Abstract
BACKGROUND
In some studies, tight glycemic control with insulin improved outcomes in adults undergoing cardiac surgery, but these benefits are unproven in critically ill children at risk for hyperinsulinemic hypoglycemia. We tested the hypothesis that tight glycemic control reduces morbidity after pediatric cardiac surgery.
METHODS
In this two-center, prospective, randomized trial, we enrolled 980 children, 0 to 36 months of age, undergoing surgery with cardiopulmonary bypass. Patients were randomly assigned to either tight glycemic control (with the use of an insulin-dosing algorithm targeting a blood glucose level of 80 to 110 mg per deciliter [4.4 to 6.1 mmol per liter]) or standard care in the cardiac intensive care unit (ICU). Continuous glucose monitoring was used to guide the frequency of blood glucose measurement and to detect impending hypoglycemia. The primary outcome was the rate of health care–associated infections in the cardiac ICU. Secondary outcomes included mortality, length of stay, organ failure, and hypoglycemia.
RESULTS
A total of 444 of the 490 children assigned to tight glycemic control (91%) received insulin versus 9 of 490 children assigned to standard care (2%). Although normoglycemia was achieved earlier with tight glycemic control than with standard care (6 hours vs. 16 hours, P<0.001) and was maintained for a greater proportion of the critical illness period (50% vs. 33%, P<0.001), tight glycemic control was not associated with a significantly decreased rate of health care–associated infections (8.6 vs. 9.9 per 1000 patient-days, P = 0.67). Secondary outcomes did not differ significantly between groups, and tight glycemic control did not benefit high-risk subgroups. Only 3% of the patients assigned to tight glycemic control had severe hypoglycemia (blood glucose <40 mg per deciliter [2.2 mmol per liter]).
CONCLUSIONS
Tight glycemic control can be achieved with a low hypoglycemia rate after cardiac surgery in children, but it does not significantly change the infection rate, mortality, length of stay, or measures of organ failure, as compared with standard care. (Funded by the National Heart, Lung, and Blood Institute and others; SPECS ClinicalTrials.gov number, NCT00443599.)
Congenital heart defects are the most common birth defects, with approximately 20,000 pediatric cardiothoracic surgical procedures performed each year in the United States.1,2 Postoperative morbidity and mortality among infants and young children remain relatively high3; thus, identification of modifiable risk factors during postoperative critical care is important for continued improvement in outcomes. Tight glycemic control has emerged as a potential approach to reduce morbidity in adult cardiac medical4,5 and surgical6,7 populations, but it has not proved to be generalizable to all critical care patients.8–11
The incidence of hyperglycemia (blood glucose level >126 mg per deciliter [7.0 mmol per liter]) after cardiac surgery in infants and young children is uniformly high, reaching more than 90% in some series.12–15 Retrospective studies of the possible association between hyperglycemia and perioperative morbidity in this population have yielded mixed results.14,16–18 One pediatric clinical trial compared tight glycemic control with standard glucose management in a mixed critical care population of medical and surgical patients, primarily those who had undergone cardiac surgery.19 Tight glycemic control was associated with several benefits but also extremely high rates of severe hypoglycemia (<40 mg per deciliter [2.2 mmol per liter]). Routine use of tight glycemic control in the pediatric cardiac intensive care unit (ICU) is controversial, owing both to contradictory results of the trials evaluating this approach in adults and concerns about potentially deleterious effects of insulin-induced hypoglycemia on the developing brain.
The Safe Pediatric Euglycemia after Cardiac Surgery (SPECS) trial reported here tested the hypothesis that tight glycemic control in the cardiac ICU would reduce perioperative morbidity — specifically, the rate of health care–associated infections — in young children after cardiac surgery with cardiopulmonary bypass. To minimize the risk of hypoglycemia associated with tight glycemic control, our study protocol specified the use of a subcutaneous continuous glucose monitor as a means of determining the frequency of blood glucose measurement and detecting impending hypoglycemia, paired with an explicit insulin-dosing algorithm.20,21
METHODS
STUDY DESIGN
This randomized, controlled trial was conducted in the cardiac ICUs at Boston Children’s Hospital and the University of Michigan C.S. Mott Children’s Hospital. Children 0 to 36 months of age who were being admitted to the cardiac ICU after undergoing cardiac surgery with cardiopulmonary bypass were included. Children with diabetes or without adequate intravascular access were excluded. The study was approved by the institutional review board at each institution, and written informed consent was obtained from parents or legal guardians. All authors vouch for the accuracy of the data and the fidelity of the study to the protocol, which is available with the full text of this article at NEJM.org.
INTERVENTIONS AND OUTCOMES
A detailed description of the study methods was published previously.22 Study participants were randomly assigned to either tight glycemic control or standard care in the cardiac ICU postoperatively according to a permuted-block design with stratification according to center. Operating-room clinicians were unaware of the study-group assignments. The preoperative plan was to administer intraoperative glucocorticoids to all children at both institutions who required hypothermic circulatory arrest and to all children at Boston Children’s Hospital who were younger than 1 year of age; preoperative glucocorticoids were not given. The study protocol was initiated immediately after postoperative admission. Bedside clinicians in the cardiac ICU were aware of the study-group assignments, given the need to manage insulin therapy and the potential harm of administering placebo fluid.
The group assigned to tight glycemic control (hereafter referred to as the glycemic-control group) received an intravenous infusion of regular human insulin at the lowest dose necessary to achieve normoglycemia (defined as a blood glucose level of 80 to 110 mg per deciliter [4.4 to 6.1 mmol per liter]). Dose adjustment was guided by frequent bedside measurements with a blood glucose meter (LifeScan SureStep Flexx in Boston and Roche ACCU-CHEK Inform in Michigan).23 Glucose levels were entered into a proportional–integral–derivative insulin-dosing algorithm20 on a Microsoft Excel spreadsheet displayed on a dedicated laptop computer at the patient’s bedside. A continuous glucose monitor (Guardian REAL-Time device, Medtronic Diabetes) was used for the duration of insulin therapy in the glycemic-control group to guide the frequency of blood glucose checks and to alert the bedside nurse to impending or actual hypoglycemia; however, no decisions regarding insulin dosing or glucose rescue were made solely on the basis of readings from continuous glucose monitors.
