Abstract
Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) is a chronic, disabling illness that affects approximately 0.2% of the population. Non-restorative sleep despite sufficient or extended total sleep time is one of the major clinical diagnostic criteria; however, the underlying cause of this symptom is unknown. This review aims to provide a comprehensive overview of the literature examining sleep in CFS/ME and the issues surrounding the current research findings. Polysomnographic and other objective measures of sleep have observed few differences in sleep parameters between CFS/ME patients and healthy controls, although some discrepancies do exist. This lack of significant objective differences contrasts with the common subjective complaints of disturbed and unrefreshed sleep by CFS/ME patients. The emergence of new, more sensitive techniques that examine the microstructure of sleep are showing promise for detecting differences in sleep between patients and healthy individuals. There is preliminary evidence that alterations in sleep stage transitions and sleep instability, and other physiological mechanisms, such as heart rate variability and altered cortisol profiles, may be evident.
Future research investigating the etiology of non-restorative sleep in CFS/ME may also help us to undercover the causes of non-restorative sleep and fatigue in other medical conditions.
Citation:
Jackson ML; Bruck D. Sleep abnormalities in chronic fatigue syndrome/myalgic encephalomyelitis: a review. J Clin Sleep Med 2012;8(6):719-728.
1.0. INTRODUCTION
Chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME) is a medically unexplained disabling illness, with prevalence estimates of between 0.007% and 2.8% of the general adult population.1–3 It is primarily characterized by persistent relapsing fatigue of at least 6 months in duration that reduces activity levels. Other key features of the disorder include post-exertion malaise of either physical or mental exertion, memory and concentration difficulties, muscle pain, headaches, tender lymph nodes, sore throat, and non-restorative sleep. Of direct relevance to the current review, patients often report daytime sleepiness, feeling unrefreshed upon waking despite sufficient or extended total sleep time, extended sleep including daytime napping, and other sleep-related symptoms, such as difficulty falling asleep and disturbed sleep.2,4,5 Since the late 1980s, research in the area of CFS/ME has begun to focus on examining and determining the cause of non-restorative sleep and sleep disturbance in CFS/ME, and how these symptoms may relate or contribute to patients' daytime fatigue. The current review aims to provide a comprehensive overview of these studies, describe where the field currently stands on this issue, and outline potential directions for future research.
2.0. HISTORICAL OVERVIEW OF DIAGNOSTIC CRITERIA
Clusters of symptoms including chronic fatigue, cognitive and mood impairments, sleep difficulties and pain have been observed in clinical practice for centuries. A collection of symptoms similar to that of CFS/ME, including fatigue, depression, headache, impotence and neuralgia, were first reported in the 19th century.6 The diagnosis “neurasthenia” was popularized by American neurologist George Beard to describe this collection of symptoms.7 With increasing sophistication of psychiatric diagnoses over time, diagnosis of neurasthenia has declined, however, the symptoms described in these early patients closely match those we now recognize in CFS/ME today.
The name “chronic fatigue syndrome” was first suggested in 1988, and most commonly used by the medical and scientific community to describe the illness. However, the name CFS has been rejected by many patients, patient advocacy groups and some doctors as it undermines and trivializes the illness. Since 1969, myalgic encephalomyelitis (ME) has been included in the World Health Organization's International Classification of Diseases (ICD-10), and although is clinically distinct from CFS, the two terms are used interchangeably. Due to the stigma of the name “chronic fatigue syndrome,” the illness is often referred to as CFS/ME.
It is important to make a distinction between CFS/ME and fibromyalgia (FM). FM is a chronic medical disorder characterized by widespread pain, a heighten pain response, and sleep disturbance,8 and is commonly comorbid with CFS/ME.9 Given the overlapping symptoms and lack of a diagnostic test, it is important for research studies to have clear criteria for distinguishing these two conditions. This review will focus on research that has specifically examined sleep in CFS/ME.
2.1. Research Definition
The first working case definition of chronic fatigue syndrome was introduced in 1988 by the United States Centers for Disease Control and Prevention (CDC).10 The development of a case definition allowed for a systematic and comprehensive approach to defining the etiology and pathophysiology of CFS/ME. These definitions, along with the 198811 and 199012 Australian definitions, and the 1991 Oxford, UK definition,13 have played an essential role in orienting clinical research, and facilitating consistency and homogeneity of samples across research studies. Although these initial criteria were considered quite restrictive, the diagnostic criteria did not specifically exclude a sleep disorder. As a result, early studies that examined the association between sleep complaints and functional disability in CFS/ME suggested that a primary sleep disorder may be the cause of unrefreshed sleep in some patients.
In 1994, a revision to the case definition and a set of research guidelines for use in studies of CFS patients was proposed by the Centers for Disease Control and Prevention.14 These guidelines (known as the Fukuda criteria) used more relaxed definitions than the 1988 criteria, requiring only four criteria beyond fatigue for diagnosis, and not excluding non-psychotic psychiatric disorders. Given that there is no laboratory test for diagnosing CFS/ME, and the etiology is typically unknown, CFS/ME was seen as a diagnosis of exclusion. This was reflected in the new criteria by specifically excluding comorbid conditions such as a treatable sleep disorder (e.g., obstructive sleep apnea [OSA] and narcolepsy) and other potential causes of fatigue (e.g., substance abuse, psychiatric disorder). Unrefreshing sleep was the only criteria relating to sleep, with other sleep disturbances not a criterion. Although alternative definitions have been proposed,15 the 1994 Fukuda criteria are considered the international accepted research definition. However, it was recognized that the Fukuda criteria and other broadly inclusive criteria15 do not adequately discriminate CFS/ME patients from those with other conditions such as major depressive disorder. Additionally, since the Fukuda criteria were primarily developed to inform clinical research excluding comorbid conditions, such as treatable sleep disorders, they may not be appropriate to use exclusively for clinical diagnoses, which are often more broadly defining.
2.2. Clinical Guidelines
In 2003, an Expert Medical Consensus Panel with extensive experience in the research and clinical management of CFS/ME developed the Canadian Clinical Case Definition.16 This document was created specifically to inform healthcare professionals. The Canadian Criteria has incorporated a larger spectrum of potential symptoms, aimed to assist recognition of the “interrelationships of each patient's symptoms and their coherence into a syndrome of related symptoms.”16 This updated clinical definition captured, in addition to chronic fatigue, the issue of post-exertion malaise. These criteria also highlight mental fatigue (loss of cognitive function and alertness) as well as physical fatigue (lack of energy and strength). Sleep disturbance is recognized as a major feature of CFS/ME in the Canadian Criteria. Specifically, sleep and circadian rhythms disturbances are listed, including early, middle, and late insomnia, and reversed or abnormal diurnal and sleep rhythms. Further, periodic limb and restless legs syndrome are reported to accompany these other changes in sleep in many cases. These criteria also recognize the importance of ruling out a treatable sleep disorder, such as upper airway resistance syndrome and OSA.
Most recently, International Consensus Criteria (ICC) have been developed.17 The ICC build from the Canadian Criteria to identify the distinct characteristic patterns of symptom clusters of CFS/ME. These new definitions relaxed the requirement for symptoms to have persisted for at least 6 months, giving the physician more temporal control of when the diagnosis of CFS/ME can be made. Sleep disturbances were divided into two categories: disturbed sleep patterns, including insomnia, prolonged sleep including naps, frequent awakenings, and vivid dreams/nightmares; and unrefreshed sleep, including excessive daytime sleepiness.17 Importantly, these updated criteria serve to not only diagnose patients in the clinical setting, but to also assist in identifying patients for research studies.
3.0. DIFFERENTIAL DIAGNOSES
The diagnostic overlap between CFS/ME and primary sleep disorders has been documented in a number of studies. Prior to the revision of the diagnostic criteria for CFS/ME in 1994, two studies reported that over half of their CFS/ME patients had a sleep disorder as assessed by overnight polysomnography (PSG).5,18 These sleep disorders include hypersomnia, sleep maintenance and sleep initiation insomnia, OSA, narcolepsy, and periodic limb movement disorder, as well as inadequate sleep hygiene. CFS/ME patients were found to have higher levels of fatigue and sleep disturbance than patients with multiple sclerosis5; those with a comorbid sleep disorder reported greater functional impairment.18 Since these studies included CFS/ME patients with a suspected sleep disorder who were attending the sleep clinic, rather than randomly selected or consecutive patients, these figures are mostly likely inflated.
