Abstract
The neural bases of empathy have been examined mainly in the context of reacting to others’ distress, while almost no attention has been paid to the mechanisms by which we share others’ joy. Using functional magnetic resonance imaging, we demonstrated that the same neural network mediates judgment of the emotional state of the other in response to both negative and positive events through empathy-related structures, such as the medial prefrontal cortex (MPFC), the insula, the superior temporal sulcus (STS) and the inferior frontal gyrus (IFG). However, the responses of the MPFC, bilateral insula and the right IFG to negative experiences occurring to the other (but not to the self) were found to be much more intense than the responses to positive experiences, indicating that humans have a remarkable ability to share the distress of others, but may react less to the joy of others.
Keywords: empathy, medial prefrontal cortex, social neuroscience, joy, distress
It is not in the human heart to put ourselves in the place of those who are happier than ourselves, but only in the place of those who are most to be pitied.
(Rousseau, Emile).
INTRODUCTION
Empathy relates to the ability to perceive and react to the emotional state of the other.
Signs of empathy are being increasingly reported in various mammals, such as chimpanzees (de Waal et al., 2008), and even in rodents (Langford et al., 2006; Grenier and Luthi, 2010), further attesting to its evolutionary roots. While empathy broadly applies to the individual's reaction to all sorts of emotional states, research on the subject to date has focused on empathy in response to negative events, such as pain and distress (Singer et al., 2004; Lamm et al., 2011). Even on the purely linguistic level, there are far more terms in most languages to specifically denote a vicarious affective response to another's distress (pity, sympathy, compassion) than there are to describe the affective response to another's joy. Moreover, the supposedly generic term empathy is, in practice, synonymous with the terms that signify our affinity with another's negative state (Rozin and Royzman, 2001).
Accordingly, most of the neuroscientific investigations of empathy are essentially based on studies examining empathy in relation to negative rather than positive events. These studies, which used tasks involving social reasoning, responding to emotional expressions and inferring mental states repeatedly demonstrate that empathy activates a neural circuit consisting of the medial prefrontal cortex (MPFC), the insula, the superior temporal sulcus (STS), the temporal parietal junction (TPJ) and the inferior frontal gyrus (IFG) as core network regions (Farrow et al., 2001; Amodio and Frith, 2006; Blair, 2007; Carrington and Bailey, 2009; Iacoboni, 2009; Singer and Lamm, 2009; Xu et al., 2009; Fan et al., 2010).
Many of these studies used paradigms in which subjects observed others in painful situations and have found the insula to be strongly involved in empathy for pain (Singer and Lamm, 2009). The MPFC has repeatedly been shown to take part in a wide variety of social cognition related tasks and monitoring of one's emotional state (Amodio and Frith, 2006; Tamir and Mitchell, 2010), while the IFG has been shown to support empathic judgments (Farrow et al., 2001) and emotion attribution (Schulte-Ruther et al., 2007). Theory of Mind tasks have been reported to activate the STS (Gallagher and Frith, 2003; Saxe, 2006), whereas the TPJ is reported to participate in the attribution of mental states (Lee and Siegle, 2009; Cheon, et al., 2011).
Given that these empathy-related brain activations have been reported primarily in negative situations, the question arises as to whether the brain reacts differently to the joy vs the distress of others.
Considering previous studies regarding valence-dependent brain responses to emotional contents (Cunningham et al., 2004), one possibility is that empathy for negative vs positive events is mediated by a distinct and separate neural network. Furthermore, it is possible that facing a protagonist's positive emotional state may trigger opposite emotions, such as envy, rather than empathy (Dvash et al., 2010), and that different neural substrates actually participate in empathy to positive vs negative events occurring to a protagonist.
A second, equally plausible, theoretical model is that both forms of empathy involve the same neural network, but activate the empathy network to a different extent. This hypothesis is supported by studies reporting similar brain responses (particularly in the MPFC) to emotions, regardless of the specific emotion (Phan et al., 2002), as well as the activation of similar empathy-related structures, such as the IFG and the insula, while sharing positive (hunger) and negative (disgust) gustatory emotions (Jabbi et al., 2007). Furthermore, it is possible that empathy for negative events activates the empathy network more prominently than would empathy for positive events, because the former has been targeted by natural selection, as empathy promotes helping behaviors in social animals (De Waal, 2007). The implication of this model would be that although similar empathy-related responses are involved in empathy to positive and negative events, the empathy network would react more to negative than to positive contents.
