Abstract
Fibrin polymerization is a necessary part of hemostasis but clots can obstruct blood vessels and cause heart attacks and strokes. The polymerization reactions are specific and controlled, involving strong knob-into-hole interactions to convert soluble fibrinogen into insoluble fibrin. It has long been assumed that clots and thrombi are stable structures until proteolytic digestion. On the contrary, using the technique of fluorescence recovery after photobleaching, we demonstrate here that there is turnover of fibrin in an uncrosslinked clot. A peptide representing the knobs involved in fibrin polymerization can compete for the holes and dissolve a preformed fibrin clot, or increase the fraction of soluble oligomers, with striking rearrangements in clot structure. These results imply that in vivo clots or thrombi are more dynamic structures than previously believed that may be remodeled as a result of local environmental conditions, may account for some embolization, and suggest a target for therapeutic intervention.
Fibrin clot formation, initiated upon vascular injury, is an essential part of hemostasis1. The precursor of fibrin, fibrinogen, is a fibrous protein made up of three pairs of polypeptide chains, Aα, Bβ, and γ, linked together by 29 disulfide bonds. The polymerization of fibrin takes place on cleavage of small peptides, called fibrinopeptides A and B, from the center of the fibrinogen molecule, converting it to fibrin monomer, which polymerizes into half-staggered oligomers2,3,4 that lengthen to form protofibrils, which aggregate laterally to make fibers. Branching, longitudinal and lateral growth of fibers leads to three-dimensional network or gel formation. The fibrin gel is stabilized through covalent bonds by a transglutaminase, blood clotting Factor XIIIa, which specifically crosslinks glutamine and lysine residues of fibrin molecules, both within and between the protofibrils, stabilizing the clot and making it more resistant to mechanical, chemical, and proteolytic actions. Disorders of hemostasis that cause changes to the fibrin gel can lead to bleeding or thrombosis.
The fibrin polymerization reactions are very specific and tightly controlled, proceeding via interaction of the knobs ‘A’ in the center of the fibrin molecule, the GPR amino-terminal sequence of the α chain exposed due to cleavage of fibrinopeptides A, with complementary holes ‘a’, resulting in a half – staggered overlap between molecules (Fig. 1)2,4,5. This is followed by cleavage of fibrinopeptides B, resulting in exposure of the GHR sequence of the β chain comprising knobs ‘B’, which likely interact with holes ‘b’ at the ends of the molecules. The two–stranded protofibrils grow linearly until they reach a length of about 600 nm3,6, at which point protofibrils aggregate laterally to form thicker and branched fibers7. The ‘A’:‘a’ association is sufficient to promote clot formation even when the ‘B’:‘b’ interaction is not present7,8. The polymerization reaction is inhibited by addition of the peptide GPRP, which acts by competing with knobs ‘A’ for holes ‘a’ (Fig. 1)9.
Figure 1. Thrombin cleaves fibrinopeptides from the fibrinogen molecule to create fibrin monomers.
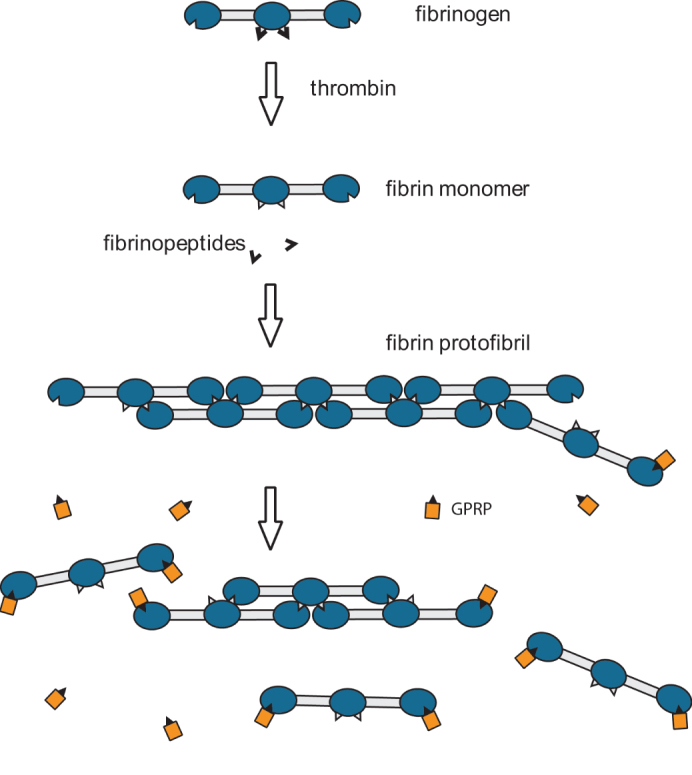
The cleavage of fibrinopeptides allows ‘knob-hole’ interactions between fibrin monomers, causing the fibrin monomers to polymerize and form protofibrils and fibers. The peptide GPRP competes for the ‘holes’ in the fibrin monomers and inhibits the ‘knob-hole’ interactions. If GPRP is present in large enough concentrations, the fibrin aggregates depolymerize and the clot dissolves. GPRP plays the role of a competitive inhibitor of fibrin polymerization, but can also push the reaction in the opposite direction.
