Abstract
Corn (Zea mays L.) root adaptation to pH 3.5 in comparison with pH 6.0 (control) was investigated in long-term nutrient solution experiments. When pH was gradually reduced, comparable root growth was observed irrespective of whether the pH was 3.5 or 6.0. After low-pH adaptation, H+ release of corn roots in vivo at pH 5.6 was about 3 times higher than that of control. Plasmalemma of corn roots was isolated for investigation in vitro. At optimum assay pH, in comparison with control, the following increases of the various parameters were caused by low-pH treatment: (a) hydrolytic ATPase activity, (b) maximum initial velocity and Michaelis constant (c) activation energy of H+-ATPase, (d) H+-pumping activity, (e) H+ permeability of plasmalemma, and (f) pH gradient across the membranes of plasmalemma vesicles. In addition, vanadate sensitivity remained unchanged. It is concluded that plasmalemma H+-ATPase contributes significantly to the adaptation of corn roots to low pH. A restricted net H+ release at low pH in vivo may be attributed to the steeper pH gradient and enhanced H+ permeability of plasmalemma but not to deactivation of H+-ATPase. Possible mechanisms responsible for adaptation of plasmalemma H+-ATPase to low solution pH during plant cultivation are discussed.
Acid soils make up to 40% of the world′s arable land (Kochian, 1995). Plant growth and development on acid soils may be affected by high levels of Al and Mn, as well as by limited availability of various nutrients (Adams, 1981). On the other hand, low pH (high H+ activity) in root medium (pHe) may directly inhibit plant growth (Islam et al., 1980; Schubert et al., 1990). Mechanisms of Al toxicity have been studied extensively during the last decade (Kochian, 1995), whereas the understanding of H+ toxicity in plants remains poor. It has been observed that root growth rate was related to net H+ release, which may be restricted at low pHe. Therefore, it has been suggested that H+ homeostasis of plant root cells may be influenced by low pHe, resulting in the reduction of root growth rate (Yan et al., 1992). Net H+ release results from H+ efflux driven by plasmalemma H+-ATPase activity and from H+ influx following the plasmalemma H+ gradient. Reduced net H+ release may be attributed to a decrease in H+ pump activity, an increase in plasmalemma H+ permeability, or both. Because of its overall importance in physiological processes, the plasmalemma H+-ATPase has been investigated extensively during the last two decades. This enzyme has been found to respond to a number of environmental factors, such as saline stress (Braun et al., 1986; Ayala et al., 1996), nutrient supply (Kuiper et al., 1991; Santi et al., 1995; Schubert and Yan, 1997), high-O2 treatment (Pinton et al., 1996; Xia and Roberts, 1996), mechanical stimulation (Bourgeade and Boyer, 1994), and fusicoccin, a fungal toxin (Marré, 1979). Although there are reports in the literature describing a response of yeast H+-ATPase to low pHe (Eraso and Gancedo, 1987), investigations concerning the response of plasmalemma H+-ATPase of higher plant cells to low pHe are surprisingly scarce. Also, it remains unknown whether the plasmalemma H+ permeability changes when roots are exposed to low pHe.
In this study we investigated the contribution of plasmalemma H+-ATPase of corn (Zea mays L.) roots to low-pHe adaptation. In a long-term experiment with low-pH treatment, pHe was gradually reduced to 3.5, whereas pHe in the control was kept constant at 6.0. H+ release by intact roots was studied in vivo. Furthermore, plasmalemma of corn roots was isolated from adapted and nonadapted plants. In vitro, plasmalemma H+-ATPase activity, activation energy, kinetic characteristics, H+-pumping activity, and H+ permeability were investigated. The aim of our investigation was to reveal the mechanisms by which plant root cells adapt to low pHe.
MATERIALS AND METHODS
Plant Cultivation
Corn (Zea mays L. cv Blizzard) seeds were soaked in aerated 0.5 mm CaSO4 for 1 d and germinated at 25°C in the dark between two layers of filter paper moistened with 0.5 mm CaSO4. After 4 d seedlings were transferred to a container with 400 L of one-fifth-concentrated nutrient solution. Plants were grown in a growth chamber under controlled conditions. Fluorescent lamps (66% L58/31 and 34% L58 W/78, Osram, Frankfurt, Germany) gave a light intensity of approximately 50 W m−2, with a day/night cycle of 16 h/8 h at 25°C. RH was 80%. After 2 and 4 d of cultivation the concentration of the nutrient solution was increased to a one-half and a full-strength concentration, respectively. The full-strength nutrient solution had the following composition: 1 mm NH4NO3, 0.2 mm NaH2PO4, 1 mm K2SO4, 2 mm CaCl2, 3 mm MgSO4, 0.2 μm H3BO3, 0.2 μm CuSO4, 0.01 μm (NH4)6Mo7O24, 5 μm MnSO4, 0.2 μm ZnSO4, and 200 μm Fe-EDTA. During the growth period the concentrations of nitrogen, phosphate, and potassium were controlled every 3 d and substituted if necessary. There was no significant depletion of other nutrients in the solution. The pH of the control solution was kept constant at pH 6.0 by continuous titration with 0.1 m NaOH using a pH stat system. In the low-pH treatment, acidity was gradually enhanced by increasing H+ activity by increments of 100 μm daily to a final pH of 3.5. Plants were allowed to grow at the final pH for 4 d and were then used for proton release studies and plasmalemma isolation at the age of about 3 weeks. To investigate the effect of low pHe on root growth, plant cultivation was extended to 33 d.
Measurement of Root Growth and Net Proton Release by Roots
To measure root growth, plants were harvested three times after cultivation for 19, 27, and 33 d. Root fresh weights and the number of laterals were determined. Main root length was measured with a ruler. Roots of intact plants used for the study of net H+ release were washed three times for 5 min each time in a solution composed of 1 mm CaSO4, 0.5 mm K2SO4, and 0.5 mm Na2SO4. In an identical solution net H+ release was quantified in 2.5 L per four plants. The initial pH of this test solution was adjusted to 5.6 and 3.4, respectively, by titration with H2SO4. Net H+ release was calculated from the pH change of the solution.