While neuroimaging studies to date have been increasingly capable of characterizing the neural networks involved in empathy to distress and pain, no study has directly compared the empathic neural responses to distress vs joy. Therefore, the first goal of the current study was to examine whether the brain structures that react to the other's distress (MPFC, IFG, insula, STS) will also react to the other's joy. To examine if these regions react specifically to negative events occurring to the other, we also compared the brain responses to negative and positive events occurring either to the self or to the other. Thus, in order to directly examine the neural basis of empathy in reaction to negative vs positive events, we used a neuroimaging protocol that compared the brain responses to positive and negative emotional events occurring to the self or to another protagonist.
METHODS
Participants
Twenty-one individuals (9 women, mean age of 29, s.d. = 3.8) participated in this study. Participants had no reported history of psychiatric or neurological disorders and were not using psychoactive drugs at the time of the study. In addition, all participants had normal or corrected-to-normal vision. All subjects gave informed consent and were paid for their participation.
Assessment of trait empathy
The Hebrew version of the Interpersonal Reactivity Index (IRI) was used to evaluate levels of empathic ability (Davis, 1980). The IRI includes 47-item subscales each tapping different aspect of empathy (Davis, 1980). (i) the perspective taking (PT) subscale, which measures the reported tendency to adopt spontaneously the psychological point of view of others; (ii) the fantasy subscale (FS) measuring the tendency to imaginatively transpose oneself into fictional situations; (iii) the empathic concern (EC) scale assesses the tendency to experience feelings of sympathy and compassion for others; and (iv) the personal distress (PD) scale assesses the tendency to experience distress and discomfort in response to others’ observed distress.
Stimuli and procedure
Prior to scanning subjects were given a short vignette describing a fictional character matching their sex. The description included some demographical and basic traits regarding the character. Subjects were asked to read the vignette and were told that the character will appear in the scanning session.
The stimuli consisted of 80 sentences depicting everyday negative (‘You lost your wallet’) or positive (‘John won a scholarship’) emotional events occurring to either the subject (‘SELF’ condition—‘You lost your wallet’) or to the fictional character which has been previously presented (‘OTHER’ condition—‘John lost his wallet’). The subjects had to read the sentence and indicate whether the event was distressful or joyful. The events were based on a larger sample of events tested in a pretest with 30 different participants, which confirmed that there were no significant differences in emotional ratings of the positive and negative events. That is, while both negative and positive events were similar in the levels of emotional ratings, the negative events were judged as distressful and the positive events were rated as joyful.
The current experiment was divided into four runs of 7.25 min each. The order of the four tasks was counterbalanced across subjects. In each run, 20 sentences were presented in a pseudo-random manner. The sentences were presented visually in a slow event-related fashion. Each event was followed by a blank screen with a fixation point. After the presentation of each sentence, participants were instructed to judge the emotional intensity of the event using a cursor that moved along a judgment bar, ranging from 5 = highest joy to −5 = highest distress. Participants were instructed to read the sentences and rate the amount of emotional intensity that would be felt by the protagonists (SELF, OTHER) in such an event. Event length varied from 2.5 to 7.5 s. During the first 2.5 s, subjects had to read the sentence and indicate whether the event was distressful or joyful by clicking on one of two keys. Following this response, the cursor started to move to the left or to the right along the judgment bar. The subjects then had to estimate the intensity of the emotion by another click, which stopped the cursor from moving at a point between 0 and 5 and ended the event. The sides of the judgment bar were switched between subjects in a counterbalanced manner. Between events there were rest époques that lasted from 5 to 10 s, depending on the length of the preceding event.
fMRI image acquisition and analysis
Scanning was performed in a 3T GE scanner with an 8-channel head coil, using a gradient echo-planar imaging (EPI) sequence of functional T2*-weighted images (repetition time (TR)/echo time (TE)/flip angle: 2500/35/90; field of view (FOV): 20 × 20 cm2; matrix size: 64 × 64) divided into 40 axial slices (thickness: 3 mm; gap: 0 mm) covering the whole cerebrum. Anatomical 3D-sequence spoiled gradient (SPGR) echo sequences were obtained with high-resolution 1-mm slice thickness (FOV: 25 × 18 cm2; matrix: 256 × 256; TR/TE: 9/3.6 ms).
