Abstract
Background. Direct comparisons of similar doses of a novel influenza virus antigen administered by the intradermal route and the intramuscular route have not been reported.
Methods. A total of 227 healthy adults aged 18–49 years were randomized to receive 2 doses 1 month apart of a subvirion inactivated influenza A virus subtype H5N1 (rgA/Vietnam/1203/2004) vaccine containing 38.7 μg of H5N1 hemagglutinin (HA), by the intramuscular route or by the intradermal route using the Mantoux technique. Clinical and serologic responses were assessed.
Results. Injection site reactions were more frequent in the intradermal group. Immune responses and the geometric mean titer of serum hemagglutination inhibition and neutralizing antibodies 1 month after receipt of the first dose were similar and low but were significantly higher after 2 doses of vaccine in both groups.
Conclusions. Intramuscular and intradermal delivery of vaccine were both well tolerated. Immune responses after 2 doses of this influenza A/H5N1 HA (38.7 μg) were low and not significantly different when given by the intradermal or intramuscular route. Evaluation of higher dosages, alternative intradermal delivery methods, and the addition of adjuvants will be needed to enhance the immunogenicity of inactivated influenza A/H5N1 vaccines by the intradermal route.
Clinical Trials Registration. NCT00439335.
Annual epidemics and periodic pandemics due to influenza A virus cause significant morbidity and mortality. The most severe recorded pandemic, the influenza A virus subtype H1N1 pandemic of 1918–1919, claimed 50–100 million lives worldwide. Vaccination is the primary method for the prevention and control of influenza. Since 1997, human infections caused by H5, H7, H9, and, more recently, 2009 H1N1 influenza A viruses have raised concerns and fueled efforts to develop safe and immunogenic vaccines and vaccine strategies that can provide an ample supply for the world's population in a timely fashion. Influenza A/H5N1 continues to cause outbreaks in poultry and sporadic infections in humans. Among 15 countries worldwide, >500 cases have been reported and confirmed, with a case-fatality rate of approximately 60% [1]. Prior studies demonstrated that 2 doses containing 90 μg of H5 hemagglutinin (HA) of a subvirion inactivated vaccine delivered intramuscularly were required to elicit immune responses in approximately 50% of people [2]. Alternative approaches, including cell culture–derived vaccines, adjuvanted vaccines, and whole-virus vaccines, continue to be investigated, with the goal of identifying more-immunogenic regimens that use lower doses of antigen to help stretch the vaccine supply [3–12].
Intradermal immunization is a potential dosage-sparing approach that is being explored for control of seasonal and pandemic influenza [13–21]. Vaccination via the intradermal route is based on the principle that the skin is rich in efficient antigen-presenting cells (ie, dendritic cells) and in blood and lymphatic vessels for circulation of immune cells. Studies of vaccines for such diseases such as hepatitis B virus infection and rabies have demonstrated that intradermal delivery using the Mantoux technique can be an effective alternative route for vaccination that uses smaller amounts of antigen [22–24]. Although lower doses of vaccine can stimulate adequate immune responses, these studies did not directly compare the same amount of antigen given by the intramuscular or intradermal routes.
Intradermal immunization is being considered as a potential antigen-sparing approach for prevention of influenza A/H5N1 infections. Our pilot study demonstrated that 3 μg and 9 μg of a monovalent, inactivated subvirion influenza A/H5N1 vaccine administered by the intradermal route using the Mantoux technique was well tolerated but poorly immunogenic as compared to 15-μg and 30-μg formulations given by the intramuscular route [15]. In studies evaluating seasonal influenza vaccines that had been published by the time this trial was started, up to 18 μg of influenza virus antigen administered by the intradermal route was reported to be well tolerated [14, 17]. The goal of this study was to directly compare the safety, reactogenicity, and immunogenicity of a higher dosage (38.7 μg HA) of a monovalent, inactivated subvirion influenza A/H5N1 vaccine administered by the intradermal or intramuscular route in healthy adults. Selection of the 38.7-μg dosage was based on the available formulation (approximately 387 μg HA/mL) of the vaccine and a volume limitation of 0.1 mL delivered in a single intradermal injection by the Mantoux technique.
MATERIALS AND METHODS
Subjects and Study Design
We conducted a single-center, phase I/II, randomized, double-blinded, placebo-controlled, clinical trial to assess the safety and immunogenicity of intradermal and intramuscular immunization with a similar dosage (38.7 μg) of an inactivated subvirion influenza A/H5N1 vaccine. Study subjects were healthy men and nonpregnant women aged 18–49 years. Subjects were excluded if they had received an influenza A/H5N1 vaccine previously; had a contraindication to receiving an influenza vaccine; had an acute illness and/or a temperature of >38.0°C within 1 week of vaccination; were immunosuppressed; were actively infected with human immunodeficiency virus, hepatitis B virus, or hepatitis C virus; or had a chronic medical condition that might interfere with the evaluation of immune responses (including diseases such as chronic liver disease, significant renal disease, and diabetes mellitus). Additional exclusion criteria also included a history of Guillain-Barré syndrome, alcohol or drug abuse in the prior 5 years, or a major psychiatric disorder. Subjects were also not allowed to receive another investigational product or to participate in another interventional trial while enrolled in this study.
