Abstract
Sexual arousal is thought to be the result of two levels of processing: conscious and unconscious. Whereas some research exists on the neural correlates related with conscious exposure to sexual stimuli, there are no parallel data regarding unconscious or subliminal exposure to such stimuli. In the present study, we therefore compared brain activation of 39 participants (20 women) as they were exposed to supraliminal vs subliminal sexual stimuli. Supraliminal exposure was associated with greater activation in areas that were previously associated with sexual arousal (e.g. caudate nucleus and thalamus) as well as areas that were previously associated with control (e.g. orbitofrontal cortex and cingulate cortex). In contrast, subliminal exposure was mainly related to activation in areas previously associated with sexual arousal. Men and women exhibited theoretically meaningful differences in patterns of activation associated with supra- and subliminal exposure. Findings are discussed with regard to sexual arousal and regulatory processes.
Keywords: sex, priming, subliminal, supraliminal, arousal, regulation
INTRODUCTION
Sexual arousal is a complex phenomenon involving psychological (cognitive and affective), behavioral, and physiological as well as neural components, which often are desynchronized and uncoordinated (Rosen & Beck, 1988; Chivers et al., 2010). For example, exposure to a sexual stimulus (e.g. a picture of a nude person), which often results with physiological arousal (blood flow to the genitals), can also result with a positive affective reaction (subjective arousal; e.g. excitement or pleasure), a neutral affective reaction, or a negative affective reaction (e.g. anxiety, shame, or guilt). When negative affect is involved, the reaction is not simply desynchronized with the physiological one, but can actually dampen or inhibit it (e.g. Chivers et al., 2010). This complexity leaves open the question of when exposure to a sexual stimulus will result with transition to the next phase in the human sex response cycle (Masters & Johnson, 1966; Basson, 2001).
Recently, Janssen and colleagues (e.g. Janssen & Bancroft, 2007) have suggested that the reaction following exposure to a sexual stimulus depends on automatic bottom-up appraisal and response-generation processes as well as on effortful top-down regulatory processes. Moreover, Janssen and colleagues have suggested that these processes take place at both subliminal and supraliminal levels of processing. Sexual reaction is thought to begin with automatic processes at the subliminal or pre-attentive level, followed by regulatory processes, taking place at a supraliminal level (see also Gillath et al., 2007).
Whereas there is ample research on the supraliminal level of sexual response, relatively little is known about processes taking place at the subliminal level. To fully understand sexual arousal and behavior, research focused on the subliminal, or automatic, level in comparison with the supraliminal level is needed (e.g. Janssen et al., 2000). The present paper, thus, is set to investigate these potentially differential processes using neuroimaging.
Janssen and colleagues’ (2007) argument and focus on the subliminal level, fits with claims by scholars such as Greenwald and colleagues (2009) who argued that using subliminal methods and assessing subliminal processes is useful when studying socially sensitive topics—such as sex or prejudice—providing higher predictive and incremental validity. Testing people's reactions to subliminal stimuli can help researchers minimize effects of social desirability, defensiveness, demand characteristics, and participants’ attempts to control or inhibit their responses, which often affect or bias studies using supraliminal stimuli. For example, supraliminal exposure to sexual cues evokes not only sexual arousal, but also associated regulatory processes. Some are related to appropriateness of the sexual response in general, and some specifically associated with the laboratory settings. This makes it difficult to study the sexual arousal response separately from regulatory processes (e.g. Beauregard et al., 2001). Using subliminal exposure might reduce participants’ tendency to activate control or regulatory processes, especially those related to the specific context. This, in turn, can help researchers better understand the human sex response cycle.
An approach that can help in elucidating the differences between the processing of subliminal and supraliminal stimuli is neuroimaging. Numerous papers from various domains such as memory (Rugg et al., 1998; Voss & Paller, 2008), self-relevant processing (Rameson et al., 2010), processing of social information (Critchley et al., 2000), and emotional processing (Scheuerecker et al., 2007) have already successfully used this approach. Moreover, neuroimaging has already been successfully applied to study the effects of exposure to supraliminal sexual stimuli (e.g. Hamann et al., 2004; Stark et al., 2005; Walter et al., 2008). To our knowledge, however, there are no parallel data on subliminal exposure to sexual stimuli (Georgiadis & Kortekaas, 2010).
Explicit vs implicit sexual arousal
Janssen et al. (2000) have provided preliminary evidence, mainly among men, to support their model. This evidence suggests that the automatic or pre-attentive processes form a major pathway to sexual arousal, which enables fast recognition of the sexual meaning of a stimulus and the generation of automatic, uncontrolled, and at least partially unconscious cognitive and physiological responses. This primary pathway is suggested to be modified by controlled, deliberate mental processes, which occur at a higher cognitive level and are thought to be relatively slow, more resource-consuming, and at least partially conscious.
Supporting and extending Janssen and colleagues’ (2000) model, Gillath et al. (2007); Gillath et al. (2008) conducted a series of experiments involving both men and women to further elucidate the mechanisms underlying cognitive and affective responses to subliminal and supraliminal sexual cues. Using well-validated social-cognitive methods (i.e. cognitive priming; Bargh, 2006), Gillath et al. (2007) exposed participants to either sexual or neutral cues and then assessed their subjective arousal and cognitive accessibility of sex-related concepts. The findings indicated that subliminal and supraliminal exposure to sexual cues result in differential responses (see also Spiering et al., 2003). Specifically, subliminal exposure resulted in higher accessibility of sex-related thoughts (assessed via a reaction-time task), but no increase—or even a decrease among women—in subjective sexual arousal. In contrast, supraliminal exposure resulted in decreased accessibility of sex-related thoughts (manifested by poorer categorization of words and images) and increased subjective sexual arousal. These findings, while providing further support for Janssen et al.'s (2000) model of the dual level processing of sexual cues, do not explain the underlying mechanisms of these processes. Learning about these mechanisms may help understand why responses to supraliminal and subliminal sexual cues differ and how these two levels interact to affect the sex response cycle.
Brain activation associated with exposure to supraliminal sexual cues
Sumich et al. (2003) reviewed the diverse literature of neural correlates associated with exposure to sexual cues. They concluded their review by highlighting some areas as central for human sexual arousal [middle occipital, left inferior parietal and right superior parietal cortexes, insula, orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), thalamus, caudate, and putamen, among others]. However, not all of these areas were identified in all of the studies they reviewed and many studies showed activation in other brain areas as well.
In addition to reviewing particular areas important in the processing of sexual stimuli, Sumich et al. (2003) suggest that activation related to sexual arousal is distinct from activation associated with regulation of the arousal response. For example, Beauregard and colleagues (2001) asked participants to either respond normally to sexual stimuli or to try to inhibit their sexual response. They found that sexual arousal was associated with activation in the limbic structures of the amygdala and hypothalamus, while the attempted inhibition of such arousal recruited the right superior frontal gyrus (SFG) and right ACC. Likewise, Stoléru et al. (1999) found regulation of the arousal, or management of the conflict resulting from being aroused and trying to inhibit the arousal to be associated with ACC activation. In a similar investigation using functional near-infrared spectroscopy, Leon-Carrion et al. (2007) found that even after erotic stimuli presentation ceased, dorsolateral pre-frontal cortex (DLPFC) activation continued, supporting the involvement of frontal areas such as the DLPFC, in the regulation of sexual arousal.
These studies suggest that two neural systems or processes might be activated when people are exposed to sexual cues: one related to automatic arousal (or appraisal) and the other related to regulation. This idea is in line with dual model approaches by Lieberman (2007) and others, suggesting that two distinct neural systems underlie automatic and controlled responses. The problem with relying on studies such as Beauregard et al. (2001) to support such a model for sexual arousal is that even in the ‘arousal’ condition, when no regulation was requested by the experimenter, participants were likely to engage in some regulation (due, for example, to the laboratory settings). Hence, some of the observed brain activation was likely due to setting-related regulation rather than arousal. To deal with the problems resulting from presenting sexual stimuli in the laboratory, and to further explicate the differences between automatic and controlled processes we compared, in the present work, neural responses to supraliminal and subliminal sexual images.
