Abstract
Oil-in-water adjuvants have been shown to improve immune responses against pandemic influenza vaccines as well as reduce the effective vaccine dose, increasing the number of doses available to meet global vaccine demand. Here, we use genome fragment phage display libraries and surface plasmon resonance to elucidate the effects of MF59 on the quantity, diversity, specificity, and affinity maturation of human antibody responses to the swine-origin H1N1 vaccine in different age groups. In adults and children, MF59 selectively enhanced antibody responses to the hemagglutinin 1 (HA1) globular head relative to the more conserved HA2 domain in terms of increased antibody titers as well as a more diverse antibody epitope repertoire. Antibody affinity, as inferred by greatly diminished (≥10-fold) off-rate constants, was significantly increased in toddlers and children who received the MF59-adjuvanted vaccine. Moreover, MF59 also improved antibody affinity maturation after each sequential vaccination against avian H5N1 in adults. For both pandemic influenza vaccines, there was a close correlation between serum antibody affinity and virus-neutralizing capacity. Thus, MF59 quantitatively and qualitatively enhances functional antibody responses to HA-based vaccines by improving both epitope breadth and binding affinity, demonstrating the added value of such adjuvants for influenza vaccines.
INTRODUCTION
The periodic introduction of novel influenza A viruses (IAVs) expressing the serologically distinct surface proteins hemagglutinin (HA) and neuraminidase (NA) can result in pandemics capable of causing millions of deaths worldwide. Inactivated influenza vaccines based on HA have been the most effective approach for controlling epidemics and diminishing the effects of pandemics. Because of the fast spread of pandemics, speed is of the essence in vaccinating the world population. Due to inherent difficulties in generating sufficient doses of vaccine, it is always an advantage to minimize the amount of antigen per effective dose. In the case of the highly pathogenic H5N1 virus, a feared potential pandemic virus, standard subunit vaccines (unadjuvanted) exhibited low immunogenicity even when used at high doses or with two or more injections (1, 2).
Improving IAV vaccines will likely require the inclusion of adjuvants, which enhance antigen presentation and innate responses (3) and boost immunogenicity. Only a few adjuvants are approved for human use. The longest used, alum, was not efficient in IAV vaccines (4, 5). On the other hand, oil-in-water adjuvants, such as MF59 and AS03, induced higher IAV-specific antibody seroconversion rates and heterosubtypic neutralization (against diverse H5N1 types), as well as allowed antigen dose sparing (4, 6–12).
We previously examined the effect of MF59 on the immunogenicity of the H5N1-inactivated vaccine in adults using whole IAV genome fragment phage display libraries (GFPDLs) expressing protein fragments from the corresponding HA and NA genes. MF59 shifted the focus of antibody responses from predominantly HA2 sequences (conserved between H5 and seasonal H1 strains) to sequences in HA1 [receptor binding domain (RBD)] and NA (sialic acid–binding site). The expanded antibody repertoires correlated with an increase in the titer of antibodies reactive with native HA and with viral neutralization (VN) (13).
Here, we describe multiple studies to evaluate the effects of MF59 on the antibody responses induced by swine-origin influenza virus (SOIV) H1N1 and H5N1 vaccines in various age groups. In addition to the antibody epitope repertoire, we have investigated antibody affinity using 7 M urea resistance and calculated antibody off-rate constants by surface plasmon resonance (SPR). Technically, because antibodies are bivalent, the proper term for their binding to multivalent antigens like viruses is avidity, but here we use the term affinity throughout because we do not describe any monovalent interactions. The contribution of affinity maturation to antiviral protection is surprisingly ill-defined, and indeed, the very existence of affinity maturation of antiviral antibodies has been questioned (14, 15).
Our findings support the importance of antibody affinity in antiviral immunity and demonstrate the effectiveness of MF59 in enhancing antibody targeting of relevant neutralizing domains and increasing antibody affinity maturation after repeated vaccination.
RESULTS
An MF59-adjuvanted H1N1 vaccine generates broader antibody epitope profiles in adults and children than the vaccine alone
Immunogenicity of the SOIV-H1N1 vaccine was evaluated in randomized studies of adults (18 to 60 years), children (3 to 8 years), and toddlers (12 to 35 months), who received either unadjuvanted vaccine (15 µg of HA per dose) or MF59-adjuvanted vaccine (7.5 µg of HA per dose). Adults received a single dose, whereas children and toddlers were boosted on day 21. Sera collected 21 days after each vaccination were tested for hemagglutination inhibition (HAI) and VN capacity. Serum samples with similar VN titers from the unadjuvanted and MF59-adjuvanted vaccine groups were tested by GFPDL to assess the specificity of anti-HA and anti-NA antibody responses (Figs. 1 to 3).
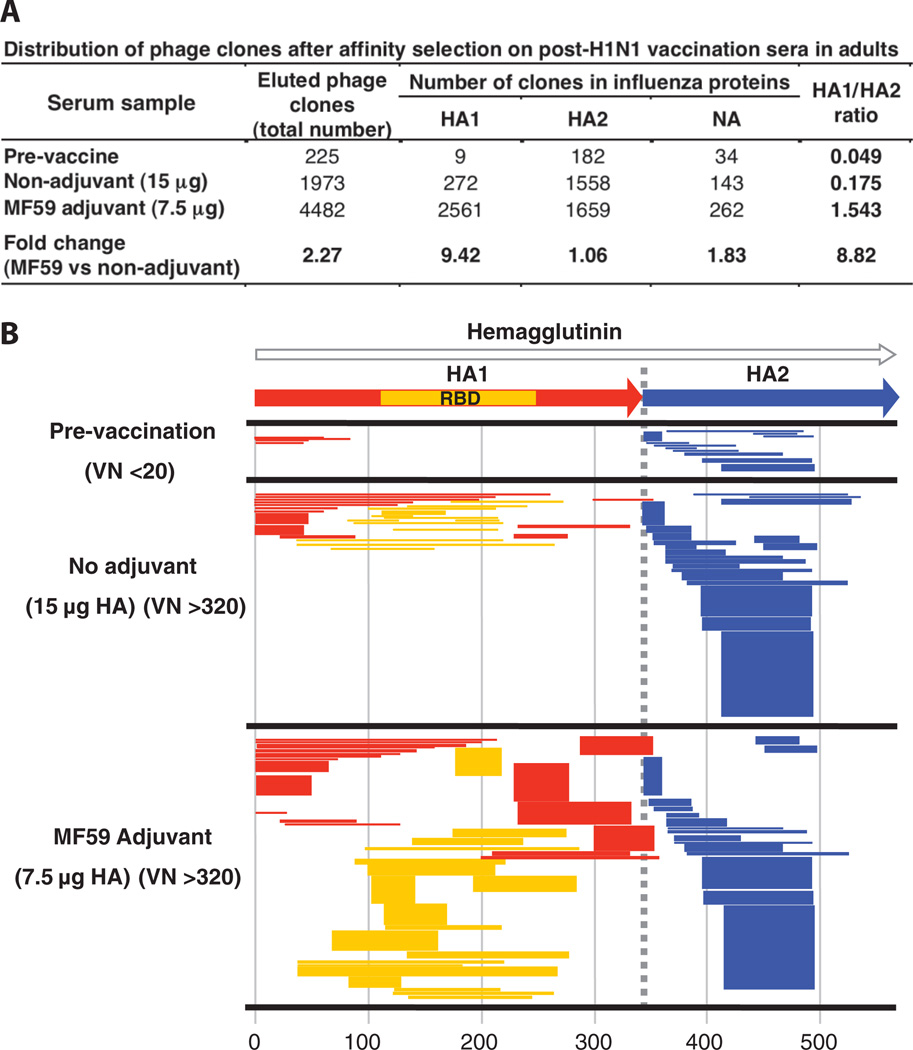
Fig. 1.
Analysis of antibody repertoires elicited in adults after vaccination with unadjuvanted and adjuvanted subunit H1N1 vaccines. (A) Distribution of phage clones after affinity selection with sera obtained from adults before and after single vaccination with subunit SOIV-H1N1 vaccine (with and without MF59 adjuvant). (B) Schematic alignment of the peptides recognized by post-first H1N1 vaccination sera in the adults, identified by panning with H1N1-GFPDL A/California/07/2009. The amino acid designation is based on the HA protein sequence (fig. S1). Bars indicate identified inserts in HA1 (red bars) and HA2 (blue bars). The thickness of each bar represents the frequencies of repetitively isolated phage inserts (only clones with a frequency of two or more are shown; sequenced clones are shown in table S1). Phage-displaying peptides from sequences within the HA1 receptor binding domain (RBD) are depicted with yellow bars.
