Abstract
Study Objectives:
Sleep disturbances, including obstructive sleep apnea (OSA), commonly limit function and quality of life in people with spondyloarthritis (SpA). Systemic inflammation has been implicated in the pathophysiology of both OSA and SpA, and suppression of inflammation with tumor necrosis factor α (TNF) inhibitors may decrease OSA severity. In this study, we compared the frequency of OSA in patients receiving and not receiving TNF-inhibitor therapy.
Methods:
Data were collected from 63 consecutively screened veterans with SpA. Participant interviews, examinations, chart reviews, and referrals to the Salt Lake City Veteran Affairs (SLCVA) Sleep Center were used to obtain demographic data, comorbidities, SpA features, therapy data, and sleep study outcomes.
Results:
OSA occurred in 76% of SpA patients. OSA was less common in patients receiving TNF-inhibitor therapy (57%), compared to patients not receiving TNF-inhibitor therapy (91%) (p = 0.01).
Conclusions:
OSA is underrecognized in veterans with SpA, and TNF-inhibition was associated with a lower frequency of OSA.
Citation:
Walsh JA; Duffin KC; Crim J; Clegg DO. Lower frequency of obstructive sleep apnea in spondyloarthritis patients taking TNF-inhibitors. J Clin Sleep Med 2012;8(6):643-648.
Keywords: Obstructive sleep apnea, spondyloarthritis, TNF-inhibitor
Normal sleep is a vital component of health and quality of life. Sleep disturbances negatively affect health, with elevated risks of neurocognitive dysfunction, coronary artery disease, hypertension, depression, stroke, motor vehicle accidents, and premature death.1 Sleep disturbances and fatigue are prominent features of SpA,2,3 and inflammatory arthritis patients with sleep inadequacies experience more pain and functional limitations than patients with healthy sleep patterns.4
The contribution of OSA to sleep disturbances in SpA is not well characterized. Two small uncontrolled studies reported OSA prevalences of 12% and 23% in ankylosing spondylitis (AS) patients, compared to 1% to 9% in the general population.5,6 Psoriatic arthritis (PsA) has been associated with sleep interference independent of body mass index (BMI),7 and a higher prevalence of OSA has been reported in psoriasis patients compared to controls.8
Various mechanisms have been proposed to explain the high prevalence of OSA in SpA patients including (1) glucocorticoid-induced weight gain, (2) compression of the oropharyngeal airway by the cervical spine, and (3) systemic inflammation.5,9 Glucocorticoids are selectively used to treat peripheral disease manifestations of SpA. Glucocorticoids cause central obesity,10 and glucocorticoid-induced deposition of fat in the thorax and neck may increase the risk of OSA in SpA patients.5,11
The second hypothesis proposes that SpA patients are at increased risk for OSA because of structural changes in the cervical spine. SpA patients frequently develop pathologic boney bridging between vertebrae called syndesmophytes. Syndesmophytes in the anterior cervical spine may contribute to OSA by compressing the oropharyngeal airway.5 This hypothesis is supported by case reports of improvement in OSA after surgical removal of excessively bulky pathologic bone from the anterior cervical spine.12
BRIEF SUMMARY
Current Knowledge/Study Rationale: Sleep disturbances and fatigue are common in patients with systemic inflammatory conditions, including spondyloarthritis. The purposes of this study were to evaluate the frequency of obstructive sleep apnea (OSA) in spondyloarthritis patients and to explore associations between TNF-inhibitor therapy and OSA severity.
Study Impact: The high frequency of OSA demonstrated that routine screening for OSA risks should be considered in spondyloarthritis patients. The association between TNF-inhibitor therapy and a lower frequency of OSA suggests that further investigation of TNF-inhibition may contribute to our understanding of OSA pathology and the development of novel therapies for OSA.
The hypothesis that systemic inflammation contributes to OSA risk is particularly intriguing because shared inflammatory pathways contribute to the pathophysiology of both SpA and OSA. OSA is characterized by repetitive airway obstructions causing intermittent hypoxia during sleep. Nuclear factor-κB is activated by intermittent hypoxia and upregulates the transcription of pro-inflammatory genes.13 The pro-inflammatory genes encode multiple inflammatory cytokines, including TNF-α. Elevated levels of TNF-α have been reported in OSA patients, independent of obesity, and TNF-α levels fall with continuous positive airway pressure (CPAP) therapy for OSA.14,15 TNF-α is a well-recognized mediator of inflammation in SpA, and inhibition of TNF-α improves symptoms of SpA, including fatigue and sleepiness.2,16,17
To explore the hypothesis that suppression of inflammation with TNF-inhibition improves sleep outcomes, Vgontzas et al. treated eight obese OSA patients with etanercept.18 They reported improvements in the apnea hypopnea index (AHI) (p < 0.05) and sleepiness (p < 0.05) after three weeks of therapy. Since inflammatory cytokine levels are higher in inflammatory arthritis patients than obese patients,19,20 we hypothesize that TNF-inhibitors may have a greater effect on OSA in SpA patients than obese patients. In this study, we explored the impact of TNF-inhibition on OSA, by comparing OSA frequencies in SpA patients receiving and not receiving TNF-inhibitor therapy.