Ethical Approval of the Study Protocol
The study was conducted in accordance with the Declaration of Helsinki, The Belmont Report ,and Good Clinical Practice regulations. The study was approved by the Baylor College of Medicine Institutional Review Board in Houston, Texas, prior to any subjects being consented. The study is registered on ClinicalTrials.gov (identifier NCT00439335).
Vaccine
The study product was an inactivated monovalent subvirion influenza A/H5N1 vaccine (rgA.Vietnam/1203/2004; batch 04-067; lot UD08854 [sanofi pasteur, Swiftwater, PA]) that was produced using methods similar to those used to manufacture Fluzone, a licensed seasonal trivalent influenza vaccine. The vaccine used in this study was provided in multidose vials containing 387 μg/mL of influenza A/H5N1 HA (H5/HA), as determined by single radial immunodiffusion. The placebo used was sodium chloride 0.9% injection, USP (lot 33-248-DK).
The intradermal injection was performed using the Mantoux technique (needle and syringe) by an experienced vaccinator, and bleb formation was confirmed. The intramuscular injection was performed using standard techniques. All injections were given in the deltoid region.
Study Procedures
After signing the informed consent document, subjects were screened for eligibility by review of inclusion/exclusion criteria, medical history, medication list, a targeted physical examination if indicated by medical history, and pregnancy testing (for females of childbearing potential). Inclusion/exclusion criteria were reviewed prior to randomization. Urine pregnancy tests were performed on the day of each vaccination for female subjects, as indicated.
Enrolled subjects were randomized at a ratio of 1:1 to one of two groups, using the Internet Data Entry System (AdvantageEDC, EMMES). Subjects in group 1 received 0.1 mL H5 HA by the intradermal route in one arm and 0.1 mL of saline placebo by the intramuscular route in the opposite arm. In group 2, subjects received 0.1 mL H5 HA by the intramuscular route in one arm and 0.1 mL of saline placebo by the intradermal route in the opposite arm.
After randomization (on day 0), all subjects received the first dose of study vaccine and placebo, as indicated. The subject and study staff assessing safety parameters and adverse events were unaware of the vaccine group assignments. The study vaccine and placebo were administered by unblinded vaccinators. To prevent unblinding of the subject, the syringes were labeled “left” or “right” and “intramuscular” or “intradermal” to designate the arm and route, respectively. In addition, the subject was asked to look in the opposite direction when the product was administered in each arm. Four weeks after the initial vaccination (on day 28), eligibility was reassessed, and eligible subjects received a second dose of study vaccine and placebo by the same route as the initial injection. Subjects remained in the clinic for at least 20 minutes after each injection and were seen in the clinic 2 and 7 days later for safety evaluations. All subjects recorded their oral temperature, the presence of any injection site or systemic symptoms, and the use of any medication(s) on a memory aid daily for 1 week after each vaccination.
Solicited and unsolicited adverse events were graded on a scale of 0–3, where 0 indicated absence of symptoms, 1 indicated mild symptoms that did not interfere with daily activities, 2 indicated moderate symptoms that had some interference with daily activities, and 3 indicated severe symptoms that were incapacitating. Solicited adverse events included injection site symptoms (pain, tenderness, induration, itching, erythema, and pigmentation) and systemic symptoms (feverishness, headache, malaise, nausea, or myalgia). Fever was an oral temperature of ≥37.8°C (≥100°F). The diameter of injection site erythema, induration, and pigmentation were graded as follows: grade 1, mild (diameter, <20 mm); grade 2, moderate (20–50 mm); and grade 3, severe (>50 mm). Unsolicited adverse events that occurred during days 0–56 were recorded. Serious adverse events (SAEs) were recorded throughout the study period (from days 0 to 208, or 6 months after the second dose of vaccine) and were defined as any event that was considered life threatening and resulted in significant or persistent disability, hospitalization, or death.
Serological Testing
Blood samples for hemagglutination inhibition (HAI) and microneutralization (Neut) antibody assays were collected prior to each vaccination (on days 0 and 28) and 4 weeks and 6 months after the second vaccination (on days 56 and 208, respectively). An HAI or Neut antibody titer of ≥40 was considered a putative protective titer. Samples that were negative (titer, <10) were assigned a titer of 5. Seroresponse was defined as increases relative to baseline in H5-specific HAI and/or Neut antibody titer of ≥4-fold to at least 40 after vaccination. HAI and Neut assays were performed at Southern Research Institute, Birmingham, Alabama, as described previously [25, 26].