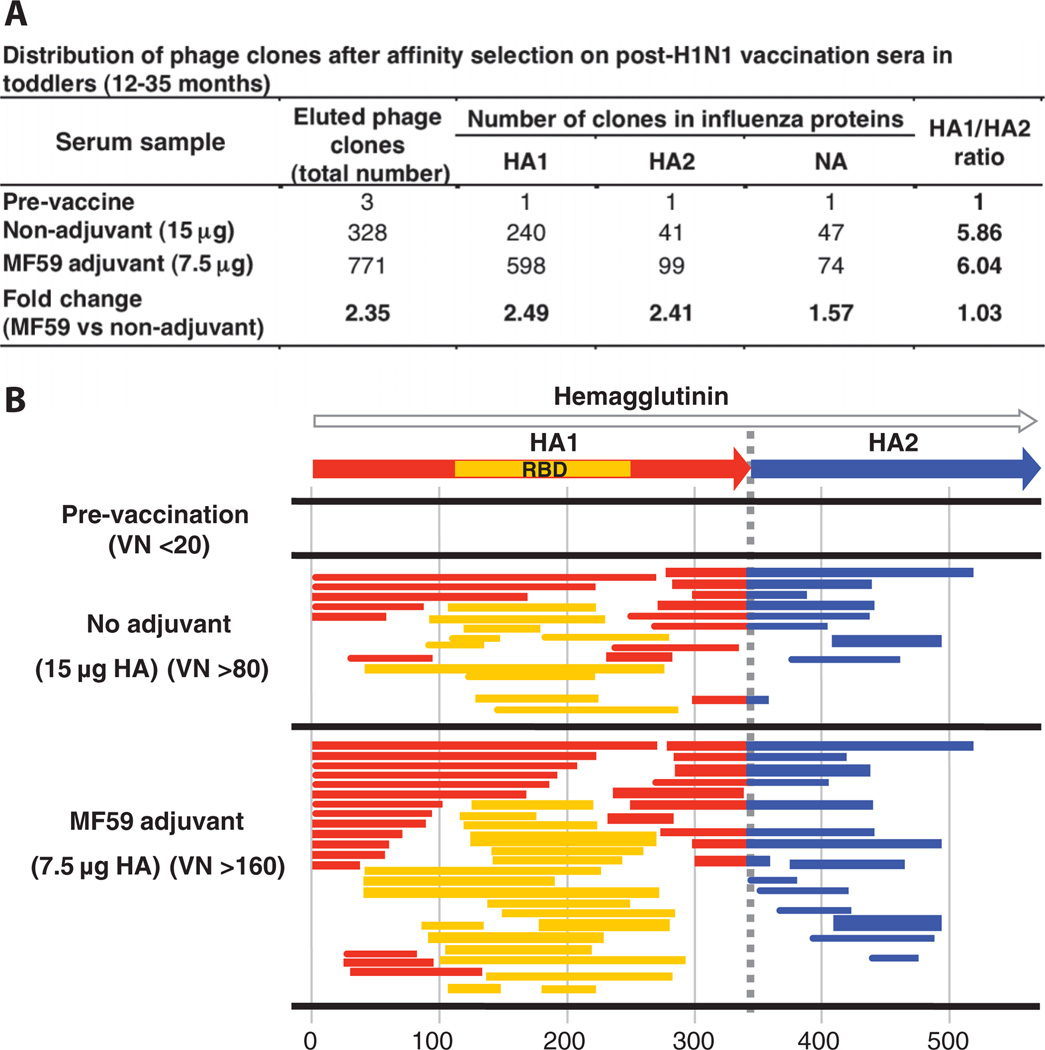
Fig. 3.
Antibody epitope profile elicited in toddlers after vaccination with an unadjuvanted or MF59-adjuvanted subunit SOIV-H1N1 vaccine. (A) Distribution of phage clones after affinity selection with sera obtained from toddlers (12 to 35 months) before and after two immunizations with subunit SOIV-H1N1 vaccine (with and without MF59 adjuvant). (B) Schematic alignment of the peptides recognized by post-second H1N1 vaccination sera in the toddlers, identified by panning with H1N1-GFPDL A/California/07/2009. Amino acid designation is based on the HA protein sequence (fig. S1). Bars indicate identified inserts in HA1 (red bars) and HA2 (blue bars). Phage-displaying peptides from sequences within the HA1 RBD are depicted with yellow bars. The thickness of bars represents the frequencies of repetitively isolated phage inserts (only clones with a frequency of two or more are shown; sequenced clones are shown in table S1).
In adults, antibodies in prevaccination sera predominantly bound to SOIV-HA epitopes in the HA2 region and in the N terminus of the HA1 region (representing conserved regions between SOIV and seasonal H1N1 viruses) (Fig. 1B and table S1). HA2 and the N terminus of HA1 together form the stem of HA trimers. None of these prevaccination sera exhibited VN or HAI activity, as expected from the low functionality of antibodies specific for these regions, which typically are not exposed for antibody binding on intact virions (16–18).
After vaccination with the unadjuvanted H1N1 vaccine, 1973 phage clones were captured by the immune sera, of which 1558 mapped to HA2, 272 to HA1, and 143 to NA. HA1 epitopes included RBD-spanning sequences, as well as N-terminal sequences (Fig. 1A). Sera from individuals vaccinated with MF59-adjuvanted vaccine bound 4482 phages (2.3-fold higher than unadjuvanted immune sera), of which 1659 mapped to HA2, 2561 to HA1, and 262 to NA. Compared to prevaccination sera, both unadjuvanted and MF59-adjuvanted vaccines expanded the numbers of HA2-binding antibodies (eight- to ninefold). Expansion of HA1 epitopes was 10-fold higher after vaccination with MF59-adjuvanted compared with unadjuvanted vaccine (280- and 30-fold increase from baseline, respectively), resulting in an 8.8-fold higher HA1/HA2 ratio (1.543 and 0.175, respectively) (Fig. 1A, right column). The number of NA-specific phage clones was also increased 2.5-fold in the adjuvanted vaccines sera compared with unadjuvanted vaccine sera.
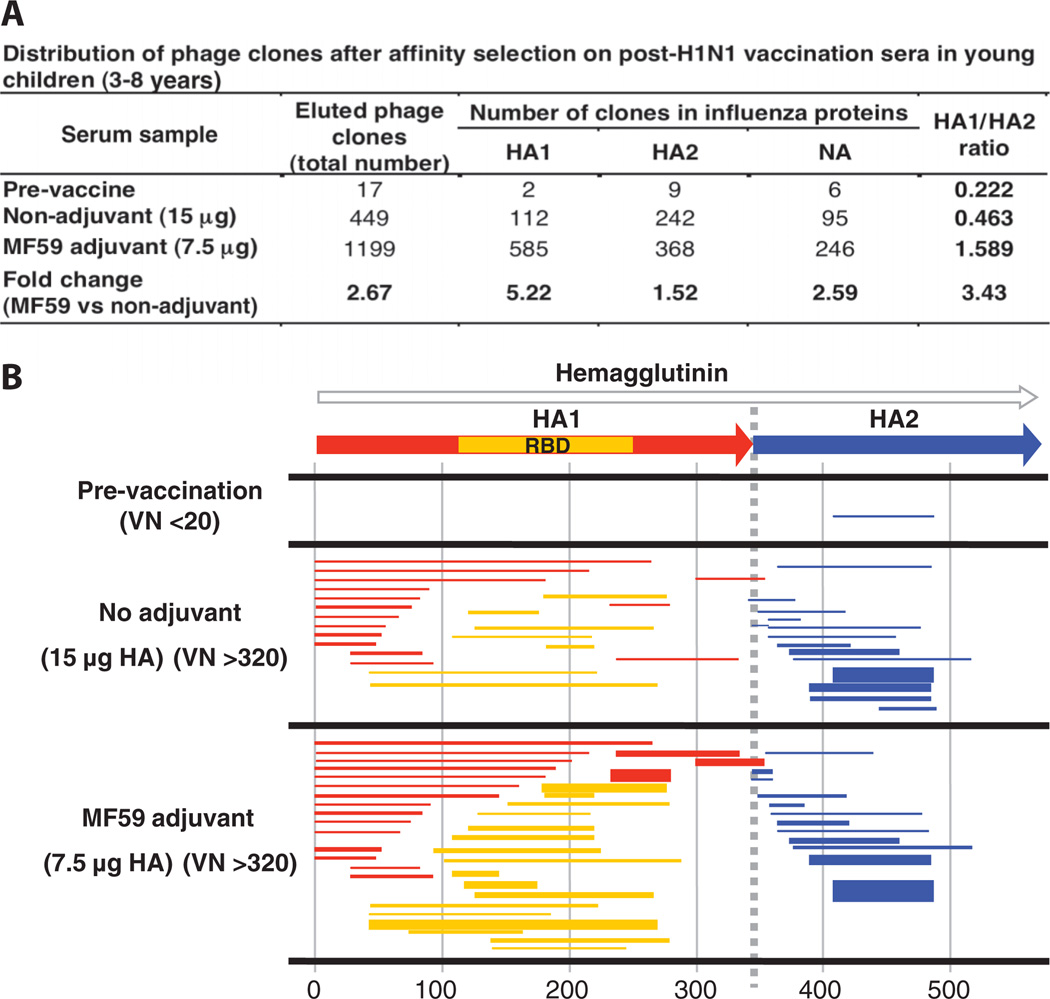
We next compared the epitope profiles of sera obtained from 3- to 8-year-old children (five per group) vaccinated with two doses of H1N1 vaccine (with or without MF59 adjuvant), with similar titers of virus-neutralizing antibodies (320 to 1280) (Fig. 2, A and B). Compared to adults, antibodies in prevaccination sera bound less than 20 phage-displayed epitopes, and all recognized inserts mapped to HA2. Vaccination increased the number of antibodies that bound HA-specific epitopes by 26-fold, and MF59 increased the number of HA fragments recognized by more than twofold. As in adults, a predominance of HA2-specific antibodies in response to the unadjuvanted vaccine was observed (Fig. 2, A and B, and table S1), and MF59 induced more expansion in the number of HA1 compared with HA2-binding antibodies (3.43 increase in the HA1/HA2 ratio). Thus, although the magnitude of the antibody response was lower in 3- to 8-year-old children than adults, the effects of MF59 in 3- to 8-year-old children were similar to those observed in adults (Figs. 1A and 2A).
