Abstract
Loss of polarity and disruption of cell junctions are common features of epithelial-derived cancer cells, and mounting evidence indicates that such defects have a direct function in the pathology of cancer. Supporting this idea, results with several different human tumor viruses indicate that their oncogenic potential depends in part on a common ability to inactivate key cell polarity proteins. For example, adenovirus (Ad) type 9 is unique among human Ads by causing exclusively estrogen-dependent mammary tumors in experimental animals and in having E4 region-encoded open reading frame 1 (E4-ORF1) as its primary oncogenic determinant. The 125-residue E4-ORF1 protein consists of two separate protein-interaction elements, one of which defines a PDZ domain-binding motif (PBM) required for E4-ORF1 to induce both cellular transformation in vitro and tumorigenesis in vivo. Most notably, the E4-ORF1 PBM mediates interactions with a selected group of cellular PDZ proteins, three of which include the cell polarity proteins Dlg1, PATJ and ZO-2. Data further indicate that these interactions promote disruption of cell junctions and a loss of cell polarity. In addition, one or more of the E4-ORF1-interacting cell polarity proteins, as well as the cell polarity protein Scribble, are common targets for the high-risk human papillomavirus (HPV) E6 or human T-cell leukemia virus type 1 (HTLV-1) Tax oncoproteins. Underscoring the significance of these observations, in humans, high-risk HPV and HTLV-1 are causative agents for cervical cancer and adult T-cell leukemia, respectively. Consequently, human tumor viruses should serve as powerful tools for deciphering mechanisms whereby disruption of cell junctions and loss of cell polarity contribute to the development of many human cancers. This review article discusses evidence supporting this hypothesis, with an emphasis on the human Ad E4-ORF1 oncoprotein.
Keywords: virus, polarity, tumor suppressor, PDZ, migration
Introduction
Cancer is a leading cause of death in developed countries, and viruses are associated with an estimated 15–20% of human malignancies worldwide (Flint et al., 2000). Research directed at determining how viruses promote tumors in experimental animals has also contributed greatly to our understanding of molecular events associated with human cancer (Flint et al., 2000). Illustrating this fundamental principle, studies of RNA tumor viruses led to the seminal concept of the oncogene, whereas studies of DNA tumor viruses led not only to the identification of the p53 tumor suppressor protein, but also proved instrumental in deciphering functions for the pRb tumor suppressor protein. Thus, tumor viruses are proven powerful tools for revealing mechanisms responsible for the development of all human cancers. This article reviews accumulating evidence suggesting that blocking functions of key cell polarity proteins represents an important new theme among tumorigenic human viruses.
Human adenovirus type 9
The E1 region codes for the oncogenic determinants of most human adenoviruses
Adenovirus (Ad) is a nonenveloped virus with an approximately 36-kb linear double-stranded DNA genome. The 51 different serotypes of human Ads are organized into six subgroups (A through F) on the basis of physical and immunological criteria and cause acute infections of the respiratory and gastrointestinal tracts, as well as the eye (Green et al., 1979; Horwitz, 2001; Shenk, 2001). Although lacking a recognized association with human cancers, all human Ads can transform cultured rodent cells (Shenk, 2001), and a subset of the viruses, including all subgroup A and B Ads and two subgroup D Ads, also induces tumors in experimentally infected rodents (Graham, 1984). As opposed to productive lytic replication in human cells, Ad instead causes an abortive, nonpermissive infection in rodent cells (Graham, 1984). Thus, in Ad-induced rodent tumors or transformed cells, all or part of the Ad genome is maintained in the host cell by rare non-homologous recombination events occurring at random chromosomal integration sites (Graham, 1984). Expression of the viral E1 region, coding for the E1A and E1B oncogenes, is both necessary and sufficient for transformation and tumorigenesis induced by subgroup A and B Ads (Graham, 1984; Stillman, 1986; Shenk and Flint, 1991). E1A alone immortalizes cells (Houweling et al., 1980), whereas E1B alone lacks transforming potential (Van den Elsen et al., 1983). Together, however, E1A and E1B cooperate to oncogenically transform primary rodent cells or established rodent lines (Graham, 1984). The tumorigenic potential of E1A and E1B stems in part from their ability to bind and inactivate the pRb and p53 tumor suppressor proteins, respectively (Levine, 1990; Dyson et al., 1992).
Unique tumorigenic properties of human adenovirus type 9
Adenovirus type 9 (Ad9) is a member of the subgroup D Ads (Green et al., 1979), some of which cause epidemic outbreaks of keratoconjunctivitis, a painful and highly contagious eye infection that may lead to corneal opacities (Horwitz, 2001). Following infection of newborn rats, however, Ad9 is tumorigenic (Ankerst et al., 1974; Javier et al., 1991). Unlike subgroup A and B Ads that induce undifferentiated sarcomas in both male and female animals (Trentin et al., 1962), Ad9 instead elicits exclusively estrogen-dependent mammary tumors in female rats (Ankerst et al., 1974; Ankerst and Jonsson, 1989; Javier et al., 1991). These females develop multiple tumors in several different mammary glands by 3-months post-infection, whereas males fail to develop tumors of any kind (Javier et al., 1991). Similar to other Ad-induced tumors, the Ad9 genome is found integrated into the chromosomal DNA of mammary tumor cells and has a copy number varying from one to multiple genomes per cell (Javier et al., 1991). As each mammary tumor exhibits a unique viral DNA integration site, the neoplasms are monoclonal (Javier et al., 1991). Therefore, Ad9 is distinct from other tumorigenic Ads in generating only estrogen-dependent mammary tumors in animals.
Ad9-induced mammary tumors
Several histologically distinct types of mammary tumors arise in Ad9-infected animals, with fibroadenoma occurring most frequently (Javier et al., 1991). Fibroadenoma, the most prevalent benign breast tumor of young women (Cotran et al., 1994), has a sparse cellular stroma embedded in a dense extracellular matrix and contains varying amounts of glandular mammary epithelium. Phyllodes tumor and solid sarcoma are two less common types of mammary tumor induced by Ad9. Phyllodes tumor histologically resembles fibroadenoma, yet the stroma shows higher cellularity or is malignant, whereas solid sarcoma is a malignant mammary tumor composed of highly atypical stromal cells with significant mitotic activity but devoid of glandular epithelial components. Phyllodes tumor and solid sarcoma of the breast are also occasionally observed in women (Javier and Shenk, 1996).
By in situ hybridization, Ad9 mRNAs are detected in fibroblasts of mammary fibroadenomas or in myoepithelial cells of mammary phyllodes tumors and solid sarcoma (Javier et al., 1991), indicating that the latter two tumors do not arise from fibroadenomas by malignant conversion. Studies of human breast fibroadenoma and phyllodes tumor are consistent with this observation (Mechtersheimer et al., 1990). Given that Ad9-induced mammary solid sarcoma histologically resembles malignant phyllodes tumor without the epithelial component, it has been proposed that the solid sarcomas likely derive from malignant phyllodes tumors through loss of the glandular epithelial component (Javier et al., 1991). In addition, the development of Ad9-induced mammary tumors strictly depends on estrogen (Javier et al., 1991). The fact that Ad9-induced mammary tumors express estrogen receptor mRNA (Javier et al., 1991) suggests a direct function for estrogen in stimulating proliferation of tumor cells. It is possible that estrogen receptor, which activates transcription in the nucleus and cell signaling from the plasma membrane (Levin, 2002; Luconi et al., 2002; McDonnell and Norris, 2002), cooperates with Ad9 gene functions to oncogenically transform mammary cells in vivo.
The Ad9 E4-ORF1 oncoprotein
E4-ORF1 is the primary oncogenic determinant of Ad9
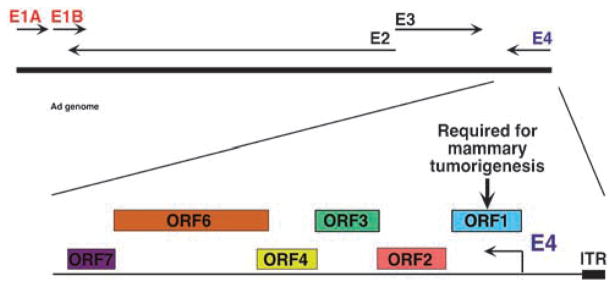
Contrary to Ad9, most subgroup D Ads (for example, Ad26) fail to generate tumors of any kind in rats (Javier et al., 1992). Exploiting this observation, analyses of Ad9-Ad26 hybrid viruses led to the surprising discovery of an Ad tumorigenic determinant genetically mapping to the viral E4 region rather than to the viral E1 region (Javier et al., 1992). The Ad E4 region is a complex transcription unit containing six different open reading frames (ORFs) (Figure 1) (Herisse et al., 1981; Hogenkamp and Esche, 1990; Javier and Shenk, 1996). Proteins encoded by E4 region ORFs regulate viral and cellular gene expression, viral DNA replication and host cell shutoff (Halbert et al., 1985; Cutt et al., 1987; Bridge and Ketner, 1989, 1990; Huang and Hearing, 1989a, b; Muller et al., 1992; Stracker et al., 2002) and also inactivate not only p53 but also Mre11, a component of the double-stranded DNA break–repair pathway (Flint and Gonzalez, 2003; Endter and Dobner, 2004; Weitzman, 2005; Weitzman and Ornelles, 2005). Several E4 proteins, including E4 region-encoded ORF1 (E4-ORF1) (Javier, 1994), E4-ORF3 (Nevels et al., 1999b), E4-ORF6 (Moore et al., 1996; Nevels et al., 1997, 1999a, 2000a, 2001) and E4-ORF6/7 (Yamano et al., 1999), also have the capacity to transform cultured cells. The transforming activities of E4-ORF6 and E4-ORF6/7 stem from an ability to inactivate p53 (Dobner et al., 1996) or to stimulate the activity of the E2F protooncogene, respectively (Huang and Hearing, 1989b; Schaley et al., 2000).
Figure 1.
The Ad genome and early transcription units with enlarged E4 region showing six open reading frames (ORFs). E4 region-encoded open reading frame (E4-ORF) 6/7 (not shown) consists of E4-ORF7 fused to the amino-terminal region of E4-ORF6.
The major E4 region oncogenic determinant of Ad9 was found to be the E4-ORF1 gene. For instance, disruption of E4-ORF1, but not E1A or E1B (Thomas et al., 1999, 2001a), abolishes the ability of Ad9 to generate mammary tumors in rats, despite the fact that Ad9 E4-ORF1 mutant viruses display no appreciable replication defects in permissive human cell lines (Javier, 1994). The E4-ORF1 protein is expressed in all Ad9-induced mammary tumors and, among the six isolated Ad9 E4 region ORFs, only E4-ORF1 is capable of inducing transformed foci on cells (Javier, 1994). Such E4-ORF1-transformed cells also form tumors in immunocompetent animals and display morphological changes, anchorage-independent growth and elevated saturation densities (Weiss et al., 1996). Furthermore, substitution of the E1 region in otherwise nontumorigenic subgroup C Ad5 with an Ad9 E4-ORF1 expression cassette confers a tumorigenic phenotype virtually identical to that of Ad9 (Thomas et al., 2001a), indicating that E4-ORF1 likewise controls the oncogenic tropism of Ad9 for mammary tissue in vivo. Although the sequence of the subgroup D Ad9 E4-ORF1 protein shares 50% identity and 70% similarity with E4-ORF1 proteins encoded by subgroup A–C Ads (Weiss et al., 1997), the Ad9 E4-ORF1 protein is uniquely tumorigenic (Javier, 1994). In transformed cells, the 14-kDa Ad9 E4-ORF1 protein accumulates in cytoplasmic punctae (Weiss et al., 1996) representing membrane vesicles (Chung et al., 2007), and some protein also localizes at the plasma membrane (Frese et al., 2003, 2006).
Evidence supports the idea that Ad E4-ORF1 genes evolved from a cellular dUTP pyrophosphatase (dUTPase) gene (Weiss et al., 1997), which codes for an essential enzyme of nucleotide metabolism. Polypeptides encoded by dUTPase genes are comparable in length to E4-ORF1 proteins and form homo-trimeric enzymes that hydrolyse dUTP to prevent detrimental incorporation of this nucleotide into replicating cellular DNA (Mol et al., 1996). Although results indicate that E4-ORF1 and dUTPase have functionally diverged (Weiss et al., 1997), the two polypeptides are predicted to share a conserved protein fold, and both form a homo-trimer dependent on a related trimerization element (Chung et al., 2008). E4-ORF1 differs from dUTPase, however, by additionally existing as a monomer in cells (Chung et al., 2008).
Ad9 E4-ORF1 consists of two separate protein-interaction elements: domain 2 and a PDZ domain-binding motif
The isolation of Ad9 E4-ORF1 mutants expressed at wild-type levels yet unable either to transform cultured cells (Lee et al., 1997; Weiss and Javier, 1997; Chung et al., 2007) or to promote mammary tumors in the context of Ad9 virus (Thomas et al., 2001a) proved instrumental for identification of crucialE4-ORF 1 functional elements. The residues altered in transformation- defective E4-ORF1 mutants cluster either within a central region of the linear E4-ORF1 polypeptide (G40A, V41A, D65A, L89Q, F91S, H93A, F97A) or at the extreme carboxyl-terminus (T123D, V125A, IIIA) (Figure 2). The central and carboxyl-terminal mutations disrupt the association of E4-ORF1 with distinct subsets of cellular factors (Weiss and Javier, 1997; Chung et al., 2007), indicating that E4-ORF1 is composed of two separate crucial protein-interaction elements.
Figure 2.
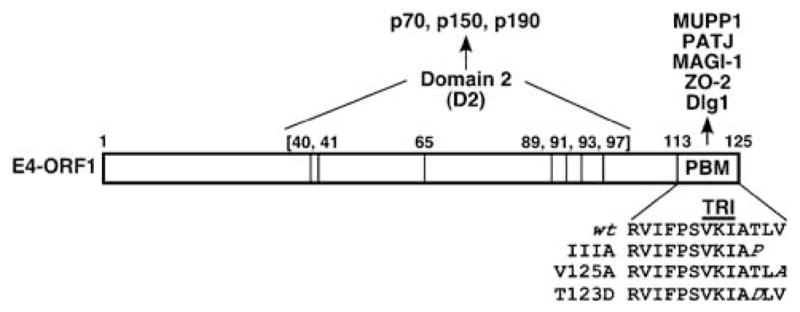
Adenovirus type 9 (Ad9) E4 region-encoded open reading frame 1 (E4-ORF1) consists of two protein-interaction elements, designated domain 2 (D2) and PDZ domain-binding motif (PBM). Centrally located D2 and the carboxyl-terminal PBM are defined by mutants G40V, V41A, D65A, L89Q, F91S, H93A and F97A or mutants IIIA, T123D and V125A, respectively. CrucialD2 residues (bracketed numbers) and crucial PBM residues (italicized) are shown. Cellular targets that bind D2 or PBM are also indicated. The E4-ORF1 trimerization (TRI) element overlaps the PBM sequence.
