Abstract
Study Objectives:
First, to determine whether serum vitamin D levels were correlated with excessive daytime sleepiness (EDS) in patients with or without vitamin D deficiency (VitDd). Second, to assess whether race affected the relation between vitamin D levels and EDS.
Methods:
Serum 25-hydroxyvitamin D (25OHD) was measured by immunoassay in a consecutive series of 81 sleep clinic patients who complained of sleep problems and nonspecific pain (25OHD < 20 ng/mL ' VitDd). Sleepiness was determined using the Epworth Sleepiness Scale score ([ESSs] ESSs ≥ 10 ' EDS). Correlations were assessed using Pearson r.
Results:
In patients without VitDd (25OHD ≥ 20 ng/mL), ESSs was inversely correlated with vitamin D concentration (r = 0.45, p < 0.05). The group consisted of 6% black patients, compared with 35% for the entire cohort. Among the patients who had VitDd (25OHD < 20 ng/mL), ESSs was directly correlated with 25OHD in black (r = 0.48, p < 0.05) but not white patients. In black patients, mean ESSs in patients with VitDd were higher and 25OHD levels were lower p < 0.05).
Conclusions:
The results suggested the novel possibility that VitDd-related disease has a yet-to-be-identified mechanistic role in the presentation of sleepiness, sleep disorders, or both. Further research is needed to clarify the mechanism(s) involved in producing the complex relationships noted.
Commentary:
A commentary on this article appears in this issue on page 699.
Citation:
McCarty DE; Reddy A; Keigley Q; Kim PY; Marino AA. Vitamin D, race, and excessive daytime sleepiness. J Clin Sleep Med 2012;8(6):693-697.
Keywords: Vitamin D, excessive daytime sleepiness, pain, sleep regulating substances
Understanding of the metabolic role of vitamin D has expanded greatly beyond its classically described effects on gut and bone, leading to reports of numerous non-classical diseases associated with insufficient supply of vitamin D.1 Recent reports showed that vitamin D had potent immunomodulatory activities2 and that low serum levels of vitamin D were linked to pulmonary disease,3 musculoskeletal pain,4 metabolic syndrome,5 hypertension,6 poor stress resilience,7 altered emotional functioning,8 and cognitive decline.9 Subjects classified as deficient in vitamin D generally exhibited more severe and/or chronic symptoms than subjects with smaller departures from the age- and gender-adjusted levels found in the clinically normal population.
More than half of the patients seen in our sleep medicine clinic who complained of sleep disruption and nonspecific somatic pain also exhibited vitamin D deficiency.10 The association was particularly strong among black patients. In a case involving idiopathic central nervous system hypersomnia, treatment of a deficiency in vitamin D resulted in a resolution of the clinical syndrome.11 These reports together with the rapidly developing perception of the ubiquitous role of vitamin D in metabolism suggest that suboptimal levels of vitamin D could cause or contribute to a pathologic level of centrally induced sleepiness, either directly or by means of chronic pain. Under the hypothesis that insufficient serum vitamin D was a causal or contributing factor in the development of excessive daytime sleepiness (EDS), we would expect to find progressively lower levels of vitamin D among patients with progressively higher levels of EDS, at least in patients who exhibited only moderate hypovitaminosis D.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Deficiency of Vitamin D is known to contribute to somatic pain symptoms and immune dysregulation (including inducing a relative elevation of circulating TNFα and NFκB, both of which can result in subjective sleepiness symptoms). It is therefore mechanistically plausible that deficiency of Vitamin D could contribute to poor quality sleep and/or symptoms of impaired wakefulness (such as excessive daytime sleepiness).
Study Impact: We found a significant, but complex, relationship between 25-hydroxyvitamin D and subjective sleepiness, as measured by the Epworth Sleepiness Scale, suggesting the possibility that Vitamin D deficiency may be a modifiable cofactor in the pathophysiology of excessive daytime sleepiness, sleep disorders, or both. Further research is needed to clarify these relationships.
Our first aim was to determine whether serum vitamin D levels were correlated with sleepiness in patients with or without VitDd. Our second aim was to assess the role of race in the relationship between vitamin D levels and sleepiness.