METHODS
Between July 2008 and July 2010, 63 consecutively encountered patients enrolled in the Program to Understand the Longterm outcomes of SpondyloARthrits (PULSAR) registry from the SLCVA Rheumatology Clinics were screened for this study. Inclusion criteria were participation in PULSAR and willingness to participate in this investigation. According to established practice at the SLCVA, current cigarette smokers were excluded because smoking confounds sleep testing and interpretation. Patients were excluded from analyses if they had sleep studies that were unavailable, incomplete, or inconsistent with the SLCVA Sleep Center's reporting of sleep apnea parameters.
In order to qualify for a sleep study, the SLCVA Sleep Center required patients to have at least one symptom of OSA. SpA patients were evaluated for symptoms of OSA by the principal investigator at the time of clinic visits. The symptom evaluations included questions about snoring, excessive daytime sleepiness, non-restorative sleep, cessation in breathing while sleeping, awakening gasping for air, restlessness at night, difficulty sleeping with frequent awakenings, concentration difficulties, falling asleep while driving, changes in mood, morning headaches, and vivid, strange, or threatening dreams. All SpA patients had ≥ 1 symptom of OSA and were offered a referral to the SLCVA Sleep Center for standard-of-care testing and therapy.
The assignment of home monitoring versus laboratory polysomnography (PSG) was determined by the SLCVA Sleep Center coordinators and was based on patient comorbidities and the availability of home monitoring devices. Home monitoring tests were conducted for 3 nights with 4-channel NovaSom QSG Diagnostic devices that measured airflow, oximetry, pulse, and respiratory effort. PSG testing was completed in sleep laboratories located in Utah and surrounding states. Apnea events were defined as cessation of airflow ≥ 10 seconds. Hypopnea events were defined as a decrease in airflow ≥ 50% and a decline in oxyhemoglobin saturation of ≥ 4% for ≥ 10 seconds. Epworth Sleepiness Scale (ESS) questionnaires were administered to patients by the SLCVA Sleep Center.
Data including demographics, comorbidities, SpA features, SpA activity, and therapies were obtained from patient interviews, examinations, and medical records. The Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) and Bath Ankylosing Spondylitis Functional Index (BASFI) are validated patient questionnaires that were used to measure SpA disease activity and severity. The erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are laboratory inflammatory markers commonly used for SpA disease activity assessments. BASFI, BASDAI, ESR, and CRP data were included only if they were completed within 2 months of the sleep study date. Treatment information was collected from clinic visit notes and verified via the SLCVA's pharmacy medication dispensing records.
Available cervical spine radiographs were evaluated by a board-certified musculoskeletal radiologist. The scoring system from the modified Stoke Ankylosing Spondylitis Spinal Score (mSASSS) was applied to quantify radiographic changes in the cervical spine consistent with SpA.21 The number of syndesmophytes in the cervical spine was also recorded. The maximum syndesmophyte size was determined by measuring the distance between the most anterior portion of the largest syndesmophyte and the vertical plane of the adjacent anterior vertebral bodies, on the lateral projection of cervical radiographs.
Continuous variables were compared with 2-tailed Student t-tests. Categorical variables were compared with Fisher exact tests. Spearman correlation coefficients were used to compare AHI to SpA features and activity measures, including cervical mSASSS, number of syndesmophytes, maximum syndesmophyte size, BASDAI, BASFI, ESR, and CRP.
RESULTS
Sixty-three consecutively encountered SpA patients were screened for participation (Figure 1); 45 patients were included in the analyses. Thirty-six participants were tested with home monitoring devices, and 9 participants received PSG in sleep laboratories. Informed consent was obtained from all participants, and approval was granted by the Institutional Review Board at the University of Utah.
Figure 1. Study profile.
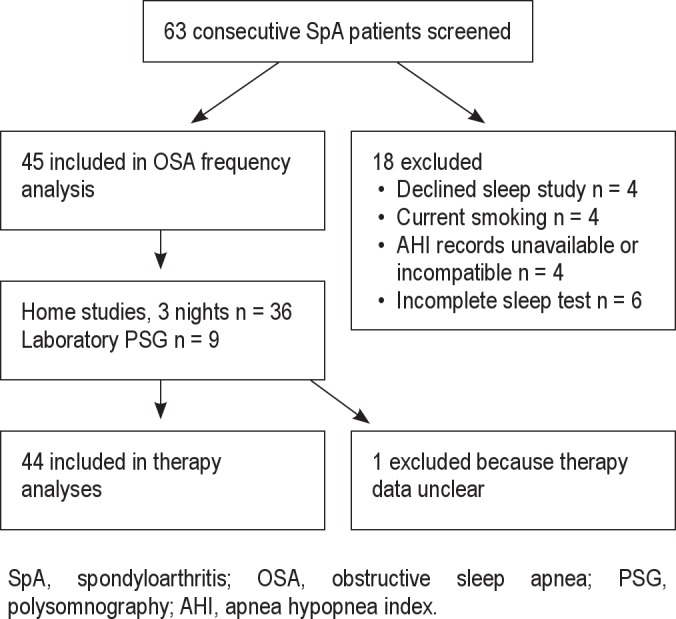
SpA, spondyloarthritis; OSA, obstructive sleep apnea; PSG, polysomnography; AHI, apnea hypopnea index.
