Abstract
Studies investigating theory of mind (ToM) abilities (i.e. ability to understand and predict others’ mental states) have revealed that affective and cognitive functions play a significant role and that each of those functions are associated with distinct neural networks. Cognitive facets of ToM have implicated the medial prefrontal cortex, temporo-parietal junction and the anterior paracingulate cortex, whereas affective facets have implicated the ventromedial prefrontal cortex (vmPFC). Although the vmPFC has repeatedly shown to be critical for affective functions, knowledge regarding the exact role of the left and right vmPFC in affective ToM is still obscure. Here, we compared performances of 30 patients with left, right and bilateral vmPFC lesions to two comparison groups (one without and one with brain injuries) on the Faux Pas Recognition task measuring the facets of ToM. We also investigated whether any deficits may be associated with other emotional measures, namely emotional empathy and emotional intelligence. Our results extend earlier findings by showing that the vmPFC is associated with abilities in affective ToM. More importantly, our results revealed that the left, and not the right vmPFC as indicated previously, is involved in affective ToM and that this deficit is associated with emotional intelligence.
Keywords: affective theory of mind, ventromedial prefrontal cortex, traumatic brain injury, emotional intelligence, empathy
INTRODUCTION
Throughout development humans acquire complex social cognitive skills that enable us to function effectively in daily human interactions. These social cognitive skills are essential for our understanding of how others’ social behaviors are motivated and how one should respond in social contexts based on those behaviors. These actions depend on individuals’ internal cognitive and affective mental states such as beliefs, desires and emotions (Wellman, 1990), and a deficit in the ability to introspect those mental states can result in inappropriate interactions. The ability to understand and predict the social behavior of other people through the process of making inferences regarding their mental states (e.g. beliefs, desires and emotions) has been termed Theory of Mind (ToM) (Premack and Woodruff, 1978; Wellman, 1990).
The inferences one makes regarding others’ mental states include not only knowledge about their thoughts and beliefs (cognitive ToM) but also knowledge regarding their emotional states (affective ToM). Cognitive ToM measures include tasks examining the ability of an individual to understand that another person can hold a belief that is mistaken (first-order false belief) or a belief about someone else's belief (second-order false belief) (Baron-Cohen et al., 1985; Perner et al., 1987; Wellman et al., 2001; Miller, 2009) whereas affective ToM measures examine, in addition, the ability of an individual to understand the emotions and feelings of others by sharing their affective mental states (Stone et al., 1998; Singer, 2006). For example, the Faux Pas task (referring to incidents where someone said something they should not have said, not knowing or not realizing that they should not have said it) examines not only a cognitive understanding between the speaker's and listener's knowledge but also an appreciation of the listener's affective state (Stone et al. 1998).
Research has consistently shown that ToM abilities are associated with the prefrontal cortex (PFC); as well as posterior brain regions such as the posterior cingulate cortex/precuneus, temporo-parietal junction, temporal poles and amygdala have been shown to also play a role in ToM (Goel et al., 1995; Baron-Cohen et al. 1999; Fine et al., 2001; Stone et al., 2003; Shaw et al., 2007). In addition, recent research has revealed that cognitive and affective ToM tasks engage dissociable underlying neural mechanisms (Shamay-Tsoory, 2007a). For example, Rowe et al. (2001) reported that individuals with either right or left PFC lesions were impaired in cognitive ToM ability (as assessed by first- and second-order false belief tests). Moreover, Stone et al., (1998) reported good performance on first- and second-order ToM tasks but impaired performance on an affective ToM task (i.e. Faux Pas task) in individuals only with bilateral orbitofrontal cortex damage. Shamay-Tsoory et al. (2003, 2005, 2006) demonstrated that vmPFC lesion patients have only difficulties in representing another person's affective mental state but not in representing cognitive mental states. Using repetitive transcranial magnetic stimulation (rTMS), Kalbe et al. (2010) demonstrated that the right dorsolateral prefrontal cortex is involved in cognitive ToM but not affective ToM performance. Finally, clinical observations and experimental studies indicate that vmPFC patient show altered emotional and social behavior by developing severe impairments in personal and social decision making, despite intact intellectual abilities (Damasio et al., 1991; Dimitrov et al., 1999; Blair and Cipolotti, 2000).
Although previous studies have repeatedly shown that the vmPFC plays a critical role in affective ToM (Shamay-Tsoory et al., 2003, 2009), knowledge regarding the exact role of the left and right vmPFC in affective ToM is still obscure. Only a recent brain lesion study revealed that impaired affective ToM is associated with right vmPFC damage (Shamay-Tsoory et al., 2005). However, the study's sample size was small (including only a total of eight vmPFC patients) and the authors did not compare patients with left and right vmPFC directly but based their reasoning only on superimposing the lesions of all ToM impaired individuals.
The goal of this study was to use a larger and homogenous sample size to directly compare performances of patients with left, right and bilateral vmPFC lesions on two ToM tasks varying in level of affective processing: (i) a cognitive (second-order false belief) ToM task without involvement of emotional representation and (ii) an affective (detecting social faux pas) ToM task requiring both cognitive and emotional representations. We investigated further whether affective ToM deficits in vmPFC lesion patients were associated with other emotional measures such as emotional empathy and emotional intelligence (EI). Our results indicate that only the left and bilateral vmPFC lesion groups were significantly impaired in affective ToM compared with the right vmPFC group. Moreover, affective ToM performances were associated with the empathy measures in the bilateral vmPFC lesion group but emotional intelligence only in the left vmPFC lesion group.
MATERIALS AND METHODS
Subjects
Participants were drawn from the W. F. Vietnam Head Injury Study (VHIS) registry, a prospective, long-term follow-up study of male veterans with focal penetrating Traumatic Brain Injuries (pTBIs) (Raymont et al., 2011). Based on existing evidence demonstrating an association between affective ToM deficits and vmPFC damage (Shamay-Tsoory et al., 2003, 2007a, 2007b), we selected 161 male combat veterans from the VHIS population and divided them into a non-brain-injured normal comparison group (NC group, n = 55), a brain-injured comparison group with damage in the posterior cortex (PC group, n = 76) and a vmPFC lesion group (vmPFC group, n = 30). The vmPFC group included three subgroups of veterans with left vmPFC damage (L vmPFC group, n = 8), right vmPFC damage (R vmPFC group, n = 7) and bilateral vmPFC damage (B vmPFC group, n = 15). The comparison and experimental groups were matched with respect to age, handedness, level of depression (BDI-II, Beck et al., 1996), verbal naming ability (Boston Naming test, BNT, Kaplanet al., 1976), working memory (WMS-III, Wechsler, 1997) and pre-injury general intelligence (Table 1). Pre-injury general intelligence was assessed with the Armed Forces Qualification Test (AFQT-7A), administered to individuals upon entry into military (Department of Defense, 1960). This test has been extensively standardized within the U.S. military and correlates highly with WAIS IQ scores (Grafman, et al., 1988). Note that although groups differed overall in education, post hoc tests revealed no significant differences between the individual groups. Although there were differences in post-injury general intelligence WAIS-III; Full-Scale, Wechsler, 1997), intelligence scores in all groups were within normal limits, and post hoc analyses revealed that the difference was driven by the B vmPFC group performing significantly worse than the NC group (P < 0.05) and the PC group (P < 0.05). In addition, groups did not differ in their ability to identify emotions through facial expression as indicated the Morphed Faces test. All participants understood the study procedures and gave their written informed consent, which was approved by the Institutional Review Board at the National Naval Medical Center and the National Institute of Neurological Disorders and Stroke.