Statistical Considerations
This study was designed to explore the immunogenicity of a monovalent inactivated influenza A/H5N1 vaccine administered in a similar dose by the intradermal or intramuscular routes. Our hypothesis was that the influenza A/H5N1 vaccine given by intradermal injection has a slightly greater but acceptable injection site reactogenicity, less systemic reactogenicity, and superior immunogenicity than the same dosage given by intramuscular injection to healthy subjects aged 18–49 years. The sample size of 113 per group (intradermal and intramuscular) was selected to detect a 20% absolute increase in seroresponse frequency in the intradermal vaccine group, assuming a 30% seroresponse frequency in the intramuscular vaccine group.
Frequencies of injection site and systemic reactions after each vaccination were based on the most severe response reported. Comparisons between vaccine groups were performed using the Fisher exact test, in which reactogenicity was dichotomized as either none to mild or moderate to severe. Logistic regression models that controlled for age and sex were used to evaluate differences between vaccine groups for injection site/systemic reactogenicity.
Immune responses were summarized in terms of H5-specific HAI and Neut antibody titers transformed to a logarithmic scale for analyses. Analyses included the distribution of titers (emphasizing the proportion of subjects achieving titers that were ≥40 and a 4-fold rise over baseline) at 28 days after each vaccination. The Fisher exact test and analysis of variance were used to test the difference between groups for dichotomous (titer ≥40, 4-fold rise) and continuous (geometric mean titer [GMT]) measures, respectively. Logistic regression and linear regression models that controlled for age and sex were developed for 4-fold responses and GMT, respectively, to examine the effect of route of administration.
RESULTS
Subjects
Two hundred fifty-nine persons were screened, and 227 were enrolled in the study (113 in the intradermal vaccine group and 114 in the intramuscular vaccine group) between 14 March 2007 and 20 June 2007. One subject in the intramuscular group did not receive the second dose of vaccine because of an exacerbation of back pain, which was a preexisting medical condition. This subject was excluded from the immunogenicity analyses following the second dose (on days 56 and 208) because of incomplete receipt of the vaccination series. Three subjects (2 in the intradermal group and 1 in the intramuscular group) did not complete the day 208 blood draw. Baseline demographic characteristics of the 227 enrolled subjects are shown in Table 1. The mean age of the study subjects was 30.1 years (range, 18–49 years), and the median age was 27.9 years. The majority were female (59%), non-Hispanic (80%), and white (63%). Age, sex, ethnicity, and race were similar in the intradermal and intramuscular groups.
Table 1.
Demographic Characteristics of Enrolled Subjects
| Characteristic | Overall (n = 227) | Intradermal H5 HA Group (n = 113) | Intramuscular H5 HA Group (n = 114) |
|---|---|---|---|
| Age, years | |||
| Mean (SD) | 30.1 (7.7) | 29.7 (7.4) | 30.5 (8.0) |
| Median (range) | 27.9 (18–49) | 27.5 (18–49) | 28.0 (20–49) |
| Sex | |||
| Male | 93 (41) | 39 (35) | 54 (47) |
| Female | 134 (59) | 74 (65) | 60 (53) |
| Ethnicity | |||
| Non-Hispanic | 182 (80) | 90 (80) | 92 (81) |
| Hispanic | 45 (20) | 23 (20) | 22 (19) |
| Race | |||
| Asian | 36 (16) | 19 (17) | 17 (15) |
| Black/African | 28 (12) | 13 (12) | 15 (13) |
| American | |||
| White | 144 (63) | 70 (62) | 74 (65) |
| Multiracial | 14 (6) | 8 (7) | 6 (5) |
Data are no. (%) of subjects, unless otherwise indicated.
Safety and Reactogenicity
The vaccine was well tolerated when delivered by each route. Four SAEs were reported during the study period; none was considered to be associated with vaccine. The SAEs were bipolar disorder, acute appendicitis, hydronephrosis, and adult-onset diabetes mellitus. None of the SAEs resulted in death.
