Abstract
This study tested whether mothers with interpersonal violence-related posttraumatic stress disorder (IPV-PTSD) vs healthy controls (HC) would show greater limbic and less frontocortical activity when viewing young children during separation compared to quiet play. Mothers of 20 children (12–42 months) participated: 11 IPV-PTSD mothers and 9 HC with no PTSD. During fMRI, mothers watched epochs of play and separation from their own and unfamiliar children. The study focused on comparison of PTSD mothers vs HC viewing children in separation vs play, and viewing own vs unfamiliar children in separation. Both groups showed distinct patterns of brain activation in response to viewing children in separation vs play. PTSD mothers showed greater limbic and less frontocortical activity (BA10) than HC. PTSD mothers also reported feeling more stressed than HC when watching own and unfamiliar children during separation. Their self-reported stress was associated with greater limbic and less frontocortical activity. Both groups also showed distinct patterns of brain activation in response to viewing their own vs unfamiliar children during separation. PTSD mothers’ may not have access to frontocortical regulation of limbic response upon seeing own and unfamiliar children in separation. This converges with previously reported associations of maternal IPV-PTSD and atypical caregiving behavior following separation.
Keywords: maternal PTSD, early childhood, functional neuroimaging, emotion regulation, corticolimbic pathways, interpersonal violence
INTRODUCTION
Adults with posttraumatic stress disorder (PTSD) have difficulty regulating their emotions when new experiences remind them of one or more aspects of their trauma (New et al., 2009). Neuromaging studies of PTSD patients have reported significant activation in brain regions implicated in the processing and regulation of emotions and arousal (Etkin and Wager, 2007; Bluhm et al., 2009). These studies have shown that limbic responses to either positively or negatively valenced emotional stimuli, such as facial expressions or evocative verbal descriptions of interpersonal conflict, are exaggerated in adults who have interpersonal violence-related PTSD (IPV-PTSD), even when the stimuli have no apparent direct connection to the traumatic events previously experienced (Shin et al., 2005). Medial prefrontal cortical (mPFC) activation that is known to regulate limbic activity within the brain is, in contrast, diminished in PTSD patients compared with controls during the processing of similar stimuli (Gilboa et al., 2004).
While there are no previously published neuroimaging studies of women with IPV-PTSD who are mothers in response to their children, behavioral studies indicate that the use of neuroimaging could help us to understand caregiving behavior that differentiates these women from healthy controls (HC) (i.e. ‘atypical caregiving behavior’), and thus help address such behavior clinically. For example, mothers who have IPV-PTSD in comparison to healthy mothers show impaired reading of child affect, less empathic responsiveness and less availability for joint attention, particularly after a social stressor such as mother–child separation (Lyons-Ruth and Block, 1996; Schechter et al., 2010). Specifically, following child separation, mothers’ emotional availability to respond to child bids for engagement in joint attention to play was negatively correlated with their IPV-PTSD symptom severity (Schechter et al., 2010). The present MRI study was, in fact, nested in that behavioral study. While, no previous MRI studies to our knowledge have directly examined the neural circuits that could underlie these in PTSD-mothers’ atypical caregiving behavior, several imaging studies investigated neural activity among healthy parents in response to infant cries and faces (Swain et al., 2007; Strathearn et al., 2008). One study that used video excerpts of toddlers during play and separation reported activation of the mPFC in healthy mothers when viewing responses of their child during separation vs play (Noriuchi et al., 2008). This study found specific coordinated neural activity in cortical (i.e. mPFC, anterior cingulate and insula) and subcortical (i.e. amygdala, anterior entorhinal and perirhinal cortex) regions that participate in the emotion processing in response to viewing the video excerpts.
We also assessed brain activity in these corticolimbic circuits, but in a sample of mothers with marked IPV-PTSD compared to those without IPV-PTSD within an inner-city community that is rarely recruited for neuroimaging studies. Our overall goal has been to understand the circuitry in a mother's brain that might hinder vs support her capacity to respond sensitively to her child's affective communication under stress. Our curiosity has been fueled by our study of maternal emotional availability for joint focus of attention as mentioned above (Schechter et al., 2010), as well as by an additional observation of IPV-PTSD mothers’ tendency to ‘misread’ child affective communication (Fraiberg et al., 1975). With respect to this latter point, we previously studied clinically referred mothers with IPV-PTSD, who prior to video feedback, frequently misread their child's anxious facial expressions as angry and controlling, and only after therapeutic intervention were able to see and describe their child's separation anxiety and helplessness (Schechter et al., 2006). In the present study, we therefore describe the functioning of neural systems that subserve emotional reactions by mothers with IPV-PTSD and non-traumatized comparison mothers while watching videos of their own and unfamiliar toddlers displaying distress responses during separation or when playing quietly.
Hypotheses
Mothers with IPV-PTSD as compared to HC will show in response to viewing their own and unfamiliar children in separation (stressful condition) as contrasted with play (non-stressful condition) the following:
greater activation of fear–response circuitry (i.e. amygdala, entorhinal and perirhinal cortices, and hippocampus). We expect that this response will be stronger for own than for unfamiliar children, but will be generalized also to unfamiliar children.
less activation of higher cortical areas [i.e. superior frontal gyrus (SFG)/Mpfc] that are involved in downregulation of the fear response (Shin et al., 2006) and
greater self-reported stress on a post-MRI scan interview.
Additionally, we expect that mothers’ self-reported stress in response to seeing their own and unfamiliar children in separation (vs play) and clinician-assessed PTSD symptom severity will both be associated with greater activation of fear–response circuitry and less activation of corticofrontal areas (i.e. mPFC) that are known to regulate limbic response when continuous analyses are applied across the entire sample. Given previous behavioral findings of less maternal emotional availability following separation, we might also expect less activation of brain regions associated with empathy (i.e. the insula) in PTSD vs HC mothers in response to viewing children during separation vs play (Decety, 2010). In testing these hypotheses, this is the first study ever to examine how IPV-PTSD, considered as a disorder of emotion regulation, affects mothers’ neural response to viewing their own and unfamiliar preschool-age children in a situation that renders the child helpless and vulnerable. It newly examines how this neural response might correspond to subjective reports of parenting stress and observations of parental interactive behavior before and after separation such as we have described in previous articles (Schechter et al., 2010). Specifically, this study will help characterize how IPV-PTSD mothers process young children's emotional communication in the context of maternal IPV-PTSD (as compared to HC) in response to seeing them in a stressful (separation) vs non-stressful (play) condition.
