Abstract
Psychopathy, a developmental disorder characterized by profound social disturbance, is associated with impaired recognition of distress cues. Since distress processing and moral socialization are closely linked, uncovering techniques to improve distress recognition could have positive treatment implications for developmental disorders that feature empathy impairments. Previous studies demonstrate that fear-recognition deficits can be remedied by redirecting attention to critical cues (the eyes for fearful faces). However, it remains unclear whether this manipulation increases activity in empathy-related brain regions, or has an alternate compensatory effect that may not promote prosocial behaviours. In this fMRI study, a community sample of individuals with high vs low callous traits completed an emotion recognition task that varied whether the most or least socially meaningful facial features were visible (the eyes were isolated or occluded). For fearful faces, individuals with high callous traits showed significantly less amygdala and medial prefrontal cortex activity than those with low callous traits when the eyes were occluded, but not when they were isolated. Consistent with recent models of the amygdala that emphasize orientation to disambiguate stimuli rather than represent distress, individuals with low trait empathy showed greater amygdala activity to the least vs most socially meaningful features of fearful faces.
Keywords: amygdala, psychopathy, empathy arousal, fear, emotion recognition, medial prefrontal cortex, coldheartedness, callous traits
INTRODUCTION
Psychopathy is a developmental disorder associated with profound behavioural and emotional disturbance. Psychopathic individuals commit a disproportionate amount of crime and violence, and exhibit a callous-unemotional and manipulative interpersonal style (Hare, 2006). A cardinal feature of the disorder is diminished empathic responding (Hare et al., 1991). One prominent neurocognitive model of psychopathy implicates dysfunction in the amygdala and functionally connected regions of ventromedial frontal cortex in its aetiology (Blair, 2010). At the cognitive level, these abnormalities are associated with impaired stimulus-reinforcement learning (Newman and Kosson, 1986; Mitchell et al., 2006), impaired decision making (Mitchell et al., 2002) and reduced sensitivity to emotional cues in others (Blair et al., 2001). In combination, these deficits are thought to disrupt the development of moral socialization, and increase the risk for developing antisocial patterns of behaviour (Blair et al., 2006).
To most humans, the presentation of distress cues such as fearful or sad facial expressions is aversive (Bandura and Rosenthal, 1966). Facial expressions of distress are thought to act as unconditioned social reinforcers that communicate the negative valence that actions have on others (Blair, 2003a). Accordingly, viewing distress has been linked with the interruption of aggression (Perry and Perry, 1974) and the initiation of prosocial behaviour (Hoffman, 1975). Relative to healthy controls, individuals with psychopathy show impaired recognition of distress cues, particularly fearful faces (Blair et al., 2001; Stevens et al., 2001). In addition, neuroimaging studies suggest that high callous-unemotional traits are associated with reduced amygdala activity to fearful facial expressions (Marsh et al., 2008; Jones et al., 2009).
Recent evidence suggests that at least one key deficit associated with psychopathy, emotional expression recognition, can be remedied through a behavioural manipulation. Neuropsychological evidence shows that, like individuals with high psychopathic traits, patients with focal amygdala lesions show fear-recognition deficits (Adolphs et al., 1994, 1999). Adolphs and colleagues (2005) have shown that the fear-recognition deficit observed in patients with amygdala lesions is associated with a failure to attend to the eye region of faces, and can be reversed by instructing the patient to focus on the eyes. Later, Dadds and colleagues (2006) revealed a strikingly similar pattern in children with callous and unemotional traits; their fear-recognition deficits were also reversed when given the same instructions. Since distress processing and moral socialization are closely linked, uncovering techniques to help individuals recognize distress could improve empathic responding and have positive implications for interventions early in development. This is particularly crucial since psychopathic individuals respond poorly to available therapeutic treatment (Ogloff et al., 1990). Despite the potential utility of attention as an empathy arousal mechanism, it remains unclear whether this manipulation is associated with recovery of function in neural regions considered critical for empathy, or reflects compensatory patterns of activity that may not have the same implications for supporting prosocial behaviour. The current study begins to address this question.
We examined the impact of isolating distinct regions of the face (i.e. the eyes vs the remaining facial features) on activity in empathy-related brain regions in a community sample of individuals with high vs low levels of callous traits as measured by the coldheartedness subscale of the Psychopathic Personality Inventory – Revised (Lilienfeld and Widows, 2005). This particular subscale was chosen because it reflects the core emotional features of psychopathy, including reduced guilt, empathy, loyalty and callous disregard to others’ suffering. One possibility is that isolating the eyes acts to augment empathy in individuals with high callous traits, and so will be associated with enhanced activity in empathy-related brain regions including the amygdala and medial prefrontal cortex. Alternatively, isolating the eyes may result in compensatory engagement of other neural regions implicated in cognitive control or attention, such as dorsal prefrontal and parietal areas (regions not traditionally associated with prosocial behaviours). A third possibility, proposed recently by Adolphs (2010), is that rather than representing the level of emotion expressed (Morris et al., 1996), the amygdala may act to direct processing resources toward the most salient elements of a stimulus in order to resolve ambiguity. According to this perspective, amygdala activity should be greatest in healthy individuals when the most ambiguous facial features are present. Thus, on the basis of this view, the prediction can be made that any existing functional amygdala abnormalities associated with high relative to low callousness traits should be most apparent when viewing fearful faces with the eyes occluded. The current study tests these dissociable predictions.
METHODS
The Psychopathic Personality Inventory-Revised
The Psychopathic Personality Inventory-Revised (PPI-R; Lilienfeld and Widows, 2005) is a 154-item self-report personality measure that includes eight subscales: Machiavellian Egocentricity, Rebellious Nonconformity, Blame Extenalization, Carefree Nonplanfulness, Social Influence, Fearlessness, Stress Immunity and Coldheartedness. Although the Psychopathy Checklist Revised (PCL-R; Hare, 2003) is considered the gold-standard in psychopathy assessment in forensic populations, the PPI is a comparable measure for examining psychopathic traits in non-incarcerated populations. It correlates (0.54) with the PCL-R (Poythress et al., 1998) and exhibits similar psychometric properties (Benning et al., 2003). Since the focus of the current study was on modulating emotional empathy (c.f. Blair, 2005), participants in the current study formed groups according to their scores on the coldheartedness subscale of the PPI-R, described as ‘a propensity towards callousness, guiltlessness and unsentimentality’ (Lilienfeld and Andrews, 1996) as well as “callous failure to sympathize with others’ suffering” (Lilienfeld and Widows, 2005: 22). Traits captured by this subscale are thought to best reflect trait empathy; coldheartedness correlates with other measures of emotional empathy (Fecteau et al., 2008) as well as Factor 1 of the PCL-R, which is thought to reflect the core features of psychopathy (Poythress et al., 1998). High coldheartedness scores in particular have been associated with empathic disturbances on physiological measures that parallel those seen in forensic populations (Harenski et al., 2009). Like the PPI-R's composite reliability (>0.90), items on the coldheartedness subscale (rated on an ordinal four-point scale), yield acceptable internal consistency (Cronbach's α > 0.80) and good test–retest reliability (r = 0.82 for coldheartedness; Lilienfeld and Widows, 2005). Following the body of literature that have utilized an extreme group approach (Mitchell et al., 2002; Murrie and Cornell, 2002), participants whose scores fell in the top and bottom 33% in coldheartedness scores of normative data for their age and gender formed the high and low callous traits groups, respectively.
