cells (PBMC) from HIV-1 infected individuals on HAART (Contreras et al., 2009 and Archin et al., 2009). We treated PBMC from patients on HAART with SAHA for 6 days. After treatment with SAHA, the frequency of latently infected resting CD4+ T cells was measured using the standard limiting dilution virus culture assay (Figure 1). Infected cells were detected in 9/9 samples at frequencies similar to those in parallel cultures set up without SAHA. These results indicate that reversal of latent infection alone is not sufficient to eliminate the latent reservoir in resting CD4+ T cells.
Figure 1.
Effect of SAHA on the frequency of latently infected cells. PBMCs from patients on HAART were isolated and cultured in basal medium with or without 500 nM SAHA for six days. Efavirenz, raltegravir and enfuvirtide were added in the culture to prevent further rounds of viral replication. Resting CD4+ T cells were then isolated from the cultures. The frequency of cells harboring replication-competent HIV-1 was then measured using a previously described co-culture assay (Siliciano and Siliciano. 2005).
Viral CPE do not cause the death of infected resting CD4+ T cells
To provide further evidence that viral CPE do not cause the death of infected resting CD4+ T cells, we used freshly isolated primary resting CD4+ T cells to assess cell killing after infection. Resting cells were infected with a replication-competent HIV-1 (NL4-3-Δnef-EGFP) with all open reading frames (ORFs) intact except nef which was disrupted by EGFP (Figure S1). Although abortive infection with incomplete reverse transcription can cause cell death in resting CD4+ T cells (Doitsh et al., 2010), steps in the life cycle through integration are already complete in latently infected cells. Therefore we focused on the fate of productively infected (GFP+) cells. Productively infected resting CD4+ T cells survived after acute infection while productively infected cells that were in an activated state rapidly died (Figure 2A and 2B). In the activated CD4+ T cell population, infected cells with higher viral gene expression died faster because the mean fluorescent intensity of GFP+ cells decreased by half from day 3 to day 4 after infection (Figure 2C). The survival of resting CD4+ T cells may reflect 20 fold lower viral gene expression than in activated cells.
Figure 2. Resting CD4+ T cells are not killed by viral CPE.
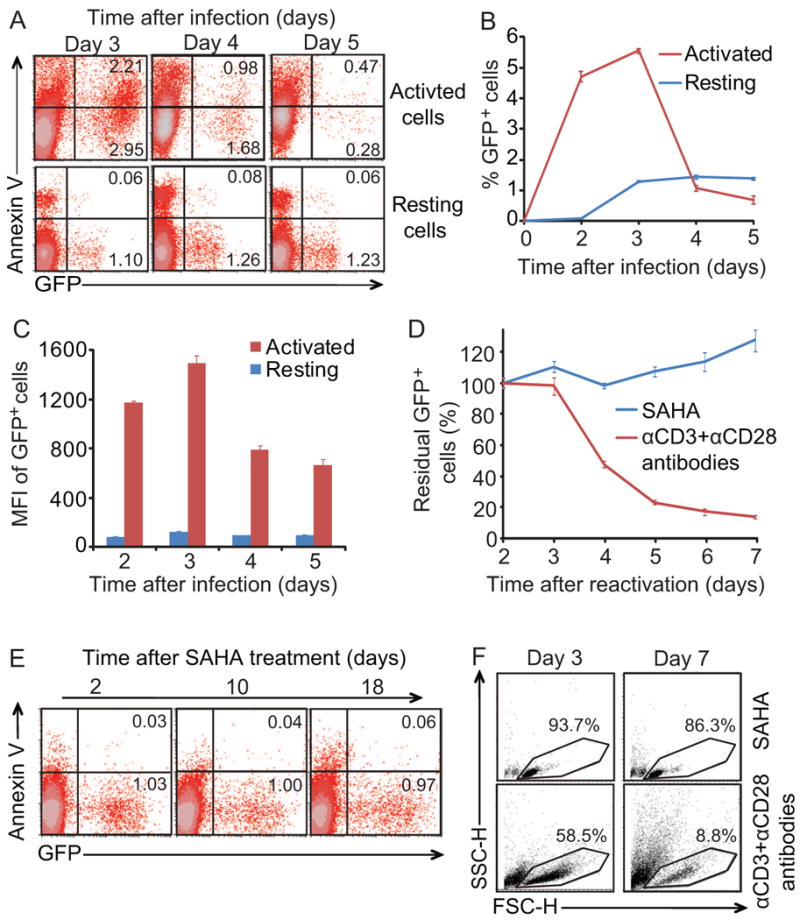
(A-C) Resting CD4+ T cells are not killed by viral CPE after acute infection. Primary resting CD4+ T cells were isolated and treated with 500 nM SAHA or anti-CD3 and anti-CD28 antibodies for one day and then infected with NL4-3-Δnef-EGFP Infected cells were either activated with anti-CD3 plus anti-CD28 antibodies, or left in a resting state. Efavirenz and enfuvirtide were added to block additional rounds of viral replication at day 3. GFP expression and annexin-V staining were analyzed by FACS. (D-F) Resting CD4+ T cells latently infected with HIV-1 are not killed by viral CPE after virus reactivation. Bcl-2 transduced CD4+ T cells were infected with NL4-3-Δnef-Δpol-EGFP to generate latent infection in vitro. To reactivate latent HIV-1, cells were treated with 500 nM SAHA or anti-CD3 and anti-CD28 antibodies. In D and E, The fraction of remaining GFP+ cells and the fraction of annexin-V-positive cells were monitored by FACS analysis. In F, GFP+ cells were purified by sorting and cultured for additional 7 days. Cell viability was monitored using forward and side scatter. Numbers in the quadrant indicate the percentage of cells.
We also evaluated the effects of reversing latency in a previously described primary cell model (Yang et al., 2009). Latently infected CD4+ T cells were generated in vitro using a replication-deficient HIV-1 reporter virus NL4-3-Δnef-Δpol-EGFP (Figure S1). This vector expresses Vif, Vpr, and Env, the HIV-1 gene products most commonly associated with CPE, and all other viral genes except pol and nef. The latter is disrupted by the insertion of the GFP coding sequence. Viral latency was then reversed with anti-CD3 plus anti-CD28 antibodies or SAHA, and cultures were monitored for GFP expression and markers of cell death (Annexin V staining). SAHA at 500 nM is not toxic to resting CD4+ or CD8+ T cells and does not activate T cells (Figures S2). With anti-CD3 plus anti-CD28 co-stimulation, a substantial fraction of GFP+ infected cells died rapidly (Figure 2D), confirming that viral CPE can be observed in this system despite transduction with Bcl-2. However, when latency was reversed without T cell activation using SAHA, GFP+ infected cells survived throughout the 18 day observation period (Figure 2D and 2E). To rule out the possibility that the stable GFP+ fraction in SAHA-treated group was due to continuous reactivation of latently infected CD4+ T cells, GFP+ fractions were purified by cell sorting from SAHA-treated and co-stimulated cultures for further analysis. No cell death was observed in purified infected resting CD4+ T cells after reversal of viral latency by SAHA. In contrast, cells treated with anti-CD3 plus anti-CD28 antibodies became activated and died rapidly (Figure 2F). Thus under conditions where viral CPE kill activated CD4+ T cells, resting CD4+ T cells survive, indicating that immune clearance may be critical for eradication strategies that reverse latency without T cell activation.