Fig. 2.
Antibody epitope repertoire elicited in young children after vaccination with unadjuvanted and adjuvanted subunit H1N1 vaccines. (A) Distribution of phage clones after affinity selection on sera obtained from young children (3 to 8 years) before and after two immunizations with subunit SOIV-H1N1 vaccine (with and without MF59 adjuvant). (B) Schematic alignment of the peptides recognized by post-second H1N1 vaccination sera in the young children (3 to 8 years) as identified with H1N1-GFPDL A/California/07/2009 (sequenced clones are shown in table S1). Phage-displaying peptides from sequences within the HA1 RBD are depicted with yellow bars, whereas identified inserts in HA1 are shown as red bars and HA2 as blue bars. The thickness of each bar represents the frequencies of repetitively isolated phage inserts (only clones with a frequency of two or more are shown; sequenced clones are shown in table S1). The amino acid designation is based on the HA protein sequence (fig. S1).
An HA2-predominant antibody repertoire is not observed in toddlers
To better understand the cause of preexisting HA2 antibodies in children and adults, we examined the specificity of antibodies in toddlers (<3 years). Toddlers responded to two doses of vaccine, and once again, MF59 increased virus-neutralizing antibody titers by a factor of ~4, from 80 to 320 against 320 to 1280 end-point titer.
Notably, prevaccination sera in this group did not contain HA2-binding antibodies (Fig. 3B). Vaccination elicited antibodies with broadened specificity for HA and NA fragments, with twofold more phage clones bound by sera from MF59-adjuvanted compared to unadjuvanted vaccine (Fig. 3A and table S1). In this age group, there was no preferential response of HA2-binding antibodies after vaccination, and the MF59-adjuvanted vaccine induced a similar increase (~2.4-fold) in the number of both HA1- and HA2-binding antibodies compared to the unadjuvanted H1N1 vaccine (Fig. 3A).
These results suggest that the abundance of HA2-specific antibodies in adults is due to repeated IAV infections or vaccinations, although antibodies that cross-react with the large number of other pathogens encountered by older individuals may be present. The selective enhancement of HA1-specific antibodies after MF59-adjuvanted vaccination may indicate a difference in the requirement for activating primary in opposition to recall antibody responses.
The MF59-adjuvanted vaccine elicits antibodies with a higher binding capacity to properly folded HA1 globular domain than unadjuvanted vaccine
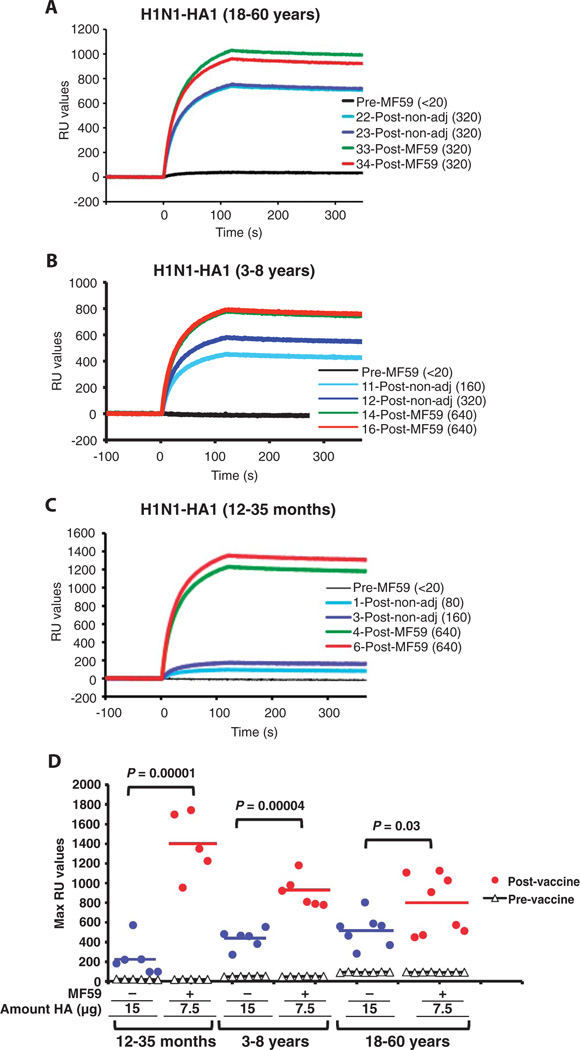
On the basis of the extensive literature on the specificity of anti-HA antibodies (17, 19, 20) and our previous study (13), we expected that increased recognition of the fragments from the globular domain would correlate with increased recognition of folded recombinant HA1 globular domain revealed by SPR. Indeed, binding to properly folded HA1 [previously described (21)] was significantly higher for antibodies from the MF59-adjuvanted vaccine sera compared with sera from unadjuvanted vaccine recipients in all three age groups (Fig. 4, A to D), with the most pronounced difference observed in the youngest age group (12 to 35 months) (Fig. 4, C and D; P = 0.00001).
Fig. 4.
Binding of post-H1N1 vaccination human serum IgG to properly folded HA1 protein. (A to C) Steady-state equilibrium analysis of the binding of human vaccine serum IgG to properly folded functional HA1 oligomers was measured with SPR and is shown for two representative samples from each group. Tenfold-diluted individual post-H1N1 vaccination sera from the three age groups [adults in (A), young children in (B), and toddlers in (C)] of the H1N1 vaccine trial were injected simultaneously onto HA1 immobilized on a sensor chip through the free amine group and onto a blank flow cell, free of peptide. Prevaccination serum (VN < 20) is included (black) and was used as control in each assay. The VN titers of each serum used in SPR are in parentheses. Binding of the antibodies to the immobilized protein is shown as resonance unit (RU) values. (D) Maximum RU values for HA1 binding by serum antibodies obtained from multiple individuals before and after vaccination with either MF59-adjuvanted (red dots) or unadjuvanted SOIV-H1N1 (blue dots) vaccination for the three age groups.
To further investigate the effect of MF59 on the quality of antibody responses, we extended our analysis to enzyme-linked immunosorbent assay (ELISA). In ELISA, as in other equilibrium-based assays (for example, HAI and VN), it is not feasible to discriminate between the contributions of antibody affinity and antibody concentration to the binding titer. It is possible, however, to approximate antibody affinity by measuring the effect of denaturants on antibody binding (22, 23), because low-affinity antibodies are more rapidly eluted.