Gender differences
The extensive literature comparing women's and men's sexuality (for reviews see Baumeister et al., 2001; Peplau, 2003; Hyde, 2005) demonstrates gender differences in the processing of sexual information. This suggests that when examining brain activation associated with exposure to sexual stimuli, gender should be considered. Whereas most studies on neural correlates of sexual arousal were done with men, there is some research comparing men's and women's responses to sexual cues. For example, Yang et al. (2007) presented erotic video as compared to neutral video while scanning people's brains. They found bilateral activation in the amygdala among women, whereas men showed activation only in the left amygdala (see also Hamann et al., 2004). In addition, men showed stronger activation in the left anterior cingulate gyrus as compared with women when viewing the erotic video. Unlike these findings, Stark et al. (2005) found no gender differences when comparing responses to pictures designed to evoke sexual arousal (potentially due to a smaller N and lower power to detect differences). Based on the broad literature and in light of these inconsistent imaging findings, we decided to include gender in our analysis.
The present study
The present work was set to extend existing findings by investigating the neural mechanisms underlying the different responses to subliminal vs. supraliminal stimuli, filling the existing gap in the literature (Georgiadis & Kortekaas, 2010). We predicted that (i) activation in sex-related regions (representing sexual arousal) would be associated with exposure to sexual stimuli at both levels (supra and sub), whereas (ii) activation in control- and/or conflict-related brain regions would be mainly associated with supraliminal exposure. Furthermore, we made an effort to recruit similar numbers of men and women so that we would be able to examine the existence of gender differences. To be able to maintain a consistent design across both subliminal and supraliminal presentation conditions, we embedded the sexual cues (as primes before each trial) within a cognitive task in which participants were asked to rate their liking of abstract images.
METHODS
Participants
Thirty-nine heterosexual men (n = 19) and women (mean age = 19.65), all right-handed, with no history of neurological or psychiatric injury or disease, no known sexual dysfunction, who had normal or corrected to normal vision, participated. Participants were all students at a mid-size American university recruited via flyers hanged around campus and were paid for their participation. Seventeen people reported being currently in a relationship (8 men and 9 women), with the remaining participants reporting being single (11 men and women). The majority of the participants reported their ethnicities as White (n = 15) and Asian (n = 15).
Materials
We used abstract pictures as our target images, and two types of pictures (sexual and neutral) as our prime images. Sexual images were of a similar age, opposite sex person. The neutral images were abstract drawings matched for size and contrast with the sexual images. The images were selected in previous studies based on extensive pretesting (for details see Gillath et al., 2007, 2008) and are similar to sexual pictures on the International Affective Picture System (IAPS; Lang et al., 2008).
Procedure
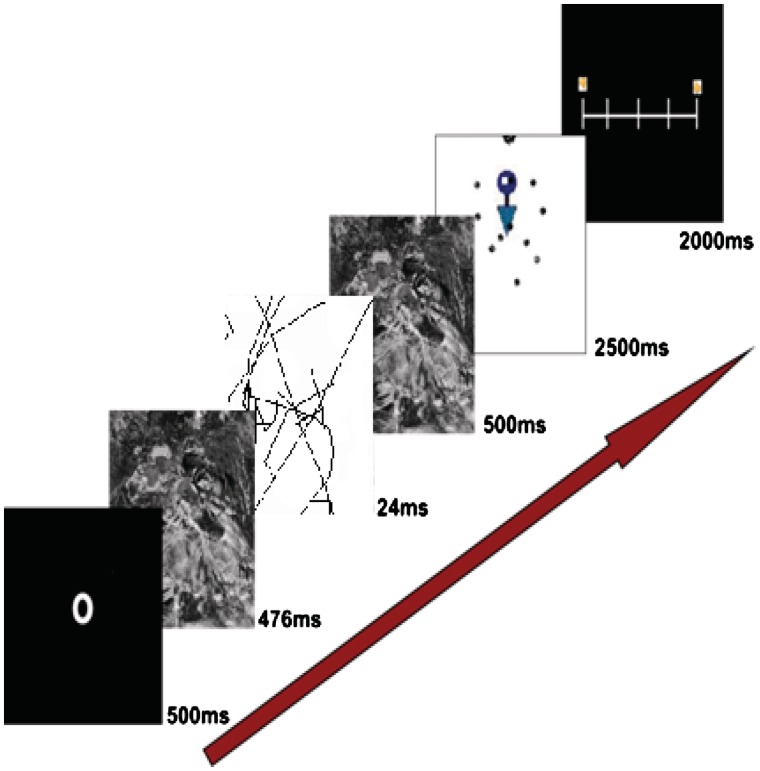
Participants were told they will be doing a cognitive task while their brain will be scanned and that they may or may not be exposed to sexual (or other) stimuli. The experiment was divided into four blocks of trials. In the first two blocks, the sexual images were presented subliminally and in the second two blocks, they were presented supraliminally. Each trial consisted of a cue directing attention to the center of the screen (500 ms), followed by a forward mask (scrambled picture; 476 ms), sexual prime (24 ms or 524 ms), a backward mask (500 ms), and then a target image for 2500 ms (Figure 1). Upon presentation of each target image, participants were instructed to rate, as quickly as possible, how much they liked the image, using one of five buttons to indicate a liking rating between 1 (do not like it at all) and 5 (like it very much). We used an event-related design consisting of stimuli presented with variable stimulus onset asynchrony (SOA ranged between 2000 and 10 000 ms) generated by Optseq (Greve, 2002). Following the scanning procedure, participants completed demographic questions, were debriefed, compensated for their time, and thanked.
Fig. 1.
A graphical depiction of the design.
Data acquisition
Brain images were acquired with a 1.5T GE Signa scanner. Head motion was minimized with comfortable padding around the participant's head. Functional images were acquired with gradient-recalled echo EPI sensitive to the blood oxygen level-dependent (BOLD) contrast [repetition time (TR) = 2.5 s, echo time (TE) = 40 ms, 24 contiguous 4 mm oblique axial slices parallel to the AC–PC line]. After the experimental scans, high-resolution anatomical images were collected for each participant (TR = 12 ms, TE = 4.5 ms, voxel dimensions = 1 × 1 × 1 mm).
fMRI data analysis
Scans were analyzed using Statistical Parametric Mapping software (SPM5, Wellcome Department of Cognitive Neurology) in Matlab 2007. The first six brain volumes of each scan were discarded from the analysis to eliminate non-equilibrium effects of magnetization. The remaining 334 volumes were used for the subsequent analyses. Images were corrected for differences in timing of slice acquisition and were then submitted to rigid body motion correction. Functional volumes were spatially normalized to EPI templates in SPM. The normalization algorithm consisted of a 12-parameter affine transformation together with a nonlinear transformation involving cosine basis functions and resampled the volumes to 2 × 2 × 2 mm cubic voxels. Functional volumes were spatially smoothed with an 8-mm FWHM isotropic Gaussian kernel. Activation was considered significant when P < 0.001, uncorrected, for five contiguous voxels, unless otherwise noted. Contrasts used in the analyses were created at the second level, with participants treated as random effects.
RESULTS
The results section consists of the following sections: (i) an analysis of the behavioral responses (liking). (ii) Whole-brain analyses (testing differences in brain activation in response to the subliminal and supraliminal presentation of both the sexual and neutral pictures); this section was further divided into four subsections: one focusing on reactions to the supraliminal primes, the second focusing on the reactions to the subliminal primes, a third section providing a direct comparison of the sexual primes at both levels, and finally the fourth section focused on gender differences. (3) Regions of interest (ROI) analysis, allowing us to test specific areas of activation.
We first explored the behavioral responses (how much people liked the images) as a function of the two within-subjects variables: prime (neutral vs sexual) and presentation level (subliminal vs supraliminal) and the between-subjects variable gender (men vs women). A repeated measures analysis of variance (RM-ANOVA) revealed a significant three-way interaction, F(1, 33) = 7.11, P = 0.012, partial η2 = 0.18. To further probe the three-way interaction, we ran the RM-ANOVA separately for men and women. The two-way interaction between prime and level of exposure was significant among men, F(1, 18) = 6.19, P = 0.023, partial η2 = 0.26, such that men showed an increased liking for images following a supraliminal sexual prime. The interaction, however, was not significant among women, F(1, 15) = 4.16, ns. Supporting previous studies (e.g. Gillath et al., 2007, 2008) subjective sexual arousal was observed only after the supraliminal cues, and mainly among men.
Whole-brain comparisons
To test our main hypothesis and determine whether there were differences in brain activation in response to the subliminal and supraliminal presentation of both the sexual and neutral pictures, we first conducted a 2 × 2 factorial analysis to assess the interaction between the two types of images (sexual and neutral) and level of presentation (subliminal and supraliminal). Areas of activation included inferior FG (IFG; BAs 13, 45 and 47), middle FG (BA 6), SFG (BA 8), middle temporal gyrus (BAs 37 and 39), supramarginal gyri (BA 40), cingulate (BA 24) and the caudate (Table 1). To further understand the findings from the factorial analysis, we used two approaches: one focused on activation from specific contrasts at each presentation level and another focused on specific regions of interest (ROI analyses). Next, we describe the results for each level of presentation separately (Table 2).