Injection Site Reactogenicity
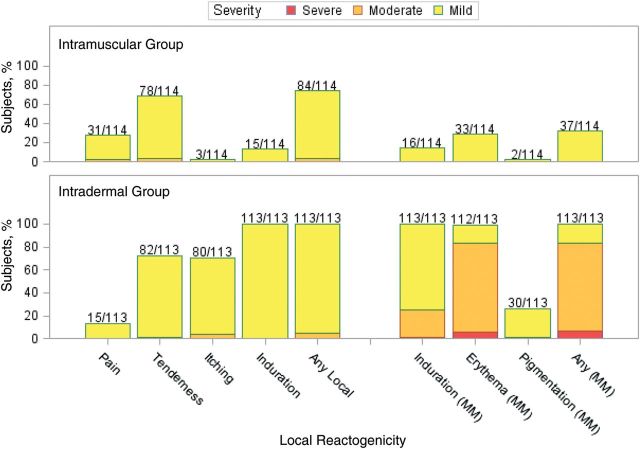
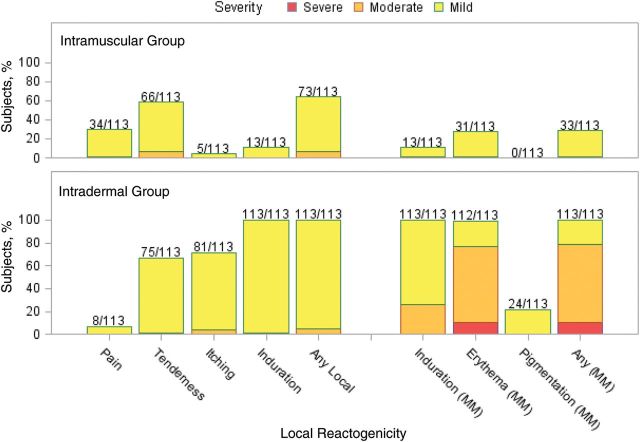
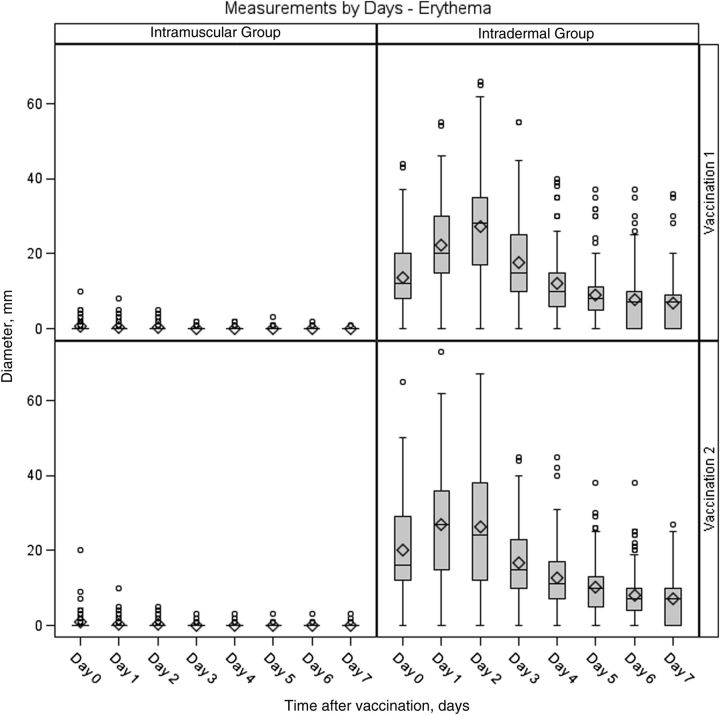
Injection site reactions were common after each vaccination. All subjects in the intradermal group reported reactions after both vaccinations, and 73% and 64% of subjects in the intramuscular group reported reactions after the first and second vaccinations, respectively (Figures 1 and 2). Most of these were mild (84% and 77% of subjects across both groups after the first and second vaccinations, respectively), peaked on days 0–1, and resolved by day 7 after vaccination. The intradermal group had a higher frequency of pain (P = .0026 for dose 1 and P < .0001 for dose 2) and itching (P < .0001 for both doses) than the intramuscular group after both vaccinations and a higher frequency of pigmentation following the vaccination (P < .0001 for dose 1). The pigmentation typically consisted of small, hyperpigmented macules at the injection site that were transient and resolved. The median time to resolution was about 89 days. The intradermal group also had a higher frequency of erythema at the injection site, compared with the intramuscular group, after both vaccinations (P < .0001 for both doses). The erythema diameter in the intradermal group ranged from 5 mm to 67 mm for the majority of subjects (1 participant had an erythema with a diameter of 73 mm on day 1). The size of the erythema typically peaked on days 1 or 2 after vaccination and resolved by day 7 (Figure 3).
Figure 1.
Maximum injection site reactogenicity among 114 subjects in the intramuscular group and 113 subjects in the intradermal group after dose 1. Numbers above the bars represent the proportion of subjects who reported the local reaction during the 7 days after dose 1. Data represent the maximum severity of local site reactogenicity during the 7 days. For injection site pain (discomfort of area at rest), tenderness (discomfort with palpation or movement of the arm), itching, and induration, yellow denotes mild (did not interfere with activity), orange denotes moderate (some interference with daily activities), and red denotes severe (incapacitating). For local induration, erythema, and pigmentation (diameter), yellow denotes mild (<20 mm), orange denotes moderate (20–50 mm), and red denotes severe (>50 mm).
Figure 2.
Maximum injection site reactogenicity among 113 subjects in the intramuscular group and 113 subjects in the intradermal group after dose 2. Numbers above the bars represent the proportion of subjects who reported the local reaction during the 7 days after dose 2. Data represent the maximum severity of local site reactogenicity during the 7 days. For local pain, tenderness, itching, and induration, yellow denotes mild (did not interfere with activity), orange denotes moderate (some interference with daily activities), and red denotes severe (incapacitating). For local induration, erythema, and pigmentation (diameter), yellow denotes mild (<20 mm), orange denotes moderate (20–50 mm), and red denotes severe (>50 mm).
Figure 3.