It has been tacitly assumed that the knob-hole interactions in fibrin are essentially irreversible. Some fibrin dissolution studies have been carried out, but these were under non-physiological conditions, such as high temperature, or 1.0 M NaBr at acidic pH10,11. A few viscoelastic studies that demonstrate the reversibility of fibrin polymerization in fine clots formed at high pH and ionic strength have been mostly ignored12,13. In the present work, we investigated the reversibility of fibrin polymerization under physiological conditions, using the technique of Fluorescence Recovery After Photobleaching (FRAP), which allows the direct measurement of any turnover that may occur in an equilibrium clot14. These conditions occur in the body during early stages of clotting or thrombosis before extensive Factor XIIIa-induced crosslinking.
Results
A portion of a single fiber of a fibrin clot prepared from fluorescently labeled purified fibrinogen was bleached, and any changes of fluorescence intensity were monitored over time by confocal microscopy (Fig. 2A–D). After bleaching, an increase of fluorescence intensity in the bleached area was observed, which must be due to mobile, labeled fibrin monomers or oligomers that moved to that area, because the bleached molecules do not recover. Our hypothesis to account for this observation is that fibrin monomers and/or oligomers dissociate and associate from fibrin fibers (Fig. 2G, H), as is the case for an equilibrium polymer15. This is a novel observation that goes against the long-standing belief that fibrin clot formation is irreversible. Increase of fluorescence in the bleached area was observed as soon as 1 second after photobleaching and the intensity increased rapidly.
Figure 2. Fluorescence recovery after photobleaching demonstrates turnover of fibrin in a clot.
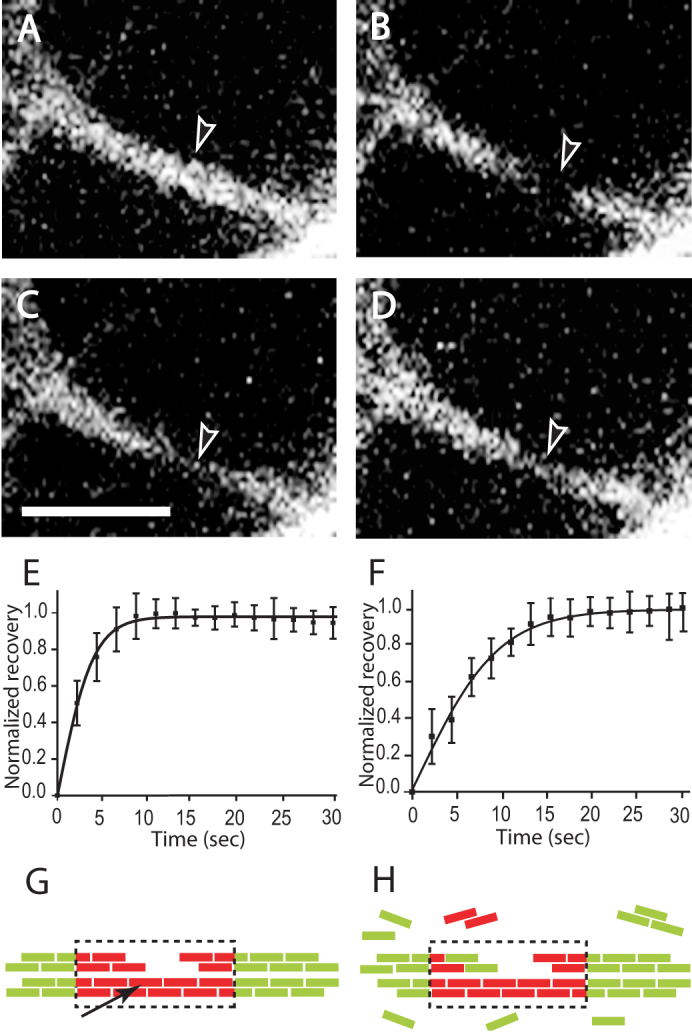
(A–D) Sequence of events during a FRAP experiment monitored by confocal microscopy. Experiments were performed on fibers in a fluorescently labeled fibrin clot made from purified fibrinogen with added thrombin. (A) Before photobleaching. (B) Photobleaching event - the fluorescently labeled fibrin in this part of the fiber has been bleached. (C) 1.6 sec after photobleaching - some recovery of the fluorescence is observed. (D) 10 sec after photobleaching - considerably more fluorescence is observed. Magnification bar = 4 µm. (E, F) Normalized recovery curves that were obtained from FRAP experiments. (E) Fibrin clot made from purified fibrinogen plus thrombin. (F) Fibrin clot made from plasma plus thrombin with addition of factor XIII inhibitor. (G, H) Schematic diagram of FRAP experiment. (G) Bleached portion of a fiber - arrow points to protofibril, broken line represents bleached area, bleached monomers are shown in red color, unbleached monomers shown in green color. (H) Fiber with partially recovered fluorescence.