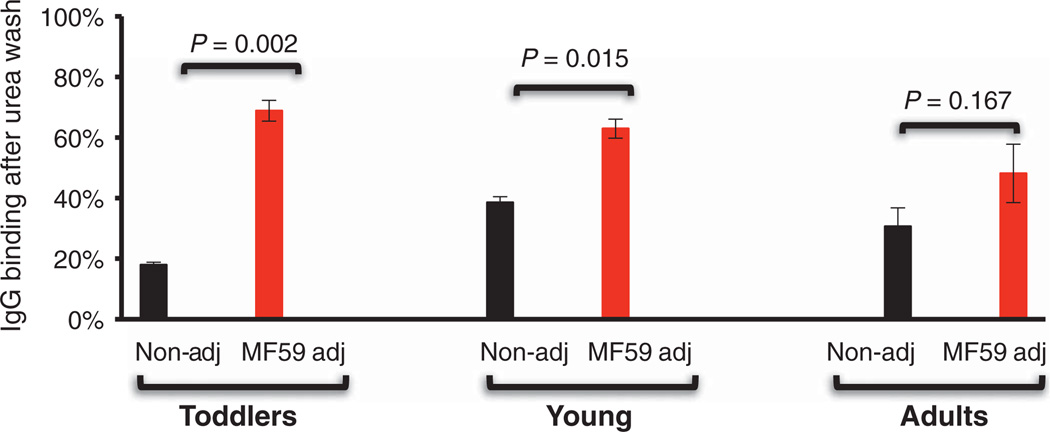
To this end, we performed a standard ELISA with immobilized properly folded recombinant HA1 (21); this assay was modified such that bound serum antibodies (from the same serum samples used in GFPDL analysis) were briefly exposed to 7 M urea before addition of the secondary labeled anti-human immunoglobulin G (IgG) antibody. MF59 increased the fraction of 7 M urea–resistant antibodies in an age-related manner (Fig. 5): The effects were highest in toddlers (<20% against >65%, P = 0.002) and lowest in adults (30 to 40% against 40 to 65%, P = 0.167), with an intermediate effect in 3 to 8 year olds (40% against >70%, P = 0.015).
Fig. 5.
Human serum IgG avidity after immunization with an MF59-adjuvanted and unadjuvanted H1N1 subunit vaccine in different age groups. H1N1-HA1–specific affinity of IgG from post-H1N1 vaccination human sera after 7 M urea wash in individuals with either MF59-adjuvanted or unadjuvanted SOIV-H1N1 vaccination for the three age groups is shown (n = 5 to 7). Data are means ± SD of three independent experiments. Differences between groups were examined for statistical significance with Student’s t test. A P value of <0.05 was considered to be significant.
Antibody avidities can also be determined by the ratio of antibody on and off rates as determined by SPR. Antibody on rates typically fall in a fairly narrow range with avidity principally resulting from decreases in off rates (24–29). Although determining on rates requires knowledge of antibody concentration (14), off-rate measurements are independent of antibody concentration and hence can be measured for serum antibodies bound to antigen (25, 30).
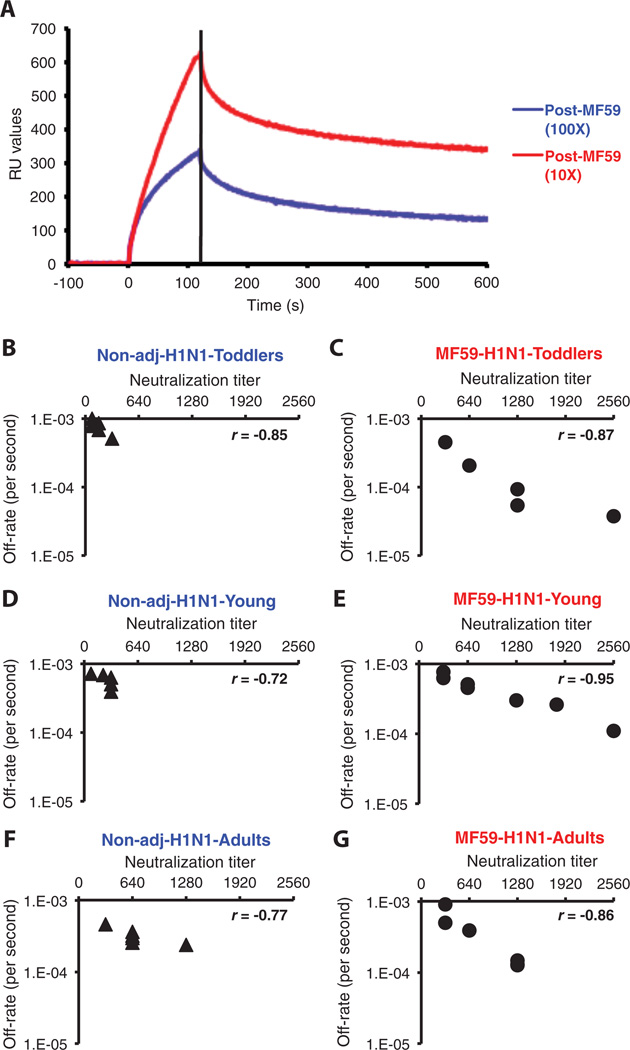
To determine polyclonal antibody off rates via SPR, we first demonstrated that although post-vaccination human sera tested in serial dilutions yielded different maximal resonance unit (RU) binding to recombinant HA1 (rHA1), the kinetics of dissociation were identical and hence independent of serum antibody concentration. As seen in Fig. 6A, antibody concentration independence was indicated by parallel lines in the dissociation phase for the 10- and 100-fold dilution for each post-vaccination human serum. Antibody off-rate constants, which describe the stability of the complex by measuring the fraction of complexes that decays per second in the disassociation phase, were determined with the Bio-Rad ProteOn manager software for the heterogeneous sample model. To improve the accuracy of measurements, we determined the off-rate constants from two independent SPR runs for each sample.
Fig. 6.
Affinity measurements and correlation between in vitro neutralizing capacity and off-rate constant in human sera after immunization with an MF59-adjuvanted and unadjuvanted H1N1 subunit vaccine in different age groups. (A) Antibody avidity measurements in polyclonal serum by off-rate constants with SPR. Antibody off-rate constants that describe the stability of the complex, which is the fraction of complexes that decays per second, were determined directly from the serum-plasma sample interaction with rHA1 protein using SPR in the dissociation phase. For accurate measurements, parallel lines in the dissociation phase for the 10- and 100-fold dilution for each post-vaccination human sera were required. The off-rate constants were determined from two independent SPR runs. (B to G) SPR analysis of post-vaccinated human sera with MF59-adjuvanted (C, E, and G) or unadjuvanted SOIV-H1N1 (B, D, and F) vaccine from three age groups of the vaccine trial was performed with properly folded SOIV-H1N1 HA1 (A/California/07/2009) (21). Serum antibody off-rate constants and serum-neutralizing antibody titers for different individual vaccinees (each symbol is one individual) were determined as described in Materials and Methods. Neutralizing titer is expressed as standardized end-point neutralizing antibody titer of post-H1N1 vaccine human sera. Antibody off-rate constants of human sera after vaccination with MF59-adjuvanted or unadjuvanted SOIV-H1N1 vaccine correlated with their in vitro neutralizing capacity. Correlation statistics of the affinity measurement and off-rate constants of the human sera between MF59-adjuvanted and unadjuvanted vaccine groups were statistically significant only for the toddlers’ samples (12 to 35 months) and young children (3 to 8 years), with P < 0.05 (t test).
The off-rate constants of individual sera supported the results in the 7 M urea treatment (Figs. 5 and 6, B to G). Lower off rates correlated with increased VN titers in the three age groups. Off-rate constants of anti-HA1 antibodies from toddlers and young children who had received nonadjuvanted vaccine ranged from 1.0 × 10−3 to 1.5 × 10−3 s−1 after two vaccinations and correlated with modest VN titers (40 to 320) (Fig. 6, B and D). In comparison, the antibodies generated by MF59-adjuvanted vaccine displayed slower off-rate constants (reaching <1 × 10−4 s−1 in some individuals) and correlated with higher VN titers (Fig. 6, C and E). As with other parameters examined, the magnitude and statistical significance of the adjuvant effects were age-dependent, with stronger effects in toddlers and the least effect seen in the adult population who also had sera with higher VN titers (320 to 1280) (Fig. 6, G and F). The inverse correlation between VN titers and off-rate constants strongly suggests that an increase in antibody affinity plays an essential role in the neutralization of the SOIV-H1N1 virus. In conclusion, MF59 greatly enhanced the affinity of antibodies specific for the HA globular domain, which was most evident in the young age groups and, in general, in subjects with limited previous exposure to the antigens present in the influenza vaccines.
