Abstract
When making a decision, humans often have to ‘coordinate’—reach the same conclusion—as another individual without explicitly communicating. Relatively, little is known about the neural basis for coordination. Moreover, previous fMRI investigations have supported conflicting hypotheses. One account proposes that individuals coordinate using a ‘gut feeling’ and that this is supported by insula recruitment. Another account proposes that individuals recruit strategic decision-making mechanisms in prefrontal cortex in order to coordinate. We investigate the neural basis for coordination in individuals with behavioral-variant frontotemporal dementia (bvFTD) who have limitations in social decision-making associated with disease in prefrontal cortex. We demonstrate that bvFTD are impaired at establishing a focal point in a semantic task (e.g. ‘Tell me any boy's name’) that requires coordination relative to a similar, control semantic task that does not. Additionally, coordination limitations in bvFTD are related to cortical thinning in prefrontal cortex. These findings are consistent with behavioral economic models proposing that, beyond a ‘gut feeling’, strategic decision-making contributes to the coordination process, including a probabilistic mechanism that evaluates the salience of a response (e.g. is ‘John’ a frequent boy's name), a hierarchical mechanism that iteratively models an opponent's likely response and a mechanism involved in social perspective taking.
Keywords: coordination, frontotemporal dementia, MRI, decision making, game theory
INTRODUCTION
Social decisions often require two individuals to reach the same conclusion despite minimal communication. These types of decisions can be formalized within a game-theoretic framework as a ‘coordination game’. By definition, coordination games contain more than one optimal strategy to solve the game, but a player cannot choose a single strategy based on the mathematical structure of the game (Dixit et al., 2009; Clark, 2011). To solve certain coordination games, players must use a ‘focal point’, defined as a salient source of information known to both players, which transcends the mathematical structure of the game. For example, Mehta et al. (1994) asked participants to select a boy's name under two conditions: ‘picking’ (pick any name) and ‘coordination’ (pick the same name as a random partner). The name ‘John’ was only given in 9% of picking responses but was given in 50% of coordination responses. In this case, players did not provide the most salient name (‘John’) in the picking condition, but in the coordination condition players established a focal point using the shared belief that ‘John’ is a common name and thus more likely to be picked by the other player than their ‘picking’ response. Mehta et al.'s results empirically demonstrated that individuals establish focal points to solve a coordination game.
Relatively, little is known about the neural mechanisms that support coordination. Kuo et al. (2009) compared fMRI activation in a coordination game relative to a solvable optimal strategy game and observed bilateral insula and anterior cingulate activation. This was attributed to mechanisms that support an intuitive ‘gut feeling’ reaction to a focal point. By comparison, Yoshida et al. (2010) reported fMRI activation in a ‘stag hunt’ game that requires two players to establish a focal point by hunting either high payoff deer or low payoff rabbits. They observed that an increase in the number of levels that a player iteratively used to model a partner's response was correlated with an increased response of dorsolateral prefrontal (DLPFC), superior frontal and superior parietal cortex activation. Yoshida et al.'s results thus suggest that strategic mechanisms supported by prefrontal and parietal cortex contribute to coordination, that is, beyond the ‘gut feeling’ mechanism suggested by Kuo et al.(2009).
fMRI demonstrates brain activation that correlates with task performance, but this technique does not reveal the neuroanatomic substrates necessary to perform a task. It is therefore important to corroborate fMRI results with converging patient studies to determine the neural basis for task performance. In this article, we investigate the neuroanatomic basis for coordination in individuals with behavioral-variant frontotemporal dementia (bvFTD). bvFTD is a rare neurodegenerative disease associated with progressive frontal and anterior–inferior temporal atrophy that results in inappropriate social behavior and executive difficulty (Rascovsky et al. 2011). Individuals with bvFTD have limitations with theory of mind (Adenzato et al., 2010), adopting the perspective of another person (Grossman et al., 2010) and appreciating the hierarchical organization of daily activities (Farag et al., 2010). Additionally, individuals with bvFTD have disease in cortical regions that may contribute to coordination, including the insula, anterior cingulate, DLPFC and orbitofrontal cortex (Grossman, 2007). Given the difficulties with strategic thinking and perspective taking associated with bvFTD, we predict limitations establishing a focal point in a coordination game, and expect this to be related to prefrontal disease.