Table 1.
2(image type) × 2(presentation level) flexible factorial analysis
| Region | ∼BA | Coordinates of peak activity | F | Z-score |
|---|---|---|---|---|
| R caudate | 10 12 0 | 13.20 | 3.37 | |
| L cingulate gyrus | 24 | −4 14 26 | 18.92 | 4.06 |
| R cingulate gyrus | 24 | 4 −16 42 | 17.55 | 3.90 |
| L inferior frontal gyrus | 45 | −42 20 10 | 14.70 | 3.56 |
| 47 | −34 18 −16 | 21.25 | 4.30 | |
| 47 | −38 28 −4 | 14.70 | 3.56 | |
| R inferior frontal gyrus | 13 | 40 22 10 | 12.77 | 3.31 |
| 47 | 28 28 −10 | 16.41 | 3.77 | |
| L middle frontal gyrus | 6 | −30 18 52 | 14.30 | 3.51 |
| L middle temporal gyrus | 37 | −56 −62 6 | 13.03 | 3.34 |
| 39 | −54 −64 20 | 15.07 | 3.61 | |
| L supramarginal gyrus | 40 | −58 −54 38 | 20.13 | 4.19 |
| R supramarginal gyrus | 40 | 64 −48 32 | 13.66 | 3.43 |
Table 2.
Whole brain analysis for contrasts of interest
| Region | ∼BA | Coordinates of peak activity | t | Z-score |
|---|---|---|---|---|
| Supraliminal sex > supraliminal neutral contrast | ||||
| L anterior cingulate | 24 | −6 20 24 | 6.68 | 5.40* |
| L inferior frontal gyrus | 47 | −32 18 −14 | 7.03 | 5.59* |
| R inferior frontal gyrus | 45 | 42 20 14 | 7.14 | 5.65* |
| L insula | 13 | −36 20 12 | 5.45 | 5.27* |
| R middle occipital gyrus | 37 | 50 −66 −6 | 5.43 | 4.65* |
| L middle temporal gyrus | 21 | −62 −40 −8 | 3.94 | 3.59 |
| 37 | −54 −66 8 | 5.90 | 4.94* | |
| L precuneus | 7 | −8 −72 38 | 4.14 | 3.73 |
| R precuneus | 7 | 6 −58 48 | 4.04 | 3.66 |
| 7 | 12−72 38 | 4.06 | 3.67 | |
| R supramarginal gyrus | 40 | 66 −46 30 | 6.19 | 5.11* |
| Subliminal sex > subliminal neutral contrast | ||||
| L inferior frontal lobe | 47 | −28 20 −10 | 3.55 | 3.28 |
| L inferior parietal | 40 | −40 −40 42 | 3.58 | 3.31 |
| R medial fronal gyrus | 10 | 6 66 12 | 3.50 | 3.24 |
| R middle frontal gyrus | 9 | 60 12 36 | 3.72 | 3.41 |
| 6 | 58 6 42 | 3.87 | 3.53 | |
| 6 | 34 4 52 | 3.85 | 3.52 | |
| R middle occipital gyrus | 18 | 38 −86 2 | 4.41 | 3.94 |
| R middle temporal gyrus | 39 | 38 −60 28 | 4.15 | 3.75 |
| R parahippocampal gyrus | 35 | 20 −34 −6 | 3.67 | 3.37 |
| R postcentral gyrus | 2 | 52 −26 50 | 3.70 | 3.40 |
| R posterior cingulate | 30 | 4 −44 18 | 3.86 | 3.52 |
| R precuneus | 7 | 12 −74 42 | 3.75 | 3.44 |
| 19 | 30 −68 40 | 5.18 | 4.48 | |
| R superior frontal gyrus | 9 | 26 44 36 | 3.71 | 3.41 |
| L superior parietal | 7 | −30 −66 52 | 3.72 | 3.42 |
| R superior parietal | 7 | 34 −54 48 | 4.48 | 3.99 |
| L thalamus | −10 −6 4 | 3.56 | 3.29 | |
| Supraliminal sex > subliminal sex contrast | ||||
| L cingulate gyrus | 32 | −8 22 32 | 4.91 | 4.29 |
| R culmen | 14 −40 −4 | 4.83 | 4.24 | |
| L cuneus | 18 | −22 −98 12 | 6.05 | 5.03* |
| R inferior frontal gyrus | 9 | 50 10 32 | 4.63 | 4.10 |
| 47 | 36 32 −8 | 4.85 | 4.25 | |
| R inferior occipital gyrus | 19 | 42 −78 −2 | 5.34 | 5.21* |
| L insula | 13 | −36 16 12 | 5.74 | 4.84* |
| R medial frontal gyrus | 8 | 4 18 46 | 4.59 | 4.07 |
| L middle frontal gyrus | 6 | −30 −8 58 | 4.58 | 4.06 |
| 11 | −32 38 −10 | 4.03 | 3.65 | |
| L middle occipital gryus | 18 | −26 −90 8 | 5.51 | 4.70* |
| 19 | −54 −68 8 | 3.66 | 3.37 | |
| R middle occipital gryus | 18 | 30 −92 6 | 6.78 | 5.45* |
| L parahippocampal gyrus | 30 | −18 −36 −6 | 5.14 | 4.45 |
| R posterior cingulate | 23 | 6 −38 24 | 3.93 | 3.58 |
| L precentral | 6 | −44 −2 36 | 5.74 | 4.84* |
| R precentral | 6 | 54 −2 52 | 6.13 | 5.08* |
| 44 | 48 16 8 | 4.61 | 4.08 | |
| L precuneus | 7 | −2 −66 46 | 3.55 | 3.28 |
| L superior frontal | 9 | −32 48 34 | 4.32 | 3.87 |
| R superior frontal | 10 | 24 48 22 | 4.34 | 3.89 |
| Subliminal sex > supraliminal sex | ||||
| No significant voxels. |
N = 39; All reported regions are significant at P < .001 uncorrected.
*P < 0.05 with FWE correction for multiple comparisons.
Supraliminal exposure
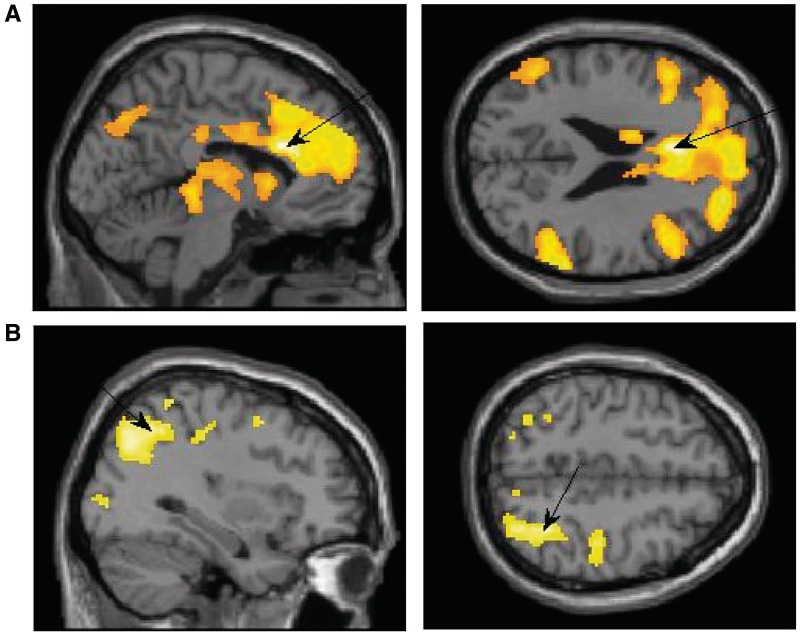
Exposure to the supraliminal sexual image as compared with the supraliminal neutral image (supraliminal sexual > supraliminal neutral) resulted with unique activation in the IFG (BA 45, 47), ACC (BA24; Figure 2A), the insula (BA 13), middle occipital (BA 37), middle temporal (BA 21, 37), supramarginal gyrus (BA 40) and the precuneus (BA 7). These activations are in line with previous research using supraliminal sexual stimuli (e.g. Redouté et al., 2000; Sumich et al., 2003).
Fig. 2.
(A) Activation in the supraliminal sexual > supraliminal neutral contrast showing peak activation in the ACC: x = −6, z = 24. (B) Activation in the subliminal sexual > subliminal neutral contrast showing peak activation in the superior parietal: x = 34, z = 48.