Table 1.
Demographical and neuropsychological variables (mean ± s.d.) for veterans with no-head injury (normal control, NC group), veterans with PC damage (brain damage control group) and veterans with left ventromedial prefrontal cortex (vmPFC) damage (L vmPFC group), right vmPFC damage (R vmPFC) damage and bilateral vmPFC damage (B vmPFC) damage
| Variables\groups | NC (n = 55) | L vmPFC (n = 8) | R vmPFC (n = 7) | B vmPFC (n = 15) | PC (n = 76) | Statistics |
|---|---|---|---|---|---|---|
| Age (years) | 59.1 ± 3.4 | 57.6 ± 1.3 | 57.0 ± 1.6 | 58.4 ± 4.6 | 58.7 ± 2.9 | F = 0.9, P = 0.492 |
| Education (years) | 15.2 ± 2.5 | 14.6 ± 1.6 | 13.2 ± 1.1 | 13.6 ± 3.2 | 15.3 ± 2.4 | F = 2.4, P < 0.05 |
| Handedness (L:R:A)a | 43:8:4 | 7:1:0 | 7:0:0 | 12:3:0 | 60:13:3 | χ2 = 4.3, P = 0.826 |
| Pre-injury IQ (AFQT)b | 65.4 ± 22.9 | 65.8 ± 17.5 | 46.7 ± 20.7 | 49.5 ± 28.3 | 65.0 ± 25.9 | F = 2.1, P = 0.090 |
| Post-injury IQ (WAIS III)c | 110.3 ± 12.3 | 101.6 ± 12.9 | 102.5 ± 6.9 | 93.8 ± 11.5 | 106.4 ± 13.7 | F = 4.8, P < 0.05 |
| Depression (BDI)d | 11.6 ± 9.6 | 8.1 ± 8.9 | 12.0 ± 9.6 | 6.5 ± 3.9 | 8.3 ± 8.1 | F = 1.8, P = 0.135 |
| Working memory (WMS III)e | 106.6 ± 13.4 | 97.6 ± 16.3 | 98.3 ± 13.0 | 97.2 ± 15.2 | 101.6 ± 12.8 | F = 2.2, P = 0.090 |
| Verbal naming ability (BNT)f | 55.4 ± 4.6 | 52.3 ± 7.3 | 55.4 ± 2.1 | 52.4 ± 5.0 | 55.5 ± 5.1 | F = 0.9, P = 0.423 |
| Morphed facesg | 37.3 ± 7.3 | 40.7 ± 13.6 | 38.9 ± 6.6 | 36.7 ± 7.2 | 38.1 ± 7.1 | F = 0.4, P = 0.778 |
aHandedness (L:R:A), left, right and ambiguous.
bPre-injury IQ (AFQT), percentile score of Armed Forces Qualification Test.
cPost-injury IQ (WAIS III), full-scale score of Wechsler Adult Intelligence Scale (3rd edition).
dDepression (BDI), total score of beck depression inventory.
eWorking memory (WMS III), primary index score of Wechsler Memory Scale (3rd edition).
fVerbal naming ability (BNT), total score of Boston Naming Test; Morphed Faces test, total percentage of correct answers.
gMorphed Faces test, total percentage of correct answers.
Materials
We employed the Faux Pas Recognition task (Stone et al. 1998) measuring affective ToM as an experimental task, the Happé Story Task (Happé, 1994) measuring cognitive ToM, the Balanced Emotional Empathy Scale (BEES) (Mehrabian, 1996) measuring emotional empathy and Mayer–Salovey–Caruso Emotional Intelligence Test (MSCEIT) (Mayer et al., 2002) measuring emotional intellectual abilities as control tasks.
Experimental task
The Faux Pas Recognition task is composed of 20 short (one paragraph) stories: 10 stories with a Faux Pas (the speaker unintentionally said something hurtful or insulting to the listener) and 10 stories without a Faux Pas. For each trial, the experimenter read the story to the participants, while they were able to read along on their own copy. To reduce memory demands, the stories remained in front of the participant during the duration of the task. After each story, participants were asked a series of questions. First, participants received a Faux Pas detection question: did anyone say something they should not have said or something awkward? If participants answered yes (and the story was a Faux Pas story) participants were asked five additional Faux Pas follow up questions. Finally, to control for verbal comprehension, participants were also always asked two control questions regardless of whether they answered the first question correctly.
Only the 10 Faux Pas stories were evaluated. Participants could receive a total of 60 points. If participants answered ‘no’ to any Faux Pas detection questions, they received 0 points for that particular story, but could still receive one point for each correctly answered control question. For each participant, two primary scores were calculated for the Faux Pas related stories: (i) Faux Pas story score [percentage correct (0–100%), sum of the scores for the six Faux Pas questions for each of the 10 Faux Pas stories divided by 60 and multiplied by 100] and (ii) Control question score [percentage correct (0–100%), sum of the scores for the two control questions for each of the 10 Faux Pas storied divided by 20 and multiplied by 100].
Control tasks
Participants completed the Happé Stories Task (Happé, 1994) to determine whether cognitive ToM impacts deficits in affective ToM. Participants were presented with 16 stories (eight ToM stories and eight physical response stories serving as controls), selected from 24 developed by Happé (1994). Stories were selected if they contained little emotional representation. After each story was presented, participants were asked to turn the story page over and to answer the question on the next page. ToM questions involved understanding the intentions of the characters in the stories, whereas physical story questions involved understanding physical inferences that can be made about the story. Participants received two points for each full and explicitly correct answer, one point for a partial and implicitly correct answer, and zero points for an incorrect answer or no response. Two primary scores were calculated for each participant: (i) ToM story score [sum of the scores (0–16) for the ToM story questions] and (ii) physical story score [sum of the scores (0–16) for the eight physical story questions]. The following represents an example of one of our selected Happé ToM stories (story number 21): Simon is a big liar. Simon's brother Jim knows this; he knows that Simon never tells the truth! Now, yesterday, Simon stole Jim's ping-pong bat, and Jim knows Simon has hidden it somewhere, though he can't find it. He is very cross. So he finds Simon and he says, “Where is my ping-pong bat? You must have hidden it either in the cupboard or under your bed, because I've looked everywhere else. Where is it, in the cupboard or under your bed?” Simon tells him the bat is under his bed. Q: Why will Jim look in the cupboard for the bat?
Moreover, participants completed the BEES (Mehrabian, 1996) to investigate whether emotional empathy is associated with deficits in affective ToM as indicated by previous research (Shamay-Tsoory et al., 2005). Participants rated for each of the 30 statements (e.g. I cannot feel much sorrow for those who are responsible for their own misery), the degree of their agreement/disagreement on a 9-point Likert scale (−4 = very strong disagreement; 4 = very strong agreement). A total z-score was calculated for each participant based on the ratings for positively and negatively worded.