Continuous glucose monitoring was used in the standard-care group only for the first 3 days in the cardiac ICU, to alert bedside clinicians to impending hypoglycemia. The standard-care group had no set target range for blood glucose management; patients were treated according to the preference of the attending cardiac intensivist. A blood-sampling system (VAMP Jr., Edwards Lifesciences) was used in both groups to reduce blood loss, sample dilution, and the risk of catheter contamination associated with frequent blood draws. The study protocol was discontinued on removal of the arterial catheter, at the time of discharge from the cardiac ICU, or 30 days after randomization, whichever came first.
The primary outcome was the number of health care–associated infections (i.e., pneumonia, bloodstream, urinary tract, and surgical-site infections, as defined by the Centers for Disease Control and Prevention24), per 1000 patient-days in the cardiac ICU. All infections were adjudicated by local infection-control clinicians who were unaware of the study-group assignments. Multiple infections in an individual patient were included in the calculation of the overall rate. Pneumonia, bloodstream, and urinary tract infections were tracked for 30 days in the cardiac ICU or until 48 hours after discharge from the cardiac ICU. Surgical-site infections were tracked for 30 days after the index procedure. Secondary outcomes were mortality, the number of days of mechanical ventilation, the length of stay in the cardiac ICU, the length of stay in the hospital, the proportion of children with hypoglycemia, and measures of organ failure. The duration of mechanical ventilation, arterial catheterization, the stay in the cardiac ICU, and the stay in the hospital was considered to be 30 days for patients with a duration of more than 30 days and for children who died in the cardiac ICU by the 30th day. Nutritional intake and indexes of glycemic control were tracked during the period of critical illness, as defined by the presence of an arterial catheter.
SERIOUS ADVERSE EVENTS
Severe hypoglycemia (blood glucose level, <40 mg per deciliter) was considered to be a serious adverse event.25 Mild hypoglycemia (blood glucose level, 50 to 59 mg per deciliter [2.8 to 3.3 mmol per liter]) and moderate hypoglycemia (blood glucose level, 40 to 49 mg per deciliter [2.2 to 2.7 mmol per liter]) were considered to be nonserious adverse events. Other tracked serious adverse events were hypokalemia (potassium level, <2.0 mmol per liter), bleeding and thrombotic complications, renal and hepatic failure, necrotizing enterocolitis, and neurologic injury, including seizures.
STATISTICAL ANALYSIS
We calculated that enrollment of 980 patients would provide a statistical power of 80% to detect a 50% difference in the rate of health care–associated infections between treatment groups at a two-sided alpha level of 0.05, assuming an average baseline rate of 10.9 infections per 1000 patient-days in the cardiac ICU. Analysis of the primary outcome variable was conducted with the use of exact Poisson regression (with adjustment for site) performed on an intention-to-treat basis. Additional analyses were performed on a per-protocol basis. A priori planned analyses were conducted for high surgical risk (defined as a Risk Adjustment in Congenital Heart Surgery [RACHS-1] category26 of ≥3 or not assignable, on a scale from 1 to 6, with higher categories indicating greater risk) and prolonged stay in the cardiac ICU (defined as ≥3 days); the latter analysis was based on a postrandomization factor. In a secondary analysis, we used logistic regression with adjustment for site to examine factors that might be associated with infection within the first 30 days, including a RACHS-1 category of 3 or higher (or not assignable), an age of 30 days or younger, the placement of an implant during surgery, hyperglycemia (blood glucose level initially or at any time during the period of critical illness, >110 mg per deciliter or >180 mg per deciliter [10.0 mmol per liter]), prolonged stay in the cardiac ICU (≥3 days), and postoperative glucocorticoid therapy.
Binary variables were analyzed with the use of stratified exact tests, with adjustment for site. Continuous and length-of-stay variables were analyzed with the use of stratified Wilcoxon rank-sum tests, with adjustment for site. P values were not adjusted for multiple comparisons. Data on glycemic control included all blood glucose concentrations measured with the use of a bedside glucose meter and in the central hospital laboratory. Time-weighted blood glucose averages were calculated from serial measurements. Glucose measurements were interpolated from available measurements, with the time to the target range (the interval between randomization and the first measured glucose level of 80 to 110 mg per deciliter) and the percentage of time in the target range calculated from half-hour values from the interpolated curves. All reported P values are two-tailed. Statistical and graphical analyses were performed with the use of SAS software, version 9.3 (SAS Institute); StatXact, version 9.0 (Cytel); and GraphPad Prism, version 6.0 (GraphPad Software).