Participants
Thirty-four participants participated in the current study. Data from two participants were excluded due to scanner malfunction or failure to follow instructions, leaving 32 participants who formed the high (n = 16) and a low (n = 16) callous traits group (see Table 1 for participant characteristics). Participants were recruited from the University of Western Ontario and the local community through flyers and newspaper advertisements. All participants were between the ages of 17 and 35 years and were screened by experienced administrators using the Structured Clinical Interview of the DSM-IV (First et al., 1997) to exclude Axis-I disorders or history of neurological disorder. All participants had normal or corrected-to-normal vision, and were right-handed as determined by the Edinburgh Handedness Inventory (Oldfield, 1971). The groups did not differ significantly on age [t(30) = 0.48, ns] or IQ [t(30) = −0.75, ns]. This study was approved by the University of Western Ontario Human Research Ethics Board.
Table 1.
Demographic information for participants in the high and low callous trait groups. For each group, columns indicate gender (number of females, number of males), and the mean (M) and standard deviation (SD) for age, IQ, percentile for scores on the Coldheartedness subscale of the PPI-R, and percentile for total PPI-R scores.
| Group | Gender (female, male) | Age M (SD) | IQ M (SD) | CH score percentile M (SD) | Total PPI-R score percentile M (SD) |
|---|---|---|---|---|---|
| High callous traits | 10, 6 | 24.44 (5.15) | 113 (6.54) | 89.94 (6.24) | 79.19 (22.48) |
| Low callous traits | 9, 7 | 25.25 (4.49) | 110 (11.31) | 15.50 (10.84) | 24.25 (18.64) |
fMRI data acquisition
Participants were scanned while performing the task in a 3.0 Tesla Siemens MRI scanner with a 32-channel head coil at Robarts Research Institute. A high-resolution T1-weighted anatomical scan was acquired at the beginning of each session (TR = 2300 ms; TE = 4.25 ms; 192 axial slices; voxel size = 1 mm isovoxels; 256 × 256 matrix; field of view = 25.6 cm). Six functional MRI runs followed to measure changes in blood oxygen-level dependent (BOLD) response. Functional images were acquired with a T2*-gradient echo-planar imaging sequence (TR = 3000 ms; TE = 30 ms; 120 × 120 matrix; flip angle 90°; field of view = 24 cm). Coverage was obtained with 45 axial brain slices (2.5 mm thickness; 2 × 2 mm in-plane resolution) acquired in an interleaved fashion. The number of volumes acquired was 147 in total.
Experimental task
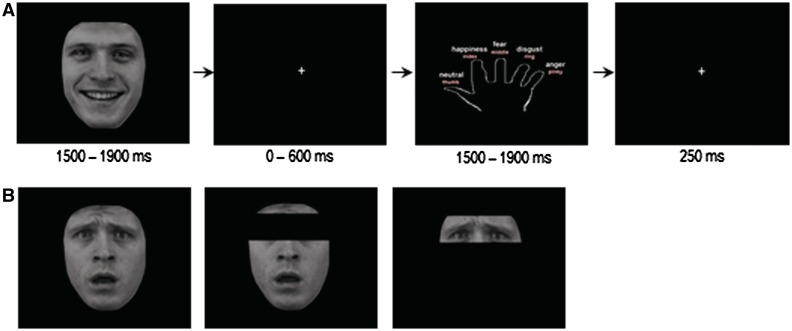
Participants completed the Partial Face Encoding (PFE) task, a novel emotion recognition task involving grey scale images of actors depicting realistic emotional faces taken from the empirically validated Karolinska Directed Emotional Faces (Lundqvist et al., 1998). Stimuli involved college-aged actors (balanced for gender), who faced the viewer while making one of five facial expressions: fear, anger, happiness, disgust and neutral. Using Adobe Photoshop Elements 4.0, all stimuli were converted to grey scale images on a black background, realigned 1–2° and resized to fit the computer screen. The hair and the ears were cropped out so that only the face remained which removes potentially distracting idiosyncratic stylistic features such as hairstyle. Three portion conditions were used for each of the five emotions: a ‘whole face’ condition that allowed the participant to free gaze, an ‘eyes-only’ condition which was cropped so that only the eye region and eyebrows could be seen, and an ‘eyes-removed’ condition in which the eye region was occluded, leaving the remainder of the face visible. To construct the eyes-only and eyes-removed stimuli, upper and lower boundaries were set at 12% of the total face length and above and below the nasion. Inter-rater reliability was high for all nasion estimates (r = 0.91, p < 0.01).
The PFE task consisted of six full runs (7 min 21 s per run) constructed and run using E-Prime software (Schneider et al. 2002). Stimuli were presented through a data projector onto a screen that was visible to a subject in the scanner via a mirror positioned above the coil in the MRI. The trial components and stimulus timings are presented in Figure 1. Instructions were projected on the screen at the beginning of each run to ensure that task administration was standardized across participants. Each run began with a fixation cross (1200 ms). Each trial consisted of a face (1500, 1700 or 1900 ms), followed by a fixation point (0, 300 or 600 ms), then a response screen (1500, 1700 or 1900 ms) and a fixation cross (250 ms). A separate response screen was constructed to collect response data to avoid undue demands on working memory and to isolate face processing from labelling and motor-related processes. This screen involved an outline of a hand, with an emotion designated to each finger. Responses were recorded using a five-button response box (Current Designs, Pennsylvania) held in the right hand. Each run consisted of 90 images, balanced for emotion (fear, anger, happiness, disgust and neutral), portion of face (whole face, eyes-only or eyes-removed) and actor's gender. Run order and emotion-finger designations for the response screen were randomized across participants. While in the scanner, participants completed two practice versions of the task before the experimental runs began. Practice A was an abbreviated version of the PFE task, in which the name of the emotion was projected on the screen in place of an emotional face. Unlike in the experimental runs, feedback was provided following their response on each trial (‘Correct!’, ‘Incorrect’ or ‘No response detected.’). Practice B was identical to the actual PFE task (novel emotional faces were used and no feedback provided), though its duration was shorter than an actual test run. Completion of these practice tasks ensured that all participants understood the objectives and were able to respond proficiently.
Fig. 1.