From analysis of the fluorescence recovery curves for single fibers, we found that the fraction of mobile fibrin structures represented an average of 14.1% ± 5.1% of total fibrin incorporated into a fiber. We also carried out similar FRAP studies of clots from platelet poor plasma in the presence of an inhibitor of Factor XIIIa (SI, p. 1)16 and in these experiments the mobile fraction was 9.8% ± 2.62%. Furthermore, we found that the half time of fluorescence recovery due to fibrin molecules incorporated into the fibers of a platelet poor plasma clot was 5.1 ± 1.1 sec (Fig. 2F), while the half time of fluorescence recovery due to fibrin molecules incorporated into fibers of a fibrin clot made from purified fibrinogen was 2.2 ± 0.5 sec (Fig. 2E). As a control, recovery of fluorescence after photobleaching was not observed for fibers crosslinked by Factor XIIIa, either normally present in platelet-poor plasma or added to purified fibrinogen (SI, p. 1). In other words, immobilization of the fibrin incorporated into fibers prevents any recovery, as expected. Conversely, preventing crosslinking by the addition of FXIII inhibitor to platelet poor plasma before initiation of polymerization made the fibrin mobile (Fig. 2F). Although there is a small concentration of fibrin still in solution in the clot (since clottability is 97%) that might bind to the bleached portion of the fiber, it is not enough to account for the magnitude of recovery. Furthermore, the exponential rate of recovery requires fibrin coming off the fibers, since an off rate of 0 would yield linear recovery (SI, p. 4).
To understand why the amount of fluorescence recovery and the half time of recovery due to fibrin molecules incorporated into fibers is different for a platelet-poor plasma clot and clot made from purified fibrinogen, we analyzed the relationship between fiber diameter and the rate of fluorescence recovery. We found that there is an inverse relationship between the percentage of fluorescence recovery and fiber diameter and also between the rate of recovery after photobleaching and fiber diameter (Fig. 3A, C). The bigger the fiber diameter, the smaller the percentage of recovery, and the bigger the fiber diameter, the slower the rate of recovery. A platelet poor plasma clot has a population of fibers with larger average diameter and hence a lower average percentage and rate of recovery, compared to the population of fibers from a purified fibrin(ogen) clot. Since fibers are three-dimensional structures, and, during a bleaching event, the whole volume, which has the shape roughly of a cylinder, is bleached, these results suggest that the fibrin on the surface of the fiber may exchange differently than fibrin deeper within the fiber. Note that the number of bleached monomers in a portion of a fiber of diameter d and length l is proportional to the fiber volume, or πd2l/4, while the number of monomers with recovered fluorescence is proportional to the surface area, or πdl, if most of the turnover occurs on the surface of the fiber. So, the fraction of monomers with recovered fluorescence should be proportional to the ratio of surface area to volume or 1/d (SI, p. 6–7). With this in mind, we plotted the percentage of fluorescence recovery as a function of 1/d and found that there is a linear relationship between the percentage of fluorescence recovery and the surface to volume ratio (Fig. 3B, SI Fig. S3). Similarly, there is a linear relationship between the rate of fluorescence recovery in the bleached volume and the surface to volume ratio (Fig. 3D and SI, p. 6–7, Fig. S4). The reason for this is as follows. The initial rate of fluorescence recovery is proportional to the product of the concentration of binding sites in the bleached region and the concentration of free monomers in solution (SI, pp. 4–5). If the turnover of fibrin monomers occurs predominantly on the surface of the fibers, then the concentration of binding sites in the bleached region is proportional to 1/d while the concentration of free monomers is fixed, so that their product varies as 1/d.
Figure 3. Relationship between fiber diameter and percentage of fibrin fluorescence recovery after photobleaching and the relationship between fiber diameter and rate of fluorescence recovery after photobleaching.
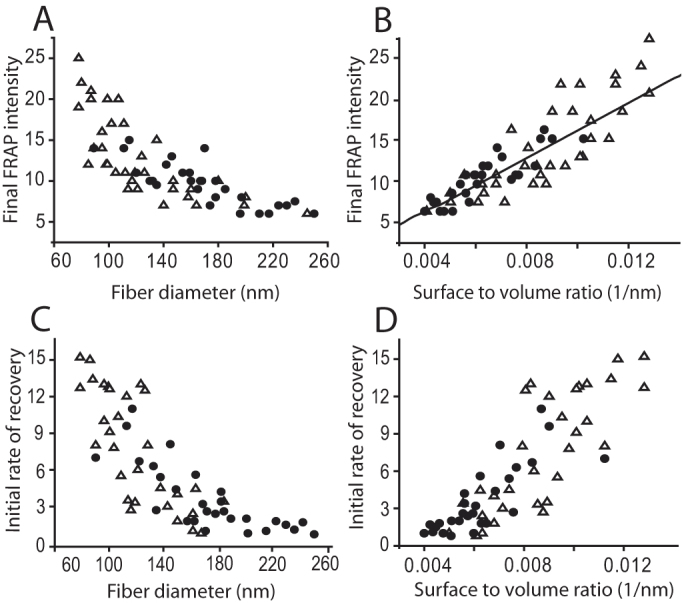
(Δ) Results of a FRAP experiment on a single fiber from clot made from purified fibrinogen and thrombin, (•) results of a FRAP experiment on a single fiber from a platelet poor plasma clot. (A) Percentage of fluorescence recovery of fibrin after photobleaching decreases as fiber diameter increases. (B) Percentage of fluorescence recovery of fibrin after photobleaching varies linearly with the surface to volume ratio - data was fit to a straight line by a least squares method (SI p. 6). (C) The initial rate of fluorescence recovery of fibrin after photobleaching varies inversely with the fiber diameter. (D) The initial rate of fluorescence recovery of fibrin after photobleaching varies linearly with the surface to volume ratio.