Finally, participants completed the MSCEIT (Mayer et al., 2002) to investigate whether emotional intelligence is associated with deficits in affective ToM. The MSCEIT consists of a 141-item scale that focuses on emotion-related competencies that can be assessed through performance-based standardized norms. Responses on the MSCEIT were scored with respect to their degree of correctness, as determined by their correspondence with the answers provided by a normative sample of the general population. Besides the Full Scale EI, the MSCEIT yields two area scores each combining two branch scores (average normalized scores between 85 and 115): (i) experiential EI, the competency of perceiving emotions (e.g. it includes the ability to accurately read facial expressions) and using emotions (e.g. anticipating another person's emotional reaction and using that knowledge to modify one's own behavior) and (ii) strategic EI, the competency of understanding emotions (e.g. understanding the relationship between sadness and loss) and managing emotions (e.g. conscious regulation of emotions both in oneself and in others).
Computed tomography acquisition and analysis
The axial computed tomography (CT) scans were acquired without contrast in helical mode on a GE Electric Medical Systems Light Speed Plus CT scanner at the Bethesda Naval Hospital. Structural neuroimaging data were reconstructed with an in-plane voxel size of 0.4 × 0.4 mm, an overlapping slice thickness of 2.5 mm and a 1-mm slice interval. Lesion location and volume from CT images were determined using the interactive Analysis of Brain Lesions (ABLe) software implemented in MED × v3.44 (Medical Numerics) (Makale, et al., 2002; Solomon, et al., 2007). Lesion volume was calculated by manually tracing the lesion in all relevant slices of the CT image in native space, and then summing the trace areas and multiplying by slice thickness. Manual tracing was performed by a trained psychiatrist (V.R.) with clinical experience of reading CT scans. The lesion tracing was then reviewed by an observer that was blind to the results of the clinical evaluation and neuropsychological testing (J.G.) enabling a consensus decision to be reached regarding the limits of each lesion. The CT image of each individual's brain was normalized to a CT template brain image in Montreal Neurological Institute (MNI) space. The spatial normalization was performed with the AIR algorithm (Woods et al., 1993), using a 12-parameter affine fit. Note that both the patient's brain and the MNI template's brain are first skull-stripped in order to maximize the efficacy of the AIR registration from native space to MNI space. In addition, voxels inside the traced lesion were not included in the spatial normalization procedure. Afterwards, the percentage of automated anatomical labeling (AAL) structures that were intersected by the lesion was determined by analyzing the overlap of the spatially normalized lesion image with the AAL atlas (Tzourio-Mazoyer et al., 2002).
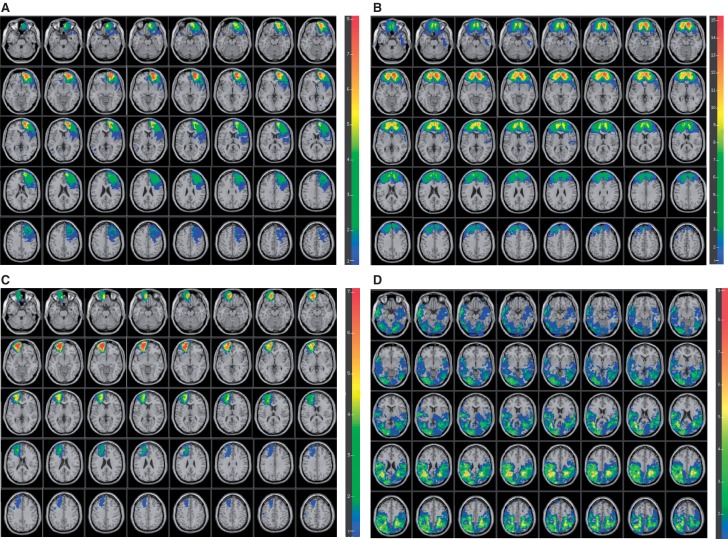
The vmPFC was defined as region of interest (ROI) based on specifying the lower and upper x, y and z coordinates in MNI space (R: 0 ≤ x ≤ 20, L: −20≤− x ≤0, z ≤ 1). No differences in lesion volume loss were observed between the L (28.0 ± 12.9) (Fig. 1A) and R (35.5 ± 11.1) (Fig. 1B) vmPFC groups (z = −1.16, P = 0.281) and between the L (39.7 ± 11.7) and R (37.6 ± 19.1) hemispheres of the B vmPFC group (z = −0.15, P = 0.901) (Fig. 1C) and lesion size was not correlated with performance on any of the ToM measures (P's > 0.153). The PC group was defined as having brain lesions outside the specified vmPFC and other PFC regions (Fig. 1D). Table 2 displays the percentage of AAL structures that were intersected by lesion for the PC group.
Fig. 1.
Overlay map of patient populations: (A) patients with left vmPFC lesions, (B) patients with right vmPFC lesions, (C) patients with bilateral vmPFC lesions and (D) patients with posterior lesions. Color indicates the number of overlapping lesions at each voxel. Red indicates more subjects and blue fewer. We restricted all analyses to a minimum overlap of two patients in a given voxels. In each slice, the right hemisphere is on the reader's left. For Figure 1A–C, z = −28 to 50 with 2-mm intervals. For Figure 1D, z = −24 to 54 with 2-mm intervals.
Table 2.