Data were presented to an independent data and safety monitoring board appointed by the National Heart, Lung, and Blood Institute at 6-month intervals. One formal analysis was conducted at 50% enrollment to consider early termination of the study for safety, efficacy, or futility.27
RESULTS
STUDY PARTICIPANTS
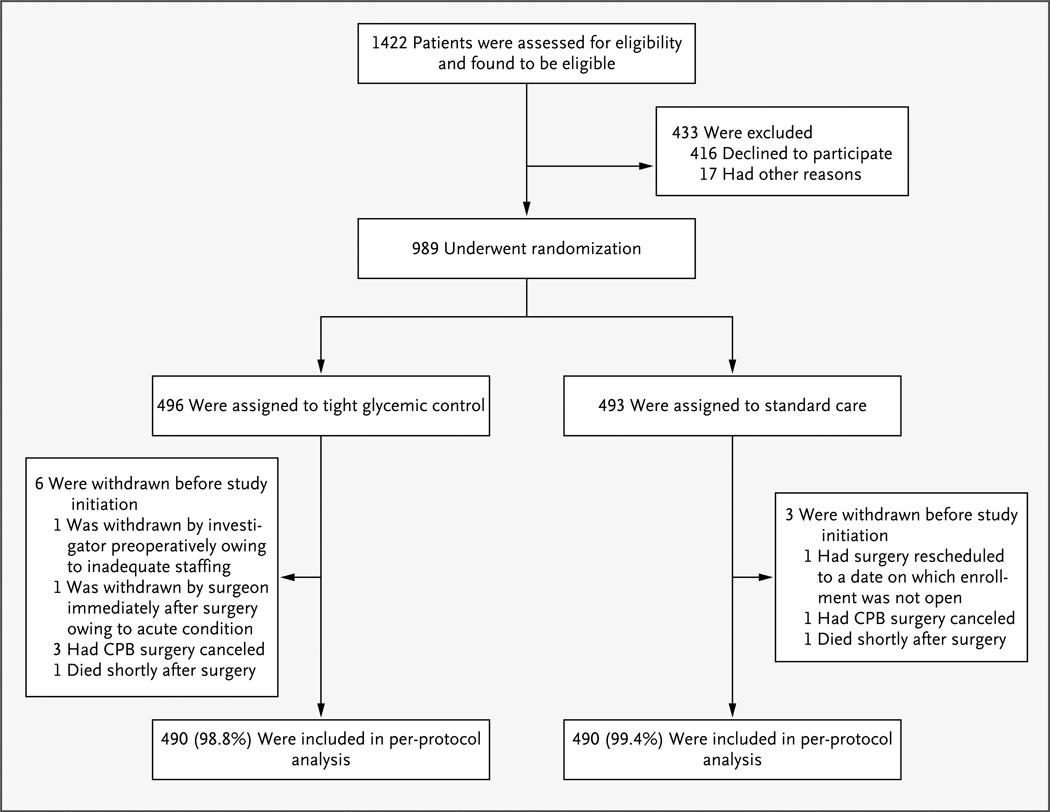
Enrollment began in September 2006 and ended in May 2012. A total of 989 children underwent randomization; 496 were assigned to tight glycemic control and 493 to standard care. Nine children were withdrawn before study initiation and were not included in the per-protocol analyses (Fig. 1).
Figure 1. Assessment, Randomization, and Follow-up of the Study Patients.
CPB denotes cardiopulmonary bypass.
The baseline characteristics of the treatment groups were similar (Table 1). There were no significant or clinically important differences between groups with regard to demographic variables, surgical complexity, or intraoperative or postoperative therapies, including cardiopulmonary bypass techniques, delayed sternal closure, and extracorporeal membrane oxygenation. No children received insulin intraoperatively.
Table 1.
Baseline Characteristics of the Study Patients.*
| Variable | Tight Glycemic Control (N = 490) |
Standard Care (N = 490) |
|---|---|---|
| Enrolled in Boston — no. (%) | 323 (66) | 325 (66) |
| Age at surgery | ||
| Median — mo | 4.3 | 4.9 |
| Interquartile range — mo | 1.8–9.7 | 2.3–10.8 |
| ≤30 days — no. (%) | 99 (20) | 90 (18) |
| Female sex — no. (%) | 241 (49) | 217 (44) |
| Preoperative weight — kg | ||
| Median | 5.3 | 5.7 |
| Interquartile range | 3.7–7.5 | 3.9–7.8 |
| RACHS-1 category — no. (%)† | ||
| 1 | 22 (4) | 33 (7) |
| 2 | 205 (42) | 207 (42) |
| 3 | 157 (32) | 147 (30) |
| 4 | 62 (13) | 60 (12) |
| 5 or 6 | 32 (7) | 29 (6) |
| Not assignable | 12 (2) | 14 (3) |
| Premature — no. (%) | 68 (14) | 71 (14) |
| Chromosomal anomaly — no. (%) | 94 (19) | 98 (20) |
| Noncardiac structural abnormality — no. (%) | 60 (12) | 69 (14) |
| Duration of cardiopulmonary bypass — min | ||
| Median | 104 | 105 |
| Interquartile range | 72–143 | 74–140 |
| Deep hypothermic circulatory arrest — no. (%) | 78 (16) | 77 (16) |
| Highest intraoperative glucose value — mg/dl‡ | ||
| Median | 186 | 192 |
| Interquartile range | 157–230 | 157–232 |
| Intraoperative glucocorticoid therapy — no. (%) | 255 (52) | 247 (50) |
| Intraoperative insulin therapy — no. | 0 | 0 |
| Implant left during surgery — no. (%) | 317 (65) | 320 (65) |
| Delayed sternal closure — no. (%) | 63 (13) | 58 (12) |
| ECMO support — no. (%) | 12 (2) | 12 (2) |
| Postoperative glucocorticoid therapy — no. (%) | 226 (46) | 213 (43) |
There were no significant differences in baseline characteristics between the treatment groups. ECMO denotes extracorporeal membrane oxygenation.
The scale for Risk Adjustment in Congenital Heart Surgery (RACHS-1) categories ranges from 1 to 6, with higher categories indicating greater risk.
To convert values for glucose to millimoles per liter, multiply by 0.05551.