Plasmalemma Isolation
A microsomal membrane fraction was prepared as described by Faraday and Spanswick (1992) with some modifications. Roots of 3-week-old plants were cut and washed three times with chilled, deionized water. Roots were cut into 1-cm segments and ground in ice-cold homogenization buffer with a mortar and pestle. The homogenization buffer contained 250 mm Suc, 2 mm EGTA, 10% (v/v) glycerol, 0.5% (w/v) BSA, 2 mm DTT, 1 mm PMSF, 5 mm 2-mercaptoethanol, and 50 mm BTP, adjusted to pH 7.8 with Mes. The homogenate (adjusted to a grinding medium/tissue ratio of 4 mL/g fresh weight) was filtered through two layers of Miracloth (Calbiochem) and centrifuged in a swinging bucket rotor at 11,500g (Sorvall AH-629 rotor, 36 mL) for 10 min at 0°C. The supernatants were centrifuged at 87,000g for 35 min. The microsomal pellets were resuspended in phase buffer (250 mm Suc, 3 mm KCl, and 5 mm KH2PO4, pH 7.8).
The microsomal membrane preparation was fractionated by two-phase partitioning in aqueous dextran T-500 (Sigma) and PEG-3350 (Sigma) according to the method of Larsson (1985). Phase separations were carried out in a series of 32-g phase systems that contained 6.2% (w/w) of each polymer dissolved in phase buffer (see above). Stock solutions of polymers were prepared with concentrations of 20 and 40% (w/w) for dextran and PEG, respectively. The concentration of dextran stock solution was determined by optical rotation (Larsson, 1985). The phase stock was weighed and diluted to 6.2% (w/w, each polymer) with phase buffer to a final weight of 32 g. Polymers in “start tubes” were, however, diluted to 26 g. Six grams of microsomal resuspension (in phase buffer) was added to the upper phase of each start tube. The tubes were sealed with Parafilm (American National Can, Greenwich, CT) and mixed by inversion (30 times). Phase separation was achieved at 4°C by centrifugation at 720g (Sorvall AH-629 rotor, 36 mL) for 23 min followed by two washing steps in identical phases. Centrifugation times for the second through fourth separation were 15, 10, and 5 min, respectively. The upper phases obtained after four separations were diluted with phase buffer (see above) and centrifuged at 151,200g for 40 min. The pellets were washed with resuspension buffer (250 mm Suc, 3 mm KCl, and 5 mm BTP/Mes, pH 7.8) and pelleted again. The pellets were resuspended in resuspension buffer, divided into aliquots, and immediately stored in liquid nitrogen. Protein was quantified according to the method of Bradford (1976) using BSA (Sigma) as a standard.
Enzyme Assays
Hydrolytic ATPase activity was determined in 0.5 mL of 30 mm BTP/Mes buffer containing 5 mm MgSO4, 50 mm KCl, 0.02% (w/v) Brij 58 (Sigma), and 5 mm disodium-ATP. Reactions were initiated by the addition of 1 μg of membrane protein, proceeded for 30 min at 30°C, and stopped with 1 mL of stopping reagent (2% [v/v] concentrated H2SO4, 5% [w/v] SDS, 0.7% [w/v] (NH4)2MoO4) followed immediately by 50 μL of 10% (w/v) ascorbic acid. After 10 min 1.45 mL of arsenite-citrate reagent (2% [w/v] sodium citrate, 2% [w/v] sodium m-arsenite, and 2% [w/v] glacial acetic acid) was added to prevent the measurement of phosphate liberated because of ATPase activity from ATP hydrolysis under acidic conditions (Baginski et al., 1967). Color development was complete after 30 min and A820 was measured by means of a spectrophotometer (U3200, Hitachi, Tokyo, Japan). ATPase activity was calculated as phosphate liberated in excess of boiled-membrane control. Plasmalemma-bound ATPase activity was determined as the difference in activity between assays with and without addition of 0.1 mm Na3VO4. The kinetic characteristics of plasmalemma ATPase were studied in the presence of an ATP-generating system that included 5 units of pyruvate kinase (Sigma) and 5 mm PEP (Boehringer Mannheim; Sekler and Pick, 1993). Vmax and Km were determined by means of a regression analysis. Activation energy of ATPase was calculated using the Arrhenius equation from Vmax values determined at 20 and 30°C, respectively.
pH Gradient
The formation of a pH gradient across the plasmalemma of inside-out vesicles was measured as the quenching of A492 by AO. The assay mixture contained 5 mm BTP/Mes (pH 6.5), 7.5 μm AO, 100 mm KCl, 1 mm NaN3, 1 mm Na2MoO4, 0.05% (w/v) Brij 58, 50 μg of membrane protein in a final volume of 1.5 mL. Brij 58 was used to create inside-out vesicles (Johansson et al., 1995). After equilibration of the membrane vesicles with the reaction medium (about 10 min), the reaction was initiated by the addition of Mg-ATP (mixture of MgSO4 and disodium-ATP, adjusted to pH 6.5 with BTP) to give a final concentration of 5 mm. The reaction temperature was 25°C.
Determination of Membrane Lipids
Total plasmalemma lipids were determined according to the work of Bligh and Dyer (1959). Lipids were extracted by adding 1.6 mL of membrane to a solution consisting of 4 mL of chloroform, 4 mL of isopropanol, and 2 mL of water. After chloroform was evaporated, lipids were determined gravimetrically. Then, lipids were digested with perchloric acid and the released phosphate was quantified with the method of Fiske and SubbaRow (Dittmer and Wells, 1969). The amount of phospholipids was calculated by assuming an average Mr of 800.
Statistical Treatment
Variation is indicated by ± se (if bars exceed symbols in figures). Significant differences between treatments were calculated by using the Student's t test.