METHODS
Participants
Seventeen individuals with bvFTD [mean age = 65.3 years (s.d. = 6.4); mean education = 15.5 years (s.d. = 3.2); 5 females, 12 males; 14 right-handed; 3 left-handed] were diagnosed by a board certified neurologist (M.G.) using a consensus procedure and published criteria (Rascovsky et al., 2011). Other causes of dementia were excluded by clinical exam, blood and neuroimaging tests. We additionally tested 22 healthy seniors [mean age = 64.6 years (s.d. = 8.3); mean education = 15 years (s.d. = 2.4); 12 females, 10 males; all right-handed] as a control group. Healthy seniors and individuals with bvFTD were demographically matched for age [t(37) < 1; P > 0.1], education [t(37) < 1; P > 0.1] and gender (χ2 = 2.64; P > 0.1). Healthy seniors and individuals with bvFTD were not matched for handedness; however, an independent-samples t-test revealed that left- and right-handed individuals with bvFTD did not differ in behavioral performance across conditions (all P > 0.3). Dementia severity was evaluated using the Mini-Mental State Examination with a 30-point scale in individuals with bvFTD [mean MMSE = 25 (s.d. = 3)] and healthy seniors [mean MMSE = 29.1 (s.d. = 1.0)]. Individuals with bvFTD had lower MMSE scores than healthy seniors [t(37) = 4.74, P < 0.001]. However, bvFTD performance falls within the range considered to be ‘not-demented’ (i.e. >24). Informed consent was provided by all participants according to a protocol approved by the Institutional Review Board at the University of Pennsylvania.
Behavioral coordination task
Participants were presented with 10 questions (e.g. ‘Tell me the name of any _____’) probing a semantic category (e.g. ‘fabric’, ‘boy's name’, ‘supermarket item’). Refer to Supplementary Data for a complete list of categories. Each question was presented in a pseudorandomized order that was consistent across participants and across two experimental conditions to every participant. In the survey condition, participants were instructed that we were conducting a survey, which they could answer; however, they wished and they were told ‘This is not a memory test’. In the coordination condition, participants were given the following instructions: ‘We are conducting a survey and would like to know your responses to the following set of questions. We are also conducting the same survey with another person just like you. We want you to give the same answer you think the other person will give. This is not a memory test.’ In an effort to ensure that participants would consider the set of possible responses to each category probe, all participants were presented with the survey condition prior to the coordination condition.
Previous investigations of coordination have calculated a ‘coordination index’ value by computing the co-occurrence of responses given the distribution of responses from a large cohort of participants (Mehta et al., 1994; Bardsley et al., 2010). Since bvFTD is a rare disease and a large sample is not available, we generated an alternative quantitative measure of coordination that we refer to as a focal index (FI) value. The FI is based on the well-established finding that semantically related words co-occur more frequently than semantically unrelated words (Landauer and Dumais, 1997). We defined co-occurrence using the Normalized Google Distance (NGD; Cilibrasi and Vitányi, 2007) algorithm, in which semantic relatedness is quantified based on the co-occurrence of each response (e.g. ‘cotton’) and category (e.g. ‘fabric’) pair in a Google search:
In the formula, f(x) and f(y) represent the number of Google hits for each search term, f(x, y) represents the number of co-occurrence hits for both terms together, and M represents the number of pages queried. To obtain an accurate value for M, we limited searches to the New York Times (nytimes.com) for all Google queries. Since the scale of NGD values varied across semantic categories we transformed each NGD-value into a FI value by generating a z-score from the set of responses for each category. A response that is more central to a semantic category (e.g. ‘cotton’ to the probe ‘type of fabric’) yields a higher FI value (e.g. ‘cotton’ and ‘fabric’ = 1.17) and is a more focal response than a response that is less central to a semantic category (e.g. ‘corduroy’ and ‘fabric’ = −2.32) and therefore less focal. We report an analysis of variance (ANOVA) and post hoc t-tests along with Cohen's d statistic for estimating the effect size of all t-tests.
Neuropsychological assessment
A subset of individuals with bvFTD (n = 15) completed a neuropsychological assessment within 1.73 months (s.d. = 3.53) of participating in the coordination task. Our neuropsychological evaluation included semantic, executive and social cognition tasks.
Category fluency
A semantic task that involves naming as many animals as possible in 60 s and is sensitive to semantic deficits in FTD (Rascovsky et al., 2007) and temporal lobe atrophy (Baldo et al., 2006; Libon et al., 2009).
Visual verbal task
An executive task that probes executive task switching by requiring participants to generate two ways of grouping three items in a four item set.
Cartoon predictions
A 10-item task that requires the prediction of the outcome of social situations depicted in short cartoon vignettes that has previously been reported in FTD samples (Eslinger et al., 2007, 2011).