CD8+ T cells from patients on HAART do not kill infected CD4+ T cells after virus reactivation
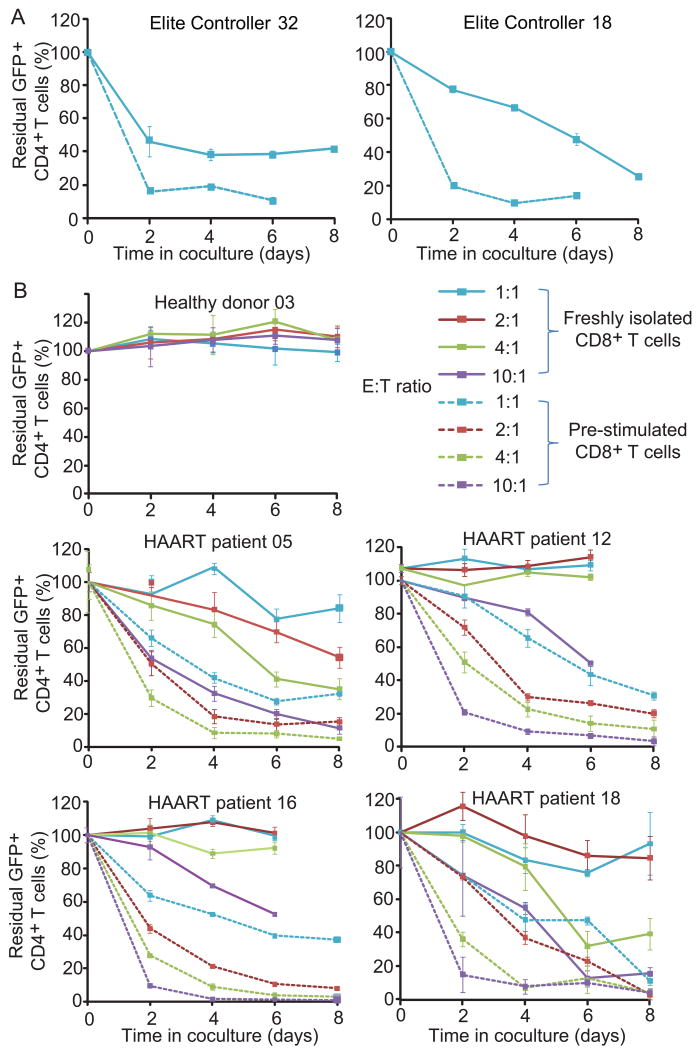
The cytolytic capacity of CD8+ T cells in patients with progressive disease is defective and is not restored with HAART (Kalams et al., 1999; Saez-Cirion et al., 2007; Migueles et al., 2008; Migueles et al., 2009 and Hersperger et al., 2010). It is unknown whether CTL from these patients can kill resting CD4+ T cells in which latent infection has been reversed. We therefore developed a coculture system to evaluate CTL-mediated killing of autologous latently infected CD4+ T cells. HIV-1 latently infected cells were generated from primary CD4+ T cells from HIV-1-infected and uninfected donors as described previously (Yang et al., 2009) using the replication-deficient HIV-1 reporter virus NL4-3-Δvpr-Δenv-drEGFP. In this reporter virus, genes important for CTL recognition such as gag are intact, but vpr is inactivated to improve the yield of latently infected cells (Figure S1). Latently infected cells were then treated with SAHA for two days and cocultured with freshly isolated autologous CD8+ T cells at a 1:1 ratio to determine whether they could be killed after the reversal of latency. In this primary cell model, SAHA reactivates latent HIV-1 to the same extent that co-stimulation with anti-CD3 and anti-CD28 antibodies does, although with slower kinetics (Figure 3A). Latently infected cells became GFP-positive after SAHA treatment and expressed HIV-1 proteins such as Gag, an important target antigen for HIV-1-specific CTL (Figure 3B).
Figure 3. Autologous CD8+ T cells from patients on HAART do not kill infected CD4+ T cells after virus reactivation.
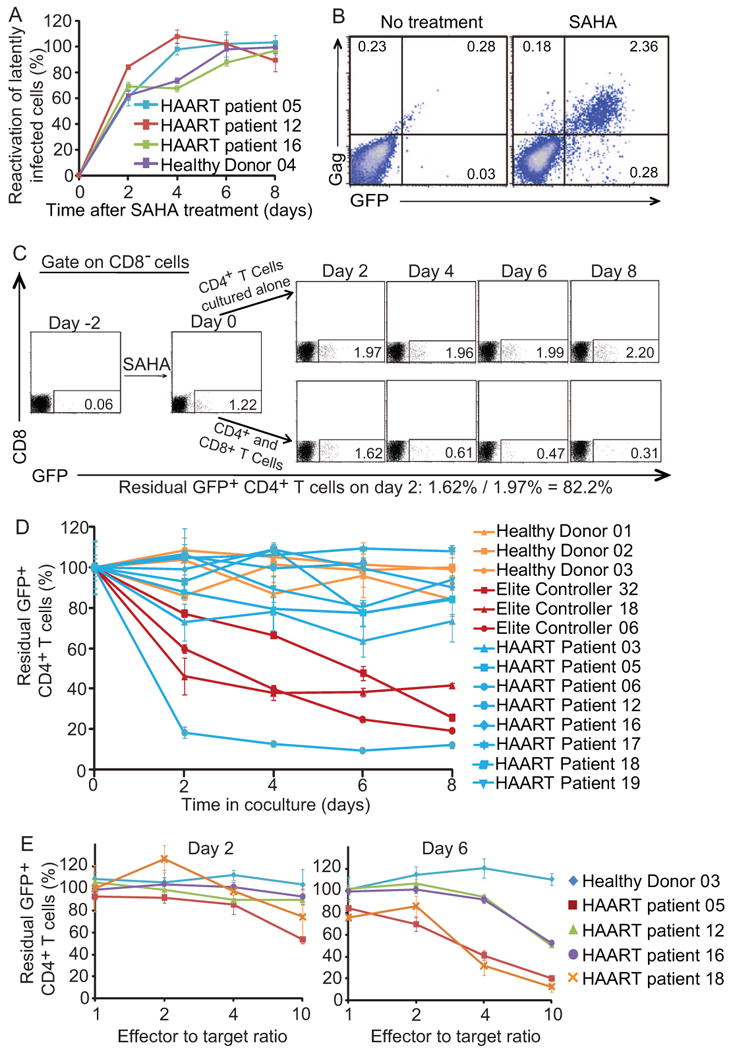
(A) 500 nM SAHA reactivates latent viruses. The effect of SAHA was normalized to effect of co-stimulation with anti-CD3 and anti-CD28 antibodies at day 2. (B) GFP expression is correlated with Gag protein expression. (C) Calculation of residual CD4+ GFP+ cells. In vitro latently infected CD4+ T cells generated from Elite controller 32 were treated with SAHA and cocultured with freshly isolated autologous CD8+ T cells at a 1:1 ratio. Numbers in the quadrant indicate the percentage of cells. (D) Effect of CD8+ T cells from different healthy donors, ECs, and patients on HAART on the survival of autologous CD4+ T cells in which latent HIV-1 infection has been reversed. E:T ratio is 1:1. Data represent the mean ± SEM, N=3. (E) Killing of latently infected cells is observed with higher E:T ratio. Freshly isolated autologous CD8+ T cells were co-cultured with SAHA-treated CD4+ T cells at different E:T ratio.