The PFE task (A) trial structure for a happy whole-face condition and (B) examples of fearful whole-face, eyes-only and eyes-removed conditions. Each trial consisted of a face, followed by a fixation point, then a response screen and ended with a fixation cross. A separate response screen was used to collect response data so as to avoid undue demands on working memory and to isolate face processing from labelling and motor-related processes. The response screen involved an outline of a hand with an emotion designated to each finger. Responses were recorded using a five-button response box held in the right hand. Each run consisted of 90 images, balanced for emotion (fear, anger, happiness, disgust and neutral), portion of face (whole-face, eyes-only or eyes-removed) and actor's gender.
Behavioural analysis
Statistical analyses of behavioural recognition accuracy data was conducted by way of four repeated measures ANOVAs (one for each emotion of interest). This was followed by Bonferroni-adjusted t-tests. Thus, the effects of group (high vs low callous traits) and stimulus portion (whole, eyes-only and eyes-removed) on recognition accuracy were examined.
fMRI analysis
Individual and group analyses were conducted using Analysis of Functional NeuroImages software (AFNI; Cox, 1996). Following motion-correction, each volume was spatially smoothed using an Isotropic 4 mm full-width half-maximum Gaussian kernel to reduce the influence of individual differences in anatomy before creating group maps. To normalize the time-series data, the signal intensity of a given voxel at each time point was divided by the mean signal intensity of that voxel for each run and multiplied by 100. Regressors were created for each of the 15 conditions (five emotions crossed with three portions) by convolving the stimulus events with a γ-variate basis function to account for the slow haemodynamic response. Errors were modelled separately.
The BOLD response was fitted to each regressor to perform linear regression modelling. To account for voxel-wise correlated drifting, baseline plus linear drift and quadratic trend were modelled to the time series of each voxel and regressor. The regression coefficients represented the percent signal change from the mean activity. To perform group analyses, each participant's data was transformed into the standard space of Talairach and Tournoux (1988). This was followed by our primary analyses of the regressors of interest, described below.
Whole-brain analysis
To test our hypotheses concerning the relative effects of isolating eye regions of the face from other facial features, a series of t-tests were conducted. These compared the whole-brain BOLD response between groups to stimuli containing only the eyes vs those conditions with the eyes-removed. Regions significantly active at a threshold of p < 0.001 were examined. To reduce the probability of Type I error, we corrected for multiple comparisons using AlphaSim, an AFNI spatial clustering operation with 1000 Monte Carlo simulations taking into account the entire echo-planar imaging matrix. Clusters that survived correction were significant at p < 0.05. The focus of the current study was on the effects of isolating eye regions; however, prior studies have examined the BOLD response in clinical populations using whole-face stimuli and contrasting the emotional expression vs a neutral facial expression baseline (Marsh et al., 2008; Jones et al., 2009). The results of that analysis can be found in the Supplementary Results section.
ROI approach to amygdala activity
Based on predictions about the amygdala's role in face processing (Adolphs, 2010) and dysfunction in psychopathy (Blair, 2003b), a region of interest (ROI) analysis was justified to further investigate activity in this region at a more liberal threshold (p < 0.01, small-volume corrected to p < 0.05).
RESULTS
Behavioural results
A two (group) by three (portion) ANOVA was conducted separately for each emotion (Greenhouse–Geisser degrees of freedom were used as appropriate). No significant main effect of group was observed. However, a significant effect of portion was evident for fearful [F(1.5,46.18) = 36.58, p < 0.001], neutral [F(2,60) = 4.01, p < 0.05], happy [F(1.14,34.23) = 32.57, p < 0.001], angry [F(1.50,44.94) = 185.92, p < 0.001] and disgusted [F(1.45,43.56) = 379.02, p < 0.001] expressions. A significant group by portion interaction emerged only for disgusted expressions [F(1.45,43.6) = 4.56, p < 0.05]. To delineate the effect of portion on each emotion, groups were collapsed together and a series of paired t-tests (Bonferroni and degrees of freedom-adjusted) were conducted comparing each condition (see Table 2 for details). In brief, when viewing fearful faces or angry faces, participants’ recognition accuracy was significantly greater for the eyes-only relative to the eyes-removed conditions. In contrast, when viewing the happy or disgusted expressions, participants’ recognition accuracy was significantly greater for the eyes-removed relative to eyes-only conditions. Because a significant interaction was observed for disgust, additional tests were performed to determine the nature of this effect (Bonferroni-adjusted to correct for multiple comparisons where appropriate). Within-group paired t-tests performed on the high and low callous trait groups separately revealed that both groups were significantly more accurate at recognizing disgust when presented with the eyes-removed vs eyes-only stimuli (p < 0.001 in each case). Similarly, both groups were significantly more accurate at recognizing disgust from the whole faces relative to the eyes-only stimuli (p < 0.001 in each case). However, only the low callous trait group showed significantly better recognition accuracy for the whole relative to eyes-removed disgusted faces (p < 0.05). For the high callous trait group, they actually showed better recognition accuracy for the eyes-removed relative to the whole face, though this difference was not significant (p > 0.1). The independent sample t-tests showed that the two groups did not differ significantly in their recognition accuracy of whole or eyes-removed disgusted faces (p > 0.1 in each case). Thus, despite the interaction driven by group differences in the recognition of disgust from the eyes, the principle effect of interest was identical in both groups; recognition accuracy was significantly better for eyes-removed relative to eyes-only conditions.
Table 2.
PFE task: post-hoc tests of emotion by portion interaction
| Comparison | dfa | t | P* |
|---|---|---|---|
| Fear | |||
| Whole > eyes only | 46 | 3.35 | <0.005 |
| Whole > eyes removed | 46 | 7.5 | <0.001 |
| Eyes only > eyes removed | 46 | 4.14 | <0.001 |
| Anger | |||
| Whole < eyes only | 45 | 1.34 | ns |
| Whole > eyes removed | 45 | 13.73 | <0.001 |
| Eyes only > eyes removed | 45 | 15.08 | <0.001 |
| Happy | |||
| Whole > eyes only | 34 | 5.71 | <0.001 |
| Whole > eyes removed | 34 | 1.02 | ns |
| Eyes only < eyes removed | 34 | 4.69 | <0.001 |
| Disgustb | |||
| Whole > eyes only | 44 | 20.37 | <0.001 |
| Whole > eyes removed | 44 | 0.11 | ns |
| Eyes only < eyes removed | 44 | 20.26 | <0.001 |
aThe degrees of freedom (df) were calculated based on the pooled Greenhouse–Geisser-adjusted whole-analysis error term.
bA significant group × portion interaction emerged for disgust characterized by significantly higher recognition accuracy in the low relative to the high callous trait group for the disgust eyes-only condition; the nature of this interaction is described fully in the Supplementary Materials along with means for each condition for each group.