We relate performance on each of these tasks to the FI score in the coordination condition using Pearson correlations.
Volumetric MRI
Volumetric MRI images were available for a subset of individuals with bvFTD [n = 12; mean age = 63.1 years (s.d. = 5.0); mean education = 15.4 years (s.d. = 2.8); 8 males, 4 females; 9 right-handed, 3 left-handed] and a cohort of 50 healthy seniors [mean age = 65.1 years (s.d. = 8.2); mean education = 15.2 years (s.d. = 2.7); 28 males, 22 females; all right-handed] matched for age, education and gender (all P > 0.4). Since groups were not matched for handedness, we included a nuisance covariate for handedness in all imaging analyses. None of the healthy seniors in our MRI sample completed the behavioral coordination task. MRI images were not available for a subset of individuals with bvFTD (n = 5) due to health and safety exclusion criteria, including claustrophobia and metallic objects in the body (e.g. pacemakers and shrapnel).
High-resolution T1-weighted three-dimensional spoiled gradient echo images were acquired with repetition time = 1620 ms, echo time = 3 ms, slice thickness 1.0 mm, flip angle 15°, matrix = 192 × 256 and in-plane resolution 0.9 × 0.9 mm. All images were preprocessed using PipeDream (https://sourceforge.net/projects/neuropipedream/) and Advanced Normalization Tools (ANTS, http://www.picsl.upenn.edu/ANTS/) to perform the most stable and reliable multivariate normalization and structure-specific processing currently available (Avants et al., 2008, 2010; Klein et al., 2009). PipeDream deforms each individual data set into a standard local template space in a canonical stereotactic coordinate system. Core processing involves mapping T1 structural MRI to a population specific, unbiased average-shape and average-appearance image derived from a representative population consisting of 50 volumes (25 healthy seniors, 25 individuals with FTD) (Kim et al., 2008). A diffeomorphic deformation was used for registration that is symmetric so that it is not biased toward the reference space for computing the mappings, and topology preserving to capture the large deformation necessary to aggregate images in a common space. These algorithms allow template-based priors to guide cortical segmentation and compute cortical thickness (Das et al., 2009). We then used SPM5 to smooth cortical thickness images using a 4 mm FWHM Gaussian kernel and to compare bvFTD relative to healthy seniors using a two-sample t-test with a FDR-corrected height threshold (q < 0.01) and a 200 adjacent voxel extent. The multiple regression module in SPM5 was used to relate cortical thinning in bvFTD to the FI-difference score of each individual with bvFTD obtained from the behavioral task. In this hypothesis-driven regression analysis we only report clusters that overlap with regions of cortical thinning that contain a peak voxel that exceeds P < 0.001 (uncorrected) and contain a minimum of 200 adjacent voxels. We do not report regions where coordination limitations were related to nonatrophied cortical regions, because it would be difficult to interpret the role that these regions contribute to coordination limitations in individuals with bvFTD. For example, we would not be able to distinguish effects due to FTD from effects due to healthy aging.
RESULTS
Behavioral results
To quantify coordination, we calculated a FI value for each response to a semantic probe in a ‘survey’ condition requiring any response and a ‘coordination’ condition requiring the same response as another random player. The FI value provides a continuous and quantitative measurement of semantic category membership. A more focal response has a higher value such as the response ‘cotton’ to the probe ‘Tell me any type of fabric’, while a less focal response like ‘corduroy’ has a lower FI value.