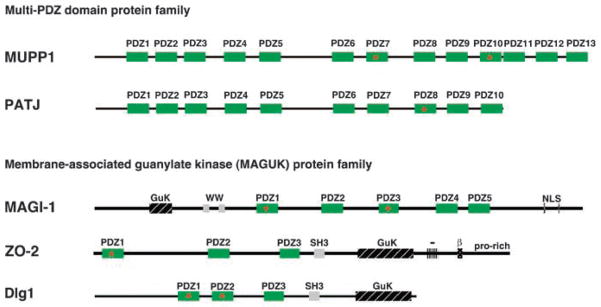
Recent evidence has revealed that critical centrally located E4-ORF1 mutations define one functional element, designated domain 2 (D2), which mediates binding to several unidentified cellular phosphoproteins (p70, p150, p190) and which is sufficient to promote association of E4-ORF1 with membrane vesicles (Chung et al., 2007). On the other hand, E4-ORF1 carboxyl-terminal mutations disrupt a separate functional element that was identified as a PSD-95, Dlg, ZO-1 (PDZ) domain-binding motif (PBM) (Lee et al., 1997). This element, which represented the first functional PBM identified in a viral protein, was found to mediate interactions with a selected group of cellular PDZ domain-containing proteins, including Dlg1, Multi-PDZ domain protein 1 (MUPP1), PATJ, MAGI-1 and ZO-2 (Lee et al., 1997, 2000; Glaunsinger et al., 2000, 2001) (Figures 2 and 3). In polarized epithelial cells, Dlg1 localizes to the adherens junction (AJ) (Laprise et al., 2004; Stucke et al., 2007), whereas MUPP1, PATJ, MAGI-1 and ZO-2 localize to the tight junction (TJ) (Jesaitis and Goodenough, 1994; Hamazaki et al., 2002; Patrie et al., 2002; Shin et al., 2005). These PBM-mediated interactions are highly specific because Ad9 E4-ORF1 fails to bind other cellular PDZ proteins (Glaunsinger et al., 2000, 2001; Latorre et al., 2005), including FAP-1, ZO-1, ZO-3, AF-6, hINADL, Par-6 and Scribble (Prasad et al., 1993; Willott et al., 1993; Sato et al., 1995; Philipp and Flockerzi, 1997; Haskins et al., 1998; Joberty et al., 2000; Nakagawa and Huibregtse, 2000). Similar to D2, the PBM is also sufficient to target E4-ORF1 to cytoplasmic membrane vesicles (Chung et al., 2007).
Figure 3.
Domain structures of E4-ORF1-associated cellular PDZ proteins. Red asterisks indicate specific PDZ domains that mediate binding to Ad9 E4-ORF1. SH3, Src homology 3 domain; GuK, guanylate kinase-homology domain; WW, WW domain; NLS, nuclear localization signal; –, acidic domain; prorich, proline-rich region.
High-risk human papillomaviruses E6 and human T-cell leukemia virus type 1 Tax oncoproteins also possess a carboxyl-terminal PBM
PDZ domains are approximately 90 amino-acid modular units that mediate protein–protein interactions (Kim, 1995; Kornau et al., 1995; Songyang et al., 1997), similar to Sarcoma (Src) homology region-2 and Src homology region-3 or phosphotyrosine-binding domains. The term PDZ derives from names of the first three proteins recognized to contain these domains (postsynaptic density protein (PSD-95), discs-large tumor suppressor (dlg) and zonula occludens protein 1 (ZO-1)). Suggesting coevolution with multicellularity, PDZ proteins are encoded primarily by metazoans, where these numerous proteins constitute 0.2–0.5% of ORFs (Harris and Lim, 2001). Such proteins typically function as scaffolds to assemble receptors and cytosolic factors into supramolecular signaling complexes and to localize them to specialized membrane regions of cell–cell contact, such as the AJ and TJ (Saras and Heldin, 1996; Sheng and Kim, 1996; Sheng, 1996).
PDZ domains bind to a specific sequence motif, or PBM, typically present at the extreme carboxyl-terminus of target proteins (Songyang et al., 1997). A class I PBM having the consensus sequence -(S/T)-X-(V/I/L)-COOH (X denotes any amino acid) (Saras and Heldin, 1996; Sheng and Kim, 1996; Sheng, 1996) was identified at the carboxyl-terminus of Ad9 E4-ORF1 (Lee et al., 1997). This observation, together with the fact that otherwise unrelated viral oncoproteins frequently share common mechanisms of cellular transformation, prompted an immediate search for additional viral oncoproteins having a carboxyl-terminal PBM. This search led to the discovery of a class 1 PBM at the carboxyl-terminus of E6 oncoproteins encoded by high-risk human papillomaviruses (HPV) and the Tax oncoprotein encoded by human T-cell leukemia virus type 1 (HTLV-1) (Lee et al., 1997). The significance of this observation is illustrated by the fact that, in humans, HTLV-1 and high-risk HPV are the causative agents of adult T-cell leukemia and cervical cancer, respectively.
Functions of the HPV E6 PBM in cellular transformation and tumorigenesis
In accordance with the demonstrated oncogenic function of the Ad9 E4-ORF1 PBM, the E6 PBM was shown to be required for E6-mediated transformation of cultured cells and tumorigenesis in transgenic mice, as well as HPV-induced pathogenesis in organotypic raft cultures of human keratinocytes (Kiyono et al., 1997; Mantovani and Banks, 2001; Watson et al., 2003; Nguyen et al., 2003a; Lee and Laimins, 2004; Simonson et al., 2005; Shai et al., 2007). Supporting an additional function of the E6 PBM in HPV-induced cervical cancer of women, E6 proteins encoded by low-risk HPVs, which are not associated with this disease, lack the carboxyl-terminal PBM (Kiyono et al., 1997; Lee et al., 1997). Also worth mentioning is that HPV E6 synergizes with E7, an additional crucial HPV oncogenic determinant, to trigger cervical carcinogenesis in transgenic mice by a mechanism requiring both the E6 PBM and estrogen (Shai et al., 2007). The dual dependence of both Ad9- and HPV-induced tumors on estrogen and a viral oncoprotein PBM hints that dysregulation of cellular PDZ proteins may commonly contribute to the development of estrogen-dependent malignancies. A detailed discussion of functions for cellular PDZ proteins in HPV-induced cancers can be found in the accompanying review by Thomas et al. (2008) in this issue.
Functions of the HTLV-1 Tax PBM in cellular transformation and tumorigenesis
Human T-cell leukemia virus type 1 is the etiological agent of adult T-cell leukemia, a rapidly progressing, clonal malignancy of CD4+ T lymphocytes in humans. An estimated 10–20 million people worldwide are infected with HTLV-1, and approximately 3% of these individuals will develop adult T-cell leukemia. The primary oncogenic determinant of HTLV-1 is the Tax gene, which encodes a nuclear and cytoplasmic phosphoprotein with pleiotropic functions that promote cell survival, cell-cycle progression, multipolar mitosis, aneuploidy and DNA damage. In addition, Tax-induced cellular transformation depends in part on its ability to activate the cyclic AMP pathway, as well as the nuclear factor κB (NFκB) and phosphatidylinositol 3-kinase (PI3K) cell survival pathways (for review, see Matsuoka and Jeang, 2007). Pertinent to this review is that the Tax PBM mediates binding to multiple cellular PDZ proteins (Lee et al., 1997; Rousset et al., 1998; Ohashi et al., 2004; Arpin-Andre and Mesnard, 2007) and that mutational disruption of the Tax PBM substantially decreases Tax-mediated cellular transformation and micronuclei formation, as well as the ability of HTLV-1 to stimulate T-cell proliferation and to cause a persistent viral infection in vivo (Suzuki et al., 1999; Endo et al., 2002; Hirata et al., 2004; Tsubata et al., 2005; Ishioka et al., 2006; Kondo et al., 2006; Xie et al., 2006; Higuchi et al., 2007). Results demonstrating that the HTLV-1 Tax PBM cooperates with Tax-induced NFκB activation to transform cells (Higuchi et al., 2007) and that HPV E6 mediates NFkB activation in a PBM-dependent manner (James et al., 2006) further hint at a potentially important interplay between the NFkB and PDZ-protein pathways in triggering cellular transformation.
As with the differences between high-risk and low-risk HPV E6 proteins, a natural example also lends strong support to the idea that the Tax PBM has an important function in promoting adult T-cell leukemia. In this regard, unlike HTLV-1, the closely related HTLV-2 is not associated with any malignant lymphoproliferative diseases in people (Mahieux and Gessain, 2003), despite the fact that the Tax proteins encoded by these two different retroviruses share the ability to activate the cyclic AMP and NFκB pathways (Wang et al., 2000). Strikingly, HTLV-2 Tax differs from HTLV-1 Tax in lacking the carboxyl-terminal PBM (Rousset et al., 1998; Suzuki et al., 1999; Hirata et al., 2004), and this difference explains the more efficient induction of cellular transformation by HTLV-1 Tax compared with HTLV-2 Tax (Endo et al., 2002; Hirata et al., 2004).
Taken together, findings with Ad9 E4-ORF1, high-risk HPV E6 and HTLV-1 Tax indicate that functional perturbation of cellular PDZ proteins is a common theme among tumorigenic human viruses and that this activity has an important function in their oncogenic potential.
The E4-ORF1 monomer and trimer possess distinct functions
In a PBM-dependent and D2-independent manner, E4-ORF1 affects the subcellular localization of its PDZ protein targets in one of two strikingly different ways by inducing either (1) translocation of AJ-associated Dlg1 to the plasma membrane (Frese et al., 2006) or (2) sequestration of TJ-associated MUPP1, PATJ, MAGI-1 and ZO-2 within insoluble complexes associated with cytoplasmic punctae (Glaunsinger et al., 2000, 2001; Lee et al., 2000; Latorre et al., 2005). The former and latter effects were recently shown to be specifically mediated by the E4-ORF1 trimer or monomer, respectively (Chung et al., 2008). In addition to selective binding to Dlg1, the E4-ORF1 trimer also specifically possesses D2 activity (Chung et al., 2007), consistent with the fact that molecular modeling of E4-ORF1 to the crystal structure of human dUTPase predicts that E4-ORF1 trimerization brings six out of seven D2 residues together at each of the three subunit interfaces (Chung et al., 2007). These molecular modeling analyses further suggest that the Ad9 E4-ORF1 gene evolved from an ancestral dUTPase gene by events that transformed the dUTPase catalytic cleft and carboxyl-terminal nucleotide-binding P-loop motif into the E4-ORF1 D2 or PBM element, respectively. The data support the idea that E4-ORF1 trimers bind both Dlg1 and D2-interacting cellular factors and promote their translocation to the plasma membrane, whereas monomers instead bind TJ-associated PDZ protein targets and sequester them within insoluble complexes associated with cytoplasmic membrane vesicles. Oligomerization of E4-ORF1 is unlikely controlled by a posttranslational modification(s) because, thus far, none has been detected. Evidence rather hints at the possibility that cholesterol present in cellular membranes triggers E4-ORF1 monomers to assemble into trimers (Chung et al., 2008). These collective findings expose a novel strategy wherein the oligomerization state of a protein not only determines the capacity to bind different cellular targets but also couples each type of interaction to a different functional consequence.
The E4-ORF1 cellular targets Dlg1, PATJ and ZO-2 are key polarity proteins
Polarity is a fundamental property of all cells and signifies the asymmetry in function, shape or content of a cell created through differential distribution of its macromolecular constituents. In metazoans, this asymmetry is central to the establishment of apical–basal polarity, planar cell polarity and anterior–posterior polarity, which have important functions in proper functioning of cells and development of organisms (Siegrist and Doe, 2007). The fundamental importance of cell polarity is further underscored by the requirement for proper polarization in a wide range of cellular processes, including morphogenesis, asymmetric division and directed migration (Siegrist and Doe, 2007). Notably, loss of apical–basal cell polarity and disruption of cell–cell junctions, such as the AJ and TJ, are common features of epithelial-derived cancer cells (Cochand-Priollet et al., 1998; Soler et al., 1999). Accumulating evidence indicates that such defects directly contribute to carcinogenesis by dysregulating normal proliferation and differentiation programs in cells (Cochand-Priollet et al., 1998; Soler et al., 1999; Bilder, 2003, 2004; Humbert et al., 2003; Lallemand et al., 2003; Matter and Balda, 2003; Aranda et al., 2006; Curto et al., 2007), suggesting that loss of cell polarity has an important function in the pathology of cancer.
Most relevant to this discussion is that three of the five E4-ORF1-interacting PDZ proteins (PATJ, ZO-2 and Dlg1) represent key components of the cell machinery that controls polarity. For example, in mammalian epithelial cells, proper apical–basal polarity establishment depends on the two evolutionarily conserved polarity complexes, Crumbs-Pals1-PATJ and Par3-Par6-aPKC (Matter and Balda, 2003; Bilder, 2004; Shin et al., 2006; Suzuki and Ohno, 2006; Siegrist and Doe, 2007), as well as the ZO-2 polarity protein (Hernandez et al., 2007). In addition, recent evidence has revealed important functions of evolutionarily conserved Scribble and Dlg1, as well as PATJ, in determination of anterior–posterior polarity establishment in astrocytes, T cells and epithelial cells (Etienne-Manneville et al., 2005; Humbert et al., 2006; Krummel and Macara, 2006; Osmani et al., 2006; Shin et al., 2007). Providing a possible link to cancer, defects in anterior–posterior polarization are reported to increase the production of stem cells, from which cancers may originate (Wodarz and Gonzalez, 2006; Wodarz and Nathke, 2007).