METHODS
Patients
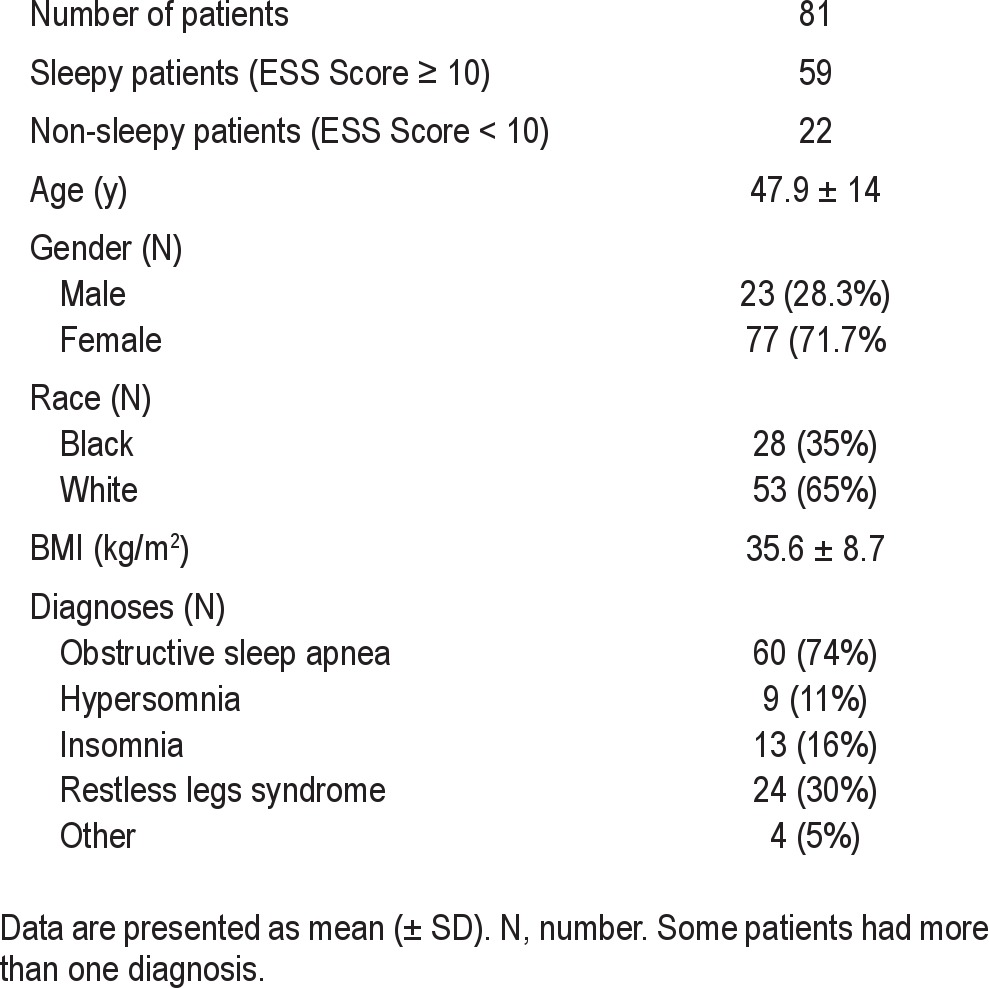
Consecutive new patients seen during routine consultation visits in an academic sleep medicine clinic between April 2008 and October 2010 were interviewed within the context of a full sleep medicine history and physical exam. During this encounter, patients completed a questionnaire in which they were queried about the presence of various symptoms potentially linked to sleep disruption, including moderate to severe musculoskeletal pain contributing to sleep disruption, daytime discomfort, or both. Affirmative responses were verified during the interview by a physician board certified in Sleep Medicine (DM). Epworth Sleepiness Scale (ESS) surveys were completed by all patients as part of the same multi-item questionnaire. Those who answered affirmatively to the presence of pain and who agreed to undergo venous blood sampling for 25-hydroxyvitamin D (25OHD) were included in the study. ESS scores (ESSs) ≥ 10 were considered to indicate excessive daytime sleepiness (EDS). A total of 81 patients, all of whom were ultimately diagnosed with various sleep disorders (mostly obstructive sleep apnea) were included in the final analysis (Table 1). All research-related procedures were approved by the institutional review board for human research.
Table 1.