In the representative experiment shown in Figure 3C, latently infected cells were generated by superinfection of primary CD4+ T cells from an elite controller (EC32), a patient who controls HIV-1 without treatment (Deeks and Walker, 2007 and O'Connell et al., 2009). Such patients generally have high HIV-1-specific CTL activity (Saez-Cirion et al., 2007; Migueles et al., 2008; Migueles et al., 2009 and Hersperger et al., 2010). SAHA induced reactivation of latent HIV-1, with 1.22% of the cells becoming GFP+ after 2 days. In the absence of CD8+ T cells, the fraction of GPF+ cells increased slightly over the next 8 days. However, when autologous CD8+ T cells were present, the number of GFP+ cells decreased dramatically. When latently infected cells were generated from HIV-negative donors, the fraction of CD4+ T cells expressing GFP did not decrease during the coculture with autologous CD8+ T cells, which lack HIV-1-specific CTL activity (Figure 3D). Three HLA-B57+ ECs with HIV-1-specific CTL activity were also included as positive controls. A substantial decrease in GFP+ cells was observed for all three (Figure 3D). This decrease is not due to non-specific killing by CD8+ T cells or spontaneous reentry into latency because no decrease was observed in cultures from HIV-negative donors. Rather, the decrease is likely to reflect HIV-1-specific killing by CD8+ T cells (see below).
We also used disulfiram to reactivate latent virus in CD4+ T cells and monitored cell death with the presence of autologous CD8+ T cells. A modest killing effect was observed at day 2 in cultures from two HLA-B57+ ECs (Figure S3A). No additional killing effect was observed after longer periods of culture in the presence of disulfiram, probably due to the general cytotoxicity of disulfiram. Therefore, disulfiram was not used for further study.
In the case of all three ECs, a notable fraction of GFP-positive cells (20% to 40%) remained after 8 days of coculture. To test whether these cells were resistant to CTL-mediated killing, we added freshly isolated autologous CD8+ T cells for the second time at day 8 of coculture and continued the culture for an additional 8 days. A further reduction of GFP-positive cells was observed (Figure S3B). Therefore, the residual GFP-positive cells were not resistant to CTL-mediated killing. A more likely explanation is that the viability of CD8+ T cells decreased after several days of culture in vitro while CD4+ T cells survived better due to Bcl-2 over-expression.
Eradication strategies will be implemented in patients with progressive disease who respond to HAART. Thus it is important to determine whether such patients have CTL that kill infected cells after reversal of latency. Of cultures from eight patients on HAART, only one had CD8+ T cells that eliminated latently infected CD4+ T cells at a 1:1 effector-to-target (E:T) ratio (Figure 3D). Despite the presence of autologous CD8+ T cells, latently infected cells from the other seven patients survived with a median half-life of 34.2 days, compared with 4.3 days in the case of EC (Figure S4). These results suggested that reactivating latent HIV-1 in patients on HAART might not lead to the elimination of this reservoir because of the weak CTL responses in these patients.
CTL responses increase at higher E:T ratios
The failure of CD8+ T cells from patients on HAART to clear latently infected cells after virus reactivation could be due to an insufficient number of HIV-1-specific cells or diminished CTL function (Migueles et al., 2008 and Migueles et al., 2009). Even at higher E:T ratios, no substantial killing of GFP+ cells was observed in cultures from patients on HAART at day 2 of co-culture (Figure 3E). Killing of GFP+ cells was observed only at high E:T ratios after a longer period of co-culture (Figure 3E). These results suggest limitations in the number and/or cytolytic capacity of HIV-1-specific CTL that can be restored after co-culture with infected target cells. We therefore hypothesized that prior antigen-specific stimulation of CD8+ T cells might facilitate the elimination of latently infected cells after virus reactivation.
Pre-stimulation of patient CD8+ T cells leads to elimination of HIV-1 latently infected cells
CD8+ T cells from infected donors were stimulated with HIV-1 Gag peptides and IL-2 for 6 days and then cocultured with autologous CD4+ T cells treated with SAHA as described above. We used B57-restricted Gag peptides to stimulate CD8+ T cells from HLA B57+ EC and a mixture of 129 Group M consensus Gag peptides to stimulate CD8+ T cells from patients on HAART. For ECs, who generally have strong CTL responses, pre-stimulation led to more rapid and effective CTL clearance of cells reactivated from latency (Figure 4A). For patients on HAART, significant killing was observed at a 1:1 E:T ratio even for patients from whom almost no CTL activity was observed without pre-stimulation (Figure 4B). The median half-life of latently infected CD4+ T cells from those patients was reduced from 34.2 days to 3.6 days (Figure S4). Increasing the E:T ratio further enhanced killing (Figure 4B). Pre-stimulation with Gag peptides and IL-2 caused modest proliferation of HIV-1 specific CTLs, but IL-2 alone did not (Figure 5A and 5B). Pre-stimulation also up-regulated granzyme B, INF-γ, CD107a and perforin production, but not IL-2 production in HIV-1-specific CTLs (Figure 5C). These results suggest that antigen-specific stimulation restored killing ability of CD8+ T cells from patients on HAART and therefore facilitated the elimination of infected resting CD4+ T cells after reversal of viral latency.
Figure 4. Pre-stimulation of CD8+ T cells enhanced CTL responses.
Before coculture, CD8+ T cells from ECs or patients on HAART were stimulated with B57-restricted Gag peptides KF11 and TW10 (A), or mixture of 129 Gag peptides (B), respectively. Solid lines: unstimulated CD8+ T cells; Dashed lines: pre-stimulated CD8+ T cells. Data represent the mean ± SEM, N=3.
Figure 5. Pre-stimulation with Gag peptides plus IL-2 induces proliferation of HIV-1 specific CD8+ T cells.
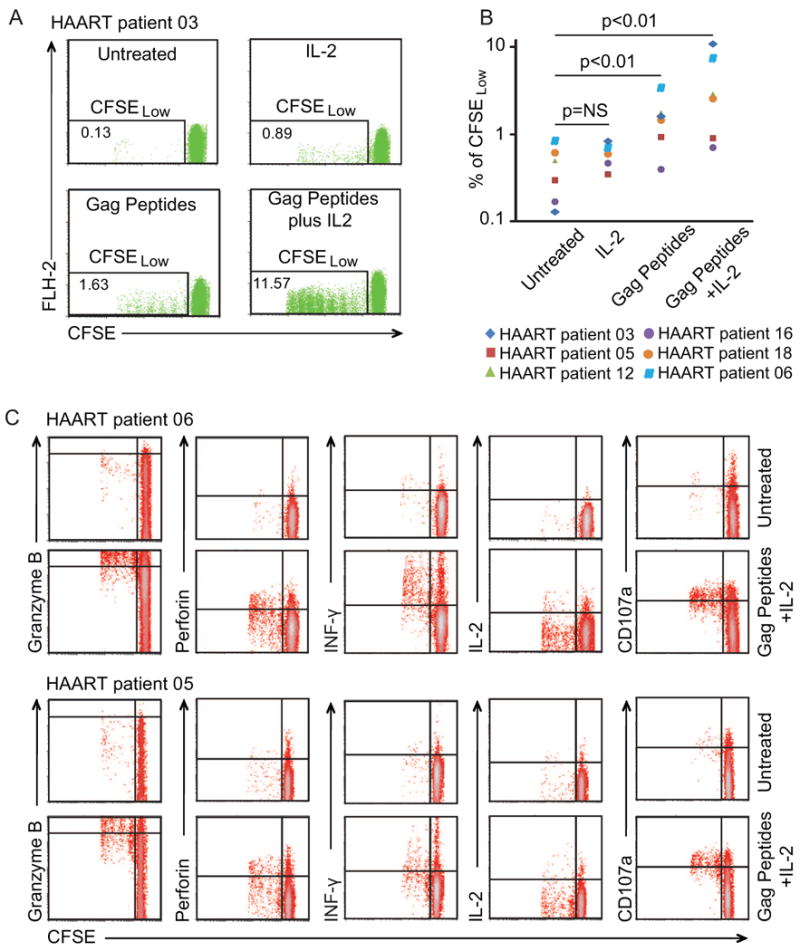
(A and B) Proliferative responses. PBMCs from patients on HAART were stained with CFSE and cultured with indicated treatments for 6 days. CD8+ T cells were then isolated and analyzed with FACS. Numbers in the quadrant indicate the percentage of cells. (C) Production of granzyme B, interferon-γ, perforin, CD107a and IL-2. PBMCs from two patients were stained with CFSE and cultured with indicated treatments for 6 days. CD8+ T cells were then isolated from PBMCs and used for intracellular staining for the indicated proteins.