Eighteen patients were excluded: 4 patients declined participation, and 4 were excluded because of current smoking. Four additional patients with a diagnosis of sleep disordered breathing were excluded because they did not have clinical indications for retesting, and medical records were unavailable or inconsistent with the SLCVA Sleep Center's reporting of OSA parameters. Six excluded patients had incomplete home sleep studies and were unwilling or unable to repeat sleep testing. No patient was excluded because of lack of OSA symptoms. One patient was excluded from therapy analyses because it was unclear if a TNF-inhibitor was used when the sleep study was completed. A comparison of patients included and excluded from this investigation demonstrated no differences in demographics, comorbidities, SpA features, SpA disease activity, TNF-inhibitors, or other SpA therapies.
The mean age of participants was 59.7 ± 11.8, and 43 (96%) were male. (Table 1) The mean BMI was 29.2 ± 5.2. Thirty-one (69%) participants had hypertension, 4 (9%) had ischemic cardiomyopathy, and 3 (7%) had a history of a stroke. Radiographic measures of cervical spine disease included a mean cervical mSASSS score of 8.5 ± 8.4, a mean number of syndesmophytes of 2.8 ± 2.0, and a maximum syndesmophyte size mean of 2.6 ± 1.4. The mean BASDAI and BASFI scores were 4.5 ± 2.5 and 4.3 ± 2.6, respectively. The mean ESR was 14.6 ± 14.2 mm/h, and the mean CRP was 10.0 ± 10.8 mg/L.
Table 1.
Characteristics of 45 participants
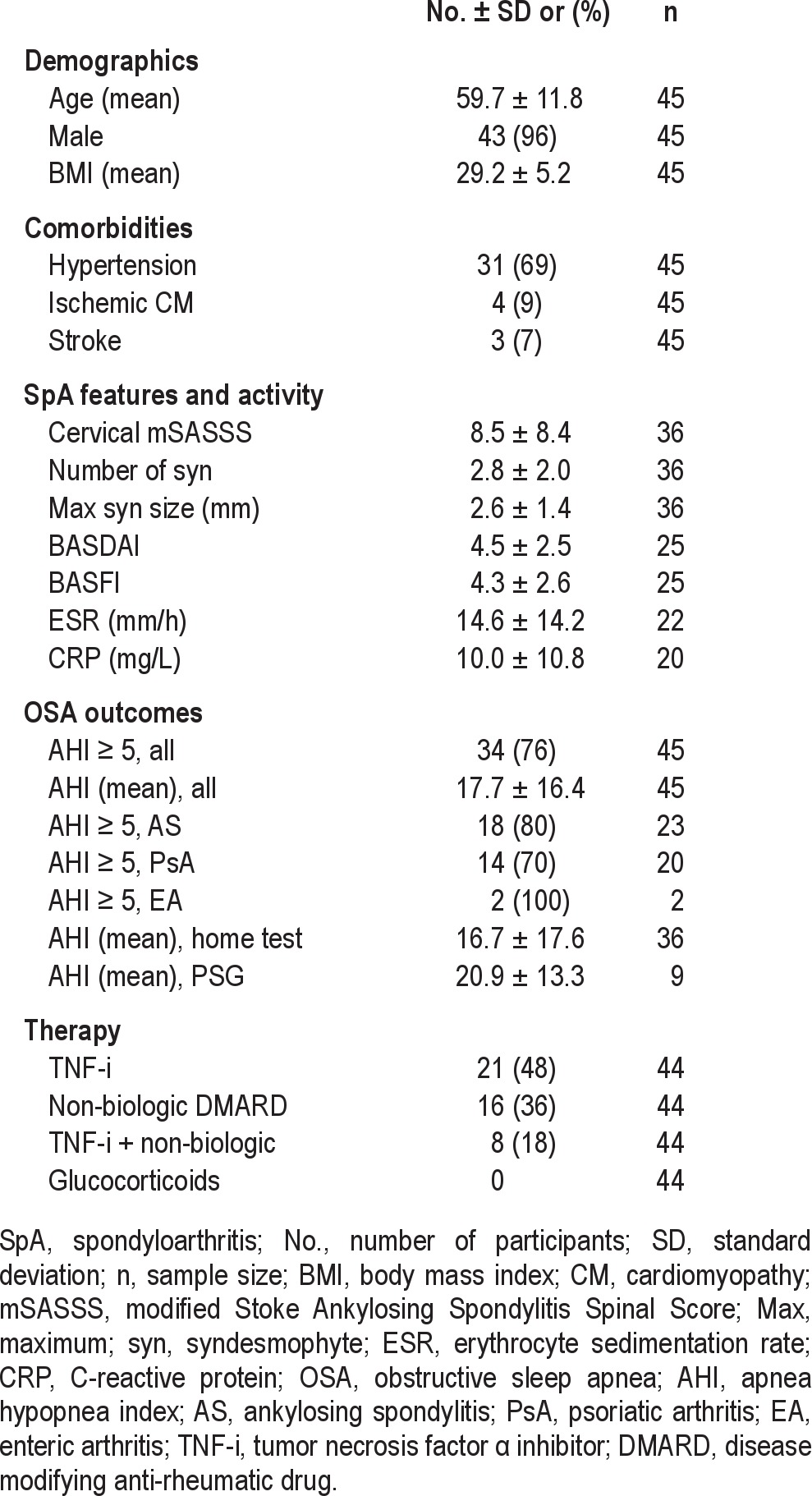
Thirty-four (76%) participants had an AHI ≥ 5. The mean AHI was 17.7 ± 16.4. The AHI was ≥ 5 in 18 (80%) AS patients, 14 (70%) PsA patients, and 2 (100%) enteric arthritis (EA) patients. The mean AHI of participants tested with home monitoring devices was not statistically different than the mean AHI of participants tested with laboratory PSG (16.7 ± 17.6 vs. 20.9 ± 13.3, respectively, p = 0.47).