Subliminal exposure
Exposure to the subliminal sexual image as compared with the subliminal neutral image (subliminal sexual > subliminal neutral) resulted with unique activation in frontal areas including the IFG (BA 47), the medial (BA 10) and middle frontal gyri (BA 6, 9) and the SFG (BA 9). In addition, there was increased activation in the superior parietal lobule (BA 7; see Figure 2B), inferior parietal (BA 40), middle occipital (BA 18), middle temporal (BA 39), and parahippocampal gyri (BA 35), the postcentral gyrus (BA 2), posterior cingulate (BA 30), the precuneus (BA 7, 19) and the thalamus. After looking at the contrast sex > neutral within each presentation level (supraliminal vs subliminal), we next compared the two sexual conditions directly.
Comparison of exposure to supraliminal and subliminal sexual images
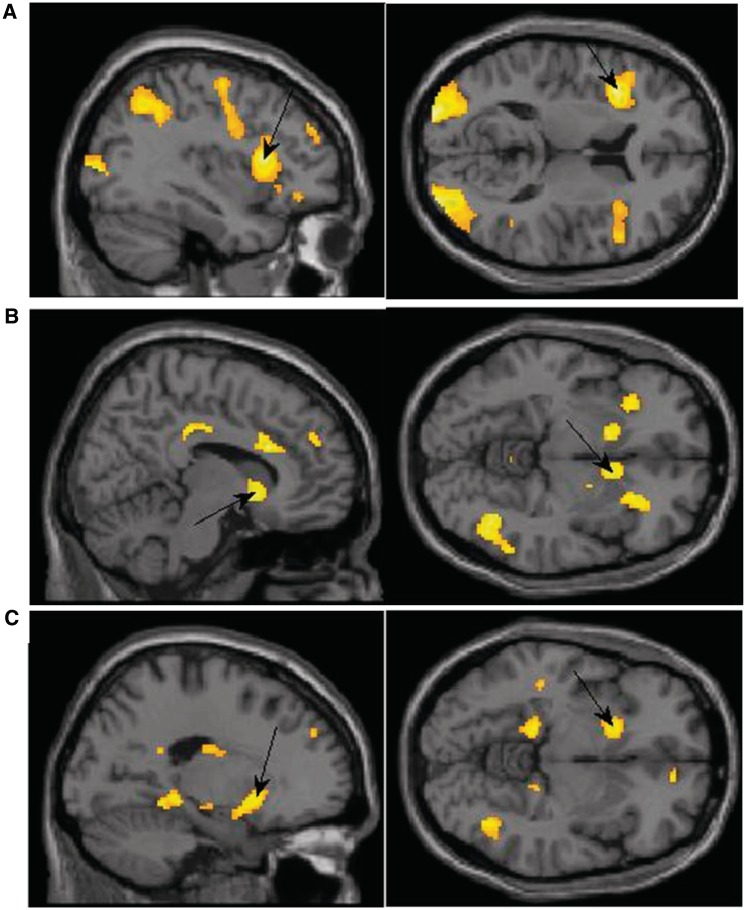
Exposure to the supraliminal sexual images as compared with exposure to subliminal sexual images was associated with higher activation in the insula (BA 13), inferior (BA 19) and middle occipital (BA 18, 19), precentral gyrus (BA6, 44), the cuneus (BA 18) and the precuneus (BA7). It was also associated with higher activation in IFG (BA 9, 47; Figure 3A), cingulate gyrus (BA 32), SFG (BA 9, 10) medial (BA 8) and middle frontal gyri (BA 6, 11), the parahippocampal gyrus (BA 30) and the culmen. Subliminal exposure to the sexual image did not result in any increased activation at the P < 0.001 level in comparison to supraliminal exposure.
Fig. 3.
(A) Activation in the supraliminal sexual > subliminal sexual contrast with peak activation in the IFG. x = −36, z = 12. (B) Activation in the supraliminal sexual > supraliminal neutral contrast for men showing peak activation in the caudate. x = 10, z = −6. (C) Activation in the subliminal sexual > subliminal neutral contrast for women showing peak activation in the putamen. x = −18, z = −6.
Based on the above-mentioned work regarding gender differences in sexual response (e.g. Baumeister et al., 2001; Peplau, 2003) and fMRI studies showing that men and women exhibit difference in the processing of sexual stimuli (e.g. Karama et al., 2002; Hamann et al., 2004), we next analyzed each contrast separately for men and women to determine if there were gender differences in activation patterns associated with exposure to supraliminal and subliminal sexual images.
Gender differences
To examine differences between men and women when viewing sexual images at the supraliminal level, we conducted a t-test with two groups (men and women) using the supraliminal sexual > neutral contrast. Direct comparison of the genders in this way did not reveal an increased activation for men or women when viewing sexual images for 500 ms at the P < 0.001 threshold. We next compared men and women's activation when exposed to subliminal sexual images (using the subliminal sex > neutral contrast). Women had higher activation than men in the middle temporal (−56 −14 −6), middle frontal (−6 44 −8), and middle occipital gyri (34 −80 10), STG (−40 −56 16), precuneus (−6 −66 18) and the putamen (−22 14 −4) when exposed to subliminal sexual images. Men, conversely, did not show increased activation in response to subliminal sexual images when compared to women.
In addition to directly comparing the genders, we examined activation patterns in response to supraliminal and subliminal sexual images separately for men and women. Although this approach is not as conclusive as the one reviewed above, it can provide more fine-tuned description of the activation associated with sexual cues among men and women. We first examined activation in response to sexual images at the supraliminal level using the supraliminal sexual > neutral contrast. For women, the supraliminal sexual images resulted in higher activation in the ACC (BA 30), the cingulate gyrus (BA 24), IFG (BA 13, 44, 45, 47), middle frontal (BA 6) and SFG (BA 8, 9). In addition there was activation in the caudate, globus pallidus, inferior occipital (BA 19), superior (BA 39) and middle temporal gyri (BA 37), supramarginal gyrus (BA 40) and inferior parietal (BA 40).
The same contrast for men showed increased activation in the IFG (BA 45, 47), the ACC (BA 32) and cingulate (BA 23, 32), culmen, globus pallidus, and in the SFG/middle frontal (BA 9, 10). Men also had activation in the thalamus, insula (BA 13), caudate (Figure 3B), supramarginal gyrus (BA 40) and putamen (Table 3).
Table 3.