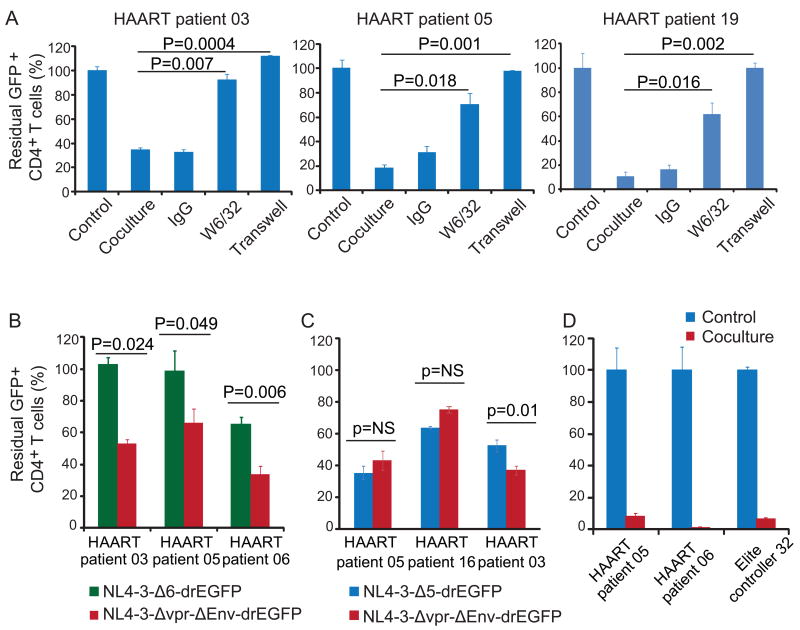
Reduction in GFP+ cells in co-culture is caused by direct killing by HIV-1-specific CTLs
CTL killing requires cell-to-cell contact and is MHC class I restricted. As shown in figure 6A, the clearance of GFP+ cells was abolished when CD4+ and CD8+ T cells were co-cultured in trans-well plates, which allow the exchange of cytokines but block cell-to-cell contact. The reduction in GFP+ cells was inhibited by the MHC class I-specific antibody W6/32, which blocks T cell recognition of class I MHC-peptide complexes (Figure 6A). W6/32 did not induce reactivation or affect activation by SAHA (Figure S5). To confirm that the observed killing required expression of viral genes, we generated latently infected cells using the virus NL4-3-A6-drEGFP which expresses only tat and rev (Figure S1). As shown in Figure 6B, there was no CTL-induced reduction in GFP+ cells in the NL4-3-Δ6-drEGFP group in two of three patients and only a partial reduction in the third. This reduction was likely due to Rev-specific CTL (data not shown). In control cultures from the same patients, there was robust killing of cells infected with NL4-3-Δvpr-Δenv-drEGFP which expresses all viral genes except vpr and env. Thus these CTL responses specifically target viral proteins. For unstimulated CD8+ T cells from patients on HAART, Gag protein is the major target antigen (Figure 6B, 6C). It remained possible that the disappearance of GFP+ cells was due to CTL-mediated inhibition of viral gene transcription or translation. Therefore, we isolated the CD4+ T cells after 8 days co-culture and activated them with anti-CD3 and anti-CD28 antibodies. No GFP+ cells were rescued (Figure 6D), indicating that the target cells had been killed. These observations confirmed that the disappearance of GFP+ cells in coculture was due to CTL-mediated killing which was contact dependent and MHC class I restricted.
Figure 6. Reduction in GFP+ cells in co-culture is caused by direct killing by HIV-1-specific CTLs.
(A) Reduction of GFP+ cells is cell contact-dependent and MHC class-I restricted. Pre-stimulated autologous CD8+ T cells from patients on HAART were co-cultured with SAHA-treated CD4+ T cells at a 4:1 ratio with 500 nM SAHA and the indicated treatment for 4 days. (B and C) Elimination of latently-infected cells requires viral gene expression. Latently infected cells were generated using indicated viruses. Unstimulated autologous CD8+ T cells were co-cultured with SAHA-treated latently infected CD4+ T cells at a 4:1 ratio with 500 nM SAHA for 4 days. (D) GFP+ cells cannot be recovered after co-culture. CD4+ T cells were isolated after 8 days of co-culture with pre-stimulated autologous CD8+ T cells at a 4:1 ratio in the presence of 500 nM SAHA, and then co-stimulated with anti-CD3 and anti-CD28 antibodies for 2 days. GFP+ cells were analyzed with FACS and normalized to the control culture set up without CD8+ T cells. Data represent the mean ± SEM, N=3.
Pre-stimulated patient CD8+ T cells eliminate CD4+ T cells infected with autologous viruses
It is important to investigate whether CD8+ T cells stimulated with Group M consensus Gag peptides can recognize viruses coming from the same patient's latent reservoir. CD4+ T cells isolated from patients on HAART were infected with viruses recovered from resting CD4+ T cells from the same patient, and then cocultured with autologous CD8+ T cells. The viruses recovered from patient resting CD4+ T cells were likely a mixture of different viral clones. In Figure 7A and 7B, most of the infected CD4+ T cells were killed three days after coculture with stimulated autologous CTL. This killing was contact dependent since not killing was observed when CD4+ T cells and CD8+ T cells were cultured in transwells. Since the killing efficiency was similar for target cells infected with patient-derived virus and HIV-1 BaL (Figure 7C), the small fraction of infected cells that are not killed are unlikely due to the resistance to CTL killing. It is more likely due to the insufficient time for CTL killing, which was limited to three days because of the viability of infected primary CD4+ T cells cultured in vitro. These results demonstrate that stimulated patient CTLs efficiently killed the target cells infected with the autologous viruses.
Figure 7. Pre-stimulated CD8+ T cells from patients on HAART eliminate CD4+ T cells infected with autologous viruses.