Percentage of AAL structures that were intersected by lesion for the PC group (brain damage control group)
| Fraction | Left labels | Fraction | Right labels |
|---|---|---|---|
| 66.53 | Precentral_L | 73.91 | Precentral_R |
| 9.67 | Frontal_Sup_L | 11.32 | Frontal_Sup_R |
| 2.08 | Frontal_Sup_Orb_L | 0.00 | Frontal_Sup_Orb_R |
| 6.19 | Frontal_Mid_L | 7.66 | Frontal_Mid_R |
| 3.27 | Frontal_Mid_Orb_L | 0.00 | Frontal_Mid_Orb_R |
| 13.49 | Frontal_Inf_Oper_L | 23.37 | Frontal_Inf_Oper_R |
| 3.12 | Frontal_Inf_Tri_L | 10.55 | Frontal_Inf_Tri_R |
| 1.72 | Frontal_Inf_Orb_L | 12.19 | Frontal_Inf_Orb_R |
| 31.41 | Rolandic_Oper_L | 93.54 | Rolandic_Oper_R |
| 10.85 | Supp_Motor_Area_L | 39.01 | Supp_Motor_Area_R |
| 0.00 | Olfactory_L | 0.00 | Olfactory_R |
| 0.00 | Frontal_Sup_Medial_L | 1.64 | Frontal_Sup_Medial_R |
| 0.00 | Frontal_Mid_Orb_L | 0.00 | Frontal_Mid_Orb_R |
| 0.00 | Rectus_L | 0.00 | Rectus_R |
| 10.66 | Insula_L | 28.31 | Insula_R |
| 0.00 | Cingulum_Ant_L | 0.00 | Cingulum_Ant_R |
| 10.97 | Cingulum_Mid_L | 8.40 | Cingulum_Mid_R |
| 13.61 | Cingulum_Post_L | 7.76 | Cingulum_Post_R |
| 42.70 | Hippocampus_L | 6.66 | Hippocampus_R |
| 45.60 | ParaHippocampal_L | 0.97 | ParaHippocampal_R |
| 10.45 | Amygdala_L | 0.00 | Amygdala_R |
| 77.55 | Calcarine_L | 58.14 | Calcarine_R |
| 91.81 | Cuneus_L | 81.25 | Cuneus_R |
| 45.97 | Lingual_L | 52.35 | Lingual_R |
| 73.13 | Occipital_Sup_L | 97.17 | Occipital_Sup_R |
| 68.38 | Occipital_Mid_L | 98.71 | Occipital_Mid_R |
| 74.28 | Occipital_Inf_L | 91.30 | Occipital_Inf_R |
| 46.97 | Fusiform_L | 16.84 | Fusiform_R |
| 85.69 | Postcentral_L | 84.41 | Postcentral_R |
| 90.99 | Parietal_Sup_L | 99.05 | Parietal_Sup_R |
| 96.77 | Parietal_Inf_L | 90.56 | Parietal_Inf_R |
| 96.50 | SupraMarginal_L | 98.63 | SupraMarginal_R |
| 93.61 | Angular_L | 100.00 | Angular_R |
| 62.41 | Precuneus_L | 67.38 | Precuneus_R |
| 80.36 | Paracentral_Lobule_L | 95.22 | Paracentral_Lobule_R |
| 0.00 | Caudate_L | 0.80 | Caudate_R |
| 5.15 | Putamen_L | 4.32 | Putamen_R |
| 0.00 | Pallidum_L | 0.00 | Pallidum_R |
| 36.64 | Thalamus_L | 9.18 | Thalamus_R |
| 52.44 | Heschl_L | 56.63 | Heschl_R |
| 73.82 | Temporal_Sup_L | 80.26 | Temporal_Sup_R |
| 69.03 | Temporal_Pole_Sup_L | 79.45 | Temporal_Pole_Sup_R |
| 75.78 | Temporal_Mid_L | 88.25 | Temporal_Mid_R |
| 41.99 | Temporal_Pole_Mid_L | 84.08 | Temporal_Pole_Mid_R |
| 53.12 | Temporal_Inf_L | 61.82 | Temporal_Inf_R |
| 87.71 | Cerebelum_Crus1_L | 88.63 | Cerebelum_Crus1_R |
| 88.49 | Cerebelum_Crus2_L | 88.00 | Cerebelum_Crus2_R |
| 1.47 | Cerebelum_3_L | 0.00 | Cerebelum_3_R |
| 40.80 | Cerebelum_4_5_L | 0.00 | Cerebelum_4_5_R |
| 84.53 | Cerebelum_6_L | 39.22 | Cerebelum_6_R |
| 86.84 | Cerebelum_7b_L | 80.34 | Cerebelum_7b_R |
| 76.42 | Cerebelum_8_L | 65.73 | Cerebelum_8_R |
| 7.13 | Cerebelum_9_L | 0.49 | Cerebelum_9_R |
| 75.69 | Cerebelum_10_L | 0.00 | Cerebelum_10_R |
| 0.00 | Vermis_1_2 | 0.00 | Vermis_3 |
| 0.60 | Vermis_4_5 | 22.64 | Vermis_6 |
| 43.30 | Vermis_7 | 11.93 | Vermis_8 |
| 0.57 | Vermis_9 | 0.00 | Vermis_10 |
Note: PC regions are defined as all regions in the table, except vmPFC and other PFC regions.
Statistical analysis
Behavioral data analysis was carried out for the following outcome measures: (i) affective ToM (Faux Pas story, Control questions) and (ii) cognitive ToM (ToM story, Physical story) (SPSS Version 14.0.1). Effect sizes (Cohen's d, d = 0.2 indicates a small effect size, d = 0.5 a medium effect size and d = 0.8 a large effect size) and confidence intervals (95% CI) for the difference between the means were calculated representing the observed difference performances between groups. Given that the outcome measures did not fulfill normality (Kolgomorov–Smirnov test) and homogeneity of variance (Bartlett homogeneity test) assumptions, non-parametric analyses were performed: first, the overall difference between the three groups (NC, PC and vmPFC) was assessed applying the Kruskal–Wallis test. Then, planned follow-up Mann–Whitney tests were performed to assess the differences between the experimental and comparison groups with the prediction that the vmPFC will perform below the other two groups (NC and PC) which, in turn, will not differ (P < 0.05, one-tailed). Next, the differences within the experimental subgroups (L vmPFC, R vmPFC and B vmPFC) were explored applying nonparametric Kruskal–Wallis tests, followed by Mann–Whitney tests (P < 0.05, two-tailed, Bonferroni correction for multiple comparisons). Finally, bivariate Spearman correlations between affective ToM performance and emotional measures (BEES and MSCEIT) within subgroups were performed (P < 0.05, two-tailed).
RESULTS
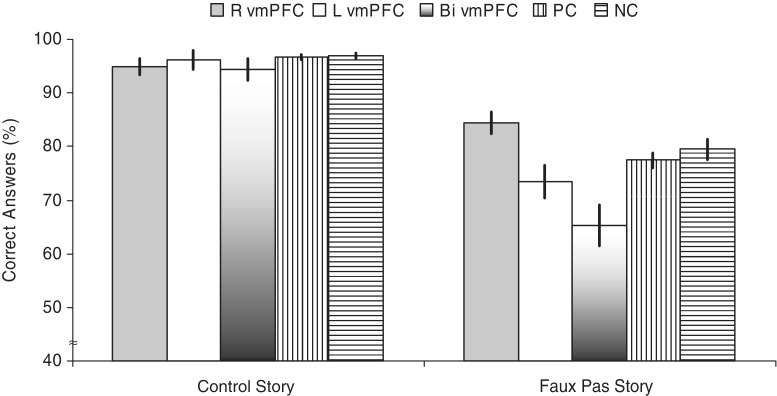
First, comparing the performance across all three groups revealed a significant overall difference for the Faux Pas story [χ2(2) = 8.3, P < 0.05], but not for the control questions [χ2(2) = 1.5, P = 0.465] and the cognitive ToM performance [ToM Story: χ2(2) = 0.1, P = 0.972, physical story: χ2(2) = 2.7, P = 0.265] (Fig. 2). Planned follow-up comparisons for the Faux Pas story score revealed that the vmPFC group performed significantly worse than the NC group (z = −2.84, P < 0.005; d = 0.53, 95% CI = 1.06–14.12) and the PC group (z = −1.93, P < 0.05; d = 0.43, 95% CI = 0.15–10.97), whereas the PC group performed as well as the NC group (z = −1.4, P = 0.160; d = 0.15, 95% CI = −3.02 to 7.08).
Fig. 2.
Performance on the Faux Pas Recognition Task Stories. Graph shows means and standard errors of the left, right and bilateral ventromedial prefrontal cortex lesion group, PC lesion group and no lesion group for the scores on the Faux Pas recognition stories. Correct Answers = Faux Pas story–faux pas question percentage correct.