Characteristics of cohort
Serum Measurements
Serum concentration of 25OHD was determined by immunoassay employing the manufacturer's specifications (DiaSorin Liason, Italy). A 25OHD < 20 ng/mL was regarded as indicating a vitamin D deficiency (VitDd).12
Statistics
The correlation between the ESS score and serum 25OHD levels was evaluated using Pearson r. Mean serum levels were compared between races using the t-test. The significance level in both cases was p < 0.05.
RESULTS
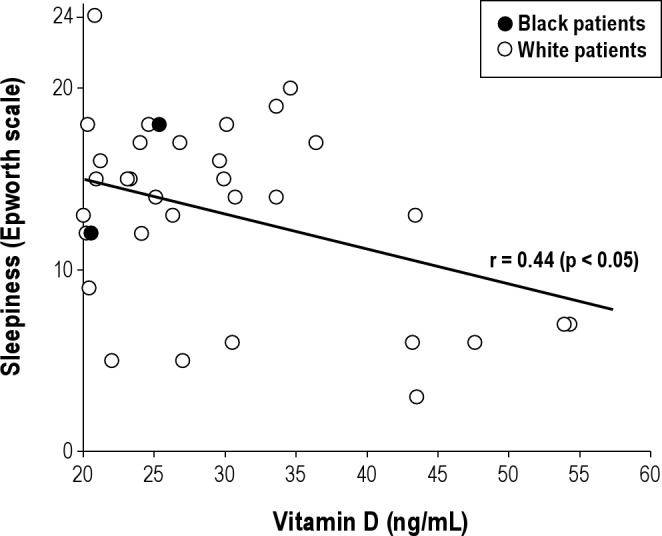
In patients without VitDd (25OHD ≥ 20 ng/mL), sleepiness was inversely correlated with 25OHD levels (Figure 1). The portion of the cohort without VitDd consisted of only 6% black patients (2 of 34), compared with 35% in the entire cohort (Table 1). In this (predominantly white) portion of the cohort, vitamin D and ESSs were significantly correlated (r = 0.44, p < 0.05).
Figure 1. Epworth Sleepiness Scale score (ESSs) as a function of vitamin D concentration in patients without vitamin D deficiency (25OHD ≥ 20 ng/mL).
Among all patients with VitDd, the ESSs and 25OHD levels were uncorrelated. The VitDd portion of the cohort consisted of 26 black and 21 white patients. Mean ESSs among the black patients was significantly greater than among the white patients, and 25OHD levels tended to be lower (Figure 2). Taken as a group, there was no relation between 25OHD and ESSs (Figure 2A), nor did such a relation exist for the white patients alone. For black patients, in contrast, a direct correlation was seen, with higher 25OHD levels associated with higher ESSs (r = 0.48, p < 0.05) (Figure 2A, black patients). On average, the ESSs was higher among blacks (Figure 2B).
Figure 2. Epworth Sleepiness Scale score (ESSs) as a function of vitamin D concentration in patients with vitamin D deficiency (25OHD < 20 ng/mL).