Subsequent to the 1994 guideline revisions, Fossey et al. (2004) observed prevalence rates > 50% of ICSD-classified primary sleep disorders (OSA and movement disorders) in CFS/ME patients.19 Similarly, Le Bon and colleagues examined the prevalence of primary sleep disorders and objective sleepiness in CFS/ME and found that 46% of the 46 unselected patients who met Fukuda criteria for CFS/ME also presented with OSA (using a criterion of AHI > 5), and a further 5% presented with periodic limb movement disorder.20 On multiple sleep latency testing (MSLT), an objective measure of sleep propensity, 30% of the patients were classified as clinically sleepy. Objective (MSLT) and subjective (Stanford Sleepiness Scale) measures of sleepiness, however, were not associated with a subjective fatigue measure, suggesting that the fatigue experienced by CFS/ME patients is separate from the expression of sleepiness. Importantly, this study compared CFS/ME patients with and without a primary sleep disorder and found that they could not be separated on clinical presentation. The authors concluded that the symptoms of CFS/ME are clearly distinct from those of primary sleep disorders, and the illness is more than simply a somatic expression of an underlying sleep disorder or sleepiness.20
Larger population-based studies of CFS/ME patients have also provided some insight into the rates of sleep disorders in CFS/ME patients over time.15,21 Approximately 20% of CFS/ME patients in these studies were found to have either OSA or narcolepsy, with one study reporting that 20% of patients were given an alternative diagnosis of sleep disorder at 3-year follow-up.21 Subclinical levels of sleep disordered breathing have also been reported in some studies.15,22
From a clinical standpoint, early studies that examined prevalence of comorbid sleep disorders provide new insights into the syndrome and potential differential diagnoses and highlight the importance of considering a potential sleep disorder as a cause of unexplained fatigue.23 While there is overwhelming evidence of the distinction between CFS/ME and sleep disorders,24 some researchers argue that a diagnosis of OSA should not be an exclusion criterion for CFS/ME. It is argued that primary sleep disorders do not influence the core symptoms of CFS/ME and therefore should be considered a comorbid condition.25 Indeed, in clinical practice many physicians treat primary sleep disorders concurrently with CFS/ME rather than exclude these patients. CPAP therapy in CFS/ME patients with comorbid OSA has been found to improve some daytime features, such as cognitive and daytime sleepiness; however, the underlying fatigue state remains.25 The magnitude of OSA in CFS/ME also depends on what diagnostic threshold is used for OSA. Le Bon and colleagues acknowledge that if an AHI cutoff of 20/h was used in their study (rather than AHI < 5), OSA would only be prevalent in 11% of their CFS/ME sample (as opposed to nearly 50%).20 These figures are comparable to the prevalence of sleep disordered breathing in the general population.26 Thus it could be argued that primary sleep disorders are a comorbid condition that occur at a similar frequency in CFS/ME to the general population and are therefore not reflective of the disorder itself.
In addition to generalized joint and muscle myalgia, which are integral features of the diagnostic criteria, CFS/ME is also associated with comorbid pain conditions including irritable bowel syndrome and migraine headaches. Pain experienced by CFS/ME patients may also play a critical role in sleep disturbance. Studies of FM have found that both subjective sleep quality measures and phasic alpha sleep are associated with pain sensitivity.27 There is a potential bidirectional relationship between sleep and pain—pain disrupts sleep, and sleep disruption enhances pain. Firstly, pain causes disruption to sleeping pattern and increases sleep onset latency. Experimental manipulations of pain stimuli to the muscles during sleep have revealed decreased delta and increased alpha activity of the sleep EEG, and impaired sleep quality.28,29 CFS/ME patients are found to have more self-reported awakenings during sleep due to pain compared to depressed patients and healthy controls.32 On the other hand, sleep disturbance also leads to reduced pain thresholds during waking.30 Pain and fatigue symptoms, similar to those reported in CFS/ME and FM, have been induced in healthy individuals by disrupting SWS.30 Thus, it has been posited that the physiological arousals that are observed during sleep reflect a vigilant nocturnal state that contributes to daytime fatigue, pain, and hypersensitivity, and subjective feelings of non-restorative sleep. The influence of myalgic symptoms on sleep disturbance in CFS/ME has received little research attention to date.
Some patients with CFS/ME have comorbid psychiatric or somatoform illness, such as depressive disorder or fibromyalgia, which do not rule out a diagnosis of CFS/ME. Given that sleep disturbance is a common symptom of psychiatric conditions,31 it could be argued that sleep disturbance in CFS/ME is a secondary consequence of comorbid depression. Studies that have explored this potential link by comparing sleep study results of CFS/ME patients with and without a psychiatric comorbidity suggest that sleep disturbances are common in both subtypes, and therefore do not appear to be solely the result of underlying depression.4,32 All of these studies mentioned above have been critical for highlighting CFS/ME as an autonomous syndrome.
4.0. MACROSTRUCTURE MEASURES OF SLEEP IN CFS/ME
Table 1 presents studies that have compared sleep parameters of CFS/ME patients to healthy controls using polysomnography. Of 24 papers reviewed, only 15 used recognized diagnostic criteria for patient recruitment, and only these 15 are included in Table 1. Of these 15, only 10 reported that they excluded patients who were on medication or asked patients to withdraw from their medication for 2 weeks prior to the study.
Table 1.
Summary of literature investigating sleep, measured polysomnographically, in CFS/ME patients compared to healthy controls, using standard diagnostic criteria
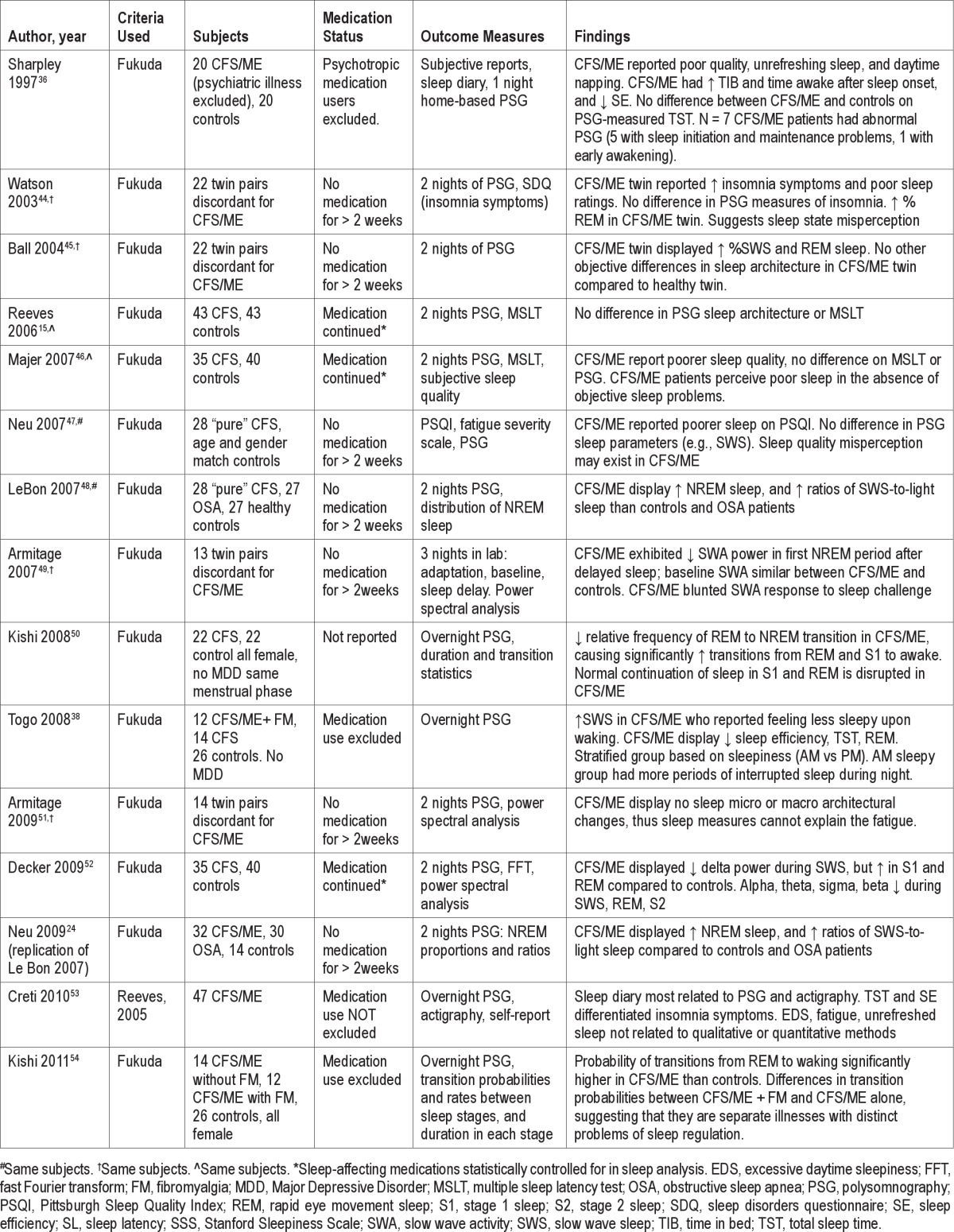
Studies examining traditional sleep parameters as measured by PSG have reported variable and nonspecific differences in sleep parameters between CFS/ME patients and controls. The reason for the discrepancy may be due in part to differences in selection criteria (e.g., medication status), the type of control group used, or characteristics of the recorded night (e.g., first night of recording only, home recordings), which also make comparisons between studies difficult. First night effects are apparent in CFS/ME patients who do not have a primary sleep disorder,33 and therefore studies that have only used one night of recording may not provide an accurate picture of the patient's typical sleep.