Therapy data were available in 44 of the 45 participants. Twenty-one (48%) were receiving TNF-inhibitor therapy at the time of sleep testing. Non-biologic disease modifying anti-rheumatic drugs (DMARDs) included methotrexate, hydroxychloroquine, sulfasalazine, and leflunomide. Sixteen (36%) patients received non-biologic DMARDs, and 8 (18%) were on a combination of a TNF-inhibitor and non-biologic DMARD(s). Glucocorticoids were not used by any participant within 12 months prior to sleep testing.
In comparing participants with and without TNF-inhibitor therapy, the demographics, SpA subtype distribution, and comorbidities were similar (Table 2). Radiographic disease of the cervical spine, as measured by the cervical mSASSS, number of syndesmophytes, and maximum syndesmophyte size, was similar in participants receiving and not receiving TNF-inhibitors. The BASDAI and BASFI disease activity scores were lower in the group receiving TNF-inhibitor therapy than the group without TNF-inhibitor therapy, but the differences were not statistically significant. There were no differences in ESR or CRP between groups.
Table 2.
Characteristics of 44 SpA patients with and without TNF-I
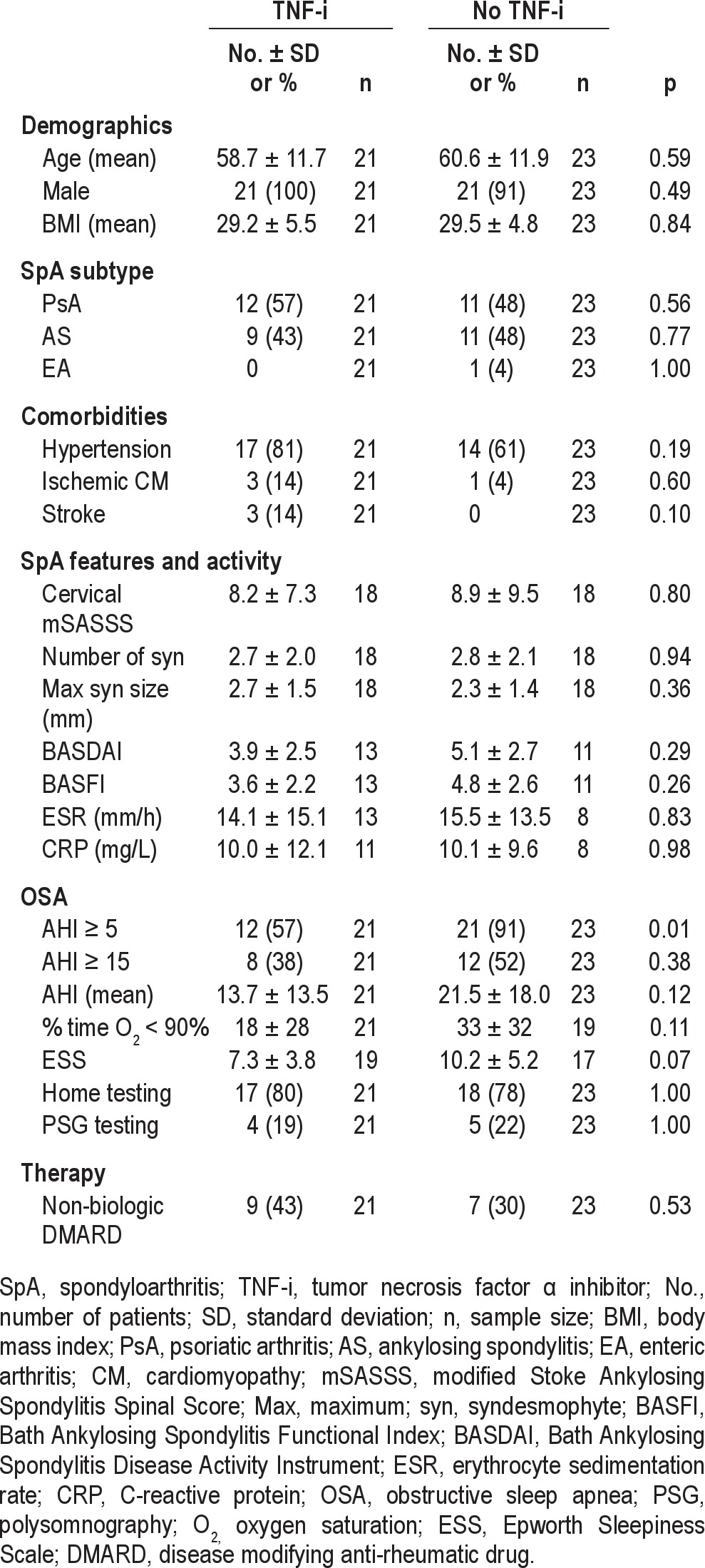
Participants using TNF-inhibitor therapy had OSA less frequently than participants without TNF-inhibitor therapy (12 [57%] vs. 21 [91%], p = 0.01). Compared to participants taking TNF-inhibitors, participants without TNF-inhibition had more abnormal OSA outcomes, including a higher proportion of participants with AHI ≥ 15, a higher mean AHI, a higher percent time with oxygen saturation < 90%, and a higher mean ESS score, but the differences were not statistically significant. The allocation of home monitoring devices and PSG testing was similar in the TNF-inhibitor and no TNF-inhibitor groups (p = 1.00). Similar proportions of participants with and without TNF-inhibition were taking non-biologic DMARDs at the time of sleep testing.