Whole brain analysis for contrasts of interest by gender
| Region | ∼BA | Coordinates of peak activity | t | Z-score |
|---|---|---|---|---|
| Supraliminal sex > supraliminal neutral contrast | ||||
| Women | ||||
| R anterior cingulate | 30 | 0 20 22 | 4.74 | 3.80 |
| R caudate | 8 8 8 | 4.71 | 3.79 | |
| 12 2 16 | 4.21 | 3.50 | ||
| L caudate | −14 −4 20 | 4.06 | 3.40 | |
| R cingulate gyrus | 24 | 4 −4 30 | 5.36 | 4.14 |
| R globus pallidus | 10 0 −2 | 4.13 | 3.44 | |
| L inferior frontal gyrus | 13 | −42 24 6 | 6.82 | 4.79 |
| 44 | −48 14 16 | 5.78 | 4.34 | |
| 47 | −32 18 −14 | 5.42 | 4.16 | |
| R inferior frontal gyrus | 45 | 58 20 16 | 5.89 | 4.39 |
| R inferior occipital gyrus | 19 | 44 −76 −4 | 3.78 | 3.22 |
| L inferior parietal | 40 | −48 −58 46 | 5.09 | 3.99 |
| L middle frontal gyrus | 6 | −44 2 52 | 3.68 | 3.15 |
| 6 | −34 −6 46 | 4.18 | 3.47 | |
| R middle frontal gyrus | 6 | 50 12 48 | 5.28 | 4.09 |
| R middle temporal gyrus | 37 | 54 −64 4 | 4.51 | 3.68 |
| L superior frontal gyrus | 8 | −24 42 46 | 4.18 | 3.48 |
| 9 | −28 52 32 | 4.10 | 3.43 | |
| R superior frontal gyrus | 8 | 6 16 52 | 4.83 | 3.85 |
| 8 | 10 48 40 | 4.58 | 3.71 | |
| 9 | 12 58 28 | 5.57 | 4.24 | |
| L superior temporal gyrus | 39 | −54 −52 10 | 5.45 | 4.18 |
| L supramarginal gyrus | 40 | −52 −56 34 | 5.90 | 4.40 |
| R supramarginal gyrus | 40 | 64 −48 30 | 4.88 | 3.88 |
| Men | ||||
| L anterior cingulate | 32 | −2 34 16 | 5.91 | 4.35 |
| R caudate | 4 10 2 | 5.02 | 3.92 | |
| 10 12 −6 | 5.21 | 4.02 | ||
| L cingulate gyrus | 32 | −4 20 26 | 6.59 | 4.64 |
| R cingulate gyrus | 23 | 2 −26 30 | 6.30 | 4.52 |
| 24 | 2 −18 34 | 6.37 | 4.55 | |
| R culmen | 2 −48 0 | 4.83 | 3.82 | |
| R globus pallidus | 20 −2 −4 | 3.98 | 3.33 | |
| R inferior frontal gyrus | 45 | 42 18 14 | 6.11 | 4.44 |
| 47 | 28 30 −10 | 5.46 | 4.14 | |
| R insula | 13 | 36 16 0 | 3.88 | 3.26 |
| R middle frontal gyrus | 9 | 42 48 28 | 4.22 | 3.27 |
| 9 | 46 12 34 | 4.08 | 3.39 | |
| 10 | 32 44 24 | 4.71 | 3.75 | |
| L putamen | −14 12 −6 | 5.06 | 3.94 | |
| R supramarginal gyrus | 40 | 66 −44 26 | 4.70 | 3.75 |
| R superior frontal gyrus | 10 | 26 48 18 | 5.09 | 3.95 |
| 9 | 6 50 30 | 4.14 | 3.42 | |
| R thalamus R | 2 −18 10 | 4.17 | 3.44 | |
| Subliminal sex > subliminal neutral contrast | ||||
| Women | ||||
| L cingulate gyrus | 31 | −8 −28 38 | 4.68 | 3.77 |
| R cingulate gyrus | 32 | 10 18 38 | 3.99 | 3.36 |
| L cuneus | 7 | −10 −72 32 | 7.03 | 4.88* |
| L culmen | −18 −44 −10 | 4.66 | 3.76 | |
| L inferior frontal gyrus | 9 | −50 6 30 | 4.37 | 3.59 |
| R inferior occipital | 18 | 38 −84 −2 | 6.19 | 4.53 |
| L inferior parietal | 40 | −42 −30 36 | 4.20 | 3.49 |
| L insula | 13 | −30 22 4 | 4.44 | 3.63 |
| L medial frontal gyrus | 10 | −4 60 12 | 4.17 | 3.47 |
| R medial frontal gyrus | 25 | 10 10 −18 | 3.90 | 3.30 |
| L parahippocampal gyrus | 34 | −20 2 −14 | 4.53 | 3.58 |
| R parahippocampal gyrus | 34 | 18 2 −16 | 5.92 | 4.40 |
| R postcentral gyrus | 2 | 54 −26 52 | 4.55 | 3.70 |
| L posterior cingulate | 23 | −4 −58 18 | 4.04 | 3.39 |
| R posterior cingulate | 30 | 6 −48 14 | 5.13 | 4.02 |
| L precentral gyrus | 6 | −34 −8 50 | 4.13 | 3.45 |
| 6 | −46 −4 58 | 4.58 | 3.71 | |
| L precuneus | 7 | −24 −56 44 | 4.30 | 3.55 |
| 19 | −10 −80 40 | 4.22 | 3.50 | |
| R precuneus | 7 | 14 −48 48 | 3.69 | 3.16 |
| 7 | 10 −60 62 | 4.13 | 3.45 | |
| L putamen | −18 14 −6 | 4.94 | 3.91 | |
| L superior frontal gyrus | 9 | −20 48 34 | 4.72 | 3.79 |
| L superior parietal | 7 | −26 −68 52 | 5.26 | 4.08 |
| R superior temporal | 38 | 30 12 −24 | ||
| Men | ||||
| No significant voxels |
Women, n = 20; men, n = 19; all reported regions are significant at P < 0.001 uncorrected.
*P < 0.05 with FWE correction for multiple comparisons.
We next examined activation in response to subliminal exposure to sexual images using the subliminal sexual > neutral contrast separately among men and women. When exposed to subliminal sexual images, women showed higher activation in the inferior parietal (BA 40), putamen (Figure 3C), insula (BA 13) and in the inferior (BA 9), medial (BA 10, 25) and superior (BA 9) frontal gyri. Women also had activation in the cingulate (BA 31, 32), posterior cingulate (BA 23, 30), precentral gyrus (BA 6), cuneus (BA 7), precuneus (BA 7, 19), culmen, parahippocampal gyrus (BA 34), superior temporal (BA 38) and inferior (BA 40) and superior parietal (BA 7). Men, on the other hand, did not show higher activation in any area at the P < 0.001 threshold.
Regions of interest
To further test our predictions regarding activation in arousal-related and control-related areas, we conducted ROI analysis on six brain areas—three known to be associated with arousal and three with control or regulation. Three of these areas were chosen out of the areas identified using the factorial analysis and the rest were chosen based on the literature and our a priori predictions. The ROIs included the right IFG, right ACC, and right caudate (from the factorial) and the left thalamus, left putamen, and left SFG. These areas were explored using anatomical ROIs provided by the Marsbar toolbox for Matlab (Brett et al., 2002; http://marsbar.sourceforge.net). Marsbar was used to extract mean contrast values for each participant for both primes (sex and neutral) at each level (supraliminal and subliminal). These values were examined using RM-ANOVA. In the RM-ANOVA, we tested for interaction effects between the prime and presentation level, as well as three-way interactions including gender.1 The RM-ANOVAs revealed no three-way interactions involving gender; thus, we report only effects of presentation level and prime type, and their interaction.2
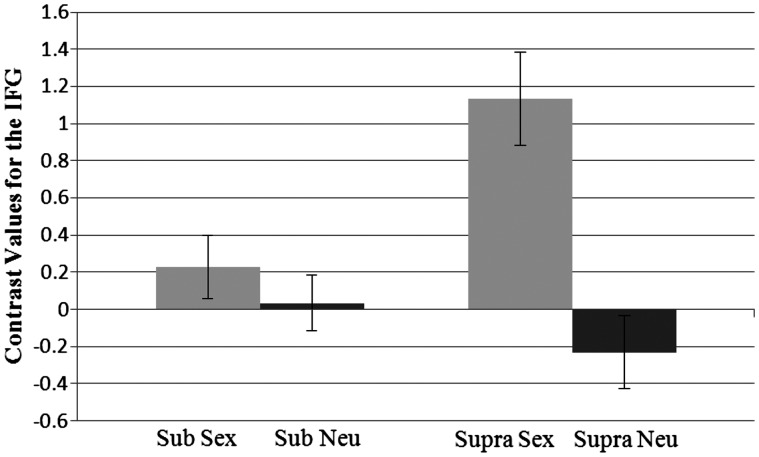
The RM-ANOVA on the right IFG revealed an effect of prime, F(1, 37) = 20.20, P < 0.001, η2 = 0.35, such that activation was higher for sexual prime (M = 0.68) than for the neutral prime (M = −0.10). There was also a significant interaction effect between prime and level of activation in the IFG, F(1, 37) = 13.00, P = 0.001, η2 = 0.26 (Figure 4). Pairwise comparisons showed that the activation in response to the sex prime (M = 1.13) was significantly higher than activation following the neutral prime (M = −0.23) at the supraliminal level, P < 0.001. No such difference was found in the subliminal level, and thus the sex prime (M = 0.23) and the neutral prime (M = 0.04) did not differ. Looking at it another way, activation in the IFG in response to the sex prime was higher at the supraliminal level, P = 0.002, as compared with the subliminal level. The presentation level did not significantly affect activation in the IFG in response to the neutral prime (P = ns).
Fig. 4.
Interaction between prime type and presentation level for contrast values at the inferior frontal gyrus. Sub, subliminal; supra, supraliminal. Standard errors shown.