To investigate further the mechanism of fluorescence recovery after photobleaching, we perfused a fibrin clot with different concentrations of the peptide GPRP, which competes with the fibrin molecules for holes ‘a’9. We found that perfusion of 0.1 mM GPRP dissolved a preformed fibrin clot, while there were no changes in the structure of a clot crosslinked by FXIIIa. Experiments were carried out with perfusion of lower concentrations of GPRP. After perfusion with 0.01 mM GPRP through the fibrin clot, the percentage of fluorescence recovery was 6.53% ± 1.7%, versus 14.1% ± 5.1% without GPRP. These findings led us to investigate the process in detail. We hypothesized that the decrease of fluorescence recovery after perfusion of GPRP through the fibrin clot could be due to an increase of dissociation of fibrin molecules and/or oligomers from fibers, because the peptide competes with fibrin knobs for the holes.
Analyses of the FRAP recovery curves showed that the concentration of the bound complex (Ceq) consisting of fibrin monomers bound to sites on the fiber (SI, p. 2) decreased with increasing GPRP concentration (Fig. 4A), while the off rate koff was not much affected (Fig. 4B). Moreover, there was a linear relationship between Ceq and the surface to volume ratio, which suggests that dissociation is more likely near the surface of fiber, and so there are more potential binding sites for GPRP at and near the surface, as we have discussed above. Inhibition of these sites and those on the freely floating oligomers by GPRP pushes the equilibrium toward dissociation rather than association reactions, which is consistent with GPRP being a competitive inhibitor of fibrin polymerization. This finding shows that the turnover of fibrin structures from fibers involves knobs ‘A’:holes ‘a’ interactions and that it depends on the concentration of binding sides.
Figure 4. Effects of GPRP, a peptide mimicking the knobs ‘A’ and competing with them for holes ‘a.’ (A) Variation of the off rate and the equilibrium concentration of bound complex as a function of the GPRP concentration and the surface to volume ratio.
The equilibrium concentration of bound complex of fibrin monomers and binding sites on the fibers varies linearly with the surface to volume ratio. ( ) FRAP recovery data obtained from experiments when no GPRP was perfused through the clots; (
) FRAP recovery data obtained from experiments when no GPRP was perfused through the clots; (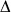 ) FRAP recovery data obtained from experiments when 0.001 mM GPRP was perfused through the clots; (
) FRAP recovery data obtained from experiments when 0.001 mM GPRP was perfused through the clots; ( ) FRAP recovery data obtained from experiments when 0. 01 mM GPRP was perfused through the clots; (B) The off rate is essentially the same for experiments with no GPRP perfused (left), 0.001 mM GPRP perfused (middle), or 0.01 mM GPRP perfused (right). (C–F). Fibrin clot network examined by confocal light microscopy and scanning electron microscopy. (C) Fibrin network before perfusion of GPRP examined by confocal microscopy. Magnification bar = 10 µm. (D) Fibrin network after perfusion of GPRP examined by confocal microscopy - white arrowheads indicate fluorescence from fibrin-related structures that have dissociated from the fibers. (E) Fibrin network before perfusion with GPRP examined by scanning electron microscopy. Magnification bar = 2 µm. (F) Fibrin network after perfusion of 0.01 mM GPRP examined by scanning electron microscopy - arrowheads indicate fiber ends. (G, H) Transmission electron microscope images of structures in the supernatant after perfusion of fibrin clot with 0.01 mM GPRP. (G) Area with monomers indicated by thin arrowheads. Magnification bar = 50 nm. (H) Area with oligomers indicated by wide arrowheads.
) FRAP recovery data obtained from experiments when 0. 01 mM GPRP was perfused through the clots; (B) The off rate is essentially the same for experiments with no GPRP perfused (left), 0.001 mM GPRP perfused (middle), or 0.01 mM GPRP perfused (right). (C–F). Fibrin clot network examined by confocal light microscopy and scanning electron microscopy. (C) Fibrin network before perfusion of GPRP examined by confocal microscopy. Magnification bar = 10 µm. (D) Fibrin network after perfusion of GPRP examined by confocal microscopy - white arrowheads indicate fluorescence from fibrin-related structures that have dissociated from the fibers. (E) Fibrin network before perfusion with GPRP examined by scanning electron microscopy. Magnification bar = 2 µm. (F) Fibrin network after perfusion of 0.01 mM GPRP examined by scanning electron microscopy - arrowheads indicate fiber ends. (G, H) Transmission electron microscope images of structures in the supernatant after perfusion of fibrin clot with 0.01 mM GPRP. (G) Area with monomers indicated by thin arrowheads. Magnification bar = 50 nm. (H) Area with oligomers indicated by wide arrowheads.
In agreement with this result, our confocal and electron microscopy images showed dramatic effects on clot structure of perfusion of GPRP through uncrosslinked clots. At the light microscopy level, optically dense areas were observed around fibers, which suggests the presence of some fibrin-related structures that have been dissociated from the fibers (Fig. 4C, D). At the scanning electron microscopy level, many free fiber ends were observed, most likely due to shifting of the equilibrium toward dissociation, and whole pieces of fibers dissolving (Fig. 4E, F). The remaining parts of the fibers associate laterally with adjacent fibers, yielding a clot with thicker fibers (Fig. 4F). We found that perfusion of GPRP increased the concentration of fibrin in the supernatant of the fibrin clot, 16.3%, versus control 2.2%, consistent with the presence of soluble fibrin products. Examination by transmission electron microscopy of the supernatant after perfusion of the fibrin clot with GPRP revealed the nature of the soluble fibrin material present. The majority of structures were monomers but oligomers and protofibrils were present as well (Fig. 4G, H). In fact, it is likely that the structures that dissociate initially are large oligomers, but dissociation into smaller structures and monomers continues during the several minutes necessary for preparation of the samples for microscopy since more surfaces and protofibril ends are created due to dissociation of oligomers. All of these observations suggest that monomers as well as larger structures dissociate from fibrin fibers.