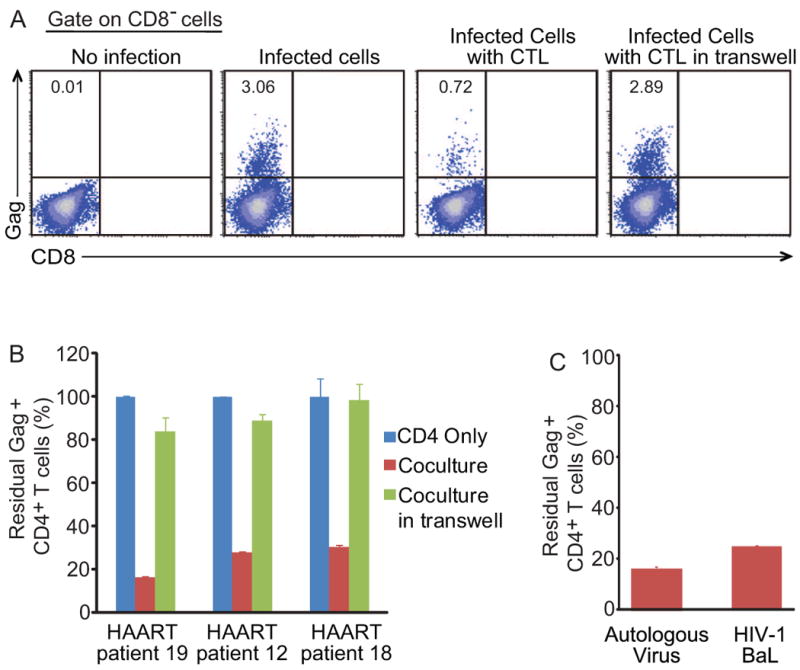
(A and B) PBMCs from patients on HAART were isolated and cultured in basal medium for 5-6 days to allow the decay of remaining intracellular triphosphorylated nucleoside/nucleotide reverse transcriptase inhibitors, and then stimulated with phytohemagglutinin. CD4+ T cells were isolated 2 days after stimulation and infected with autologous viruses. Pre-stimulated autologous CD8+ T cells were added into the culture 4 hours after infection at the effector-to-target ratio 1:1. Raltegravir and enfuvirtide were added in the culture to prevent further rounds of viral replication. The fraction of residual Gag+ CD4+ T cells was measured after 3 days of co-culture. Numbers in the quadrant indicate the percentage of cells. Data represent the mean ± SEM, N=3. (C) Patient autologous viruses or HIV-1 BaL were used to infected CD4+ T cells. Infected cells were then cocultured with CD8+ T cells as described above. The fraction of residual Gag+ CD4+ T cells was measured after 3 days of co-culture. Data represent the mean ± SEM, N=3.
Discussion
Purging the latent reservoir for HIV-1 requires reactivation of latent virus and then elimination of infected host cells. Recent studies have identified several pharmacologic agents that reverse latency without inducting global T cell activation (Ylisastigui et al., 2004; Contreras et al., 2009; Archin et al., 2009; Yang et al., 2009 and Xing et al., 2011). In our study, SAHA was tested for the reactivation of latent virus and was shown to be as effective as general T cell activators in our primary cell model, although with slower kinetics. SAHA is currently used in the treatment of cutaneous T-cell lymphoma but is associated with common gastrointestinal and hematologic adverse effects. Following the development of several human primary cell models of HIV-1 latency (Sahu et al., 2006; Marini et al., 2008; Bosque and Planelles, 2009; Yang et al., 2009 and Tyagi et al., 2010), other agents that reactivate latent virus have been identified (Yang et al., 2009 and Xing et al., 2011), and it is likely that many additional agents will be found in these screens. Mechanisms of HIV-1 latency are also being extensively studied in these primary cell models and cell line models (Jordan et al., 2003). Eventually, combination of agents affecting different pathways will likely be used to maximally reactivate latent viruses in vivo. However, the reactivation of latent virus is only the first step to viral eradication. Elimination of the infected cells after virus reactivation has not been previously studied.
It is often assumed that the latently infected CD4+ T cells will die after the virus is reactivated because of viral CPE or host immunity, or both. Here we demonstrate that neither viral CPE nor CD8+ T cells from patients on HAART are sufficient to cause immediate death of latently infected resting CD4+ T cells after virus reactivation with agents that do not cause T cell activation. In our in vitro model of HIV-1 latency, Bcl-2 is over-expressed in order to promote long-term survival of primary CD4+ T cells. Bcl-2 over-expression in our system imposes some limitations and could potentially interfere with the study of viral CPE or the CTL response. To address this concern, freshly isolated primary resting CD4+ T cells were also used for the study of viral CPE and similar effects were observed. Specifically, we showed that reactivation of latent HIV-1 in freshly isolated, un-transduced resting CD4+ T cells from patients on HAART does not result in death to the infected cells. With regard to studies of CTL-mediated killing of infected cells, we showed that over-expression of Bcl-2 does not protect infected target cells from cytolytic effects of CD8+ T cells, which was consistent with other studies (Zhang et al., 2001; Packard et al., 2007 and Goping et al., 2008). Therefore, the lack of cell death after virus reactivation in our in vitro model is unlikely to be due to over-expression of Bcl-2.
Resting memory CD4+ T cells may be resistant to viral CPE for several reasons. First, viral gene transcription and translation are much less efficient in resting cells. Second, resting memory CD4+ T cells are in general less prone to cell death than activated CD4+ T cells (Stockinger et al., 2006; Surh et al., 2006; van Leeuwen et al., 2009 and Taylor and Jenkins, 2011). Third, resting memory CD4+ T cells remain at a quiescent G0 state after treatment with agents like SAHA, and in this state are not affected by the viral proteins Vpr and Vif (Roshal et al., 2001; Sakai et al., 2006 and Shedlock et al., 2008), which cause cell cycle arrest and cell death in activated cells. Thus reversing latency with agents that do not induce global T cell activation will not eliminate the latent reservoir. Finding a way to eliminate the cellular reservoir after virus reactivation is thus an important step to viral eradication.
While HIV-1-specific CTL are presumed to be capable of eliminating latently infected cells after the reversal of latency, we found that freshly isolated CD8+ T cells from patients on HAART were only effective at high E:T ratios and with a prolonged period of coculture. Little killing of infected target cells was observed within the first two days of coculture. One explanation is that the frequency of HIV-1-specific CD8+ T cells in patients on HAART is diminished as a result of the lack of antigen stimulation. In addition, the CTL dysfunction seen in patients with progressive disease is not fully restored on HAART (Kalams et al., 1999; Saez-Cirion et al., 2007; Migueles et al., 2008; Migueles et al., 2009 and Hersperger et al., 2010).
CD8+ T cells from one of eight patients (HAART patient 06) on HAART retained strong ability to kill infected target cells without in vitro stimulation. We used Gag peptides to stimulate CD8+ T cells from all eight patients on HAART and found that this patient had more Gag-specific CD8+ T cells and/or better antigen-driven proliferation than other patients. We also found that this patient had higher Granzyme B and INF-γ production than a representative control patient after in vitro stimulation with Gag peptides. This patient also had a notable CTL response against other HIV-1 proteins. It appears that this patient has a larger number of HIV-1-specific CD8+ T cells and has broader recognition of viral epitopes. Unfortunately, the majority of the patients on HAART does not have such strong CTL response and may not be able to eliminate the latent reservoir after virus reactivation.