The fMRI data were processed using the BrainVoyager QX 2 software package (http://www.brainvoyager.com). Pre-processing of functional scans included head movement assessment (scans with head movement >1.5 mm were rejected), high-frequency temporal filtering and removal of low-frequency linear trends. In order to allow for T2*-equilibration effects, the first six images of each functional scan were rejected. Pre-processed functional images were incorporated into the 3D data sets through tri-linear interpolation. The complete data set was transformed into Talairach space. Three-dimensional statistical parametric maps were calculated separately for each subject using a general linear model (GLM) in which all stimuli conditions were positive predictors, with a lag of 3–6 s (individual account for the hemodynamic response delay), and spatially smoothing the data (full width at half maximum: 4 mm). A random-effects GLM analysis was conducted on the fMRI signal during the emotional judgment task with the predictors SELF and OTHER. The predictors were convolved with a standard canonical hemodynamic response function.
Regions of interest
Regions of interests (ROIs) were identified based on prior hypotheses regarding four regions (MPFC, IFG, insula, STS) that have been repeatedly reported to play a key role in empathy (Farrow et al., 2001; Amodio and Frith, 2006; Blair, 2007; Carrington and Bailey, 2009; Iacoboni, 2009; Shamay-Tsoory et al., 2009; Fan et al., 2010). Additionally, the role of these ROIs in empathy was functionally confirmed by the initial contrast between negative vs positive conditions (random, P < 0.0008, uncorrected). For each region, a 53 voxels ROI was selected around the voxel of peak activation for each of the ROIs. The ROIs were verified anatomically according to Talairach coordinates.
Correlation between estimated activation in ROIs
Due to the major role of the MPFC in the empathy-related neural network and has been repeatedly activated in metal state inferences tasks (Gallagher and Frith, 2003; Singer, 2006), the relationship between the MPFC and the other ROIs were further explored using correlation analysis.
RESULTS
Behavioral results
Mean emotional rating for each condition was as follows: SELF negative: M = 3.08, s.d. = 0.46; SELF positive: M = 9.38, s.d. = 0.47; OTHER negative: 3.14, s.d. = 0.55, OTHER positive: M = 9.31, s.d. = 0.59. To examine the differences in emotional ratings for the different protagonists/subject (SELF and OTHER), and event's valence (‘positive’ and ‘negative’), a repeated measures ANOVA was conducted. A main effect of valence was found [F(3,−17) = 1260, P < 0.0001] confirming that selected positive events were rated on the judgment bar as related to joy and the negative events as related to distress. There was no main effect for the protagonist [F(3,17) = 0.001, P = 0.99] nor was there any interaction found between valence and protagonist [F(3,17) = 0.38, P = 0.54].
To ensure that the emotional rating task indeed involved empathy, a correlation analysis was carried out (with Bonferroni corrections) between subjects’ emotional ratings for the OTHER condition and the individuals’ empathy trait scores (i.e. IRI scores). Results indicated that the subjects’ IRI total scores were correlated significantly with the emotional ratings for the OTHER only in the case of negative events (r = 0.441, P < 0.045). Specifically, the FS scale correlated significantly with the OTHER negative events (r = 0.474, P < 0.03) while the correlations of the other scales with the OTHER condition did not reach significance (PT: r = 0.306, P < 0.177; EC: r = 0.241, P < 0.292; PD: r = −0.035, P < 0.88). In contrast, for positive contents, the correlation was not significant for the IRI total scores (r = −0.021, P < 0.9 ns), neither for each scale separately (FS: r = −0.055, P < 0.814; PT: r = −0.167, P < 0.477; EC: r = 0.001, P < 0.995; PD: r = 0.200, P < 0.3896), suggesting that empathic abilities as measured by the total IRI scores are predicted by the level of emotional ratings for negative, rather than for positive, experiences of the OTHER protagonist.
Neuroimaging results
To characterize the neural network mediating the emotional value judgment about the self and the other, separate brain activity maps of the emotional ratings for SELF and for OTHER (Figure 1 and Table 1) were identified as follows:
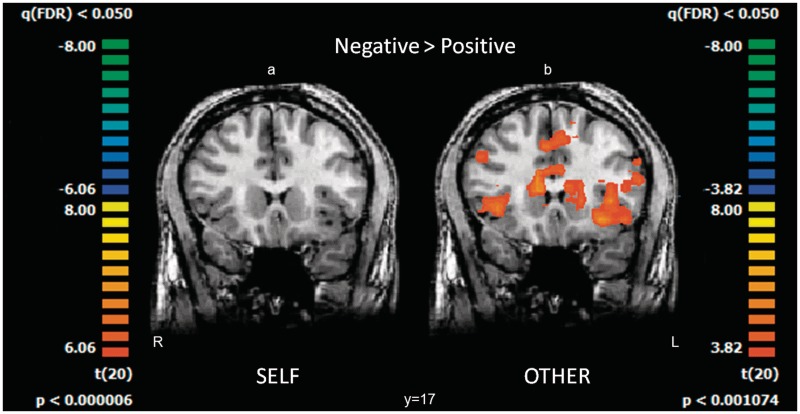
Fig. 1.