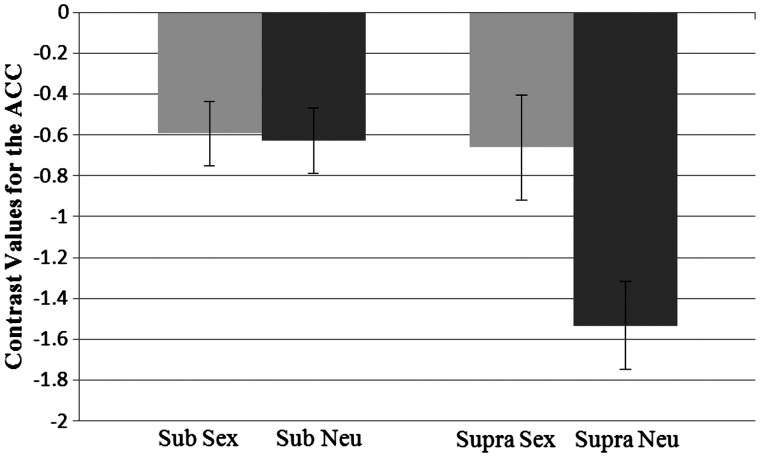
The analysis looking at activation in the ACC also revealed a main effect showing that activation was higher for sexual primes (M = −0.63) than neutral primes (M = −1.08), F(1, 37) = 9.24, P = 0.004, η2 = 0.20. There was also an effect of presentation level, such that there was less difference in activation in response to the subliminal primes (M = −0.61) than the supraliminal primes (M = −1.10), F(1, 37) = 5.71, P = 0.022, η2 = 0.13. In addition, there was a significant interaction between prime and level, F(1, 37) = 13.58, P < 0.001, η2 = 0.28 (Figure 5). Pairwise comparisons showed that the activation in response to the sex prime (M = −.66) was significantly higher than for the neutral prime (M = −1.53) at the supraliminal level, P < 0.001. However, at the subliminal level the activation following the sex prime (M = −0.59) and the neutral prime (M = −0.63) did not differ. Activation for the sex prime did not differ by presentation level. However, activation for the neutral prime was lower in the supraliminal level vs the subliminal level, P < 0.001.
Fig. 5.
Interaction between prime type and presentation level for contrast values for the anterior cingulate cortex. Sub, subliminal; supra, supraliminal. Standard errors shown.
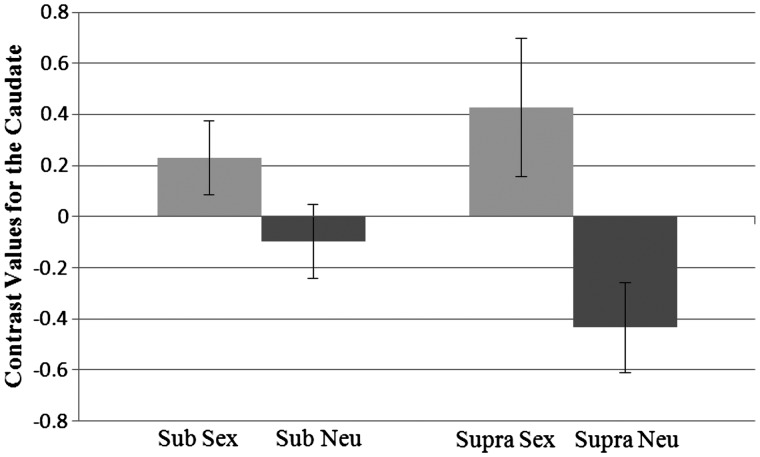
Likewise, the analysis of activation in the caudate, revealed a main effect showing that activation was higher for sexual primes (M = 0.33) than neutral primes (M = −0.27), F(1, 37) = 17.72, P < 0.001, η2 = 0.32. There was also a significant two-way interaction between prime and level, F(1, 37) = 4.70, P = 0.037, η2 = 0.11 Figure 6). Pairwise comparisons revealed that at the subliminal level activation was higher for the sex prime (M = 0.23) than the neutral prime (M = −0.10), P = 0.024. In addition, at the supraliminal level activation was higher for the sex prime (M = 0.43) than the neutral prime (M = −0.43), P < 0.001. Within each prime, the activation between the levels did not differ.
Fig. 6.
Interaction between prime type and presentation level for contrast values for the caudate. Sub, subliminal; supra, supraliminal. Standard errors shown.
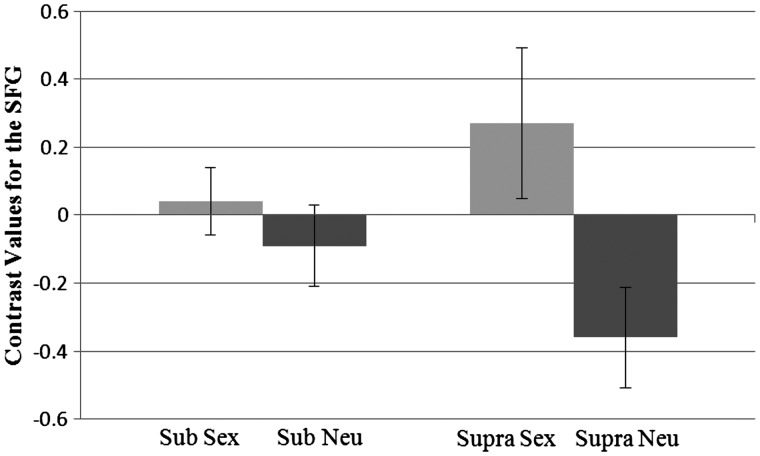
We next examined our three a priori predicted areas. For the SFG, there was an effect of prime, such that activation was higher for sexual primes (M = 0.15) than neutral primes (M = −0.22), F(1, 37) = 14.02, P = 0.001, η2 = 0.28. There was also a significant interaction between prime and level, F(1, 37) = 6.83, P = 0.013, η2 = 0.16 (Figure 7). Pairwise comparisons revealed that at the supraliminal level activation was higher for the sex prime (M = 0.27) than the neutral prime (M = −0.36), P = 0.001. At the subliminal level, activation did not differ between the sex prime (M = 0.04) and the neutral prime (M = −0.09). In addition, within each prime, the activation between the levels did not differ.
Fig. 7.
Interaction between prime type and presentation level for contrast values for the superior frontal gyrus. Sub, subliminal; supra, supraliminal. Standard errors shown.
The analysis for the thalamus and the putamen revealed no significant interactions between presentation level and prime. However, there were main effects of the prime. In the putamen, the sex prime (M = 0.52, s.d. = 0.15) resulted in increased activation in comparison to the neutral prime (M = 0.19, s.d. = 0.12), F(1, 37) = 4.83, P = 0.034, η2 = 0.12. Likewise, in the thalamus the sex prime (M = 1.76, s.d. = 0.25) had increased activation in comparison to the neutral prime (M = 1.36, s.d. = 0.21), F(1, 37) = 5.76, P = 0.022, η2 = 0.14. The level of presentation was also significant in the thalamus, in that there was increased activation at the supraliminal (M = 2.01) in comparison to the subliminal (M = 1.11) level, F(1, 37) = 6.93, P = 0.012, η2 = 0.16.
DISCUSSION
Human beings are sexual organisms (Williams, 1975), who depend on sexual reproduction for genetic variation and adaptation to changes in the environment. Being dependent on sex for reproduction and survival makes the role of sex central in people's lives. Such importance manifests in sexual cues being highly salient and their processing being rapid and potentially automatic (e.g. Gillath et al., 2007, 2008; Janssen et al., 2000). This processing results with the formation of often implicit attitudes and emotions that can shape (e.g. reduce arousal) the explicit processes to follow.
Whereas a substantial literature exists regarding the neural correlates of exposure to supraliminal sexual cues (e.g. Sumich et al., 2003; Georgiadis & Kortekaas, 2010), there are no data to date on the neural correlates of exposure to subliminal sexual cues, and in turn, there is little insight into the earliest stages of sexual information processing. Based on existing literature regarding discrepancies between implicit and explicit processes (e.g. Greenwald et al., 2009), we predicted that these implicit processes would be different from reactions to supraliminal sexual cues. Supraliminal exposure to sexual cues, especially in the context of an experiment, is likely to result with not only sexual arousal, but also other processes, such as inhibition (of urges) and regulation (of the arousal), which in turn might generate conflict and require conflict management. Conversely, subliminal presentation of sexual cues is less likely to activate control- and regulation-related processes (at least those associated with being aware of the sexual nature of the study) and their associated brain areas.
Overall our results regarding the differences between supraliminal and subliminal exposure to sexual images were in line with our predictions. Exposure to supraliminal sexual images was associated with higher activation in both arousal-related areas (such as the insula and caudate; Arnow et al., 2002) and control-related areas (such as the OFC and the SFG). Conversely, exposure to subliminal sexual images was associated mainly with activation in arousal-related areas (though women had increased activation in some control regions as well). These findings support Janssen et al's (2000) claim about two levels of processing: one pre-attentive involving appraisal and response generation, and the other involving regulation.
This idea of two levels involving two distinct processes (or two systems) is similar to existing neuro-cognitive models regarding regulatory processes in general (e.g. Lieberman, 2007) and emotion regulation process specifically (e.g. Ochsner, 2007). It is also in line with previous findings related to regulation of sexual material (e.g. Stoléru et al., 1999; Beauregard et al., 2001), showing activation in the IFG and ACC when participants try to control their reactions to sexual material.
