Abstract
Infant crying can elicit sensitive caregiving as well as hostility and harsh parenting responses. In the current study (N = 42 females) with a double-blind experimental design, we tested the effect of intranasal oxytocin administration on the use of excessive force using a hand-grip dynamometer during listening to infant cry sounds. Participants’ experiences with harsh parental discipline during childhood were found to moderate the effect of oxytocin administration on the use of excessive force. Participants’ whose parents did not discipline them harshly used less excessive force in the oxytocin condition, but for participants who were disciplined harshly there was no difference between the oxytocin and placebo condition. Such effects were not found during listening to infant laughter. We conclude that early caregiving experiences constitute an important moderator of the prosocial and/or stress-reducing effects of oxytocin. Oxytocin administration may increase trust and cooperation in individuals with supportive backgrounds, but not generate this effect in individuals who as a consequence of unfavorable early caregiving experiences may have a bias toward negative interpretation of social cues.
Keywords: oxytocin, infant crying, handgrip force, parenting, harsh discipline
INTRODUCTION
Infant crying is an important social cue that alerts parents to the needs of the infant. It elicits parental proximity and sensitive caregiving, but it is also an aversive stimulus (Murray, 1985), which can bring about irritation and harsh or abusive parenting responses (Frodi, 1985; Barr et al., 2006; Del Vecchio et al., 2009). Mothers’ prenatal negative emotional responses to 1-min videotapes of crying infants were predictive of lower sensitivity to their infant's distress at 6 months (Leerkes, 2010) and of punitive/minimizing responses to their toddlers’ negative emotions at 16 months (Leerkes et al., 2011). In a recent fMRI study, we found that experimentally induced oxytocin levels reduced activation in the neural circuitry for anxiety and aversion when listening to infant crying, and increased activation in regions involved in empathy (Riem et al., 2011). As a natural sequel to these findings, in the current study, we tested the effect of oxytocin administration on the use of excessive force using a hand-grip dynamometer during listening to infant crying sounds.
Hand-grip dynamometer data have been previously used as a measure of the use of excessive force in pseudo-parenting contexts. For example, Crouch et al. (2008) had parents at high and low risk for child physical abuse use a hand dynamometer when they watched video segments of an infant in quiet, smiling and crying states. After negative priming, at risk parents tended to use more excessive force when asked to produce a half-strength grip. In a study on punitive force, mothers used the dynamometer to provide feedback to a child in a simulated computer interaction (Bugental et al., 1999). Women who perceived themselves as low in power used more excessive force than other women when children were ambiguously responsive.
The use of excessive power in reaction to child signals may result from early experiences with harsh discipline. Children who are forcefully punished by their parents show more aggression to peers (Strassberg et al., 1994). Adults with a history of forceful and violent punishment have been suggested to have more problems with power in the relationship with their children (Bugenthal et al., 1999) and are more likely to use coercive or abusive force in their interactions with children (Bugenthal et al., 1989; Sroufe et al., 2005).
Recent correlational studies indicate that parenting may be related to oxytocin levels in humans as well as in other mammals (Carter and Altemus, 1997; Insel, 1997; Feldman et al., 2007; Strathearn et al., 2009). The neuropeptide oxytocin, produced in the hypothalamus, has been the focus of various studies on the influence of hormonal functioning on feelings, attitudes, behavior and neural responses (for a review see Campbell, 2010). Experiments with intranasal oxytocin administration documented its positive effect on observed parenting (Naber et al., 2010), interpersonal trust (Baumgartner et al., 2008; Theodoridou et al., 2009; Declerck et al., 2010) and emotional empathy (Hurlemann et al., 2010; for a review, see Bartz et al., 2011; for a meta-analysis see Van IJzendoorn and Bakermans-Kranenburg, in press). Oxytocin sniffs increase oxytocin levels in saliva for several hours (Huffmeijer et al., submitted for publication), and in the same study oxytocin administration was found to change event-related potentials in response to facial feedback stimuli. More positive vertex positive potential (VPP) and late positive potential (LPP) amplitudes after oxytocin compared to placebo administration were found, suggesting that oxytocin increased attention to the feedback stimuli (LPP) and enhanced the processing of emotional faces (VPP) (Huffmeijer et al., submitted for publication).