Reflecting the disturbed sleep reported by many CFS/ME patients, an increased number18,34,35 and duration18,34,36 of intermittent awakenings have been reported. However, using an adaptation night prior to the main sleep recording night, Reeves et al. found no difference in sleep efficiency between CFS/ME patients and controls.15 This study, however, enforced bed and wake times for both groups, and as such may not have captured true sleep efficiencies that the participants would typically experience. When patients and controls have been allowed to go to bed at their usual bed time, reduced total sleep time (TST) and sleep efficiency are observed in the CFS/ME group, both in single-night PSG recording in the laboratory,22,37,38 and in some,18,35 but not all36 at-home studies. Togo et al. (2008) examined this observation further by stratifying their patients into those who reported sleepiness upon waking, and those who reported more sleepiness in the evening. Using this distinction, they found that those who reported morning sleepiness had lower sleep efficiencies and more periods of interrupted sleep.38
Sleep onset latencies are often longer in CFS/ME patients compared to healthy control subjects, suggesting that some patients may have difficulties initiating sleep.4,37 It has been reported that CFS/ME patients may have differential parasympathetic activity at sleep onset, which may contribute to delayed sleep latency.39 This finding could also reflect poor sleep hygiene or extended sleep periods and napping, which may reduce sleep drive at night.
Actigraphy studies potentially provide a more ecologically valid assessment of TST, sleep efficiency, and sleep onset latency parameters, as measurements are made in a naturalistic setting where patients can follow their usual sleep routines. A study in children with CFS/ME reported that continuous sleep of > 10 h, measured with actigraphy, was not uncommon.40 Impaired daily sleep/wake rhythms and disturbed sleep were observed in those children who displayed an irregular sleep type. These studies can also examine whether changes in the circadian timing of sleep are evident, such as a delayed sleep phase. Most actigraphy studies to date have examined diurnal activity patterns in CFS/ME patients,41 and there are currently mixed findings relating to circadian rhythm disturbances.42,43
The sleep architecture of CFS/ME patients may differ from that of healthy individuals. Stage 3 sleep or slow wave sleep (SWS) is typically observed for approximately a fifth of the sleep period in young healthy individuals.55 A number of studies have reported reduced time in SWS in CFS/ME patients relative to controls4,38 and between monozygotic twins discordant for CFS/ME.41 These effects are independent of depression and FM.4,24
Alpha-delta sleep is an atypical electroencephalographic (EEG) pattern recorded during NREM sleep. In normal sleep, alpha activity is characteristic of drowsy wakefulness, and delta activity indicates restorative NREM sleep. Alpha-delta sleep was first observed during studies of sleep EEG of patients with psychiatric illness who presented with fatigue. The appearance of alpha-delta sleep, or alpha intrusions, during SWS has been reported in some early studies of CFS/ME patients.37,56 The appearance of alpha-delta sleep in these patients was initially thought to be the cause of non-restorative sleep. However, whether the patients in these studies had a “pure” diagnosis of CFS/ME or potentially some feature of FM is unclear. Later studies failed to find this phenomenon,51,57 and the role of alpha-delta sleep in the pathophysiology of CFS/ME has since been questioned.
The amount of SWS and slow wave activity (SWA; power density in the delta frequency) are determined by prior wakefulness. For example, SWS and SWA are found to increase following periods of sleep deprivation or restriction, when there is a build-up of homeostatic sleep pressure.58 As such, SWA during NREM sleep is often used as a marker of sleep homeostasis. A study by Armitage and colleagues exploited this phenomenon by exposing 13 monozygotic twins discordant for CFS/ME to a sleep delay schedule.49 After 2 baseline nights in the laboratory, a mild sleep challenge was imposed, involving a sleep delay of 4 hours, followed by a regular sleep length “recovery” period. Although no differences in SWA during baseline sleep were found, the CFS/ME twin expressed significantly less SWA in the first NREM period after the sleep delay. Additionally, the time course of dissipation of SWA across the night was altered in the CFS/ME twin. The authors concluded that CFS/ME is associated with impaired sleep homeostasis and basic sleep drive. Interestingly, the cytokine systems are intimately involved with sleep regulation; increased SWA also occurs in response to acute infection, with proinflammatory cytokines increasing SWS.59 Alteration in SWA may therefore be associated with the systemic inflammation found in CFS/ME.60 Further research examining SWA and immune dysfunction in CFS/ME would be valuable for understanding this potential link.
The distribution and amount of REM sleep in CFS/ME is not as clearly defined as that of SWS. Reduced REM sleep in CFS/ME patients is reported in some studies,35,38 whereas others have observed a higher percentage of REM sleep in CFS/ME relative to controls.37,45,61 When statistically controlling for medication use, no difference in REM sleep latency was observed between controls and CFS/ME patients,15 suggesting that medications used by these patients may play a role in some sleep architecture differences previously reported. Perhaps the clearest findings have been derived from twin studies, as described earlier. In these studies, no differences in REM sleep are observed between the CFS/ME twin and the healthy twin.51
To date, approximately 273 clinically diagnosed CFS/ME patients have been assessed using PSG, with less than half of these patients studied not using medication. Based on these limited data, there appear to be very few differences in sleep architecture or TST between CFS/ME patients and healthy individuals, with mixed findings for SWA and SWS. The distribution of SWS and the frequency of sleep stage transitions appear to differ between CFS/ME patients, healthy controls, and other sleep disorders. Of the 4 twin studies published, increased SWS and REM sleep are typically reported in the CFS twin, with evidence for an impaired sleep homeostatic response, but no differences on power spectral analysis were observed. Studies that have had utilized co-twin control methodology have the added benefit of controlling for many genetic and environmental factors that are typically not accounted for in either CFS/ME or sleep research, making for a more powerful and robust design.44
Based on these data, it appears that CFS/ME does not have a characteristic objective sleep disturbance found across all patients. As a result, some researchers have concluded that CFS/ME patients do not have abnormal sleep, and objective sleep measures do not account for subjective reports of non-restorative sleep. Studies using traditional PSG measures have been unable to shed light on a cause for the experience of non-restorative sleep.
5.0. DISCREPANCY BETWEEN OBJECTIVE AND SUBJECTIVE REPORTS OF SLEEPINESS
While CFS/ME patients present with fatigue as their primary symptom, whether they also experience excessive daytime sleepiness is less clear cut. The consensus from a number of studies is that pathological sleepiness objectively measured using multiple sleep latency tests (MSLT) is not observed in CFS/ME patients.15,46,62 This is despite CFS/ME patients reporting higher levels of subjective sleepiness and poorer sleep quality than healthy controls.24,53,61
There are a few possible explanations for this discrepancy between objective and subjective sleepiness measures. Firstly, it has been suggested by some authors that CFS/ME patients have sleep quality misperception.15,61 Interestingly, poor self-rated health and depressive symptoms have been found to be associated with over reporting of sleep difficulties and underestimation of sleep efficiency.63 However, it is unlikely that this discord is truly a global phenomenon across all CFS/ME patients, and perhaps reflects more of a generalized issue among all patients experiencing sleep disturbance, such as insomnia.64 It may be that sleep disturbed individuals more closely monitor their sleep habits and patterns and are more in tune to changes in their sleep.