MF59 enhances antibody affinity maturation after repeat dosing with the H5N1 subunit vaccine
The adjuvant effect on antibody affinity maturation as measured by either 7 M urea resistance or SPR was most pronounced in the influenzanaïve groups. This suggested that MF59 might have a greater effect on the affinity of HA-specific antibodies elicited by the H5N1 vaccine in adults, because H5N1 viruses do not circulate in humans.
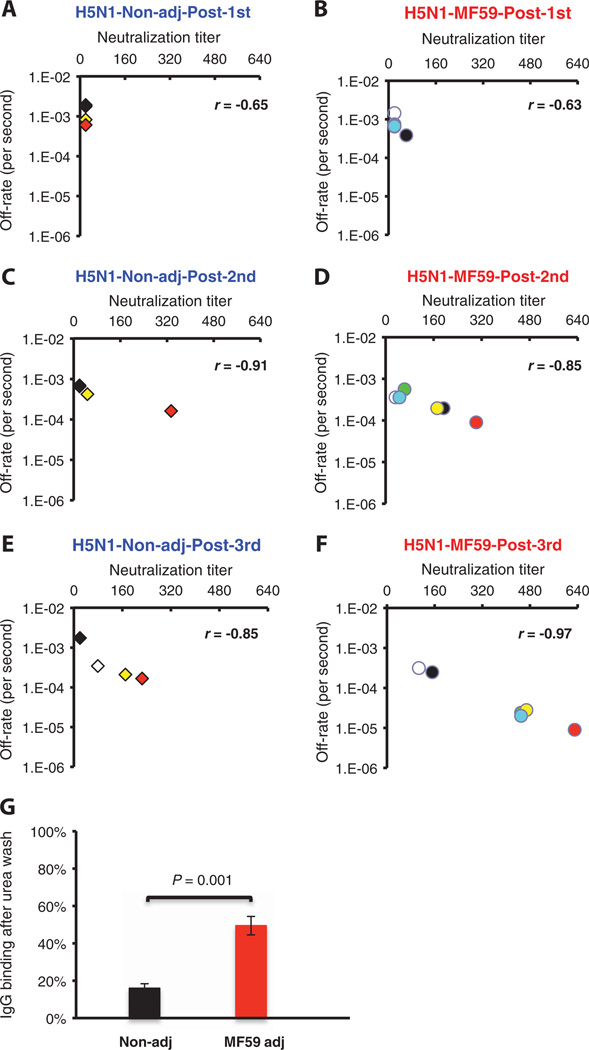
The H5N1 antibody responses in adults were typical of a primary immune response in a naïve population as previously described (13) (Fig. 7). To assess antibody affinity maturation after repeated vaccination and its effect on VN, we followed the serum VN titer and corresponding polyclonal serum off-rate constants after every vaccination for each individual. The values for each individual vaccinee are depicted with the same colored symbol after the first, second, and third immunizations in the three corresponding figures of each arm (Fig. 7, A to F). After the first vaccination, VN titers were below detectable limits, and off-rate constants of native H5-binding antibodies were high (1 × 10−3 s−1; that is low affinity). Second and third doses of unadjuvanted vaccine elicited neutralizing antibodies in the 40 to 320 range with a parallel decrease in antibody off-rate constants. In the MF59-adjuvanted vaccine group, a clear improvement of antibody affinity maturation was observed after repeated vaccination between the second and third dose, with off-rate constants reaching 1 × 10−5 s−1 and VN titers ranging from 160 to 640. As with the SOIV-H1N1 vaccine, there was a tight inverse correlation between VN titer and off-rate constants. ELISA revealed similar behavior of antibody affinity as measured by 7 M urea resistance (Fig. 7G).
Fig. 7.
Kinetics of antibody affinity maturation to HA after multiple immunizations with an MF59-adjuvanted and unadjuvanted H5N1 subunit vaccine and its correlation with in vitro VN. (A to F) Sequential SPR analysis of vaccine sera (after the first, second, and third vaccination with unadjuvanted or MF59-adjuvanted H5N1 vaccine) was performed with properly folded H5N1 HA1 (A/Vietnam/1203/2204) (13, 47). Tenfold-diluted individual sera from three arms of the NVD vaccine trial at 28 days after each immunization were evaluated. After binding of the sera to the immobilized ligand, antibody off-rate constants were calculated with a heterogeneous sample model as described in Materials and Methods. Values on the x axis denote the end-point VN titers (mean of three replicates) with individual sera in a VN assay performed with A/Vietnam/1203/2004-rgH5N1×PR8 reassorted virus. Data plotted are shown for four individuals from the unadjuvanted arm (A, C, and E) and six individuals from the MF59- adjuvanted H5N1 vaccine arm (B, D, and F) in the NVD trial after first, second, and third immunizations. To follow the serum VN titer and corresponding polyclonal serum off-rate constants after every vaccination, the values for each individual vaccinee are depicted with the same colored symbol after the first, second, and third immunization in the three corresponding figures of each arm. Statistical analysis of the off-rate constants between MF59-adjuvanted and unadjuvanted vaccine groups after each vaccination showed statistical significance betweenMF59-adjuvanted and unadjuvanted vaccine groups only for samples after the third immunization, with P<0.05 (t test). (G)H5N1-HA1–specific affinity of serum IgG after 7 M urea wash in individuals after the third vaccination with either MF59-adjuvanted or unadjuvanted H5N1 subunit vaccine (n = 6). Data are means ± SD of three independent experiments. Differences between groups were examined for statistical significance with Student’s t test. A P value of <0.05 was considered to be significant.
These findings clearly demonstrated that MF59 improved the immunogenicity of both the SOIV-H1N1 and H5N1 vaccines by increasing antibody avidity. Such improvements to antibody qualities are predicted to improve protective immunity in vivo.
DISCUSSION
The insufficient immunogenicity of the original H5N1 vaccine led to the inclusion of MF59 and AS03 adjuvants in influenza vaccines, which enabled the vaccine to achieve U.S. Food and Drug Administration and European Union criteria of seroconversion and seroprotection rates. At the onset of the SOIV-H1N1 pandemic in Europe, these adjuvants were combined with the SOIV-H1N1 vaccines to enhance immunogenicity and allow dose sparing (8–11).
Here, we provide the initial characterization of the effects of oil-in-water adjuvants on the quality of antibodies induced by the SOIV-H1N1 vaccine in different age populations. Our data clearly show that MF59 enhances VN responses by both quantitative and qualitative improvement in HA-specific antibody responses by increasing the diversity of epitope repertoire recognized in the HA1 globular domain and by enhancing the affinity of antibodies specific for native HA. On the basis of the increased magnitude of the effect of MF59 on anti–SOIV-H1N1 responses in younger individuals, and similar findings with the H5N1 vaccine in H5N1-naïve adults, it is likely that the major effect of MF59 is exerted on naïve B cells, increasing the rate of somatic hypermutation. Therefore, MF59 and similar adjuvants have the potential to overcome the tendency in some individuals to generate low-affinity antibodies with pathological potential.
We show that in the face of a novel IAV pandemic, most preexisting serum antibodies are specific for HA2. These antibodies would be induced by previous clinically overt or subclinical infections with influenza viruses or by previous immunizations (mainly in adults) with conventional, nonadjuvanted seasonal influenza vaccines. This is consistent with previous findings that HA2 antibodies are highly cross-reactive, which is expected because of the much higher sequence conservation in HA2 compared to HA1 (13). The RBD of HA1, where antigenic drift is most extensive, is particularly diverse.
A number of groups have recently described antibodies in mice, ferrets, monkeys, and humans specific for a discontinuous antigenic site in the HA stem region that cross-react between related subtypes (for example, H1-H2-H5) with post-attachment virus-neutralizing activity (31–36). Representative monoclonal antibodies (mAbs) have been shown to prevent and treat IAV infections in animal models, raising the prospect of generating vaccines that provide broader immunity within and even across related subtypes (35, 36). It will be of great interest to examine the effect of MF59 on eliciting such antibodies, and particularly on increasing their affinity, which may be critical in enhancing their in vivo activity.