Here we demonstrated that CTL activity could be restored through in vitro stimulation with Gag peptides for every patient on HAART studied. This suggests that HIV-1-specific CTL responses in patients on HAART are defective but can be restored to effectively eliminate latently infected resting CD4+ T cells after virus reactivation. Boosting CTL responses and then reactivating latent HIV-1 may be an efficient strategy to eradicate HIV-1. Stimulation with other viral proteins could provide a broader CTL response in some of the patients. Since reactivation strategies will likely be implemented with the presence of HAART, further rounds of viral replication are inhibited and de novo CTL escape mutations cannot arise. The pre-existing CTL escape variants archived in latent reservoir can be overcome by inducing strong and broad CTL responses against multiple viral epitopes, and are not an obstacle to elimination.
Other strategies for promoting the death of infected cells have been proposed. For example, elegant studies have shown that cells expressing the HIV-1 Env protein could be targeted using antibodies conjugated bacterial toxins (Brooks et al., 2003). The application of this approach may be limited because of the high variability of the Env protein, drug bioavailability, and potential adverse effects.
In conclusion, resting CD4+ T cells latently infected with HIV-1 will not be efficiently killed by either viral CPE, or host CTL responses after virus reactivation. Our study strongly suggests that boosting CTL responses through vaccination prior to virus reactivation may be essential for eradication of HIV-1 infection.
Experimental Procedures
Study subjects
Peripheral blood for the isolation of primary CD4+ and CD8+ T cells was obtained from HIV-1-infected donors (Supplementary table 1) and healthy adult volunteers. This study was approved by the Johns Hopkins Institutional Review Board. Written informed consent was provided by all study participants. For CTL study using human primary cell models of HIV-1 latency, each patient and healthy adult was recruited for at least two visits. CD4+ T cells were isolated in the first visit for Bcl-2 transduction and then HIV-1 reporter virus infection. CD8+ T cells were isolated in the second visit to coculture with in vitro infected autologous CD4+ T cells.
Generation of latently infected CD4+ T cells in vitro
Primary CD4+ T cells from HIV/AIDS patients or healthy donors were isolated to generate HIV-1 latent infection in vitro as previously described (Yang et al., 2009). Briefly, primary CD4+ T cells were transduced with Bcl-2 to allow long term culture. Bcl2-transduced primary CD4+ T cells return to a quiescent state but respond normally to T cell activating stimuli (Yang et al., 2009). Bcl-2-transduced cells were infected with HIV-1 reporter viruses. The infected cells were then cultured for several weeks without activating stimuli to allow establishment of latency in surviving cells. Flow cytometric cell sorting was used to remove residual GFP+ cells. This approach produces cultures in which 0.5-3% of the cells are latently infected, with the remaining cells (>97%) being uninfected. All HIV-1 reporter viruses used in this study are listed in figure S1. HIV-1 reporter viruses NL4-3-Δnef-Δpol-EGFP and NL4-3-Δnef-EGFP were used for viral CPE study. HIV-1 reporter virus NL4-3-Δvpr-Δenv-drEGFP was used for CTL studies unless otherwise specified.
Coculture of autologous CD4+ and CD8+ T cells
After cell sorting, the purified GFP- cells including latently infected cells were treated with 500 nM SAHA for 2 days before coculture. SAHA treated CD4+ T cells were cocultured with autologous CD8+ T cells in the presence of 500 nM SAHA in 24-well or 48-well plate. The fraction of residual GFP+ CD4+ T cells was measured by FACS. For analysis of contact dependence, CD4+ T cells and CD8+ T cells were placed in separate chambers of trans-well plates (0.4 μm, Costar). For analysis of MHC-I restriction, the class-I specific antibody W6/32 was added to the coculture medium at 5 μg/ml.
Pre-stimulation of CD8+ T cells
PBMCs from B57+ ECs were isolated and cultured in the presence of B57-restricted peptides KF11 and TW10 (5 μg/ml for each) and IL-2 (100 U/ml). PBMCs from patients on HAART were isolated and cultured in the presence of a mixture of 129 Gag peptides (80 ng/ml for each) (NIH AIDS Reagent Program) and IL-2 (100 U/ml). CD8+ T cells were isolated 6 days after stimulation for coculture with infected CD4+ T cells.
Recovery of patient virus from resting CD4+ T cells
Resting CD4+ T cells were isolated from patients on HAART. Coculture assay was performed to recover and amplify replication-competent viruses as previously described (Siliciano and Siliciano, 2005). The viruses were recovered from 5 to 10 million resting CD4+ T cells and were probably a mixture of different viral clones.
Supplementary Material
Highlights.
Resting CD4+ T cells latently infected with HIV-1 do not die after virus reactivation
Viral CPE or patient CTLs do not cause death of HIV-1 infected resting CD4+ T cells
Stimulated patient CTLs kill infected resting CD4+ T cells after reversal of latency
Autologous viruses recovered from latent reservoir do not escape from patient CTLs
Acknowledgments
We thank L. Alston for coordinating patient recruitment. This work was supported by NIH grant AI43222, by the Howard Hughes Medical Institute, and by an ARCHE grant from amfAR – The Foundation for AIDS Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Archin NM, Cheema M, Parker D, Wiegand A, Bosch RJ, Coffin JM, Eron J, Cohen M, Margolis DM. Antiretroviral intensification and valproic acid lack sustained effect on residual HIV-1 viremia or resting CD4+ cell infection. PLoS One. 2010;5:e9390. doi: 10.1371/journal.pone.0009390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archin NM, Eron JJ, Palmer S, Hartmann-Duff A, Martinson JA, Wiegand A, Bandarenko N, Schmitz JL, Bosch RJ, Landay AL, Coffin JM, Margolis DM. Valproic acid without intensified antiviral therapy has limited impact on persistent HIV infection of resting CD4+ T cells. AIDS. 2008;22:1131–1135. doi: 10.1097/QAD.0b013e3282fd6df4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archin NM, Espeseth A, Parker D, Cheema M, Hazuda D, Margolis DM. Expression of latent HIV induced by the potent HDAC inhibitor suberoylanilide hydroxamic acid. AIDS Res Hum Retroviruses. 2009;25:207–212. doi: 10.1089/aid.2008.0191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton DL, Hahn BI, Park EA, Lehnhoff LL, Hornung F, Lenardo MJ. Death of CD4(+) T-cell lines caused by human immunodeficiency virus type 1 does not depend on caspases or apoptosis. J Virol. 2002;76:5094–5107. doi: 10.1128/JVI.76.10.5094-5107.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borrow P, Lewicki H, Wei X, Horwitz MS, Peffer N, Meyers H, Nelson JA, Gairin JE, Hahn BH, Oldstone MB, Shaw GM. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat Med. 1997;3:205–211. doi: 10.1038/nm0297-205. [DOI] [PubMed] [Google Scholar]