METHODS
Participants
Recruitment
We obtained informed consent from participants both for the non-MRI and nested MRI study reported here according to procedures approved by the Columbia University Medical Center's Institutional Review Board. Recruitment from pediatric community clinics is described in further detail in the Supplementary Materials (see also Schechter et al., 2010). Twenty mothers who either had a diagnosis of IPV-PTSD (PTSD mothers) or who did not (HC) and who had both joined the larger mother–child study and were eligible for imaging (i.e. not pregnant) were scanned.
Sample characteristics
Mothers (19 right-handed, 1 left-handed) of 20 children (10 boys, 10 girls), 12–42 (mean 24 ± 8) months of age, participated. The single left-handed subject, when subtracted and then re-added to the sample, did not significantly alter results within or between groups.
Procedures
Procedures for visits prior to MRI scan
The protocol consisted of two 2-h videotaped visits following informed consent and screening (Schechter et al., 2010). During an initial videotaped interview, PTSD and HC mothers underwent a variety of psychometric examinations to assess their life-events history and psychopathology. Most importantly, lifetime and current diagnoses of PTSD was determined using the Clinician Administered PTSD Scale (CAPS) (Blake et al., 1995—see Supplementary Data and Schechter et al., 2010), which has extremely high interrater reliability (Cicchetti et al., 2009). The diagnosis of current PTSD at the time of participation was confirmed by self-report using the PTSD symptom checklist-short version (PCL-S) (Weathers et al., 1996—see Supplementary Data and Schechter et al., 2010).
One to 2 weeks after the initial videotaped maternal interview, the mothers and their children returned for a videotaped interaction. The protocol used to structure this visit was the Crowell procedure (Crowell et al., 1988) as modified by Zeanah et al. (2000) It consisted of two repetitions of the following mother–child interactions: (i) playing together as they did at home using a range of toys provided (8 min), (ii) separating (3 min) and (iii) reuniting (3 min). fMRI stimuli were drawn from interaction sequences (i) and (ii).
Stimuli
Mother viewed six different, silent, 40-s video excerpts of three different children, each during the two conditions: separation and play: (i) own child, (ii) unfamiliar boy and (iii) unfamiliar girl. The unfamiliar children were of similar mean age, drawn from a similar Hispanic and African–American inner-city community, and were filmed in a similar parent–child interaction protocol to ours, but at another institution.
Selection of stimuli
A research assistant who was blind to case–control status among mothers’ own children selected the silent excerpts for the fMRI stimulus in play and separation: mothers viewed the play excerpt observed to show the most joy and reciprocally the separation excerpt with the strongest child emotional response.
Selection of separation video excerpts
The two separation segments during the videotaped interaction protocol were rated without sound on a 5-point Separation Distress Scale (SDS; Schechter and McCaw, 2005) by two graduate-student raters independently, who were both naïve to group membership (rating 0 = no observable distress, 4 = agitation). The consistency of the child's response to separation across the both episodes of videotaped separation, as a measure of test–retest reliability was robust (Cronbach's α = 0.82). The episode in which the child was more distressed was shown in the scanner. Interrater reliability was excellent (ICC = 0.95, P < 0.001). For own child, raters found the mean stress on separation to be 2.31 (s.d. = 1.35; range 1.5–4.0); unfamiliar boy was rated ‘2’ and girl ‘4’. Concordance between raters’ and mothers’ estimation of child distress was high (Cronbach's α = 0.83). Both genders of unfamiliar children were included in an effort to control for gender mix among own-child stimuli.
Study design
The study design (Supplementary Figure S1) consisted of four runs, each lasting 11 min 12 s, and each containing two blocks during which mothers viewed six 40-s video excerpts per block, displayed in a pseudorandom order, counterbalanced within and across runs. Thus, mothers viewed each of the six 40-s film clips eight times. Eye tracking and vocal reminders by the technician to pay attention to the screen between runs were used to ensure that mothers were attending to stimuli.
Postscan interviews of mothers about their response to stimuli
A postscan interview developed by the authors assessed subjective (self-reported) ‘stress’ (displeasure) and ‘fun’ (pleasure) experienced in response to viewing each of the six excerpts on a 5-point rating scale from ‘0’ as ‘least’ to ‘4’ as ‘most’.
Image acquisition and pre-processing
Briefly, imaging was performed under GE 3T scanner. Standard fMRI preprocessing procedures including slice timing and motion corrections, and spatial normalization and smoothing were performed under SPM and MATLAB. See Supplementary Data for details.
Hypothesis-driven analyses
The Blood Oxygen-Level Dependent (BOLD) response from each run was first assessed using a General Linear Model containing seven time-dependent functions representing each stimulus type (resting gaze fixation and six videos) and one constant (baseline BOLD signal). Contrast images representing the differential effect of the video presentations on voxel-wise BOLD signals were created for each subject. A contrast specifying ‘separation vs play’ was the difference in estimated neural activity during the viewing of the child in the separation condition compared with estimated activity during the viewing of the child during free play (condition). Results for this are not discussed but can be found in the Supplementary Table S3. To examine hypotheses 1 and 2 (see page 4), we performed paired-samples t-tests that compare BOLD activation in subjects with PTSD to the BOLD activation in HC when watching scenes of separation vs play, once for own children and once for unfamiliar children. To examine within-group differences in activation associated with the familiarity of the children (i.e. own vs unfamiliar), we performed paired-sample t-tests comparing activity when viewing their own child vs unfamiliar children for the separation relative to play contrast. We report significant changes in voxels using a P ≤ 0.025 threshold (i.e. a two-tailed P ≤ 0.05) together with the requirement that the activation occurred in an empirically derived spatial cluster greater than 25 adjacent voxels. This conjoint cluster-size requirement which is based on an approximation formula (Friston et al., 1994), yields a conservative effective P < 0.000005. The combined application of a statistical threshold and cluster filter reduces substantially the false-positive identification of activated voxels at any given threshold (Forman et al., 1995).
Group differences with respect to maternal self-reported stress experienced when looking at their own and unfamiliar children during separation were tested using independent t-tests.