Here, we revisited the central question of the role of affinity maturation in antiviral immunity. In earlier mouse studies using a panel of mouse mAbs specific for vesicular stomatitis virus (VSV) glycoprotein, protection against VSV infection was independent of immunoglobulin class, affinity, and VN rate constant. Antibody affinity above a minimal threshold (about 2 × 10−7 M−1) provided complete protection from acute infection, and protection depended simply on serum antibody concentration (37, 38). Furthermore, mAbs generated either early or late after VSV hyperimmunization demonstrated similar high affinity, with no evidence of time-dependent affinity maturation (14). The authors proposed that for acute viruses, there is little need for affinity maturation because the virus is cleared long before higher-affinity antibodies reach effective concentrations. However, these studies focused on the mouse responses to VSV, for which it appears that a germ-line antibody offers high neutralizing titers against the virus (15, 39). This is probably an unusual situation whereby very limited affinity maturation is needed, and does not reflect normal antiviral antibody responses. Recent studies demonstrate that acute viral infections (including SOIV) and formalin-inactivated vaccines for measles and respiratory syncytial virus may elicit low-affinity antibodies that form immune complexes in the lungs and activate complement. These non-neutralizing antibodies may contribute to enhanced lung pathology with severe disease and even death after infection (22, 23, 40, 41).
Here, we confirm the importance of antibody affinity in anti-influenza immunity by demonstrating strong correlation between 7 M urea–resistant serum antibodies and off-rate constants with VN titers in response to SOIV-H1N1 and H5N1 vaccination. We found that MF59 adjuvant greatly enhanced affinity maturation especially in naïve populations.
How is an oil-in-water adjuvant such as MF59 able to increase antibody affinity? The enhancement observed by MF59 of HA1-specific antibodies could be due to a difference in the requirements for activating naïve HA1-specific as opposed to recall HA2-specific B cells. Indeed, previous findings have shown that vaccination with the MF59-adjuvanted H5N1 vaccine, but not with unadjuvanted vaccine, primes a potent and rapid antigen-specific CD4+ T cell response that is predictive of the high VN antibody levels found after booster immunization (42, 43).
Together, our data clearly demonstrate that antibody affinity maturation occurs after influenza vaccination, particularly in naïve individuals. Most importantly, MF59 enhances affinity maturation and offers the promise of improved protection in vivo. These findings provide support for use of adjuvants in influenza vaccines, especially when targeting naïve populations.
MATERIALS AND METHODS
Description of clinical trials and collection of vaccination samples
Serum or plasma samples were obtained from subjects enrolled in clinical trials with either the SOIV-H1N1 or the H5N1 vaccine in three different clinical trials sponsored by Novartis Vaccines and Diagnostics. All study protocols were in accordance with the Helsinki Declaration and with Good Clinical Practice principles and were approved by the local ethical committees.
The SOIVA/H1N1 trials were two randomized, single-blind, dose-ranging, multicenter studies aimed at evaluating immunogenicity, safety, and tolerability of various amounts (3.5 or 7.5 µg of HA) of MF59-adjuvanted and non-adjuvanted, egg-derived, inactivated (15 µg of HA) SOIV A/H1N1 monovalent subunit vaccine in healthy adults and elderly, age 18 years and above (n = 656, ClinicalTrials.gov no. NCT00971906), and in 720 toddlers, children, and adolescents with ages ranging from 6 months to 17 years (ClinicalTrials.gov no. NTC00971542). The composition and the presentation of the vaccine have been already reported in previous publication (44).
The third study used MF59-adjuvanted or non-adjuvanted H5N1 subunit vaccine (ClinicalTrials.gov no. NCT00382187), and the results obtained have already been reported in details elsewhere (13, 42, 45).
Pre- and post-vaccination serum samples were tested (i) from 40 adults (18 to 60 years old), 20 young children (3 to 8 years old), and 20 toddlers (12 to 35 months) receiving the Novartis Vaccine and Diagnostics (NVD) A/H1N1 (A/H1N1/California/07/2009) vaccine not adjuvanted or adjuvanted with MF59 and given as ready-to-use formulations in prefilled syringes, and (ii) from 40 adults immunized with the A/H5N1 subunit vaccine (A/Vietnam/1194/2004) prepared by NVD with and without MF59, and given as ready-to-use formulations in prefilled syringes (42).
All samples were de-identified. The protocols were evaluated by CBER (Center for Biologics Evaluation and Research) Research Involving Human Subjects Committee and were conducted under Research Involving Human Subjects Committee exemption #03-118B.
Construction of H1N1 GFPDLs and panning of H1N1 GFPDLs with polyclonal human vaccine sera
Complementary DNA corresponding to all eight gene segments of the A/H1N1/California/07/2009 was generated from the RNA isolated from egg-grown virus strain and was henceforth used for cloning. The phage display libraries used in the current study expressed inserts spanning the HA (fig. S1) and NA genes as previously described for H5N1 GFPDL (13, 46).
For each round of panning, an equal volume of sera was used for each group. Before panning of GFPDL, serum components, which might nonspecifically interact with phage proteins, were removed by incubation with ultraviolet-killed M13K07 phage-coated petri dishes. Subsequent GFPDL selection was performed in solution (with protein A/G). Inserts of bound phages were polymerase chain reaction (PCR)–amplified and sequenced.
Affinity measurements for 7 M urea–resistant antibodies
IgG affinity was determined directly for each individual post-vaccination serum that was used in GFPDL analysis by a modified ELISA method as previously described (22, 23). Briefly, ELISA plates were coated with purified HA1 proteins from either SOIV-H1N1 or H5N1 (A/Vietnam/1203/2004) (22, 23) or subunit influenza vaccines. After blocking with 2% bovine serum albumin (BSA)–phosphate-buffered saline with 0.1% Tween 20 (PBST), serial dilutions of human sera in 2% BSA–PBST were incubated for 1 hour. Bound IgG was detected with horseradish peroxidase–conjugated antibodies specific to human IgG-Fc and OPD (o-phenylenediamine dihydrochloride) as substrate. Samples were read at 490 nm. Affinity was determined by modifying this immunoassay to include a 10-min wash with 7 M urea after incubation of sera with the HA-coated wells. Percent of urea-resistant antibodies was calculated by dividing the optical density (OD) of the urea-washed samples by the OD of the unwashed samples.
Affinity measurements by SPR
Steady-state equilibrium binding of post-H1N1 or post-H5N1 human vaccine sera was monitored at 25°C with a ProteOn SPR biosensor (Bio-Rad). The HA1-His6 for the respective influenza strains (21, 47) was coupled to a GLC sensor chip with amine coupling with 500 RUs in the test flow cells. Samples of 60 µl of freshly prepared sera at 10- and 100-fold dilutions were injected at a flow rate of 30 µl/min (120-s contact time) for association, and disassociation was performed over a 600-s interval (at a flow rate of 30 µl/min). Responses from the protein surface were corrected for the response from a mock surface and for responses from a separate buffer-only injection. mAb 2D7 (anti-CCR5) was used as a negative control in the experiments. Binding kinetics for the human vaccine sera and the data analysis were calculated with Bio-Rad ProteOn manager software (version 2.0.1).
Antibody off-rate constants that describe the stability of the complex, which is the fraction of complexes that decays per second, were determined directly from the serum-plasma sample interaction with rHA1 protein using SPR in the disassociation phase (as described above) and calculated with the Bio-Rad ProteOn manager software for the heterogeneous sample model. For accurate measurements, it was important to have parallel lines in the dissociation phase for the 10- and 100-fold dilution for each post-vaccination human sera. To improve the measurements, we determined the off-rate constants from two independent SPR runs.
Statistical analyses
Differences between groups were examined for statistical significance with Student’s t test. An unadjusted P value of <0.05 was considered to be significant.
Supplementary Material
Acknowledgments
We thank M. Eichelberger and M. Zaitseva for a thorough evaluation of the manuscript, P. Dormitzer (Novartis Vaccines and Diagnostics) for precious comments and valuable discussions, and G. Corsi for his help with the preparation of figures.
Funding: This study was partly supported by IAA 224-10-1006 from the Division of Microbiology and Infectious Diseases, NIH. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
SUPPLEMENTARY MATERIAL
www.sciencetranslationalmedicine.org/cgi/content/full/3/85/85ra48/DC1
Fig. S1. The A/H1N1/California/07/2009 HA protein sequence.
Table S1. Frequency of selected phage clones with SOIV-H1N1–vaccinated individuals using H1N1 GFPDL in different age groups.