USE OF INSULIN AND BLOOD GLUCOSE VALUES
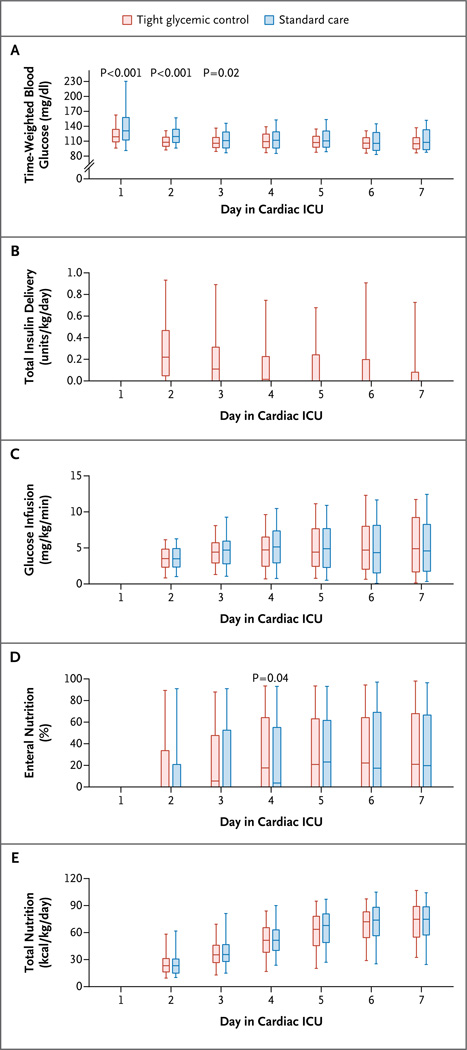
Ninety-seven percent of the children (954 of 980) had at least one glucose measurement above 110 mg per deciliter. Ninety-one percent of the children in the glycemic-control group (444 of 490) received insulin therapy per protocol, as compared with 2% of the children in the standard-care group (9 of 490), who received insulin at the discretion of the treating physician (P<0.001) (Table 2). The median amount of insulin administered during the first full day in the cardiac ICU was 0.2 units per kilogram in the glycemic-control group. There was adherence to 98% of protocol recommendations. In addition to continuous glucose monitoring, bedside nurses performed a median of 14.2 blood glucose measurements (interquartile range, 11.4 to 17.2) per 24-hour period in children in the glycemic-control group and 3.6 measurements (interquartile range, 3.0 to 4.4) in children in the standard-care group. The two groups had similar blood glucose values on admission (Table 2; and Fig. S1 in the Supplementary Appendix, available at NEJM.org); however, normoglycemia was achieved significantly earlier in the glycemic-control group than in the standard-care group (Table 2, and Fig. S1 in the Supplementary Appendix). In addition, children in the glycemic-control group had lower time-weighted glucose averages in each of the first 3 days in the cardiac ICU (Fig. 2A) and had lower time-weighted glucose averages overall (Table 2).
Table 2.
Blood Glucose Management and Insulin Therapy, According to Study Group.*
| Variable | Tight Glycemic Control (N = 490) |
Standard Care (N = 490) |
P Value† |
|---|---|---|---|
| Blood glucose at postoperative admission to the cardiac ICU | 0.71 | ||
| Median — mg/dl | 135 | 136 | |
| Interquartile range — mg/dl | 107–173 | 106–171 | |
| >110 mg/dl — no. (%) | 352 (72) | 356 (73) | 0.83 |
| Treated with insulin therapy — no. (%) | 444 (91) | 9 (2) | <0.001 |
| Duration of insulin therapy — days | |||
| Median | 2 | 0 | |
| Interquartile range | 2–4 | 0–0 | |
| Adherence to protocol recommendations — no./total no. (%)‡ | 27,080/27,736 (98) | NA | |
| Time to the target range — hr§ | <0.001 | ||
| Median | 6 | 16 | |
| Interquartile range | 4–10 | 7–26 | |
| Time in the target range — % | <0.001 | ||
| Median | 50 | 33 | |
| Interquartile range | 34–63 | 11–54 | |
| Time-weighted blood glucose average — mg/dl | <0.001 | ||
| Median | 112 | 121 | |
| Interquartile range | 104–120 | 109–136 | |
| Hypoglycemia — no. (%)¶ | |||
| Severe | 16 (3) | 5 (1) | 0.03 |
| Any | 93 (19) | 45 (9) | <0.001 |
| Hypokalemia — no. (%)‖ | 15 (3) | 19 (4) | 0.60 |
ICU denotes intensive care unit, and NA not applicable.
P values for the comparison between treatment groups were calculated with the use of stratified exact tests or stratified Wilcoxon rank-sum tests with adjustment for site, as appropriate.
Protocol recommendations were defined as individual dosing changes that were recommended by the insulin-dosing algorithm. Nonadherence was noted when a bedside nurse overrode a recommendation.
The time to the target range was defined as the interval between randomization and the first measured blood glucose level of 80 to 110 mg per deciliter (4.4 to 6.1 mmol per liter). The analysis included patients with a blood glucose level of more than 110 mg per deciliter at admission to the cardiac ICU.
Severe hypoglycemia was defined as a blood glucose level below 40 mg per deciliter (2.2 mmol per liter) and any hypoglycemia as a blood glucose level below 60 mg per deciliter (3.3 mmol per liter).
Hypokalemia was defined as a potassium level below 2.0 mmol per liter.
Figure 2. Glucose, Insulin, and Nutrition, According to Treatment Group.
Data in all the panels are for full 24-hour days during the period of critical illness. Panel A shows time-weighted blood glucose averages calculated from all blood glucose samples on the day of postoperative admission to the cardiac intensive care unit (ICU) (day 1) and the subsequent 6 days (7 a.m. to 6:59 a.m.). Panel B shows total daily insulin delivery. Panel C shows average daily glucose infusion rates. Panel D shows the daily percentage of nutrition delivered through the enteral route. Panel E shows total kilocalories of nutrition per kilogram of body weight per day. In each panel, the boxes represent the interquartile range (25th percentile to 75th percentile) and the horizontal lines the median; the whiskers extend to the 5th and 95th percentiles. To convert the values for glucose to millimoles per liter, multiply by 0.05551.
The rate of severe hypoglycemia (<40 mg per deciliter) was 3% in the glycemic-control group (16 of 490 patients) as compared with 1% in the standard-care group (5 of 490 patients) (P = 0.03), and the rate of total hypoglycemia (<60 mg per deciliter [3.3 mmol per liter]) was 19% versus 9% (P<0.001). No episode of hypoglycemia was associated with seizures, hemodynamic instability, arrhythmia, or any other identifiable symptoms. Rates of hypokalemia (potassium level, <2.0 mmol per liter) were similar in the two groups (3% in the glycemic-control group and 4% in the standard-care group, P = 0.60).