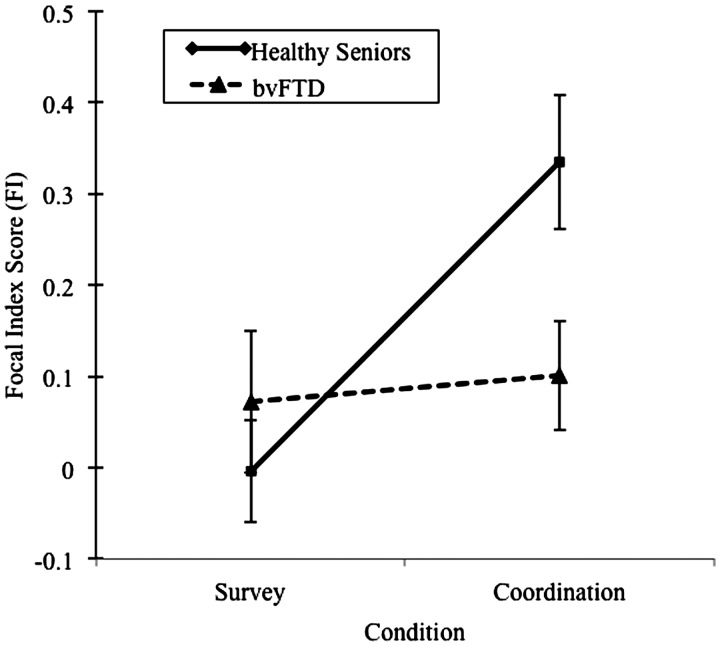
A two-way ANOVA with condition (survey, coordination) as a within-participants factor and group (healthy seniors, bvFTD) as a between-participants factor revealed a significant main effect for condition [F(1,37) = 6.49; P < 0.05] and a condition × group interaction [F(1,37) = 4.64; P < 0.05]. We did not observe a significant main effect for group [F(1,37) = 1.53; P > 0.1]. Individuals with bvFTD and healthy seniors provide equally focal responses in the survey condition [t(37) < 1; d = 0.25]. However, in the coordination condition, individuals with bvFTD provided significantly less focal responses relative to healthy seniors [t(37) = 2.36; P < 0.05; d = 0.77]. Within-group comparisons revealed that individuals with bvFTD did not provide responses that differed in focality across the survey and coordination conditions [t(16) < 1; d = 0.16], while healthy seniors' responses were significantly more focal in the coordination condition relative to the survey condition [t(21) = 3.19; P < 0.005; d = 1.09]. See Figure 1 for a summary of results.
Fig. 1.
Mean Focal Index (FI) scores for responses to survey and coordination conditions. Note: Error bars represent standard error mean (s.e.m.).
To determine whether the coordination limitations observed in individuals with bvFTD were due to a lack of changing responses between conditions (perseverative responses); we compared the percentage of responses in which each participant switched their response between the survey and coordination conditions. This analysis revealed that both healthy seniors [mean = 73.2% (s.d. = 23.3%)] and individuals with bvFTD [mean = 65% (s.d. = 25.5%)] switched their responses equally often between conditions [t(37) < 1; not signficant (ns); d = 0.34] demonstrating that, while individuals with bvFTD switched their response across conditions equally as often as healthy seniors, they did not switch to a more focal, or coordinating, response.
To determine whether coordination limitations in bvFTD are associated with semantic, executive or social limitations, we correlated performance on each neuropsychological task with the FI value in the coordination condition for each individual with bvFTD. We observed that coordination limitations were associated with executive limitations from the visual–verbal task [r2 = −0.57; P < 0.05; mean = 18.54 (s.d. = 1.33)], and not with semantic performance in category fluency [r2 = 0.28; P > 0.1; mean = 12 (s.d. = 6.78)] or social performance in the cartoons prediction task [r2 = 0.08; P > 0.1; mean = 7.93 (s.d. = 1.59)].
Volumetric MRI results
Since volumetric MRI images were only available for a subset of individuals with bvFTD (n = 12), we first evaluated whether this subset exhibited the same limitations with coordination observed in the larger sample of individuals with bvFTD. The subset of individuals with bvFTD, like the larger sample, exhibited limitations with coordination by providing equally focal responses in the survey condition [mean = 0.08 (s.d. = 0.27)] and the coordination condition [mean = 0.14 (s.d. = 0.21); t(11) < 1; d = 0.25].