Nevertheless, oxytocin might not promote positive feelings and suppress aggression for all people in all circumstances (Bartz et al., 2011). Oxytocin administration may drive a ‘tend and defend’ response promoting in-group trust and cooperation, but at the same time enhancing defensive aggression toward competing out-groups (De Dreu et al., 2010). Males and females were more willing to continue a social-interactive computer game after oxytocin administration, unless they were confronted with a manipulation that made them feel belonging to a rejected out-group (Alvares et al., 2010). Bartz et al. (2010a) found that effects of oxytocin administration were moderated by participants’ attachment representations, with less anxiously attached individuals remembering their mother as more caring and close after oxytocin (vs placebo) but more anxiously attached individuals remembering their mother as less caring after oxytocin (vs placebo).
In one of our own studies, oxytocin administration appeared to increase participants’ willingness to donate money to a charity after having watched a promo film clip presenting a deprived child, but only when they had experienced low levels of parental love withdrawal (Van IJzendoorn et al., in press). Love withdrawal is a parental disciplinary strategy that involves withholding love and affection when a child misbehaves or fails at a task. When used excessively, it is considered psychological maltreatment (Euser et al., 2010). Participants who experienced high levels of love withdrawal tended to donate even less in the oxytocin condition.
Any positive effects of oxytocin may thus be altered or hindered by experiences of harsh discipline in childhood. We tested the effect of oxytocin administration on hand-grip dynamometer strength during listening to infant crying sounds in a study with a between-subjects design with (mainly) twins, females who were perfectly matched on age and global child rearing experiences. We expected that oxytocin administration would reduce the use of excessive force as indicated by grip strength on a hand-grip dynamometer when exposed to infant crying in particular in individuals whose parents did not (often) use harsh discipline methods. In case of high levels of harsh discipline, the effects of oxytocin might be impeded, with no reduction of excessive power as a result.
METHODS
Participants
A group of 44 right-handed females were recruited, half of them from monozygotic (MZ) twin pairs and half of them from dizygotic (DZ) twin pairs, without children of their own, in good health, without hearing impairments, pregnancy, psychiatric or neurological disorders, and screened for alcohol and drug use (see Riem et al., 2011). One participant failed to perform the dynamometer task, and one participant had incomplete questionnaire data, resulting in a total sample of 42 participants: 30 participants from twin pairs (16 MZ, 14 DZ) and 12 participants without a participating twin sibling (5 MZ, 7 DZ). The mean age of the participants was 29.3 years (s.d. = 7.5, range 22–49). Their educational level was on average 3.7 (s.d. = 0.8) on a scale ranging from 1 to 5, with 3 equivalent to 16 years of education and 4 equivalent to 20 years of education. Sixty-seven percent of the participants used oral contraceptives. Permission for this study was obtained from the Medical Ethics Committee of the Leiden University Medical Center and all participants gave informed consent.
Procedure
Participants were invited preferably in the luteal phase of their menstrual cycle. They were instructed to abstain from alcohol and excessive physical activity during the 24 h before the start of each session, and from caffeine on the day the session took place. At the start of each session, a saliva sample was collected and participants completed a number of questionnaires, e.g. on their use of oral contraceptives and menstrual cycle phase, which might influence the effect of oxytocin administration. The participants then received six puffs of nasal spray containing 4 IU/puff of oxytocin (16 IU total, RVG number 03716, Sandoz b.v.) or six puffs of a placebo-spray (NaCl solution) under supervision of the experimenter. One sibling from each twin pair was randomly assigned to the oxytocin condition and the other sibling to the placebo condition. Participants without a twin sibling were also randomly assigned to the oxytocin and placebo condition. Drug administration was double blind.
Approximately 19 min after the administration of oxytocin or placebo the hand-grip task was administered. In a previous study, we showed that salivary oxytocin levels remain strongly elevated in a stable way up to (at least) 2¼ h after administration of nasal spray containing 16 IU of oxytocin (Huffmeijer et al., submitted for publication), which is longer than the time period between oxytocin administration and completion of the hand-grip task in the current study. Indeed, immediately before the start of the hand-grip task we found significantly higher salivary oxytocin levels in the oxytocin group compared to the placebo group (oxytocin group: M = 206.11, s.d. = 178.10; placebo group: M = 78.52, s.d. = 159.02; P = 0.02, d = 0.78). Participants were not informed about the effects of oxytocin under investigation, only about the possible side effects they might experience (which was required by the ethical committee).