- Bosque A, Planelles V. Induction of HIV-1 latency and reactivation in primary memory CD4+ T cells. Blood. 2009;113:58–65. doi: 10.1182/blood-2008-07-168393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DG, Hamer DH, Arlen PA, Gao L, Bristol G, Kitchen CM, Berger EA, Zack JA. Molecular characterization, reactivation, and depletion of latent HIV. Immunity. 2003;19:413–423. doi: 10.1016/s1074-7613(03)00236-x. [DOI] [PubMed] [Google Scholar]
- Chun TW, Carruth L, Finzi D, Shen X, DiGiuseppe JA, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn TC, et al. Quantification of latent tissue reservoirs and total body viral load in HIV-1 infection. Nature. 1997a;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- Chun TW, Engel D, Mizell SB, Hallahan CW, Fischette M, Park S, Davey RT, Jr, Dybul M, Kovacs JA, Metcalf JA, et al. Effect of interleukin-2 on the pool of latently infected, resting CD4+ T cells in HIV-1-infected patients receiving highly active anti-retroviral therapy. Nat Med. 1999;5:651–655. doi: 10.1038/9498. [DOI] [PubMed] [Google Scholar]
- Chun TW, Finzi D, Margolick J, Chadwick K, Schwartz D, Siliciano RF. In vivo fate of HIV-1-infected T cells: quantitative analysis of the transition to stable latency. Nat Med. 1995;1:1284–1290. doi: 10.1038/nm1295-1284. [DOI] [PubMed] [Google Scholar]
- Chun TW, Justement JS, Lempicki RA, Yang J, Dennis G, Jr, Hallahan CW, Sanford C, Pandya P, Liu S, McLaughlin M, et al. Gene expression and viral prodution in latently infected, resting CD4+ T cells in viremic versus aviremic HIV-infected individuals. Proc Natl Acad Sci U S A. 2003;100:1908–1913. doi: 10.1073/pnas.0437640100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proc Natl Acad Sci U S A. 1997b;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras X, Schweneker M, Chen CS, McCune JM, Deeks SG, Martin J, Peterlin BM. Suberoylanilide hydroxamic acid reactivates HIV from latently infected cells. J Biol Chem. 2009;284:6782–6789. doi: 10.1074/jbc.M807898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Doitsh G, Cavrois M, Lassen KG, Zepeda O, Yang Z, Santiago ML, Hebbeler AM, Greene WC. Abortive HIV infection mediates CD4 T cell depletion and inflammation in human lymphoid tissue. Cell. 2010;143:789–801. doi: 10.1016/j.cell.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein LC, Micheva-Viteva S, Phelan BD, Dougherty JP. Short communication: activation of latent HIV type 1 gene expression by suberoylanilide hydroxamic acid (SAHA), an HDAC inhibitor approved for use to treat cutaneous T cell lymphoma. AIDS Res Hum Retroviruses. 2009;25:883–887. doi: 10.1089/aid.2008.0294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, et al. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- Gandhi RT, Walker BD. Immunologic control of HIV-1. Annu Rev Med. 2002;53:149–172. doi: 10.1146/annurev.med.53.082901.104011. [DOI] [PubMed] [Google Scholar]
- Goping IS, Sawchuk T, Rieger A, Shostak I, Bleackley RC. Cytotoxic T lymphocytes overcome Bcl-2 inhibition: target cells contribute to their own demise. Blood. 2008;111:2142–2151. doi: 10.1182/blood-2007-08-105221. [DOI] [PubMed] [Google Scholar]
- Hermankova M, Siliciano JD, Zhou Y, Monie D, Chadwick K, Margolick JB, Quinn TC, Siliciano RF. Analysis of human immunodeficiency virus type 1 gene expression in latently infected resting CD4+ T lymphocytes in vivo. J Virol. 2003;77:7383–7392. doi: 10.1128/JVI.77.13.7383-7392.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersperger AR, Migueles SA, Betts MR, Connors M. Qualitative features of the HIV-specific CD8+ T-cell response associated with immunologic control. Curr Opin HIV AIDS. 2011;6:169–173. doi: 10.1097/COH.0b013e3283454c39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersperger AR, Pereyra F, Nason M, Demers K, Sheth P, Shin LY, Kovacs CM, Rodriguez B, Sieg SF, Teixeira-Johnson L, et al. Perforin expression directly ex vivo by HIV-specific CD8 T-cells is a correlate of HIV elite control. PLoS Pathog. 2010;6:e1000917. doi: 10.1371/journal.ppat.1000917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan A, Bisgrove D, Verdin E. HIV reproducibly establishes a latent infection after acute infection of T cells in vitro. EMBO J. 2003;22:1868–1877. doi: 10.1093/emboj/cdg188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalams SA, Goulder PJ, Shea AK, Jones NG, Trocha AK, Ogg GS, Walker BD. Levels of human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte effector and memory responses decline after suppression of viremia with highly active antiretroviral therapy. J Virol. 1999;73:6721–6728. doi: 10.1128/jvi.73.8.6721-6728.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, Farthing C, Ho DD. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–4655. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkosky J, Nunnari G, Otero M, Calarota S, Dornadula G, Zhang H, Malin A, Sullivan J, Xu Y, DeSimone J, et al. Intensification and stimulation therapy for human immunodeficiency virus type 1 reservoirs in infected persons receiving virally suppressive highly active antiretroviral therapy. J Infect Dis. 2002;186:1403–1411. doi: 10.1086/344357. [DOI] [PubMed] [Google Scholar]
- Lehrman G, Hogue IB, Palmer S, Jennings C, Spina CA, Wiegand A, Landay AL, Coombs RW, Richman DD, Mellors JW, et al. Depletion of latent HIV-1 infection in vivo: a proof-of-concept study. Lancet. 2005;366:549–555. doi: 10.1016/S0140-6736(05)67098-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini A, Harper JM, Romerio F. An in vitro system to model the establishment and reactivation of HIV-1 latency. J Immunol. 2008;181:7713–7720. doi: 10.4049/jimmunol.181.11.7713. [DOI] [PubMed] [Google Scholar]
- Migueles SA, Osborne CM, Royce C, Compton AA, Joshi RP, Weeks KA, Rood JE, Berkley AM, Sacha JB, Cogliano-Shutta NA, et al. Lytic granule loading of CD8+ T cells is required for HIV-infected cell elimination associated with immune control. Immunity. 2008;29:1009–1021. doi: 10.1016/j.immuni.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migueles SA, Weeks KA, Nou E, Berkley AM, Rood JE, Osborne CM, Hallahan CW, Cogliano-Shutta NA, Metcalf JA, McLaughlin M, et al. Defective human immunodeficiency virus-specific CD8+ T-cell polyfunctionality, proliferation, and cytotoxicity are not restored by antiretroviral therapy. J Virol. 2009;83:11876–11889. doi: 10.1128/JVI.01153-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell KA, Bailey JR, Blankson JN. Elucidating the elite: mechanisms of control in HIV-1 infection. Trends Pharmacol Sci. 2009;30:631–637. doi: 10.1016/j.tips.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Packard BZ, Telford WG, Komoriya A, Henkart PA. Granzyme B activity in target cells detects attack by cytotoxic lymphocytes. J Immunol. 2007;179:3812–3820. doi: 10.4049/jimmunol.179.6.3812. [DOI] [PubMed] [Google Scholar]