Author contributions: International Committee of Medical Journal Editors criteria for authorship were read and met. S.K., N.V., J.W.Y., A.K.H., F.C., M.L., G.D.G., R.R., and H.G. agree with the manuscript’s results and conclusions. S.K., N.V., and H.G. designed the experiments and conducted the study. S.K. and H.G. analyzed the data. M.L. was responsible for the clinical trials at Novartis Vaccines. S.K., H.G., J.W.Y., A.K.H., F.C., M.L., G.D.G., and R.R. contributed to the writing of the manuscript.
Competing interests: The authors declare that they have no competing interests.
REFERENCES AND NOTES
- 1.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N. Engl. J. Med. 2006;354:1343–1351. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 2.Beigel JH, Voell J, Huang CY, Burbelo PD, Lane HC. Safety and immunogenicity of multiple and higher doses of an inactivated influenza A/H5N1 vaccine. J. Infect. Dis. 2009;200:501–509. doi: 10.1086/599992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coffman RL, Sher A, Seder RA. Vaccine adjuvants: Putting innate immunity to work. Immunity. 2010;33:492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernstein DI, Edwards KM, Dekker CL, Belshe R, Talbot HK, Graham IL, Noah DL, He F, Hill H. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J. Infect. Dis. 2008;197:667–675. doi: 10.1086/527489. [DOI] [PubMed] [Google Scholar]
- 5.Zhu FC, Wang H, Fang HH, Yang JG, Lin XJ, Liang XF, Zhang XF, Pan HX, Meng FY, Hu YM, Liu WD, Li CG, Li W, Zhang X, Hu JM, Peng WB, Yang BP, Xi P, Wang HQ, Zheng JS. A novel influenza A (H1N1) vaccine in various age groups. N. Engl. J. Med. 2009;361:2414–2423. doi: 10.1056/NEJMoa0908535. [DOI] [PubMed] [Google Scholar]
- 6.Stephenson I, Bugarini R, Nicholson KG, Podda A, Wood JM, Zambon MC, Katz JM. Cross-reactivity to highly pathogenic avian influenza H5N1 viruses after vaccination with nonadjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: A potential priming strategy. J. Infect. Dis. 2005;191:1210–1215. doi: 10.1086/428948. [DOI] [PubMed] [Google Scholar]
- 7.Stephenson I, Nicholson KG, Hoschler K, Zambon MC, Hancock K, DeVos J, Katz JM, Praus M, Banzhoff A. Antigenically distinct MF59-adjuvanted vaccine to boost immunity to H5N1. N. Engl. J. Med. 2008;359:1631–1633. doi: 10.1056/NEJMc0805274. [DOI] [PubMed] [Google Scholar]
- 8.Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, Groth N, Stephenson I. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. N. Engl. J. Med. 2009;361:2424–2435. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 9.Roman F, Vaman T, Gerlach B, Markendorf A, Gillard P, Devaster JM. Immunogenicity and safety in adults of one dose of influenza A H1N1v 2009 vaccine formulated with and without AS03A-adjuvant: Preliminary report of an observer-blind, randomised trial. Vaccine. 2010;28:1740–1745. doi: 10.1016/j.vaccine.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Carmona A, Omeñaca F, Tejedor JC, Merino JM, Vaman T, Dieussaert I, Gillard P, Arístegui J. Immunogenicity and safety of AS03-adjuvanted 2009 influenza A H1N1 vaccine in children 6–35 months. Vaccine. 2010;28:5837–5844. doi: 10.1016/j.vaccine.2010.06.065. [DOI] [PubMed] [Google Scholar]
- 11.Nicholson KG, Abrams KR, Batham S, Clark TW, Hoschler K, Lim WS, Medina MJ, Nguyen-Van-Tam JS, Read RC, Warren FC, Zambon M. Immunogenicity and safety of a two-dose schedule of whole-virion and AS03A-adjuvanted 2009 influenza A (H1N1) vaccines: A randomised, multicentre, age-stratified, head-to-head trial. Lancet Infect. Dis. 2011;11:91–101. doi: 10.1016/S1473-3099(10)70296-6. [DOI] [PubMed] [Google Scholar]
- 12.Galli G, Hancock K, Hoschler K, DeVos J, Praus M, Bardelli M, Malzone C, Castellino F, Gentile C, McNally T, Del Giudice G, Banzhoff A, Brauer V, Montomoli E, Zambon M, Katz J, Nicholson K, Stephenson I. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc. Natl. Acad. Sci. U.S.A. 2009;106:7962–7967. doi: 10.1073/pnas.0903181106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khurana S, Chearwae W, Castellino F, Manischewitz J, King LR, Honorkiewicz A, Rock MT, Edwards KM, Del Giudice G, Rappuoli R, Golding H. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci. Transl. Med. 2010;2 doi: 10.1126/scitranslmed.3000624. 15ra15. [DOI] [PubMed] [Google Scholar]
- 14.Roost HP, Bachmann MF, Haag A, Kalinke U, Pliska V, Hengartner H, Zinkernagel RM. Early high-affinity neutralizing anti-viral IgG responses without further overall improvements of affinity. Proc. Natl. Acad. Sci. U.S.A. 1995;92:1257–1261. doi: 10.1073/pnas.92.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hangartner L, Zinkernagel RM, Hengartner H. Antiviral antibody responses: The two extremes of a wide spectrum. Nat. Rev. Immunol. 2006;6:231–243. doi: 10.1038/nri1783. [DOI] [PubMed] [Google Scholar]
- 16.Wilson IA, Cox NJ. Structural basis of immune recognition of influenza virus hemagglutinin. Annu. Rev. Immunol. 1990;8:737–771. doi: 10.1146/annurev.iy.08.040190.003513. [DOI] [PubMed] [Google Scholar]
- 17.Wiley DC, Wilson IA, Skehel JJ. Structural identification of the antibody-binding sites of Hong Kong influenza haemagglutinin and their involvement in antigenic variation. Nature. 1981;289:373–378. doi: 10.1038/289373a0. [DOI] [PubMed] [Google Scholar]
- 18.Roberts PC, Garten W, Klenk HD. Role of conserved glycosylation sites in maturation and transport of influenza A virus hemagglutinin. J. Virol. 1993;67:3048–3060. doi: 10.1128/jvi.67.6.3048-3060.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knossow M, Gaudier M, Douglas A, Barrère B, Bizebard T, Barbey C, Gigant B, Skehel JJ. Mechanism of neutralization of influenza virus infectivity by antibodies. Virology. 2002;302:294–298. doi: 10.1006/viro.2002.1625. [DOI] [PubMed] [Google Scholar]
- 20.Knossow M, Skehel JJ. Variation and infectivity neutralization in influenza. Immunology. 2006;119:1–7. doi: 10.1111/j.1365-2567.2006.02421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khurana S, Verma S, Verma N, Crevar CJ, Carter DM, Manischewitz J, King LR, Ross TM, Golding H. Properly folded bacterially expressed H1N1 hemagglutinin globular head and ectodomain vaccines protect ferrets against H1N1 pandemic influenza virus. PLoS One. 2010;5 doi: 10.1371/journal.pone.0011548. e11548. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Monsalvo AC, Batalle JP, Lopez MF, Krause JC, Klemenc J, Hernandez JZ, Maskin B, Bugna J, Rubinstein C, Aguilar L, Dalurzo L, Libster R, Savy V, Baumeister E, Aguilar L, Cabral G, Font J, Solari L, Weller KP, Johnson J, Echavarria M, Edwards KM, Chappell JD, Crowe JE, Jr, Williams JV, Melendi GA, Polack FP. Severe pandemic 2009 H1N1 influenza disease due to pathogenic immune complexes. Nat. Med. 2011;17:195–199. doi: 10.1038/nm.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polack FP, Hoffman SJ, Crujeiras G, Griffin DE. A role for nonprotective complement-fixing antibodies with low avidity for measles virus in atypical measles. Nat. Med. 2003;9:1209–1213. doi: 10.1038/nm918. [DOI] [PubMed] [Google Scholar]
- 24.Zahnd C, Spinelli S, Luginbühl B, Amstutz P, Cambillau C, Plückthun A. Directed in vitro evolution and crystallographic analysis of a peptide-binding single chain antibody fragment (scFv) with low picomolar affinity. J. Biol. Chem. 2004;279:18870–18877. doi: 10.1074/jbc.M309169200. [DOI] [PubMed] [Google Scholar]