*All p-values are Bonferroni-adjusted for multiple comparisons.
Together, the behavioural results indicate that the eye region contains critical (i.e. most socially meaningful) information for identifying angry and fearful facial expressions. Conversely, the lower region of the face, which is isolated in the eyes-removed stimuli, appears to contain critical information used for the accurate recognition of happiness and disgust. This key pattern of results was the same for the high and low callous trait groups, and validated the analytic strategy planned for the fMRI results based on pilot work (Alders, 2010). The means and standard error for each condition by group are available as Supplementary Results.
fMRI results
Between-group contrasts
Contrast tests were performed between the conditions of each emotional face that was shown to be most vs least socially meaningful (contained vs did not contain information critical for accurate recognition, respectively).
Fear: eyes-removed–eyes-only
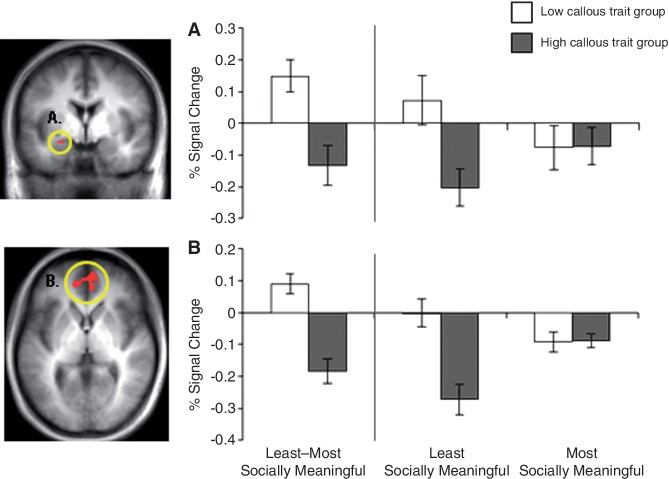
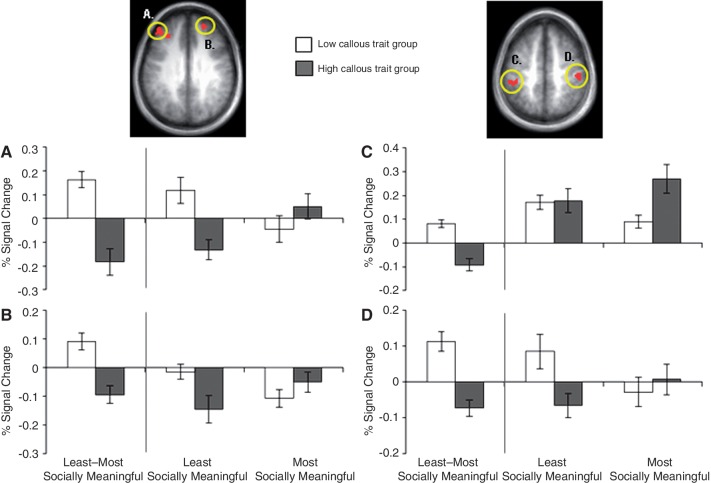
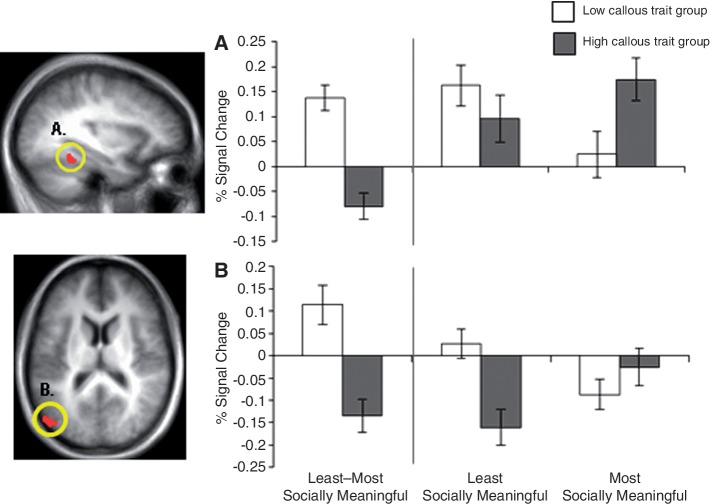
When the least socially meaningful portion of fearful faces (eyes-removed) was contrasted with the most meaningful one (eyes-only), individuals with low, relative to high, callous traits showed significantly greater activity in the bilateral medial frontal gyrus, bilateral inferior parietal lobule, bilateral superior frontal gyrus, bilateral middle frontal gyrus and right cingulate gyrus (p < 0.001; p < 0.05 corrected; Table 3). The ROI analysis revealed that low callous traits were associated with significantly greater left amygdala activity (p < 0.01, p < 0.05 small-volume corrected). Noteworthy regions are shown in Figures 2 and 3.
Table 3.
Areas showing significantly greater activity in individuals with low relative to high callous traits when the least-informative portion of an emotional face was contrasted with the most informative portion
| Anatomical location | L/R | BA | x | y | z | t-value |
|---|---|---|---|---|---|---|
| Fear (eyes-removed > eyes-only) | ||||||
| Medial frontal gyrus/ anterior cingulate | L/R | 10, 32 | 6.3 | 55.3 | 4.3 | 5.7 |
| Inferior parietal lobule | L | 40 | −39.1 | −31.9 | 45.9 | 4.9 |
| Superior frontal gyrus | L | 9 | −41.7 | 35.8 | 33.2 | 4.7 |
| Inferior parietal lobule/ post-central gyrus | R | 2 | 46.7 | −26.7 | 46.2 | 5.9 |
| Middle frontal gyrus | L | 9 | −26.5 | 30.3 | 41.0 | 4.4 |
| Middle frontal gyrus | R | 8 | 24.0 | 30.2 | 43.8 | 4.7 |
| Cingulate gyrus | R | 1.3 | −18.9 | 43.9 | 5.2 | |
| Superior frontal gyrus | R | 9 | 21.5 | 43.3 | 39.0 | 4.6 |
| Amygdala* | L | −24.0 | −0.7 | −13.4 | 3.5 | |
| Happy (eyes-only > eyes-removed) | ||||||
| Fusiform gyrus | L | 37 | −31.6 | −46.9 | −19.1 | 5.7 |
| Middle temporal gyrus | L | 19/39 | −49.2 | −76.8 | 13.6 | 4.4 |
| Amygdala* | R | 18.9 | −0.2 | −25.3 | 5.0 | |
| Amygdala* | L | −26.5 | 4.7 | −19.1 | 3.6 |
Thresholded at p < 0.001; p < 0.05 corrected.
*Thresholded at p < 0.01; p < 0.05, small-volume corrected.
Table displays the anatomical location, hemispheric location (L, left; R, right), Brodmann's area (BA), MNI coordinates (x, y, z) and the maximum neural activity for the peak of that cluster (t-value).