Maximum diameter of erythema at the injection site among subjects in the intradermal and intramuscular groups 0–7 days after doses 1 and 2. In the box plots, the center horizontal line is drawn at the 50th percentile (median); the bottom and top edges of the box are located at the 25th and 75th percentiles, respectively; the mean value is denoted by the diamond; the vertical lines extend from the box to the 5th and 95th percentiles; and extreme values are marked with a plot symbol.
Systemic Reactogenicity
Similar frequencies of systemic reactions were observed following each dose of vaccine, and there were no significant differences in the frequencies of systemic reactions observed between the intradermal and intramuscular groups. Among both groups, systemic reactions were reported by 54% of subjects after dose 1 and by 30% of subjects after dose 2; most of these were mild. One subject in the intradermal group experienced a severe headache after dose 2. The headache resolved within 24 hours and was not associated with vaccine. The most frequently reported systemic symptoms among both groups were headache (34% after dose 1 and 21% after dose 2) and malaise (29% after dose 1 and 17% after dose 2). Most symptoms were reported within 4 days of vaccination and resolved within 7 days.
Immunogenicity
Serum antibody levels before and after each dose of vaccine are shown in Table 2. Preimmunization levels of HAI and Neut antibodies were low and similar in the intradermal and intramuscular groups (GMT, 5.0–6.1). The highest serum antibody levels were observed 1 month after the second vaccine dose. The GMT of HAI antibody 1 month after the second intradermal dose (25.2; 95% confidence interval [CI], 18.3–34.5) was significantly higher than that observed after the first dose (13.1; 95% CI, 9.9–17.3); however, titers were similar to those achieved after 2 doses by the intramuscular route (18.1; 95% CI, 13.6–24.1). By 6 months after dose 2, the HAI GMTs decreased significantly and remained similar in the 2 groups. For Neut GMT responses, antibody levels were significantly higher for both groups after the second vaccine dose as compared to levels after the first dose and at the 6-month time point.
Table 2.
Serum Hemagglutination Inhibition (HAI) and Neutralizing (Neut) Antibody Responses Before and After Each Dose of Vaccine
| Day 0 |
Day 28 |
Day 56 |
Day 280 |
|||||
|---|---|---|---|---|---|---|---|---|
| Vaccine Group | No. Tested | Before Dose 1 | No. Tested | 28 Days After Dose 1 | No. Tested | 28 Days After Dose 2 | No. Tested | 6 Months After Dose 2 |
| HAI | ||||||||
| GMT (95% CI) | ||||||||
| Intramuscular H5 HA | 114 | 5.2 (5.0–5.4) | 114 | 11.9 (9.1–15.4) | 113 | 18.1 (13.6–24.1) | 112 | 8.9 (7.3–10.9) |
| Intradermal H5 HA | 113 | 5.4 (5.0–5.9) | 113 | 13.1 (9.9–17.3) | 113 | 25.2 (18.3–34.5) | 111 | 10.0 (8.1–12.4) |
| Percentage achieving titer ≥40 (95% CI) | ||||||||
| Intramuscular H5 HA | 114 | 0.9 (0–4.8) | 114 | 25.4 (17.7–34.4) | 113 | 35.4 (26.6–45.0) | 112 | 14.3 (8.4–22.2) |
| Intradermal H5 HA | 113 | 1.8 (.2–6.2) | 113 | 23.0 (15.6–31.9) | 113 | 42.5 (33.2–52.1) | 111 | 18.0 (11.4–26.4) |
| Percentage achieving ≥4-fold rise (95% CI) | ||||||||
| Intramuscular H5 HA | … | NA | 114 | 25.4 (17.7–34.4) | 113 | 35.4 (26.6–45.0) | 112 | 14.3 (8.4–22.2) |
| Intradermal H5 HA | … | NA | 113 | 22.1 (14.9–30.9) | 113 | 41.6 (32.4–51.2) | 111 | 15.3 (9.2–23.4) |
| Neut | ||||||||
| GMTs (95% CI) | ||||||||
| Intramuscular H5 HA | 114 | 5.4 (5.1–5.8) | 114 | 13.3 (10.5–16.7) | 113 | 33.1 (26.5–41.3) | 112 | 11.0 (9.3–13.1) |
| Intradermal H5 HA | 113 | 5.6 (5.1–6.1) | 113 | 14.3 (11.4–18.0) | 113 | 42.7 (34.3–53.0) | 111 | 13.3 (11.1–16.0) |
| Percentage achieving titer ≥40 (95% CI) | ||||||||
| Intramuscular H5 HA | 114 | 0.9 (0–4.8) | 114 | 21.9 (14.7–30.6) | 113 | 51.3 (41.7–60.8) | 112 | 12.5 (7.0–20.1) |
| Intradermal H5 HA | 113 | 1.8 (.2–6.2) | 113 | 23.9 (16.4–32.8) | 113 | 60.2 (50.5–69.3) | 111 | 17.1 (10.6–25.4) |
| Percentage achieving ≥4-fold rise (95% CI) | ||||||||
| Intramuscular H5 HA | … | NA | 114 | 21.1 (14.0–29.7) | 113 | 51.3 (41.7–60.8) | 112 | 12.5 (7.0–20.1) |
| Intradermal H5 HA | … | NA | 113 | 22.1 (14.9–30.9) | 113 | 56.6 (47.0–65.9) | 111 | 15.3 (9.2–23.4) |
Abbreviations: CI, confidence interval; GMT, geometric mean titer; HA, hemagglutinin; NA, not applicable.