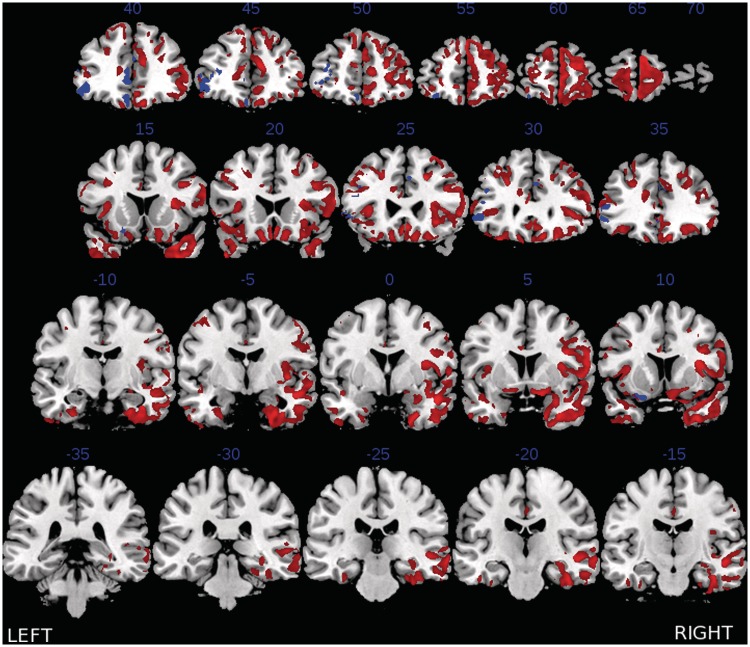
A comparison of gray matter thickness in bvFTD relative to healthy seniors revealed significant cortical thinning throughout frontal cortex (see Table 1 and colored regions in Figure 2). To determine how coordination limitations are related to cortical thinning, we calculated FI-difference scores (coordination minus survey) based on each individual's set of responses, where a higher value represents more focal responses in the coordination condition relative to the survey condition. These FI-differences scores were generated to allow us to relate coordination limitations in individuals with bvFTD to cortical thinning using a univariate regressor. A regression analysis using the FI-difference scores revealed that coordination limitations in individuals with bvFTD were associated with cortical thinning in to DLPFC, rostral prefrontal cortex (rPFC), ventromedial prefrontal cortex (vmPFC) and anterior cingulate (see Table 1 and blue regions in Figure 2).
Table 1.
Regions of cortical thinning in individuals with bvFTD relative to healthy seniors and overlapping regions that are related to impaired coordination in bvFTD
| Neuroanatomic region (BA) | L/R | Coordinates |
z-score | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Peaks of cortical thinning in individuals with bvFTD | |||||
| Anterior temporal (38) | R | 47 | 1 | −22 | 6.23 |
| Anterior temporal (38) | L | −46 | 20 | −39 | 5.43 |
| Anterior cingulate (32) | M | −6 | 47 | 13 | 5.42 |
| Dorsolateral prefrontal (9) | L | −48 | 21 | 33 | 4.88 |
| Anterior temporal (20) | L | −29 | −8 | −34 | 4.86 |
| Anterior cingulate (24) | M | −4 | 11 | 25 | 4.62 |
| Rostral prefrontal (10) | L | −47 | 48 | −11 | 4.48 |
| Subgenual cingulate (25), ventromedial prefrontal (11) | L | −14 | 7 | −12 | 4.26 |
| Dorsal inferior frontal (6) | L | −44 | −5 | 54 | 3.90 |
| Regression relating coordination limitations to cortical thickness | |||||
| Ventromedial prefrontal (11/25) | L | −17 | 13 | −19 | 3.72 |
| Ventromedial prefrontal (11) | L | −28 | 58 | −16 | 3.56 |
| Dorsolateral prefrontal (46) | L | −42 | 28 | 24 | 3.52 |
| Rostral prefrontal (47/10) | L | −47 | 37 | −5 | 3.43 |
| Ventromedial prefrontal (11) | M | −3 | 43 | −20 | 3.24 |
| Anterior cingulate (32) | M | 3 | 25 | 36 | 3.22 |
| Anterior cingulate (32) | M | −4 | 39 | 1 | 3.11 |
Fig. 2.
Significant cortical thinning in bvFTD relative to healthy seniors (all colored areas) and regions of cortical thinning that are related to impaired coordination (FI-difference score) in bvFTD (blue). Note: Error bars represent standard error mean (s.e.m). Blue numbers indicate y-axis coordinate.
DISCUSSION
The current study demonstrated that individuals with bvFTD, who have social and executive limitations associated with disease in frontal cortex, experience difficulty establishing a focal point needed to coordinate behavior. We found that healthy seniors regularly take into account frequency in the coordination condition, providing more focal responses than had been provided in the survey condition. By comparison, individuals with bvFTD provide less focal responses than healthy seniors in a coordination game, and these coordination failures are related to cortical thinning in DLPFC, rPFC, vmPFC and anterior cingulate.
Our observations of healthy seniors are more consistent with a strategic approach rather than an intuitive ‘gut feeling’ to establishing a focal point for coordination. A gut feeling may be driven by a salient property associated with coordination, but this alone does not capture the need to create a mental model of the population when trying to coordinate. Such a model balances salience with the frequency of occurrence in order to establish focality, as we observed.