Fig. 2.
Between-group contrast of the least vs the most socially meaningful condition of fearful faces produces dissociable activity in regions of emotion processing. The low callous trait group showed significantly greater activity relative to the high callous trait group in (A) left amygdala (threshold of p < 0.01; small-volume corrected) to p < 0.05 and (B) bilateral medial prefrontal cortex (threshold of p < 0.001; p < 0.05 corrected) when fear eyes-removed was contrasted with fear eyes-only. Follow-up comparisons revealed that the low callous trait group showed greater activity in these regions when viewing the least socially meaningful condition (fear eyes-removed) relative to the most (fear eyes-only), whereas the high callous trait group showed greater activity in these regions for the most socially meaningful condition relative to the least.
Fig. 3.
Between-group contrast of least vs most socially meaningful condition of fearful faces displays differential activity in a frontoparietal network. The low callous trait group showed significantly greater activity relative to the high callous trait group in (A) left superior frontal gyrus (p < 0.001; p < 0.05 corrected), (B) right superior frontal gyrus (p < 0.001; p < 0.05 corrected), (C) left inferior parietal lobule (p < 0.001; p < 0.05 corrected) and (D) right inferior parietal lobule (p < 0.001; p < 0.05 corrected) when fear eyes-removed was contrasted with fear eyes-only. Follow-up comparisons revealed that the low callous trait group showed greater activity in these regions when viewing the least socially meaningful condition (fear eyes-removed) relative to the most (fear eyes-only), whereas the high callous trait group showed greater activity in these regions for the most socially meaningful condition relative to the least.
Happy: eyes-only–eyes-removed
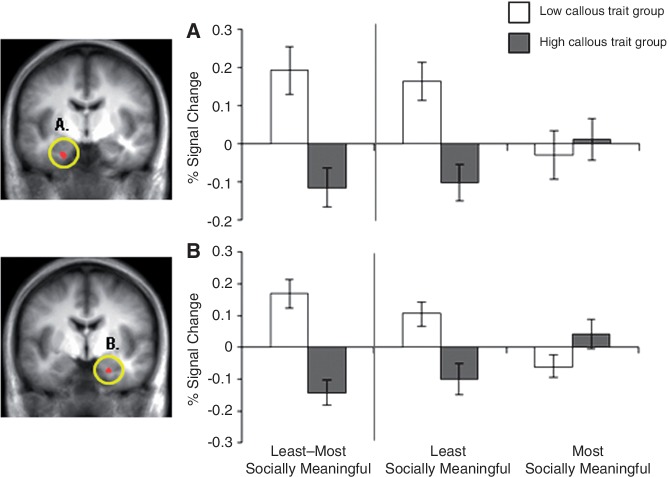
Relative to the high callous trait group, individuals with low callous traits showed significantly greater activity in the left fusiform gyrus and left middle temporal gyrus when viewing the least-informative portion (eyes-only) of happy faces (p < 0.001; p < 0.05 corrected, see Table 3 and Figures 4 and 5). In addition, the low callous trait group showed significantly greater activity in bilateral amygdala (p < 0.01; p < 0.05 small-volume corrected).
Fig. 4.
Between-group contrast of least vs most socially meaningful condition of happy faces produces distinct activity in the amygdala. The low callous trait group showed significantly greater activity relative to the high callous trait group in (A) left amygdala (p < 0.01; small-volume corrected to p < 0.05) and (B) right amygdala (p < 0.01; small-volume corrected to p < 0.05), when happy eyes-only was contrasted with happy eyes-removed. Follow-up comparisons revealed that the low callous trait group showed greater activity in these regions when viewing the least socially meaningful condition (happy eyes-only) relative to the most (happy eyes-removed), whereas the high callous trait group showed greater activity in these regions for the most socially meaningful condition relative to the least.
Fig. 5.
Between-group contrast of least vs most socially meaningful condition of happy faces reveals differential activity in the ventral visual system. The low callous trait group showed significantly greater activity relative to the high callous trait group in (A) left fusiform gyrus (p < 0.001; p<0.05 corrected) and (B) left middle temporal gyrus (p < 0.001; p<0.05 corrected), when happy eyes-only was contrasted with happy eyes-removed. Follow-up comparisons revealed that the low callous trait group showed greater activity in these regions when viewing the least socially meaningful condition (happy eyes-only) relative to the most (happy eyes-removed), whereas the high callous trait group showed greater activity in these regions for the most socially meaningful condition relative to the least.
Other emotions
Between-group contrasts were also performed on the least vs most socially meaningful portions for anger (eyes-removed vs eyes-only) and disgust (eyes-only vs eyes-removed). This contrast revealed no significant clusters of activation in a corrected whole-brain contrast or the ROI analysis involving left and right amygdala.
Follow-up analysis
The between-group contrasts did not reveal any patterns of activity consistent with the function of representing emotional clarity (e.g. increased activity for the eyes-only condition relative to eyes-removed for fear). This pattern of activity may have existed within groups, but was obscured in the between-group analysis. To determine whether this was the case, additional within-group analyses were conducted involving this contrast. No neural regions survived correction showing greater activity to the most relative to least socially meaningful conditions.
DISCUSSION
It has been recently demonstrated that fear-recognition deficits in youths with callous traits can be remedied through attentional means (Dadds et al., 2006). However, it remains unclear whether focusing on the eyes leads to a normalization of activity in brain regions associated with the facilitation of empathy and prosocial behaviour. In the current study, fMRI was used to examine how individuals with high vs low callous traits responded at the neural level to a novel emotion recognition task that isolated distinct components of emotional expressions. At a behavioural level, recognition accuracy between the two groups did not differ significantly. In both groups, the recognition of fearful and angry faces was lowest when the eyes were occluded, consistent with the idea that the eye region is the most ‘socially meaningful’ portion for recognizing these emotions. Happy and disgusted faces showed the opposite pattern; recognition accuracy was lowest when participants were presented with the eyes-only portion, suggesting that socially meaningful aspects of happy expressions are located away from the eyes. At a functional level, robust group differences in neural activity were revealed when comparing stimuli that contained the least vs the most socially meaningful facial features. When the eyes were occluded, individuals with low relative to high callous traits showed significantly greater activity in emotion-related brain regions including the amygdala and attention-related frontoparietal regions. The results involving individuals with low callous traits are consistent with the idea that the amygdala directs attention to socially informative features. Furthermore, our results suggest that individuals with high callous traits exhibit functional brain abnormalities in neurocognitive systems associated with orienting attention to socially meaningful components of stimuli.