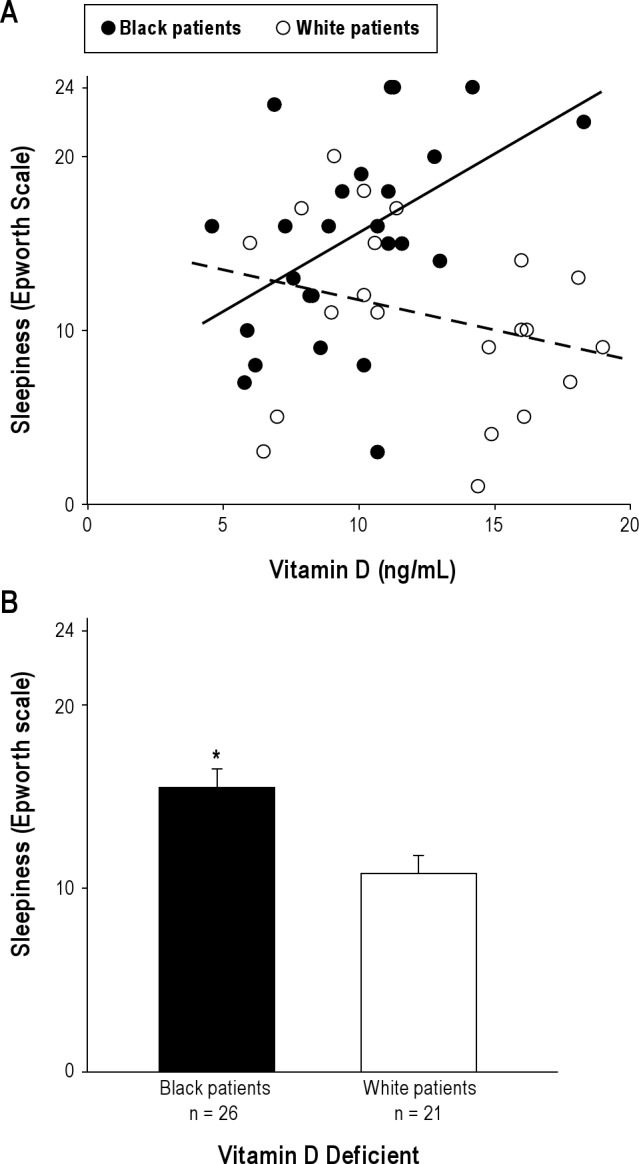
(A) Individual patients stratified by race. Black patients, r = 0.48 (p < 0.05). White patients (dashed line), r = −0.27 (NS). (B) Mean sleepiness ± SD. *p < 0.05.
DISCUSSION
Increasing evidence from clinical and basic research suggests that a suboptimal level of vitamin D constitutes a condition that disposes patients to the development of various diseases apart from the classical descriptions of bony demineralization.1,13 The spectrum of disease associated with low vitamin D is likely to include diseases of immune dysregulation, one manifestation of which could be excessive daytime sleepiness mediated by components of inflammatory cascades (see below). We therefore expected that progressively lower levels of 25OHD would be correlated with increased sleepiness. Further, because increased skin pigmentation is an established risk factor for low vitamin D14—and logically would increase risk for diseases associated with low vitamin D—we also expected that the relation between 25OHD and sleepiness would depend materially on race.
Patients with a chief complaint of chronic nonspecific musculoskeletal pain have been shown to have low vitamin D levels in some,15–18 but not all19,20 studies. We chose to study the relationship between 25OHD and sleepiness in a group of patients we felt would have an elevated likelihood for low vitamin D. Our cohort consisted of patients seen in a sleep medicine specialty clinic for various reasons, but who all admitted to the presence of moderate to severe pain either disrupting sleep, impairing daytime function, or both. This criterion for inclusion has important clinical implications, in that patients were not required to seek care for the pain symptoms, nor were patients required to independently offer the complaint, but instead were only required to admit to its presence when asked. This not only creates a lower threshold for inclusion in the study, it also produces a cohort that is clinically heterogeneous, both in terms of the sleep-related diagnoses and with respect to the anatomic localization, duration, and severity of pain symptoms. In this admittedly diverse group, we asked whether sleepiness (as ascertained by ESS) was associated in a race-dependent manner with circulating 25OHD, and we found that it was. This is the first evidence of such a relationship which, if confirmed, has significant public health implications.
Sleepiness was inversely correlated with vitamin D concentration among those with 25OHD levels ≥ 20 ng/mL, a threshold traditionally felt to represent a lower risk for the classical diseases of bone demineralization (Figure 1).1 Black patients were scarcely present in this group, which prevented drawing conclusions regarding the relation between sleepiness and 25OHD among black patients. Among white patients, however, the observed inverse relationship between 25OHD and ESSs in a range assumed to carry low risk for classical deficiency disease (Figure 1) suggested that the spectrum of non-classical illness may include magnification of hypersomnia symptoms.
The cumulative burden of VitDd may influence how this deficiency state interacts with sleepiness symptoms. This concept was supported by the results from those with 25OHD < 20 ng/mL—the threshold considered to increase the risk for classical disease of vitamin D deficiency (Figure 2). This portion of our cohort was composed of 55% black patients, even though they represented only 35% of the cohort. Among all patients with 25OHD < 20 ng/mL, there was no significant relationship between 25OHD and ESSs. Among black patients, however, a significant direct relationship was found (r = 0.48, p < 0.05). The black patients also were significantly sleepier (Figure 2B).