RESULTS
Adaptation of Corn Roots to Low pHe
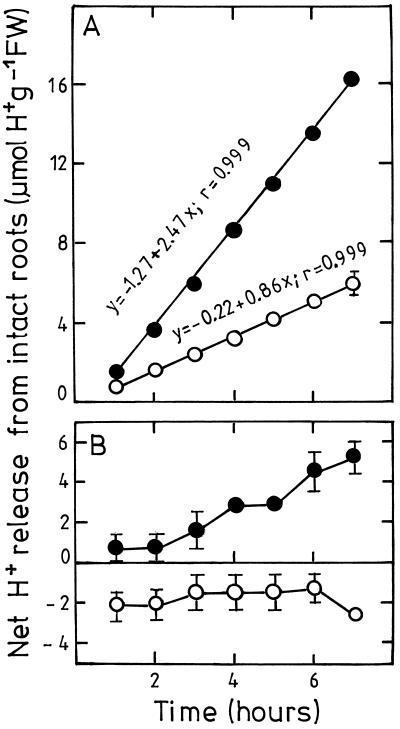
We reported previously that an abrupt decrease in solution pH markedly reduced the root growth rate of corn seedlings (Yan et al., 1992). In contrast, in the present study we found that corn roots were capable of growing normally even at pHe 3.5 when nutrient solution pH was decreased gradually. During an experimental period from 19 to 33 d no difference between pHe 3.5 and 6.0 was found for root fresh weight, main root length, or the number of laterals (Table I). In addition, roots grown at pHe 3.5 were harder and looked brighter than those grown at pHe 6.0. In a simple test solution (pH 5.6) roots grown at pHe 3.5 showed about 3 times higher net H+ release than control roots (Fig. 1A). This could not be attributed to passive release of protons from the apoplast because roots were thoroughly washed with the test solution three times before being transferred into test solution. Also, the fact that substantial H+ release lasted for more than 8 h indicates enhanced active H+ pumping of corn root cells after adaptation to low pHe. This holds true even when the test solution pH was initially adjusted to 3.4 (Fig. 1B). Under this condition control roots showed net H+ uptake.
Table I.
Effect of adaptation to low pHe on corn root growth
| Solution pHe | Root
Fresh Wt
|
Main Root Length at 33 d | Laterals at 33 d | ||
|---|---|---|---|---|---|
| 19 d | 27 d | 33 d | |||
| g plant−1 | cm | no. plant−1 | |||
| 6.0 | 2.68 (±0.04) | 4.56 (±0.08) | 6.06 (±0.60) | 76.6 (±2.1) | 1271 (±108) |
| 3.5 | 2.54 (±0.12) | 4.60 (±0.16) | 6.04 (±0.40) | 71.8 (±3.3) | 1508 (±130) |
Main root length and the number of laterals were determined after 27 d of cultivation at different nutrient solution pH. In the low-pHe treatment nutrient solution pH was gradually reduced to 3.5 within the first 14 d of cultivation. The values represent means (± se) of six plants.
Figure 1.
Effect of low nutrient-solution pH during cultivation on the time course of net H+ release by roots of intact corn plants. Plants were adapted for 3 weeks in nutrient solution at pHe 6.0 (○) and 4 d at pHe 3.5 after gradually decreasing pHe (•). Test medium consisted of 1 mm CaSO4, 0.5 mm K2SO4, and 0.5 mm Na2SO4 with pH 5.6 (A) and 3.4 (B). Negative values denote H+ uptake. Values represent means ± se of six replications. FW, Fresh weight.
Effect of Low pHe on the Isolation of Plasmalemma from Corn Roots
A summary of relative ATPase-specific activities associated with phase-partitioned corn root plasmalemma is presented in Table II. At assay pH 6.5 both nitrate and azide showed no inhibition effect on the ATPase activities. Similar results were obtained at assay pH 8.0 (not shown). Molybdate inhibited specific ATPase activity by about 5%, which indicates the presence of soluble phosphatase. On the other hand, specific ATPase activity of the isolated membrane fraction was highly sensitive to vanadate. Fifty percent inhibition was measured at 3.8 (± 0.2) and 3.3 (±0.2) μm vanadate for adapted and nonadapted membrane, respectively, and more than 95% inhibition was achieved at 500 μm vanadate (not shown). These data indicate that the isolated membrane fraction from corn roots is highly enriched with plasmalemma. This is in agreement with results of Faraday and Spanswick (1992). Furthermore, it is also evident from Table II that pHe during plant cultivation did not affect the isolation of plasmalemma from corn roots by aqueous polymer two-phase partitioning.
Table II.
Effect of corn root adaptation to low pHe on relative ATPase activities after treatment with various inhibitors
| Treatment | Relative ATPase Activity
|
|
|---|---|---|
| pHe 6.0 | pHe 3.5 | |
| Control | 100.0 | 100.0 |
| Nitrate (50 mm) | 109.4 (±2.9) | 109.0 (±4.1) |
| Azide (1 mm) | 102.4 (±0.9) | 102.7 (±1.0) |
| Molybdate (1 mm) | 94.8 (±2.7) | 90.9 (±0.8) |
| Vanadate (0.1 mm) | 17.6 (±1.5) | 17.1 (±0.5) |
Assays were conducted at pH 6.5. Membranes were isolated from corn roots cultivated at pHe 6.0 and 3.5, respectively. The values represent means (± se) of four isolations.
Effect of Low pHe during Plant Cultivation on Plasmalemma ATPase Activity
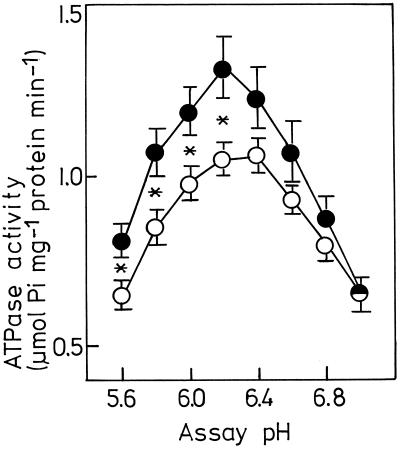
To investigate the effect of low pHe on plasmalemma ATPase activity, we measured the difference in specific ATPase activities with and without 100 μm vanadate and defined this difference as plasmalemma ATPase activity. In an assay pH range from 5.6 to 6.2 the plasmalemma derived from corn roots grown at pHe 3.5 showed about 20% higher ATPase activity than that grown at pHe 6.0 (Fig. 2). This increase in plasmalemma ATPase activity by low pHe during plant cultivation was less pronounced at higher assay pH and vanished at assay pH 7.0. Figure 2 also indicates a small variation of pH optimum for ATPase activity after adaptation to low pH. Maximum ATPase activity for roots grown at pHe 3.5 was reached at assay pH 6.2, whereas ATPase activity for control roots showed an optimum between 6.2 and 6.4. This is very close to the optimum pH 6.5 reported earlier for plasmalemma H+-ATPase activity of corn roots (De Michelis and Spanswick, 1986; Cowan et al., 1993). In addition, plasmalemma ATPase of corn roots grown at pHe 3.5 showed higher sensitivity to a change in assay pH from 7.0 to 6.4. In this range the pH dependence of ATPase activity was 0.40 and 0.58 μmol Pi mg−1 protein min−1 for control and adapted roots, respectively.