Whole-brain activation patterns obtained for the contrast: (a) ‘SELF negative > SELF positive’ (b) ‘OTHER negative > OTHER positive’ superimposed on coronal anatomical slices (n = 21, random effect, GLM analysis).
Table 1.
Peak of activation obtained from whole-brain contrasts
| Contrast | Region | BA | Voxel of peak activation (x, y, z) | t | P |
|---|---|---|---|---|---|
| OTHER’ contrasts | |||||
| OTHER negative > OTHER positive | R insula | 13 | 35, 16, 4 | 6.12 | 0.000006 |
| L insula | 47 | −31, 16, −2 | 5.7 | 0.00001 | |
| MPFC | 8 | −1, 28, 51 | 6.12 | 0.000006 | |
| L LPFC | 10 | −43, 40, 12 | 6.26 | 0.000004 | |
| R ACC | 11, 19, 18 | 6.59 | 0.000002 | ||
| R IFG | 45 | 47, 13, 12 | 5.95 | 0.000008 | |
| L insula | 13 | −43, 10, 12 | 5.26 | 0.00003 | |
| L IFG | 9 | −43, 10, 30 | 5.95 | 0.000008 | |
| L STS | −43, −62, 15 | 6.35 | 0.000003 | ||
| OTHER positive > OTHER negative | NS | ||||
| SELF vs OTHER contrasts | |||||
| SELF > OTHER | MPFC | 10 | −3, 53, 10 | 8.05 | 1.04-7 |
| OTHER > SELF | PCC | 7 | 6, −67, 34 | 7.77 | 1.8-7 |
| SELF and OTHER | |||||
| Negative > positive | R Insula | 13 | 32, 16, 9 | 5.89 | 0.000009 |
| L insula | 13 | −31, 19, 9 | 6.7 | 0.000002 | |
| MPFC | 8 | −1, 33, 48 | 4.7 | 0.0001 | |
| L IFG | 44 | −45, 7, 9 | 4.79 | 0.0001 | |
| R IFG | 44 | 50, 10, 15 | 5.03 | 0.00006 | |
| L STS | 37 | −46, −59, 6 | 5.13 | 0.00005 | |
| L LPFC | 9 | −49, 28, 33 | 5.79 | 0.00001 | |
| L LPFC | 46 | −43, 34, 18 | 4.78 | 0.00009 | |
| Caudate | 5, 13, 15 | 4.69 | 0.0001 | ||
| Positive > negative | NS |
Talairach coordinates of regions extracted from the: clusters taken from random effect, FDR corrected GLM, clusters taken from random effect, FDR corrected GLM, (SELF and OTHER) negative—(SELF and OTHER) positive contrasts. Clusters taken from random effect, FDR uncorrected GLM. Stereotactic coordinates and t-values are provided for local voxel maxima.
L = left; R = right; M = middle; LPFC = lateral prefrontal cortex, ACC = anterior cingulated cortex; NS = not significant.
‘OTHER negative’ > ‘OTHER positive’.
This whole-brain analysis revealed a markedly greater activation for the negative than positive events. Prominent loci for differential ‘OTHER negative’ were found in the MPFC, bilateral IFG, bilateral insula and left STS (see selected views obtained from the whole-brain analysis for this contrast, P < 0.001, random, FDR corrected).
‘SELF negative’ > ‘SELF positive’.
The same contrast between the ‘SELF negative’ and the ‘SELF positive’ events yielded a very weak brain response with no activations surviving the FDR correction.
SELF (negative and positive) > OTHER (negative and positive).
This analysis revealed a significant dissociation in midline brain regions so that the self activated strongly the MPFC, whereas the OTHER activated the posterior cingulate cortex (PCC) more prominently (P < 0.0007, random, FDR corrected).
(SELF + OTHER) negative > (SELF + OTHER) positive.
In order to compare negative and positive events, we contrasted negative and positive trials across protagonist type. This contrast revealed activations in a few brain regions, mostly for negative events, including the MPFC, the bilateral insula, the bilateral IFG, the lateral PFC and the STS (Table 1). Selected views from this contrast are presented in Figure 2 (random, P < 0.0008, uncorrected).