Consider in this context the performance of individuals with bvFTD. Behavioral economic models propose two levels of establishing a focal point (Bacharach, 1999; Camerer et al., 2004). In the first level, each player must evaluate the possible set of responses (e.g. ‘John’, ‘Bill’, ‘Alfred’, …) and determine which possible response has ‘primary salience’. Salience in this sense can be defined as the most probabilistically likely response (e.g. ‘John’). Neuroimaging investigations have implicated DLPFC in a variety of domains requiring probabilistic evaluation, including probabilistic category learning (Fera et al., 2005), discriminating between advantageous and disadvantageous choices (Christakou et al., 2009), and evaluating a probabilistic distribution in a decision-making task (Casey et al., 2001; Huettel et al., 2005). Individuals with bvFTD have difficulty establishing a focal point, and our imaging findings related this in part to DLPFC disease. This suggests that the deficit in bvFTD is due in part to an impairment at the level of establishing primary salience, that is, difficulty estimating the probabilities needed to solve a coordination problem. Critically, the coordination limitations observed in individuals with bvFTD were not associated with semantic performance on a category fluency task, which suggests that their limitation cannot be attributed to difficulty considering the set of possible responses but is instead related to the evaluation of the salience of alternative responses. Additional work is needed to distinguish between establishing the salience of items within a set of possible responses (e.g. ‘John’ is more frequent than ‘Alfred’; Camerer et al., 2004) or choosing a selection rule (e.g. ‘Choose the most frequent boy's name’; Bacharach, 1999) to establish a focal point.
In the second level of establishing a focal point, players of a game build a representation of another player's response. This level, called ‘secondary salience’, involves player A guessing what player B will do. This can extend to include iterative hierarchical representations of each player's response. For example, player A guesses what they think player B thinks that player A will respond. Neuroimaging investigations of executive control have implicated a rostral–caudal axis of hierarchical control extending across the lateral surface of prefrontal cortex (Badre, 2008). fMRI studies associate more rostral activation patterns with increased levels of hierarchical abstraction required to make a decision (Badre and D'Esposito, 2007), and converging evidence from individuals with frontal lesions suggests that the more rostral the lesion, the fewer levels of hierarchical abstraction a patient can perform (Badre et al., 2009). We found that difficulty establishing a focal point in bvFTD is related to atrophy in rPFC. This suggests that their impairment is also due in part to a deficit building a hierarchical representation of another player's response, that is, an impairment in secondary salience.
We additionally observed that vmPFC was related to the coordination limitations observed in individuals with bvFTD. vmPFC is often implicated in fMRI tasks that require theory of mind (Van Overwalle, 2011) and individuals with bvFTD who have disease in vmPFC have difficulty taking the perspective of another individual (Eslinger et al., 2005, 2007, 2011). Our observation that a theory of mind mechanism is implicated in coordination, in conjunction with a hierarchical mechanism, suggests that individuals do not simply establish a hierarchical representation without taking the perspective of another individual. Instead, this finding is consistent with the hypothesis that successful coordination requires taking the perspective of another individual. We did not observe that coordination in individuals with bvFTD was related to their performance on a social cartoons prediction task, but this may be due to the fact that this task probes the evaluation of social situations in a way that does not require perspective taking.
Together, our observation relating the magnitude of dlPFC, rPFC and vmPFC cortical thinning in bvFTD to difficulty establishing a focal point is consistent with a strategic account of coordination. Our results thus resemble Yoshida et al.'s (2010) fMRI investigation which demonstrated that the magnitude of dlPFC activation in young adults was related to increased levels of strategic thinking during a coordination task. While individuals with bvFTD patients have atrophy in the insula, as illustrated in Figure 2, we did not find that coordination difficulty is related to atrophy in the insula. Our findings thus are less consistent with the claim that individuals are simply relying on a ‘gut-feeling’, but instead appear to recruit decision-making mechanisms in order to strategically establish a focal point.
The final region related to limitations establishing a focal point in individuals with bvFTD was the anterior cingulate. The anterior cingulated has been implicated ina mechanism that contributes to the resolution of conflicting responses (Botvinick et al., 2001). This region may therefore play a role in the resolution of competing alternative responses (e.g. Should I respond ‘cotton’, ‘wool’, or ‘corduroy’). The anterior cingulate has additionally been implicated as part of a ‘mentalizing’ or theory-of-mind network (Gallagher and Frith, 2003; Van Overwalle, 2011).
Our observation that individuals with bvFTD have coordination limitations provides the first study, to our knowledge, with patient-based evidence for the neural mechanisms involved in coordination. With this foundation, future research that addresses additional features of coordination and economic behavior in this population may prove fruitful for not only the advancement of neuroeconomic theory but also for improving our understanding of the social and executive disorder resulting from bvFTD. For example, an improved understanding of the role of mutual information—that is, the extent to which coordination may differ between individuals with shared knowledge compared to individuals who are strangers—would shed light on anecdotal differences between patient–stranger and patient–caregiver interaction in bvFTD. Moreover, studies that investigate the extension of coordination limitations observed in the laboratory to facets of daily life—such as successful communication and financial decision making—may contribute to improved care of individuals with bvFTD.