To investigate the neural correlates of this same variable of maternal self-reported stress, we entered into a regression model BOLD signal change, detected by the contrast of separation relative to free play as the dependent variable and as a predictor, the maternal self-rated stress score from the post-MRI interview. Similarly, to investigate the neural correlates of maternal PTSD symptom severity, we entered into a similar regression model as a predictor, a composite of z-scores based on the CAPS score from the initial interview with mother—and the maternal self-reported PCL-S that was administered during the scanning visit. These analyses also use a P ≤ 0.025 threshold with a minimum cluster size of 25 voxels.
RESULTS
Characteristics of PTSD vs HC groups
The IPV-PTSD case group (n = 11) was rigorously defined by clinician rating on the CAPS and PCL-S scores (Supplementary materials; Schechter et al., 2010): 11 PTSD mothers (30 ± 6 years old) and 9 HC mothers (31 ± 7 years old), 5 of whom had limited violence exposure that they did not rate as traumatic and to which they did not attribute PTSD symptoms (one with prior domestic violence and four with histories of excessive corporal punishment for a defined period). These nine HC mothers had neither the diagnosis of PTSD nor subthreshold PTSD, and were not suffering from major depression.
As expected (Shea et al., 2004), PTSD was significantly more often comorbid with major depressive disorder (MDD) and greater severity of depressive symptoms. Overall dissociative symptom scores (DESs) were similarly elevated for PTSD (27 ± 20) and minimal for HC (5 ± 1.5; t1 = 3.6, P < 0.01). The degree of naively rated child distress on silent video excerpts depicting children during separation also did not differ between groups (Table 1).
Table 1.
Demographic and clinical characteristics of subjectsa
| PTSD cases (n = 11) | HC (n = 9) |
t-test and χ2 statistics (df = 18) |
||
|---|---|---|---|---|
| t-value or χ2 | Significance (P) | |||
| Mean age of mother | 29.5 (7.1) | 30.4 (7.2) | 0.31 | 0.76 |
| Mean age of child (months) | 25.4 (7.9) | 21.9 (7.8) | −0.98 | 0.34 |
| Number of boys | 6 | 4 | −1.34 | 0.20 |
| Number of mothers with high school diploma or equivalent (GED) | 6 | 7 | 1.17 | 0.37 |
| Mean years of the mothers education | 11.9 (1.4) | 14.3 (3.4) | 2.15 | 0.05 |
| Mean income in $1000 increments | 25.5 (18.6) | 54.4 (32.1) | 2.53 | 0.02 |
| Mean number of violent events | 25 (17.4) | 8.7 (8.9) | −2.71 | 0.02 |
| Recent domestic violence (%) | 64 | 0 | −4.18 | 0.002 |
| Mothers who attempted suicide (%) | 45 | 0 | −2.89 | 0.02 |
| History of child protection involvement (%) | 45 | 0 | −2.90 | 0.02 |
| PSI-SF mean score | 47.6 (15.4) | 20 (12.4) | 3.38 | 0.006 |
| CAPS mean score (lifetime PTSD) | 88.5 (15.6) | 18.3 (16) | −9.40 | <0.001 |
| Baseline mean PCLS (current PTSD) | 43.3 (17.2) | 19.9 (9.7) | −3.80 | <0.001 |
| Scan mean PCLS (current PTSD) | 43.7 (13.5) | 13.2 (10.2) | −5.58 | <0.001 |
| MDD diagnosis on MINI | 7.0 (63.6) | 0.0 (0.0) | 5.87 | 0.02 |
| Baseline mean BDI | 18.7 (13.2) | 5.2 (4.8) | −3.91 | 0.004 |
| Scan mean BDI | 16.2 (12.6) | 3.3 (4.6) | −3.77 | 0.002 |
| DES mean score | 25.8 (21.4) | 5 (5.2) | −3.12 | 0.009 |
| Borderline personality screen (PDQ-IV) | 3 (27.2%) | 2 (22.2%) | 0.27 | 0.54 |
| Mean child distress (independently rated) | 2.5 (1.4) | 2.1 (1.3) | −0.56 | 0.58 |
aMeans are reported with standard deviations; 19/20 subjects were right-handed.
PSI-SF, parenting stress index-short form; PCLS, post-traumatic stress symptoms checklist-short version; MINI, Mini International Neuropsychiatric Interview; BDI, Beck Depression Inventory; DES, Dissociative Experiences Scale; PDQIV, Personality Disorders Questionnaire.
PTSD and HC groups did not differ significantly in terms of child age or gender (Table 1). Cases differed only from controls on background variables with respect to lower household income and number of years of education (P < 0.05), despite no group difference in attainment of a high-school diploma or General Educational Diploma (GED) (P > 0.3). Consequently, we included household income and number of years of education as covariates in the model for neural activation during the viewing of separation vs play and noted no significant effects.
Imaging results
Mothers viewing their own children during separation vs free play
Neural activity when viewing their own children during separation vs free play (‘separation-play’) was compared across two groups: for 11 PTSD mothers vs 9 HC (group-by-condition inter action, see Figure 1A).
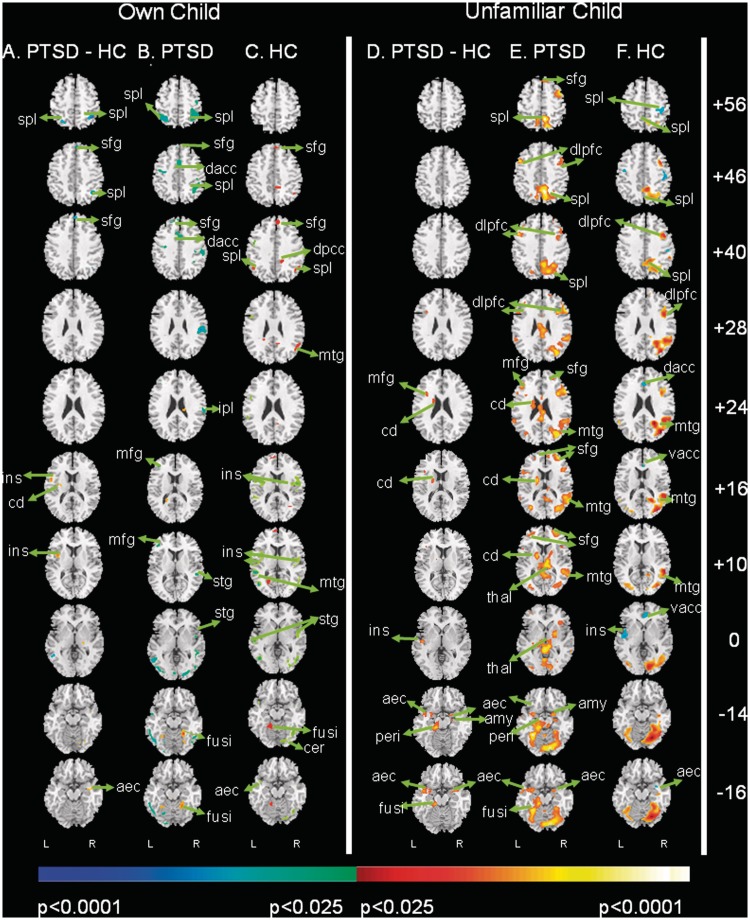
Fig. 1.