The Dlg1 polarity protein
Dlg1/SAP97, a mammalian homolog of the Drosophila discs-large (dlg) protein (Woods and Bryant, 1991), was the first cellular PDZ-protein target identified for Ad9 E4-ORF1, as well as HTLV-1 Tax and high-risk HPV E6 (Lee et al., 1997). Both Dlg1 and dlg are members of the membrane-associated guanylate kinase (MAGUK) family of proteins that contain, in addition to multiple PDZ domains, an Src homology region-3 or WW domain and a guanylate kinase-homology domain (Kim et al., 1995) (Figure 3), all of which function as protein–protein interaction modules (Gonzalez-Mariscal et al., 2000). In Drosophila imaginal disc epithelia, dlg localizes to the septate junction, which is analogous to the TJ of mammalian cells (Woods et al., 1996). Homozygous dlg mutations in Drosophila cause embryonic lethality due to disruption of cell junctions, cell shape and apical–basal cell polarity, as well as neoplastic overgrowth of imaginal disc epithelia and hyperplastic growth within larval brains (Woods and Bryant, 1989). Furthermore, ectopic expression of mammalian Dlg1 reverses these dlg mutant phenotypes, including neoplastic cell overgrowth (Thomas et al., 1997). In addition to showing that dlg is a tumor suppressor, these findings are significant by linking the loss of cell polarity directly to neoplastic cellular transformation.
It has been postulated that mammalian Dlg1 likewise is a tumor suppressor and that the tumorigenic potential of Ad9 E4-ORF1, HTLV-1 Tax and high-risk HPV E6 depends in part on an ability to inactivate this cellular factor. In support of this idea, HPV E6 targets Dlg1 for proteosome-mediated degradation in cells (Gardiol et al., 1999, 2002; Kuhne et al., 2000; Pim et al., 2000, 2002; Mantovani and Banks, 2001; Thomas et al., 2001b; Massimi et al., 2004, 2006; Matsumoto et al., 2006; Kuballa et al., 2007), and siRNA-mediated downregulation of Dlg1 augments HTLV-1 Tax-induced T-cell transformation (Ishioka et al., 2006). Although Dlg1+/− mice are not reported to exhibit a heightened tumor incidence, increased proliferation of ocular lens cells is observed in Dlg1−/− mice (Nguyen et al., 2003b), which die perinatally and display abnormal craniofacial, kidney and urogenital development (Caruana and Bernstein, 2001; Naim et al., 2005; Mahoney et al., 2006; Iizuka-Kogo et al., 2007). Consistent with Dlg1 localization to the AJ of polarized epithelial cells (Muller et al., 1995; Reuver and Garner, 1998; Wu et al., 1998; Laprise et al., 2004), cells treated with Dlg1 siRNAs show defects in AJ formation, as well as TJ function (Laprise et al., 2004; Stucke et al., 2007). Unlike Drosophila dlg, mammalian Dlg1 does not appear to determine establishment of apical–basal cell polarity. Instead, findings indicate that Dlg1 has an important function in the establishment of anterior–posterior cell polarization required for directed migration of astrocytes and epithelial cells (Etienne-Manneville et al., 2005; Mimori-Kiyosue et al., 2007) and for activation of T-cells (Xavier et al., 2004; Round et al., 2005, 2007; Krummel and Macara, 2006; Rebeaud et al., 2007; Rincon and Davis, 2007). Thus, Dlg1 is an evolutionarily conserved polarity protein.
Notably, recent data indicate that Ad9 E4-ORF1 inhibits both directed migration and anterior–posterior polarity establishment in cells and that both E4-ORF1 D2 and PBM are required for these activities (L Waldron and RTJ, unpublished data). These observations suggest that Ad9 E4-ORF1 directly interferes with the cell polarity function of Dlg1. As this function depends on the interaction of Dlg1 with the adenomatous polyposis coli (APC) tumor suppressor (Etienne-Manneville and Hall, 2003a; Etienne-Manneville et al., 2005; Gomes et al., 2005), one interesting possibility is that Ad9 E4-ORF1 blocks anterior–posterior polarity establishment by disrupting the Dlg1–APC complex in cells. Consistent with this idea, the Dlg1 PDZ1+2 domain tandem mediates binding to the carboxyl-terminal PBM of both APC and E4-ORF1 (Matsumine et al., 1996; Frese et al., 2006; Chung et al., 2008). A detailed discussion of anterior–posterior cell polarity and directed cell migration can be found in the accompanying review article by Etienne-Manneville in this issue.
Given evidence suggesting that HTLV-1 Tax and HPV E6 likewise bind and inactivate Dlg1, as well as Scribble that acts upstream of Dlg1 in the anterior–posterior polarity pathway (Nakagawa and Huibregtse, 2000; Etienne-Manneville and Hall, 2001, 2002, 2003b; Qin et al., 2005; Osmani et al., 2006; Arpin-Andre and Mesnard, 2007; Dow et al., 2007), it is anticipated that these two viral oncoproteins also will be found to prevent anterior–posterior polarity establishment and directed migration of cells. Consistent with this prediction, unlike normal T cells, HTLV-1-infected T cells specifically fail to establish proper anterior–posterior polarity upon stimulation of the CD3 or CD28 receptor involved in T-cell receptor-mediated activation (Barnard et al., 2005). Thus, considering that HTLV-1 infection activates human T cells (Gazzolo and Duc Dodon, 1987), Tax-mediated inactivation of the cell growth-inhibitory Dlg1–APC complex (Matsumine et al., 1996; Ishidate et al., 2000; Hirata et al., 2004; Ishioka et al., 2006) may cause a loss of polarity that, in conjunction with Tax1-mediated activation of the cyclic AMP, NFkB and PI3K pathways, provokes abnormal proliferation of human T cells. Because the Dlg1 and Scribble polarity proteins also control immune synapse formation, migration and signaling in T cells (Xavier et al., 2004; Ludford-Menting et al., 2005; Round et al., 2005, 2007; Krummel and Macara, 2006; Rebeaud et al., 2007; Rincon and Davis, 2007), an interesting possibility is that the Tax oncoprotein additionally prevents host-mediated viral clearance by blocking these important T-cell functions, thereby aiding HTLV-1 establishment of persistent T-cell infections in people.
Also worth mentioning is the subversion of cell migration in cancer, where increased migration contributes to tumor invasion and metastasis. Consistent with this idea, loss or reduction of Scribble or Dlg1 expression is correlated with more invasive and aggressive human tumors (Humbert et al., 2003; Nakagawa et al., 2004; Navarro et al., 2005; Gardiol et al., 2006). At first glance, these observations seem at odds with compelling evidence showing that loss or reduction in Scribble or Dlg1 expression in normal mammalian cells instead inhibits migration (Etienne-Manneville et al., 2005; Ludford-Menting et al., 2005; Wada et al., 2005; Osmani et al., 2006; Dow et al., 2007). In a recent review (Humbert et al., 2006), this apparent discrepancy was proposed to reflect context-dependent functions of these tumor suppressors in migration (Goode and Perrimon, 1997; Abdelilah-Seyfried et al., 2003; Pagliarini and Xu, 2003; Qin et al., 2005). On the basis of this idea, it may be postulated that functional loss of Scribble or Dlg1 in normal cells contributes to the early stages of neoplastic transformation by preventing anterior–posterior polarity establishment (Wodarz and Nathke, 2007) and thereby directed migration, yet, in the context of additional oncogenic insults involved in late stage malignant progression, loss of Scribble or Dlg1 function instead acts to increase the migration of cancer cells.
E4-ORF1 binding to the Dlg1 polarity protein also promotes constitutive growth factor-independent PI3K activation
Phosphatidylinositol 3-kinase represents a key component of a signaling pathway triggered by activated tyrosine kinase and heterotrimeric G protein-coupled membrane receptors or Ras (Blume-Jensen and Hunter, 2001). These factors recruit PI3K to the plasma membrane, where this lipid kinase phosphorylates 4,5-phosphoinositides at the D3 position. The resulting 3,4,5-phosphoinositide products act as second messengers to recruit Akt/protein kinase B (PKB) to the membrane, where PDK1 and the TORC2 complex activate PKB by phosphorylating threonine residue 308 (T308) or serine residue 473 (S473), respectively (Bhaskar and Hay, 2007). Activated PKB promotes cell survival and proliferation through its ability to control the activities of multiple downstream effectors, including pro-apoptotic Forkhead transcription factors, translation and cell-cycle progression regulator p70S6-kinase (S6K) and cyclin-dependent kinase inhibitor p27Kip1 (Yu and Sato, 1999; Medema et al., 2000). A critical antagonist of PI3K is the PTEN tumor suppressor protein, a lipid phosphatase that removes D3 phosphates from 3,4,5-phosphoinositides (Yamada and Araki, 2001). Significantly, human cancers are frequently associated with activating mutations in PI3K or PKB or loss-of-function mutations in PTEN (Engelman et al., 2006), thereby widely implicating dysregulated PI3K–PKB signaling in the development of many human malignancies.
Findings have shown that Ad9 E4-ORF1 promotes constitutive growth factor-independent activation of PI3K, as well as its downstream effectors PKB and S6K, but not components of several other signaling pathways (ERK, β-catenin/TCF, JNK, NFκB, Notch, Stat3) (Frese et al., 2003). In addition, the PI3K inhibitor LY294002 or mammalian target of rapamycin inhibitor, which blocks transformation by constitutively activated forms of PI3K and PKB but not 11 other oncoproteins (Aoki et al., 2001), abrogates soft agar growth and focus formation by Ad9 E4-ORF1-expressing cells and reverses the transformed state of Ad9-induced mammary tumor cells (Frese et al., 2003). Hence, cellular transformation induced by Ad9 E4-ORF1 depends on its capacity to dysregulate cellular PI3K signaling, an activity shared by all Ad E4-ORF1 proteins (Frese et al., 2003; O’Shea et al., 2005a). As expression of a constitutively activated PI3K or PKB mutant fails to recapitulate Ad9 E4-ORF1-induced cellular transformation (Frese et al., 2003), it also must be concluded that PI3K activation is necessary but not sufficient for the full oncogenic potential of Ad9 E4-ORF1. The capacity of E4-ORF1 to stimulate the PI3K pathway is abolished by an inactivating mutation in either the D2 or PBM (Frese et al., 2003), identical to Ad9 E4-ORF1-induced disruption of anterior–posterior cell polarization. The fact that, unlike wild-type Ad9 virus, Ad9 viruses encoding PBM or D2 E4-ORF1 mutants fail to activate PI3K during a viral infection and to elicit mammary tumors in rats (Frese et al., 2003) additionally links PI3K activation, as well as disruption of anterior–posterior cell polarization, to Ad9-induced mammary tumorigenesis.
The finding that Ad9 E4-ORF1, high-risk HPV E6 and HTLV-1 Tax independently evolved to target the Dlg1 polarity protein have provided compelling evidence implicating this cellular factor in human cancer. Despite the general belief that these interactions solely function to inactivate this cell polarity protein, results showed that Dlg1−/− mouse embryo fibroblasts fail to support E4-ORF1-induced PI3K activation and cellular transformation (Frese et al., 2006). This defect was specific to Dlg1−/− mouse embryo fibroblasts, as MUPP1−/− or MAGI-1−/− mouse embryo fibroblasts retain the capacity to support this E4-ORF1 activity. Moreover, growth factor-induced PI3K activation remained normal in Dlg1−/− mouse embryo fibroblasts, revealing a specific defect in E4-ORF1-induced PI3K activation. As expected, this Dlg1- and D2 element-dependent E4-ORF1 activity is mediated specifically by E4-ORF1 trimers (Chung et al., 2007, 2008) and also depends on several different Dlg1 domains, including PDZ1+2, US3, Src homology region-3 and I3 (Frese et al., 2006). These findings revealed the first known function for Dlg1 in virus-mediated cellular transformation and also exposed an unexpected oncogenic activity for this suspected cellular tumor suppressor protein.
The oncogenes encoded by DNA tumor viruses, such as Ad, evolved to promote quiescent cells to progress from G0 to S phase of the cell cycle to provide an optimal environment for viral DNA replication. As this aberrant proliferative signal often induces a cellular antiviral response that triggers apoptosis, DNA tumor virus oncoproteins also promote cell survival to ensure robust viral replication. Thus, it will be important to determine whether the perturbation of cell polarity proteins by Ad9 E4-ORF1 serves to promote both cell cycle progression and cell survival during the viral life cycle. This outcome seems likely given that the related Ad type 5 E4-ORF1 protein activates the cellular PI3K effector mammalian target of rapamycin and, in so doing, enhances S-phase entry and viral replication in quiescent primary cells under nutrient and growth factor-limiting conditions (O’Shea et al., 2005a, b).
The PATJ and ZO-2 polarity proteins
The development of epithelial-derived cancers is commonly linked to a failure of tumor cells to form TJs and to establish proper apical–basal polarity (Cochand-Priollet et al., 1998; Soler et al., 1999). Evidence further suggests that such defects directly trigger neoplastic cellular transformation (Bilder, 2003, 2004; Humbert et al., 2003; Matter and Balda, 2003; Aranda et al., 2006). Given that MUPP1, PATJ, MAGI-1 and ZO-2 localize to the TJ (Beatch et al., 1996; Hamazaki et al., 2002; Hirabayashi et al., 2003; Shin et al., 2005) and become aberrantly sequestered by E4-ORF1 in the cytoplasm (Glaunsinger et al., 2000, 2001; Lee et al., 2000; Latorre et al., 2005), a reasonable hypothesis would be that Ad9 E4-ORF1 binds and inactivates these PDZ proteins and, in so doing, blocks TJ formation and causes a loss of apical–basal polarity in epithelial cells. In support of this idea, both PATJ and ZO-2 are polarity proteins required for both TJ formation and proper apical–basal polarity establishment in epithelial cells (Shin et al., 2005; Umeda et al., 2006; Hernandez et al., 2007) (Figure 3). Moreover, in Madin-Darby Canine Kidney (MDCK) epithelial cells, it was reported that Ad9 E4-ORF1 blocks proper TJ localization of PATJ and ZO-2, as well as their interacting partners, and also disrupts both the TJ barrier and apical–basal polarity (Latorre et al., 2005). In contrast, E4-ORF1 fails to interfere with the proper AJ localization of Dlg1 or β-catenin in the same cells. In addition, E4-ORF1 D2 mutants, which cannot activate PI3K (Frese et al., 2003; Chung et al., 2007), retain a wild-type capacity to disrupt the TJ in MDCK cells, indicating that TJ disruption and PI3K activation are separable E4-ORF1 activities. The fact that siRNA-mediated downregulation of either PATJ or ZO-2 similarly blocks TJ formation and proper apical–basal polarity establishment in MDCK cells (Shin et al., 2005; Hernandez et al., 2007) argues strongly that analogous effects caused by E4-ORF1 are due to its direct inactivation of these two PDZ proteins. This work represented the first demonstration that PBM-mediated interactions of a viral oncoprotein with cell polarity proteins functionally disrupt the TJ and cause a loss of polarity.