Second, the performances among L, R and B vmPFC groups were compared. For the affective ToM ability, a significant difference among subgroups was only found for the Faux Pas story [χ2(2) = 10.7, P < 0.005], but not for the control questions [χ2(2) = 0.5, P = 0.508] (Fig. 2). Follow-up comparisons for the Faux Pas story condition revealed that the L vmPFC group performed significantly worse than the R vmPFC group (z = −2.7, P < 0.007; d = 1.71, 95% CI = 5.04–17.07), the B vmPFC group performed significantly worse than the R vmPFC group (z = −2.9, P < 0.003, d = 1.69, 95% CI = 6.74–31.58) and the L vmPFC group performed as worse as the B vmPFC group (z = −1.1, P = 0.286, d = 0.68, 95% CI = −3.79 to 20.01). Additional analyses comparing the performance of the L and R vmPFC group on the control stories (L vmPFC: 7.62; R vmPFC: 8.43; z=−0.42; P = 0.779, d = 0.26, 95% CI =−11.68 to 7.34) and the corresponding control questions revealed no differences (L vmPFC: 9.00; R vmPFC: 6.86; z = −0.96; P = 0.397, d = 0.23, 95% CI = −7.64 to 11.57).
Third, overall comparisons (mean ± s.e.m) showed that the groups differed on strategic EI [χ2(2) = 11.0, P < 0.005; vmPFC: 83.4 ± 1.8; PC: 90.6 ± 1.4, NC: 95.7 ± 2.3], but not experiential EI [χ2(2)=0.1, P = 0.929; vmPFC: 99.8 ± 3.8; PC: 99.6 ± 2.3, NC: 101.7 ± 2.5], total EI [χ2(2) = 2.9, P = 0.236; vmPFC: 87.9 ± 2.7; PC: 92.0 ± 1.8, NC: 95.8 ± 2.1] and emotional empathy [χ2(2) = 3.9, P = 0.139; vmPFC: −0.65 ± 0.17; PC: −0.49 ± 0.13, NC: −0.88 ± 0.19]. Planned follow-up comparisons for the strategic EI scores revealed that the vmPFC group performed significantly worse than the NC group (z = −3.25, P < 0.005; d = 0.92, 95% CI = 5.20–19.30) and the PC group (z = −2.61, P < 0.01; d = 0.69, 95% CI = 1.80–12.62), whereas the PC group performed as well as the NC group (z = −1.12, P = 0.262, d = 0.35, 95% CI = −0.39–10.48).
Finally, correlational analyses between ToM abilities and emotional measures (BEES and MSCEIT) revealed significant positive associations between affective ToM ability and emotional empathy in the B vmPFC group (r = 0.58, P < 0.023) and Strategic EI in the L vmPFC group (r = 0.75, P < 0.033), indicating that the higher the affective ToM ability, the higher the emotional empathy in the B vmPFC group and the higher the Strategic EI in the L vmPFC group.
DISCUSSION
The goal of this study was to investigate neural mechanism engaged in affective ToM abilities by comparing the performance of patients with left, right and the bilateral vmPFC lesions on the Faux Pas task. This measure taxes both cognitive (i.e. a person saying something does not know that he/she should not say it) and affective subcomponents (i.e. a person hearing it would feel insulted or hurt). We investigated further whether affective ToM deficits in vmPFC lesion patients were associated with other emotional measures, namely emotional empathy and emotional intelligence. Consistent with previous research, we found that patients with lesions in the vmPFC were significantly impaired in affective ToM abilities compared to normal controls and patients with lesions in the PC. The PC group included patients with lesions in areas outside the vmPFC and other prefrontal regions but included lesions areas that have been associated previously with ToM abilities, such as precuneus, temporal poles and amygdala (Goel et al., 1995; Baron-Cohen et al. 1999; Fine et al., 2001; Stone et al., 2003; Shaw et al., 2007). Importantly, our results show that the vmPFC is a key region and that other regions do not influence ToM abilities.
Finally, we found that only damage to the left vmPFC and bilateral vmPFC leads to significantly impaired affective ToM ability (detecting social faux pas) but not cognitive ToM ability (second-order false belief) compared with damage to the right vmPFC. Furthermore, damage to the bilateral vmPFC was specifically associated with expression of emotional empathy, whereas damage to the left vmPFC was specifically associated with strategic aspects of emotional intelligence.
In this study, patients with vmPFC lesions performed without difficulty a task that requires appreciating the cognitive aspects of belief but were impaired in tasks that involved understanding belief about emotions. This deficit was not due to difficulty in identifying emotions per se, as patients with lesions in the vmPFC did not differ from other individuals in their performance of the Morphed Faces test. The test requires individuals to recognize emotions whereas recognizing a social faux pas involves beyond emotion recognition, an ability to ascribe thoughts, feelings, ideas and intentions to others and to then use that information to anticipate behavior of others (Premack and Woodruff, 1978), thus representing a more complex assessment of affective abilities. Since groups did not differ in object naming ability (Boston naming test), deficits in Faux Pas task performance could not be attributed to simple word finding impairments. Deficits in language comprehension can also not have influenced our results, given that all groups performed similarly well on the control questions of the faux pas task and that no differences were found in the performance on the Happé stories, which also require verbal comprehension. Nevertheless, we did compare performance on the Faux Pas control stories between the patient subgroups to provide an additional measure of story comprehension. Given that patients with lesions in the left and right vmPFC performed equally well on those stories, differences in faux pas recognition cannot be attributed to verbal comprehension deficits. Differences could also not be explained by depression levels. Even though the vmPFC was suggested to be critically and causally involved in depression by diminishing depression symptoms (Koenigs et al., 2008; Koenigs and Grafman, 2009a, 2009b), our data showed no differences between groups on depression scores. Additionally, it is highly unlikely that the patients’ affective ToM performance was due to difficulties in coping with memory demands given the control over such demands and the lack of difference between the groups on measures of working memory. It is also worth pointing out that it is unlikely that the sex of our participants may have led to our finding of left-sided deficits. Rather, it has been shown by Tranel et al. (2005) that men exhibit a right-hemisphere bias in the context of emotional processing. Therefore, our findings, indicating a left vmPFC lesion association with a deficit in affective processing are probably attributable to factors other than sex.
Recently, it has been suggested that while performance on cognitive ToM measures (i.e. second- and first-order ToM) is associated with widespread PFC function, impairment in affective ToM, as assessed by the faux pas task, may be more closely associated with vmPFC damage specifically (Shamay-Tsoory et al., 2003, 2007). This finding is supported by the suggestion that the vmPFC is an area that is necessary for the normal regulation of emotion. In fact, the most robust clinical findings associated with vmPFC damage have been defective modulation of emotion (Koenigs and Tranel, 2007; Koenigs and Grafman 2009a, 2009b). Lesions in the vmPFC have shown to result in a syndrome characterized by disinhibition, lack of tact (Eslinger and Damasio, 1985), impaired empathy (Eslinger, 1998; Barrash et al., 2000; Shamay-Tsoory et al., 2003), deficits in emotional intelligence (Krueger et al., 2009) and poorly regulated anger (Anderson et al., 2006; Strenziok et al., 2011), as well as irritability, emotional outbursts and tantrums, particularly in social situations involving frustration or provocation (Grafman et al., 1996; Berlin et al., 2004; Anderson et al., 2006).