The direct relationship between 25OHD and ESSs in black patients was unexpected, and ran counter to our original hypothesis of increasing sleepiness with lower 25OHD. One possible mechanistic explanation for this result involves a greater degree of sympathetic stimulation and/or activation of the hypothalamic-pituitary-adrenal stress response axis, possibly due to increased pain in the context of a more severe burden of vitamin D deficiency-related disease. Another possibility involves an interaction between VitDd and other disorders, particularly obstructive sleep apnea (OSA). VitDd is correlated with chronic rhinitis,21 tonsillar hypertrophy,22,23 and nonspecific myopathy24–26—all of which are known to increase the risk for OSA—and therefore represents a plausible novel factor that could lead to more severe OSA, as ascertained by frequency of apneas or hypopneas or severity of intermittent hypoxia. We had no reason to speculate on such a relationship a priori, and consequently we did not collect data pertinent to OSA severity. The issue remains to be addressed.
The observation that ESSs and 25OHD levels < 20 ng/mL were correlated in black but not white patients suggests that a single blood draw may yield an incomplete picture of the true burden of disease. We previously showed that season of blood draw (summer vs. winter) was uncorrelated with VitDd in (~35% black, ~65% white) patients who admitted to nonspecific musculoskeletal pain during evaluation at a sleep medicine specialty clinic.27 In that cohort, however, white patients had higher mean summer 25OHD levels compared with winter levels (28.0 ± 13.5 and 23.1 ± 12.7 ng/mL, respectively). In contrast, there was no seasonal variation seen in black patients (12.7 ± 9.4 and 13.9 ± 6.0 ng/mL, respectively). These observations suggest the possibility that a year-round burden of chronic VitDd may be more severe in black patients, thereby providing a basis for understanding different presentations and/or consequences of chronic diseases.
VitDd may contribute to symptoms of sleepiness via known sleep regulating substances, for example, TNFα, whose importance has been shown in clinical and experimental studies.28,29 VitDd is also associated with upregulation of NFκB,30 which functions as a master switch for inflammation and a trigger of the cellular inflammatory cascade resulting from intermittent hypoxia associated with OSA.31 NFκB is also responsible for the regulation of numerous substances known to exert homeostatic sleep pressure, including prostaglandin D2.32,33 Thus VitDd may not only be a cofactor for the development of cardiovascular morbidity associated with OSA, but may also play a role in the pathogenesis of EDS associated with the disease.
The study limitations included the small cohort size and the relative clinical heterogeneity of the patients. The post hoc recognition of a potential relationship between vitamin D and OSA highlighted the need for data pertinent to this diagnosis. The lack of uniformity may have distorted the subjective experience of excessive sleepiness in an unpredictably variable manner from patient to patient, thereby obscuring the role of VitDd in the development of EDS symptoms. Further studies involving narrowed sleep disorder entry criteria (including quantification of severity of OSA) and quantification of pain location and severity levels would help clarify the connections between sleep disorders, EDS, vitamin D levels, and race.
This study was not designed to identify mechanisms. Low 25OHD may cause daytime impairment, but it is also possible that patients with severe sleepiness may exhibit behaviors that increase the likelihood of low 25OHD. For example, sun avoidance may lessen the biosynthesis of vitamin D, which is related to direct sunlight exposure. In this view, however, a linear inverse relationship would be expected for all patients, with higher ESS scores correlated with lower 25OHD values. Our discovery of a direct relationship among those with a greater burden of deficiency (i.e., blacks with 25OHD < 20 ng/mL) does not support the notion of sleepiness-related behavior provoking deficiency. Further studies would help clarify the biologic underpinnings relating 25OHD to sleepiness.
In conclusion, this is the first study to demonstrate a significant relationship between sleepiness and vitamin D. Though ESSs and 25OHD are related, the relationship is complex; the presence of VitDd changes the nature of this relationship compared to subjects without VitDd; among subjects with VitDd, the relationship between ESSs and 25OHD is markedly affected by race.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
This work was supported by the Department of Neurology, LSUHSC-Shreveport.