Providing possible links for these observations to cancer, ZO-2 is a candidate human tumor suppressor gene (Chlenski et al., 1999a, b, 2000; Sato et al., 2003; Fink et al., 2006), and recent data suggest that ZO-2+/− mutant mice exhibit an elevated tumor incidence (I Latorre and RTJ, unpublished data). In addition, overexpressed ZO-2 interferes with focus formation induced by Ad9 E4-ORF1, as well as by the polyomavirus middle T (mT) and activated RasV12 oncoproteins (Glaunsinger et al., 2001). Likewise, tumorigenic Ad9 E4-ORF1 and nontumorigenic Ad E4-ORF1 proteins interact with MUPP1, PATJ, MAGI-1 and Dlg1, whereas Ad9 E4-ORF1 uniquely binds ZO-2 (Glaunsinger et al., 2001), thereby linking this particular interaction to the distinct Ad9 E4-ORF1 tumorigenic properties. Evidence further shows that high-risk HPV E6 binds PATJ (Latorre et al., 2005) and targets it for degradation in cells (Storrs and Silverstein, 2007), suggesting that inactivation of this polarity protein may contribute to the development of cervical carcinoma in women.
Inactivation of TJ-associated polarity proteins likewise can be envisioned as contributing to Ad9-induced mammary tumorigenesis, as well as human breast cancer. Malignant Ad9-induced mammary tumor cells express myoepithelial cell markers (Javier et al., 1991), implying an origin from polarized mammary stem cells of the lumenal epithelial lineage that form TJs (Gudjonsson et al., 2002b). Notably, most breast carcinomas arise from this epithelial lineage, which functions to maintain normal mammary tissue polarity, a property lost during breast neoplasia (Pechoux et al., 1999; Gudjonsson et al., 2002a, 2005; Bissell and Bilder, 2003; Adriance et al., 2005; Lakhani and Bissell, 2005; Polyak and Hu, 2005). Thus, the tumorigenic potential of Ad9 E4-ORF1 in mammary epithelial cells could conceivably stem in part from its capacity to prevent TJ formation and proper apical–basal polarity establishment by inactivating TJ-associated polarity proteins in polarized mammary stem cells.
Although TJ-associated MAGI-1 and MUPP1, a paralog of the PATJ polarity protein (Lemmers et al., 2002; Michel et al., 2005; Shin et al., 2005), are involved in the assembly of membrane signaling complexes (Barritt et al., 2000; Becamel et al., 2001; Hamazaki et al., 2002; Kimber et al., 2002; Laura et al., 2002; Patrie et al., 2002; Hirabayashi et al., 2003; Jeansonne et al., 2003; Murata et al., 2005; Heydecke et al., 2006; Sakurai et al., 2006; Balasubramanian et al., 2007; Sugihara-Mizuno et al., 2007), it is not yet known whether these two PDZ proteins likewise function in TJ assembly and apical–basal polarity establishment (Figure 3). In addition to their aberrant sequestration by Ad9 E4-ORF1 in insoluble cytoplasmic complexes, MAGI-1 and MUPP1 bind to and are targeted for proteasome-mediated degradation by high-risk HPV E6 (Glaunsinger et al., 2000; Lee et al., 2000). These observations, coupled with the requirement for MAGI-1 in localization and stabilization of the PTEN tumor suppressor at the plasma membrane (Kotelevets et al., 2005; Valiente et al., 2005) and the association of reduced MUPP1 levels with a poor prognosis in breast cancer patients (Martin et al., 2004), hint at possible functions of MAGI-1 and MUPP1 in cancer. On the basis of these observations, it seems conceivable that, similar to PATJ and ZO-2, MAGI-1 and MUPP1 are also cell polarity proteins targeted for functional inactivation by Ad9 E4-ORF1 and high-risk HPV E6 in cells.
Model for Ad9 E4-ORF1-induced cellular transformation through perturbation of cell polarity proteins
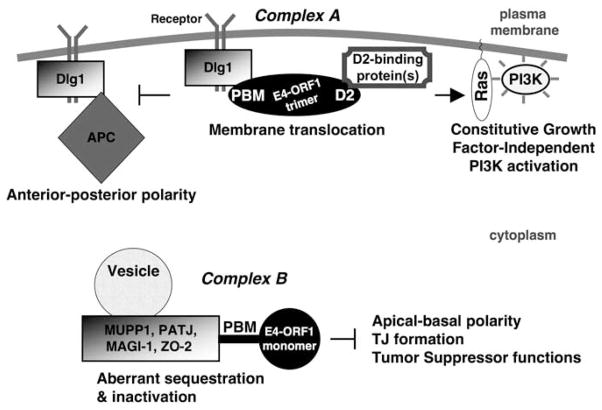
Figure 4 presents a model on the basis of available data indicating that the Ad9 E4-ORF1 oncoprotein promotes cellular transformation in large part through interactions with the cell polarity proteins Dlg1, PATJ and ZO-2. The model shows that E4-ORF1 exists in two oligomeric forms, which assemble different general types of protein complexes (complex A and complex B) and produce distinct functional consequences.
Figure 4.
Molecular model for polarity disruption and PI3K activation by the adenovirus type 9 E4 region-encoded open reading frame 1 (E4-ORF1) oncoprotein. See text for additional details about the model and E4-ORF1 complexes A and B.
Complex A is formed through interactions of the E4-ORF1 trimer with Dlg1 and D2-interacting cellular proteins (Figure 4). TJ-associated PDZ proteins are excluded from complex A, as they cannot bind the E4-ORF1 trimer (Chung et al., 2008). This specificity is determined in part by the unique capacity of E4-ORF1 trimers to bind cooperatively to the Dlg1 PDZ1+2 domain tandem and to form functionalD2 elements. Upon complex formation, Dlg1 mediates translocation of complex A to the plasma membrane (Frese et al., 2006), in agreement with numerous studies implicating Dlg1 in receptor trafficking to the plasma membrane (Chetkovich et al., 2002; Wu et al., 2002; Lee et al., 2003; Leonoudakis et al., 2004; Cai et al., 2006; Inoue et al., 2006; Gardner et al., 2007; Marcello et al., 2007; Mauceri et al., 2007). Complex A functions both to promote Dlg1-mediated PI3K activation by a Ras-dependent mechanism and to block Dlg1-mediated establishment of anterior–posterior cell polarity. This dual function of complex A interestingly suggests that subversion of Dlg1 for E4-ORF1-induced PI3K activation simultaneously blocks the Dlg1 cell polarity function, perhaps by disrupting the Dlg1–APC complex. Also worth mentioning is that proper anterior–posterior polarity establishment depends not only on Dlg1–APC complex formation at the leading edge membrane, but also on spatially restricted PI3K activation that stabilizes the polarized microtubule network at the same membrane site (Higuchi et al., 2001; Procko and McColl, 2005; Onishi et al., 2007; Primo et al., 2007). Thus, an intriguing scenario would be that PI3K activation at the leading edge membrane is normally mediated by Dlg1 through an APC-independent mechanism and that E4-ORF1 usurps and dysregulates this additional Dlg1 polarity function to promote oncogenic PI3K activation in cells. Consistent with this hypothesis, Dlg1 has been shown to promote lamellipodia formation at the leading edge membrane by an APC-independent mechanism (Etienne-Manneville et al., 2005; Humbert et al., 2006).
Complex B, on the other hand, is formed by interaction of the E4-ORF1 monomer with individual TJ-associated PDZ protein targets (MUPP1, PATJ, MAGI-1 and ZO-2) (Figure 4) (Chung et al., 2008). In contrast to soluble plasma membrane-associated complex A, complex B is insoluble and causes aberrant sequestration of TJ-associated PDZ proteins on cytoplasmic membrane vesicles. As PDZ proteins often link membrane proteins to the cortical cytoskeleton, the insolubility of these complexes may reflect an association with cytoskeletal proteins. Dlg1 and D2-interacting cellular proteins are excluded from complex B because they cannot bind the E4-ORF1 monomer (Chung et al., 2007, 2008). Notably, complex B-mediated sequestration of PATJ and ZO-2 blocks their ability to promote TJ formation and proper apical–basal cell polarity (Latorre et al., 2005). As the full oncogenic potential of E4-ORF1 depends not only on Dlg1-dependent PI3K activation, but also on other undetermined E4-ORF1 activities (Frese et al., 2003), a reasonable hypothesis is that functional inactivation of the polarity proteins PATJ and ZO-2, as well as Dlg1, represents these additional crucial activities.
Conclusion
Findings suggest that key PDZ domain-containing polarity proteins are common cellular targets for inactivation by human virus oncoproteins and that such interactions contribute to virus-mediated tumorigenesis. In fact, available data support the notion that inhibition of proper cell polarity establishment may be the primary mechanism whereby the Ad9 E4-ORF1 oncoprotein induces mammary tumors in experimental animals. With respect to this idea, identification of Ad9 E4- ORF1 D2-interacting cellular proteins may reveal that they too are required for establishment of proper cell polarity and that the Ad9 E4-ORF1 oncoprotein likewise blocks their function. In addition, the essential function of Dlg1 in E4-ORF1-induced oncogenic PI3K activation predicts that Dlg1-mediated establishment of anterior–posterior cell polarity in part may depend on an ability of Dlg1 to promote spatially restricted PI3K activation at the plasma membrane of cells. Future experiments should explore this hypothesis.
Also worth mentioning is the suggestion that viral oncoproteins have evolved diverse mechanisms to perturb cell polarity pathways, as evidenced by the report of TJ disruption in polarized epithelial cells expressing the SV40 polyomavirus small t antigen oncoprotein (Nunbhakdi-Craig et al., 2002, 2003), which due to the lack of a PBM is unable to interact with cellular PDZ proteins. This observation hints that cell polarity loss may have an even greater function in virus-mediated tumorigenesis than was thought previously. In this regard, it will be important to determine whether small t antigen expressed by the newly discovered Merkel cell polyomavirus associated with Merkel cell carcinoma (Feng et al., 2008), a rare but aggressive human skin cancer of neuroendocrine origin, disrupts the tight junction and causes a loss of apical–basal polarity in epithelial cells. Additionally, although research to date has primarily focused on functions of polarity loss in the development of epithelial-derived carcinomas, the fact that Ad9, high-risk HPV and HTLV-1 promote sarcomas, carcinomas or leukemias, respectively, may indicate that loss of cell polarity is a contributing factor not only for carcinoma development, but also for the development of many other types of malignancies. This idea clearly warrants investigation.
It is important to mention that cell polarity loss is likewise implicated in the development of human cancers lacking an association with viral agents. For example, several different signaling pathways (for example, ErbB2, transforming growth factor-β, mammalian target of rapamycin) with involvement in human cancers coordinately regulate both apical–basal cell polarity and cellular proliferation, and evidence suggests that combined dysregulation of these processes cooperates to instigate malignant cellular transformation (Wodarz and Nathke, 2007). Moreover, the loss of apical–basal cell polarity and disruption of cell junctions is a hallmark of malignant human tumors. Thus, the development of drugs designed to inhibit cellular pathways that promote the loss of polarity represents a promising area for future cancer research.
It is presently unclear how cell polarity loss contributes to the development of cancer, although some reports have provided potentially important clues. For example, TJ disruption and loss of polarity in epithelial cells can activate a basolateral membrane-localized growth factor receptor by permitting inappropriate intermixing with its cognate apical membrane-localized growth factor (Vermeer et al., 2003) and can also release growth-stimulatory transcription factors from the TJ and promote their translocation into the nucleus (Balda et al., 2003; Betanzos et al., 2004). Exciting results in Drosophila further show that perturbations in the establishment of anterior–posterior polarity required for asymmetric cell division increase the production of cancer stem cells (Wodarz and Nathke, 2007). Clearly, future research must explore the mechanisms by which polarity loss contributes to abnormal cellular proliferation that provokes the development of cancer. Studies of human tumor viruses and their oncogenic determinants promise to serve as powerful tools to understand this important disease process.