A second possibility is that the discrepancy reflects issues with definitions and measurement tools used to determine fatigue and sleepiness. Outside of the sleep arena, fatigue and sleepiness are ill-defined concepts with overlap in their definitions and are often used interchangeably. This can make it difficult for both patients and clinicians to correctly distinguish between the two states.65 Further, currently there is a lack of a clear and reliable subjective measure that differentiates the two states. One study examined whether current measures correctly distinguish between sleepiness and fatigue, by comparing CFS/ME and OSA patients whose primary symptom is daytime sleepiness.66 In this study, a clear distinction between subjective measures between the 2 patient groups was observed, with CFS/ME reporting the most fatigue and OSA patients reporting higher levels of sleepiness. However, there was some overlap in the levels of subjective sleepiness between the 2 groups, combined with the well-recognized discordance between objective and subjective sleepiness in the CFS/ME group. This study highlights the need for more precise tools and analyses for distinguishing these 2 states.
Another explanation for differences in objective and subjective measures of sleep in CFS/ME is that potential nocturnal neurophysiological disturbances that result in the non-restorative sensation following sleep in CFS/ME patients are not detected by traditional sleep variables or sleep stage distributions measures. More sensitive micro-analyses of the sleep EEG and other nocturnal parameters are currently being explored in this population.
6.0. MICROSTRUCTURE MEASURES OF SLEEP IN CFS/ME
While objective sleep parameters do not clearly predict subjective reports of sleep disturbance, other physiological measures may have more promise for detection of alterations in CFS/ME patients' sleep. Stage shifts4,34 and dynamic stage transitions50 have been shown to discriminate CFS/ME patients and healthy controls. The relative frequency of REM to NREM transition is lower in CFS/ME, while there are significantly more transitions from REM and stage 1 sleep to wakefulness.50 Normal continuation of sleep in stage 1 and REM is disrupted in CFS/ME, which may contribute to feelings of non-restorative sleep upon waking.
More recently, studies have utilized alternative methods for quantifying EEG in CFS/ME patients, which provide a more sensitive method for evaluation of sleep abnormalities. Power spectral analysis using fast Fourier transform (FFT) has been used in a few recent studies,51,52 with conflicting results. Decker reported differences in delta power, with reduced delta power observed in SWS and increased delta power during stage 1 and REM sleep in 35 CFS/ME patients relative to 40 healthy controls.52 Reductions in alpha power were observed most strongly during REM but were also seen in SWS and stage 2 in the CFS/ME subjects. The finding of attenuated delta power complements the reduced SWA reported previously following sleep delay,49 providing further evidence of an altered sleep homeostat in CFS/ME. In contrast, Armitage found no difference in any frequency band between twins discordant for CFS/ME.51 There is a clear need for future research utilizing this method of sleep EEG analysis to clarify these findings.
It is also plausible that CFS/ME patients may experience arousals during sleep that are not detected using traditional scoring methods.67 Supporting this idea, more microarousals have been observed in the sleep of CFS/ME patients than healthy control subjects.24,66 Recently, other methods of arousability or sleep instability have been developed and used in this population. Cyclic alternating pattern (CAP) is an EEG-derived measure of sleep instability, which is reflected as periodic EEG activity during NREM sleep.68,69 In contrast, periods of Non-CAP are indicative of consolidated sleep. CAP is somewhat distinct from typically measured arousals from sleep, both as a phenomenon and in terms of how they are scored.69 Clinical studies of insomniacs have found strong correlations between CAP rate (ratio of total CAP time to total NREM sleep time) and subjective reports of sleep quality.70 This measure may therefore provide a more specific marker of sleep quality that the arousal index derived from PSG. Only one study to date has examined CAP rate in CFS/ME patients.22 Despite having similar NREM sleep times, CFS/ME patients had higher CAP rates than matched controls, indicating higher NREM sleep instability in their CFS/ME patients. Abnormal CAP rate was also accompanied by an increase in slow wave delta power. Interestingly, there was no difference in arousal indices between the patient and control groups. A number of CFS/ME patients in this study, however, were found to have nasal cannula flow limitation, indicative of upper airway resistance syndrome (UARS), which may have influenced the findings. CAP has also been associated with UARS and other sleep related breathing disorders previously.71 Whether the higher CAP rate observed in CFS/ME patients was solely a result of UARS is unclear. Importantly, CAP was associated with both subjective fatigue and sleepiness ratings, as has been found previously in other sleep disordered populations.70
Taken together, these studies indicate that conventional sleep stage scoring methods use may not be sensitive enough to detect microstructural changes in CFS/ME patients. Consideration of these microstructure methods for analyzing the sleep EEG may provide a fruitful alternative for uncovering subtle differences during sleep in individuals reporting non-restorative sleep and daytime fatigue.
7.0. OTHER MEASURES OF SLEEP DISTURBANCE
7.1. Autonomic Activity Measures in CFS/ME
Autonomic activity alterations, such as hypotension and reduced heart rate variability (HRV), are a common feature of CFS/ME,72 and are a feature of the diagnostic criteria. In healthy individuals, autonomic nervous system dynamics also have characteristic profiles during sleep onset,73 and different sleep stages and depths of sleep.74 In some CFS/ME patients, the autonomic dysfunction observed during waking also transfers into sleep.75 HRV during sleep is consistently found to be significantly lower in CFS/ME patients compared to well-matched controls, reflecting a reduction in nocturnal parasympathetic activity.39,43,75,76 Decreased HRV is thought to reflect a persistent state of autonomic hypervigilance. The influence of daytime physical activity, however, should not be dismissed as a potential confounder. Regression analyses have also demonstrated that HRV is the best predictor of subjective sleep quality in CFS/ME patients in one study.39
Cardiopulmonary coupling (CPC) is another emerging technique, used as a measure of sleep quality and stability based solely on the electrocardiogram (ECG) signal.77 This technique records both heart rate (R-R interval) and respiration dynamics to generate a spectrogram of cardiopulmonary coupling. Based on this output, one can determine periods of high-frequency and low-frequency coupling, indicative of high and low sleep stability. CPC has been used to demonstrate sleep instability in sleep disordered populations,77 major depressive disorder,78 and fibromyalgia.79 There is preliminary evidence that sleep quality and stability measured by CPC is poor in CFS/ME patients, with reduced high-frequency coupling and increased low-frequency coupling.80 Studies utilizing such autonomic activity techniques indicate that autonomic measures during sleep may be a promising mechanism associated with non-restorative sleep in CFS/ME.43
7.2. Hormone Profiles
Substantial research examining the pathophysiology of CFS/ME indicates that the hypothalamic-pituitary axis (HPA) may be implicated in this disorder.81,82 The HPA also plays an important role in sleep regulation.83 Dysregulation of the HPA has been examined using salivary cortisol profiling in a few studies.84–86 Morning cortisol levels upon awakening, recognized as an indicator of the HPA response to stress, were attenuated in CFS/ME patients compared to healthy controls,85 with this difference most evident in females with CFS/ME.84 Given that CFS/ME is 2 to 3 times more prevalent in women, it has been proposed that sex differences in hypocortisolism may explain the increased risk of CFS in women.84
Heightened IL-6 plasma levels have also been reported in CFS/ME patients,87 possibly reflecting the low levels of systemic inflammation associated with CFS/ME. However, whether increased levels of IL-6 in this study were directly related to CFS/ME or other confounders such as BMI and underlying sleep disorders is unclear. IL-6 has been implicated in the pathogenesis of psychological and physiological fatigue in healthy individuals,88 and has been reported in other patient groups that suffer debilitating fatigue, such as cancer patients.89 Of direct relevance to this review, altered levels of IL-6 are also associated with somnolence and chronic sleep restriction.59 It is plausible that a link between IL-6 or other inflammatory markers and sleep disturbance in CFS/ME exists; this remains an interesting hypothesis that warrants further investigation.
7.3. Neuroimaging
Neuroimaging studies have allowed researchers to examine the sleeping brain in healthy subjects. These techniques have also applied to clinical populations, including primary insomnia,90 OSA,91,92 and depression.93 Particularly in primary insomnia, functional neuroimaging studies during sleep have helped understand the neurophysiological underpinnings of sleep dysregulation in these patients. In line with the hyperarousal theory of primary insomnia, patients were found to have abnormally high regional brain activity during sleep compared to controls subjects.94 This is proposed as one of the mechanisms contributing to sleep state misperception and sleep disturbance found in such patients. A growing number of studies are using functional neuroimaging to examine CFS/ME patients during wake (see Lange95 for a review). These studies have typically observed altered neural activity during performance of a fatiguing cognitive task in CFS/ME patients relative to controls, in the absence of performance impairment, potentially reflecting greater perceived cognitive effort.96,97 Future studies may also benefit from examining neural function and cerebral blood flow in CFS/ME patients during sleep.