These results, however, were moderated by gender, such that women showed control-related activation even in the subliminal exposure blocks. This is in line with previous studies regarding gender-related differences in brain structure and function (e.g. for a review see Cosgrove et al., 2007). When looking at the supraliminal exposure, whereas many activations among men had parallels among women (in line with Hyde, 2005; Georgiadis & Kortekaas, 2010), only men showed activation in areas such as the putamen, thalamus and insula, all known to be positively associated with subjective sexual arousal. These activations, however, did not survive the direct gender comparison. One potential reason for that is that men, although experiencing an initial strong arousal, quickly regulated it. This idea is supported by the activation men showed in the ACC (BA 24 and 32) and previous work by Beauregard et al. (2001) and others.
Activation in the ACC was previously found to be associated with conflict monitoring (Botvinick et al., 1999). This is consistent with the idea that when people (like in the present study) are consciously aroused and potentially having the urge to act based on this arousal, but cannot act on the basis of this urge, they experience conflict (e.g. Georgiadis & Kortekaas, 2010). As men are presumably feeling stronger sexual arousal (Baumeister et al., 2001), they may also have a stronger need to regulate it. This need fits with the activations males have in BA 10 and 47, both associated with regulatory processes (Beauregard et al., 2001; Kim & Hamann, 2007). The combination of high arousal and high regulation leads to men's activation level in sex-related areas being comparable (or not different) from that of women. Further support to this idea comes from the positive correlations between the ROIs representing activation in sex-related and control-related areas (Table 4). Future studies using methods with a better temporal resolution (like EEG) could help shed light on this issue.
Table 4.
Correlations among the ROI activation in response to the supraliminal sex prime
| 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|
| 1. IFG | – | |||||
| 2. ACC | 0.73** | – | ||||
| 3. SFG | 0.80** | 0.80** | – | |||
| 4. Caudate | 0.75** | 0.78** | 0.88** | – | ||
| 5. Putamen | 0.70** | 0.57** | 0.71** | 0.83** | – | |
| 6. Thalamus | 0.61** | 0.41* | 0.64** | 0.69** | 0.64** | – |
Note. IFG, inferior frontal gyrus; ACC, anterior cingulate cortex; SFG, superior frontal gyrus. n = 39
*P < 0.05 **P < 0.001
In the subliminal exposure blocks, men showed no differences between the sexual and neutral images, almost as if the sexual images they were exposed to had no effect on them. As men did show activation in the supraliminal condition, one might conclude that men may have higher threshold or lower sensitivity to such rapid cues. However, our own previous work (e.g. Gillath et al., 2007, 2008) on cognitive accessibility following sexual cues showed that men are actually affected by subliminal sexual cues (higher accessibility of sex-related words). This contradiction supports previous claims regarding the complex and desynchronized sexual response (e.g. Chivers et al., 2010). An alternative explanation may have to do with the characteristics of the stimuli. The subliminal pictures used may perhaps have been less arousing for men than for women (although our pretest of the stimuli, using a separate sample, did not show such a tendency).
Women, unlike men, showed activation in both arousal and control-related areas for both supraliminal (e.g., IFG, inferior parietal) and subliminal (e.g., putamen, superior parietal, and BA 32) levels of image presentation. This pattern of activation may suggest two things: first, women may be more sensitive than men to sexual stimuli and second, once perceived, sexual stimuli are associated with a conflicted reaction (both arousal and control or both negative and positive affective response) among women, even in the subliminal level. These results regarding the possible higher sensitivity to sexual cues women displayed are in line with literature regarding the different threats, costs and benefits associated with sex for each gender (e.g. Buss, 1989). Thus, various outcomes associated with sex, such as pregnancy, abuse, and rape, all pose a bigger potential threat to women than men. Perhaps this is why women were found to be more sensitive to sexual images in our study (as well as in previous studies; e.g. Gillath et al., 2007).
Limitations
There are a few limitations to the current article. First, as suggested in previous studies, exposure to a sexual stimulus can result with positive emotions such as desire and arousal, but also with negative ones such as anxiety and embarrassment (Mosher, Barton-Henry, & Green, 1988). Although our results do not provide direct evidence regarding negative emotions, some of the activation we found was in areas previously associated with embarrassment (e.g. SFG; Takahashi, et al., 2004). Future studies will have to further examine negative emotions potentially generated by supra- and subliminal exposure to sexual stimuli. Second, we do not provide in the present article subjective ratings of arousal (beyond the liking of the target images following the primes). Previously, it was suggested that lacking those ratings may hamper the interpretation of gender differences related to sexuality (Hamann et al., 2004; Georgiadis & Kortekaas, 2010). In our own experience, there is a complex and inconsistent pattern of correlations between subliminal exposure to sexual stimuli and subjective ratings (Gillath et al., 2007; Janssen et al., 2000); hence, we decided not to include links with subjective arousal in this initial work on the neural correlates of subliminal sexual exposure. Future studies, as suggested above, should combine the investigation of neural correlates of supraliminal and subliminal exposure with subjective ratings. A final limitation relates to exposure time. In the present study, we compared exposure for 500 ms with exposure for 24 ms, whereas in most other studies researchers used a much longer exposure time (e.g. 3500 ms). It might be worthwhile to compare our two presentation times with a longer exposure and examine the effects on activation pattern and strength.
Future directions and implications
Despite the limitations mentioned above, the present article provides, for the first time, a coherent and consistent theoretically based set of findings regarding the neural processes related with subliminal exposure to sexual stimuli. Furthermore, it sheds light on gender differences in sexual response. It would be interesting in future studies to include a combination of measures (cognitive, physiological and self-reports), and various stimuli (e.g. own partner vs. stranger; old vs. young; a dressed rather than a naked person), to better understand the associations between the sexual system components, and the sexual response cycle.
Although preliminary, our findings, which are in line with existing literature on regulatory processes and emotion-regulation, put us one step closer to understanding sexual arousal, regulation, and the sex response cycle. They also contribute to the understanding of the early stages of sexual information processing. The differences between implicit and explicit priming highlight the need to study both levels and consider their unique contributions. To practitioners and clinicians our findings suggest that people can be sexually aroused and react to it, even if they have no conscious awareness of the arousing stimulus or the arousal itself. Furthermore, when people are unaware of their sexual arousal, they are less likely to activate regulatory processes. This, in turn, could open them up to various impulsive, careless behaviors. That said, women seem to be in control even when sexual cues are very subtle, which can serve a protective function, but also may hamper women's ability to completely let go and experience unregulated arousal. This may explain gender differences found in other (i.e. non neural) studies, where women seem more restricted than or not as aroused as men (Baumeister et al., 2001). For clinicians or practitioners, our findings may also suggest a new approach to therapy. Having access to the implicit processes as they manifest in the brain, which are outside the client's awareness, can potentially help the therapist to provide better treatment options.
Acknowledgments
We would like to thank Silvia Bunge, Carter Wendelken and the Bunge lab for their tremendous support and for comments on previous versions of this article.
Footnotes
The original version was incorrect. The captions on Figure 3 were given incorrectly. This has now been corrected to read:
“Figure 3a. Activation in the supraliminal sexual > subliminal sexual contrast with peak activation in the IFG. x = -36, z = 12.
Figure 3b. Activation in the supraliminal sexual > supraliminal neutral contrast for men showing peak activation in the caudate. x = 10, z = -6.
Figure 3c. Activation in the subliminal sexual > subliminal neutral contrast for women showing peak activation in the putamen. x = -18, z = -6.”
1Since previous research has demonstrated that whether or not a person is in a relationship can affect responses to attractive opposite-sex others, even at an implicit level (Maner et al., 2009), we also examine effects of relationship status. The RM-ANOVA revealed no two-way interactions among prime type and status and no three-way interactions with prime type, status and gender for any of the six regions explored in the ROI analysis.
2Mean contrast values for gender can be attained by contacting the first author.