Figure 2.
Effect of low nutrient-solution pH during plant cultivation on pH-dependent plasmalemma ATPase activity. Plasmalemma was isolated from 3-week-old corn roots grown at pHe 6.0 (○) and 3.5 (•). Plasmalemma ATPase activity is expressed as the difference of activity assayed with and without 0.1 mm Na3VO4. Values represent means ± se of four replications. *, Significant difference at 5% level.
Effect of Low pHe during Plant Cultivation on the Kinetic Characteristics and the Activation Energy of Plasmalemma ATPase
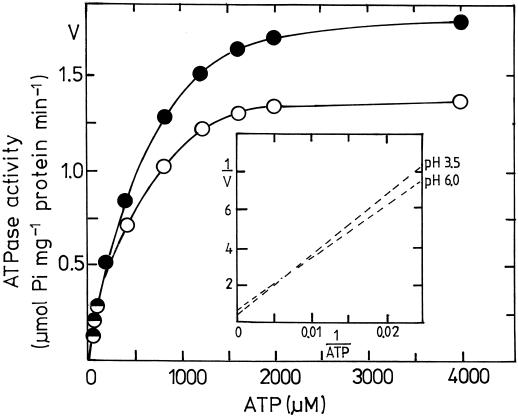
To gain a deeper insight into the effect of low pHe on the enzymatic properties of corn root ATPase, we analyzed the kinetic characteristics of plasmalemma ATPase at ATP concentrations from 20 to 4000 μm. In this concentration range plasmalemma ATPase revealed typical Michaelis-Menten kinetics (Fig. 3), as found before by other authors (Cowan et al., 1993). At an assay temperature of 30°C, plasmalemma ATPase from low-pHe (adapted) roots showed higher Vmax and Km than that from high-pHe (control) roots (Table III). This was found both at assay pH 6.5 and 7.0. However, at an assay temperature of 20°C, the difference between treatments (pHe 3.5 and 6.0) became insignificant. Because quantification of ATPase activity was based on total membrane protein but not pure ATPase enzyme protein, it was not evident whether the increase in Vmax was caused by a relative increase in the amounts of ATPase molecules or in the hydrolytic efficiency of the enzyme per se.
Figure 3.
Effect of low nutrient-solution pH during plant cultivation on the kinetic characteristics of corn root plasmalemma ATPase. Plasmalemma was isolated from 3-week-old corn roots grown at pHe 6.0 (○) and 3.5 (•). Activity of plasmalemma ATPase was determined in an assay solution of pH 6.5, consisting of 100 mm KNO3, 1 mm NaN3, and 1 mm Na2MoO4. The concentration of Mg-ATP in the assay solution was kept constant in the range of 20 to 4000 μm. The inset represents a double-reciprocal plot of the effect of ATP concentration on ATPase activity. A representative example of four replications is presented.
Table III.
Effect of corn root adaptation to low pHe on the kinetic characteristics of plasmalemma ATPase
| Kinetic Characteristic and Assay Temperature (°C) | pHe | Assay pH
|
|
|---|---|---|---|
| 6.5 | 7.0 | ||
| μmol Pi mg−1 protein min−1 | |||
| Vmax | |||
| 20 | 6.0 | 0.35 (±0.03) | 0.21 (±0.02) |
| 20 | 3.5 | 0.40 (±0.05) | 0.24 (±0.03) |
| 30 | 6.0 | 1.25 (±0.15) | 0.91 (±0.07) |
| 30 | 3.5 | 1.78 (±0.37) | 1.31 (±0.25) |
| μm | |||
| Km | |||
| 20 | 6.0 | 242 (±19) | 277 (±22) |
| 20 | 3.5 | 298 (±28) | 328 (±24) |
| 30 | 6.0 | 377 (±23) | 472 (±36) |
| 30 | 3.5 | 582 (±79) | 724 (±137) |
Membranes were isolated from 3-week-old corn plants grown at pHe 6.0 and 3.5. Vmax and Km were determined using ATP concentrations from 20 to 4000 μm. The values represent means (± se) of four isolations.
Therefore, we calculated the activation energy of plasmalemma ATPase according to the Arrhenius equation using Vmax determined at both 20 and 30°C. At assay pH 6.5 the activation energy of the plasmalemma ATPase derived from corn roots grown at pHe 3.5 (107.0 ± 5.4 kJ mol−1) was significantly higher than that from control roots (93.5 ± 3.1 kJ mol−1). Similar to ATPase activity (Fig. 2), the difference in activation energy between plasmalemma ATPase from corn roots grown at pHe 3.5 and those grown at pHe 6.0 almost disappeared at assay pH 7.0 (not shown). The fact that, despite increased activation energy, at assay pH 6.5 hydrolytic ATPase activity from pHe 3.5 roots under saturating substrate concentrations was higher than ATPase activity from pHe 6.0 roots implies that adaptation of roots to low pHe increased the number of ATPase molecules in the plasmalemma.
Phospholipids have been reported to stimulate plasmalemma ATPase activity (Palmgren and Sommarin, 1989; Kasamo and Yamanishi, 1991). Therefore, we determined the concentration of phospholipids and the lipid-to-protein ratio of the corn root plasmalemma after differential pHe treatment. The results presented in Table IV reveal no difference in the lipid-to-protein ratio or in the phospholipid-to-protein ratio. Also, there was no significant effect on the phospholipid-to-lipid ratio (Table IV).
Table IV.