8.0. DIRECTIONS FOR FUTURE RESEARCH
There are still many unanswered questions regarding the pathogenesis and nature of sleep disturbance and unrefreshed sleep in CFS/ME. Sleep disturbance may precipitate CFS/ME, may alter or complicate its course by worsening fatigue, pain, or mood, or may represent an independent factor unrelated to fatigue itself. With the development of new standardized criteria for diagnosing CFS/ME, more homogenous patient samples and comparability across studies will be afforded for future research. Standardized research designs with less ridged imposition of sleep-wake times, and control over medication use and symptoms and comorbid conditions (such as pain and depression) will assist in understanding the state of sleep in CFS/ME.
Non-restorative sleep has a fairly distinct subjective definition; however, the physiological markers that underlie this experience and the extent to which alterations in sleep relate to the experience of non-restorative sleep in these patients are relatively unknown. Does non-restorative sleep stem from hyperarousability, sleep hygiene, or circadian rhythm disturbance, or is it biologically driven in some other way? Is the nature of non-restorative sleep in CFS/ME similar to that experienced in other clinical conditions and by healthy individuals on occasion, or is it a heterogeneous phenomenon? There may also be other symptoms that CFS/ME patients experience that could cause non-restorative sleep, such as upper airway resistance syndrome, food intolerance, or immunological and metabolic changes. Emergence of new methods for analyzing the microstructure of sleep have allowed researchers to detect more subtle EEG changes in CFS/ME patients during sleep. Given that very little is known about the nature and cause of non-restorative sleep, these studies have opened up new avenues for investigating sleep disturbance and non-restorative sleep, not only in CFS/ME but other clinical conditions such as insomnia.
Having a clearer understanding about the pathophysiology of non-restorative sleep in CFS/ME may lead to better treatment options for patients. For example, one theory for the non-restorative sleep experienced in insomnia suggests that negative cognitions trigger autonomic arousal during wakefulness that transfers into sleep.98 Nocturnal arousal is often treated by reducing hyperarousability and cognitions during the day, using cognitive behavioral therapy99 or mindfulness based techniques,100 with the aim of reducing arousal at night. If a similar arousal phenomenon is found during sleep in CFS/ME, then insomnia-based treatments have the potential to be utilized in CFS/ME patients.
More precise tools and analyses for differentiating fatigue and sleepiness are needed. Distinguishing these two states remains a diagnostic challenge for clinicians. While there are validated objective and subjective measures of sleepiness, only subjective measures currently exist for assessing fatigue. Development of an objective measure of fatigue and a questionnaire that assesses both fatigue and sleepiness would be useful not only in the context of CFS/ME, but for assessing other disorders in which both sleepiness and fatigue are a feature. In addition, further research into the relationship between sleep and fatigue is greatly needed.
Patients commonly report improved sleep following some treatment regimes, such as graded exercise therapy and antibiotic treatment for imbalances in gut microbial flora. There are avenues for research into objective assessments of sleep before and after treatment, and to follow the time course of changes in sleep as patients' symptoms improve. Future studies are needed to explore the interaction of sleep homeostasis, SWA, and immune system activation in CFS/ME, given the changes in immune function that are observed in these patients.6
The issue surrounding possible sleep state misperception in CFS/ME raises the question of whether abnormalities in sleep associated with CFS/ME are similar to those found in other populations that experience sleep misperception, such as insomnia, or whether they are specific to CFS/ME. This also raises the question of whether CFS/ME patients report a bigger discrepancy between objective and subjective measures of sleep compared to other medical conditions, and is this relative to the experience of non-restorative sleep? Understanding the association between these two conditions may provide insight into the mechanisms of sleep misperception. Future studies will benefit by incorporating subjective, physiological and behavioral measures of sleep to gain a broader insight into the nature of sleep disturbance of CFS/ME.
Finally, CFS/ME may provide a unique insight into the link between non-restorative sleep and fatigue. Other clinical conditions also experience non-restorative sleep and fatigue, such as FM, narcolepsy, and coronary heart disease,101,102 as well as a high number of otherwise healthy individuals.103 It is plausible that there may be subgroups of such people with fatigue and non-restorative sleep with similar underlying symptoms and etiologies, or, alternatively, there may be a continuum of such symptoms with CFS/ME being at the upper end of the spectrum. Given the constellation of physiological and psychological symptoms characterizing CFS/ME, examining sleep and fatigue in CFS/ME patients may allow us to better understand the neurobiology and etiology of fatigue in other patient populations.
SUMMARY AND CONCLUSIONS
CFS/ME is a complex and severely debilitating condition. Non-restorative sleep, reduced sleep quality, and extended periods of sleep are commonly reported, however the basis of these symptoms are unclear. The heterogeneities associated with this disorder make it challenging for researchers to study and make cross-study comparisons difficult. While there is little evidence of sleep architecture differences between CFS/ME and healthy individuals, many patients subjectively report sleep disturbance and unrefreshed sleep, highlighting a potential disconnect between objective and subjective measures of sleep. There is preliminary evidence that alteration in sleep stage transitions and sleep instability, and other physiological mechanisms such as heart rate variability and altered cortisol profiles, may be implicated in the sleep difficulties of this population. Further research is required to investigate the cause of non-restorative sleep and fatigue in CFS/ME, which may aid understanding of this symptom in other medical conditions.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. Bruck received industry support from Bioscreen Inc. over three years 2011–2013 for research involving Chronic Fatigue Syndrome patients. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Work for this study was performed at Victoria University, Melbourne, Australia.
ABBREVIATIONS
- CAP
cyclical alternating pattern
- CDC
United States Centers for Disease Control and Prevention
- CFS
chronic fatigue syndrome
- CPAP
continuous positive airway pressure
- CPC
cardiopulmonary coupling
- ECG
electrocardiogram
- EDS
excessive daytime sleepiness
- EEG
electroencephalogram
- FFT
fast Fourier transform
- FM
fibromyalgia
- HPA
hypothalamic-pituitary axis
- HRV
heart rate variability
- ICC
International Consensus Criteria
- ICD-10
International Classification of Diseases
- ICSD
International Classification of Sleep Disorders
- IL-6
interleukin 6
- MDD
Major Depressive Disorder
- ME
myalgic encephalomyelitis
- MSLT
multiple sleep latency test
- OSA
obstructive sleep apnea
- PI
Primary Insomnia
- PSG
polysomnography
- PSQI
Pittsburgh Sleep Quality Index
- S1
stage 1 sleep
- S2
stage 2 sleep
- SDQ
Sleep Disorder Questionnaire
- SE