In addition, the vmPFC's intimate connections to limbic, hypothalamic and brainstem regions and the bidirectional connections with the hippocampus and the amygdala (Van Hoesen et al., 1975), places it in a suitable position for integrating diverse cognitive and emotional processes (Koenigs and Tranel, 2007; Krueger et al., 2009), thus facilitating inferences about affective components accompanying social conduct. Existing research points to the medial PFC's role in ToM but our findings may highlight a differential involvement of regions within the medial PFC for affective and cognitive ToM. Affective ToM may largely involve the ventromedial PFC via an ‘outcome pathway’, in which outcome knowledge about an event enables inferences about affective states (Krueger et al., 2009), whereas cognitive ToM may largely involve the dorsomedial PFC (dmPFC) via a ‘goal pathway’. The dmPFC with its connections to posterior and subcortical brain regions thus may be well suited for knowledge inferences regarding goal-directed actions.
However, even though previous findings have shed light on the neural correlates of affective ToM, it is still unclear whether the left or right hemispheres play distinct roles. Our results showed that those patients with lesions in the left vmPFC and bilateral vmPFC performed significantly worse than those with lesions in the right vmPFC, suggesting that the left hemisphere may play a larger role in affective ToM. Our finding is in contrast with studies conducted by Shamay-Tsoory et al. (2003, 2005), who found that the right hemisphere may play a larger role. In their first study, however, the authors did not compare the left and right vmPFC specifically but showed deficits in patients with lesions in the right PFC compared to the PC. They found the same result when doing a similar comparison in the later study. These studies had restricted sample sizes that did not allow for a direct comparison of performance in patients with left and right vmPFC damage. Given our larger sample size, we were able to directly compare the performance between the two groups, which revealed greater deficits for subjects with left vmPFC lesions. In support, other studies have also shown involvement of the left hemisphere in ToM. In a PET study conducted by Goel et al. (1995), the authors asked subjects to make inferences that required modeling another person's mental state and found selective activation in the left medial frontal cortex and left temporal lobe. Similarly, in an fMRI study Gallagher et al. (2000) found left medial PFC activation in participants while performing story and cartoon tasks. Furthermore, whereas left hemisphere lesion patients’ performance did not differ from that of right hemisphere lesion patients in a study conducted by Shamay-Tsoory et al., (2005), in a later study, Shamay-Tsoory and Aharon-Peretz (2007) did show impairment in patients with left PFC damage in an affective ToM eye gaze condition.
Importantly, Sabbagh (2004) has suggested that differences in findings between left and right hemisphere contribution can be attributed to varying processes involved in making appropriate ToM judgments. Thus, ToM ability can be separated either into detecting or decoding others mental states based on immediately available observable information or reasoning about mental states in the service of explaining or predicting others’ actions. Decoding others’ mental states has suggested to be lateralized to the right hemisphere, whereas reasoning about mental states has been associated with the left hemisphere (Sabbagh, 1999, Siegal and Varley, 2002; Sabbagh, 2004). Accordingly, it has been argued that the left hemisphere may function as an ‘interpreter’ (Gazzaniga, 2000) that makes sense of the environment by locking into and extrapolating patterns (Goel, 2007). A series of lesion studies (e.g., Goel et al. 2004; Reverberi et al. 2005) and neuroimaging studies (Goel et al. 2000; Acuna et al. 2002; Goel and Dolan 2003; Knauff et al. 2003) support this assumption by having implicated a system in the left PFC involved in reasoning tasks, and our results are consistent with these findings.
Finally, results from our correlational analyses revealed that ToM may involve empathic abilities as well as emotional intelligence. The positive correlation between the performance of the affective ToM tasks and emotional empathy was found for patients with bilateral vmPFC lesions only, and not for patients with unilateral left vmPFC lesions. Thus, more research is needed to determine if empathic processes or other mechanisms may be involved in affective ToM deficits in this patient group. More importantly, emotional intelligence, specifically strategic EI, which measures individuals’ competency in understanding emotions, was positively correlated with affective ToM for patients with left vmPFC lesions. This finding is consistent with other studies showing that vmPFC damage impairs EI in general (Bar-On et al., 2003) and strategic EI specifically (Krueger et al., 2009). Furthermore, it may suggest a neural mechanism for the behavioral and emotional dysfunctions observed in vmPFC lesion patients. As mentioned previously, these patients show deficits despite preservation of relatively intact intellectual abilities and even though we found differences in intelligence scores between our control and experimental groups, further analysis showed that this was due to bilateral vmPFC patients’ low performance. Patients with left vmPFC lesions, however, did not show impairments in cognitive intelligence compared to any other group. Thus, their deficit in affective ToM abilities cannot be explained by overall intelligence impairments but rather, the deficits may be due to decreased competencies underlying EI, which may complement cognitive intelligence and facilitate appropriate emotional and social processes (Krueger et al., 2009). Thus, strategic emotional intelligence appears to be essential for understanding and predicting behaviors through the analysis of emotions, the appreciation of their probable trends over time and the understanding of their outcomes (Mayer et al., 2004).
In conclusion, our results support the theory that the vmPFC is associated with abilities in affective ToM. Moreover, our findings highlight that the left, and not the right vmPFC as indicated previously, is involved in affective ToM. Despite those contrary findings, they are in line with theories that implicate the left PFC in reasoning about mental states and the right hemisphere in decoding others’ mental states. Most importantly, the mechanism underlying this function may be Strategic Emotional Intelligence competencies as well as emotional empathic abilities. Thus, future research on ToM should be careful in specifying which subcomponents of ToM are being investigated and which measures are being used to identify the neural correlates associated with those components. In addition, our findings call for the use of fine-grained measurements of subcomponents of empathy to provide insight into the results highlighted by our study.
Conflict of Interest
None declared.
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, the Department of Defense, or the U.S. Government. The authors are grateful to all the Vietnam veterans who participated in this study. Without their long-term commitment to improving the health care of veterans, this study could not have been completed. We thank the National Naval Medical Center for their support and provision of their facilities. We are grateful to S. Bonifant, B. Cheon, C. Ngo, A. Greathouse, K. Reding and G. Tasick for their invaluable help with the testing of participants and organization of this study. The work was supported by the U.S. National Institute of Neurological Disorders and Stroke intramural research program and a project grant from the U.S. Army Medical Research and Material Command administrated by the Henry M. Jackson Foundation (Vietnam Head Injury Study Phase III: a 30-year post-injury follow-up study), grant number: DAMD17-01-1-0675.