Effect of corn root adaptation to low pHe on the lipid-to-protein, the phospholipid-to-protein, and the phospholipid-to-lipid ratios in the plasmalemma
| pHe | Lipid:Protein | Phospholipid:Protein | Phospholipid:Lipid |
|---|---|---|---|
| w/w | % | ||
| 6.0 | 2.28 (±0.12) | 0.82 (±0.03) | 35.9 (±1.0) |
| 3.5 | 2.34 (±0.10) | 0.87 (±0.03) | 37.5 (±2.1) |
Ratios were calculated from concentrations determined in the plasmalemma of 3-week-old corn roots grown at pHe 6.0 and 3.5, respectively. The values represent means (± se) of four isolations.
Effect of Low pHe during Plant Cultivation on H+-Pumping Activity and Plasmalemma H+ Permeability
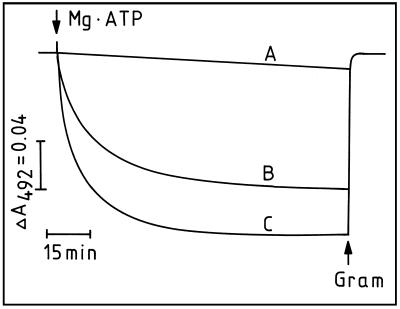
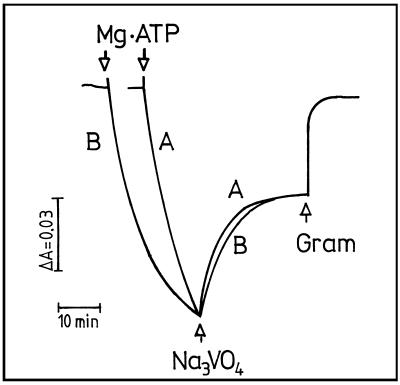
Plasmalemma H+-pumping activity was monitored by the ΔA492 of AO. After initiation of H+ pumping by addition of Mg-ATP there was rapid quenching and it eventually reached a constant level and completely collapsed by 5 μm gramicidin (Fig. 4). In addition, this H+-pumping activity was almost completely inhibited by 500 μm vanadate (Fig. 4A). Compared with control (Fig. 4B), absorbance quenching of AO caused by plasmalemma vesicles from low pHe roots was more rapid at the beginning and reached a higher level after 100 min (Fig. 4C). We used initial rate and maximum quenching (pH gradient) to characterize the plasmalemma H+ pump. The initial rate of H+ pumping was determined according to the quenching rate within the 1st min, which may reflect active H+ influx into plasmalemma vesicles. Maximum quenching was measured 100 min after initiation of the H+ pump. At this time net H+ transport across the plasmalemma was 0 and H+ influx due to active pumping and H+ efflux because of leakage reached equilibrium. This parameter indicates the steepest pH gradient that can be created by H+-pumping activity.
Figure 4.
Effect of low nutrient-solution pH during plant cultivation on H+ transport of plasmalemma vesicles of 3-week-old corn roots. Plasmalemma vesicles were isolated from corn roots grown at pHe 6.0 (B) and 3.5 (C). The pH gradient formation was monitored by ΔA492 of AO. At assay solution pH 6.5, intravesicular acidification was initiated by addition of 5 mm Mg-ATP. The pH gradient formation was almost completely inhibited by 500 μm Na3VO4 (A). The established pH gradient was completely collapsed by 5 μm gramicidin (Gram).
At assay pH 6.5 the initial rate of H+ pumping by plasmalemma ATPase from low-pHe-adapted roots was increased by 42.4% in comparison with control. This increase became less pronounced when assay pH was increased to 7.0 (Table V). Furthermore, at assay pH 6.5 the plasmalemma from low-pHe roots created a 35.2% steeper H+ gradient than control roots (Table V). A less pronounced increase in pH gradient of low-pHe root plasmalemma in comparison with control was measured when assayed at pH 7.0, a similar trend as found for initial rate of H+ pumping.
Table V.
Effect of corn root adaptation to low pHe on H+ transport across plasma membrane
| Assay pH | Active H+
Transport
|
Passive H+ Transport
|
||||
|---|---|---|---|---|---|---|
| Initial rate
|
pH
gradient
|
Initial rate
|
t1/2
|
|||
| pH 6.5 | pH 7.0 | pH 6.5 | pH 7.0 | pH 6.5 | pH 6.5 | |
| ΔA492 mg−1 protein min−1 | ΔA492 mg−1 protein | ΔA492mg−1 protein min−1 | min | |||
| pHe 6.0 | 0.12 ± 0.01 | 0.15 ± 0.01 | 2.08 ± 0.07 | 2.26 ± 0.11 | 0.17 ± 0.01 | 3.35 ± 0.26 |
| pHe 3.5 | 0.28 ± 0.00 | 0.19 ± 0.02 | 2.93 ± 0.25 | 2.55 ± 0.21 | 0.25 ± 0.01 | 2.18 ± 0.18 |
Membranes were isolated from 3-week-old corn roots grown at solution pHe 6.0 and 3.5. The assay was conducted at 25°C and at pH 6.5 and pH 7.0. The values represent means (± se) of four independent isolations.
For inside-out vesicles the establishment of a pH gradient is determined by both active H+ influx (pumping) and passive H+ efflux (leakage). To determine H+ efflux from plasmalemma vesicles, we measured the degradation of the pH gradient after stopping H+ pumping by addition of 500 μm vanadate. Because the degradation rate of pH gradient depends on the gradient itself, a comparison between treatments should be made at the same pH gradient. Therefore, we stopped H+ pumping when the pH gradient of plasmalemma vesicles reached 0.0900 A units. After addition of vanadate the established pH gradient was degraded quickly and then reached a relative constant level. This resting pH gradient was completely collapsed by gramicidin (Fig. 5). It is evident from Figure 5 that the gradient degradation was faster for low-pHe roots (Fig. 5A) than for control roots (Fig. 5B). Two parameters were used to characterize the pH gradient degradation. The degradation rate within the 1st min after addition of vanadate was measured as the initial rate to describe how rapidly the pH gradient degradation starts, and t1/2, the time in which half of the established pH gradient was degraded, was determined to characterize the time course of degradation. Compared with control, the initial degradation rate for plasmalemma vesicles from low-pHe roots was increased by 42%. Furthermore, a 35% decrease in t1/2 was caused by low-pHe treatment (Table V). Both parameters indicate a higher H+ permeability of plasmalemma derived from low-pHe roots.