Group comparisons of activation when mothers view their own and unfamiliar children during separation vs play. Two panels each containing three columns of contrast images are presented that test the a priori hypothesis that PIV-PTSD mothers exhibit reduced mPFC activation and greater limbic activation vs control mothers when viewing child mental states during separation vs during play, in both their own and in unfamiliar children. Each of the two panels is divided into three columns that represent the following: (A and D) group differences in fMRI signal with greater activation in PTSD mothers compared to controls in red and greater activation in controls in blue (i.e. the contrast of the activations in column B with the activations in column C. (B and E) group average fMRI signal in PTSD mothers when viewing separation compared with play and (C and F) HC mothers when viewing separation compared with play. For columns, B and E, and C and F, increases in signal while viewing separation relative to play are shown in red and decreases are in blue. The left-most three columns of this figure (A–C) show neural activity in PTSD and HC mothers while viewing their own children during separation relative to play. The right-most three columns (D–F) show neural activity in PTSD and HC mothers while viewing unfamiliar children during separation relative to play. These are transaxial views with Talairach z-coordinates shown to the far right of the row of corresponding slices, representing the slices positioned from superiorly to inferiorly (top to bottom). The left side of the brain (L) is displayed on the left side of the image. We report voxels using a P-value threshold <0.05 together with the requirement that the activation occurred in a spatial cluster >25 adjacent pixels, a conjoint requirement which, based on an approximation formula, yields a conservative effective P < 0.000005. The color bars depict P-values for the threshold of the respective statistical contrast without adjustment for the conjoint requirement of the special cluster. aec, anterior entorhinal cortex; amy, amygdala; cd, caudate; dacc, dorsal anterior cingulate cortex; dorsolateral prefrontal cortex; fusi, fusiform gyrus; ins, insula; peri, perirhinal cortex; mfg, middle frontal gyrus; mtg, medial temporal gyrus; sfg, superior frontal gyrus; spl, superior parietal lobe; stg, superior temporal gyrus; thal, thalamus; vacc, ventral anterior cingulate cortex.
PTSD mothers vs HC (Figure 1A and B) exhibited significantly greater activation in the bilateral anterior entorhinal cortex (AEC, BA34) and the left caudate.
HC compared to PTSD mothers (Figure 1A and C) exhibited significantly greater activation in higher cortical regions (SFG; BA8,10) and bilateral superior parietal lobes (SPL; BA7).
Maps for separation vs rest (‘separation-rest’) and play vs rest (‘play-rest’) suggested that the group difference in these activations originated in response to separation rather than to play (Supplementary Figure S2).
In response to seeing their own children in separation vs play (Figure 1A), we noted apparent activation of the left insula (BA13) in the PTSD mothers compared to HC, which actually represents ‘deactivation’ among HC (i.e. activation while seeing their own child in play, which is also a control condition, as confirmed by separation vs rest and play vs rest maps (Supplementary Table S1 and Figure S2).
Mothers viewing unfamiliar children during separation vs with free play
PTSD vs HC mothers (Figure 1D and E) exhibited significantly greater activation in limbic regions: the right amygdala, bilateral AEC (BA34), left perirhinal cortex (BA35); as well as the left caudate and the L > R fusiform gyrus (BA37).
HC vs PTSD mothers (Figure 1D and F) when viewing unfamiliar children in separation vs play did not exhibit significantly greater activation in the SFG (BA8,10). However, PTSD mothers activated the L middle frontal gyrus (MFG; BA10) significantly more than HC.
Maps for separation vs rest and play vs rest suggested that this group difference originated in response to separation rather than to play (Supplementary Figure S2).
In response to seeing unfamiliar children in separation-play (Figure 1D), we noted activation of the left insula (BA13) in the PTSD vs HC mothers that was similar to that which we noted in response to mothers viewing their own child. The origin of this apparent activation is again a ‘deactivation’ among HC (i.e. activation in response to viewing play), as was true in response to mothers viewing their own child. Separation- and play-rest maps showed that HC mothers activated the insular cortex more than PTSD mothers when seeing their own and unfamiliar children play (Supplementary Table S2 and Supplementary Figure S2).
Within-group differences in viewing children in separation by own vs unfamiliar child
We assessed group differences in activation associated with the familiarity of the children (i.e. own vs unfamiliar) during separation only as per our a priori hypothesis (familiarity-by-condition interaction) separately in PTSD and HC mothers (Figure 2).
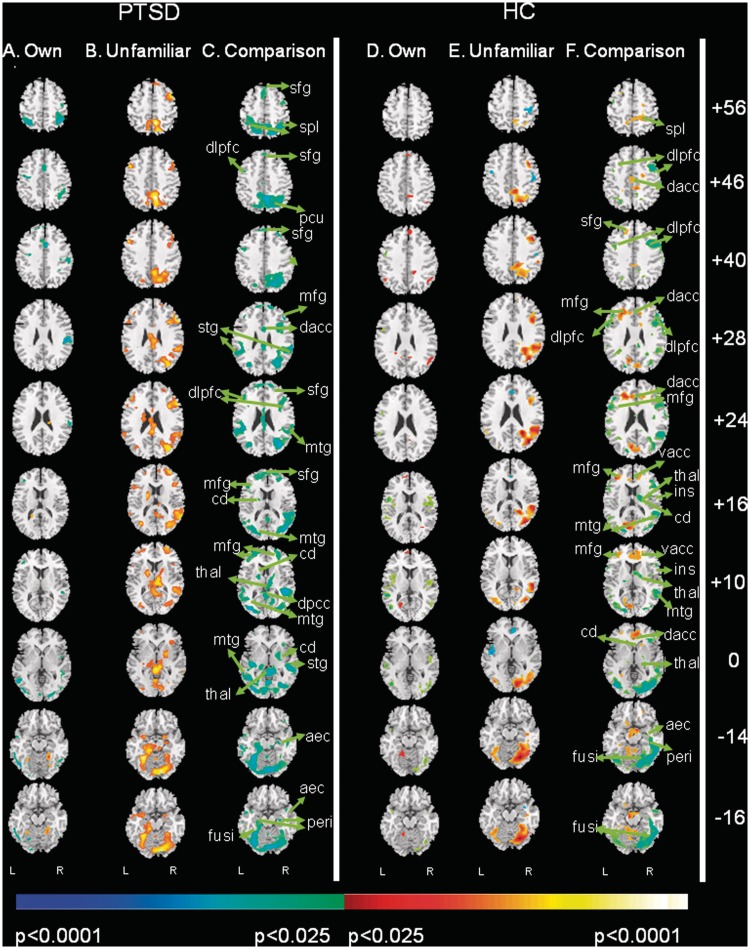
Fig. 2.