Discussion
FRAP experiments demonstrate turnover of fibrin oligomers and monomers from fibers in a pre-formed clot, so that blood clotting is a reversible reaction. Remarkably, a peptide representing the knobs involved in fibrin polymerization can compete for the holes and dissolve a preformed fibrin clot. Lower concentrations of this peptide increase the fraction of soluble oligomers, with striking rearrangements in clot structure. Thus, fibrin is an equilibrium polymer.
Both the extent of recovery and the rate vary as a function of the surface to volume ratio of the fibers, indicating that fibrin monomers or larger structures located on or near the surface of the fibers are more likely to dissociate and re-associate. There are two potential mechanisms that may account for this observation. The first and simplest explanation is that fibrin structures that are located on the outside of the fiber will have fewer steric restrictions to dissociation and association than those inside the fiber, which could imply that most structures dissociating are oligomers rather than monomers, since there are large spaces within fibers that would permit easy diffusion of monomers and other small structures. Second, fibrin fibers have a twisted structure due to twisted protofibrils that laterally aggregate, so that an increase of fiber diameter yields more tension on the surface and compression near the center of the fibers17. This is related to the idea of frustration due to chirality’18 in the packing of twisted filaments where the diameter of the packed assembly is self-limited by the competition between the decrease of free energy due to aggregation of protofibrils and the increase of free energy due to elastic deformation of the twisted protofibrils. Therefore, binding energy considerations make fibrin structures on the surface of the fiber more likely to dissociate and re-associate.
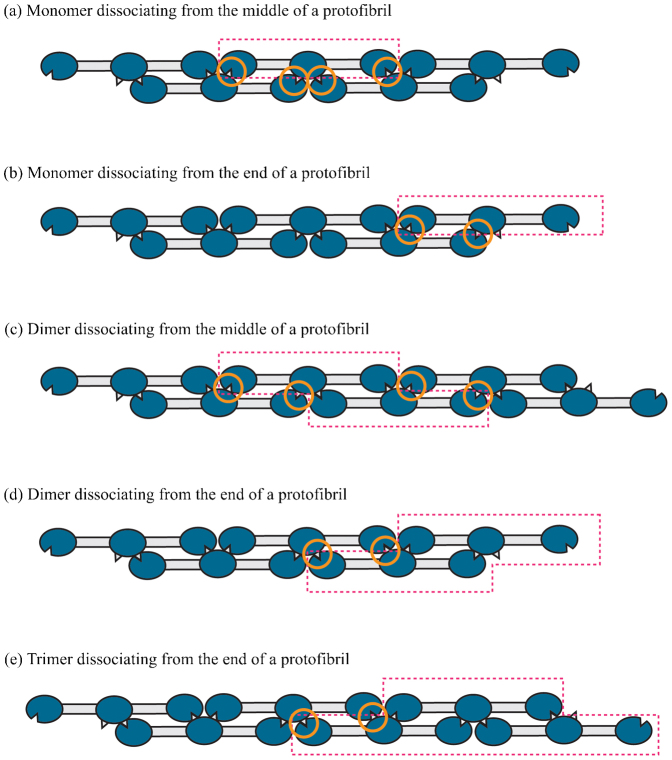
The turnover of fibrin monomers or oligomers from the fiber requires dissociation of several non-covalent bonds, potentially including ‘A’:‘a’, ‘B’:‘b’, end-to-end, and those involved in lateral aggregation1. Dissociation of a monomer from the middle of a protofibril would require breaking four ‘A’:‘a’ bonds, in addition to the weaker bonds, whereas dissociation of a monomer from a protofibril end only requires breaking two ‘A’:‘a’ bonds (Fig. 5). Therefore, it is considerably more likely for dissociation to occur from protofibril ends on the surface of the fibers (Fig. 6). Although protofibril ends in fibers have not been demonstrated, they are likely from what we know of mechanisms of fibrin polymerization19,20. Furthermore, dissociation of oligomers also only requires breaking two ‘A’:‘a’ bonds, in addition to the weaker bonds. Thus, the only penalty for dissociation of larger structures is an increased number of weaker bonds and slower diffusion rate, consistent with our observation of dissociated oligomers.
Figure 5. Bond breaking in the depolymerization of protofibrils.
The dissociating monomers, dimers, etc. are shown in boxes bounded by dashed lines. Four bonds (shown in orange circles) need to be broken if a monomer (a) or a dimer (c) is to dissociate from the middle of a protofibril. Only two bonds need to be broken if a monomer (b), dimer (d) or trimer (e) is to dissociate from the end of a protofibril.
Figure 6. Schematic diagram of the turnover of fibrin monomers or oligomers from the fiber.