Figure 5.
Effect of low nutrient-solution pH during plant cultivation on passive H+ transport across the plasmalemma of corn roots. Plasmalemma was isolated from 3-week-old corn roots grown at pH 3.5 (A) and 6.0 (B). The change of pH gradient across the plasmalemma was monitored by ΔA492 of AO. Plasmalemma ATPase hydrolytic activity was initiated by addition of Mg-ATP (5 mm) to create a pH gradient across plasmalemma vesicles. For a reliable comparison, ATPase activity was stopped by addition of Na3VO4 (500 μm) after quenching had reached 0.0900 A units for both membranes. The resting pH gradient was collapsed by gramicidin (Gram, 5 μm).
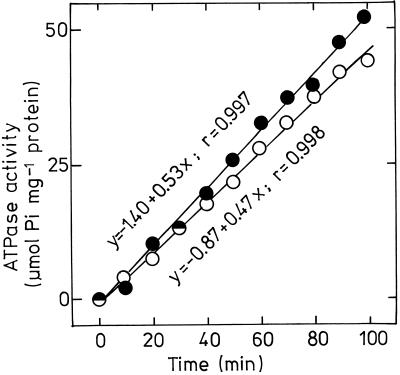
To investigate the coupling of ATPase hydrolytic activity with pumping activity, we determined hydrolytic ATPase activity for 100 min under the same conditions as for H+-pumping measurement. Within 100 min the activity of plasmalemma H+-ATPase was very constant (Fig. 6), although there must have been a large increase in pH gradient during this time (Fig. 4). This result indicates that the hydrolytic activity of plasmalemma H+-ATPase is independent of the pH gradient across the membrane of plasmalemma vesicles. Furthermore, under this condition only 13% stimulation of hydrolytic ATPase activity (0.53 and 0.47 μmol Pi mg−1 min−1 for pHe 3.5 and 6.0 vesicles, respectively) was estimated, a result similar to that found for the kinetic parameters of the enzyme assayed at 20°C (Table III). The fact that low-pHe treatment caused differential stimulation in H+-pumping activity (42% increase) and hydrolytic activity (13% increase) suggests that low-pHe treatment of corn root cells improved transport-coupling efficiency of plasmalemma H+-ATPase.
Figure 6.
Time course of plasmalemma H+-ATPase activity of 3-week-old corn roots grown at pH 6.0 (○) and 3.5 (•). The H+-ATPase activity of plasmalemma was assayed at 25°C in a solution with 5 mm BTP/Mes (pH 6.5), 5 mm Mg-ATP, 100 mm KCl, 7.5 μm AO, and 0.05% (w/v) Brij 58. A representative example of four replications is presented.
DISCUSSION
Adaptation of Corn Roots to Low Solution pH
In a previous study we reported that the growth rate of corn roots was markedly inhibited by an abrupt decrease of pHe (Yan et al., 1992). When, however, corn roots were adapted to low pHe by gradually increasing H+ activity in the root medium, corn roots attained a growth rate at pHe 3.5, which was comparable to that at pHe 6.0 (Table I). After this adaptation period no apparent damage to corn root physiology or morphology was observed. This is in contrast to faba beans, which showed a marked decrease in root growth after identical adaptation conditions (not shown). Therefore, it may be concluded that species differences between corn and faba beans with respect to low-pHe tolerance are not only constitutive but also adaptive. As shown previously for constitutive low-pHe tolerance (Yan et al., 1992), adaptive low-pHe tolerance was associated with the ability of corn roots to release higher amounts of protons into the external medium (Fig. 1). Although it cannot be excluded that there was additional release of protons that were stored in vacuoles during the cultivation at low pHe (Fig. 1A), the fact that higher net H+ extrusion was also observed at pHe 3.4 (Fig. 1B) indicates that the major contribution to low-pHe tolerance may be an improvement of H+ pumping at low pHe. This is in agreement with a tight relationship between net H+ release and root growth rate (Schubert et al., 1990; Yan et al., 1992).
Involvement of Plasmalemma H+-ATPase during Adaptation of Corn Roots to Low Solution pH
In the present study we demonstrated the involvement of plasmalemma H+-ATPase in adaptation of corn root cells to low solution pH. This conclusion is supported by the following results obtained at optimum assay pH in vitro: compared with control, (a) the hydrolytic activity of plasmalemma H+-ATPase of adapted root cells was increased by about 20% (Fig. 2); (b) the initial rate of H+-pumping activity of adapted plasmalemma was enhanced by 42% (Table V; Fig. 4); (c) adapted plasmalemma H+-ATPase created and maintained a 35% higher pH gradient (Fig. 4; Table V); and (d) this higher pH gradient was not explained in terms of a reduced H+ permeability of this membrane; rather, low-pHe plasmalemma showed 42% higher H+ permeability (Fig. 5; Table V).
The possible mechanisms involved in the adaptation of plasmalemma H+-ATPase to low-pHe conditions may include (a) an increase in the number of H+-ATPase enzymes per unit membrane, (b) a modulation of the turnover rate of hydrolysis of this enzyme by lipid environment change (Cooke and Burden, 1990), (c) a modification of the autoinhibitory domain in the C terminus of H+-ATPase (Palmgren et al., 1991), and (d) differential expression of isoforms of this enzyme (Palmgren and Christensen, 1994).
Although an increase in plasmalemma H+-ATPase activity has been reported under a number of environmental conditions, in most cases it is not clear whether the observed changes in H+-ATPase activity reflect modulation of either the amount or the turnover rate of hydrolysis of the enzyme (Serrano, 1989). Identifying these two components is a prerequisite for understanding plant responses to environmental conditions on a molecular basis. Therefore, in the present study we attempted to separate these two components by additional measurement of activation energy of this enzyme. Higher activation energy of H+-ATPase in low-pHe plasmalemma indicates a reduced substrate turnover rate of this enzyme. The increase in hydrolytic activity of plasmalemma ATPase under acid-stress conditions (Fig. 2) may thus be attributed to higher abundance of H+-ATPase molecules in adapted plasmalemma, not to higher substrate turnover rate per mole of enzyme. This conclusion can be supported by a recent finding that the amount of the H+-ATPase protein in plasmalemma and the level of its mRNA transcript in Dunaliella acidophila (an acid-tolerant alga) are far higher than in Dunaliella salina (a salt-tolerant alga) and that the level of mRNA transcript of H+-ATPase in both algae displays pHe dependence (Weiss and Pick, 1996).