References
- Abdelilah-Seyfried S, Cox DN, Jan YN. Bazooka is a permissive factor for the invasive behavior of discs large tumor cells in Drosophila ovarian follicular epithelia. Development. 2003;130:1927–1935. doi: 10.1242/dev.00420. [DOI] [PubMed] [Google Scholar]
- Adriance MC, Inman JL, Petersen OW, Bissell MJ. Myoepithelial cells: good fences make good neighbors. Breast Cancer Res. 2005;7:190–197. doi: 10.1186/bcr1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankerst J, Jonsson N. Adenovirus type 9-induced tumorigenesis in the rat mammary gland related to sex hormonal state. J Natl Cancer Inst. 1989;81:294–298. doi: 10.1093/jnci/81.4.294. [DOI] [PubMed] [Google Scholar]
- Ankerst J, Jonsson N, Kjellen L, Norrby E, Sjogren HO. Induction of mammary fibroadenomas in rats by adenovirus type 9. Int J Cancer. 1974;13:286–290. doi: 10.1002/ijc.2910130303. [DOI] [PubMed] [Google Scholar]
- Aoki M, Blazek E, Vogt PK. A role of the kinase mTOR in cellular transformation induced by the oncoproteins P3k and Akt. Proc Natl Acad Sci USA. 2001;98:136–141. doi: 10.1073/pnas.011528498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda V, Haire T, Nolan ME, Calarco JP, Rosenberg AZ, Fawcett JP, et al. Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat Cell Biol. 2006;8:1235–1245. doi: 10.1038/ncb1485. [DOI] [PubMed] [Google Scholar]
- Arpin-Andre C, Mesnard JM. The PDZ domain-binding motif of the human T cell leukemia virus type 1 tax protein induces mislocalization of the tumor suppressor hScrib in T cells. J Biol Chem. 2007;282:33132–33141. doi: 10.1074/jbc.M702279200. [DOI] [PubMed] [Google Scholar]
- Balasubramanian S, Fam SR, Hall RA. GABAB receptor association with the PDZ scaffold Mupp1 alters receptor stability and function. J Biol Chem. 2007;282:4162–4171. doi: 10.1074/jbc.M607695200. [DOI] [PubMed] [Google Scholar]
- Balda MS, Garrett MD, Matter K. The ZO-1-associated Y-box factor ZONAB regulates epithelial cell proliferation and cell density. J Cell Biol. 2003;160:423–432. doi: 10.1083/jcb.200210020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard AL, Igakura T, Tanaka Y, Taylor GP, Bangham CR. Engagement of specific T-cell surface molecules regulates cytoskeletal polarization in HTLV-1-infected lymphocytes. Blood. 2005;106:988–995. doi: 10.1182/blood-2004-07-2850. [DOI] [PubMed] [Google Scholar]
- Barritt DS, Pearn MT, Zisch AH, Lee SS, Javier RT, Pasquale EB, et al. The multi-PDZ domain protein MUPP1 is a cytoplasmic ligand for the membrane-spanning proteoglycan NG2. J Cell Biochem. 2000;79:213–224. doi: 10.1002/1097-4644(20001101)79:2<213::aid-jcb50>3.0.co;2-g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatch M, Jesaitis LA, Gallin WJ, Goodenough DA, Stevenson BR. The tight junction protein ZO-2 contains three PDZ (PSD-95/discs–large/ZO-1) domains and an alternatively spliced region. J Biol Chem. 1996;271:25723–25726. doi: 10.1074/jbc.271.42.25723. [DOI] [PubMed] [Google Scholar]
- Becamel C, Figge A, Poliak S, Dumuis A, Peles E, Bockaert J, et al. Interaction of serotonin 5-hydroxytryptamine type 2C receptors with PDZ10 of the multi-PDZ domain protein MUPP1. J Biol Chem. 2001;276:12974–12982. doi: 10.1074/jbc.M008089200. [DOI] [PubMed] [Google Scholar]
- Betanzos A, Huerta M, Lopez-Bayghen E, Azuara E, Amerena J, Gonzalez-Mariscal L. The tight junction protein ZO-2 associates with Jun, Fos and C/EBP transcription factors in epithelial cells. Exp Cell Res. 2004;292:51–66. doi: 10.1016/j.yexcr.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. doi: 10.1016/j.devcel.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Bilder D. PDZ domain polarity complexes. Curr Biol. 2003;13:R661–R662. doi: 10.1016/s0960-9822(03)00599-2. [DOI] [PubMed] [Google Scholar]
- Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–1925. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- Bissell MJ, Bilder D. Polarity determination in breast tissue: desmosomal adhesion, myoepithelial cells, and laminin 1. Breast Cancer Res. 2003;5:117–119. doi: 10.1186/bcr579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume-Jensen P, Hunter T. Oncogenic kinase signalling. Nature. 2001;411:355–365. doi: 10.1038/35077225. [DOI] [PubMed] [Google Scholar]
- Bridge E, Ketner G. Redundant control of adenovirus late gene expression by early region 4. J Virol. 1989;63:631–638. doi: 10.1128/jvi.63.2.631-638.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridge E, Ketner G. Interaction of adenoviralE4 and E1b products in late gene expression. Virology. 1990;174:345–353. doi: 10.1016/0042-6822(90)90088-9. [DOI] [PubMed] [Google Scholar]
- Cai C, Li H, Rivera C, Keinanen K. Interaction between SAP97 and PSD-95, two Maguk proteins involved in synaptic trafficking of AMPA receptors. J Biol Chem. 2006;281:4267–4273. doi: 10.1074/jbc.M505886200. [DOI] [PubMed] [Google Scholar]
- Caruana G, Bernstein A. Craniofacial dysmorphogenesis including cleft palate in mice with an insertional mutation in the discs large gene. Mol Cell Biol. 2001;21:1475–1483. doi: 10.1128/MCB.21.5.1475-1483.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chetkovich DM, Bunn RC, Kuo SH, Kawasaki Y, Kohwi M, Bredt DS. Postsynaptic targeting of alternative postsynaptic density-95 isoforms by distinct mechanisms. J Neurosci. 2002;22:6415–6425. doi: 10.1523/JNEUROSCI.22-15-06415.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlenski A, Ketels KV, Engeriser JL, Talamonti MS, Tsao MS, Koutnikova H, et al. zo-2 gene alternative promoters in normal and neoplastic human pancreatic duct cells. Int J Cancer. 1999a;83:349–358. doi: 10.1002/(sici)1097-0215(19991029)83:3<349::aid-ijc10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Chlenski A, Ketels KV, Korovaitseva GI, Talamonti MS, Oyasu R, Scarpelli DG. Organization and expression of the human zo-2 gene (tjp-2) in normal and neoplastic tissues. Biochim Biophys Acta. 2000;1493:319–324. doi: 10.1016/s0167-4781(00)00185-8. [DOI] [PubMed] [Google Scholar]
- Chlenski A, Ketels KV, Tsao MS, Talamonti MS, Anderson MR, Oyasu R, et al. Tight junction protein ZO-2 is differentially expressed in normal pancreatic ducts compared to human pancreatic adenocarcinoma. Int J Cancer. 1999b;82:137–144. doi: 10.1002/(sici)1097-0215(19990702)82:1<137::aid-ijc23>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Chung SH, Frese KK, Weiss RS, Prasad BV, Javier RT. A new crucial protein-interaction element that targets the adenovirus E4-ORF1 oncoprotein to membrane vesicles. J Virol. 2007;81:4787–4797. doi: 10.1128/JVI.02855-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SH, Weiss RS, Prasad BVV, Javier RT. Functionally distinct monomers and trimers produced by a viral oncoprotein. Oncogene. 2008;27:1412–1420. doi: 10.1038/sj.onc.1210784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochand-Priollet B, Raison D, Molinie V, Guillausseau PJ, Wassef M, Bouchaud C. Altered gap and tight junctions in human thyroid oncocytic tumors: a study of 8 cases by freeze-fracture. Ultrastruct Pathol. 1998;22:413–420. doi: 10.3109/01913129809032276. [DOI] [PubMed] [Google Scholar]
- Cotran RS, Robbins SL, Kumar V. Robbins Pathological Basis of Disease. The WB Saunders Co; Philadelphia, PA: 1994. [Google Scholar]
- Curto M, Cole BK, Lallemand D, Liu CH, McClatchey AI. Contact-dependent inhibition of EGFR signaling by Nf2/Merlin. J Cell Biol. 2007;177:893–903. doi: 10.1083/jcb.200703010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutt JR, Shenk T, Hearing P. Analysis of adenovirus early region 4-encoded polypeptides synthesized in productively infected cells. J Virol. 1987;61:543–552. doi: 10.1128/jvi.61.2.543-552.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobner T, Horikoshi N, Rubenwolf S, Shenk T. Blockage by adenovirus E4orf6 of transcriptional activation by the p53 tumor suppressor. Science. 1996;272:1470–1473. doi: 10.1126/science.272.5267.1470. [DOI] [PubMed] [Google Scholar]
- Dow LE, Kauffman JS, Caddy J, Peterson AS, Jane SM, Russell SM, et al. The tumour-suppressor Scribble dictates cell polarity during directed epithelial migration: regulation of Rho GTPase recruitment to the leading edge. Oncogene. 2007;26:2272–2282. doi: 10.1038/sj.onc.1210016. [DOI] [PubMed] [Google Scholar]
- Dyson N, Guida P, McCall C, Harlow E. Adenovirus E1A makes two distinct contacts with the retinoblastoma protein. J Virol. 1992;66:4606–4611. doi: 10.1128/jvi.66.7.4606-4611.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo K, Hirata A, Iwai K, Sakurai M, Fukushi M, Oie M, et al. Human T-cell leukemia virus type 2 (HTLV-2) Tax protein transforms a rat fibroblast cell line but less efficiently than HTLV-1 Tax. J Virol. 2002;76:2648–2653. doi: 10.1128/JVI.76.6.2648-2653.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endter C, Dobner T. Cell transformation by human adenoviruses. Curr Top Microbiol Immunol. 2004;273:163–214. doi: 10.1007/978-3-662-05599-1_6. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003a;421:753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Cell polarity: Par6, aPKC and cytoskeletal crosstalk. Curr Opin Cell Biol. 2003b;15:67–72. doi: 10.1016/s0955-0674(02)00005-4. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Manneville JB, Nicholls S, Ferenczi MA, Hall A. Cdc42 and Par6-PKCzeta regulate the spatially localized association of Dlg1 and APC to control cell polarization. J Cell Biol. 2005;170:895–901. doi: 10.1083/jcb.200412172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng H, Shuda M, Chang Y, Moore PS. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science. 2008;319:1096–1100. doi: 10.1126/science.1152586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink C, Weigel R, Hembes T, Lauke-Wettwer H, Kliesch S, Bergmann M, et al. Altered expression of ZO-1 and ZO-2 in Sertoli cells and loss of blood-testis barrier integrity in testicular carcinoma in situ. Neoplasia. 2006;8:1019–1027. doi: 10.1593/neo.06559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint SJ, Enquist LW, Krug RM, Racaniello VR, Shalka AM. Principles of Virology: Molecular Biology, Pathogenesis, and Control. ASM Press; Washington DC: 2000. pp. 552–5593. [Google Scholar]
- Flint SJ, Gonzalez RA. Regulation of mRNA production by the adenoviralE1B 55-kDa and E4 Orf6 proteins. Curr Top Microbiol Immunol. 2003;272:287–330. doi: 10.1007/978-3-662-05597-7_10. [DOI] [PubMed] [Google Scholar]
- Frese KK, Latorre IJ, Chung SH, Caruana G, Bernstein A, Jones SN, et al. Oncogenic function for the Dlg1 mammalian homolog of the Drosophila discs-large tumor suppressor. EMBO J. 2006;25:1406–1417. doi: 10.1038/sj.emboj.7601030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frese KK, Lee SS, Thomas DL, Latorre IJ, Weiss RS, Glaunsinger BA, et al. Selective PDZ protein-dependent stimulation of phosphatidylinositol 3-kinase by the adenovirus E4-ORF1 oncoprotein. Oncogene. 2003;22:710–721. doi: 10.1038/sj.onc.1206151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiol D, Galizzi S, Banks L. Mutational analysis of the discs large tumour suppressor identifies domains responsible for human papillomavirus type 18 E6-mediated degradation. J Gen Virol. 2002;83:283–289. doi: 10.1099/0022-1317-83-2-283. [DOI] [PubMed] [Google Scholar]
- Gardiol D, Kuhne C, Glaunsinger B, Lee SS, Javier R, Banks L. Oncogenic human papillomavirus E6 proteins target the discs large tumour suppressor for proteasome-mediated degradation. Oncogene. 1999;18:5487–5496. doi: 10.1038/sj.onc.1202920. [DOI] [PubMed] [Google Scholar]
- Gardiol D, Zacchi A, Petrera F, Stanta G, Banks L. Human discs large and scrib are localized at the same regions in colon mucosa and changes in their expression patterns are correlated with loss of tissue architecture during malignant progression. Int J Cancer. 2006;119:1285–1290. doi: 10.1002/ijc.21982. [DOI] [PubMed] [Google Scholar]
- Gardner LA, Naren AP, Bahouth SW. Assembly of an SAP97-AKAP79-cAMP-dependent protein kinase scaffold at the type 1 PSD-95/DLG/ZO1 motif of the human beta(1)-adrenergic receptor generates a receptosome involved in receptor recycling and networking. J Biol Chem. 2007;282:5085–5099. doi: 10.1074/jbc.M608871200. [DOI] [PubMed] [Google Scholar]
- Gazzolo L, Duc Dodon M. Direct activation of resting T lymphocytes by human T-lymphotropic virus type I. Nature. 1987;326:714–717. doi: 10.1038/326714a0. [DOI] [PubMed] [Google Scholar]
- Glaunsinger BA, Lee SS, Thomas M, Banks L, Javier R. Interactions of the PDZ-protein MAGI-1 with adenovirus E4-ORF1 and high-risk papillomavirus E6 oncoproteins. Oncogene. 2000;19:5270–5280. doi: 10.1038/sj.onc.1203906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaunsinger BA, Weiss RS, Lee SS, Javier R. Link of the unique oncogenic properties of adenovirus type 9 E4-ORF1 to a select interaction with the candidate tumor suppressor protein ZO-2. EMBO J. 2001;20:5578–5586. doi: 10.1093/emboj/20.20.5578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–463. doi: 10.1016/j.cell.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Betanzos A, Avila-Flores A. MAGUK proteins: structure and role in the tight junction. Semin Cell Dev Biol. 2000;11:315–324. doi: 10.1006/scdb.2000.0178. [DOI] [PubMed] [Google Scholar]
- Goode S, Perrimon N. Inhibition of patterned cell shape change and cell invasion by discs large during Drosophila oogenesis. Genes Dev. 1997;11:2532–2544. doi: 10.1101/gad.11.19.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham FL. Transformation by and oncogenicity of human adenoviruses. In: Ginsberg HS, editor. The Adenoviruses: The Viruses. Plenum Press; New York: 1984. pp. 339–398. [Google Scholar]