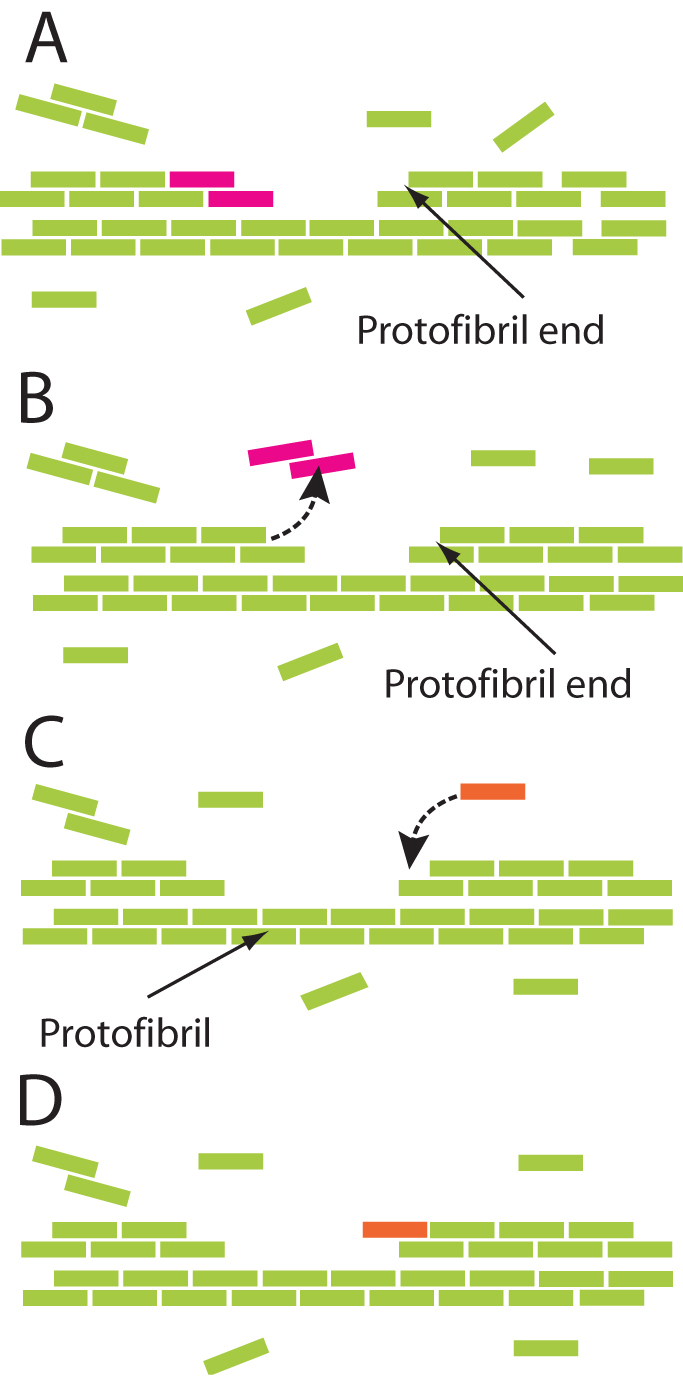
Fibrin monomers are represented schematically as colored rods assembled in a half-staggered manner into two-stranded protofibrils. (A) Fiber in equilibrium with oligomers in solution. The solid arrow indicates a protofibril end. Purple dimer at the end of another protofibril is about to dissociate. (B) Purple dimer goes into solution as shown by the dashed arrow. (C) Association of monomer to fiber. The solid arrow indicates a protofibril. Dashed arrow in (C) shows the path of association of the orange monomer. Both monomers and oligomers can associate and dissociate. (D) Fiber with orange monomer in equilibrium (as in (A)) with oligomers in solution.
The implications of this research are that clots and thrombi appear to be much more dynamic structures than has been previously believed. The observed modulation of clot structure in vitro suggests that in vivo clots and thrombi can respond to the changing environmental conditions surrounding them. Furthermore, the flow of blood will carry away any fibrin structures that dissociate, increasing the rate of turnover, which is more relevant for arterial blood flow with higher shear rates. However, it is necessary to remember that crosslinking by Factor XIIIa prevents turnover of fibrin, so any of these effects are more likely to be important early in the formation of clots or thrombi than later. Nevertheless, the presence of high concentrations of fibrin degradation products (containing either knobs or holes) in disseminated intravascular coagulation could act as competitors for the knob-hole interactions holding the clot together and induce some transient instability or depolymerization. These findings may also be relevant to in vivo embolization, which could be a consequence of the reversibility of polymerization. Furthermore, such modulation of clot structure could be a target for potential therapeutic intervention, such as dissolution of thrombi and prevention of obstruction.
Methods
Fluorescence recovery after photobleaching (FRAP)
A FRAP technique was used to discover and characterize a mobile fraction of fibrin in clots and the kinetic parameters of fibrin turnover. FRAP was examined for two types of fibrin clots, one made from purified fibrinogen and another made from platelet-poor plasma. The former represents a simpler system, while the latter is more physiological.
To make clots of purified fibrinogen, polymerization was carried out in 50 mM Tris-HCl, 140 mM NaCl, pH 7.4, 2.5 mM CaCl2 by the mixing of 1.5 mg/ml purified human plasminogen-free fibrinogen (Hyphen BioMed, Andresy, France) and 0.1 U/ml thrombin (American Diagnostica Inc, Stamford, CT), final concentrations. After all components were mixed, the sample was placed for 30 min in a chamber with constant high humidity.