After the experiment, participants received some questionnaires, including a questionnaire on experiences of parental harsh discipline, which they completed at home and returned by mail within two weeks of the experiment.
Harsh discipline
Participants completed an 18-item questionnaire on experiences of parental harsh discipline (Van IJzendoorn and Bakermans-Kranenburg, unpublished data) that was based on the Parent–Child Conflict Tactics Scales (Straus et al., 1998). Items included experiences such as being spanked on the bottom, being kicked hard or hit with a fist, and threats to be sent away or kicked out of the house. Items were scored on a rating scale ranging from 1 = (almost) never to 5 = (almost) always. Scores on two items (‘explained why something was wrong’ and ‘given something else to do instead of what was wrong’) were reversed. Reliability of the scale was satisfactory (α = 0.78). Standardized item scores were added to yield a total score for experiences of parental harsh discipline. Scores were normally distributed, and did not differ for participants in the oxytocin or placebo condition, t(40) = 0.37, P = 0.71.
Handgrip-force task
An adult hand dynamometer was used as an indicator of the use of excessive force during listening to infant laughter and infant crying. The dynamometer (model TSD121C) weighed 315 g and was 185-mm long, 42-mm wide and 30-mm thick, with an isometric range from 0 to 100 kg. Squeeze intensities (in kg) were transferred directly from the dynamometer to the AcqKnowledge software program (version 3.8; Biopac Systems, 2004). Matlab (version 7.8.0, Mathworks, MA, USA) was used to identify peak intensities for each squeeze.
Participants were asked to squeeze the handgrip dynamometer as hard as possible and then at 50% of their maximal handgrip strength. They performed as many trials as necessary for training, with their performance displayed on a monitor to check the 50% level of each second handgrip, until they were able to modulate the force of their second squeeze to half the strength of their first squeeze. Then the monitor was directed away from the participant in order to prevent them from receiving feedback regarding their performance during the remainder of the task.
The handgrip-force task was administered on a laptop using E-Prime software (version 2.0; Psychology Software Tools, Inc., PA, USA). During the task participants were seated in front of a computer screen wearing headphones (type König CMP). As a prompt, the words ‘squeeze maximally’ were displayed briefly in the middle of the screen, after 2 s followed by the prompt ‘squeeze at half strength’, thus prompting the participants to perform a brief firm squeeze followed by a brief squeeze half the strength. After baseline squeezing (no sound), participants were requested to squeeze the handgrip dynamometer eight times at full and half strength, respectively, the first four times listening to infant laughter and then four times listening to infant crying. The infant laughter sound (duration = 2 min, average fundamental frequency = 215.96 Hz, constant volume) and the infant crying sound (duration = 2 min, average fundamental frequency = 360.06, constant volume) from Groh and Roisman (2009) were used. The intervening time between full- and half-strength prompts was 2 s; the intervening time period between half-strength and the next full-strength prompt was 25 s.
Grip strength modulation was calculated by dividing the half-strength squeeze intensity by the full-strength squeeze intensity, so that scores of over 0.50 indicated excessive force on the half-strength squeeze attempt. As a result of fatigue the last trial yielded too many missing data. Therefore we decided to use the first three trials per infant sound condition (laughter and cry), for which we added the numbers of trials with too much physical force (>0.50). Since only few participants never used more than half strength, we distinguished between using excessive force never or only once vs using excessive force two or three times per condition.
RESULTS
Twelve participants (29%) used excessive force once or never during listening to infant laughter, and 16 (38%) used excessive force once or never when exposed to infant cry sounds. Handgrip force was unrelated to participants’ age and educational level (P > 0.23). Similarly, age (P = 0.31) and educational level (P = 0.44) were not associated with experiences of harsh discipline. Menstrual cycle phase and use of oral contraceptives were not related to any of the predictor or outcome variables (P's > 0.16). The oxytocin and placebo group did not show different baseline salivary oxytocin levels (before taking the puffs of oxytocin or placebo, P = 0.31), nor were baseline oxytocin levels related to experiences of harsh discipline (P = 0.41).