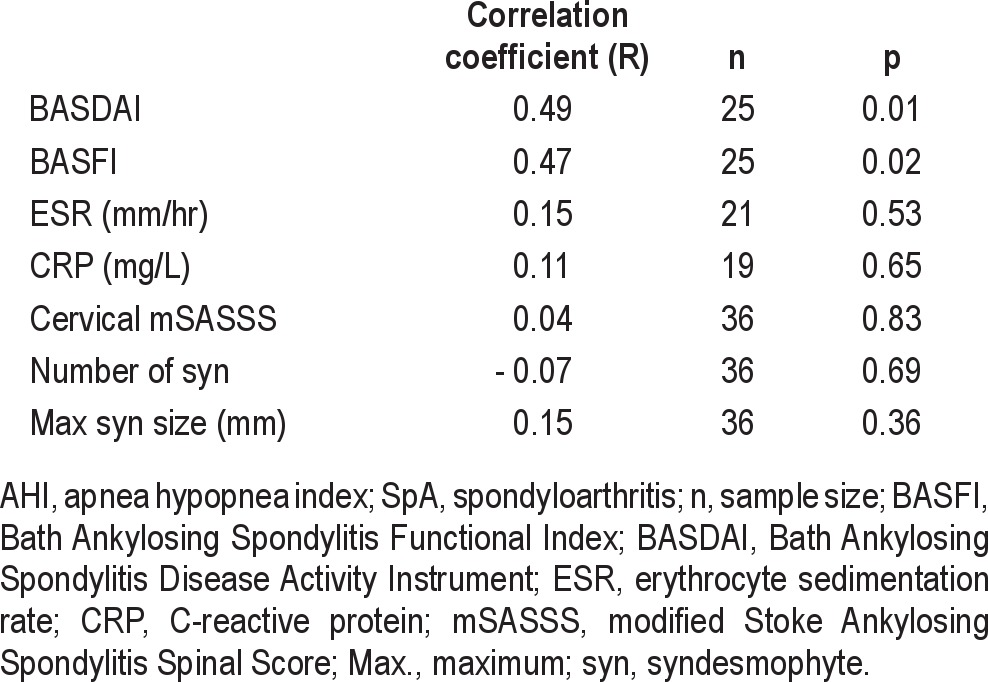
Correlation coefficients were calculated between AHI and SpA features and between AHI and SpA activity measures in all participants, regardless of TNF-inhibitor therapy (Table 3). There were moderate correlations between AHI and BASDAI (R = 0.49) and between AHI and BASFI (R = 0.47). The ESR, CRP, cervical mSASSS, number of syndesmophytes, and maximum syndesmophyte size did not significantly correlate with AHI.
Table 3.
Correlations between AHI and SpA features and activity measures in all participants
DISCUSSION
We found that OSA was less frequent in participants receiving TNF-inhibitor therapy, compared to participants not receiving TNF-inhibitor therapy. Additionally, there were nonsignificant trends toward less severe OSA in the TNF-inhibitor group, with respect to AHI, oxygen saturation, and sleepiness. These data suggest that TNF-inhibition may protect against the development of OSA and decrease OSA severity. The improvement in AHI and sleepiness in obese patients treated with a TNF-inhibitor reported by Vgontzas et al. also suggested that TNF-inhibition may reduce OSA severity and sleepiness.18 These findings are conceptually supported by the growing body of evidence implicating TNF-α and other inflammatory mediators in the pathophysiology of OSA.13–15
The mechanistic relationship between inflammation and hypoxia in OSA is unclear. It has been proposed that intermittent hypoxia stimulates inflammatory cytokine production, and resultant inflammation contributes to OSA comorbidities, such as cardiovascular disease.22 This theory suggests that suppression of inflammation may improve comorbidity-related outcomes for OSA patients, but fails to explain the possible protective effects of TNF-inhibition on OSA development and severity. Research in children and adults with OSA has demonstrated that intranasal steroids improve AHI, presumably by diminishing inflammation in hypertrophied lymphoid tissue adjacent to airways.23,24 An alternative hypothesis is that alterations of cholinergic activity with TNF-inhibition may reduce airway collapse during sleep. Acetylcholine increases muscle contraction and sympathetic autonomic activity. It is also involved in the regulation of inflammatory cytokines, including TNF-α.25,26 TNF-inhibition may alter the balance of acetylcholine and inflammatory cytokines. These changes may positively influence airway musculature and reduce hypoxic events in OSA patients. A pilot study, reporting reductions in AHI after therapy with a cholinesterase inhibitor, supports this hypothesis.27
Since systemic inflammation is suspected to contribute to the severity of OSA, we hypothesized that AHI would correlate with measures of SpA disease activity. BASDAI and BASFI scores had moderate correlations with AHI, suggesting that higher burdens of inflammation are positively associated with OSA severity. In contrast, ESR and CRP did not correlate with AHI. This was unexpected, since CRP levels have been demonstrated to correlate with both OSA and SpA disease activity.28 However, other uncontrolled factors may have influenced ESR and CRP levels, including non-biologic DMARD use. Several non-biologic DMARDs were used by participants, and these DMARDS are known to affect ESR and CRP levels.