The proportion of subjects achieving a serum antibody level of 40 and the proportion achieving an increase of ≥4-fold were significantly higher after the second dose as compared to after the first dose for the intradermal group, as measured by the HAI and Neut assays. The proportions by both measures were highest after the second dose (on day 56), as measured by the Neut assay. Logistic regression models were built on the 4-fold rise responses for the HAI and Neut assays and for each vaccination. Sex and age were included in the models. No clear effect of route of delivery was found on the 4-fold rise responses in any of these models.
Overall, Neut antibody response rates were higher than the HAI response rates at most time points. At 6 months after the second dose of vaccine, GMTs and seroprotection rates had decreased in both groups.
DISCUSSION
We evaluated the safety and immunogenicity of the same dosage (38.7 μg) of a subvirion influenza A/H5N1 vaccine in young adults that was administered by the standard intramuscular route or by the intradermal route using the Mantoux technique. Overall, the vaccine was well tolerated regardless of route of administration. Intradermal vaccination was associated with higher frequencies of transient injection site redness and pigmentation, compared with intramuscular administration. Systemic reactogenicity was similar between the groups. No vaccine-associated SAEs were observed, and no subjects discontinued vaccinations because of vaccine intolerability.
Prior to vaccination, all but 3 healthy adults between the ages of 18–49 years in the study were H5N1 seronegative, which is comparable to data from prior H5N1 vaccine studies [4, 8, 9]. Among all subjects, a 38.7-μg dosage of a subvirion inactivated influenza A/H5N1 vaccine administered by the intradermal or the intramuscular route yielded GMTs, seroprotection rates, and seroresponse rates that were low but comparable between both the intradermal and intramuscular groups. The immune responses were significantly higher after 2 doses of vaccine, regardless of route of administration. The responses after 2 doses did not meet the primary immunogenicity end points outlined in the guidance for licensure for pandemic influenza vaccines provided by the US Food and Drug Administration (end point for individuals <65 years of age: HAI antibody titer ≥1:40 in ≥70% of subjects) [27].
There remains a need for timely delivery of an adequate supply of immunogenic influenza vaccines for the global population. Development of ideal vaccine strategies remains challenging. Along with the development of various types of vaccines and manufacturing methods, dosage-sparing strategies will help stretch vaccine supply for pandemic and seasonal influenza. The intradermal route delivers the antigen directly into the skin, which is rich in antigen-presenting dendritic cells. In the majority of the previous intradermal studies, a standard intramuscular or subcutaneous dosage was compared to a reduced intradermal dosage of influenza vaccines in a population that was primed to the vaccine subtype (seasonal influenza vaccines). Findings from many of the earlier studies are difficult to generalize because of differences in study populations, dosages, and study design. More recently, a seasonal influenza vaccine administered by the intradermal route using a microinjection system was shown to elicit superior immune responses among healthy subjects aged ≥60 years when compared with a similar dose of vaccine given by the intramuscular route [28]. In another study, a lower dose (9 μg) of a seasonal influenza vaccine given intradermally using the same microinjection system elicited similar responses when compared with those observed after a 15-μg dose given intramuscular among healthy subjects aged 18–64 years [29]. The use of a microinjection device may help to control the location and the amount of vaccine delivered, thus resulting in consistent results.
Intradermal delivery of seasonal influenza vaccines has been studied and shown to be noninferior to intramuscular delivery among primed individuals, but limited studies have evaluated the intradermal route for novel strains of influenza in an unprimed population. Our study is among a few reported that has delivered a novel influenza virus antigen by the intradermal route to an unprimed population [15, 19]. McCarroll et al evaluated 6 different immunization regimens involving the novel Asian Flu vaccine in hospital employees in the 1950s. Study arms consisted of direct comparisons of similar dosages of vaccine (2 doses of 0.1 or 0.2 mL given 7 days apart, either subcutaneously or intradermally). The responses were similar between the subcutaneous and intradermal groups, regardless of amount (0.1 or 0.2 mL), and 2 doses of vaccine were superior to a single dose of vaccine, regardless of route [19].