- 25.Zahnd C, Sarkar CA, Plückthun A. Computational analysis of off-rate selection experiments to optimize affinity maturation by directed evolution. Protein Eng. Des. Sel. 2010;23:175–184. doi: 10.1093/protein/gzp087. [DOI] [PubMed] [Google Scholar]
- 26.Batista FD, Neuberger MS. Affinity dependence of the B cell response to antigen: A threshold, a ceiling, and the importance of off-rate. Immunity. 1998;8:751–759. doi: 10.1016/s1074-7613(00)80580-4. [DOI] [PubMed] [Google Scholar]
- 27.Maynard JA, Maassen CB, Leppla SH, Brasky K, Patterson JL, Iverson BL, Georgiou G. Protection against anthrax toxin by recombinant antibody fragments correlates with antigen affinity. Nat. Biotechnol. 2002;20:597–601. doi: 10.1038/nbt0602-597. [DOI] [PubMed] [Google Scholar]
- 28.Yang WP, Green K, Pinz-Sweeney S, Briones AT, Burton DR, Barbas CF., III CDR walking mutagenesis for the affinity maturation of a potent human anti-HIV-1 antibody into the picomolar range. J. Mol. Biol. 1995;254:392–403. doi: 10.1006/jmbi.1995.0626. [DOI] [PubMed] [Google Scholar]
- 29.Jackson H, Bacon L, Pedley RB, Derbyshire E, Field A, Osbourn J, Allen D. Antigen specificity and tumour targeting efficiency of a human carcinoembryonic antigen-specific scFv and affinity-matured derivatives. Br. J. Cancer. 1998;78:181–188. doi: 10.1038/bjc.1998.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finlay WJ, Cunningham O, Lambert MA, Darmanin-Sheehan A, Liu X, Fennell BJ, Mahon CM, Cummins E, Wade JM, O’Sullivan CM, Tan XY, Piche N, Pittman DD, Paulsen J, Tchistiakova L, Kodangattil S, Gill D, Hufton SE. Affinity maturation of a humanized rat antibody for anti-RAGE therapy: Comprehensive mutagenesis reveals a high level of mutational plasticity both inside and outside the complementarity-determining regions. J. Mol. Biol. 2009;388:541–558. doi: 10.1016/j.jmb.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 31.Corti D, Suguitan AL, Jr, Pinna D, Silacci C, Fernandez-Rodriguez BM, Vanzetta F, Santos C, Luke CJ, Torres-Velez FJ, Temperton NJ, Weiss RA, Sallusto F, Subbarao K, Lanzavecchia A. Heterosubtypic neutralizing antibodies are produced by individuals immunized with a seasonal influenza vaccine. J. Clin. Invest. 2010;120:1663–1673. doi: 10.1172/JCI41902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okuno Y, Isegawa Y, Sasao F, Ueda S. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J. Virol. 1993;67:2552–2558. doi: 10.1128/jvi.67.5.2552-2558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ekiert DC, Bhabha G, Elsliger MA, Friesen RH, Jongeneelen M, Throsby M, Goudsmit J, Wilson IA. Antibody recognition of a highly conserved influenza virus epitope. Science. 2009;324:246–251. doi: 10.1126/science.1171491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sui J, Hwang WC, Perez S, Wei G, Aird D, Chen LM, Santelli E, Stec B, Cadwell G, Ali M, Wan H, Murakami A, Yammanuru A, Han T, Cox NJ, Bankston LA, Donis RO, Liddington RC, Marasco WA. Structural and functional bases for broad-spectrum neutralization of avian and human influenza A viruses. Nat. Struct. Mol. Biol. 2009;16:265–273. doi: 10.1038/nsmb.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wei CJ, Boyington JC, Dai K, Houser KV, Pearce MB, Kong WP, Yang ZY, Tumpey TM, Nabel GJ. Cross-neutralization of 1918 and 2009 influenza viruses: Role of glycans in viral evolution and vaccine design. Sci. Transl. Med. 2010;2 doi: 10.1126/scitranslmed.3000799. 24ra21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wrammert J, Koutsonanos D, Li GM, Edupuganti S, Sui J, Morrissey M, McCausland M, Skountzou I, Hornig M, Lipkin WI, Mehta A, Razavi B, Del Rio C, Zheng NY, Lee JH, Huang M, Ali Z, Kaur K, Andrews S, Amara RR, Wang Y, Das SR, O’Donnell CD, Yewdell JW, Subbarao K, Marasco WA, Mulligan MJ, Compans R, Ahmed R, Wilson PC. Broadly cross-reactive antibodies dominate the human B cell response against 2009 pandemic H1N1 influenza virus infection. J. Exp. Med. 2011;208:181–193. doi: 10.1084/jem.20101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bachmann MF, Kalinke U, Althage A, Freer G, Burkhart C, Roost H, Aguet M, Hengartner H, Zinkernagel RM. The role of antibody concentration and avidity in antiviral protection. Science. 1997;276:2024–2027. doi: 10.1126/science.276.5321.2024. [DOI] [PubMed] [Google Scholar]
- 38.Bachmann MF, Zinkernagel RM. Neutralizing antiviral B cell responses. Annu. Rev. Immunol. 1997;15:235–270. doi: 10.1146/annurev.immunol.15.1.235. [DOI] [PubMed] [Google Scholar]
- 39.Kalinke U, Oxenius A, Lopez-Macias C, Zinkernagel RM, Hengartner H. Virus neutralization by germ-line vs. hypermutated antibodies. Proc. Natl. Acad. Sci. U.S.A. 2000;97:10126–10131. doi: 10.1073/pnas.97.18.10126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, Batalle JP, Diaz L, Trento A, Chang HY, Mitzner W, Ravetch J, Melero JA, Irusta PM, Polack FP. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat. Med. 2009;15:34–41. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Polack FP, Teng MN, Collins PL, Prince GA, Exner M, Regele H, Lirman DD, Rabold R, Hoffman SJ, Karp CL, Kleeberger SR, Wills-Karp M, Karron RA. A role for immune complexes in enhanced respiratory syncytial virus disease. J. Exp. Med. 2002;196:859–865. doi: 10.1084/jem.20020781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galli G, Medini D, Borgogni E, Zedda L, Bardelli M, Malzone C, Nuti S, Tavarini S, Sammicheli C, Hilbert AK, Brauer V, Banzhoff A, Rappuoli R, Del Giudice G, Castellino F. Adjuvanted H5N1 vaccine induces early CD4+ T cell response that predicts long-term persistence of protective antibody levels. Proc. Natl. Acad. Sci. U.S.A. 2009;106:3877–3882. doi: 10.1073/pnas.0813390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dormitzer PR, Galli G, Castellino F, Golding H, Khurana S, Del Giudice G, Rappuoli R. Influenza vaccine immunology. Immunol. Rev. 2011;239:167–177. doi: 10.1111/j.1600-065X.2010.00974.x. [DOI] [PubMed] [Google Scholar]
- 44.Gasparini R, Schioppa F, Lattanzi M, Barone M, Casula D, Pellegrini M, Veitch K, Gaitatzis N. Impact of prior or concomitant seasonal influenza vaccination on MF59-adjuvanted H1N1v vaccine (Focetria) in adult and elderly subjects. Int. J. Clin. Pract. 2010;64:432–438. doi: 10.1111/j.1742-1241.2009.02309.x. [DOI] [PubMed] [Google Scholar]
- 45.Alberini I, Del Tordello E, Fasolo A, Temperton NJ, Galli G, Gentile C, Montomoli E, Hilbert AK, Banzhoff A, Del Giudice G, Donnelly JJ, Rappuoli R, Capecchi B. Pseudoparticle neutralization is a reliable assay to measure immunity and cross-reactivity to H5N1 influenza viruses. Vaccine. 2009;27:5998–6003. doi: 10.1016/j.vaccine.2009.07.079. [DOI] [PubMed] [Google Scholar]
- 46.Khurana S, Suguitan AL, Jr, Rivera Y, Simmons CP, Lanzavecchia A, Sallusto F, Manischewitz J, King LR, Subbarao K, Golding H. Antigenic fingerprinting of H5N1 avian influenza using convalescent sera and monoclonal antibodies reveals potential vaccine and diagnostic targets. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000049. e1000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Khurana S, Verma S, Verma N, Crevar CJ, Carter DM, Manischewitz J, King LR, Ross TM, Golding H. Bacterial HA1 vaccine against pandemic H5N1 influenza virus: Evidence of oligomerization, hemagglutination, and cross-protective immunity in ferrets. J. Virol. 2011;85:1246–1256. doi: 10.1128/JVI.02107-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.