sleep efficiency
- SL
sleep latency
- SSS
Stanford Sleepiness Scale
- SWA
slow wave activity
- SWS
slow wave sleep
- TIB
time in bed
- TST
total sleep time
REFERENCES
- 1.Jason LA, King CP, Frankenberry EL, et al. Chronic fatigue syndrome: assessing symptoms and activity level. J Clin Psychol. 1999;55:411–24. doi: 10.1002/(sici)1097-4679(199904)55:4<411::aid-jclp6>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 2.Afari N, Buchwald D. Chronic fatigue syndrome: a review. Am J Psychiatry. 2003;160:221–36. doi: 10.1176/appi.ajp.160.2.221. [DOI] [PubMed] [Google Scholar]
- 3.Ranjith G. Epidemiology of chronic fatigue syndrome. Occup Med. 2005;55:13–9. doi: 10.1093/occmed/kqi012. [DOI] [PubMed] [Google Scholar]
- 4.Fischler B. Review of clinical and psychobiological dimensions of the chronic fatigue syndrome: differentiation from depression and contribution of sleep dysfunctions. Sleep Med Rev. 1999;3:131–46. doi: 10.1016/s1087-0792(99)90020-5. [DOI] [PubMed] [Google Scholar]
- 5.Krupp LB, Jandorf L, Coyle PK, Mendelson WB. Sleep disturbance in chronic fatigue syndrome. J Psychosom Res. 1993;37:325–31. doi: 10.1016/0022-3999(93)90134-2. [DOI] [PubMed] [Google Scholar]
- 6.Lorusso L, Mikhaylova SV, Capelli E, Ferrari D, Ngonga GK, Ricevuti G. Immunological aspects of chronic fatigue syndrome. Autoimmunity Rev. 2009;8:287–91. doi: 10.1016/j.autrev.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 7.Beard G. Neurasthenia, or nervous exhaustion. Boston Med Surg J. 1869;80:217–20. [Google Scholar]
- 8.Wolfe F, Smythe HA, Yunus MB, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 9.Friedberg F, Jason LA. Chronic fatigue syndrome and fibromyalgia: Clinical assessment and treatment. J Clin Psychol. 2001;57:433–55. doi: 10.1002/jclp.1040. [DOI] [PubMed] [Google Scholar]
- 10.Holmes GP, Kaplan JE, Gantz NM, et al. Chronic fatigue syndrome: a working case definition. Ann Intern Med. 1988;108:387. doi: 10.7326/0003-4819-108-3-387. [DOI] [PubMed] [Google Scholar]
- 11.Lloyd AR, Wakefield D, Boughton CR, Dwyer J. What is myalgic encephalomyelitis? Lancet. 1988;8597:1286–7. doi: 10.1016/s0140-6736(88)92107-1. [DOI] [PubMed] [Google Scholar]
- 12.Lloyd AR, Hickie I, Boughton CR, Spencer O, Wakefield D. Prevalence of chronic fatigue syndrome in an Australian population. Med J Australia. 1990;153:522–8. doi: 10.5694/j.1326-5377.1990.tb126191.x. [DOI] [PubMed] [Google Scholar]
- 13.Sharpe MC, Archard LC, Banatvala JE, et al. A report—chronic fatigue syndrome: guidelines for research. J Royal Soc Med. 1991;84:118. doi: 10.1177/014107689108400224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. Ann Intern Med. 1994;121:953–9. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 15.Reeves WC, Heim C, Maloney EM, et al. Sleep characteristics of persons with chronic fatigue syndrome and non-fatigued controls: results from a population-based study. BMC Neurol. 2006;6:41. doi: 10.1186/1471-2377-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carruthers BM, Jain AK, Meirleir KLD, et al. Myalgic encephalomyelitis/chronic fatigue syndrome: clinical working case definition, diagnostic and treatment protocols. J Chronic Fatigue Syndr. 2003;11:7–116. [Google Scholar]
- 17.Carruthers BM, van de Sande MI, De Meirleir KL, et al. Myalgic encephalomyelitis: International Consensus Criteria. J Intern Med. 2011;270:327–38. doi: 10.1111/j.1365-2796.2011.02428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morriss RK, Sharpe M, Sharpley AL, Cowen PJ, Hawton K, Morris J. Abnormalities of sleep in patients with the chronic fatigue syndrome. BMJ. 1993;306:1161–4. doi: 10.1136/bmj.306.6886.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fossey M, Libman E, Bailes S, et al. Sleep quality and psychological adjustment in chronic fatigue syndrome. J Behav Med. 2004;27:581–605. doi: 10.1007/s10865-004-0004-y. [DOI] [PubMed] [Google Scholar]
- 20.Le Bon O, Fischler B, Hoffmann G, et al. How significant are primary sleep disorders and sleepiness in the chronic fatigue syndrome? Sleep Res. 2000;3:43–8. [PubMed] [Google Scholar]
- 21.Nisenbaum R, Jones J, Unger E, Reyes M, Reeves W. A population-based study of the clinical course of chronic fatigue syndrome. Health Qual Life Outcomes. 2003;1:49. doi: 10.1186/1477-7525-1-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guilleminault C, Poyares D, Da Rosa A, Kirisoglu C, Almeida T, Lopes MC. Chronic fatigue, unrefreshing sleep and nocturnal polysomnography. Sleep Med. 2006;7:513–20. doi: 10.1016/j.sleep.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Buchwald D, Pascualy R, Bombardier C, Kith P. Sleep disorders in patients with chronic fatigue. Clin Infect Dis. 1994;18:S68–S72. doi: 10.1093/clinids/18.supplement_1.s68. [DOI] [PubMed] [Google Scholar]
- 24.Neu D, Cappeliez B, Hoffmann G, Verbanck P, Linkowski P, Le Bon O. High slow-wave sleep and low-light sleep: Chronic fatigue syndrome is not likely to be a primary sleep disorder. J Clin Neurophysiol. 2009;26:207–12. doi: 10.1097/WNP.0b013e3181a1841b. [DOI] [PubMed] [Google Scholar]
- 25.Libman E, Creti L, Baltzan M, Rizzo D, Fichten CS, Bailes S. Sleep apnea and psychological functioning in chronic fatigue syndrome. J Health Psychol. 2009;14:1251–67. doi: 10.1177/1359105309344895. [DOI] [PubMed] [Google Scholar]
- 26.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 27.Osorio CD, Gallinaro AL, Lorenzi-Filho G, et al. Sleep quality in patients with fibromyalgia using the Pittsburgh Sleep Quality Index. J Rheumatol. 2006;33:1863–5. [PubMed] [Google Scholar]
- 28.Lavigne G, Brousseau M, Kato T, et al. Experimental pain perception remains equally active over all sleep stages. Pain. 2004;110:646–55. doi: 10.1016/j.pain.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 29.Modofsky, H The significance, assessment, and management of nonrestorative sleep in fibromyalgia syndrome. CNS Spectrums. 2008;13:22–6. doi: 10.1017/s1092852900026808. [DOI] [PubMed] [Google Scholar]
- 30.Older SA, Battafarano DF, Danning CL. The effects of delta wave sleep interruption on pain thresholds and fibromyalgia-like symptoms in healthy subjects; correlations with insulin-like growth factor I. J Rheumatol. 1998;25:1180–6. [PubMed] [Google Scholar]
- 31.Benca RM, Obermeyer WH, Thisted RA, Gillin JC. Sleep and psychiatric disorders: A meta-analysis. Arch Gen Psychiatry. 1992;49:651–68. doi: 10.1001/archpsyc.1992.01820080059010. [DOI] [PubMed] [Google Scholar]
- 32.Morriss RK, Wearden AJ, Battersby L. The relation of sleep difficulties to fatigue, mood and disability in chronic fatigue syndrome. J Psychosom Res. 1997;42:597–605. doi: 10.1016/s0022-3999(97)89895-9. [DOI] [PubMed] [Google Scholar]
- 33.Le Bon O, Minner P, Van Moorsel C, et al. First-night effect in the chronic fatigue syndrome. Psychiatry Res. 2003;120:191–9. doi: 10.1016/s0165-1781(03)00185-9. [DOI] [PubMed] [Google Scholar]
- 34.Fischler B, Le Bon O, Hoffmann G, Cluydts R, Kaufman L, De Meirleir K. Sleep anomalies in the chronic fatigue syndrome. Neuropsychobiology. 1997;35:115–22. doi: 10.1159/000119331. [DOI] [PubMed] [Google Scholar]
- 35.Stores G, Fry A, Crawford C. Sleep abnormalities demonstrated by home polysomnography in teenagers with chronic fatigue syndrome. J Psychosom Res. 1998;45:85–91. doi: 10.1016/s0022-3999(98)00024-5. [DOI] [PubMed] [Google Scholar]
- 36.Sharpley A, Clements A, Hawton K, Sharpe M. Do patients with pure chronic fatigue syndrome (neurasthenia) have abnormal sleep? Psychosom Med. 1997;59:592–6. doi: 10.1097/00006842-199711000-00006. [DOI] [PubMed] [Google Scholar]
- 37.Whelton CL, Salit I, Moldofsky H. Sleep, Epstein-Barr virus infection, musculoskeletal pain, and depressive symptoms in chronic fatigue syndrome. J Rheumatol. 1992;19:939. [PubMed] [Google Scholar]