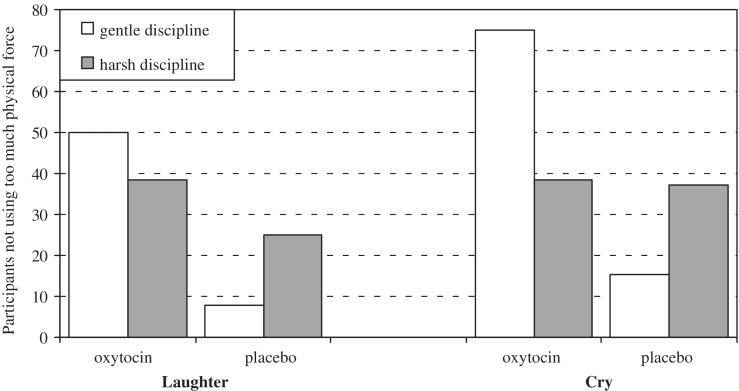
General loglinear regression analyses were conducted to predict the use of excessive force during infant laughter and cry sound with condition (oxytocin vs placebo) and experienced harsh discipline (median split) as well as the interaction between condition and discipline as predictors. The regression model for excessive force during infant laughter was not significant (likelihood ratio 8.20, P = 0.09; no significant predictors), but the model predicting excessive force during infant crying was significant (likelihood ratio 10.16, P = 0.04, d = 0.66). Participants in the oxytocin condition with low scores on harsh discipline less often used excessive force when exposed to infant cry sounds. The results are presented in Figure 1, showing an overrepresentation of these participants among those who rarely used excessive physical force during infant crying (75%, adjusted standardized residual +2.4). For those participants who reported being disciplined harshly, it made no difference whether they were in the oxytocin or in the placebo condition (adjusted standardized residual 0.0).
Fig. 1.
Percentages of participants rarely using too much physical force during infant laughter and crying, with and without oxytocin administration, taking into account their experiences with parental harsh discipline. Gray bars represent participants with high scores on experienced harsh discipline.
DISCUSSION
Oxytocin decreased the use of excessive force when listening to infant crying, but only for those participants who experienced little harsh discipline in their childhood. Our results support and extend previous studies showing differential effects of oxytocin administration, with the quality of childhood caregiving experiences as an important moderating factor (Bartz et al., 2010; Huffmeijer et al., submitted for publication; Van IJzendoorn et al., in press).
The significance of infant crying and the variety in responses to infant crying cannot be overstated. Infant crying signals the infant's need for safety, protection and comfort, and from an evolutionary perspective, crying serves the purpose of eliciting parental proximity and care. Mothers’ affective responses to infant crying were found predictive of their sensitive care of the infant when distressed, which in turn predicted attachment security with more explanatory power than maternal sensitivity to their infants when they were not distressed (McElwain and Booth-LaForce, 2006). On the dark side, using reports on age-specific incidences of hospitalized cases of Shaken Baby Syndrome from California hospitals in the period 1996–2001, Barr et al. (2006) presented evidence for crying as a trigger to violent shaking, resulting in head trauma or fractures of the long bones or ribs. In the Netherlands, 5.6% of parents with a 6-month-old infant reported having smothered, slapped or shaken their baby at least once because of its crying (Reijneveld et al., 2004).
In our previous study, we found activation in the amygdala when participants listened to infant crying compared to the control sounds—an activation that was reduced for participants with experimentally induced oxytocin levels (Riem et al., 2011). Decreased amygdala activation might promote sensitive responsiveness to infant crying rather than more impatient or harsh responses by preventing parents from being overwhelmed by anxious or aversive feelings. Stress-reducing effects of oxytocin in lactating mothers have been documented as well (Heinrichs et al., 2001; Heinrichs et al., 2002). Whereas oxytocin reduced neural activation in the amygdala, it increased activation in regions associated with empathy and mother–infant bonding, the insula and the inferior frontal gyrus pars triangularis (Riem et al., 2011). While annoyance and anxiety may lead parents to punish the infant for displaying negative affect in order to reduce their exposure to distress (Out et al., 2010; Leerkes et al., 2011) increased empathy and reduced anxiety and aversion may effectively prevent the use of excessive force in response to infant crying.