Plant plasmalemma H+-ATPase has an absolute requirement for a lipid environment (Serrano et al., 1988). Although the mechanism by which ATPase activity is activated is still uncertain, it has been suggested that a direct lipid-protein binding is involved (Cooke and Burden, 1990). It has been demonstrated that a stimulation of plasmalemma H+-ATPase may be achieved by phospholipids (Palmgren et al., 1988; Serrano et al., 1988; Kasamo and Yamanishi, 1991). However, in the present study the stimulation of H+-ATPase and H+-pumping activities induced by low pHe was not mediated by changes in lipid composition. Neither the ratio of phospholipid to protein nor the ratio of phospholipid to total lipid of corn root plasmalemma was changed by low-pHe treatment (Table IV). This, of course, does not rule out the possibility that the composition of individual phospholipids or fatty acids may be changed by low-pHe treatment, which may be involved in the modulation of H+-ATPase activity (Palmgren et al., 1988).
It is well established that a part of the C-terminal region of H+-ATPase constitutes an autoinhibitory domain. Removal of this domain by fusicoccin, lysophospholipids, and trypsin can activate the enzyme (Palmgren et al., 1991; Johansson et al., 1993). The activation effect of lysophospholipids on the plasmalemma H+-ATPase is of physiological relevance because a phospholipase that generates lysophosphatidylcholine from phosphatidylcholine is found in the plant plasmalemma (Palmgren et al., 1988; Palmgren and Sommarin, 1989). Therefore, an attractive hypothesis for adaptation of corn root cells to low pHe may be a stimulation of phospholipase in plasmalemma by low pH, resulting in the release of lysophospholipids that may interact with H+-ATPase to remove the autoinhibitory domain. Lysophospholipids may induce several effects on plasmalemma H+-ATPase (Palmgren et al.,1988; Palmgren and Sommarin, 1989): (a) a higher degree of stimulation in H+ pumping than in hydrolytic ATPase activity, (b) an increase in Vmax and a decrease in Km, (c) a shift of pH optimum toward more alkaline values, or (d) sensitivity of the enzyme to vanadate remains unchanged. After adaptation to low-pHe, plasmalemma H+-ATPase showed a 20% higher hydrolytic activity and 42% higher H+-pumping activity (Figs. 2 and 4; Table V). This may indicate a changed degree of coupling between ATP hydrolysis and H+ pumping (Baunsgaard et al., 1996). In addition, adapted H+-ATPase displayed the same sensitivity to vanadate as the control (Table II). These results are compatible with effects (a) and (d) induced by lysophospholipids. However, in comparison with control, adapted plasmalemma H+-ATPase showed increased Km (Table III) and a slight shift of pH optimum toward more acid values (Fig. 2). These changes in H+-ATPase in adapted plasmalemma are not consistent with effects (b) and (c), which are caused by lysophospholipids.
Alternatively, differential expression of H+-ATPase isoforms may be responsible for the adaptation of plasmalemma H+-ATPase to low pHe. Palmgren and Christensen (1994) compared functions between plant plasmalemma H+-ATPase isoforms expressed in yeast and found a significant difference in Km, turnover rate for ATP hydrolysis, and vanadate sensitivity between investigated isoforms. Existence of biochemical heterogeneity was reported for native corn root plasmalemma H+-ATPase (Gallagher and Leonard, 1987; Grouzis et al., 1990). The increase in Km of low-pHe plasmalemma H+-ATPase may imply differential expression of H+-ATPase isoforms in the plasmalemma as a response of the root cells to low pHe. With higher ATP supply in vivo (Yan et al., 1992) more efficient isoforms may significantly contribute to H+ extrusion against a steeper H+ gradient across the plasmalemma of corn root cells exposed to low pHe.
Abbreviations:
- AO
acridine orange
- BTP
1,3-bis[tris(hydroxylmethyl)-methylamino]propane
- pHe
external pH
Footnotes
This work was supported by German Science Foundation grant Schu 589/5–1.