For platelet-poor plasma clots, 150 μl of pooled platelet-poor plasma from 6 healthy volunteers with 10% of total volume of fibrinogen fluorescently labeled as described below was clotted for 30 minutes by recalcification with 26 mM CaCl2 and addition of 0.5 U/ml thrombin in 50 mM Tris-HCl, 140 mM NaCl, pH 7.4. Clots were made in the presence of 1 mM 1,3-dimethyl-4,5-diphenyl-2[2(oxopropyl)thio]imidazolium trifluoromethyl-sulfonate, a highly specific active-site-directed inhibitor of Factor XIII16, to prevent crosslinking. The sample was prepared by mixing CaCl2 with thrombin and adding it to the plasma. After all components were mixed, the sample was inserted into a glass chamber made from a microscope slide and a cover slip separated by a spacer made of two strips of double-stick tape, such that the thickness of the chamber was ~350 μm. To carry out photobleaching experiments, Alexa 488 labeled fibrinogen (Invitrogen, Carlsbad, CA) with an average of 6 molecules of dye per molecule of protein was added to the polymerization mixture at a final concentration of 0.03 mg/ml for both types of clots.
FRAP experiments were carried out with a Zeiss 510 LSM confocal microscope, using the LSMacquisition software (Carl Zeiss, Thornwood, NY), equipped with a 63 X 1.4 NA Plan Apo objective lens, and an argon ion 488 nm laser. The gain and offset of the detector are the most critical parameters to set up before collecting FRAP images. The offset was set to see background pixels slightly higher than zero to catch all possible changes in the sample signal. The detector gain was set to a level such that very few or no pixels were saturated. Areas of about 0.3 × 0.9 × 0.3 μm were photobleached with 100% output of the 488 nm laser for 20 µseconds/pixel. Five pre-bleached images were collected, and those images were used as a reference for the steady-state distribution of the fluorescent molecules for FRAP analyses. Images of fluorescence recovery were collected at 2% of excitation laser power.
Analysis of FRAP time – lapse images
For each FRAP experiment, fluorescence intensity measurements were performed by measuring the total intensity in the region of interest by using Image J software (NIH, Bethesda, MD). The raw intensity measurements were imported into Excel (Microsoft, Redmond, WA) for further analyses, including background subtraction, normalization and plotting of fluorescence changes as a function of time. Those data were fitted by using Origin version 7.5 software (OriginLab Corp., Northampton, MA) and kinetic parameters, such as the half-time of recovery and mobile fraction of fibrin were extracted from the curves.
Effect of crosslinking on FRAP of fibrin
To examine the effect of crosslinking on FRAP of fibrin, clots were made from purified fibrinogen with added thrombin as described above in the presence of 0.34 mg/ml Factor XIII (American Diagnostica Inc, Stamford, CT). FRAP experiments were carried out as described, but in the presence of FXIII. The same results were also obtained with physiological concentrations of FXIII (22 μg/ml).
Experiments on the effects of the peptide GPRP on clot structure
Two concentrations of GPRP were used for perfusion through the fibrin clot, 0.01 mM and 0.001 mM, in TBS buffer (50 mM Tris-HCl, 140 mM NaCl, pH 7.4). The fibrin clot was made from purified fibrinogen as described above and 100 μl of peptide was perfused for 10–15 min. The effects of perfusion of the peptide on clot structure were monitored in the Zeiss 510 LSM confocal microscope. After perfusion, FRAP experiments were performed, data were collected and analyzed as described above.
Measurement of the soluble fibrin concentration in the clot after perfusion of GPRP
A 100 µl aliquot of 0.01 mM of GPRP was perfused through the fibrin clot. After perfusion, the clot was removed with a glass rod, and dissolved in 0.1 M NaOH. The remaining liquid after removal of the fibrin clot was collected and put into 0.1 M NaOH as well. The concentration of protein in the clot and the liquid was calculated from measurements of the optical density at 280 nm.
Transmission electron microscopy
Fibrin clots were made as described above and 0.01 mM GPRP was perfused through them. The first 5 fractions of perfusate of 20 µl each were collected. Each fraction was immediately diluted with a volatile buffer, 0.05 M ammonium formate, pH 7.4, with 35% glycerol final concentration21. After spraying diluted samples onto freshly cleaved mica, they were rotary-shadowed with tungsten in a vacuum evaporator (Denton Vacuum, Cherry Hill, NJ). Specimens were examined in a FEI/Philips 400 electron microscope (FEI, Hillsboro, OR). Images were taken at 80 kV with a magnification of 36,000 ×. More than 10 micrographs were analyzed for each fraction.
Scanning electron microscopy
To determine the effects of the peptide GPRP on clot structure, fibrin clots after perfusion were examined by scanning electron microscopy. Clots prepared as described above were perfused with 0.01 mM GPRP for 20 min. Both control and GPRP perfused clots were fixed in 2% glutaraldehyde, dehydrated in ethanol, dried with hexamethyldisilazane, and sputter coated with gold-palladium22. Samples were examined in a Philips/FEI XL20 scanning electron microscope (FEI, Hillsboro, OR).
Author Contributions
I.N.C., P.K.P. and J.W.W. designed experiments and analyzed data. I.N.C. and C.N. performed experiments. P.K.P. did all of the theoretical analysis. The manuscript was written by I.N.C., P.K.P. and J.W.W.