NUTRITIONAL MANAGEMENT
During the first 7 days of the study protocol, total nutritional intake and percentage of intake as enteral nutrition were similar in the two treatment groups (Fig. 2). During the period of critical illness, children in the glycemic-control group received a median of 41% (interquartile range, 19 to 65) of their total caloric intake enterally, and those in the standard-care group received 38% (interquartile range, 11 to 66) enterally (P = 0.24). Dextrose accounted for a median of 100% (interquartile range, 71 to 100) of total parenteral caloric intake in the glycemic-control group and for 100% (interquartile range, 72 to 100) in the standard-care group (P = 0.94).
OUTCOMES
In the intention-to-treat analysis, which included data from all 989 patients, the rate of health care–associated infections at 30 days after randomization was 8.9 per 1000 patient-days in the cardiac ICU in the glycemic-control group and 9.8 per 1000 patient-days in the standard-care group (P = 0.78). In the per-protocol analysis, which included data from 980 patients, the infection rate was 8.6 per 1000 patient-days in the cardiac ICU in the glycemic-control group and 9.9 per 1000 patient-days in the standard-care group (P = 0.67; relative risk of infection with tight glycemic control vs. standard care, 0.88; 95% confidence interval [CI], 0.48 to 1.59) (Table 3). There were no significant differences between the two groups with respect to any of the four types of infection that were tracked. Furthermore, tight glycemic control did not confer any benefit relative to standard care with regard to 30-day or in-hospital mortality, length of stay in the cardiac ICU, length of stay in the hospital, duration of mechanical ventilation, duration of vasoactive support, or other measures of organ failure (Table 3). Thirty-day and in-hospital mortality were 1% and 2%, respectively, across both treatment groups and in each group. Subgroup analyses did not reveal significant differences in outcomes for patients with a RACHS-1 category of 3 or higher (or not assignable) or a length of stay in the cardiac ICU of 3 days or more.
Table 3.
Study Outcomes and Adverse Events, According to Study Group.
| Variable | Tight Glycemic Control (N = 490) |
Standard Care (N = 490) |
P Value* |
|---|---|---|---|
| 30-day rate of health care–associated infections — no. of infections/1000 patient-days in the cardiac ICU† | 8.6 | 9.9 | 0.67 |
| Infections — no. of patients (%) | |||
| Any infections | 1.00 | ||
| Yes | 24 (5) | 24 (5) | |
| No | 466 (95) | 466 (95) | |
| No. of infections | 0.78 | ||
| 0 | 466 (95) | 466 (95) | |
| 1 | 24 (5) | 22 (4) | |
| 2 | 0 | 2 (<1) | |
| Type of infection — no. | |||
| Pneumonia | 3 | 3 | |
| Bloodstream | 3 | 4 | |
| Urinary tract | 2 | 6 | |
| Surgical site | 16 | 13 | |
| 30-Day mortality — no./total no. (%)‡ | 5/488 (1) | 6/484 (1) | 0.77 |
| In-hospital mortality — no./total no. (%) | 11/490 (2) | 11/489 (2) | 1.00 |
| Length of stay in the cardiac ICU — days§ | 0.24 | ||
| Median | 3 | 3 | |
| Interquartile range | 2–6 | 2–6 | |
| Length of stay in the hospital — days§ | 0.20 | ||
| Median | 8 | 7 | |
| Interquartile range | 5–15 | 5–13 | |
| Arterial catheter — days§ | 0.55 | ||
| Median | 2 | 2 | |
| Interquartile range | 1–5 | 1–5 | |
| Readmission to the hospital within 30 days — no./total no. (%) | 44/483 (9) | 34/478 (7) | 0.29 |
| Mechanical ventilation — days§ | 0.61 | ||
| Median | 3 | 2 | |
| Interquartile range | 2–5 | 1–5 | |
| Cardiac index on day 2 — liters/min/m2¶ | 0.61 | ||
| Median | 2.0 | 1.8 | |
| Interquartile range | 1.1–2.8 | 1.2–2.6 | |
| Cardiopulmonary resuscitation — no. (%) | 9 (2) | 10 (2) | 1.00 |
| Vasoactive support — days | 0.78 | ||
| Median | 2 | 2 | |
| Interquartile range | 0–5 | 0–5 | |
| Maximum inotrope score on day 1 in cardiac ICU‖ | 1.00 | ||
| Median | 3 | 3 | |
| Interquartile range | 0–8 | 0–8 | |
| Any tachyarrhythmia — no. (%) | 81 (17) | 87 (18) | 0.61 |
| Days of tachyarrhythmia/1000 patient-days in the cardiac ICU | 66 | 76 | 0.18 |
| Serum lactate 6 hr after admission to the cardiac ICU — mmol/liter | 0.45 | ||
| Median | 1.6 | 1.5 | |
| Interquartile range | 1.1–2.4 | 1.0–2.4 | |
| Dialysis-dependent renal failure — no. (%) | 5 (1) | 6 (1) | 0.77 |
| Time to first 12-hr negative fluid balance — hr | 0.71 | ||
| Median | 34.8 | 34.0 | |
| Interquartile range | 18.5–42.1 | 18.4–41.3 | |
| Seizures — no. (%) | 3 (<1) | 6 (1) | 0.34 |
| Red-cell transfusion — no. (%) | 270 (55) | 252 (51) | 0.27 |
P values for the comparison between treatment groups were calculated with the use of stratified exact tests or stratified Wilcoxon rank-sum tests with adjustment for site, as appropriate.
Infections include pneumonia, bloodstream, and urinary tract infections, which were tracked for up to 30 days in the cardiac ICU or until 48 hours after discharge from the cardiac ICU, and surgical site infections, which were tracked for 30 days after the index procedure. For patients who remained in the cardiac ICU for more than 30 days, the number of patient-days in the cardiac ICU was considered to be 30.