Implications for views on the role of the amygdala in emotional empathy
These findings have important implications for two views concerning the amygdala's involvement in emotional empathy. The traditional view has been that the amygdala plays a role in representing the intensity of negative emotions such as fear (Morris et al., 1996). On the basis of this suggestion, it can be predicted that amygdala activity should be greatest when fear is most apparent (for eyes-only relative to eyes-removed stimuli in the current study). Furthermore, given evidence that focusing attention on the eyes alleviates fear-recognition impairments in clinical populations, isolating the eyes for individuals with high callous traits might result in normalized amygdala activity, or the recruitment of a compensatory network of brain regions. Our results were not consistent with this prediction. Individuals with low callous traits showed enhanced amygdala activity when the most diagnostic region of a distressed face was missing (fear eyes-removed). Although high callous traits were associated with the opposite pattern (greater amygdala activity to the eyes-only relative to eyes-removed), their amygdala activity remained below baseline levels. Furthermore, this activity was significantly less than that elicited in the low callous traits group by the least socially meaningful condition.
The current results are in accordance with recent conceptualizations concerning the functional contribution of the amygdala to fearful face recognition. Adolphs (2010) has proposed that the amygdala is critical for detecting and directing attention onto features that are particularly socially meaningful, ‘a mechanism that could engage even more so when the eyes are covered in an attempt to glean whatever information possible from the region’ (Adolphs, 2010). In line with this perspective, we found that the individuals with low callous traits showed greater amygdala activity when viewing fearful and happy faces with the most socially meaningful regions removed, relative to when that region was isolated. In sharp contrast, individuals with high callous traits showed greater amygdala activity when the most socially meaningful region of fearful and happy faces were isolated relative to when these regions were missing. This pattern was not driven by an effect of a specific facial feature, since the most socially meaningful facial feature differs for fearful (the eyes) and happy (the mouth) faces. To our knowledge, this is the first imaging study to characterize the amygdala abnormality associated with callous features of psychopathy in terms of a deficiency in orienting attention to the most socially meaningful components of a stimulus.
Although consistent with recent models of amygdala function that emphasize orienting attention to critical social cues, the present study may initially seem at odds with some other recent findings. For example, in a study examining neural activity to parts of faces, Benuzzi and colleagues (2007) report greater amygdala activity to whole faces relative to parts of faces. Whalen and colleagues (2004) have noted robust amygdala activity to fearful eye-whites when compared with happy eye-whites. However, these studies differ from the present one in a number of important respects. First, Benuzzi et al.'s (2007) study involves only neutral facial expressions. As the current study suggests, the most socially informative region of the face varies depending on the emotion. In addition, like most prior imaging studies of emotional expression processing, neither study involved an explicit emotion recognition task. It is possible that the pattern of amygdala activation elicited by fragmented facial expressions is influenced by task objectives (e.g. whether emotion identification is explicit or implicit). Whalen et al.'s (2004) study involved very rapid (17 ms) subliminal presentations involving only the eye-whites. The combined rapid presentation and limited exposure to facial components may have created an ambiguous stimulus representation (e.g. wide eyes are also associated with surprised facial expressions), which contributed to the robustness of the effects. Indeed, a similar interpretation of these results was developed further by the author (Whalen, 2007). One possibility is that the amygdala not only represents fear, but does so more robustly in ambiguous or unpredictable contexts (c.f. Herry et al., 2007; Whalen, 2007). Further work will be required to delineate the role of the amygdala with reference to representing relative to disambiguating emotion.
It is important to note that the pattern of activity observed in the amygdala extended to other neural regions including medial prefrontal cortex, dorsolateral prefrontal cortex and inferior parietal cortex. One possibility is that these areas work in concert with the amygdala to direct attention towards the critical features of a stimulus. For example, medial prefrontal cortex has been linked to emotion processing, has dense connections with the amygdala (Ghashghaei et al., 2007) and plays a role in regulating amygdala output during emotional learning (Mitchell, 2011) and during emotional conflict (Bishop et al., 2004; Etkin et al., 2006; Amting et al., 2010; Mitchell and Greening, 2011). A frontoparietal network has also been associated with cognitive control in emotional contexts (Mitchell et al., 2007, 2009) and these regions may be involved in providing top–down support when disambiguating a stimulus becomes particularly challenging.
Implications for neurocognitive models of psychopathy
Although the pattern of results observed in the low callous traits group are consistent with recent theories of the role of the amygdala in emotional expression processing, a clearly divergent pattern was observed in the high callous trait group. The high callous trait group showed an inverse pattern of activity relative to the low callous trait group, with greater activity in the amygdala and prefrontal cortex for the most relative to least-informative condition of fearful and happy faces. These empathy-related brain regions may have failed to adapt to emotional ambiguity by seeking out the most socially meaningful stimulus components. We also observed reduced activity in dorsal prefrontal cortex and inferior parietal lobe in the high callous trait group. This was an unanticipated effect given strong evidence that these areas are functionally intact in psychopathy (Blair et al., 2005; Blair and Mitchell, 2009). It seems likely therefore that the observed abnormalities resulted from a downstream effect of dysfunction in the amygdala and medial prefrontal cortex. For example, in the low callous trait group, attention-related areas like inferior parietal cortex may be recruited in response to emotional conflict signalled by frustrated attempts of the amygdala and medial prefrontal cortex to locate disambiguating information. In contrast, the high callous trait group may not have generated these emotional conflict-related signals in response to the least-informative conditions and, as a result, showed relatively less recruitment of secondary attention-related brain regions.
Future directions
Our findings raise several additional questions which will guide future research. Despite clear group differences in brain function, we did not observe impaired recognition accuracy in our subclinical sample. It remains unclear what compensatory mechanism might have allowed intact task performance despite these functional abnormalities. In addition, our results are consistent with the idea that the amygdala does not merely represent fearful stimuli or embody fear, but rather, directs resources to disambiguating socially meaningful stimuli (Adolphs, 2010), perhaps particularly for threat-related stimuli (c.f. Frewen et al., 2008; White et al., 2010). However, we did not identify neural regions involved in representing the intensity or clarity of a fearful stimulus, a function that might be critical for supporting prosocial behaviour. Supplementary Analyses revealed no significant clusters of activity consistent with this function. One possibility is that areas involved in either compensating for functional abnormalities, or in representing the emotion, went undetected due to signal drop-out. For example, two regions implicated in social cognition, frontopolar and ventral prefrontal cortex, are particularly susceptible to signal loss in typical fMRI scan parameters (Osterbauer et al., 2006). Lastly, although a number of studies have observed neurocognitive deficits in community samples that parallel those observed in institutionalized ones (Lynam et al., 1999; Gordon et al., 2004; Rilling et al., 2007; Fullam et al., 2009), particularly in association with the coldheartedness subscale (Harenski et al., 2009), it remains unclear the extent to which our findings can be generalized to more severely affected samples with higher levels of psychopathy. Future studies can help disentangle these issues by applying neuroimaging parameters optimized for detecting signal in orbitofrontal cortex, in conjunction with studies involving patients with focal lesions and forensic populations of individuals with psychopathy.