Supplementary Material
Supplementary Information
Acknowledgments
The authors thank Drs. Ritesh Agarwal, Yale Goldman, and Rustem I. Litvinov for their comments and valuable discussions in preparing this manuscript. We thank Dr. Klara Stefflova for synthesis of the Factor XIII inhibitor. We acknowledge the support of NIH grants HL030954 and HL090774 and NSF grant CMMI-1066787 and the Nano/Bio Interface Center at the University of Pennsylvania through grant NSEC DMR08-32802.
References
- Weisel J. W. Fibrinogen. and fibrin. Adv Protein Chem 70, 247–99 (2005). [DOI] [PubMed] [Google Scholar]
- Doolittle R. F. Fibrinogen and fibrin. Annu Rev Biochem 53, 195–229 (1984). [DOI] [PubMed] [Google Scholar]
- Hantgan R. R. & Hermans J. Assembly of fibrin. A light scattering study. J Biol Chem 254, 11272–81 (1979). [PubMed] [Google Scholar]
- Litvinov R. I., Gorkun O. V., Owen S. F., Shuman H. & Weisel J. W. Polymerization of fibrin: specificity, strength, and stability of knob-hole interactions studied at the single-molecule level. Blood 106, 2944–51 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budzynski A. Z. Fibrinogen and fibrin: biochemistry and pathophysiology. Crit Rev Oncol Hematol 6, 97–146 (1986). [DOI] [PubMed] [Google Scholar]
- Fowler W. E., Erickson H. P., Hantgan R. R., McDonagh J. & Hermans J. Cross-linked fibrinogen dimers demonstrate a feature of the molecular packing in fibrin fibers. Science 211, 287–9 (1981). [DOI] [PubMed] [Google Scholar]
- Weisel J. W. The electron microscope band pattern of human fibrin: various stains, lateral order, and carbohydrate localization. J Ultrastruct Mol Struct Res 96, 176–88 (1986). [DOI] [PubMed] [Google Scholar]
- Mosesson M. W. et al. Studies on the ultrastructure of fibrin lacking fibrinopeptide B (beta-fibrin). Blood 69, 1073–81 (1987). [PubMed] [Google Scholar]
- Laudano A. P. & Doolittle R. F. Studies on synthetic peptides that bind to fibrinogen and prevent fibrin polymerization. Structural requirements, number of binding sites, and species differences. Biochemistry 19, 1013–9 (1980). [DOI] [PubMed] [Google Scholar]
- Donnelly T. H., Laskowski M. Jr, Notley N. & Scheraga H. A. Equilibria in the fibrinogen-fibrin conversion. II. Reversibility of the polymerization steps. Arch Biochem Biophys 56, 369–87 (1955). [DOI] [PubMed] [Google Scholar]
- Endres G. F. & Scheraga H. A. Equilibria in the fibrinogen--fibrin conversion. 8. Polymerization of acceptor-modified fibrin monomer. Biochemistry 7, 4219–26 (1968). [DOI] [PubMed] [Google Scholar]
- Bale M. D., Muller M. F. & Ferry J. D. Effects of fibrinogen-binding tetrapeptides on mechanical properties of fine fibrin clots. Proc Natl Acad Sci U S A 82, 1410–3 (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu A., Schindlauer G. & Ferry J. D. Interaction of the fibrinogen-binding tetrapeptide Gly-Pro-Arg-Pro with fine clots and oligomers of alpha-fibrin; comparisons with alpha beta-fibrin. Biopolymers 27, 775–88 (1988). [DOI] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E. & Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J 16, 1055–69 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson G., Howard J. & Hammett A. L. Use and production of solid sawn timbers in the United States. Forest Products Journal 51, 23–28 (2001). [Google Scholar]
- Freund K. F. et al. Transglutaminase inhibition by 2-[(2-oxopropyl)thio]imidazolium derivatives: mechanism of factor XIIIa inactivation. Biochemistry 33, 10109–19 (1994). [DOI] [PubMed] [Google Scholar]
- Weisel J. W., Nagaswami C. & Makowski L. Twisting of fibrin fibers limits their radial growth. Proc Natl Acad Sci U S A 84, 8991–5 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grason G. M. Braided bundles and compact coils: the structure and thermodynamics of hexagonally packed chiral filament assemblies. Phys Rev E Stat Nonlin Soft Matter Phys 79, 041919 (2009). [DOI] [PubMed] [Google Scholar]
- Houser J. R. et al. Evidence that alphaC region is origin of low modulus, high extensibility, and strain stiffening in fibrin fibers. Biophys J 99, 3038–47 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falvo M. R., Gorkun O. V. & Lord S. T. The molecular origins of the mechanical properties of fibrin. Biophys Chem 152, 15–20 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veklich Y. I., Gorkun O. V., Medved L. V., Nieuwenhuizen W. & Weisel J. W. Carboxyl-terminal portions of the alpha chains of fibrinogen and fibrin. Localization by electron microscopy and the effects of isolated alpha C fragments on polymerization. J Biol Chem 268, 13577–85 (1993). [PubMed] [Google Scholar]
- Weisel J. W., Nagaswami C., Vilaire G. & Bennett J. S. Examination of the platelet membrane glycoprotein IIb-IIIa complex and its interaction with fibrinogen and other ligands by electron microscopy. J Biol Chem 267, 16637–43 (1992). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information