REFERENCES
- Anderson SW, Barrash J, Bechara A, Tranel D. Impairments of emotion and real-world complex behavior following childhood- or adult-onset damage to ventromedial prefrontal cortex. Journal of International Neuropsychology Society. 2006;12(2):224–35. doi: 10.1017/S1355617706060346. [DOI] [PubMed] [Google Scholar]
- Anonymous. Armed Forces Qualification Test (AFQT-7A) 1960 Department of Defense Form 1293. [Google Scholar]
- Acuna BD, Eliassen JC, Donoghue J, Sanes JN. Frontal and parietal lobe activation during transitive inference in humans. Cerebral Cortex. 2002;12(12):1312–21. doi: 10.1093/cercor/12.12.1312. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Leslie AM, Frith U. Does the autistic child have a “theory of mind”! Cognition. 1985;21(1):37–46. doi: 10.1016/0010-0277(85)90022-8. [DOI] [PubMed] [Google Scholar]
- Bar-On R, Tranel D, Denburg NL, Bechara A. Exploring the neurological substrate of emotional and social intelligence. Brain. 2003;126:1790–800. doi: 10.1093/brain/awg177. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, O'Riordan M, Stone V, Jones R, Plaisted K. Recognition of faux pas by normally developing children and children with Asperger syndrome or high-functioning autism. Journal of Autism and Developmental Disorders. 1999;29(5):407–18. doi: 10.1023/a:1023035012436. [DOI] [PubMed] [Google Scholar]
- Barrash J, Tranel D, Anderson SW. Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Developmental Neuropsychology. 2000;18(3):355–81. doi: 10.1207/S1532694205Barrash. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory II (BDI-II) San Antonio, TX: Psychology Corporation; 1996. [Google Scholar]
- Berlin HA, Rolls ET, Kischka U. Impulsivity, time perception, emotion, and reinforcement sensitivity in patients with orbitofrontal cortex lesions. Brain. 2004;127(5):1108–26. doi: 10.1093/brain/awh135. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Cipolotti L. Impaired social response reversal: a case of ‘acquired sociopathy’. Brain. 2000;123(6):1122–41. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- Burgess PW. Neuropsychology of behaviour disorders following brain injury. In: Wood RL, editor. Neurobehavioural Sequelae of Traumatic Brain Injury. New York: Taylor and Francis; 1990. pp. 110–133. [Google Scholar]
- Critchley HD, Elliott R, Mathias CJ, et al. Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. Journal of Neuroscience. 2000;20(8):3033–40. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Somatic markers and the guidance of behaviour: theory and preliminary testing. In: Levin HS, Eisenberg HM, Benton AL, editors. Frontal Lobe Function and Dysfunction. New York: Oxford University Press; 1991. pp. 217–229. [Google Scholar]
- Dimitrov M, Phipps M, Zahn T, Grafman J. A thoroughly modern Gage. Neurocase. 1999;5:345–54. [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35(12):1731–41. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ. Neurological and neuropsychological bases of empathy. European Neurology. 1998;39(4):193–9. doi: 10.1159/000007933. [DOI] [PubMed] [Google Scholar]
- Fine C, Lumsden J, Blair RJ. Dissociation between ‘theory of mind’ and executive functions in a patient with early left amygdala damage. Brain. 2001;124(2):287–98. doi: 10.1093/brain/124.2.287. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happé F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of ‘theory of mind’ in verbal and nonverbal tasks. Neuropsychologia. 2000;38(1):11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gazzaniga MS. Cerebral specialization and interhemispheric communication: Does the corpus callosum enable the human condition? Brain. 2000;123(7):1293–326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- Goel V, Buchel C, Frith C, Dolan R. Dissociation of Mechanisms Underlying Syllogistic Reasoning. NeuroImage. 2000;12(5):504–14. doi: 10.1006/nimg.2000.0636. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ. Reciprocal neural response within lateral and ventral prefrontal cortex during hot and cold cognition. NeuroImage. 2003;20(4):2314–21. doi: 10.1016/j.neuroimage.2003.07.027. [DOI] [PubMed] [Google Scholar]
- Goel V, Grafman J, Sadato N, Hallett M. Modeling other minds. Neuroreport. 1995;6(13):1741–6. doi: 10.1097/00001756-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Goel V, Shuren J, Sheesley L, Grafman J. Asymmetrical involvement of frontal lobes in social reasoning. Brain. 2004;127:783–90. doi: 10.1093/brain/awh086. [DOI] [PubMed] [Google Scholar]
- Goel V, Tierney M, Sheesley L, Bartolo A, Vartanian O, Grafman J. Hemispheric specialization in human prefrontal cortex for resolving certain and uncertain inferences. Cerebral Cortex. 2007;17:2245–50. doi: 10.1093/cercor/bhl132. [DOI] [PubMed] [Google Scholar]
- Grafman J, Jonas BS, Martin A, et al. Intellectual function following penetrating head injury in Vietnam veterans. Brain. 1988;111(1):169–84. doi: 10.1093/brain/111.1.169. [DOI] [PubMed] [Google Scholar]
- Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM. Frontal lobe injuries, violence, and aggression: a report of the Vietnam Head Injury Study. Neurology. 1996;46(5):1231–8. doi: 10.1212/wnl.46.5.1231. [DOI] [PubMed] [Google Scholar]
- Happé F. An advanced test of theory of mind: understanding of story characters’ thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. Journal of Autism and Development Disorders. 1994;24(2):129–54. doi: 10.1007/BF02172093. [DOI] [PubMed] [Google Scholar]
- Kalbe E, Schlegel M, Sack AT, et al. Dissociating cognitive from affective theory of mind: a TMS study. Cortex. 2010;46(6):769–80. doi: 10.1016/j.cortex.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming Test. Philadelphia: Lea & Febiger; 1976. [Google Scholar]
- Knauff M, Fangmeier T, Ruff CC, Johnson-Laird PN. Reasoning, models, and images: behavioral measures and cortical activity. Journal of Cognitive Neuroscience. 2003;15(4):559–73. doi: 10.1162/089892903321662949. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. The functional neuroanatomy of depression: distinct roles for ventromedial and dorsolateral prefrontal cortex. Behavioural Brain Research. 2009a;201(2):239–43. doi: 10.1016/j.bbr.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Grafman J. Prefrontal asymmetry in depression? The long-term effect of unilateral brain lesions. Neuroscience Letters. 2009b;459(2):88–90. doi: 10.1016/j.neulet.2009.04.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Tranel D. Irrational economic decision-making after ventromedial prefrontal damage: evidence from the Ultimatum Game. The Journal of Neuroscience. 2007;27(4):951–6. doi: 10.1523/JNEUROSCI.4606-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Huey ED, Calamia M, Raymont V, Tranel D, Grafman J. Distinct regions of prefrontal cortex mediate resistance and vulnerability to depression. The Journal of Neuroscience. 2008;28(47):12341–8. doi: 10.1523/JNEUROSCI.2324-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger F, Barbey AK, Grafman J. The medial prefrontal cortex mediates social event knowledge. Trends in Cognition Science. 2009;13(3):103–9. doi: 10.1016/j.tics.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Krueger F, Barbey AK, McCabe K, Strenziok M, Zamboni G, Solomon J, Raymont V, Grafman J. The neural bases of key competencies of emotional intelligence. Journal of Proceedings of the National Academy of Science. 2009;106(52):22486–91. doi: 10.1073/pnas.0912568106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makale M, Solomon J, Patronas NJ, Danek A, Butman JA, Grafman J. Quantification of brain lesions using interactive automated software. Behavior Research Methods, Instruments, and Computers. 2002;34(1):6–18. doi: 10.3758/bf03195419. [DOI] [PubMed] [Google Scholar]