LITERATURE CITED
- Adams F. Nutritional imbalances and constraints to plant growth on acid soils. J Plant Nutr. 1981;4:81–87. [Google Scholar]
- Ayala F, Leary JWO, Schumaker KS. Increased vacuolar and plasma membrane H+-ATPase activities in Salicornia bigelovii Torr. in response to NaCl. J Exp Bot. 1996;47:25–32. [Google Scholar]
- Baginski ES, Foa PP, Zak B. Determination of phosphate: study of labile organic phosphate interference. Clin Chim Acta. 1967;15:155–158. [Google Scholar]
- Baunsgaard F, Venema K, Axelsen KB, Villaba JM, Welling A, Wollenweber B, Palmgren MG. Modified plant plasma membrane H+ ATPase with improved transport coupling efficiency identified by mutant selection in yeast. Plant J. 1996;10:451–458. doi: 10.1046/j.1365-313x.1996.10030451.x. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bourgeade P, Boyer N. Plasma membrane H+-ATPase activity in response to mechanical stimulation of Bryonia dioica internodes. Plant Physiol Biochem. 1994;32:661–668. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braun Y, Hassidim M, Lerner HR, Reinhold L. Studies on H+-translocation ATPase in plants of varying resistance to salinity. I. Salinity during growth modulates the proton pump in the halophyte Atriplex nummularia. Plant Physiol. 1986;81:1050–1056. doi: 10.1104/pp.81.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke DT, Burden RS. Lipid modulation of plasma membrane-bound ATPases. Physiol Plant. 1990;78:153–159. [Google Scholar]
- Cowan DSC, Clarkson DT, Hall JL. A comparison between the ATPase and proton pumping activities of plasma membranes isolated from the stele and cortex of Zea mays roots. J Exp Bot. 1993;44:983–989. [Google Scholar]
- De Michelis M, Spanswick RM. H+-pumping driven by the vanadate-sensitive ATPase in membrane vesicles from corn roots. Plant Physiol. 1986;81:542–547. doi: 10.1104/pp.81.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer JC, Wells MA. Quantitative and qualitative analysis of lipid components. Methods Enzymol. 1969;14:482–530. [Google Scholar]
- Eraso P, Gancedo G. Activation of yeast plasma membrane ATPase by acid pH during growth. FEBS Lett. 1987;224:187–192. doi: 10.1016/0014-5793(87)80445-3. [DOI] [PubMed] [Google Scholar]
- Faraday CD, Spanswick RM. Maize root plasma membranes isolated by aqueous polymer two-phase partitioning: assessment of residual tonoplast ATPase and pyrophosphatase activities. J Exp Bot. 1992;43:1583–1590. [Google Scholar]
- Gallagher SR, Leonard RT. Electrophoretic characterization of a detergent-treated plasma membrane fraction from corn roots. Plant Physiol. 1987;83:265–271. doi: 10.1104/pp.83.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grouzis JP, Gibrat R, Rigaud J, Ageorges A, Grignon C. Potassium stimulation of corn root plasmalemma ATPase. I. Hydrolytic activity of native vesicles and purified enzyme. Plant Physiol. 1990;93:1175–1182. doi: 10.1104/pp.93.3.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam AKMS, Edwards DG, Asher CJ. pH optima for crop growth. Results of a flowing solution culture experiment with six species. Plant Soil. 1980;54:339–357. [Google Scholar]
- Johansson F, Olbe M, Sommarin M, Larsson C. Brij 58, a polyoxythylene acyl ether, creates membrane vesicles of uniform sidedness. A new tool to obtain inside-out (cytoplasmic side-out) plasma membrane vesicles. Plant J. 1995;7:165–173. doi: 10.1046/j.1365-313x.1995.07010165.x. [DOI] [PubMed] [Google Scholar]
- Johansson F, Sommarin M, Larsson C. Fusicoccin activates the plasma membrane H+-ATPase by a mechanism involving the C-terminal inhibitory domain. Plant Cell. 1993;5:321–327. doi: 10.1105/tpc.5.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamo K, Yamanishi H. Functional reconstitution of plasma membrane H+-ATPase from mung bean (Vigna radiata L.) hypocotyls in liposomes prepared with various molecular species of phospholipids. Plant Cell Physiol. 1991;32:1219–1225. [Google Scholar]
- Kochian LV. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol. 1995;46:237–260. [Google Scholar]
- Kuiper D, Sommarin M, Kylin A. The effects of mineral nutrition and benzyladenine on the plasmalemma ATPase activity from roots of wheat and Plantago major ssp. pleiosperma. Physiol Plant. 1991;81:169–174. [Google Scholar]
- Larsson C. Plasma membrane. In: Linskens HF, Jackson JF, editors. Modern Methods of Plant Analysis, New Series Vol 1: Cell Components. Berlin: Springer-Verlag; 1985. pp. 85–104. [Google Scholar]
- Marré E. Fusicoccin: a tool in plant physiology. Annu Rev Plant Physiol. 1979;30:273–288. [Google Scholar]
- Palmgren MG, Christensen G. Functional comparisons between plant plasma membrane H+-ATPase isoforms expressed in yeast. J Biol Chem. 1994;269:3027–3033. [PubMed] [Google Scholar]
- Palmgren MG, Sommarin M. Lysophosphatidylcholine stimulates ATP dependent proton accumulation in isolated oat root plasma membrane vesicles. Plant Physiol. 1989;90:1009–1014. doi: 10.1104/pp.90.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren MG, Sommarin M, Serrano R, Larsson Ch. Identification of an autoinhibitory domain in the C-terminal region of the plant plasma membrane H+-ATPase. J Biol Chem. 1991;266:20470–20475. [PubMed] [Google Scholar]
- Palmgren MG, Sommarin M, Ulvskov P, Jφrgensen PL. Physiol Plant. 1988;74:11–19. [Google Scholar]
- Pinton R, Poles A, Cesco S, Varanini Z. Changes in plasma membrane H+ ATPase activity during aeration of maize roots. J Plant Physiol. 1996;147:511–515. [Google Scholar]
- Santi S, Locci G, Pinton R, Cesco S, Varanini Z. Plasma membrane H+-ATPase in maize roots induced for NO3− uptake. Plant Physiol. 1995;109:1277–1283. doi: 10.1104/pp.109.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubert S, Schubert E, Mengel K. Effect of low pH of the root medium on proton release, growth, and nutrient uptake of field beans (Vicia faba) Plant Soil. 1990;124:239–244. [Google Scholar]
- Schubert S, Yan F. Nitrate and ammonium nutrition of plants: effects on acid/base balance and adaptation of root cell plasmalemma H+ ATPase. Z Pflanzenernaehr Bodenk. 1997;160:275–281. [Google Scholar]
- Sekler I, Pick U. Purification and properties of a plasma membrane H+-ATPase from the extremely acidophilic alga Dunaliella acidophila. Plant Physiol. 1993;101:1055–1061. doi: 10.1104/pp.101.3.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R. Structure and function of plasma membrane ATPase. Annu Rev Plant Physiol Plant Mol Biol. 1989;40:61–94. [Google Scholar]
- Serrano R, Montesinos C, Sanchez J. Lipid requirements of the plasma membrane ATPase from oat roots and yeast. Plant Sci. 1988;56:117–122. [Google Scholar]
- Weiss M, Pick U. Primary structure and effect of pH on the expression of the plasma membrane H+-ATPase from Dunaliella acidophila and Dunaliella salina. Plant Physiol. 1996;112:1693–1702. doi: 10.1104/pp.112.4.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia JH, Roberts JKM. Regulation of H+ extrusion and cytoplasmic pH in maize root tips acclimated to a low-oxygen environment. Plant Physiol. 1996;111:227–233. doi: 10.1104/pp.111.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F, Schubert S, Mengel K. Effect of low root medium pH on net proton release, root respiration, and growth of corn (Zea mays L.) and broad bean (Vicia faba L.) Plant Physiol. 1992;99:415–421. doi: 10.1104/pp.99.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]