Within-group comparison of activation when mothers view their own and unfamiliar children. This figure shows group differences in activation associated with the familiarity of the children (i.e. own vs unfamiliar) during separation compared with play (i.e. testing a familiarity-by-condition interaction) separately in PTSD and HC mothers. Two panels each containing three columns of contrast images are presented that test the a priori hypothesis that PTSD mothers compared to HC would display greater neural activation in response to unfamiliar children in separation vs play. The two panels representing these separate comparisons are: (A) PTSD and (B) HC. Each panel is subdivided into three columns: (A and D) own child in separation vs play, (B and E) unfamiliar children in separation vs play and (C and F) own child in contrast to unfamiliar children in separation vs play. Increases in signal while viewing own child relative to unfamiliar children are shown in red and decreases are in blue. Orientation and statistical thresholding are the same as in Figure 1. aec, anterior entorhinal cortex; amy, amygdala; cd, caudate; dacc, dorsal anterior cingulate cortex; dlpfc, dorsolateral prefrontal cortex; fusi, fusiform gyrus; ins, insula; mfg, middle frontal gyrus; mtg, medial temporal gyrus; pcu, precuneus; peri, perirhinal cortex; sfg, superior frontal gyrus; spl, superior parietal lobe; stg, superior temporal gyrus; thal, thalamus; vacc, ventral anterior cingulate cortex.
Among PTSD mothers, activation differed significantly when viewing their own vs unfamiliar children (Figure 2C), with greater activation in response to viewing unfamiliar children as compared to own children in AEC and perirhinal cortices, as well as caudate and cerebellum, fusiform gyrus; as well as higher cortical areas (SFG [BA8,10]).
Among HC mothers, we noted greater activation in response to viewing unfamiliar children than own children in the higher cortical regions [bilateral SPL (BA7) and MFG (L > R (BA10)] (Figure 2C). This pattern of activity in HC mothers derived from more prominent deactivation when viewing their own children in separation compared to unfamiliar children (Figure 2F vs C).
In general, PTSD mothers activated more strongly to viewing unfamiliar children as compared to their own and HC mothers reacted more strongly to viewing their own children as compared to unfamiliar as supported by group comparisons (i.e. omnibus test) (Supplementary Table S2).
Postscan maternal interview data
The analysis of post-MRI interviews revealed a main effect of condition on the mothers’ level of self-reported stress [F(1,20) = 138.02, P < 0.001), indicating that both groups experienced less stress when watching children (either their own or unfamiliar) during play than during separation. We also noted an interaction of group-by-condition [F(1,20) = 11.37, P = 0.002), which derived from significantly less stress in HC than in PTSD mothers when watching their own and unfamiliar children during separation but disproportionately greater stress in PTSD mothers when viewing their own child during separation as examined by independent t-tests [t-own child (1,18) = 2.59, P < 0.05; t-unfamiliar(1,18) = 2.15, P < 0.05], see Supplementary Table S4. These group differences cannot be accounted for by differences in independently rated child behavior during separation [t(1,18) = −0.56; P = 0.58].
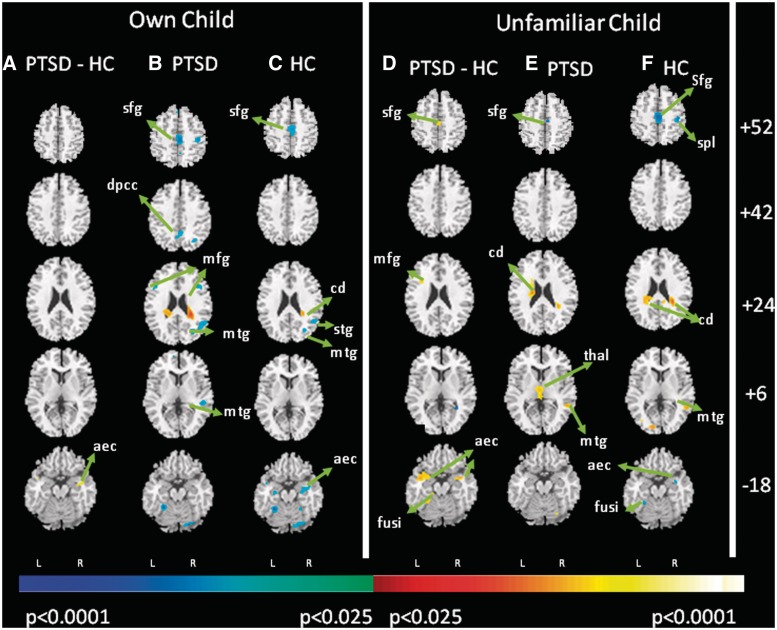
To evaluate relations between self-report measures of stress and neuroimaging, we assessed correlations of within-group BOLD signal changes with post-MRI interview responses. In both HC and PTSD mothers, when seeing their own and unfamiliar children during separation (Figure 3), self-reported levels of stress correlated inversely with activation of dorsal SFG (BA10), and positively with activation of the caudate. The more stress HC mothers reported in response to seeing their own and unfamiliar children during separation the more they deactivated the bilateral AEC. PTSD mothers—who reported more stress than HC in the same conditions—differed significantly from this pattern of activation.
Fig. 3.