- Mayer JD, Salovey P, Caruso DR. Emotional Intelligence: theory, findings, and implications. Psychological Inquiry. 2004;15:197–215. [Google Scholar]
- Mayer JD, Salovey P, Caruso DR. Emotional intelligence: new ability or eclectic traits? American Psychology. 2008;63:503–17. doi: 10.1037/0003-066X.63.6.503. [DOI] [PubMed] [Google Scholar]
- Mayer JD, Salovey P, Caruso DR, Sitarenios G. Emotional intelligence as a standard intelligence. Emotion. 2001;1:232–42. [PubMed] [Google Scholar]
- Mayer JD, Salovey P, Caruso DR, Sitarenios G. Test (Version 2.0) User's Manual. Toronto (Canada): Multi-Health Systems; 2002. Mayer-Salovey-Caruso Emotional Intelligence. [Google Scholar]
- Miller SA. Children's understanding of second-order mental states. Psychological Bulletin. 2009;135(5):749–73. doi: 10.1037/a0016854. [DOI] [PubMed] [Google Scholar]
- Pandya DN, old D, Berger T. Interhemispheric connections of the precentral motor cortex in the rhesus monkey. Brain Research. 1969;15:594–6. doi: 10.1016/0006-8993(69)90193-0. [DOI] [PubMed] [Google Scholar]
- Perner J, Leekam SR, Wimmer H. Three-year olds’ difficulty with false belief: the case for a conceptual deficit. British Journal of Developmental Psychology. 1987;5:125–37. [Google Scholar]
- Premack D, Woodruff G. Does the Chimpanzee Have a Theory of Mind? Behavioral and Brain Sciences. 1978;1(4):515–26. [Google Scholar]
- Raymont V, Salazar AM, Krueger F, Grafman J. “Studying injured minds”—the Vietnam head injury study and 40 years of brain injury research. Frontiers Neurotrauma. 2011;2(15):1–13. doi: 10.3389/fneur.2011.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reverberi C, Lavaroni A, Gigli GL, Skrap M, Shallice T. Specific impairments of rule induction in different frontal lobe sub- groups. Neuropsychologia. 2005;43(3):460–72. doi: 10.1016/j.neuropsychologia.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Rowe A, Bullock P, Polkey C, Morris RG. ‘Theory of mind’ impairments and their relationship to executive functioning following frontal lobe excisions. Brain. 2001;124(3):600–616. doi: 10.1093/brain/124.3.600. [DOI] [PubMed] [Google Scholar]
- Sabbagh MA. Communicative intentions and language: evidence from right-hemisphere damage and autism. Brain and Language. 1999;70(10):29–69. doi: 10.1006/brln.1999.2139. [DOI] [PubMed] [Google Scholar]
- Sabbagh MA. Understanding orbitofrontal contributions to theory-of-mind reasoning: implications for autism. Brain and Cognition. 2004;55(1):209–19. doi: 10.1016/j.bandc.2003.04.002. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J. Dissociable prefrontal networks for cognitive and affective theory of mind: a lesion study. Neuropsychologia. 2007a;45(13):3054–67. doi: 10.1016/j.neuropsychologia.2007.05.021. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy for inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132(3):617–27. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Shur S, Barcai-Goodman L, Medlovich S, Harari H, Levkovitz Y. Dissociation of cognitive from affective components of theory of mind in schizophrenia. Psychiatry Research. 2007b;149(1–3):11–23. doi: 10.1016/j.psychres.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tibi-Elhanany Y, Aharon-Peretz J. The ventromedial prefrontal cortex is involved in understanding affective but not cognitive theory of mind stories. Social Neuroscience. 2006;1(3–4):149–66. doi: 10.1080/17470910600985589. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Aharon-Peretz J. Characterization of empathy deficits following prefrontal brain damage: the role of the right ventromedial prefrontal cortex. Journal of Cognitive Neuroscience. 2003;15(3):324–37. doi: 10.1162/089892903321593063. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Goldsher D, Aharon-Peretz J. Impaired “affective theory of mind” is associated with right ventromedial prefrontal damage. Cognitive Behavioral Neurology. 2005;18(1):55–67. doi: 10.1097/01.wnn.0000152228.90129.99. [DOI] [PubMed] [Google Scholar]
- Shaw P, Lawrence E, Bramham J, Brierly B, Radbourne C, David AS. A prospective study of the effects of anterior temporal lobectomy on emotion recognition and theory of mind. Neuropsychologia. 2007;45(12):2783–90. doi: 10.1016/j.neuropsychologia.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Siegal M, Varley R. Neural systems involved in “theory of mind”. Natural Review of Neuroscience. 2002;3(6):463–71. doi: 10.1038/nrn844. [DOI] [PubMed] [Google Scholar]
- Singer T. The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neuroscience and Biobehavioral Reviews. 2006;30:855–63. doi: 10.1016/j.neubiorev.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Solomon J, Raymont V, Braun A, Butman JA, Grafman J. User-friendly software for the analysis of brain lesions (ABLe) Computer Methods and Programs in Biomedicine. 2007;86(3):245–54. doi: 10.1016/j.cmpb.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone VE, Baron-Cohen S, Calder A, Keane J, Young A. Acquired theory of mind impairments in individuals with bilateral amygdala lesions. Neuropsychology. 2003;41(2):209–20. doi: 10.1016/s0028-3932(02)00151-3. [DOI] [PubMed] [Google Scholar]
- SPSS for Windows, Rel. 14.0.1 Chicago: SPSS Inc; 2006. [Google Scholar]
- Stone VE, Baron-Cohen S, Knight RT. Frontal lobe contributions to theory of mind. Journal of Cognitive Neuroscience. 1998;10(5):640–56. doi: 10.1162/089892998562942. [DOI] [PubMed] [Google Scholar]
- Strenziok M, Krueger F, Heinecke A, Lenroot RK, Knutson KM, Van der Meer E, Grafman J. Developmental effects of aggressive behavior in male adolescents assessed with structural and functional brain imaging. Soc Cognition Affect Neuroscience. 2011;6(1):2–11. doi: 10.1093/scan/nsp036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Denburg NL, Bechara A. Does gender play a role in functional assymetry of ventromedial prefrontal cortex? Brain. 2005;128:2872–81. doi: 10.1093/brain/awh643. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Van Hoesen G, Pandya DN, Butters N. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. II. Frontal lobe afferents. Brain Research. 1975;95:25–38. doi: 10.1016/0006-8993(75)90205-x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. The Measurement of Adult Intelligence. 3rd edn. Baltimore: Williams & Wilkins; 1997. [Google Scholar]
- Wechsler D. Wechsler Memory Scale. 3rd edn. San Antonio, TX: Harcourt Assessment; 1997. [Google Scholar]
- Wellman HM. The Child's Theory of Mind. Cambridge, MA: MIT Press; 1990. The MIT Press series in learning, development, and conceptual change. [Google Scholar]
- Wellman HM, Cross D, Watson J. Meta-analysis of theory-of-mind development: The truth about false belief. Child Development. 2001;72:655–84. doi: 10.1111/1467-8624.00304. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. Journal of Computer Assisted Tomography. 1993;17:536–46. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]