Eight patients were lost to follow-up between hospital discharge and day 30.
The duration of the stay in the cardiac ICU, the stay in the hospital, arterial catheterization, and mechanical ventilation were considered to be 30 days for patients with a duration of more than 30 days and for the 11 patients who died in the cardiac ICU by the 30th day.
The cardiac index on day 2 was measured in 191 patients at Boston Children’s Hospital.
The inotrope score quantifies the amount of cardiovascular support received by children after cardiac surgery, with higher scores indicating a greater requirement for pressor agents.28
On the basis of logistic regression with adjustment for site, factors significantly associated with 30-day infection were a RACHS-1 category of 3 or higher (or not assignable) (P = 0.002), an initial blood glucose level of more than 180 mg per deciliter (P = 0.02), a blood glucose level that was more than 180 mg per deciliter at any time during the period of critical illness (P = 0.006), a prolonged stay in the cardiac ICU (≥3 days, P<0.001), an age of 30 days or younger (P = 0.02), and post-operative glucocorticoid therapy (P = 0.03). After adjustment for a prolonged stay in the cardiac ICU (odds ratio for infection with a prolonged ICU stay as compared with an ICU stay that was not prolonged, 6.7; 95% CI, 2.9 to 15.2), no other factor, including treatment group, was significantly associated with 30-day infection.
DISCUSSION
This study showed that tight glycemic control, as compared with standard care in the cardiac ICU, did not change the rate of health care–associated infections, mortality, the length of stay in the cardiac ICU, or several organ-specific end points. Glucose control was achieved in the intervention group quickly and with low rates of severe hypoglycemia in our study as compared with previous studies.
The present trial assessed the benefits of tight glycemic control in infants and young children recovering from cardiac surgery, on the basis of published studies indicating that tight glycemic control improved important clinical outcomes in adult cardiac medical and surgical patients.4–7 We enrolled high-risk, relatively homogeneous, critically ill children in our study because this is the pediatric population that would be most likely to benefit from tight glycemic control. Mortality was an inadequate end point for this trial because in this patient population, deaths are largely attributable to underlying cardiac anatomy and the technical quality of surgical repair.3,29 Furthermore, low mortality at high-volume pediatric cardiac surgical centers would necessitate a prohibitively large sample to achieve adequate power with this end point. The rate of health care–associated infections was chosen as the primary outcome because of its relevance with respect to clinical outcomes and health care costs.30 Our infection rate proved to be similar to the rates in other cohorts.31,32 The biologic plausibility of reducing infection rates with tight glycemic control is supported by previous clinical trials and in vitro models.33–35
In contrast to previous trials involving adults, our study showed no benefit of tight glycemic control in critically ill children who had undergone cardiac surgery, though the reasons are unclear. Unlike the findings in adults, normoglycemia was achieved in virtually all the children in our standard-care group without insulin therapy in the first 48 hours after surgery. This is a limited window to produce a benefit of tight glycemic control as compared with standard care. Whether there are age-related differences in the biologic sequelae of hyperglycemia in pediatric and adult populations of cardiac surgical patients is unknown. One meta-analysis suggested that the high proportion of nutrition delivered parenterally as dextrose was an important positive predictor of a beneficial effect of tight glycemic control.36 However, despite extensive use of dextrose for parenteral nutrition in our study population, similar benefits were not observed.
Our results also differ from those of the one previous randomized trial of tight glycemic control in children, which showed reductions in mortality, length of stay in the ICU, and rate of infection in a mixed critical care population of medical and surgical patients.19 This study did not lead to widespread adoption of tight glycemic control in children, in part because of an unacceptably high rate of severe hypoglycemia resulting from the extremely low target glucose values specified by the study protocol. We chose normal, accepted glucose targets for our study, achieved with the use of a structured, explicit, and easily replicable insulin-dosing algorithm that incorporated data from continuous subcutaneous glucose monitoring. Though not directly comparable, the time-weighted glucose average in our study was similar to the average of all values measured in the intensive-insulin cohort in the previous study, in which the glucose target was 70 to 100 mg per deciliter (3.9 to 5.6 mmol per liter).19 As in the previous trial, the children in our study had a relatively high reliance on parenteral nutrition, and the majority of the children received all parenteral calories as dextrose at similar rates of infusion in the two trials. Marked differences between the two studies in the rate of infection (33% in the previous study vs. 5% in our study) and 30-day mortality (4% vs. 1%) and in the amount of insulin administered in the glycemic-control groups (median amount during the first full day in the ICU, approximately 1.5 units per kilogram vs. 0.2 units per kilogram). These differences suggest important dissimilarities between study populations and settings in these two trials.
The strengths of our study include high adherence to the study protocol and the fact that nearly all patients received the treatment to which they were randomly assigned. To minimize bias, the study investigators, intraoperative care teams, and adjudicators of the primary outcome were unaware of the treatment-group assignments until the study had been completed. Blood glucose management was relatively uniform in the intervention group, as guided by a detailed dosing algorithm, and very few patients in the standard-care group received insulin. The rate of severe hypoglycemia was the lowest reported in any prospective trial to date, at or below the background rate among critically ill children not enrolled in a trial.37–39 The key features of our trial design that were implemented specifically to minimize hypoglycemic episodes were an explicit insulin-dosing algorithm, continuous glucose monitoring, and the use of a blood-sampling device to eliminate dilution as a source of measurement error. Investigators in future trials might consider incorporating these components to maximize safety, reproducibility, and success.