Neural correlates of mothers’ self-reported stress in response to separation. The figure displays the maps of correlations of the mothers’ self-reported level of stress with neural activation when viewing their own and unfamiliar children during separation-play in control and PTSD groups. Red indicates a positive correlation and blue indicates an inverse correlation. The correlations are compared across the two diagnostic groups in the right-most columns. Orientation and statistical thresholding is the same as in Figure 1. aec, anterior entorhinal cortex; amy, amygdala; cd, caudate; dlpfc, dorsolateral prefrontal cortex; fusi, fusiform gyrus; dacc, dorsal anterior cingulate cortex; ins, insula; mfg, middle frontal gyrus; mtg, medial temporal gyrus; peri, perirhinal cortex; sfg, superior frontal gyrus; spl, superior parietal lobe; thal, thalamus; vacc, ventral anterior cingulate cortex.
We also entered into a regression model BOLD signal change, detected by the contrast of separation relative to free play as the dependent variable, and as a predictor, a composite of z-scores based on the CAPS and PCL-S scores of the mother (Table 2). Across the entire sample, limbic regions were activated in response to viewing separation of mothers own and unfamiliar children (i.e. bilateral amygdala, perirhinal, AEC and hippocampus), as well as motor planning areas (i.e. bilateral caudate and cerebellum). Scatter plots were checked to ensure that no outliers had skewed these results.
Table 2.
Interactions of familiarity (own vs unfamiliar children) by condition (separation vs free play) by group (PTSD vs controls)
| Region of interest | Side | Brodmann's area | MNI coordinates |
t-value | z-score | P | Origins | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Cortical regions | |||||||||
| STG, temporal pole | Left > Right | 38,22,21 | −44 | 0 | −24 | 3.46 | 2.99 | 0.001 | Separation |
| Limbic areas | |||||||||
| Amygdala, entorhinal cortex | Left/Right | 34,28 | −28 | 2 | −16 | 2.81 | 2.52 | 0.006 | Separation |
| Hippocampus, perirhinal cortex | Left > Right | 28,35 | −16 | −8 | −12 | 2.68 | 2.43 | 0.008 | Separation |
| Other regions | |||||||||
| Cerebellum | Left > Right | 0 | −44 | −44 | 2.86 | 2.57 | 0.005 | Separation | |
DISCUSSION
Most mothers would say that viewing their child placed in a vulnerable situation such as separation from mother in the laboratory would not be a pleasurable experience. Yet what we have found in this study is that mothers with IPV-PTSD find it particularly stressful to view young children during separation; while they differed less than HC when viewing young children during play. IPV-PTSD mothers compared to HC respond to seeing both their own and unfamiliar toddlers during separation with significantly greater (i) activation of specific fear circuit-related regions [for own child (Figure 1A): AEC; for unfamiliar children (Figure 1D): amygdala, AEC and perirhinal cortex] and (ii) self-report feeling stressed (Supplementary Table S4). Support for our a priori hypotheses as per these group findings is strengthened by the results of the continuous analyses in which maternal self-reported stress during separation was also associated with failure to deactivate the AEC in PTSD mothers compared to HC (Figure 3). Continuous standardized maternal PTSD symptom severity scores were also correlated with activation of the fear circuit-related regions: amygdala, AEC, perirhinal cortex and hippocampus in response to viewing both own and unfamiliar children during separation (Table 2.).
Only HC mothers showed activation of the SFG (i.e. mPFC) in response to seeing their own children during separation (Figure 1A). Thus, PTSD mothers displayed greater activation of fear circuit-related regions with less top-down regulation by the SFG. This finding also converges with higher levels of maternal self-reported stress among PTSD mothers in response to separation and may provide the neural underpinnings for our prior behavioral observations; namely, mothers’ emotional availability for coordinated joint attention during play upon reunion after separation negatively correlated to the severity of their IPV-PTSD symptoms (Schechter et al., 2010).
We speculate that the present neuroimaging study's findings as well as those of our previous behavioral study may be, in part, explained by ‘the polyvagal theory’ (Porges, 2007), which postulates that the subject goes into a ‘survival’ mode rather than ‘affiliative’ mode when confronted with a traumatic reminder (i.e. for an IPV-PTSD mother—as we have speculated, viewing her own and other toddlers left alone and helpless). In the ‘survival mode’, the brain and sympathetic nervous system focus on the preparation for fight or flight—including related motor planning (i.e. caudate and cerebellum activation) at the expense of social–emotional availability and parasympathetic nervous system activity (Schechter et al., 2005; Austin et al., 2007).
As an alternative hypothesis, this activation pattern may also represent a difference of the degree of normative hypervigilance that is observed among mothers of infants and very young children—which in exaggerated form has been hypothesized to be associated with parental anxiety disorders (Leckman et al., 2004). We think that such potentially exaggerated hypervigilance might be related to increased maternal emotion dysregulation among the PTSD mothers that is triggered by seeing the child in a helpless position during separation. This state of mind may be reminiscent of traumatized mother's state of mind during interpersonal violence exposure. It has been shown in multiple studies that individuals with IPV-PTSD have difficulty downregulating their own fear and helplessness in the face of negative and arousing emotional stimuli (Gilboa et al., 2004; New et al., 2009; Jovanovic and Ressler, 2010; Schechter et al., 2010). The findings of increased activation in fear circuitry-related areas (i.e. limbic), motor planning areas (i.e. caudate) and facial emotion processing areas (i.e. fusiform gyrus) among PTSD mothers compared to HC, when viewing own and unfamiliar children during separation, support this interpretation. Decreased activation in the SFG among PTSD mothers in the same conditions further supports this view.
The SFG has been implicated previously in the regulation of fear and anxiety states in response to viewing negatively valenced, arousing emotional stimuli (Numan and Insel, 2003; Leckman et al., 2004; Shin et al., 2005; Marsh et al., 2009). The absence of SFG activation in response to negative arousing emotional stimuli has also been associated with the presence of increased anticipatory anxiety, alexithymia and dissociative phenomena, and reduced self-awareness (Gilboa et al., 2004; New et al., 2009). These explanations do not account for the group difference between responses to own vs unfamiliar child during viewing of separation among mothers with PTSD as compared to HC. The amount and intensity of activation to viewing unfamiliar vs own children during separation were greater particularly among IPV-PTSD mothers. We wondered whether this might be due to greater child distress in at least one of the unfamiliar children than in most of the mothers’ own children.