In conclusion, the process of establishing a focal point appears to be supported in part by a strategic decision-making network in prefrontal cortex. The results of our investigation of individuals with bvFTD are less consistent with a ‘gut feeling’ approach than with behavioral economic models that propose a strategic approach to coordination. Future research that investigates the relative roles of mechanisms involved in probability estimation, hierarchical processing, social perspective taking and cognitive control mechanisms may elucidate our understanding of the neural basis of coordination.
SUPPLEMENTARY DATA
Supplementary Data are available at SCAN online.
Conflict of Interest
None declared.
Acknowledgments
The National Institutes of Health (HD060406, NS44266, AG17586, AG15116, AG32953 and NS53488).
REFERENCES
- Adenzato M, Cavallo M, Enrici I. Theory of mind ability in the behavioural variant of frontotemporal dementia: an analysis of the neural, cognitive, and social levels. Neuropsychologia. 2010;48(1):2–12. doi: 10.1016/j.neuropsychologia.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Medical Image Analysis. 2008;12(1):26–41. doi: 10.1016/j.media.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, Gee JC. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2010;54(3):2033–44. doi: 10.1016/j.neuroimage.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacharach M. Interactive team reasoning: a contribution to the theory of cooperation. Research in Economics. 1999;53(2):117–47. [Google Scholar]
- Badre D. Cognitive control, hierarchy, and the rostro-caudal organization of the frontal lobes. Trends in Cognitive Sciences. 2008;12(5):193–200. doi: 10.1016/j.tics.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Badre D, D'Esposito M. Functional magnetic resonance imaging evidence for a hierarchical organization of the prefrontal cortex. Journal of Cognitive Neuroscience. 2007;19(12):2082–99. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- Badre D, Hoffman J, Cooney JW, D'Esposito M. Hierarchical cognitive control deficits following damage to the human frontal lobe. Nature Neuroscience. 2009;12(4):515–22. doi: 10.1038/nn.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldo JV, Schwartz S, Wilkins D, Dronkers NF. Role of frontal versus temporal cortex in verbal fluency as revealed by voxel-based lesion symptom mapping. Journal of the International Neuropsychological Society. 2006;12(6):896–900. doi: 10.1017/S1355617706061078. [DOI] [PubMed] [Google Scholar]
- Bardsley N, Mehta J, Starmer C, Sugden R. Explaining focal points: cognitive hierarchy theory versus team reasoning. Economic Journal. 2010;120(543):40–79. [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Camerer C, Ho TH, Chong JK. A cognitive hierarchy model of games. Quarterly Journal of Economics. 2004;119(3):861–98. [Google Scholar]
- Casey BJ, Forman SD, Franzen P, Berkowitz A, Braver TS, Nystrom LE, et al. Sensitivity of prefrontal cortex to changes in target probability: a functional MRI study. Human Brain Mapping. 2001;13(1):26–33. doi: 10.1002/hbm.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakou A, Brammer M, Giampietro V, Rubia K. Right ventromedial and dorsolateral prefrontal cortices mediate adaptive decisions under ambiguity by integrating choice utility and outcome evaluation. Journal of Neuroscience. 2009;29(35):11020–8. doi: 10.1523/JNEUROSCI.1279-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cilibrasi RL, Vitányi PMB. The Google similarity distance. IEEE Transactions on Knowledge and Data Engineering. 2007;19(3):370–83. [Google Scholar]
- Clark R. Meaningful Games: Exploring Language with Game Theory. Cambridge, MA: MIT Press; 2011. [Google Scholar]
- Das SR, Avants BB, Grossman M, Gee JC. Registration based cortical thickness measurement. Neuroimage. 2009;45(3):867–79. doi: 10.1016/j.neuroimage.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit AK, Skeath S, Reiley D. Games of Strategy. 3rd edn. New York: W.W. Norton & Co; 2009. [Google Scholar]