We also explored the hypothesis that structural changes in the cervical spine may increase the risk of OSA in SpA patients. Our data did not support this premise, with no correlations between AHI and any measure of radiographic disease of the cervical spine. We were unable to comment on the relationship between glucocorticoid use and OSA because glucocorticoids were not used by any participant. The lack of support for these alternative hypotheses strengthens our position that suppression of inflammation contributed to the lower frequency of OSA in participants on TNF-inhibitor therapy.
The markedly elevated frequency of OSA in veterans with SpA suggests that the scope of this medical condition may be greater than previously recognized. The 80% frequency of OSA in the 20 veterans with AS is much higher than the 12% and 23% prevalences reported in AS patients in German and Turkish studies.5,6 The older mean age of 60 in this study, compared to 47 and 33 in the respective aforementioned studies, may account for some of the differences in OSA prevalence. Age was established as a risk factor for OSA in a study of 741 men, in which the OSA prevalence was 24.8% in men ≥ 65 years old compared to 7.9% in men 20–44 years old, when OSA was defined as AHI ≥ 5, regardless of symptoms.29 Since body weight is the strongest predictor of OSA,1 the higher mean BMI of 29.3 in this study, compared to 26.4 and 24.5 in the German and Turkish studies, also likely contributed to the high OSA frequency in this investigation.
There may have been additional characteristics affecting the risk for OSA in this veteran population that differed from the German and Turkish populations. The prevalence of OSA in 4,060,504 U.S. veterans according to ICD-9 codes was 2.9%,30 but these patients likely represent only the most symptomatic veterans. The prevalence of OSA was 27% among 26 randomly selected veteran inpatients, when OSA was defined by > 30 episodes of apnea per night.31 A matched control group of outpatient veterans without SpA would be required to quantify the differential risk for OSA with and without SpA. However, the very high frequency of OSA in this population suggests that providers should consider routine screening for OSA risks in SpA patients.
Enhanced awareness of the elevated risk of OSA in SpA will create opportunities to improve patients' quality of life and potentially survival. In a study evaluating the relative importance of disease features, approximately 50% of AS and PsA patients ranked fatigue among their top 3 priorities for disease improvement.3 OSA is strongly associated with fatigue, and CPAP therapy improves energy levels and quality of life.23,32,33 Importantly, long-term CPAP is also associated with decreased mortality.34,35
Despite the benefits of CPAP, approximately 30% of OSA patients stop CPAP within 10 years, primarily because of intolerance.36 The only available nonsurgical alternatives involve oral and nasal appliances, which are generally less effective.37,38 OSA patients would benefit from additional therapeutic options, and improving our understanding of TNF-inhibition in OSA may lead to the development of novel therapies.
This study is limited by the small sample size. An additional limitation involves the use of different types of sleep apnea testing. While PSG in a sleep laboratory is the gold standard, portable home monitoring devices are increasingly used to improve access and reduce cost. Comparisons of home monitoring to laboratory PSG have demonstrated strong correlations between the diagnostic results of these testing modalities.39 Additionally, equivalent outcomes have been reported in patients tested with home studies and in-laboratory PSG.40
In conclusion,this investigation demonstrated a high frequency of OSA in veterans with SpA and suggests that OSA is underrecognized in this population. SpA patients currently treated with TNF-inhibitors had OSA less frequently than untreated patients. Further investigation of TNF-inhibition in OSA may provide mechanistic insights into the pathogenesis of OSA,and TNF-inhibition should be explored as an alternative therapy to CPAP.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This research was conducted at the George E. Wahlen Veteran Affairs Medical Center in Salt Lake City, Utah. We gratefully acknowledge Jane Elizabeth Bell, APRN, John Shigeoka, M.D., and Sarah Richey, M.D.,for their contributions with sleep study coordination and manuscript preparation.