- Green M, Mackey JK, Wold WSM, Rigden P. Thirty-one human adenovirus serotypes (Ad1–Ad31) form five groups (A–E) based upon DNA genome homologies. Virology. 1979;93:481–492. doi: 10.1016/0042-6822(79)90251-4. [DOI] [PubMed] [Google Scholar]
- Gudjonsson T, Adriance MC, Sternlicht MD, Petersen OW, Bissell MJ. Myoepithelial cells: their origin and function in breast morphogenesis and neoplasia. J Mammary Gland Biol Neoplasia. 2005;10:261–272. doi: 10.1007/s10911-005-9586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson T, Ronnov-Jessen L, Villadsen R, Rank F, Bissell MJ, Petersen OW. Normal and tumor-derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J Cell Sci. 2002a;115:39–50. doi: 10.1242/jcs.115.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson T, Villadsen R, Nielsen HL, Ronnov-Jessen L, Bissell MJ, Petersen OW. Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes Dev. 2002b;16:693–706. doi: 10.1101/gad.952602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halbert DN, Cutt JR, Shenk T. Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J Virol. 1985;56:250–257. doi: 10.1128/jvi.56.1.250-257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamazaki Y, Itoh M, Sasaki H, Furuse M, Tsukita S. Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J Biol Chem. 2002;277:455–461. doi: 10.1074/jbc.M109005200. [DOI] [PubMed] [Google Scholar]
- Harris BZ, Lim WA. Mechanism and role of PDZ domains in signaling complex assembly. J Cell Sci. 2001;114:3219–3231. doi: 10.1242/jcs.114.18.3219. [DOI] [PubMed] [Google Scholar]
- Haskins J, Gu L, Wittchen ES, Hibbard J, Stevenson BR. ZO-3, a novel member of the MAGUK protein family found at the tight junction, interacts with ZO-1 and occludin. J Cell Biol. 1998;141:199–208. doi: 10.1083/jcb.141.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herisse J, Rigolet M, de Dinechin SD, Galibert F. Nucleotide sequence of adenovirus 2 DNA fragment encoding for the carboxylic region of fiber protein and the entire E4 region. Nucleic Acids Res. 1981;9:4023–4042. doi: 10.1093/nar/9.16.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez S, Chavez Munguia B, Gonzalez-Mariscal L. ZO-2 silencing in epithelial cells perturbs the gate and fence function of tight junctions and leads to an atypical monolayer architecture. Exp Cell Res. 2007;313:1533–1547. doi: 10.1016/j.yexcr.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Heydecke D, Meyer D, Ackermann F, Wilhelm B, Gudermann T, Boekhoff I. The multi PDZ domain protein MUPP1 as a putative scaffolding protein for organizing signaling complexes in the acrosome of mammalian spermatozoa. J Androl. 2006;27:390–404. doi: 10.2164/jandrol.05166. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Masuyama N, Fukui Y, Suzuki A, Gotoh Y. Akt mediates Rac/Cdc42-regulated cell motility in growth factor-stimulated cells and in invasive PTEN knockout cells. Curr Biol. 2001;11:1958–1962. doi: 10.1016/s0960-9822(01)00599-1. [DOI] [PubMed] [Google Scholar]
- Higuchi M, Tsubata C, Kondo R, Yoshida S, Takahashi M, Oie M, et al. Cooperation of NF-{kappa}B2/p100 activation and the PDZ domain binding motif signal in human T-cell leukemia virus type 1 (HTLV) Tax1 but not HTLV-2 Tax2 is crucial for interleukin-2 independent growth transformation of a T cell line. J Virol. 2007;81:11900–11907. doi: 10.1128/JVI.00532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi S, Tajima M, Yao I, Nishimura W, Mori H, Hata Y. JAM4, a junctional cell adhesion molecule interacting with a tight junction protein, MAGI-1. Mol Cell Biol. 2003;23:4267–4282. doi: 10.1128/MCB.23.12.4267-4282.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Higuchi M, Niinuma A, Ohashi M, Fukushi M, Oie M, et al. PDZ domain-binding motif of human T-cell leukemia virus type 1 Tax oncoprotein augments the transforming activity in a rat fibroblast cell line. Virology. 2004;318:327–336. doi: 10.1016/j.virol.2003.10.006. [DOI] [PubMed] [Google Scholar]
- Hogenkamp T, Esche H. Nucleotide sequence of the right 10% of adenovirus type 12 DNA encoding the entire region E4. Nucleic Acids Res. 1990;18:3065–3066. doi: 10.1093/nar/18.10.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz MS. Adenoviruses. In: Knipe DM, Howley PM, editors. Fields Virology. Vol. 2. Lippincott, Williams and Wilkins; Philadelphia: 2001. pp. 2301–2326. [Google Scholar]
- Houweling A, van den Elsen PJ, van der Eb AJ. Partial transformation of primary rat cells by the left-most 4.5% fragment of adenovirus 5 DNA. Virology. 1980;105:537–550. doi: 10.1016/0042-6822(80)90054-9. [DOI] [PubMed] [Google Scholar]
- Huang MM, Hearing P. Adenovirus early region 4 encodes two gene products with redundant effects in lytic infection. J Virol. 1989a;63:2605–2615. doi: 10.1128/jvi.63.6.2605-2615.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MM, Hearing P. The adenovirus early region 4 open reading frame 6/7 protein regulates the DNA binding activity of the cellular transcription factor, E2F, through a direct complex. Genes Dev. 1989b;3:1699–1710. doi: 10.1101/gad.3.11.1699. [DOI] [PubMed] [Google Scholar]
- Humbert P, Russell S, Richardson H. Dlg, Scribble and Lgl in cell polarity, cell proliferation and cancer. Bioessays. 2003;25:542–553. doi: 10.1002/bies.10286. [DOI] [PubMed] [Google Scholar]
- Humbert PO, Dow LE, Russell SM. The Scribble and Par complexes in polarity and migration: friends or foes? Trends Cell Biol. 2006;16:622–630. doi: 10.1016/j.tcb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Iizuka-Kogo A, Ishidao T, Akiyama T, Senda T. Abnormal development of urogenital organs in Dlgh1-deficient mice. Development. 2007;134:1799–1807. doi: 10.1242/dev.02830. [DOI] [PubMed] [Google Scholar]
- Inoue M, Chiang SH, Chang L, Chen XW, Saltiel AR. Compartmentalization of the exocyst complex in lipid rafts controls Glut4 vesicle tethering. Mol Biol Cell. 2006;17:2303–2311. doi: 10.1091/mbc.E06-01-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishidate T, Matsumine A, Toyoshima K, Akiyama T. The APC-hDLG complex negatively regulates cell cycle progression from the G0/G1 to S phase. Oncogene. 2000;19:365–372. doi: 10.1038/sj.onc.1203309. [DOI] [PubMed] [Google Scholar]
- Ishioka K, Higuchi M, Takahashi M, Yoshida S, Oie M, Tanaka Y, et al. Inactivation of tumor suppressor Dlg1 augments transformation of a T-cell line induced by human T-cell leukemia virus type 1 Tax protein. Retrovirology. 2006;3:71. doi: 10.1186/1742-4690-3-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MA, Lee JH, Klingelhutz AJ. Human papillomavirus type 16 E6 activates NF-kappaB, induces cIAP-2 expression, and protects against apoptosis in a PDZ binding motif-dependent manner. J Virol. 2006;80:5301–5307. doi: 10.1128/JVI.01942-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javier R, Raska K, Jr, Macdonald GJ, Shenk T. Human adenovirus type 9-induced rat mammary tumors. J Virol. 1991;65:3192–3202. doi: 10.1128/jvi.65.6.3192-3202.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javier R, Raska K, Jr, Shenk T. Requirement for the adenovirus type 9 E4 region in production of mammary tumors. Science. 1992;257:1267–1271. doi: 10.1126/science.1519063. [DOI] [PubMed] [Google Scholar]
- Javier R, Shenk T. Mammary tumors induced by human adenovirus type 9: a role for the viral early region 4 gene. Breast Cancer Res Treat. 1996;39:57–67. doi: 10.1007/BF01806078. [DOI] [PubMed] [Google Scholar]
- Javier RT. Adenovirus type 9 E4 open reading frame 1 encodes a transforming protein required for the production of mammary tumors in rats. J Virol. 1994;68:3917–3924. doi: 10.1128/jvi.68.6.3917-3924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeansonne B, Lu Q, Goodenough DA, Chen YH. Claudin-8 interacts with multi-PDZ domain protein 1 (MUPP1) and reduces paracellular conductance in epithelial cells. Cell Mol Biol (Noisyle-grand) 2003;49:13–21. [PubMed] [Google Scholar]
- Jesaitis LA, Goodenough DA. Molecular characterization and tissue distribution of ZO-2, a tight junction protein homologous to ZO-1 and the Drosophila discs-large tumor suppressor protein. J Cell Biol. 1994;124:949–961. doi: 10.1083/jcb.124.6.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2:531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- Kim SK. Tight junctions, membrane-associated guanylate kinases and cell signaling. Curr Opin Cell Biol. 1995;7:641–649. doi: 10.1016/0955-0674(95)80105-7. [DOI] [PubMed] [Google Scholar]
- Kimber WA, Trinkle-Mulcahy L, Cheung PC, Deak M, Marsden LJ, Kieloch A, et al. Evidence that the tandem-pleckstrin-homology-domain-containing protein TAPP1 interacts with Ptd(3,4)P2 and the multi-PDZ-domain-containing protein MUPP1 in vivo. Biochem J. 2002;361:525–536. doi: 10.1042/0264-6021:3610525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyono T, Hiraiwa A, Fujita M, Hayashi Y, Akiyama T, Ishibashi M. Binding of high-risk human papillomavirus E6 oncoproteins to the human homologue of the Drosophila discs large tumor suppressor protein. Proc Natl Acad Sci USA. 1997;94:11612–11616. doi: 10.1073/pnas.94.21.11612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo R, Higuchi M, Takahashi M, Oie M, Tanaka Y, Gejyo F, et al. Human T-cell leukemia virus type 2 Tax protein induces interleukin 2-independent growth in a T-cell line. Retrovirology. 2006;3:88. doi: 10.1186/1742-4690-3-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Kotelevets L, van Hengel J, Bruyneel E, Mareel M, van Roy F, Chastre E. Implication of the MAGI-1b/PTEN signalosome in stabilization of adherens junctions and suppression of invasiveness. FASEB J. 2005;19:115–117. doi: 10.1096/fj.04-1942fje. [DOI] [PubMed] [Google Scholar]
- Krummel MF, Macara I. Maintenance and modulation of T cell polarity. Nat Immunol. 2006;7:1143–1149. doi: 10.1038/ni1404. [DOI] [PubMed] [Google Scholar]
- Kuballa P, Matentzoglu K, Scheffner M. The role of the ubiquitin ligase E6-AP in human papillomavirus E6-mediated degradation of PDZ domain-containing proteins. J Biol Chem. 2007;282:65–71. doi: 10.1074/jbc.M605117200. [DOI] [PubMed] [Google Scholar]
- Kuhne C, Gardiol D, Guarnaccia C, Amenitsch H, Banks L. Differential regulation of human papillomavirus E6 by protein kinase A: conditional degradation of human discs large protein by oncogenic E6. Oncogene. 2000;19:5884–5891. doi: 10.1038/sj.onc.1203988. [DOI] [PubMed] [Google Scholar]
- Lakhani S, Bissell M. Introduction: the role of myoepithelial cells in integration of form and function in the mammary gland. J Mammary Gland Biol Neoplasia. 2005;10:197–198. doi: 10.1007/s10911-005-9580-x. [DOI] [PubMed] [Google Scholar]
- Lallemand D, Curto M, Saotome I, Giovannini M, McClatchey AI. NF2 deficiency promotes tumorigenesis and metastasis by destabilizing adherens junctions. Genes Dev. 2003;17:1090–1100. doi: 10.1101/gad.1054603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprise P, Viel A, Rivard N. Human homolog of disc-large is required for adherens junction assembly and differentiation of human intestinal epithelial cells. J Biol Chem. 2004;279:10157–10166. doi: 10.1074/jbc.M309843200. [DOI] [PubMed] [Google Scholar]
- Latorre IJ, Roh MH, Frese KK, Weiss RS, Margolis B, Javier RT. Viral oncoprotein-induced mislocalization of select PDZ proteins disrupts tight junctions and causes polarity defects in epithelial cells. J Cell Sci. 2005;118:4283–4293. doi: 10.1242/jcs.02560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laura RP, Ross S, Koeppen H, Lasky LA. MAGI-1: a widely expressed, alternatively spliced tight junction protein. Exp Cell Res. 2002;275:155–170. doi: 10.1006/excr.2002.5475. [DOI] [PubMed] [Google Scholar]
- Lee C, Laimins LA. Role of the PDZ domain-binding motif of the oncoprotein E6 in the pathogenesis of human papillomavirus type 31. J Virol. 2004;78:12366–12377. doi: 10.1128/JVI.78.22.12366-12377.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee OK, Frese KK, James JS, Chadda D, Chen ZH, Javier RT, et al. Discs-large and strabismus are functionally linked to plasma membrane formation. Nat Cell Biol. 2003;5:987–993. doi: 10.1038/ncb1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Glaunsinger B, Mantovani F, Banks L, Javier RT. Multi-PDZ domain protein MUPP1 is a cellular target for both adenovirus E4-ORF1 and high-risk papillomavirus type 18 E6 oncoproteins. J Virol. 2000;74:9680–9693. doi: 10.1128/jvi.74.20.9680-9693.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Weiss RS, Javier RT. Binding of human virus oncoproteins to hDlg/SAP97, a mammalian homolog of the Drosophila discs large tumor suppressor protein. Proc Natl Acad Sci USA. 1997;94:6670–6675. doi: 10.1073/pnas.94.13.6670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmers C, Medina E, Delgrossi MH, Michel D, Arsanto JP, Le Bivic A. hINADl/PATJ, a homolog of discs lost, interacts with crumbs and localizes to tight junctions in human epithelial cells. J Biol Chem. 2002;277:25408–25415. doi: 10.1074/jbc.M202196200. [DOI] [PubMed] [Google Scholar]
- Leonoudakis D, Conti LR, Radeke CM, McGuire LM, Vandenberg CA. A multiprotein trafficking complex composed of SAP97, CASK, Veli, and Mint1 is associated with inward rectifier Kir2 potassium channels. J Biol Chem. 2004;279:19051–19063. doi: 10.1074/jbc.M400284200. [DOI] [PubMed] [Google Scholar]
- Levin ER. Cellular functions of plasma membrane estrogen receptors. Steroids. 2002;67:471–475. doi: 10.1016/s0039-128x(01)00179-9. [DOI] [PubMed] [Google Scholar]
- Levine AJ. The p53 protein and its interactions with the oncogene products of the small DNA tumor viruses. Virology. 1990;177:419–426. doi: 10.1016/0042-6822(90)90505-l. [DOI] [PubMed] [Google Scholar]
- Luconi M, Forti G, Baldi E. Genomic and nongenomic effects of estrogens: molecular mechanisms of action and clinical implications for male reproduction. J Steroid Biochem Mol Biol. 2002;80:369–381. doi: 10.1016/s0960-0760(02)00041-9. [DOI] [PubMed] [Google Scholar]