In an effort to address this potential confounder, we controlled for the different levels of child distress among the unfamiliar children during separation (boy with score distress score of ‘2’ and girl with ‘4’) by repeating the contrasts post hoc with each child separately. Results concerning the brain regions involved in our hypotheses were remarkably similar. Thus, we think that the novelty of seeing the unfamiliar children contributed to this greater overall activation, with certain specific brain areas affected in relation to the recognition of the unfamiliar children as novel stimuli particularly among the PTSD mothers as follows: upon viewing unfamiliar children, PTSD mothers access the MFG (BA10) likely in the service of autobiographical memory retrieval perhaps to differentiate novel from familiar stimuli (Burianova and Grady, 2007).
HC as compared to PTSD mothers deactivated the insular cortex upon viewing their own and unfamiliar children in play vs separation. While beyond the scope of this article, this observation raises a question that merits further study; namely, how the two groups differed in recruitment of emotional processing circuitry in response to play, as a likely nonstressful child stimulus that evokes positive affect (Strathearn et al., 2009).
Limitations
This study has several limitations. These include a relatively small sample size and the inherent limitations in statistical power that follow. Of note, this inner-city sample of violence-exposed mothers with PTSD is rarely represented in the affective neuroscience literature and is difficult to recruit into a multivisit study including neuroimaging.
An additional limitation included unexpected group differences with respect to household income and number of years of maternal education—despite no significant group differences with respect to mothers’ having completed a high school education or its equivalent. We covaried these sociodemographic factors with no significant change in results. However, it is clearly beyond the scope of this study to test whether these sociodemographic factors are predictive of PTSD caseness and/or whether having PTSD increases impairment that in turn limits the affected individual's likelihood of social, economic and academic success. Larger epidemiologic studies have shown that poverty and poor education can be both risk factors for and effects of chronic violence exposure and related PTSD (Koenen et al., 2007).
Another important limitation pertains to the stimuli used. The naturalistic video clips of subjects own children, while having the advantage of providing more ecologically valid stimuli that were individualized for each mother, contained differing levels of distress across children. Even though naïve raters found no significant group differences in levels of child distress during separation, greater rigor could be applied to creating the unfamiliar child stimuli and in further constraining the breadth of ages among own and unfamiliar children. And as mentioned above, the levels of distress among the two unfamiliar children differed significantly from one another. Despite our post hoc analyses to control for this potential confounder, we cannot fully dismiss the possibility that these differing distress levels altered the results with regards to response to child separation stimuli.
The present study demands replication and expansion both with a larger sample and with two control groups: one with IPV exposure but no PTSD and one with neither PTSD nor IPV exposure (Adenauer et al., 2010). Additionally, given the characteristic failure among IPV-PTSD patients to extinguish hyperarousal in response to evocative stimuli, further studies might also test whether IPV-PTSD mothers habituate less to video-stimuli depicting their own and unfamiliar children in separation as compared to play (Wessa and Flor, 2007).
CONCLUSIONS
This article reports findings from the first study ever to characterize neural activation of violence-exposed parents with PTSD compared to HC in response to viewing very young children during separation as compared to free play. The study suggests that IPV-PTSD mother's viewing their own and unfamiliar children during this interpersonally stressful condition of separation triggers patterns of neural activation that are similar to those triggered in response to traumatic reminders among adult PTSD patients (Francati et al., 2007; New et al., 2009). Namely, activation of the amygdala and surrounding input and output regions (i.e. AEC and perirhinal cortex) in response to seeing very young children in separation is associated with greater PTSD symptom severity. While the SFG was not recruited by IPV-PTSD mothers in response to viewing their own child in separation, it was, in response to viewing unfamiliar children. The opposite was true for HC (i.e. the SFG was activated when seeing own children but not when seeing unfamiliar ones). The latter group activated this region in response to viewing their own children but not unfamiliar children. Given these findings, this study may help to clarify the neural underpinnings of atypical maternal behavior of IPV-PTSD mothers during interactions with their young children following interpersonal stressors such as separation (Fraiberg et al., 1975; Lyons-Ruth et al., 1999; Schechter et al., 2010). Understanding the neural basis for parental stress and its impact on caregiving behavior is particularly important given that longitudinal follow-up studies have suggested that the children of IPV-PTSD mothers are at risk for long-term disturbances in their capacity to self-regulate their emotions, arousal and behavior (Lieberman et al., 2005; Schechter et al., 2007; Dutra et al., 2009).
SUPPLEMENTARY DATA
Supplementary Data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
The authors acknowledge the following funding sources that have made the work reported in this article possible: Grants awarded to Daniel S. Schechter, M.D. from: the National Institute of Mental Health (K23 MH068405); International Psychoanalytical Association Research Advisory Board; Sackler Institute of Developmental Psychobiology at Columbia University; Bender-Fishbein Fund at Columbia University; Swiss National Science Foundation, National Centre for Competence in Research on the Synaptic Bases of Psychiatric Disorders (SYNAPSY- CC4); Gertrude von Meissner Foundation, University of Geneva Faculty of Medicine; Grant awarded to Rachel Marsh, M.D. from the National Institute of Mental Health (K02 MH746771). Grant awarded to Bradley S. Peterson, M.D. from the National Institute of Drug Abuse (DA017820).