REFERENCES
- 1.Punjabi NM. The epidemiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5:136–43. doi: 10.1513/pats.200709-155MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deodhar A, Braun J, Inman RD, et al. Golimumab reduces sleep disturbance in patients with active ankylosing spondylitis: results from a randomized, placebo-controlled trial. Arthritis Care Res (Hoboken) 2010;62:1266–71. doi: 10.1002/acr.20233. [DOI] [PubMed] [Google Scholar]
- 3.Heiberg T, Lie E, van der Heijde D, Kvien TK. Sleep problems are of higher priority for improvement for patients with ankylosing spondylitis than for patients with other inflammatory arthropathies. Ann Rheum Dis. 2011;70:872–3. doi: 10.1136/ard.2010.133793. [DOI] [PubMed] [Google Scholar]
- 4.Lee YC, Chibnik LB, Lu B, et al. The relationship between disease activity, sleep, psychiatric distress and pain sensitivity in rheumatoid arthritis: a cross-sectional study. Arthritis Res Ther. 2009;11:R160. doi: 10.1186/ar2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erb N, Karokis D, Delamere JP, Cushley MJ, Kitas GD. Obstructive sleep apnoea as a cause of fatigue in ankylosing spondylitis. Ann Rheum Dis. 2003;62:183–4. doi: 10.1136/ard.62.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solak O, Fidan F, Dündar U, et al. The prevalence of obstructive sleep apnoea syndrome in ankylosing spondylitis patients. Rheumatology (Oxford) 2009;48:433–5. doi: 10.1093/rheumatology/kep021. [DOI] [PubMed] [Google Scholar]
- 7.Callis Duffin K, Wong B, Horn EJ, Krueger GG. Psoriatic arthritis is a strong predictor of sleep interference in patients with psoriasis. J Am Acad Dermatol. 2009;60:604–8. doi: 10.1016/j.jaad.2008.10.059. [DOI] [PubMed] [Google Scholar]
- 8.Kim N, Thrash B, Menter A. Comorbidities in psoriasis patients. Semin Cutan Med Surg. 2010;29:10–5. doi: 10.1016/j.sder.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Yamamoto J, Okamoto Y, Shibuya E, Nishimura M, Kawakami Y. Obstructive sleep apnea syndrome induced by ossification of the anterior longitudinal ligament with ankylosing spondylitis. Nihon Kokyuki Gakkai Zasshi. 2000;38:413–6. [PubMed] [Google Scholar]
- 10.Peckett AJ, Wright DC, Riddell MC. The effects of glucocorticoids on adipose tissue lipid metabolism. Metabolism. 2011;60:1500–10. doi: 10.1016/j.metabol.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 11.Isono S. Obesity and obstructive sleep apnoea: mechanisms for increased collapsibility of the passive pharyngeal airway. Respirology. 2012;17:32–42. doi: 10.1111/j.1440-1843.2011.02093.x. [DOI] [PubMed] [Google Scholar]
- 12.Fuerderer S, Eysel-Gosepath K, Schröder U, Delank KS, Eysel PJ. Retro-pharyngeal obstruction in association with osteophytes of the cervical spine. Bone Joint Surg Br. 2004;86:837–40. doi: 10.1302/0301-620x.86b6.14933. [DOI] [PubMed] [Google Scholar]
- 13.Ryan S, Taylor CT, McNicholas WT. Predictors of elevated nuclear factor-kappaB-dependent genes in obstructive sleep apnea syndrome. Am J Respir Crit Care Med. 2006;174:824–30. doi: 10.1164/rccm.200601-066OC. [DOI] [PubMed] [Google Scholar]
- 14.Garvey JF, Taylor CT, McNicholas WT. Cardiovascular disease in obstructive sleep apnoea syndrome: the role of intermittent hypoxia and inflammation. Eur Respir J. 2009;33:1195–205. doi: 10.1183/09031936.00111208. [DOI] [PubMed] [Google Scholar]
- 15.Kapsimalis F, Basta M, Varouchakis G, Gourgoulianis K, Vgontzas A, Kryger M. Cytokines and pathological sleep. Sleep Med. 2008;9:603–14. doi: 10.1016/j.sleep.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Herenius MM, Hoving JL, Sluiter JK, et al. Improvement of work ability, quality of life, and fatigue in patients with rheumatoid arthritis treated with adalimumab. J Occup Environ Med. 2010;52:618–21. doi: 10.1097/JOM.0b013e3181de8357. [DOI] [PubMed] [Google Scholar]
- 17.Rudwaleit M, Gooch K, Michel B, et al. Adalimumab improves sleep and sleep quality in patients with active ankylosing spondylitis. J Rheumatol. 2011;38:79–86. doi: 10.3899/jrheum.100213. [DOI] [PubMed] [Google Scholar]
- 18.Vgontzas AN, Zoumakis E, Lin HM, Bixler EO, Trakada G, Chrousos GP. Marked decrease in sleepiness in patients with sleep apnea by etanercept, a tumor necrosis factor-alpha antagonist. J Clin Endocrinol Metab. 2004;89:4409–13. doi: 10.1210/jc.2003-031929. [DOI] [PubMed] [Google Scholar]
- 19.Edrees AF, Misra SN, Abdou NI. Anti-tumor necrosis factor (TNF) therapy in rheumatoid arthritis: correlation of TNF-alpha serum level with clinical response and benefit from changing dose or frequency of infliximab infusions. Clin Exp Rheumatol. 2005;23:469–74. [PubMed] [Google Scholar]