- Ludford-Menting MJ, Oliaro J, Sacirbegovic F, Cheah ET, Pedersen N, Thomas SJ, et al. A network of PDZ-containing proteins regulates T cell polarity and morphology during migration and immunological synapse formation. Immunity. 2005;22:737–748. doi: 10.1016/j.immuni.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Mahieux R, Gessain A. HTLV-1 and associated adult T-cell leukemia/lymphoma. Rev Clin Exp Hematol. 2003;7:336–361. [PubMed] [Google Scholar]
- Mahoney ZX, Sammut B, Xavier RJ, Cunningham J, Go G, Brim KL, et al. Discs-large homolog 1 regulates smooth muscle orientation in the mouse ureter. Proc Natl Acad Sci USA. 2006;103:19872–19877. doi: 10.1073/pnas.0609326103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani F, Banks L. The human papillomavirus E6 protein and its contribution to malignant progression. Oncogene. 2001;20:7874–7887. doi: 10.1038/sj.onc.1204869. [DOI] [PubMed] [Google Scholar]
- Marcello E, Gardoni F, Mauceri D, Romorini S, Jeromin A, Epis R, et al. Synapse-associated protein-97 mediates alpha-secretase ADAM10 trafficking and promotes its activity. J Neurosci. 2007;27:1682–1691. doi: 10.1523/JNEUROSCI.3439-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin TA, Watkins G, Mansel RE, Jiang WG. Loss of tight junction plaque molecules in breast cancer tissues is associated with a poor prognosis in patients with breast cancer. Eur J Cancer. 2004;40:2717–2725. doi: 10.1016/j.ejca.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Massimi P, Gammoh N, Thomas M, Banks L. HPV E6 specifically targets different cellular pools of its PDZ domain-containing tumour suppressor substrates for proteasome-mediated degradation. Oncogene. 2004;23:8033–8039. doi: 10.1038/sj.onc.1207977. [DOI] [PubMed] [Google Scholar]
- Massimi P, Narayan N, Cuenda A, Banks L. Phosphorylation of the discs large tumour suppressor protein controls its membrane localisation and enhances its susceptibility to HPV E6-induced degradation. Oncogene. 2006;25:4276–4285. doi: 10.1038/sj.onc.1209457. [DOI] [PubMed] [Google Scholar]
- Matsumine A, Ogai A, Senda T, Okumura N, satoh K, Baeg GH, et al. Binding of APC to the human homolog of the Drosophila discs large tumor suppressor protein. Science. 1996;272:1020–1023. doi: 10.1126/science.272.5264.1020. [DOI] [PubMed] [Google Scholar]
- Matsumoto Y, Nakagawa S, Yano T, Takizawa S, Nagasaka K, Nakagawa K, et al. Involvement of a cellular ubiquitin-protein ligase E6AP in the ubiquitin-mediated degradation of extensive substrates of high-risk human papillomavirus E6. J Med Virol. 2006;78:501–507. doi: 10.1002/jmv.20568. [DOI] [PubMed] [Google Scholar]
- Matsuoka M, Jeang KT. Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation. Nat Rev Cancer. 2007;7:270–280. doi: 10.1038/nrc2111. [DOI] [PubMed] [Google Scholar]
- Matter K, Balda MS. Signalling to and from tight junctions. Nat Rev Mol Cell Biol. 2003;4:225–236. doi: 10.1038/nrm1055. [DOI] [PubMed] [Google Scholar]
- Mauceri D, Gardoni F, Marcello E, Di Luca M. Dual role of CaMKII-dependent SAP97 phosphorylation in mediating trafficking and insertion of NMDA receptor subunit NR2A. J Neurochem. 2007;100:1032–1046. doi: 10.1111/j.1471-4159.2006.04267.x. [DOI] [PubMed] [Google Scholar]
- McDonnell DP, Norris JD. Connections and regulation of the human estrogen receptor. Science. 2002;296:1642–1644. doi: 10.1126/science.1071884. [DOI] [PubMed] [Google Scholar]
- Mechtersheimer G, Kruger KH, Born IA, Moller P. Antigenic profile of mammary fibroadenoma and cystosarcoma phyllodes. A study using antibodies to estrogen- and progesterone receptors and to a panel of cell surface molecules. Pathol Res Pract. 1990;186:427–438. doi: 10.1016/S0344-0338(11)80460-7. [DOI] [PubMed] [Google Scholar]
- Medema RH, Kops GJ, Bos JL, Burgering BM. AFX-like Forkhead transcription factors mediate cell-cycle regulation by Ras and PKB through p27kip1. Nature. 2000;404:782–787. doi: 10.1038/35008115. [DOI] [PubMed] [Google Scholar]
- Michel D, Arsanto JP, Massey-Harroche D, Beclin C, Wijnholds J, Le Bivic A. PATJ connects and stabilizes apical and lateral components of tight junctions in human intestinal cells. J Cell Sci. 2005;118:4049–4057. doi: 10.1242/jcs.02528. [DOI] [PubMed] [Google Scholar]
- Mimori-Kiyosue Y, Matsui C, Sasaki H, Tsukita S. Adenomatous polyposis coli (APC) protein regulates epithelial cell migration and morphogenesis via PDZ domain-based interactions with plasma membranes. Genes Cells. 2007;12:219–233. doi: 10.1111/j.1365-2443.2007.01045.x. [DOI] [PubMed] [Google Scholar]
- Mol CD, Harris JM, McIntosh EM, Tainer JA. Human dUTP pyrophosphatase: uracil recognition by a beta hairpin and active sites formed by three separate subunits. Structure. 1996;4:1077–1092. doi: 10.1016/s0969-2126(96)00114-1. [DOI] [PubMed] [Google Scholar]
- Moore M, Horikoshi N, Shenk T. Oncogenic potential of the adenovirus E4orf6 protein. Proc Natl Acad Sci USA. 1996;93:11295–11301. doi: 10.1073/pnas.93.21.11295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller BM, Kistner U, Veh RW, Cases-Langhoff C, Becker B, Gundelfinger ED, et al. Molecular characterization and spatial distribution of SAP97, a novel presynaptic protein homologous to SAP90 and the Drosophila discs-large tumor suppressor protein. J Neurosci. 1995;15:2354–2366. doi: 10.1523/JNEUROSCI.15-03-02354.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller U, Kleinberger T, Shenk T. Adenovirus E4orf4 protein reduces phosphorylation of c-Fos and E1A proteins while simultaneously reducing the level of AP-1. J Virol. 1992;66:5867–5878. doi: 10.1128/jvi.66.10.5867-5878.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata M, Kojima T, Yamamoto T, Go M, Takano K, Chiba H, et al. Tight junction protein MAGI-1 is up-regulated by transfection with connexin 32 in an immortalized mouse hepatic cell line: cDNA microarray analysis. Cell Tissue Res. 2005;319:341–347. doi: 10.1007/s00441-004-1017-0. [DOI] [PubMed] [Google Scholar]
- Naim E, Bernstein A, Bertram JF, Caruana G. Mutagenesis of the epithelial polarity gene, discs large 1, perturbs nephrogenesis in the developing mouse kidney. Kidney Int. 2005;68:955–965. doi: 10.1111/j.1523-1755.2005.00489.x. [DOI] [PubMed] [Google Scholar]
- Nakagawa S, Huibregtse JM. Human scribble (Vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin-protein ligase. Mol Cell Biol. 2000;20:8244–8253. doi: 10.1128/mcb.20.21.8244-8253.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa S, Yano T, Nakagawa K, Takizawa S, Suzuki Y, Yasugi T, et al. Analysis of the expression and localisation of a LAP protein, human scribble, in the normal and neoplastic epithelium of uterine cervix. Br J Cancer. 2004;90:194–199. doi: 10.1038/sj.bjc.6601465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro C, Nola S, Audebert S, Santoni MJ, Arsanto JP, Ginestier C, et al. Junctional recruitment of mammalian Scribble relies on E-cadherin engagement. Oncogene. 2005;24:4330–4339. doi: 10.1038/sj.onc.1208632. [DOI] [PubMed] [Google Scholar]
- Nevels M, Rubenwolf S, Spruss T, Wolf H, Dobner T. The adenovirus E4orf6 protein can promote E1A/E1B-induced focus formation by interfering with p53 tumor suppressor function. Proc Natl Acad Sci USA. 1997;94:1206–1211. doi: 10.1073/pnas.94.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevels M, Rubenwolf S, Spruss T, Wolf H, Dobner T. Two distinct activities contribute to the oncogenic potential of the adenovirus type 5 E4orf6 protein. J Virol. 2000;74:5168–5181. doi: 10.1128/jvi.74.11.5168-5181.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevels M, Spruss T, Wolf H, Dobner T. The adenovirus E4orf6 protein contributes to malignant transformation by antagonizing E1A-induced accumulation of the tumor suppressor protein p53. Oncogene. 1999a;18:9–17. doi: 10.1038/sj.onc.1202284. [DOI] [PubMed] [Google Scholar]
- Nevels M, Tauber B, Kremmer E, Spruss T, Wolf H, Dobner T. Transforming potential of the adenovirus type 5 E4orf3 protein. J Virol. 1999b;73:1591–1600. doi: 10.1128/jvi.73.2.1591-1600.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevels M, Tauber B, Spruss T, Wolf H, Dobner T. ‘Hit-and-run’ transformation by adenovirus oncogenes. J Virol. 2001;75:3089–3094. doi: 10.1128/JVI.75.7.3089-3094.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen ML, Nguyen MM, Lee D, Griep AE, Lambert PF. The PDZ ligand domain of the human papillomavirus type 16 E6 protein is required for E6’s induction of epithelial hyperplasia in vivo. J Virol. 2003a;77:6957–6964. doi: 10.1128/JVI.77.12.6957-6964.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MM, Nguyen ML, Caruana G, Bernstein A, Lambert PF, Griep AE. Requirement of PDZ-containing proteins for cell cycle regulation and differentiation in the mouse lens epithelium. Mol Cell Biol. 2003b;23:8970–8981. doi: 10.1128/MCB.23.24.8970-8981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunbhakdi-Craig V, Craig L, Machleidt T, Sontag E. Simian virus 40 small tumor antigen induces deregulation of the actin cytoskeleton and tight junctions in kidney epithelial cells. J Virol. 2003;77:2807–2818. doi: 10.1128/JVI.77.5.2807-2818.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunbhakdi-Craig V, Machleidt T, Ogris E, Bellotto D, White CL, III, Sontag E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J Cell Biol. 2002;158:967–978. doi: 10.1083/jcb.200206114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea C, Klupsch K, Choi S, Bagus B, Soria C, Shen J, et al. Adenoviral proteins mimic nutrient/growth signals to activate the mTOR pathway for viral replication. EMBO J. 2005a;24:1211–1221. doi: 10.1038/sj.emboj.7600597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea CC, Choi S, McCormick F, Stokoe D. Adenovirus overrides cellular checkpoints for protein translation. Cell Cycle. 2005b;4:883–888. doi: 10.4161/cc.4.7.1791. [DOI] [PubMed] [Google Scholar]
- Ohashi M, Sakurai M, Higuchi M, Mori N, Fukushi M, Oie M, et al. Human T-cell leukemia virus type 1 Tax oncoprotein induces and interacts with a multi-PDZ domain protein, MAGI-3. Virology. 2004;320:52–62. doi: 10.1016/j.virol.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Onishi K, Higuchi M, Asakura T, Masuyama N, Gotoh Y. The PI3K-Akt pathway promotes microtubule stabilization in migrating fibroblasts. Genes Cells. 2007;12:535–546. doi: 10.1111/j.1365-2443.2007.01071.x. [DOI] [PubMed] [Google Scholar]
- Osmani N, Vitale N, Borg JP, Etienne-Manneville S. Scrib controls Cdc42 localization and activity to promote cell polarization during astrocyte migration. Curr Biol. 2006;16:2395–2405. doi: 10.1016/j.cub.2006.10.026. [DOI] [PubMed] [Google Scholar]
- Pagliarini RA, Xu T. A genetic screen in Drosophila for metastatic behavior. Science. 2003;302:1227–1231. doi: 10.1126/science.1088474. [DOI] [PubMed] [Google Scholar]
- Patrie KM, Drescher AJ, Welihinda A, Mundel P, Margolis B. Interaction of two actin-binding proteins, synaptopodin and alpha-actinin-4, with the tight junction protein MAGI-1. J Biol Chem. 2002;277:30183–30190. doi: 10.1074/jbc.M203072200. [DOI] [PubMed] [Google Scholar]
- Pechoux C, Gudjonsson T, Ronnov-Jessen L, Bissell MJ, Petersen OW. Human mammary luminal epithelial cells contain progenitors to myoepithelial cells. Dev Biol. 1999;206:88–99. doi: 10.1006/dbio.1998.9133. [DOI] [PubMed] [Google Scholar]
- Philipp S, Flockerzi V. Molecular characterization of a novel human PDZ domain protein with homology to INAD from Drosophila melanogaster. FEBS Lett. 1997;413:243–248. doi: 10.1016/s0014-5793(97)00877-6. [DOI] [PubMed] [Google Scholar]
- Pim D, Thomas M, Banks L. Chimaeric HPV E6 proteins allow dissection of the proteolytic pathways regulating different E6 cellular target proteins. Oncogene. 2002;21:8140–8148. doi: 10.1038/sj.onc.1206026. [DOI] [PubMed] [Google Scholar]
- Pim D, Thomas M, Javier R, Gardiol D, Banks L. HPV E6 targeted degradation of the discs large protein: evidence for the involvement of a novel ubiquitin ligase. Oncogene. 2000;19:719–725. doi: 10.1038/sj.onc.1203374. [DOI] [PubMed] [Google Scholar]
- Polyak K, Hu M. Do myoepithelial cells hold the key for breast tumor progression? J Mammary Gland Biol Neoplasia. 2005;10:231–247. doi: 10.1007/s10911-005-9584-6. [DOI] [PubMed] [Google Scholar]
- Prasad R, Gu Y, Alder H, Nakamura T, Canaani O, Saito H, et al. Cloning of the ALL-1 fusion partner, the AF-6 gene, involved in acute myeloid leukemias with the t(6;11) chromosome translocation. Cancer Res. 1993;53:5624–5628. [PubMed] [Google Scholar]
- Primo L, di Blasio L, Roca C, Droetto S, Piva R, Schaffhausen B, et al. Essential role of PDK1 in regulating endothelial cell migration. J Cell Biol. 2007;176:1035–1047. doi: 10.1083/jcb.200607053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Procko E, McColl SR. Leukocytes on the move with phosphoinositide 3-kinase and its downstream effectors. Bioessays. 2005;27:153–163. doi: 10.1002/bies.20157. [DOI] [PubMed] [Google Scholar]
- Qin Y, Capaldo C, Gumbiner BM, Macara IG. The mammalian Scribble polarity protein regulates epithelial cell adhesion and migration through E-cadherin. J Cell Biol. 2005;171:1061–1071. doi: 10.1083/jcb.200506094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeaud F, Hailfinger S, Thome M. Dlgh1 and Carma1 MAGUK proteins contribute to signal specificity downstream of TCR activation. Trends Immunol. 2007;28:196–200. doi: 10.1016/j.it.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Reuver SM, Garner CC. E-cadherin mediated cell adhesion recruits SAP97 into the cortical cytoskeleton. J Cell Sci. 1998;111:1071–1080. doi: 10.1242/jcs.111.8.1071. [DOI] [PubMed] [Google Scholar]