The effects of oxytocin were confined to the exposure to infant crying sounds and not significant during infant laughter. Strathearn and colleagues found that dopaminergic reward-related brain regions were activated specifically in response to happy, but not sad, infant faces (Strathearn et al., 2008). On the other hand, similar physiological overreactions to crying and smiling infant stimuli in adults at risk for maltreatment have been reported (Frodi and Lamb, 1980; Pruitt and Erickson, 1985). Future studies may unravel the convergence and divergence of the processes underlying adult response to laughter and crying—the two infant cues that create and maintain caregiver proximity and are both essential to survival (Bowlby, 1969/1982; Van Hooff, 1972).
One of the limitations of our study is the use of a between-subjects design, implying the risk of pre-existing differences between the oxytocin and placebo group. However, the majority of our participants were mono- and dizygotic twin pairs, perfectly matched on age and global child rearing experiences, and even on genotype in monozygotic twin pairs. Indeed, we did not find any difference on baseline salivary oxytocin levels or harsh discipline experiences between the oxytocin and placebo groups. Second, our sample consisted of women without children, and the generalizability to mothers’ responses to their own and other infants’ cry sounds should be examined in future studies. The advantage of our sample is the homogeneity in terms of parental exposure to infant laughter and caregiving responsibilities for (excessively) crying infants. Moreover, effects of oxytocin have been found to be gender specific, and studies on human females are underrepresented (see for a review Bos et al., in press).
In line with our expectations, the positive effects of oxytocin were observed only for participants without experiences of parental harsh discipline. This points to early caregiving experiences as an important moderator of the stress-reducing effect of oxytocin. In the majority of experimental studies with intranasal oxytocin administration, this factor has not been taken into account (with the noteworthy exceptions of Bartz et al., 2010a, 2010b; Huffmeijer et al., submitted for publication; Van IJzendoorn et al., in press), leaving open the possibility that reported main effects of oxytocin administration on enhanced interpersonal trust, empathic concern and positive parenting are actually underestimates of the effects in specific subgroups.
At the same time these findings underscore the idea that oxytocin is not an all-purpose ‘love hormone’ or attachment panacea (Bartz et al., 2010). At least two factors should be taken into account. First, oxytocin may enhance aggression rather than trust with regard to out-groups (the ‘tend and defend’ response, De Dreu et al., 2010), though we did not find such an effect in the current study (converging with a recent meta-analysis of the extant literature by Van IJzendoorn and Bakermans-Kranenburg, in press). Oxytocin was found to increase jealousy or gloating when an unknown (putative) other player gained or lost money (Shamay-Tsoory et al., 2009). Testing the effect of prior social contact, DeClerck et al. (2010) found that oxytocin stimulated cooperation in participants who socially interacted before the cooperation game, but reduced cooperation when they had not met other participant before.
Second, oxytocin may increase trust and positive affect only in individuals with relatively favorable childhood relationship experiences. Meinlschmidt and Heim (2007) found that oxytocin administration reduced cortisol levels, indexing reduced stress levels, in adults who had not experienced any major childhood adversity, but did not bring about this effect in adults who endured parental separation early in life. We found in a previous study that oxytocin increased donating to a charity only in individuals who did not feel rejected by their parents (Van IJzendoorn et al., in press). In case of high love withdrawal, oxytocin administration may be ineffective (as in the current study with experiences of harsh parenting) or even aggravate the negative feelings about self and significant others, leading to smaller donations (Van IJzendoorn et al., in press), decreased trust (Bartz et al., 2010b) or more negative memories (Bartz et al., 2010a).
These two moderating factors (the perspective of the out-group and the quality of early social relationships) may not be independent. Individuals with unfavorable early caregiving experiences are more inclined to attribute hostile intentions to the social environment that is not considered a warm in-group but a potentially untrustworthy out-group (Bowlby, 1969/1982; Belsky 1988). The latent aggressive tendencies that are called for in an unsafe environment may not be lessened or even be intensified by experimentally induced oxytocin levels. On the other hand, oxytocin may increase trust and cooperation in individuals with more supportive backgrounds when they perceive the situation as unthreatening—which they naturally tend to do on the basis of their positive experiences with social interaction. Hormones yield different behavioral effects in different or differently perceived conditions and contexts (Bartz et al., 2011; Bos et al., in press); so from an evolutionary perspective these different results point in the same direction.