CONCLUSIONS
In the present study, functional abnormalities in the amygdala and medial prefrontal cortex were associated with high callous traits, consistent with dominant neurocognitive models of psychopathy that emphasize dysfunction in these regions (Blair, 2010). However, the effects observed in the amygdala do not implicate the amygdala specifically in representing or embodying fearful stimuli in the context of emotional expression recognition. Instead, the results support the theory that the amygdala helps orient attention to cues necessary to disambiguate a stimulus (Adolphs, 2010), a function that was impaired in individuals with high callous traits. Our findings also raise the possibility that regions outside the amygdala may play a role in orienting attention to socially meaningful cues; medial prefrontal cortex and a frontoparietal attention network were also activated to a greater extent in the low callous trait group when these cues were occluded. Together, our results offer further clues to the functional nature of the amygdala impairment associated with callous traits, and highlight the need for further work to disentangle the neurocognitive systems that encode the clarity or intensity of distress cues.
SUPPLEMENTARY DATA
Supplementary Data are available at SCAN online.
FUNDING
This work was funded by a Standard Research Grant from the Social Sciences and Humanities Research Council of Canada.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Social Sciences and Humanities Research Council of Canada. The authors would like to thank Betsy Schaefer for assistance with participant recruitment and screening, and Stephanie Nantes for help with the final stages of article preparation. The authors would also like to thank Dr. Bruce Morton for fruitful discussion over the course of the study.
REFERENCES
- Adolphs R. What does the amygdala contribute to social cognition? Annals of The New York Academy of Sciences. 2010;1191:42–61. doi: 10.1111/j.1749-6632.2010.05445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Gosselin F, Buchanan TW, Tranel D, Schyns P, Damasio AR. A mechanism for impaired fear recognition after amygdala damage. Nature. 2005;433:68–72. doi: 10.1038/nature03086. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372(6507):669–72. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Hamann S, et al. Recognition of facial emotion in nine individuals with bilateral amygdala damage. Neuropsychologia. 1999;37(10):1111–7. doi: 10.1016/s0028-3932(99)00039-1. [DOI] [PubMed] [Google Scholar]
- Alders GL. University of Western Ontario, London 100; 2010. The influence of isolating facial features on behavioural and neural indices of empathy. Graduate Program in Neuroscience. Master of Science Thesis. [Google Scholar]
- Amting JM, Greening SG, Mitchell DGV. Multiple mechanisms of consciousness: the neural correlates of emotional awareness. Journal of Neuroscience. 2010;30(20):10039–47. doi: 10.1523/JNEUROSCI.6434-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A, Rosenthal T. Vicarious classical conditioning as a function of arousal level. Journal of Personality and Social Psychology. 1966;3:54–62. doi: 10.1037/h0022639. [DOI] [PubMed] [Google Scholar]
- Benning SD, Patrick CJ, Hicks BM, Blonigen DM, Krueger RF. Factor structure of the Psychopathic Personality Inventory: validity and implications for clinical assessment. Psychological Assessment. 2003;15(3):340–50. doi: 10.1037/1040-3590.15.3.340. [DOI] [PubMed] [Google Scholar]
- Benuzzi F, Pugnaghi M, Meletti S, et al. Processing the socially relevant parts of faces. Brain Research Bulletin. 2007;74(5):344–56. doi: 10.1016/j.brainresbull.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Bishop SJ, Duncan J, Lawrence AD. State anxiety modulation of the amygdala response to unattended threat-related stimuli. Journal of Neuroscience. 2004;24(46):10364–8. doi: 10.1523/JNEUROSCI.2550-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ. Facial expressions, their communicatory functions and neuro-cognitive substrates. Philosophical Transactions of The Royal Society of London. 2003a;B, 358:561–72. doi: 10.1098/rstb.2002.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ. Neurobiological basis of psychopathy. British Journal of Psychiatry. 2003b;182:5–7. doi: 10.1192/bjp.182.1.5. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Responding to the emotions of others: dissociating forms of empathy through the study of typical and psychiatric populations. Conscious Cognition. 2005;14(4):698–718. doi: 10.1016/j.concog.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Psychopathy, frustration, and reactive aggression: the role of ventromedial prefrontal cortex. British Journal of Psychology. 2010;101(Pt 3):383–99. doi: 10.1348/000712609X418480. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Colledge E, Murray L, Mitchell DGV. A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. Journal of Abnormal Child Psychology. 2001;29(6):491–8. doi: 10.1023/a:1012225108281. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Mitchell DGV. Psychopathy, attention, and emotion. Psychological Medicine. 2009;39:543–55. doi: 10.1017/S0033291708003991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ, Mitchell DGV, Blair KS. The Psychopath: Emotion and the Brain. Oxford: Blackwell Publishing; 2005. [Google Scholar]
- Blair RJ, Peschardt KS, Budhani S, Mitchell DGV, Pine DS. The development of psychopathy. Journal of Child Psychology and Psychiatry. 2006;47(3):262–75. doi: 10.1111/j.1469-7610.2006.01596.x. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dadds MR, Perry Y, Hawes DJ, et al. Attention to the eyes and fear-recognition deficits in child psychopathy. British Journal of Psychiatry. 2006;189:280–1. doi: 10.1192/bjp.bp.105.018150. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51(6):871–82. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Pascual-Leone A, Theoret H. Psychopathy and the mirror neuron system: preliminary findings from a non-psychiatric sample. Psychiatry Research. 2008;160(2):137–144. doi: 10.1016/j.psychres.2007.08.022. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M. Structured Clinical Interview for DSM-IV Axis I Disorders-Clinician Version (SCID-CV) Washington DC: American Psychiatric Press; 1997. [Google Scholar]
- Frewen PA, Dozois DJ, Joanisse MF, Neufeld RW. Selective attention to threat versus reward: meta-analysis and neural-network modeling of the dot-probe task. Clinical Psychology Review. 2008;28(2):307–37. doi: 10.1016/j.cpr.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Fullam RS, McKie S, Dolan MC. Psychopathic traits and deception: functional magnetic resonance imaging study. British Journal of Psychiatry. 2009;194(3):229–35. doi: 10.1192/bjp.bp.108.053199. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34(3):905–23. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon HL, Baird AA, End A. Functional differences among those high and low on a trait measure of psychopathy. Biological Psychiatry. 2004;56:516–21. doi: 10.1016/j.biopsych.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Hare RD. Manual for the Hare Psychopathy Checklist-Revised. 2nd edn. Toronto: Multi-Health Systems; 2003. [Google Scholar]