- 20.Winkler G, Lakatos P, Salamon F, et al. Elevated serum TNF-alpha level as a link between endothelial dysfunction and insulin resistance in normotensive obese patients. Diabet Med. 1999;16:207–11. doi: 10.1046/j.1464-5491.1999.00052.x. [DOI] [PubMed] [Google Scholar]
- 21.Braun J, Baraliakos X. Imaging of axial spondyloarthritis including ankylosing spondylitis. Ann Rheum Dis. 2011;70(Suppl 1):i97–103. doi: 10.1136/ard.2010.140541. [DOI] [PubMed] [Google Scholar]
- 22.Kent BD, Ryan S, McNicholas WT. Obstructive sleep apnea and inflammation: Relationship to cardiovascular co-morbidity. Respir Physiol Neurobiol. 2011 Mar 23; doi: 10.1016/j.resp.2011.03.015. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 23.Kuhle S, Urschitz MS. Anti-inflammatory medications for obstructive sleep apnea in children. Cochrane Database Syst Rev. 2011:CD007074. doi: 10.1002/14651858.CD007074.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Smith I, Lasserson TJ, Wright J. Drug therapy for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;19:CD003002. doi: 10.1002/14651858.CD003002.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Huston JM, Tracey KJ. The pulse of inflammation: heart rate variability, the cholinergic anti-inflammatory pathway and implications for therapy. J Intern Med. 2011;269:45–53. doi: 10.1111/j.1365-2796.2010.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Yang Z, Xue B, Shi H. Activation of the cholinergic antiinflammatory pathway ameliorates obesity-induced inflammation and insulin resistance. Endocrinology. 2011;152:836–46. doi: 10.1210/en.2010-0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedner J, Kraiczi H, Peker Y, Murphy P. Reduction of sleep-disordered breathing after physostigmine. Am J Respir Crit Care Med. 2003;168:1246–51. doi: 10.1164/rccm.200211-1344OC. [DOI] [PubMed] [Google Scholar]
- 28.Mermigkis C, Bouloukaki I, Mermigkis D, et al. Sleep Breath. Epub ahead of print; 2011. Sep 1, CRP evolution patter in CPAP-treated obstructive sleep apnea patients. Does gender play a role? [DOI] [PubMed] [Google Scholar]
- 29.Bixler EO, Vgontzas AN, Ten Have T, Tyson K, Kales A. Effects of age on sleep apnea in men: Prevalence and severity. Am J Respir Crit Care Med. 1998;157:144–8. doi: 10.1164/ajrccm.157.1.9706079. [DOI] [PubMed] [Google Scholar]
- 30.Sharafkhaneh A, Richardson P, Hirshkowitz M. Sleep apnea in a high risk population: a study of Veterans Health Administration beneficiaries. Sleep Med. 2004;5:345–50. doi: 10.1016/j.sleep.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 31.Kreis P, Kripke DF, Ancoli-Israel S. Sleep apnea: a prospective study. West J Med. 1983;139:171–3. [PMC free article] [PubMed] [Google Scholar]
- 32.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 33.Sanner BM, Klewer J, Trumm A, Randerath W, Kreuzer I, Zidek W. Long-term treatment with continuous positive airway pressure improves quality of life in obstructive sleep apnoea syndrome. Eur Respir J. 2000;16:118–22. doi: 10.1034/j.1399-3003.2000.16a21.x. [DOI] [PubMed] [Google Scholar]
- 34.Young T, Finn L, Peppard PE, et al. Sleep disordered breathing and mortality: eighteen-year follow-up of the Wisconsin sleep cohort. Sleep. 2008;31:1071–8. [PMC free article] [PubMed] [Google Scholar]
- 35.Jennum P, Kjellberg J. Health, social and economic consequences of sleep-disordered breathing: a controlled national study. Thorax. 2011;66:560–6. doi: 10.1136/thx.2010.143958. [DOI] [PubMed] [Google Scholar]
- 36.Kohler M, Smith D, Tippett V, Stradling JR. Predictors of long-term compliance with continuous positive airway pressure. Thorax. 2010;65:829–32. doi: 10.1136/thx.2010.135848. [DOI] [PubMed] [Google Scholar]
- 37.Randerath WJ, Verbraecken J, Andreas S, et al. Non-CPAP therapies in obstructive sleep apnoea. Eur Respir J. 2011;37:1000–28. doi: 10.1183/09031936.00099710. [DOI] [PubMed] [Google Scholar]
- 38.Woodson BT. Non-pressure therapies for obstructive sleep apnea: surgery and oral appliances. Respir Care. 2010;55:1314–21. discussion 1321. [PubMed] [Google Scholar]
- 39.Santos-Silva R, Sartori DE, Truksinas V, et al. Validation of a portable monitoring system for the diagnosis of obstructive sleep apnea syndrome. Sleep. 2009;32:629–36. doi: 10.1093/sleep/32.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collop NA. Portable monitoring for the diagnosis of obstructive sleep apnea. Curr Opin Pulm Med. 2008;14:525–9. doi: 10.1097/MCP.0b013e328312ed4a. [DOI] [PubMed] [Google Scholar]