In future oxytocin administration studies, the oxytocin receptor genotype (OXTR) might be taken into account as OXTR has been shown to affect sensitivity to infant signals in a direct way (Bakermans-Kranenburg and Van IJzendoorn, 2008), and OXTR might also influence the effectiveness of intranasally administered oxytocin. With respect to treatment implications, we note that the importance of prior attachment experiences moderating the effects of oxytocin should tone down high expectations of oxytocin administration as a stand-alone means of intervention to increase parental sensitivity or social trust in general. The positive effects of oxytocin may be confined to those individuals who are least in need of such an intervention because of their favorable backgrounds. This scenario illustrates the so-called Matthew effect (Bakermans-Kranenburg et al., 2005) that those who fare well, tend to gain more from the intervention, whereas those who are already at a disadvantage would not profit. However, one fruitful strategy might be to combine oxytocin pharmacotherapy with psychosocial intervention (Bartz et al., 2011; Van IJzendoorn and Bakermans-Kranenburg, in press). Oxytocin might serve as a catalyst of intervention efforts in the context of a supportive therapeutic relationship (Bowlby, 1988), which is a crucial component of any effective psychosocial intervention.
Conflict of Interest
None declared.
Acknowledgments
The Netherlands Organization for Scientific Research (VICI Grant No. 453-09-003, to M.J.B.-K.); SPINOZA prize (to M.H.vI.J.)
REFERENCES
- Alvares GA, Hickie IB, Guastella AJ. Acute effects of intranasal oxytocin on subjective and behavioral responses to social rejection. Experimental and Clinical Psychopharmacology. 2010;18:316–21. doi: 10.1037/a0019719. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Social Cognitive and Affective Neuroscience. 2008;3:128–34. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, Van IJzendoorn MH, Bradley RH. Those who have, receive: the Matthew effect in early childhood intervention in the home environment. Review of Educational Research. 2005;75:1–26. [Google Scholar]
- Barr RG, Trent RB, Cross J. Age-related incidence curve of hospitalized shaken baby syndrome: convergent evidence for crying as a trigger to shaking. Child Abuse and Neglect. 2006;30:7–16. doi: 10.1016/j.chiabu.2005.06.009. [DOI] [PubMed] [Google Scholar]
- Bartz J, Simeon D, Hamilton H, et al. Oxytocin can hinder trust and cooperation in borderline personality disorder. Social Cognitive and Affective Neuroscience. 2010b;6:556–63. doi: 10.1093/scan/nsq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Bolger N, Ochsner KN. Social effects of oxytocin in humans: context and person matter. Trends in Cognitive Sciences. 2011;15:301–309. doi: 10.1016/j.tics.2011.05.002. [DOI] [PubMed] [Google Scholar]
- Bartz JA, Zaki J, Ochsner KN, et al. Effects of oxytocin on recollections of maternal care and closeness. PNAS. 2010a;107(50):21371–5. doi: 10.1073/pnas.1012669107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner T, Heinrichs M, Vonlanthen A, Fischbacher U, Fehr E. Oxytocin shapes the neural circuitry of trust and trust adaptation in humans. Neuron. 2008;58:639–50. doi: 10.1016/j.neuron.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Belsky J. Modern evolutionary theory and patters of attachment. In: Cassidy J, Shaver PR, editors. Handbook of Attachment. Theory, Research, and Clinical Applications. New York: Guilford; 1988. pp. 141–61. [Google Scholar]
- Bos PA, Panksepp J, Bluthé RM, Honk JV. Acute effects of steroid hormones and neuropeptide on human social-emotional behavior: a review of administration studies. Frontiers in Neuroendocrinology. in press doi: 10.1016/j.yfrne.2011.01.002. [DOI] [PubMed] [Google Scholar]
- Bowlby J. Attachment and Loss. Vol. 1. New York: Basic Books; 1969/1982. [Google Scholar]
- Bowlby J. London: Routledge; 1988. A secure base: clinical applications of attachment theory. [Google Scholar]
- Bugental DB, Blue J, Cruzcosa M. Perceived control over caregiving outcomes: implications for child abuse. Developmental Psychology. 1989;25:532–9. [Google Scholar]