- Hare RD. Psychopathy: a clinical and forensic overview. Psychiatric Clinics of North America. 2006;29(3):709–24. doi: 10.1016/j.psc.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Hare RD, Hart SD, Harpur TJ. Psychopathy and the DSM-IV criteria for antisocial personality disorder. Journal of Abnormal Psychology. 1991;100(3):391–8. doi: 10.1037//0021-843x.100.3.391. [DOI] [PubMed] [Google Scholar]
- Harenski CL, Kim SH, Hamann S. Neuroticism and psychopathy predict brain activation during moral and nonmoral emotion regulation. Cognitive, Affective, & Behavioral Neuroscience. 2009;9(1):1–15. doi: 10.3758/CABN.9.1.1. [DOI] [PubMed] [Google Scholar]
- Herry C, Bach DR, Esposito F, et al. Processing of temporal unpredictability in human and animal amygdala. Journal of Neuroscience. 2007;27(22):5958–66. doi: 10.1523/JNEUROSCI.5218-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman ML. Developmental synthesis of affect and cognition and its implications for altruistic motivation. Developmental Psychology. 1975;11:607–22. [Google Scholar]
- Jones AP, Laurens KR, Herba CM, Barker GJ, Viding E. Amygdala hypoactivity to fearful faces in boys with conduct problems and callous-unemotional traits. American Journal of Psychiatry. 2009;166(1):95–102. doi: 10.1176/appi.ajp.2008.07071050. [DOI] [PubMed] [Google Scholar]
- Lilienfeld SO, Andrews BP. Development and preliminary validation of a self-report measure of psychopathic personality traits in noncriminal populations. Journal of Personality Assessment. 1996;66:488–524. doi: 10.1207/s15327752jpa6603_3. [DOI] [PubMed] [Google Scholar]
- Lilienfeld SO, Widows MR. Prefessional Manual for the Psychopathic Personality Inventory-Revised: (PPI-R) Lutz: Psychological Assessment Resources; 2005. [DOI] [PubMed] [Google Scholar]
- Lundqvist D, Flykt A, Ohman A. Sweden: Department of Clinical Neuroscience, Psychology section, Karolinska Institut; 1998. Karolinska Directed Emotional Faces. Stockholm. [Google Scholar]
- Lynam DR, Whiteside S, Jones S. Self-reported psychopathy: a validation study. Journal of Personality Assessement. 1999;73(1):110–32. doi: 10.1207/S15327752JPA730108. [DOI] [PubMed] [Google Scholar]
- Marsh AA, Finger EC, Mitchell DGV, et al. Reduced amygdala response to fearful expressions in children and adolescents with callous-unemotional traits and disruptive behaviour disorders. American Journal of Psychiatry. 2008;165(6):712–20. doi: 10.1176/appi.ajp.2007.07071145. [DOI] [PubMed] [Google Scholar]
- Mitchell DG. The nexus between decision making and emotion regulation: a review of convergent neurocognitive substrates. Behavioural Brain Research. 2011;217(1):215–31. doi: 10.1016/j.bbr.2010.10.030. [DOI] [PubMed] [Google Scholar]
- Mitchell DG, Colledge E, Leonard A, Blair RJ. Risky decisions and response reversal: is there evidence of orbitofrontal cortex dysfunction in psychopathic individuals? Neuropsychologia. 2002;40(12):2013–22. doi: 10.1016/s0028-3932(02)00056-8. [DOI] [PubMed] [Google Scholar]
- Mitchell DGV, Fine C, Richell RA, et al. Instrumental learning and relearning in individuals with psychopathy and in patients with lesions involving the amygdala or orbitofrontal cortex. Neuropsychology. 2006;20(3):280–9. doi: 10.1037/0894-4105.20.3.280. [DOI] [PubMed] [Google Scholar]
- Mitchell DGV, Greening SG. Conscious perception of emotional stimuli: Brain mechanisms. The Neuroscientist. 2011 doi: 10.1177/1073858411416515. September 9 (doi:10.1177/1073858411416515; epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Mitchell DG, Luo Q, Avny SB, et al. Adapting to dynamic stimulus-response values: differential contributions of inferior frontal, dorsomedial, and dorsolateral regions of prefrontal cortex to decision making. Journal of Neuroscience. 2009;29(35):10827–34. doi: 10.1523/JNEUROSCI.0963-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DGV, Nakic M, Fridberg D, Kamel N, Pine DS, Blair RJR. The impact of processing load on emotion. NeuroImage. 2007;34:1299–309. doi: 10.1016/j.neuroimage.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383(6603):812–5. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Murrie DC, Cornell DG. Psychopathy screening of incarcerated juveniles: a comparison of measures. Psychological Assessement. 2002;14(4):390–6. [PubMed] [Google Scholar]
- Newman JP, Kosson DS. Passive avoidance learning in psychopathic and nonpsychopathic offenders. Journal of Abnormal Psychology. 1986;95:252–6. [PubMed] [Google Scholar]
- Ogloff JR, Wong S, Greenwood A. Treating criminal psychopaths in a therapeutic community program. Behavioral Sciences and the Law. 1990;8:181–90. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Osterbauer RA, Wilson JL, Calvert GA, Jezzard P. Physical and physiological consequences of passive intra-oral shimming. Neuroimage. 2006;29(1):245–53. doi: 10.1016/j.neuroimage.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Perry DG, Perry LC. Denial of suffering in the victim as a stimulus to violence in aggressive boys. Child Development. 1974;45:55–62. [PubMed] [Google Scholar]
- Poythress NG, Edens JF, Lilienfeld SO. Criterion-related validity of the psychopathic personality inventory in a prison sample. Psychological Assessment. 1998;10:426–30. [Google Scholar]
- Rilling JK, Glenn AL, Jairam MR, et al. Neural correlates of social cooperation and non-cooperation as a function of psychopathy. Biol Psychiatry. 2007;61:1260–71. doi: 10.1016/j.biopsych.2006.07.021. [DOI] [PubMed] [Google Scholar]
- Schneider W, Eschman A, Zuccolotto A. E-Prime reference guide. Pittsburgh: Psychology Software Tools, Inc; 2002. [Google Scholar]
- Stevens D, Charman T, Blair RJ. Recognition of emotion in facial expressions and vocal tones in children with psychopathic tendencies. The Journal of Genetic Psychology. 2001;162(2):201–11. doi: 10.1080/00221320109597961. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotactic atlas of the human brain. New York: Thieme Medical Publishers, Inc; 1988. [Google Scholar]
- Whalen PJ. The uncertainty of it all. Trends in Cognitive Science. 2007;11(12):499–500. doi: 10.1016/j.tics.2007.08.016. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Kagan J, Cook RG, et al. Human amygdala responsivity to masked fearful eye whites. Science. 2004;306(5704):2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]
- White CN, Ratcliff R, Vasey MW, McKoon G. Anxiety enhances threat processing without competition among multiple inputs: a diffusion model analysis. Emotion. 2010;10(5):662–77. doi: 10.1037/a0019474. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.