REFERENCES
- 1.Zittermann A, Gummert JF. Nonclassical vitamin D actions. Nutrients. 2010;2:408–25. doi: 10.3390/nu2040408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khoo AL, Chai L, Koenen H, Joosten I, Netea M, van der Ven A. Translating the role of vitamin D(3) in infectious diseases. Crit Rev Microbiol. 2012 doi: 10.3109/1040841X.2011.622716. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 3.Black PN, Scragg R. Relationship between serum 25-hydroxyvitamin D and pulmonary function in the third national health and nutrition examination survey. Chest. 2005;128:3792–8. doi: 10.1378/chest.128.6.3792. [DOI] [PubMed] [Google Scholar]
- 4.Turner MK, Hooten WM, Schmidt JE, Kerkvliet JL, Townsend CO, Bruce BK. Prevalence and clinical correlates of vitamin D inadequacy among patients with chronic pain. Pain Med. 2008;9:979–84. doi: 10.1111/j.1526-4637.2008.00415.x. [DOI] [PubMed] [Google Scholar]
- 5.Botella-Carretero JI, Alvarez-Blasco F, Villafruela JJ, Balsa JA, Vázquez C, Escobar-Morreale HF. Vitamin D deficiency is associated with the metabolic syndrome in morbid obesity. Clin Nutr. 2007;26:573–80. doi: 10.1016/j.clnu.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 6.Forman JP, Giovannucci E, Holmes MD, et al. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension. 2007;49:1063–9. doi: 10.1161/HYPERTENSIONAHA.107.087288. [DOI] [PubMed] [Google Scholar]
- 7.Thomas MK, Lloyd-Jones DM, Thadhani RI, et al. Hypovitaminosis D in medical inpatients. N Engl J Med. 1998;338:777–83. doi: 10.1056/NEJM199803193381201. [DOI] [PubMed] [Google Scholar]
- 8.Dean AJ, Bellgrove MA, Hall T, et al. Effects of vitamin D supplementation on cognitive and emotional functioning in young adults—a randomised controlled trial. PLoS One. 2011;6:e25966. doi: 10.1371/journal.pone.0025966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Annweiler C, Fantino B, Schott AM, Krolak-Salmon P, Allali G, Beauchet O. Vitamin D insufficiency and mild cognitive impairment: cross-sectional association. Eur J Neurol. 2012 doi: 10.1111/j.1468-1331.2012.03675.x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.McCarty DE, Reddy A. Prevalence of vitamin D insufficiency/deficiency among sleep medicine patients complaining of somatic pain and correlation with daytime sleepiness. Sleep. 2011;35(Abstract Supplement):A230. [Google Scholar]
- 11.McCarty DE. Resolution of hypersomnia following identification and treatment of vitamin D deficiency. J Clin Sleep Med. 2010;6:605–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–81. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 13.Hoeck AD, Pall ML. Will vitamin D supplementation ameliorate diseases characterized by chronic inflammation and fatigue? Med Hypotheses. 2011;76:208–13. doi: 10.1016/j.mehy.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 14.Clemens TL, Adams JS, Henderson SL, Holick MF. Increased skin pigment reduces the capacity of skin to synthesise vitamin D3. Lancet. 1982;1:74–6. doi: 10.1016/s0140-6736(82)90214-8. [DOI] [PubMed] [Google Scholar]
- 15.Lotfi A, Abdel-Nasser AM, Hamdy A, Omran AA, El-Rehany MA. Hypovitaminosis D in female patients with chronic low back pain. Clin Rheumatol. 2007;26:1895–901. doi: 10.1007/s10067-007-0603-4. [DOI] [PubMed] [Google Scholar]
- 16.Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003;78:1463–70. doi: 10.4065/78.12.1463. [DOI] [PubMed] [Google Scholar]
- 17.Heidari B, Shirvani JS, Firouzjahi A, Heidari P, Hajian-Tilaki KO. Association between nonspecific skeletal pain and vitamin D deficiency. Int J Rheum Dis. 2010;13:340–6. doi: 10.1111/j.1756-185X.2010.01561.x. [DOI] [PubMed] [Google Scholar]