- Prins JM, Jurriaans S, van Praag RM, Blaak H, van Rij R, Schellekens PT, ten Berge IJ, Yong SL, Fox CH, Roos MT, et al. Immuno-activation with anti-CD3 and recombinant human IL-2 in HIV-1-infected patients on potent antiretroviral therapy. AIDS. 1999;13:2405–2410. doi: 10.1097/00002030-199912030-00012. [DOI] [PubMed] [Google Scholar]
- Richman DD, Margolis DM, Delaney M, Greene WC, Hazuda D, Pomerantz RJ. The challenge of finding a cure for HIV infection. Science. 2009;323:1304–1307. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- Roshal M, Zhu Y, Planelles V. Apoptosis in AIDS. Apoptosis. 2001;6:103–116. doi: 10.1023/a:1009636530839. [DOI] [PubMed] [Google Scholar]
- Saez-Cirion A, Lacabaratz C, Lambotte O, Versmisse P, Urrutia A, Boufassa F, Barre-Sinoussi F, Delfraissy JF, Sinet M, Pancino G, Venet A Agence Nationale de Recherches sur le Sida EP36 HIV Controllers Study Group. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci U S A. 2007;104:6776–6781. doi: 10.1073/pnas.0611244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagot-Lerolle N, Lamine A, Chaix ML, Boufassa F, Aboulker JP, Costagliola D, Goujard C, Pallier C, Delfraissy JF, Lambotte O ANRS EP39 study. Prolonged valproic acid treatment does not reduce the size of latent HIV reservoir. AIDS. 2008;22:1125–1129. doi: 10.1097/QAD.0b013e3282fd6ddc. [DOI] [PubMed] [Google Scholar]
- Sahu GK, Lee K, Ji J, Braciale V, Baron S, Cloyd MW. A novel in vitro system to generate and study latently HIV-infected long-lived normal CD4+ T-lymphocytes. Virology. 2006;355:127–137. doi: 10.1016/j.virol.2006.07.020. [DOI] [PubMed] [Google Scholar]
- Sakai K, Dimas J, Lenardo MJ. The Vif and Vpr accessory proteins independently cause HIV-1-induced T cell cytopathicity and cell cycle arrest. Proc Natl Acad Sci U S A. 2006;103:3369–3374. doi: 10.1073/pnas.0509417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, Racz P, Tenner-Racz K, Dalesandro M, Scallon BJ, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- Shedlock DJ, Hwang D, Choo AY, Chung CW, Muthumani K, Weiner DB. HIV-1 viral genes and mitochondrial apoptosis. Apoptosis. 2008;13:1088–1099. doi: 10.1007/s10495-008-0239-0. [DOI] [PubMed] [Google Scholar]
- Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, Siliciano RF. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- Siliciano JD, Lai J, Callender M, Pitt E, Zhang H, Margolick JB, Gallant JE, Cofrancesco J, Jr, Moore RD, Gange SJ, Siliciano RF. Stability of the latent reservoir for HIV-1 in patients receiving valproic acid. J Infect Dis. 2007;195:833–836. doi: 10.1086/511823. [DOI] [PubMed] [Google Scholar]
- Siliciano JD, Siliciano RF. Enhanced culture assay for detection and quantitation of latently infected, resting CD4+ T-cells carrying replication-competent virus in HIV-1-infected individuals. Methods Mol Biol. 2005;304:3–15. doi: 10.1385/1-59259-907-9:003. [DOI] [PubMed] [Google Scholar]
- Steel A, Clark S, Teo I, Shaunak S, Nelson M, Gazzard B, Kelleher P. No change to HIV-1 latency with valproate therapy. AIDS. 2006;20:1681–1682. doi: 10.1097/01.aids.0000238421.36313.fa. [DOI] [PubMed] [Google Scholar]
- Stellbrink HJ, van Lunzen J, Westby M, O'Sullivan E, Schneider C, Adam A, Weitner L, Kuhlmann B, Hoffmann C, Fenske S, et al. Effects of interleukin-2 plus highly active antiretroviral therapy on HIV-1 replication and proviral DNA (COSMIC trial) AIDS. 2002;16:1479–1487. doi: 10.1097/00002030-200207260-00004. [DOI] [PubMed] [Google Scholar]
- Stockinger B, Bourgeois C, Kassiotis G. CD4+ memory T cells: functional differentiation and homeostasis. Immunol Rev. 2006;211:39–48. doi: 10.1111/j.0105-2896.2006.00381.x. [DOI] [PubMed] [Google Scholar]
- Strain MC, Gunthard HF, Havlir DV, Ignacio CC, Smith DM, Leigh-Brown AJ, Macaranas TR, Lam RY, Daly OA, Fischer M, et al. Heterogeneous clearance rates of long-lived lymphocytes infected with HIV: intrinsic stability predicts lifelong persistence. Proc Natl Acad Sci U S A. 2003;100:4819–4824. doi: 10.1073/pnas.0736332100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- Taylor JJ, Jenkins MK. CD4+ memory T cell survival. Curr Opin Immunol. 2011;23:319–323. doi: 10.1016/j.coi.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Tyagi M, Pearson RJ, Karn J. Establishment of HIV latency in primary CD4+ cells is due to epigenetic transcriptional silencing and P-TEFb restriction. J Virol. 2010;84:6425–6437. doi: 10.1128/JVI.01519-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Leeuwen EM, Sprent J, Surh CD. Generation and maintenance of memory CD4(+) T Cells. Curr Opin Immunol. 2009;21:167–172. doi: 10.1016/j.coi.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag RM, Prins JM, Roos MT, Schellekens PT, Ten Berge IJ, Yong SL, Schuitemaker H, Eerenberg AJ, Jurriaans S, de Wolf F, et al. OKT3 and IL-2 treatment for purging of the latent HIV-1 reservoir in vivo results in selective long-lasting CD4+ T cell depletion. J Clin Immunol. 2001;21:218–226. doi: 10.1023/a:1011091300321. [DOI] [PubMed] [Google Scholar]
- Walker BD, Chakrabarti S, Moss B, Paradis TJ, Flynn T, Durno AG, Blumberg RS, Kaplan JC, Hirsch MS, Schooley RT. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature. 1987;328:345–348. doi: 10.1038/328345a0. [DOI] [PubMed] [Google Scholar]
- Wong JK, Hezareh M, Gunthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- Xing S, Bullen CK, Shroff NS, Shan L, Yang HC, Manucci JL, Bhat S, Zhang H, Margolick JB, Quinn TC, et al. Disulfiram reactivates latent HIV-1 in a Bcl-2-transduced primary CD4+ T cell model without inducing global T cell activation. J Virol. 2011;85:6060–6064. doi: 10.1128/JVI.02033-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HC, Xing S, Shan L, O'Connell K, Dinoso J, Shen A, Zhou Y, Shrum CK, Han Y, Liu JO, et al. Small-molecule screening using a human primary cell model of HIV latency identifies compounds that reverse latency without cellular activation. J Clin Invest. 2009;119:3473–3486. doi: 10.1172/JCI39199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylisastigui L, Archin NM, Lehrman G, Bosch RJ, Margolis DM. Coaxing HIV-1 from resting CD4 T cells: histone deacetylase inhibition allows latent viral expression. AIDS. 2004;18:1101–1108. doi: 10.1097/00002030-200405210-00003. [DOI] [PubMed] [Google Scholar]
- Zhang D, Beresford PJ, Greenberg AH, Lieberman J. Granzymes A and B directly cleave lamins and disrupt the nuclear lamina during granule-mediated cytolysis. Proc Natl Acad Sci U S A. 2001;98:5746–5751. doi: 10.1073/pnas.101329598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Shen L, Yang HC, Siliciano RF. Preferential cytolysis of peripheral memory CD4+ T cells by in vitro X4-tropic human immunodeficiency virus type 1 infection before the completion of reverse transcription. J Virol. 2008;82:9154–9163. doi: 10.1128/JVI.00773-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.