- Rincon M, Davis RJ. Choreography of MAGUKs during T cell activation. Nat Immunol. 2007;8:126–127. doi: 10.1038/ni0207-126. [DOI] [PubMed] [Google Scholar]
- Round JL, Humphries LA, Tomassian T, Mittelstadt P, Zhang M, Miceli MC. Scaffold protein Dlgh1 coordinates alternative p38 kinase activation, directing T cell receptor signals toward NFAT but not NF-kappaB transcription factors. Nat Immunol. 2007;8:154–161. doi: 10.1038/ni1422. [DOI] [PubMed] [Google Scholar]
- Round JL, Tomassian T, Zhang M, Patel V, Schoenberger SP, Miceli MC. Dlgh1 coordinates actin polymerization, synaptic T cell receptor and lipid raft aggregation, and effector function in T cells. J Exp Med. 2005;201:419–430. doi: 10.1084/jem.20041428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousset R, Fabre S, Desbois C, Bantignies F, Jalinot P. The C-terminus of the HTLV-1 Tax oncoprotein mediates interaction with the PDZ domain of cellular proteins. Oncogene. 1998;16:643–654. doi: 10.1038/sj.onc.1201567. [DOI] [PubMed] [Google Scholar]
- Sakurai A, Fukuhara S, Yamagishi A, Sako K, Kamioka Y, Masuda M, et al. MAGI-1 is required for Rap1 activation upon cell-cell contact and for enhancement of vascular endothelial cadherin-mediated cell adhesion. Mol Biol Cell. 2006;17:966–976. doi: 10.1091/mbc.E05-07-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saras J, Heldin CH. PDZ domains bind carboxy-terminal sequences of target proteins. Trends Biochem Sci. 1996;21:455–458. doi: 10.1016/s0968-0004(96)30044-3. [DOI] [PubMed] [Google Scholar]
- Sato N, Fukushima N, Maitra A, Matsubayashi H, Yeo CJ, Cameron JL, et al. Discovery of novel targets for aberrant methylation in pancreatic carcinoma using high-throughput microarrays. Cancer Res. 2003;63:3735–3742. [PubMed] [Google Scholar]
- Sato T, Irie S, Kitada S, Reed JC. FAP-1: a protein tyrosine phosphatase that associates with Fas. Science. 1995;268:411–415. doi: 10.1126/science.7536343. [DOI] [PubMed] [Google Scholar]
- Schaley J, O’Connor RJ, Taylor LJ, Bar-Sagi D, Hearing P. Induction of the cellular E2F-1 promoter by the adenovirus E4-6/7 protein. J Virol. 2000;74:2084–2093. doi: 10.1128/jvi.74.5.2084-2093.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shai A, Brake T, Somoza C, Lambert PF. The human papillomavirus E6 oncogene dysregulates the cell cycle and contributes to cervical carcinogenesis through two independent activities. Cancer Res. 2007;67:1626–1635. doi: 10.1158/0008-5472.CAN-06-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M. PDZs and receptor/channel clustering: rounding up the latest suspects. Neuron. 1996;17:575–578. doi: 10.1016/s0896-6273(00)80190-7. [DOI] [PubMed] [Google Scholar]
- Sheng M, Kim E. Ion channel associated proteins. Curr Opin Neurobiol. 1996;6:602–608. doi: 10.1016/s0959-4388(96)80091-2. [DOI] [PubMed] [Google Scholar]
- Shenk T. Adenoviridae: the viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Vol. 2. Lippincott, Williams and Wilkins; Philadelphia: 2001. pp. 2265–2300. [Google Scholar]
- Shenk T, Flint J. Transcriptional and transforming activities of the adenovirus E1A proteins. Adv Cancer Res. 1991;57:47–85. doi: 10.1016/s0065-230x(08)60995-1. [DOI] [PubMed] [Google Scholar]
- Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235. doi: 10.1146/annurev.cellbio.22.010305.104219. [DOI] [PubMed] [Google Scholar]
- Shin K, Straight S, Margolis B. PATJ regulates tight junction formation and polarity in mammalian epithelial cells. J Cell Biol. 2005;168:705–711. doi: 10.1083/jcb.200408064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K, Wang Q, Margolis B. PATJ regulates directional migration of mammalian epithelial cells. EMBO Rep. 2007;8:158–164. doi: 10.1038/sj.embor.7400890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegrist SE, Doe CQ. Microtubule-induced cortical cell polarity. Genes Dev. 2007;21:483–496. doi: 10.1101/gad.1511207. [DOI] [PubMed] [Google Scholar]
- Simonson SJ, Difilippantonio MJ, Lambert PF. Two distinct activities contribute to human papillomavirus 16 E6’s oncogenic potential. Cancer Res. 2005;65:8266–8273. doi: 10.1158/0008-5472.CAN-05-1651. [DOI] [PubMed] [Google Scholar]
- Soler AP, Miller RD, Laughlin KV, Carp NZ, Klurfeld DM, Mullin JM. Increased tight junctional permeability is associated with the development of colon cancer. Carcinogenesis. 1999;20:1425–1431. doi: 10.1093/carcin/20.8.1425. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Fanning AS, Fu C, Xu J, Marfatia SM, Chishti AH, et al. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science. 1997;275:73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- Stillman B. Functions of the adenovirus E1B tumour antigens. Cancer Surv. 1986;5:389–404. [PubMed] [Google Scholar]
- Storrs CH, Silverstein SJ. PATJ, a tight junction-associated PDZ protein, is a novel degradation target of high-risk human papillomavirus E6 and the alternatively spliced isoform 18 E6. J Virol. 2007;81:4080–4090. doi: 10.1128/JVI.02545-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracker TH, Carson CT, Weitzman MD. Adenovirus oncoproteins inactivate the Mre11-Rad50-NBS1 DNA repair complex. Nature. 2002;418:348–352. doi: 10.1038/nature00863. [DOI] [PubMed] [Google Scholar]
- Stucke VM, Timmerman E, Vandekerckhove J, Gevaert K, Hall A. The MAGUK protein MPP7 binds to the polarity protein hDlg1 and facilitates epithelial tight junction formation. Mol Biol Cell. 2007;18:1744–1755. doi: 10.1091/mbc.E06-11-0980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugihara-Mizuno Y, Adachi M, Kobayashi Y, Hamazaki Y, Nishimura M, Imai T, et al. Molecular characterization of angiomotin/JEAP family proteins: interaction with MUPP1/Patj and their endogenous properties. Genes Cells. 2007;12:473–486. doi: 10.1111/j.1365-2443.2007.01066.x. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J Cell Sci. 2006;119:979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Ohsugi Y, Uchida-Toita M, Akiyama T, Yoshida M. Tax oncoprotein of HTLV-1 binds to the human homologue of Drosophila discs large tumor suppressor protein, hDLG, and perturbs its function in cell growth control. Oncogene. 1999;18:5967–5972. doi: 10.1038/sj.onc.1203008. [DOI] [PubMed] [Google Scholar]
- Thomas DL, Schaack J, Vogel H, Javier R. Several E4 region functions influence mammary tumorigenesis by human adenovirus type 9. J Virol. 2001a;75:557–568. doi: 10.1128/JVI.75.2.557-568.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DL, Shin S, Jiang BH, Vogel H, Ross MA, Kaplitt M, et al. Early region 1 transforming functions are dispensable for mammary tumorigenesis by human adenovirus type 9. J Virol. 1999;73:3071–3079. doi: 10.1128/jvi.73.4.3071-3079.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Glaunsinger B, Pim D, Javier R, Banks L. HPV E6 and MAGUK protein interactions: determination of the molecular basis for specific protein recognition and degradation. Oncogene. 2001b;20:5431–5439. doi: 10.1038/sj.onc.1204719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Narayan N, Pim D, Tomaic V, Massimi P, Nagasaka K, et al. Human papillomaviruses, cervical cancer and cell polarity. Oncogene. 2008;27:7018–7030. doi: 10.1038/onc.2008.351. [DOI] [PubMed] [Google Scholar]
- Thomas U, Phannavong B, Muller B, Garner CC, Gundelfinger ED. Functional expression of rat synapse-associated proteins SAP97 and SAP102 in Drosophila dlg-1 mutants: effects on tumor suppression and synaptic bouton structure. Mech Dev. 1997;62:161–174. doi: 10.1016/s0925-4773(97)00658-8. [DOI] [PubMed] [Google Scholar]
- Trentin JJ, Yabe Y, Taylor G. The quest for human cancer viruses. Science. 1962;137:835–841. doi: 10.1126/science.137.3533.835. [DOI] [PubMed] [Google Scholar]
- Tsubata C, Higuchi M, Takahashi M, Oie M, Tanaka Y, Gejyo F, et al. PDZ domain-binding motif of human T-cell leukemia virus type 1 Tax oncoprotein is essential for the interleukin 2 independent growth induction of a T-cell line. Retrovirology. 2005;2:46. doi: 10.1186/1742-4690-2-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, et al. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Valiente M, Andres-Pons A, Gomar B, Torres J, Gil A, Tapparel C, et al. Binding of PTEN to specific PDZ domains contributes to PTEN protein stability and phosphorylation by microtubule-associated serine/threonine kinases. J Biol Chem. 2005;280:28936–28943. doi: 10.1074/jbc.M504761200. [DOI] [PubMed] [Google Scholar]
- Van den Elsen P, Houweling A, Van der Eb A. Expression of region E1b of human adenoviruses in the absence of region E1a is not sufficient for complete transformation. Virology. 1983;128:377–390. doi: 10.1016/0042-6822(83)90264-7. [DOI] [PubMed] [Google Scholar]
- Vermeer PD, Einwalter LA, Moninger TO, Rokhlina T, Kern JA, Zabner J, et al. Segregation of receptor and ligand regulates activation of epithelial growth factor receptor. Nature. 2003;422:322–326. doi: 10.1038/nature01440. [DOI] [PubMed] [Google Scholar]
- Wada H, Iwasaki M, Sato T, Masai I, Nishiwaki Y, Tanaka H, et al. Dual roles of zygotic and maternal Scribble1 in neural migration and convergent extension movements in zebrafish embryos. Development. 2005;132:2273–2285. doi: 10.1242/dev.01810. [DOI] [PubMed] [Google Scholar]
- Wang TG, Ye J, Lairmore MD, Green PL. In vitro cellular tropism of human T cell leukemia virus type 2. AIDS Res Hum Retroviruses. 2000;16:1661–1668. doi: 10.1089/08892220050193119. [DOI] [PubMed] [Google Scholar]
- Watson RA, Thomas M, Banks L, Roberts S. Activity of the human papillomavirus E6 PDZ-binding motif correlates with an enhanced morphological transformation of immortalized human keratinocytes. J Cell Sci. 2003;116:4925–4934. doi: 10.1242/jcs.00809. [DOI] [PubMed] [Google Scholar]
- Weiss RS, Javier RT. A carboxy-terminal region required by the adenovirus type 9 E4 ORF1 oncoprotein for transformation mediates direct binding to cellular polypeptides. J Virol. 1997;71:7873–7880. doi: 10.1128/jvi.71.10.7873-7880.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RS, Lee SS, Prasad BV, Javier RT. Human adenovirus early region 4 open reading frame 1 genes encode growth-transforming proteins that may be distantly related to dUTP pyrophosphatase enzymes. J Virol. 1997;71:1857–1870. doi: 10.1128/jvi.71.3.1857-1870.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RS, McArthur MJ, Javier RT. Human adenovirus type 9 E4 open reading frame 1 encodes a cytoplasmic transforming protein capable of increasing the oncogenicity of CREF cells. J Virol. 1996;70:862–872. doi: 10.1128/jvi.70.2.862-872.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman MD. Functions of the adenovirus E4 proteins and their impact on viral vectors. Front Biosci. 2005;10:1106–1117. doi: 10.2741/1604. [DOI] [PubMed] [Google Scholar]
- Weitzman MD, Ornelles DA. Inactivating intracellular antiviral responses during adenovirus infection. Oncogene. 2005;24:7686–7696. doi: 10.1038/sj.onc.1209063. [DOI] [PubMed] [Google Scholar]
- Willott E, Balda MS, Fanning AS, Jameson B, Van Itallie C, Anderson JM. The tight junction protein ZO-1 is homologous to the Drosophila discs-large tumor suppressor protein of septate junctions. Proc Natl Acad Sci USA. 1993;90:7834–7838. doi: 10.1073/pnas.90.16.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Gonzalez C. Connecting cancer to the asymmetric division of stem cells. Cell. 2006;124:1121–1123. doi: 10.1016/j.cell.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nathke I. Cell polarity in development and cancer. Nat Cell Biol. 2007;9:1016–1024. doi: 10.1038/ncb433. [DOI] [PubMed] [Google Scholar]
- Woods DF, Bryant PJ. Molecular cloning of the lethal(1) discs large-1 oncogene of Drosophila. Dev Biol. 1989;134:222–235. doi: 10.1016/0012-1606(89)90092-4. [DOI] [PubMed] [Google Scholar]
- Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- Woods DF, Hough C, Peel D, Callaini G, Bryant PJ. Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J Cell Biol. 1996;134:1469–1482. doi: 10.1083/jcb.134.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Nash JE, Zamorano P, Garner CC. Interaction of SAP97 with minus-end-directed actin motor myosin VI. Implications for AMPA receptor trafficking. J Biol Chem. 2002;277:30928–30934. doi: 10.1074/jbc.M203735200. [DOI] [PubMed] [Google Scholar]
- Wu H, Reuver SM, Kuhlendahl S, Chung WJ, Garner CC. Subcellular targeting and cytoskeletal attachment of SAP97 to the epithelial lateral membrane. J Cell Sci. 1998;111:2365–2376. doi: 10.1242/jcs.111.16.2365. [DOI] [PubMed] [Google Scholar]
- Xavier R, Rabizadeh S, Ishiguro K, Andre N, Ortiz JB, Wachtel H, et al. Discs large (Dlg1) complexes in lymphocyte activation. J Cell Biol. 2004;166:173–178. doi: 10.1083/jcb.200309044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Yamamoto B, Haoudi A, Semmes OJ, Green PL. PDZ binding motif of HTLV-1 Tax promotes virus-mediated T-cell proliferation in vitro and persistence in vivo. Blood. 2006;107:1980–1988. doi: 10.1182/blood-2005-03-1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KM, Araki M. Tumor suppressor PTEN: modulator of cell signaling, growth, migration and apoptosis. J Cell Sci. 2001;114:2375–2382. doi: 10.1242/jcs.114.13.2375. [DOI] [PubMed] [Google Scholar]
- Yamano S, Tokino T, Yasuda M, Kaneuchi M, Takahashi M, Niitsu Y, et al. Induction of transformation and p53-dependent apoptosis by adenovirus type 5 E4orf6/7 cDNA. J Virol. 1999;73:10095–10103. doi: 10.1128/jvi.73.12.10095-10103.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Sato JD. MAP kinases, phosphatidylinositol 3-kinase, and p70 S6 kinase mediate the mitogenic response of human endothelial cells to vascular endothelial growth factor. J Cell Physiol. 1999;178:235–246. doi: 10.1002/(SICI)1097-4652(199902)178:2<235::AID-JCP13>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]