REFERENCES
- Adenauer H, Pinösch S, Catani C, et al. Early processing of threat cues in posttraumatic stress disorder: evidence for a cortical vigilance-avoidance reaction. Biological Psychiatry. 2010;68:451–8. doi: 10.1016/j.biopsych.2010.05.015. [DOI] [PubMed] [Google Scholar]
- Austin MA, Riniolo TC, Porges SW. Borderline personality disorder and emotion regulation: insights from the Polyvagal Theory. Brain Cognitive. 2007;65(1):69–76. doi: 10.1016/j.bandc.2006.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, et al. The development of a clinician-administered PTSD scale. Journal of Traumatic Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bluhm RL, Williamson PC, Osuch EA, et al. Alterations in default network connectivity in posttraumatic stress disorder related to early-life trauma. Journal of Psychiatry and Neuroscience. 2009;34(3):187–94. [PMC free article] [PubMed] [Google Scholar]
- Burianova H, Grady CL. Common and unique neural activations in autobiographical, episodic, and semantic retrieval. Journal of Cognitive Neuroscience. 2007;19(9):1520–34. doi: 10.1162/jocn.2007.19.9.1520. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Fontana A, Showalter D. Evaluating the reliability of multiple assessments of PTSD symptomatology: multiple examiners, one patient. Psychiatry Research. 2009;166(2–3):269–80. doi: 10.1016/j.psychres.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Crowell JA, Feldman SS, Ginsberg N. Assessment of mother-child interaction in preschoolers with behavior problems. Journal of the American Academy of Child and Adolescent Psychiatry. 1988;27(3):303–11. doi: 10.1097/00004583-198805000-00007. [DOI] [PubMed] [Google Scholar]
- Decety J. The neurodevelopment of empathy in humans. Developmental Neuroscience. 2010;32(4):257–67. doi: 10.1159/000317771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra L, Bureau JF, Holmes B, Lyubchik A, Lyons-Ruth K. Quality of early care and childhood trauma: a prospective study of developmental pathways to dissociation. The Journal of Nervous and Mental Disease. 2009;197(6):383–90. doi: 10.1097/NMD.0b013e3181a653b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164(10):1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fraiberg S, Adelson E, Shapiro V. Ghosts in the nursery. A psychoanalytic approach to the problems of impaired infant-mother relationships. Journal of the American Academy of Child Psychiatry. 1975;14(3):387–421. doi: 10.1016/s0002-7138(09)61442-4. [DOI] [PubMed] [Google Scholar]
- Francati V, Vermetten E, Bremner JD. Functional neuroimaging studies in posttraumatic stress disorder : A review of current methods and findings. Depression and Anxiety. 2007;24(3):202–18. doi: 10.1002/da.20208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Human Brain Mapping. 1994;1:210–20. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Shalev AY, Laor L, et al. Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biological Psychiatry. 2004;55(3):263–72. doi: 10.1016/j.biopsych.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. American Journal of Psychiatry. 2010;167(6):648–62. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Moffitt TE, Poulton R, Martin J, Caspi A. Early childhood factors associated with the development of post-traumatic stress disorder: results from a longitudinal birth cohort. Psychological Medicine. 2007;37(2):181–92. doi: 10.1017/S0033291706009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckman JF, Feldman R, Swain JE, Eicher V, Thompson N, Mayes LC. Primary parental preoccupation: circuits, genes, and the crucial role of the environment. Journal of Neural Transmission. 2004;111(7):753–71. doi: 10.1007/s00702-003-0067-x. [DOI] [PubMed] [Google Scholar]
- Lieberman AF, Van Horn P, Ozer EJ. Preschooler witnesses of marital violence: predictors and mediators of child behavior problems. Development and Psychopathology. 2005;17(2):385–96. doi: 10.1017/s0954579405050182. [DOI] [PubMed] [Google Scholar]
- Lyons-Ruth K, Block D. The disturbed caregiving system: relations among childhood trauma, maternal caregiving, and infant affect and attachment. Infant Mental Health Journal. 1996;17:257–75. [Google Scholar]
- Lyons-Ruth K, Bronfman E, Parsons E. Atypical attachment in infancy and early childhood among children at developmental risk. IV. Maternal frightened, frightening, or atypical behavior and disorganized infant attachment patterns. Monographs of the Society for Research in Child Development. 1999;64(3):67–96. doi: 10.1111/1540-5834.00034. discussion 213–20. [DOI] [PubMed] [Google Scholar]
- Marsh R, Maia TV, Peterson BS. Functional disturbances within frontostriatal circuits across multiple childhood psychopathologies. The American Journal of Psychiatry. 2009;166(6):664–74. doi: 10.1176/appi.ajp.2009.08091354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- New AS, Fan J, Murrough JW, Liu X, Liebman RE, Guise KG, et al. A functional magnetic resonance imaging study of deliberate emotion regulation in resilience and posttraumatic stress disorder. Biological Psychiatry. 2009;66(7):656–64. doi: 10.1016/j.biopsych.2009.05.020. [DOI] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Senoo A. The functional neuroanatomy of maternal love: mother's response to infant's attachment behaviors. Biological Psychiatry. 2008;63(4):415–23. doi: 10.1016/j.biopsych.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Numan M, Insel TR. The Neurobiology of Parental Behavior. New York: Springer-Verlag; 2003. [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychiatry. 2007;74(2):116–43. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter D, McCaw J. Separation Distress Scale (SDS). Columbia University Department of Psychiatry: Unpublished Instrument. 2005 [Google Scholar]
- Schechter DS, Myers MM, Brunelli SA, Coates SW, Zeanah CH, Davies M, et al. Traumatized mothers can change their minds about their toddlers: understanding how a novel use of video feedback supports positive change of maternal attributions. Infant Mental Health Journal. 2006;27(5):429–48. doi: 10.1002/imhj.20101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter D, Willheim E, Hinojosa C, et al. Subjective and objective measures of parent-child relationship dysfunction, child separation distress, and joint attention. Psychiatry. 2010;73(2):130–44. doi: 10.1521/psyc.2010.73.2.130. [DOI] [PubMed] [Google Scholar]
- Schechter DS, Zygmunt A, Davies M, et al. Caregiver traumatization adversely impacts young children's mental representations on the MacArthur Story Stem Battery. Attachment and Human Development. 2007;9(3):187–205. doi: 10.1080/14616730701453762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea MT, Stout RL, Yen S, et al. Associations in the course of personality disorders and Axis I disorders over time. Journal of Abnormal Psychology. 2004;113(4):499–508. doi: 10.1037/0021-843X.113.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Archives of General Psychiatry. 2005;62(3):273–81. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of the New York Academy of Sciences. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Read Montague P. Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology. 2009;34:2655–66. doi: 10.1038/npp.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR. What's in a smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122(1):40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Lorberbaum JP, Kose S, Strathearn L. Brain basis of early parent-infant interactions: psychology, physiology, and in vivo functional neuroimaging studies. Journal of Child Psychology and Psychiatry. 2007;48(3–4):262–87. doi: 10.1111/j.1469-7610.2007.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Ford J. Psychometric properties of the PTSD Checklist (PCL-C, PCL-S, PCL-M, PCL-PR) In: Stamm BH, editor. Measurement of Stress, Trauma, and Adaptation. Lutherville, MD: Sidran Foundation & Press; 1996. pp. 250–52. [Google Scholar]
- Wessa M, Flor H. Failure of extinction of fear responses in posttraumatic stress disorder: evidence from second-order conditioning. American Journal of Psychiatry. 2007;164(11):1684–92. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
- Zeanah CH, Larrieu JA, Heller SS, Valliere J. Infant-parent relationship assessment. In: Zeanah CH, editor. Handbook of Infant Mental Health. New York: Guilford Press; 2000. pp. 222–35. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.