- Eslinger PJ, Dennis K, Moore P, Antani S, Hauck R, Grossman M. Metacognitive deficits in frontotemporal dementia. Journal of Neurology Neurosurg Psychiatry. 2005;76(12):1630–5. doi: 10.1136/jnnp.2004.053157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslinger PJ, Moore P, Anderson C, Grossman M. Social cognition, executive functioning, and neuroimaging correlates of empathic deficits in frontotemporal dementia. The Journal of Neuropsychiatry and Clinical Neurosciences. 2011;23(1):74–82. doi: 10.1176/appi.neuropsych.23.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslinger PJ, Moore P, Troiani V, Antani S, Cross K, Kwok S, et al. Oops! Resolving social dilemmas in frontotemporal dementia. Journal of Neurology Neurosurg Psychiatry. 2007;78(5):457–60. doi: 10.1136/jnnp.2006.098228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farag C, Troiani V, Bonner M, Powers C, Avants B, Gee J, et al. Hierarchical organization of scripts: converging evidence from FMRI and frontotemporal degeneration. Cerebral Cortex. 2010;20(10):2453–63. doi: 10.1093/cercor/bhp313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fera F, Weickert TW, Goldberg TE, Tessitore A, Hariri A, Das S, et al. Neural mechanisms underlying probabilistic category learning in normal aging. Journal of Neuroscience. 2005;25(49):11340–8. doi: 10.1523/JNEUROSCI.2736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends in Cognitive Sciences. 2003;7(2):77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Grossman M. Imaging frontotemporal dementias. In: Miller BL, Cummings JL, editors. The human frontal lobes: Functions and Disorders. 2nd edn. New York: The Guilford Press; 2007. [Google Scholar]
- Grossman M, Eslinger PJ, Troiani V, Anderson C, Avants B, Gee JC, et al. The role of ventral medial prefrontal cortex in social decisions: converging evidence from fMRI and frontotemporal lobar degeneration. Neuropsychologia. 2010;48(12):3505–12. doi: 10.1016/j.neuropsychologia.2010.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G. Decisions under uncertainty: probabilistic context influences activation of prefrontal and parietal cortices. Journal of Neuroscience. 2005;25(13):3304–11. doi: 10.1523/JNEUROSCI.5070-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Avants B, Patel S, Whyte J, Coslett BH, Pluta J, et al. Structural consequences of diffuse traumatic brain injury: a large deformation tensor-based morphometry study. Neuroimage. 2008;39(3):1014–26. doi: 10.1016/j.neuroimage.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein A, Andersson J, Ardekani BA, Ashburner J, Avants B, Chiang MC, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46(3):786–802. doi: 10.1016/j.neuroimage.2008.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo WJ, Sjostrom T, Chen YP, Wang YH, Huang CY. Intuition and deliberation: two systems for strategizing in the brain. Science. 2009;324(5926):519–22. doi: 10.1126/science.1165598. [DOI] [PubMed] [Google Scholar]
- Landauer TK, Dumais ST. A solution to Plato's problem. The Latent Semantic Analysis theory of the acquisition, induction, and representation of knowledge. Psychological Review. 1997;104:211–40. [Google Scholar]
- Libon D, McMillan C, Gunawardena D, Powers C, Massimo L, Khan A, et al. Neurocognitive contributions to verbal fluency defecits in frontotemporal lobar degeneration. Neurology. 2009;73(7):535–42. doi: 10.1212/WNL.0b013e3181b2a4f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta J, Starmer C, Sugden R. The nature of salience - an experimental investigation of pure coordination games. American Economic Review. 1994;84(3):658–73. [Google Scholar]
- Rascovsky K, Hodges JR, Kipps CM, Johnson JK, Seeley WW, Mendez MF, et al. Diagnostic criteria for the behavioral variant of frontotemporal dementia (bvFTD): current limitations and future directions. Alzheimer Disease and Associated Disorders. 2007;21(4):S14–18. doi: 10.1097/WAD.0b013e31815c3445. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Neuhaus J, et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain. 2011;134(9):2456–77. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascovsky K, Salmon DP, Hansen LA, Thal LJ, Galasko D. Disparate letter and semantic category fluency deficits in autopsy-confirmed frontotemporal dementia and Alzheimer's disease. Neuropsychology. 2007;21(1):20–30. doi: 10.1037/0894-4105.21.1.20. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F. A dissociation between social mentalizing and general reasoning. Neuroimage. 2011;54(2):1589–99. doi: 10.1016/j.neuroimage.2010.09.043. [DOI] [PubMed] [Google Scholar]
- Yoshida W, Seymour B, Friston KJ, Dolan RJ. Neural mechanisms of belief inference during cooperative games. Journal of Neuroscience. 2010;30(32):10744–51. doi: 10.1523/JNEUROSCI.5895-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]