- 38.Togo F, Natelson BH, Cherniack N, FitzGibbons J, Garcon C, Rapoport DM. Sleep structure and sleepiness in chronic fatigue syndrome with or without coexisting fibromyalgia. Arthiritis Res Ther. 2008;10:R56. [Google Scholar]
- 39.Burton A, Rahman K, Kadota Y, Lloyd A, Vollmer-Conna U. Reduced heart rate variability predicts poor sleep quality in a case-control study of chronic fatigue syndrome. Exp Brain Res. 2010;204:71–8. doi: 10.1007/s00221-010-2296-1. [DOI] [PubMed] [Google Scholar]
- 40.Ohinata J, Suzuki N, Araki A, Takahashi S, Fujieda K, Tanaka H. Actigraphic assessment of sleep disorders in children with chronic fatigue syndrome. Brain Dev. 2008;30:329–33. doi: 10.1016/j.braindev.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Evering RMH, van Weering MGH, Groothuis-Oudshoorn KCGM, Vollenbroek-Hutten MMR. Daily physical activity of patients with the chronic fatigue syndrome: a systematic review. Clin Rehabil. 2011;25:112–33. doi: 10.1177/0269215510380831. [DOI] [PubMed] [Google Scholar]
- 42.Tryon WW, Jason L, Frankenberry E, Torres-Harding S. Chronic fatigue syndrome impairs circadian rhythm of activity level. Physiol Behav. 2004;82:849–53. doi: 10.1016/j.physbeh.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 43.Rahman K, Burton A, Galbraith S, Lloyd AR, Vollmer-Conna U. Sleep-wake behavior in chronic fatigue syndrome. Sleep. 2011;34:671–8. doi: 10.1093/sleep/34.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watson NF, Kapur V, Arguelles LM, et al. Comparison of subjective and objective measures of insomnia in monozygotic twins discordant for chronic fatigue syndrome. Sleep. 2003;26:324–8. doi: 10.1093/sleep/26.3.324. [DOI] [PubMed] [Google Scholar]
- 45.Ball N, Buchwald DS, Schmidt D, Goldberg J, Ashton S, Armitage R. Monozygotic twins discordant for chronic fatigue syndrome: Objective measures of sleep. J Psychosom Res. 2004;56:207–12. doi: 10.1016/S0022-3999(03)00598-1. [DOI] [PubMed] [Google Scholar]
- 46.Majer M, Jones J, Unger E, et al. Perception versus polysomnographic assessment of sleep in CFS and non-fatigued control subjects: results from a population-based study. BMC Neurol. 2007;7:40. doi: 10.1186/1471-2377-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Neu D, Mairesse O, Hoffmann G, et al. Sleep quality perception in the chronic fatigue syndrome: correlations with sleep efficiency, affective symptoms and intensity of fatigue. Neuropsychobiology. 2007;56:40–6. doi: 10.1159/000110727. [DOI] [PubMed] [Google Scholar]
- 48.Le Bon O, Neu D, Valente F, Linkowski P. Paradoxical NREMS distribution in “pure” chronic fatigue patients: a comparison with sleep apnea-hypopnea patients and healthy control subjects. J Chronic Fatigue Syndrome. 2007;14:2. [Google Scholar]
- 49.Armitage R, Landis C, Hoffmann R, et al. The impact of a 4-hour sleep delay on slow wave activity inb twins discordant for chronic fatigue syndrome. Sleep. 2007;30:657–62. doi: 10.1093/sleep/30.5.657. [DOI] [PubMed] [Google Scholar]
- 50.Kishi A, Struzik ZR, Natelson BH, Togo F, Yamamoto Y. Dynamics of sleep stage transitions in healthy humans and patients with chronic fatigue syndrome. Am J Physiol. 2008;294:R1980–R7. doi: 10.1152/ajpregu.00925.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Armitage R, Landis C, Hoffmann R, et al. Power spectral analysis of sleep EEG in twins discordant for chronic fatigue syndrome. J Psychosom Res. 2009;66:51–7. doi: 10.1016/j.jpsychores.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Decker M, Tabassum H, Lin J-M, Reeves W. Electroencephalographic correlates of chronic fatigue syndrome. Behav Brain Funct. 2009;5:1–8. doi: 10.1186/1744-9081-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Creti L, Libman E, Baltzan M, Rizzo D, Bailes S, Fichten CS. Impaired sleep in chronic fatigue syndrome. J Health Psychol. 2010;15:596–607. doi: 10.1177/1359105309355336. [DOI] [PubMed] [Google Scholar]
- 54.Kishi A, Natelson BH, Togo F, Struzik ZR, Rapoport DM, Yamamoto Y. Sleep stage transitions in chronic fatigue syndrome patients with or without fibromyalgia. Engineering in Medicine and Biology Society (EMBC). Annual International Conference of the IEEE. 2010:5391–4. doi: 10.1109/IEMBS.2010.5626478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Cauter E, Leproult R, Plat L. Age-related changes in slow wave sleep and REM sleep and relationship with growth hormone and cortisol levels in healthy men. JAMA. 2000;284:861–8. doi: 10.1001/jama.284.7.861. [DOI] [PubMed] [Google Scholar]
- 56.Manu P, Lane TJ, Matthews DA, Castriotta RJ, Watson RK, Abeles M. Alpha-delta sleep in patients with a chief complaint of chronic fatigue. South Med J. 1994;87:465–70. doi: 10.1097/00007611-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 57.Flanigan MJ, Morehouse RL, Shapiro CM. Determination of observer-rated alpha activity during sleep. Sleep. 1995;18:702–6. [PubMed] [Google Scholar]
- 58.Dijk D-J, Brunner DP, Beersma D, Borbely AA. Electroencephalogram power density and slow wave sleep as a function of prior waking and circadian phase. Sleep. 1990;13:430–40. doi: 10.1093/sleep/13.5.430. [DOI] [PubMed] [Google Scholar]
- 59.Madje JA, Krueger JM. Links between the innate immune system and sleep. J Allergy Clin Immunol. 2005;116:1188–98. doi: 10.1016/j.jaci.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 60.Majde JA, Krueger JM. Neuroimmunology of sleep. In: D'haenen H, editor. Textbook of biological psychiatry. London: John Wiley – Sons, Ltd; 2002. pp. 1247–57. [Google Scholar]
- 61.Watson NF, Kapur V, Arguelles LM, et al. Comparison of subjective and objective measures of insomnia in monozygotic twins discordant for chronic fatigue syndrome. Sleep. 2003;26:324–8. doi: 10.1093/sleep/26.3.324. [DOI] [PubMed] [Google Scholar]
- 62.Watson NF, Jacobsen C, Goldberg J, Kapur V, Buchwald DS. Subjective and objective sleepiness in monozygotic twins discordant for chronic fatigue syndrome. Sleep. 2004;27:973–7. doi: 10.1093/sleep/27.5.973. [DOI] [PubMed] [Google Scholar]
- 63.Jackowska M, Dockray S, Hendrickx H, Steptoe A. Psychosocial factors and sleep efficiency: discrepancies between subjective and objective evaluations of sleep. Psychosom Med. 2011;73:810–6. doi: 10.1097/PSY.0b013e3182359e77. [DOI] [PubMed] [Google Scholar]
- 64.Edinger JD, Krystal AD. Subtyping primary insomnia: is sleep state misperception a distinct clinical entity? Sleep Med Rev. 2003;7:203–14. doi: 10.1053/smrv.2002.0253. [DOI] [PubMed] [Google Scholar]
- 65.Pigeon WR, Sateia MJ, Ferguson RJ. Distinguishing between excessive daytime sleepiness and fatigue: Toward improved detection and treatment. J Psychosom Res. 2003;54:61–9. doi: 10.1016/s0022-3999(02)00542-1. [DOI] [PubMed] [Google Scholar]
- 66.Neu D, Hoffmann G, Moutrier R, Verbanck P, Linkowski P, Le Bon O. Are patients with chronic fatigue syndrome just ‘tired’ or also ‘sleepy’? J Sleep Res. 2008;17:427–31. doi: 10.1111/j.1365-2869.2008.00679.x. [DOI] [PubMed] [Google Scholar]
- 67.Rechtschaffen A, Kales A. U.S. Government Printing Office: Public Health Service; 1968. A manual of standardized terminology, techniques and scoring system for sleep stages of human subjects. [Google Scholar]
- 68.Terzano MG, Mancia D, Salati MR, Costani G. The cyclic alternating pattern as a physiologic component of normal NREM sleep. Sleep. 1985;8:137–45. doi: 10.1093/sleep/8.2.137. [DOI] [PubMed] [Google Scholar]
- 69.Terzano MG, Parrino L. Origin and significance of the cyclic alternating pattern (CAP) Sleep Med Rev. 2000;4:101–23. doi: 10.1053/smrv.1999.0083. [DOI] [PubMed] [Google Scholar]