Certain limitations of this trial must be considered. Bedside clinicians in the cardiac ICU were aware of the study-group assignments because of the requirement to closely monitor blood glucose concentrations during insulin infusion. It was not feasible to use a placebo in the standard-care group, owing to potential harm with excess fluid administration. In addition, the protocol did not specify glucose control or fluid administration in the standard-care cohort; instead, we monitored practice patterns, which were reported on a regular basis to the data and safety monitoring board, and did not detect any changes in terms of either an increase in insulin use or a reduction in dextrose administration. Bedside glucose meters, though helpful in maximizing timeliness and minimizing cost, are less accurate than blood gas analyzers or central laboratory devices40 and may have detracted from our ability to achieve ideal glycemic control.
In summary, our trial showed that tight glycemic control targeting a glucose level of 80 to 110 mg per deciliter did not change the rate of health care–associated infections, mortality, or length of stay in the cardiac ICU as compared with standard care. Postoperative pediatric patients who have undergone cardiopulmonary bypass surgery, although perhaps the most likely among critically ill children to benefit from tight glycemic control, are unique; thus, results from this study cannot be extrapolated to other pediatric critical care populations. Moreover, the study was conducted in two large pediatric cardiovascular programs, and the findings may not be generalizable to other centers, where infection and complication rates and mortality may differ.
Supplementary Material
Acknowledgments
Supported by grants from the National Heart, Lung, and Blood Institute, National Institutes of Health (R01HL088448, to Dr. Agus), the American Recovery and Reinvestment Act Supplement (R01HL088448-02S1, to Dr. Agus), and the Harvard Catalyst Clinical and Translational Research Center (National Center for Advancing Translational Sciences grant UL1 RR 05758).
Footnotes
The Safe Pediatric Euglycemia after Cardiac Surgery (SPECS) study investigators are listed in the Supplementary Appendix, available at NEJM.org.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
REFERENCES
- 1.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 2.Reller MD, Strickland MJ, Riehle-Colarusso T, Mahle WT, Correa A. Prevalence of congenital heart defects in metropolitan Atlanta, 1998–2005. J Pediatr. 2008;153:807–813. doi: 10.1016/j.jpeds.2008.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Brien SM, Clarke DR, Jacobs JP, et al. An empirically based tool for analyzing mortality associated with congenital heart surgery. J Thorac Cardiovasc Surg. 2009;138:1139–1153. doi: 10.1016/j.jtcvs.2009.03.071. [DOI] [PubMed] [Google Scholar]
- 4.Malmberg K, Rydén L, Efendic S, et al. Randomized trial of insulin-glucose infusion followed by subcutaneous insulin treatment in diabetic patients with acute myocardial infarction (DIGAMI study): effects on mortality at 1 year. J Am Coll Cardiol. 1995;26:57–65. doi: 10.1016/0735-1097(95)00126-k. [DOI] [PubMed] [Google Scholar]
- 5.Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 6.Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:1007–1021. doi: 10.1067/mtc.2003.181. [DOI] [PubMed] [Google Scholar]
- 7.van den Berghe G, Wouters P, Weekers F, et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359–1367. doi: 10.1056/NEJMoa011300. [DOI] [PubMed] [Google Scholar]
- 8.Arabi YM, Dabbagh OC, Tamim HM, et al. Intensive versus conventional insulin therapy: a randomized controlled trial in medical and surgical critically ill patients. Crit Care Med. 2008;36:3190–3197. doi: 10.1097/CCM.0b013e31818f21aa. [DOI] [PubMed] [Google Scholar]
- 9.De La Rosa Gdel C, Donado JH, Restrepo AH, et al. Strict glycaemic control in patients hospitalised in a mixed medical and surgical intensive care unit: a randomised clinical trial. Crit Care. 2008;12:R120. doi: 10.1186/cc7017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The NICE-SUGAR Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 11.Preiser JC, Devos P, Ruiz-Santana S, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the Glucontrol study. Intensive Care Med. 2009;35:1738–1748. doi: 10.1007/s00134-009-1585-2. [DOI] [PubMed] [Google Scholar]
- 12.Ballweg JA, Wernovsky G, Ittenbach RF, et al. Hyperglycemia after infant cardiac surgery does not adversely impact neurodevelopmental outcome. Ann Thorac Surg. 2007;84:2052–2058. doi: 10.1016/j.athoracsur.2007.06.099. [DOI] [PubMed] [Google Scholar]
- 13.Moga MA, Manlhiot C, Marwali EM, McCrindle BW, Van Arsdell GS, Schwartz SM. Hyperglycemia after pediatric cardiac surgery: impact of age and residual lesions. Crit Care Med. 2011;39:266–272. doi: 10.1097/CCM.0b013e3181fee88e. [DOI] [PubMed] [Google Scholar]
- 14.Polito A, Thiagarajan RR, Laussen PC, et al. Association between intraoperative and early postoperative glucose levels and adverse outcomes after complex congenital heart surgery. Circulation. 2008;118:2235–2242. doi: 10.1161/CIRCULATIONAHA.108.804286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ulate KP, Lima Falcao GC, Bielefeld MR, Morales JM, Rotta AT. Strict glycemic targets need not be so strict: a more permissive glycemic range for critically ill children. Pediatrics. 2008;122(4):e898–e904. doi: 10.1542/peds.2008-0871. [DOI] [PubMed] [Google Scholar]
- 16.DeCampli WM, Olsen MC, Munro HM, Felix DE. Perioperative hyperglycemia: effect on outcome after infant congenital heart surgery. Ann Thorac Surg. 2010;89:181–185. doi: 10.1016/j.athoracsur.2009.08.062. [DOI] [PubMed] [Google Scholar]