- Bugental DB, Lewis JC, Lin E, Lyon J, Kopeikin H. In charge but not in control: the management of teaching relationships by adults with low perceived power. Developmental Psychology. 1999;35:1367–78. doi: 10.1037//0012-1649.35.6.1367. [DOI] [PubMed] [Google Scholar]
- Campbell A. Oxytocin and human social behavior. Personality and Social Psychology Review. 2010;14:281–95. doi: 10.1177/1088868310363594. [DOI] [PubMed] [Google Scholar]
- Carter CS, Altemus M. Integrative functions of lactational hormones in social behavior and stress management. Annals of the New York Academy of Science. 1997;807:164–74. doi: 10.1111/j.1749-6632.1997.tb51918.x. [DOI] [PubMed] [Google Scholar]
- Crouch JL, Skowronski JJ, Milner JS, Harris B. Parental responses to infant crying: the influence of child physical abuse risk and hostile priming. Child Abuse and Neglect. 2008;23:702–10. doi: 10.1016/j.chiabu.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Declerck CH, Boone C, Kiyonari T. Oxytocin and cooperation under conditions of uncertainty: the modulating role of incentives and social information. Hormones and Behavior. 2010;57:368–74. doi: 10.1016/j.yhbeh.2010.01.006. [DOI] [PubMed] [Google Scholar]
- De Dreu CKW, Greer LL, Handgraaf MJJ, et al. The neuropeptide oxytocin regulates parochial altruism in intergroup conflict among humans. Science. 2010;328:1408–11. doi: 10.1126/science.1189047. [DOI] [PubMed] [Google Scholar]
- Del Vecchio T, Walter A, O'Leary SG. Affective and physiological factors predicting maternal response to infant crying. Infant Behavior and Development. 2009;32:117–22. doi: 10.1016/j.infbeh.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Euser ME, Van IJzendoorn MH, Prinzie P, Bakermans-Kranenburg MJ. Prevalance of child abuse in the Netherlands. Child Maltreatment. 2010;15:5–17. doi: 10.1177/1077559509345904. [DOI] [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendrocrinological foundation of human affiliation. Psychological Science. 2007;18:965–70. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Frodi AM. When empathy fails: aversive infant crying and child abuse. In: Lester BM, Boukydis CFZ, editors. Infant Crying: Theoretical and Research Perspectives. New York: Plenum Press; 1985. pp. 263–78. [Google Scholar]
- Frodi AM, Lamb ME. Child abusers’ responses to infant smiles and cries. Child Development. 1980;51:238–41. [PubMed] [Google Scholar]
- Groh AS, Roisman GI. Adults’ autonomic and subjective responses to Infant vocalizations: the role of secure base script knowledge. Developmental Psychology. 2009;45:889–93. doi: 10.1037/a0014943. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Meinlschmidt G, Neumann I, Wagner S, Kirschbaum, Ehlert U, et al. Effects of suckling on hypothalamicpituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. The Journal of Clinical Endocrinology and Metabolism. 2001;86:4798–804. doi: 10.1210/jcem.86.10.7919. [DOI] [PubMed] [Google Scholar]
- Heinrichs M, Neumann I, Ehlert U. Lactation and stress: protective effects of breast-feeding in humans. Stress. 2002;5:195–203. doi: 10.1080/1025389021000010530. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, et al. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. Journal of Neuroscience. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. A neurobiological basis of social attachment. American Journal of Psychiatry. 1997;154:726–35. doi: 10.1176/ajp.154.6.726. [DOI] [PubMed] [Google Scholar]
- Leerkes EM. Predictors of maternal sensitivity to infant distress. Parenting Science and Practice. 2010;10:219–39. doi: 10.1080/15295190903290840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leerkes EM, Parade SH, Gudmundson JA. Mothers’ emotional reactions to crying pose risk for subsequent attachment insecurity. Journal of Family Psychology. 2011;25:635–43. doi: 10.1037/a0023654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElwain NL, Booth-LaForce C. Maternal sensitivity to infant distress and nondistress as predictors of infant-mother attachment security. Journal of Family Psychology. 2006;20:247–55. doi: 10.1037/0893-3200.20.2.247. [DOI] [PubMed] [Google Scholar]