Our study has some limitations. First, the sample size was based on detecting a 20% absolute increase in seroresponse frequency in the intradermal vaccine group, assuming a 30% seroresponse frequency in the intramuscular vaccine group. The parameters set for the sample size calculation were based on clinically advantageous immunogenicity goals of the intradermal route over the intramuscular route. To determine whether the intradermal route has an advantage over the intramuscular route, a larger sample size would be needed to detect smaller differences between the groups. Second, the amount administered by the intradermal route was limited by the available formulation and the volume (0.1 mL) that could be administered in a single intradermal injection; however, increasing the dosage would compromise the potential for intradermal delivery to be a dosage-sparing strategy. Third, the method of delivery for the intradermal administration involved the Mantoux technique. Although, in our study a single, very experienced vaccinator conducted all the vaccinations and bleb formation was confirmed, use of a microneedle injection system may have yielded different results.
In conclusion, our study is the first to directly compare intradermal and intramuscular delivery of the same dosage of a candidate influenza A/H5N1 pandemic subvirion vaccine. The vaccine was well tolerated regardless of route. No evidence of enhanced immunogenicity was observed when administering 38.7 μg of vaccine via the intradermal route by the Mantoux technique, compared with delivery via the traditional intramuscular route. The vaccine administered in this study was A/H5N1, which, for reasons that are not completely understood, has required higher dosages when delivered by the intramuscular route (2 doses of 90 μg), compared with other novel influenza virus HA antigens. This particular antigen may be poorly immunogenic as compared to other novel antigens. To improve vaccine strategies and possible dosage-sparing strategies, further investigations of intradermal vaccination with higher concentrations of this subvirion H5 HA influenza vaccine would not be ideal. Future investigations of intradermal vaccination with this novel influenza virus antigen (H5) should include adjuvants and a lower antigen content and comparison of intradermal administration via an intradermal delivery system. In addition, future investigations may include evaluation of other novel HA antigens, such as H7 or H9, which may not be as poorly immunogenic as the H5 antigen.
Notes
Acknowledgments. We thank all study participants, for their dedication, and the following persons, for their contributions: Thomas R. Cate, MD, Robert Couch, MD, Coni Cheesman, PA, Diane Nino, and the staff of the Baylor College of Medicine Vaccine Research Center; our colleagues at EMMES; the staff at PPD and the Southern Research Institute; and our colleagues at the Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health.
Disclaimer. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US government.
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (Public Health Service Contract NO1–AI–300390.
Potential conflicts of interest. H. M. E. has received research support from Protein Sciences and GlaxoSmithKline for unrelated research projects. W. A. K. has received research support from Novartis Vaccines and Diagnostics. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.World Health Organization. Cumulative number of confirmed human cases of avian influenza A/(H5N1) reported to WHO. 2008 http://wwwwhoint/csr/disease/avian_influenza/country/cases_table_2008_01_15/en/indexhtml . accessed on January 26, 2012. [Google Scholar]
- 2.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;354:1343–51. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 3.Crowe BA, Bruhl P, Gerencer M, et al. Evaluation of the cellular immune responses induced by a non-adjuvanted inactivated whole virus A/H5N1/VN/1203 pandemic influenza vaccine in humans. Vaccine. 2010;29:166–73. doi: 10.1016/j.vaccine.2010.10.065. [DOI] [PubMed] [Google Scholar]
- 4.Keitel WA, Campbell JD, Treanor JJ, et al. Safety and immunogenicity of an inactivated influenza A/H5N1 vaccine given with or without aluminum hydroxide to healthy adults: results of a phase I-II randomized clinical trial. J Infect Dis. 2008;198:1309–16. doi: 10.1086/592172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langley JM, Frenette L, Ferguson L, et al. Safety and cross-reactive immunogenicity of candidate AS03-adjuvanted prepandemic H5N1 influenza vaccines: a randomized controlled phase 1/2 trial in adults. J Infect Dis. 2010;201:1644–53. doi: 10.1086/652701. [DOI] [PubMed] [Google Scholar]
- 6.Keitel W, Groth N, Lattanzi M, et al. Dose ranging of adjuvant and antigen in a cell culture H5N1 influenza vaccine: safety and immunogenicity of a phase 1/2 clinical trial. Vaccine. 2010;28:840–8. doi: 10.1016/j.vaccine.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Lambert LC, Fauci AS. Influenza vaccines for the future. N Engl J Med. 2010;363:2036–44. doi: 10.1056/NEJMra1002842. [DOI] [PubMed] [Google Scholar]