- 17.Rossano JW, Taylor MD, Smith EO, et al. Glycemic profile in infants who have undergone the arterial switch operation: hyperglycemia is not associated with adverse events. J Thorac Cardiovasc Surg. 2008;135:739–745. doi: 10.1016/j.jtcvs.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 18.Yates AR, Dyke PC, II, Taeed R, et al. Hyperglycemia is a marker for poor outcome in the postoperative pediatric cardiac patient. Pediatr Crit Care Med. 2006;7:351–355. doi: 10.1097/01.PCC.0000227755.96700.98. [DOI] [PubMed] [Google Scholar]
- 19.Vlasselaers D, Milants I, Desmet L, et al. Intensive insulin therapy for patients in paediatric intensive care: a prospective, randomised controlled study. Lancet. 2009;373:547–556. doi: 10.1016/S0140-6736(09)60044-1. [DOI] [PubMed] [Google Scholar]
- 20.Steil GM, Deiss D, Shih J, Buckingham B, Weinzimer S, Agus MS. Intensive care unit insulin delivery algorithms: why so many? How to choose? J Diabetes Sci Technol. 2009;3:125–140. doi: 10.1177/193229680900300114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wintergerst KA, Deiss D, Buckingham B, et al. Glucose control in pediatric intensive care unit patients using an insulin-glucose algorithm. Diabetes Technol Ther. 2007;9:211–222. doi: 10.1089/dia.2006.0031. [DOI] [PubMed] [Google Scholar]
- 22.Gaies MG, Langer M, Alexander J, et al. Design and rationale of Safe Pediatric Euglycemia after Cardiac Surgery (SPECS): a randomized controlled trial of tight glycemic control after pediatric cardiac surgery. Pediatr Crit Care Med. 2012 Jul 14; doi: 10.1097/PCC.0b013e31825b549a. (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Steil GM, Langer M, Jaeger K, Alexander J, Gaies M, Agus MS. Value of continuous glucose monitoring for minimizing severe hypoglycemia during tight glycemic control. Pediatr Crit Care Med. 2011;12:643–648. doi: 10.1097/PCC.0b013e31821926a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horan TC, Gaynes RP. Surveillance of nosocomial infections. In: Mayhall CG, editor. Hospital epidemiology and infection control. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 1659–1702. [Google Scholar]
- 25.Faustino EV, Hirshberg EL, Bogue CW. Hypoglycemia in critically ill children. J Diabetes Sci Technol. 2012;6:48–57. doi: 10.1177/193229681200600107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenkins KJ, Gauvreau K. Center-specific differences in mortality: preliminary analyses using the Risk Adjustment in Congenital Heart Surgery (RACHS-1) method. J Thorac Cardiovasc Surg. 2002;124:97–104. doi: 10.1067/mtc.2002.122311. [DOI] [PubMed] [Google Scholar]
- 27.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–556. [PubMed] [Google Scholar]
- 28.Wernovsky G, Wypij D, Jonas RA, et al. Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants: a comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation. 1995;92:2226–2235. doi: 10.1161/01.cir.92.8.2226. [DOI] [PubMed] [Google Scholar]
- 29.Shuhaiber J, Gauvreau K, Thiagarjan R, et al. Congenital heart surgeon’s technical proficiency affects neonatal hospital survival. J Thorac Cardiovasc Surg. 2012 Mar 14; doi: 10.1016/j.jtcvs.2012.02.007. (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 30.Hirshberg E, Lacroix J, Sward K, Willson D, Morris AH. Blood glucose control in critically ill adults and children: a survey on stated practice. Chest. 2008;133:1328–1335. doi: 10.1378/chest.07-2702. [DOI] [PubMed] [Google Scholar]
- 31.Edwards JR, Peterson KD, Mu Y, et al. National Healthcare Safety Network (NHSN) report: data summary for 2006 through 2008, issued December 2009. Am J Infect Control. 2009;37:783–805. doi: 10.1016/j.ajic.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Sarvikivi E, Lyytikäinen O, Nieminen H, Sairanen H, Saxén H. Nosocomial infections after pediatric cardiac surgery. Am J Infect Control. 2008;36:564–569. doi: 10.1016/j.ajic.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Dandona P, Chaudhuri A, Ghanim H, Mohanty P. Insulin as an anti-inflammatory and antiatherogenic modulator. J Am Coll Cardiol. 2009;53(Suppl):S14–S20. doi: 10.1016/j.jacc.2008.10.038. [DOI] [PubMed] [Google Scholar]
- 34.Furnary AP, Zerr KJ, Grunkemeier GL, Starr A. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67:352–362. doi: 10.1016/s0003-4975(99)00014-4. [DOI] [PubMed] [Google Scholar]
- 35.Gauglitz GG, Toliver-Kinsky TE, Williams FN, et al. Insulin increases resistance to burn wound infection-associated sepsis. Crit Care Med. 2010;38:202–208. doi: 10.1097/CCM.0b013e3181b43236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marik PE, Preiser JC. Toward understanding tight glycemic control in the ICU: a systematic review and metaanalysis. Chest. 2010;137:544–551. doi: 10.1378/chest.09-1737. [DOI] [PubMed] [Google Scholar]
- 37.Hirshberg E, Larsen G, Van Duker H. Alterations in glucose homeostasis in the pediatric intensive care unit: hyperglycemia and glucose variability are associated with increased mortality and morbidity. Pediatr Crit Care Med. 2008;9:361–366. doi: 10.1097/PCC.0b013e318172d401. [DOI] [PubMed] [Google Scholar]
- 38.Srinivasan V, Drott H, Hutchins L, Roth C, Helfaer M, Nadkarni V. Hypoglycemia in critically ill children has increased in association with the practice of glycemic control in the ICU. Pediatr Crit Care Med. 2006;7:516. abstract. [Google Scholar]
- 39.Srinivasan V, Spinella PC, Drott HR, Roth CL, Helfaer MA, Nadkarni V. Association of timing, duration, and intensity of hyperglycemia with intensive care unit mortality in critically ill children. Pediatr Crit Care Med. 2004;5:329–336. doi: 10.1097/01.pcc.0000128607.68261.7c. [DOI] [PubMed] [Google Scholar]
- 40.Scott MG, Bruns DE, Boyd JC, Sacks DB. Tight glucose control in the intensive care unit: are glucose meters up to the task? Clin Chem. 2009;55:18–20. doi: 10.1373/clinchem.2008.117291. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.