- 18.Badsha H, Daher M, Ooi Kong K. Myalgias or non-specific muscle pain in Arab or Indo-Pakistani patients may indicate vitamin D deficiency. Clin Rheumatol. 2009;28:971–3. doi: 10.1007/s10067-009-1146-7. [DOI] [PubMed] [Google Scholar]
- 19.de Rezende Pena C, Grillo LP, das Chagas Medeiros MM. Evaluation of 25-hydroxyvitamin D serum levels in patients with fibromyalgia. J Clin Rheumatol. 2010;16:365–9. doi: 10.1097/RHU.0b013e3181fe8a90. [DOI] [PubMed] [Google Scholar]
- 20.Tandeter H, Grynbaum M, Zuili I, Shany S, Shvartzman P. Serum 25-OH vitamin D levels in patients with fibromyalgia. Isr Med Assoc J. 2009;11:339–42. [PubMed] [Google Scholar]
- 21.Abuzeid W, Akbar N, Zacharek M. Vitamin D and chronic rhinitis (publish ahead of print) Curr Opin Allergy Clin Immunol. 2012;12:13–7. doi: 10.1097/ACI.0b013e32834eccdb. [DOI] [PubMed] [Google Scholar]
- 22.Nunn JD, Katz DR, Barker S, et al. Regulation of human tonsillar T-cell proliferation by the active metabolite of vitamin D3. Immunology. 1986;59:479–84. [PMC free article] [PubMed] [Google Scholar]
- 23.Reid D, Morton R, Salkeld L, Bartley J. Vitamin D and tonsil disease--preliminary observations. Int J Pediatr Otorhinolaryngol. 2011;75:261–4. doi: 10.1016/j.ijporl.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 24.Glerup H, Mikkelsen K, Poulsen L, et al. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif Tissue Int. 2000;66:419–24. doi: 10.1007/s002230010085. [DOI] [PubMed] [Google Scholar]
- 25.Prabhala A, Garg R, Dandona P. Severe myopathy associated with vitamin D deficiency in western New York. Arch Intern Med. 2000;160:1199–203. doi: 10.1001/archinte.160.8.1199. [DOI] [PubMed] [Google Scholar]
- 26.Russell JA. Osteomalacic myopathy. Muscle Nerve. 1994;17:578–80. doi: 10.1002/mus.880170603. [DOI] [PubMed] [Google Scholar]
- 27.McCarty DE, Reddy A, Kiegley Q, Kim PY, Cohen S, Marino A. Vitamin D deficiency is common in southern U.S. ambulatory sleep medicine clinic patients who complain of somatic pain, irrespective of season. Poster presented at: Southern Sleep Society; 2012; 2012; Miramar Beach, Florida. [Google Scholar]
- 28.Churchill L, Rector DM, Yasuda K, et al. Tumor necrosis factor alpha: activity dependent expression and promotion of cortical column sleep in rats. Neuroscience. 2008;156:71–80. doi: 10.1016/j.neuroscience.2008.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson CA, Heffernan ME. Serum tumor necrosis factor-alpha concentrations are negatively correlated with serum 25(OH)D concentrations in healthy women. J Inflamm (Lond) 2008;5:10. doi: 10.1186/1476-9255-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jablonski KL, Chonchol M, Pierce GL, Walker AE, Seals DR. 25-Hydroxyvitamin D deficiency is associated with inflammation-linked vascular endothelial dysfunction in middle-aged and older adults. Hypertension. 2011;57:63–9. doi: 10.1161/HYPERTENSIONAHA.110.160929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan S, Taylor CT, McNicholas WT, Ryan S, Taylor CT, McNicholas WT. Selective activation of inflammatory pathways by intermittent hypoxia in obstructive sleep apnea syndrome. Circulation. 2005;112:2660–7. doi: 10.1161/CIRCULATIONAHA.105.556746. [DOI] [PubMed] [Google Scholar]
- 32.Chen Z, Gardi J, Kushikata T, Fang J, Krueger JM. Nuclear factor-kappaB-like activity increases in murine cerebral cortex after sleep deprivation. Am J Physiol. 1999;276:R1812–8. doi: 10.1152/ajpregu.1999.276.6.R1812. [DOI] [PubMed] [Google Scholar]
- 33.Krueger JM, Szentirmai E, Kapas L. Biochemistry of sleep function: a paradigm for brain organization of sleep. In: Amlaner CJ, Fuller PM, editors. Basics of sleep guide. 2nd ed. Westchester, IL: Sleep Research Society; 2009. pp. 69–74. [Google Scholar]