- 8.Keitel WA, Dekker CL, Mink C, et al. Safety and immunogenicity of inactivated, Vero cell culture-derived whole virus influenza A/H5N1 vaccine given alone or with aluminum hydroxide adjuvant in healthy adults. Vaccine. 2009;27:6642–8. doi: 10.1016/j.vaccine.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brady RC, Treanor JJ, Atmar RL, et al. Safety and immunogenicity of a subvirion inactivated influenza A/H5N1 vaccine with or without aluminum hydroxide among healthy elderly adults. Vaccine. 2009;27:5091–5. doi: 10.1016/j.vaccine.2009.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El Sahly HM, Keitel WA. Pandemic H5N1 influenza vaccine development: an update. Expert Rev Vaccines. 2008;7:241–7. doi: 10.1586/14760584.7.2.241. [DOI] [PubMed] [Google Scholar]
- 11.Keitel WA, Atmar RL. Preparing for a possible pandemic: influenza A/H5N1 vaccine development. Curr Opin Pharmacol. 2007;7:484–90. doi: 10.1016/j.coph.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Lin J, Zhang J, Dong X, et al. Safety and immunogenicity of an inactivated adjuvanted whole-virion influenza A (H5N1) vaccine: a phase I randomised controlled trial. Lancet. 2006;368:991–7. doi: 10.1016/S0140-6736(06)69294-5. [DOI] [PubMed] [Google Scholar]
- 13.Nicholson KG, Colegate AE, Podda A, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet. 2001;357:1937–43. doi: 10.1016/S0140-6736(00)05066-2. [DOI] [PubMed] [Google Scholar]
- 14.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med. 2004;351:2295–301. doi: 10.1056/NEJMoa043540. [DOI] [PubMed] [Google Scholar]
- 15.Patel SM, Atmar RL, El Sahly HM, Cate TR, Keitel WA. A phase I evaluation of inactivated influenza A/H5N1 vaccine administered by the intradermal or the intramuscular route. Vaccine. 2010;28:3025–9. doi: 10.1016/j.vaccine.2009.10.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnou R, Icardi G, De Decker M, et al. Intradermal influenza vaccine for older adults: a randomized controlled multicenter phase III study. Vaccine. 2009;27:7304–12. doi: 10.1016/j.vaccine.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 17.Belshe RB, Newman FK, Wilkins K, et al. Comparative immunogenicity of trivalent influenza vaccine administered by intradermal or intramuscular route in healthy adults. Vaccine. 2007;25:6755–63. doi: 10.1016/j.vaccine.2007.06.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Halperin W, Weiss WI, Altman R, et al. A comparison of the intradermal and subcutaneous routes of influenza vaccination with A/New Jersey/76 (swine flu) and A/Victoria/75: report of a study and review of the literature. Am J Public Health. 1979;69:1247–51. doi: 10.2105/ajph.69.12.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCarroll J, Kilbourne E. Immunization with Asian-strain influenza vaccine: equivalence of the subcutaneous and intradermal routes. N Engl J Med. 1958;259:618–21. doi: 10.1056/NEJM195809252591304. [DOI] [PubMed] [Google Scholar]
- 20.Van Gelder DW, Greenspan FS, Dufresne NE. Influenza vaccination: comparison of intracutaneous and subcutaneous methods. U S Nav Med Bull. 1947;47:197–206. [PubMed] [Google Scholar]
- 21.Weller TH, Cheever FS, Enders JF. Immunologic reactions following the intradermal inoculation of influenza A and B vaccine. Proc Soc Exp Biol and Med. 1948;67:96–101. doi: 10.3181/00379727-67-16216. [DOI] [PubMed] [Google Scholar]
- 22.Laurent PE, Bourhy H, Fantino M, Alchas P, Mikszta JA. Safety and efficacy of novel dermal and epidermal microneedle delivery systems for rabies vaccination in healthy adults. Vaccine. 2010;28:5850–6. doi: 10.1016/j.vaccine.2010.06.062. [DOI] [PubMed] [Google Scholar]
- 23.Sangare L, Manhart L, Zehrung D, Wang CC. Intradermal hepatitis B vaccination: a systematic review and meta-analysis. Vaccine. 2009;27:1777–86. doi: 10.1016/j.vaccine.2009.01.043. [DOI] [PubMed] [Google Scholar]
- 24.Yanagisawa N, Takayama N, Nakayama E, Suganuma A. [Pre-exposure intradermal rabies vaccination using Japanese rabies vaccine following WHO recommended schedule] Kansenshogaku Zasshi. 2010;84:313–4. doi: 10.11150/kansenshogakuzasshi.84.313. [DOI] [PubMed] [Google Scholar]
- 25.Noah DL, Hill H, Hines D, White EL, Wolff MC. Qualification of the hemagglutination inhibition assay in support of pandemic influenza vaccine licensure. Clin Vaccine Immunol. 2009;16:558–66. doi: 10.1128/CVI.00368-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rowe T, Abernathy RA, Hu-Primmer J, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37:937–43. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.US Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research. Guidance for industry: clinical data needed to support the licensure of pandemic influenza vaccines. 2007 http://www.fda.gov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm074786.htm. accessed on January 26, 2012. [Google Scholar]
- 28.Holland D, Booy R, De Looze F, et al. Intradermal influenza vaccine administered using a new microinjection system produces superior immunogenicity in elderly adults: a randomized controlled trial. J Infect Dis. 2008;198:650–8. doi: 10.1086/590434. [DOI] [PubMed] [Google Scholar]
- 29.Frenck RW, Jr, Belshe R, Brady RC, et al. Comparison of the immunogenicity and safety of a split-virion, inactivated, trivalent influenza vaccine (Fluzone®) administered by intradermal and intramuscular route in healthy adults. Vaccine. 2011;29:5666–74. doi: 10.1016/j.vaccine.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]