REFERENCES
- Arnow BA, Desmond JE, Banner LL, et al. Brain activation and sexual arousal in healthy, heterosexual males. Brain. 2002;125:1014–23. doi: 10.1093/brain/awf108. [DOI] [PubMed] [Google Scholar]
- Bargh JA. What have we been priming all these years? On the development, mechanisms, and ecology of nonconscious social behaviour. European Journal of Social Psychology. 2006;36:147–68. doi: 10.1002/ejsp.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson R. Human sex-response cycles. Journal of Sex and Marital Therapy. 2001;27:33–43. doi: 10.1080/00926230152035831. [DOI] [PubMed] [Google Scholar]
- Baumeister RF, Catanese KR, Vohs KD. Is there a gender difference in strength of sex drive? Theoretical views, conceptual distinctions, and a review of relevant evidence. Personality and Social Psychology Review. 2001;5:242–73. [Google Scholar]
- Beauregard M, Lévesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402:179–81. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. Paper presented at the 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan. 2002 [Google Scholar]
- Buss DM. Sex differences in human mate preferences: Evolutionary hypotheses tested in 37 cultures. Behavioral & Brain Sciences. 1989;12:1–49. [Google Scholar]
- Chivers ML, Seto MC, Lalumière ML, Laan E, Grimbos T. Agreement of self-reported and genital measures of sexual arousal in men and women: a meta-analysis. Archives of Sexual Behavior. 2010;39:5–56. doi: 10.1007/s10508-009-9556-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biological Psychiatry. 2007;62:847–55. doi: 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley H, Daly E, Phillips M, et al. Explicit and implicit neural mechanisms for processing of social information from facial expressions: A functional magnetic resonance imaging study. Human Brain Mapping. 2000;9:93–105. doi: 10.1002/(SICI)1097-0193(200002)9:2<93::AID-HBM4>3.0.CO;2-Z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiadis JR, Kortekaas R. The sweetest taboo: functional neurobiology of human sexuality in relation to pleasure. In: Kringelbach ML, Berridge KC, editors. Pleasures of the Brain. New York: Oxford University Press; 2010. pp. 178–201. [Google Scholar]
- Gillath O, Mikulincer M, Birnbaum GE, Shaver PR. Does subliminal exposure to sexual stimuli have the same effects on men and women? Journal of Sex Research. 2007;44:111–21. doi: 10.1080/00224490701263579. [DOI] [PubMed] [Google Scholar]
- Gillath O, Mikulincer M, Birnbaum G, Shaver PR. When sex primes love: Subliminal sexual priming motivates relational goal pursuit. Personality and Social Psychology Bulletin. 2008;34:1057–69. doi: 10.1177/0146167208318141. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Poehlman TA, Uhlmann E, Banaji MR. Understanding and using the Implicit Association Test: III. Meta-analysis of predictive validity. Journal of Personality and Social Psychology. 2009;97:17–41. doi: 10.1037/a0015575. [DOI] [PubMed] [Google Scholar]
- Hamann S, Herman RA, Nolan CL, Wallen K. Men and women differ in amygdala response to visual sexual stimuli. Nature Neuroscience. 2004;7:325–6. doi: 10.1038/nn1208. [DOI] [PubMed] [Google Scholar]
- Hyde JS. The gender similarities hypothesis. American Psychologist. 2005;60:581–92. doi: 10.1037/0003-066X.60.6.581. [DOI] [PubMed] [Google Scholar]
- Janssen E, Everaerd W, Spiering M, Janssen J. Automatic processes and the appraisal of sexual stimuli: towards an information processing model of sexual arousal. Journal of Sex Research. 2000;37:8–23. [Google Scholar]
- Janssen E, Bancroft J. The dual-control model: the role of sexual inhibition and excitation in sexual arousal and behavior. In: Janssen E, editor. The Psychophysiology of Sex. Bloomington, IN: Indiana University Press; 2007. pp. 197–222. [Google Scholar]
- Karama S, Lecours AR, Leroux JM, et al. Areas of brain activation in males and females during viewing of erotic film excerpts. Human Brain Mapping. 2002;16:1–13. doi: 10.1002/hbm.10014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Hamann S. Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience. 2007;19:776–98. doi: 10.1162/jocn.2007.19.5.776. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Affective Ratings of Pictures and Instruction Manual. Technical Report A-8. University of Florida, Gainesville, FL; 2008. [Google Scholar]
- Leon-Carrion J, Martín-Rodríguez JF, Damas-López J, et al. Does dorsolateral prefrontal cortex (DLPFC) activation return to baseline when sexual stimuli cease? The role of DLPFC in visual sexual stimulation. Neuroscience Letters. 2007;416:55–60. doi: 10.1016/j.neulet.2007.01.058. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. The X- and C-systems: the neural basis of automatic and controlled social cognition. In: Harmon-Jones E, Winkielman P, editors. Fundamentals of Social Neuroscience. New York: Guilford Press; 2007. pp. 290–315. [Google Scholar]
- Masters WH, Johnson VE. Human Sexual Response. Boston: Little, Brown; 1966. [Google Scholar]
- Maner JK, Gailliot MT, Miller SL. The implicit cognition of relationship maintenance: inattention to attractive alternatives. Journal of Experimental Social Psychology. 2009;45:174–9. [Google Scholar]
- Mosher DL, Barton-Henry M, Green SE. Subjective sexual arousal and involvement: development of multiple indicators. Journal of Sex Research. 1988;25:412–25. [Google Scholar]
- Ochsner KN. How thinking controls feeling: a social cognitive neuroscience approach. In: Harmon-Jones E, Winkielman P, editors. Fundamentals of Social Neuroscience. New York: Guilford Press; 2007. pp. 106–33. [Google Scholar]
- Peplau LA. Human sexuality: How do men and women differ. Current Directions in Psychological Science. 2003;12:37–40. [Google Scholar]
- Rameson LT, Satpute AB, Lieberman MD. The neural correlates of implicit and explicit self-relevant processing. NeuroImage. 2010;50:701–8. doi: 10.1016/j.neuroimage.2009.12.098. [DOI] [PubMed] [Google Scholar]
- Redouté J, Stoléru S, Grégoire MC, et al. Brain processing of visual sexual stimuli in human males. Human Brain Mapping. 2000;11:162–77. doi: 10.1002/1097-0193(200011)11:3<162::AID-HBM30>3.0.CO;2-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen RC, Beck JG. Patterns of Sexual Arousal: Psychophysiological Processes and Clinical Applications. New York: Guilford Press; 1988. [Google Scholar]
- Rugg MD, Mark RE, Walla P, Schloerscheidt AM, Birch CS, Allan K. Dissociation of the neural correlates of implicit and explicit memory. Nature. 1998;392:595–8. doi: 10.1038/33396. [DOI] [PubMed] [Google Scholar]
- Scheuerecker J, Frodl T, Koutsouleris N, et al. Cerebral differences in explicit and implicit emotional processing—an fMRI study. Neuropsychobiology. 2007;56:32–9. doi: 10.1159/000110726. [DOI] [PubMed] [Google Scholar]
- Spiering M, Everaerd W, Janssen E. Priming the sexual system: implicit versus explicit activation. Journal of Sex Research. 2003;40:134–45. doi: 10.1080/00224490309552175. [DOI] [PubMed] [Google Scholar]
- Stark R, Schienle A, Girod C, et al. Erotic and disgust-inducing pictures: differences in the hemodynamic responses of the brain. Biological Psychology. 2005;70:19–29. doi: 10.1016/j.biopsycho.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Stoléru S, Grégoire MC, Gérard D, et al. Neuroanatomical correlates of visually evoked sexual arousal in human males. Archives of Sexual Behavior. 1999;28:1–21. doi: 10.1023/a:1018733420467. [DOI] [PubMed] [Google Scholar]
- Sumich AL, Kumari V, Sharma T. Neuroimaging of sexual arousal: research and clinical utility. Hospital Medicine. 2003;64:28–33. doi: 10.12968/hosp.2003.64.1.2378. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Yahata N, Koeda M, Matsuda T, Asai K, Okubo Y. Brain activation associated with evaluative processes of guilt and embarrassment: An fMRI study. Neuroimage. 2004;23:967–74. doi: 10.1016/j.neuroimage.2004.07.054. [DOI] [PubMed] [Google Scholar]
- Voss JL, Paller KA. Brain substrates of implicit and explicit memory: the importance of concurrently acquired neural signals of both memory types. Neuropsychologia. 2008;46:3021–9. doi: 10.1016/j.neuropsychologia.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter M, Bermpohl F, Mouras H, et al. Distinguishing specific sexual and general emotional effects in fMRI-subcortical and cortical arousal during erotic picture viewing. Neuroimage. 2008;40:1482–94. doi: 10.1016/j.neuroimage.2008.01.040. [DOI] [PubMed] [Google Scholar]
- Williams GC. Sex and Evolution. Princeton, NJ: Princeton University Press; 1975. [Google Scholar]
- Yang B, Zhang JS, Wang T, Zhou YC, Liu JH, Ma L. An fMRI study on brain activation patterns of males and females during video sexual stimulation. Zhonghua Nan Ke Xue. 2007;13:718–22. [PubMed] [Google Scholar]