- Meinlschmidt G, Heim C. Sensitivity to intranasal oxytocin in adult men with early parental separation. Biological Psychiatry. 2007;61:1109–11. doi: 10.1016/j.biopsych.2006.09.007. [DOI] [PubMed] [Google Scholar]
- Murray AD. Aversiveness is in the mind of the beholder: perception of infant crying by adults. In: Lester B, Boukydis CF, editors. Infant Crying. New York: Plenum Press; 1985. pp. 217–39. [Google Scholar]
- Naber F, Van IJzendoorn MH, Deschamps P, Van Engeland H, Bakermans-Kranenburg MJ. Intranasal oxytocin increases fathers’ observed responsiveness during play with their children: a double-blind within-subject experiment. Psychoneuroendocrinology. 2010;35:1583–6. doi: 10.1016/j.psyneuen.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Out D, Pieper S, Bakermans-Kranenburg MJ, Zeskind PS, Van IJzendoorn MH. Intended sensitive and harsh caregiving responses to infant crying: the role of cry pitch and perceived urgency in an adult twin sample. Child Abuse and Neglect. 2010;34:863–73. doi: 10.1016/j.chiabu.2010.05.003. [DOI] [PubMed] [Google Scholar]
- Pruitt DL, Erickson MT. The child abuse potential inventory: a study of concurrent validity. Journal of Clinical Psychology. 1985;41:104–11. doi: 10.1002/1097-4679(198501)41:1<104::aid-jclp2270410119>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Reijneveld SA, Van der Wal MF, Brugman E, Sing RA, Verloove-Vanhorick SP. Infant crying and child abuse. Lancet. 2004;364:1340–2. doi: 10.1016/S0140-6736(04)17191-2. [DOI] [PubMed] [Google Scholar]
- Riem MME, Bakermans-Kranenburg MJ, Pieper S, et al. Oxytocin modulates amygdala, insula and inferior frontal gyrus responses to infant crying: a randomized controlled trial. Biological Psychiatry. in press;70:291–97. doi: 10.1016/j.biopsych.2011.02.006. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Fischer M, Dvash J, Harari H, Perach-Bloom N, Levkovitz Y. Intranasal administration of oxytocin increases envy and Schadenfreude (Gloating) Biological Psychiatry. 2009;66:864–70. doi: 10.1016/j.biopsych.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Sroufe LA, Egeland B, Carlson E, Collins WA. Placing early attachment experiences in developmental context: the Minnesota longitudinal study. In: Grossmann KE, Grossmann K, Waters E, editors. Attachment from Infancy to Adulthood. New York: Guilford Press; 2005. pp. 48–70. [Google Scholar]
- Strassberg Z, Dodge KA, Pettit GS, Bates JE. Spanking in the home and children s subsequent aggression toward kindergarten peers. Development and Psychopathology. 1994;6:445–61. [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34:2655–66. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR. What's in a smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122:40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus MA, Hamby SL, Finkelhor D, Moore DW, Runyan D. Identification of child maltreatment with the parent-child conflict tactics scales: development and psychometric data from a national sample of American parents. Child Abuse and Neglect. 1998;22:249–70. doi: 10.1016/s0145-2134(97)00174-9. [DOI] [PubMed] [Google Scholar]
- Theodoridou A, Rowe AC, Penton-Voak IS, Rogers PJ. Oxytocin and social perception: oxytocin increases perceived facial trustworthiness and attractiveness. Hormones and Behavior. 2009;56:128–32. doi: 10.1016/j.yhbeh.2009.03.019. [DOI] [PubMed] [Google Scholar]
- Van Hooff J. A comparative approach to the phylogeny of laughter and smiling. In: Hinde RA, editor. Non-Verbal Communication. Oxford, England: Cambridge University Press; 1972. pp. 208–41. [Google Scholar]
- Van IJzendoorn MH, Bakermans-Kranenburg MJ. A sniff of trust: meta-analysis of the effects of intranasal oxytocin administration on face recognition, trust to in-group, and trust to out-group. Psychoneuroendocrinology. in press doi: 10.1016/j.psyneuen.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Van IJzendoorn MH, Huffmeijer R, Alink LRA, Tops M, Bakermans-Kranenburg MJ. The impact of oxytocin administration on donating behavior: moderation by parental love-withdrawal. Frontiers in Developmental Psychology. in press doi: 10.3389/fpsyg.2011.00258. [DOI] [PMC free article] [PubMed] [Google Scholar]