- 70.Parrino L, Milioli G, De Paolis F, Grassi A, Terzano MG. Paradoxical insomnia: The role of CAP and arousals in sleep misperception. Sleep Med. 2009;10:1139–45. doi: 10.1016/j.sleep.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 71.Guilleminault C, Lopes MC, Hagen CC, Da Rosa A. The cyclic alternating pattern demonstrates increased sleep instability and correlates with fatigue and sleepiness in adults with upper airway resistance syndrome. Sleep. 2007;30:641–7. doi: 10.1093/sleep/30.5.641. [DOI] [PubMed] [Google Scholar]
- 72.Yamamoto Y, LaManca JJ, Natelson BH. A measure of heart rate variability is sensitive to orthostatic challenge in women with chronic fatigue syndrome. Exp Biol Med. 2003;228:167–74. doi: 10.1177/153537020322800206. [DOI] [PubMed] [Google Scholar]
- 73.Shinar Z, Akselrod S, Dagan Y, Baharav A. Autonomic changes during wake-sleep transition: A heart rate variability based approach. Auton Neurosci. 2006;130:17–27. doi: 10.1016/j.autneu.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 74.Trinder J, Kleiman J, Carrington M, et al. Autonomic activity during human sleep as a function of time and sleep stage. J Sleep Res. 2001;10:253–64. doi: 10.1046/j.1365-2869.2001.00263.x. [DOI] [PubMed] [Google Scholar]
- 75.Boneva RS, Decker MJ, Maloney EM, et al. Higher heart rate and reduced heart rate variability persist during sleep in chronic fatigue syndrome: A population-based study. Auton Neurosci. 2007;137:94–101. doi: 10.1016/j.autneu.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 76.Tajima S, Kuratsune H, Yamaguti K, et al. Estimation of fatigue state in patient with CFS using actigraph and R-R interval power spectrum analysis. Jpn J Clin Med. 2007;65:1057–64. [PubMed] [Google Scholar]
- 77.Thomas RJ, Mietus JE, Peng CK, Goldberger AL. An electrocardiogram-based technique to assess cardiopulmonary coupling during sleep. Sleep. 2005;28:1151–61. doi: 10.1093/sleep/28.9.1151. [DOI] [PubMed] [Google Scholar]
- 78.Yang AC, Yang C-H, Hong C-J, et al. Sleep state instabilities in major depressive disorder: Detection and quantification with electrocardiogram-based cardiopulmonary coupling analysis. Psychophysiol. 2011;48:285–91. doi: 10.1111/j.1469-8986.2010.01060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Thomas RJ, Mietus JE, Peng C-K, Goldberger AL, Crofford LJ, Chervin RD. Impaired sleep quality in fibromyalgia: Detection and quantification with ECG-based cardiopulmonary coupling spectrograms. Sleep Med. 2010;11:497–8. doi: 10.1016/j.sleep.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cunnington D, Buccella D, Bastiampillai S, Swieca J. Sleep architecture and sleep stability in chronic fatigue syndrome. J Sleep Res. 2011;20:25. [Google Scholar]
- 81.Demitrack M, Dale J, Straus S, et al. Evidence for impaired activation of the hypothalamic-pituitary-adrenal axis in patients with chronic fatigue syndrome. J Clin Endocrinol Metab. 1991;73:1224–34. doi: 10.1210/jcem-73-6-1224. [DOI] [PubMed] [Google Scholar]
- 82.Di Giorgio A, Hudson M, Jerjes W, Cleare AJ. 24-Hour pituitary and adrenal hormone profiles in Chronic Fatigue Syndrome. Psychosom Med. 2005;67:433–40. doi: 10.1097/01.psy.0000161206.55324.8a. [DOI] [PubMed] [Google Scholar]
- 83.Vgontzas A, Chrousos G. Sleep, the hypothalamic-pituitary-adrenal axis, and cytokines: multiple interactions and disturbances in sleep disorders. Endocrinol Metab Clin North Am. 2002;31:15–36. doi: 10.1016/s0889-8529(01)00005-6. [DOI] [PubMed] [Google Scholar]
- 84.Nater UM, Maloney E, Boneva RS, et al. Attenuated morning salivary cortisol concentrations in a population-based study of persons with chronic fatigue syndrome and well controls. J Clin Endocrinol Metab. 2008;93:703–9. doi: 10.1210/jc.2007-1747. [DOI] [PubMed] [Google Scholar]
- 85.Roberts ADL, Wessely S, Chalder T, Papadopoulos A, Cleare AJ. Salivary cortisol response to awakening in chronic fatigue syndrome. Br J Psychiatry. 2004;184:136–41. doi: 10.1192/bjp.184.2.136. [DOI] [PubMed] [Google Scholar]
- 86.Jerjes WK, Cleare AJ, Wessely S, Wood PJ, Taylor NF. Diurnal patterns of salivary cortisol and cortisone output in chronic fatigue syndrome. J Affect Disord. 2005;87:299–304. doi: 10.1016/j.jad.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 87.Nater UM, Youngblood LS, Jones JF, et al. Alterations in diurnal salivary cortisol rhythm in a population-based sample of cases with chronic fatigue syndrome. Psychosom Med. 2008;70:298–305. doi: 10.1097/PSY.0b013e3181651025. [DOI] [PubMed] [Google Scholar]
- 88.Singh A, Petrides JS, Gold PW, Chrousos GP, Deuster PA. Differential hypothalamic-pituitary-adrenal axis reactivity to psychological and physical stress. J Clin Endocrinol Metab. 1999;84:1944–8. doi: 10.1210/jcem.84.6.5746. [DOI] [PubMed] [Google Scholar]
- 89.Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: a quantitative review. Brain Behav Immunol. 2007;21:413–27. doi: 10.1016/j.bbi.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 90.Smith MT, Perlis ML, Chengazi VU, et al. Neuroimaging of NREM sleep in primary insomnia: a Tc-99-HMPAO single photon emission computed tomography study. Sleep. 2002;25:325–35. [PubMed] [Google Scholar]
- 91.Morrell MJ, Jackson ML, Twigg GL, et al. Changes in brain morphology in patients with obstructive sleep apnoea. Thorax. 2010;65:908–14. doi: 10.1136/thx.2009.126730. [DOI] [PubMed] [Google Scholar]
- 92.O'Donoghue FJ, Wellard RM, Rochford PD, et al. Magnetic resonance spectroscopy and neurocognitive dysfunction in obstructive sleep apnea before and after CPAP treatment. Sleep. 2012;35:41–8. doi: 10.5665/sleep.1582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nofzinger EA, Price JC, Meltzer CC, Buysee DJ, Villemagne VL, Miewald JM. Towards a neurobiology of dysfunctional arousal in depression: the relationship between beta EEG power and regional cerebral glucose metabolism during NREM sleep. Psychiatry Res. 2000;98:71–91. doi: 10.1016/s0925-4927(00)00045-7. [DOI] [PubMed] [Google Scholar]
- 94.Nofzinger EA, Buysee DJ, Germaine A, Price JC, Miewald JM, Kupfer DJ. Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry. 2004;161:2126–8. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- 95.Lange G, Wang S, DeLuca J, Natelson BH. Neuroimaging in chronic fatigue syndrome. Am J Med. 1998;105:50S–3S. doi: 10.1016/s0002-9343(98)00175-2. [DOI] [PubMed] [Google Scholar]
- 96.Cook DB, O'Connor PJ, Lange G, Steffener J. Functional neuroimaging correlates of mental fatigue induced by cognition among chronic fatigue syndrome patients and controls. Neuroimage. 2007;36:108–22. doi: 10.1016/j.neuroimage.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 97.Schmaling KB, Lewis DH, Fiedelak JI, Mahurin R, Buchwald DS. Single-photon emission computerized tomography and neurocognitive function in patients with chronic fatigue syndrome. Psychosom Med. 2003;65:129–36. doi: 10.1097/01.psy.0000038942.33335.9b. [DOI] [PubMed] [Google Scholar]
- 98.Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40:869–93. doi: 10.1016/s0005-7967(01)00061-4. [DOI] [PubMed] [Google Scholar]
- 99.Harvey AG. A cognitive theory and therapy for chronic insomnia. J Cogn Psychother. 2005;19:41–59. [Google Scholar]
- 100.Lundh LG. The role of acceptance and mindfulness in the treatment of insomnia. J Cogn Psychother. 2005;19:29–39. [Google Scholar]
- 101.Modofsky H. The significance, assessment, and management of nonrestorative sleep in fibromyalgia syndrome. Sleep Med. 2008;13:22–6. doi: 10.1017/s1092852900026808. [DOI] [PubMed] [Google Scholar]
- 102.Droogleever Fortuyn HA, Fronczek R, Smitshoek M, et al. Severe fatigue in narcolepsy with cataplexy. J Sleep Res. 2012;21:163–9. doi: 10.1111/j.1365-2869.2011.00943.x. [DOI] [PubMed] [Google Scholar]
- 103.Ohayon MM. Prevalence and correlates of nonrestorative sleep complaints. Arch Intern Med. 2005;165:35–41. doi: 10.1001/archinte.165.1.35. [DOI] [PubMed] [Google Scholar]