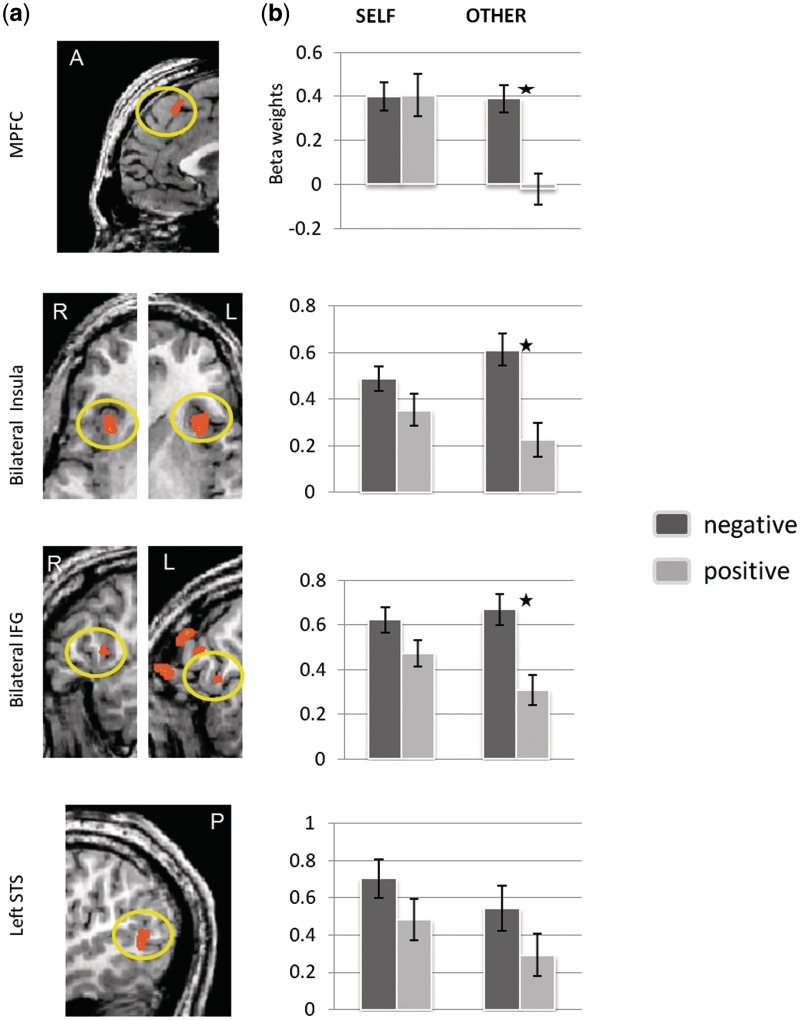
Fig. 2.
(a) ROIs derived from the negative > positive whole-brain contrast. (b) Beta weights obtained from these regions
Four a-priori defined ROIs were then analyzed in order to estimate the magnitude of activation. For these regions, a repeated measures ANOVA was conducted with protagonist (SELF vs OTHER) and valence of emotional ratings (positive vs negative) as the independent variables and the beta weights derived from the ROIs as the dependent variables. As shown in Table 2, a main effect for protagonist was found in the MPFC, the left IFG and the STS, such that SELF contributed more than OTHER to the activity in these ROIs. In addition, a main effect for emotional valence (negative > positive) was found in all of these ROIs.
Table 2.
Repeated measures ANOVA for selected ROIs
| Main effect for protagonist | Main effect for Valence | Interaction protagonist x valence | |
|---|---|---|---|
| MPFC | F = 6.6, P < 0.018 | F = 17.93, P < 0.0001 | F = 9.96, P < 0.005 |
| SELF > OTHER | Negative > positive | OTHER negative > OTHER positive, t = 5.12, P < 0.0001 | |
| Left insula | NS | F = 23.54, P < 0.0001 | F = 5.5, P < 0.029 |
| Negative > positive | OTHER negative > OTHER positive, t = 5.10, P < 0.0001 | ||
| Right insula | NS | F = 24.32, P < 0.0001 | F = 5.29, P < 0.032 |
| Negative > positive | OTHER negative > OTHER positive, t = 4.59 P < 0.0001 | ||
| Left IFG | F = 5.33, P < 0.032 | F = 16.97, P < 0.001 | NS |
| SELF > OTHER | Negative > positive | ||
| Right IFG | NS | F = 17.11, P < 0.001 | F = 6.09, P < 0.023 |
| Negative > positive | OTHER negative > OTHER positive, t = 4.53, P < 0.0001 | ||
| Left STS | F = 9.68, P < 0.006 | F = 22.87, P < 0.0001 | NS |
| SELF > OTHER | Negative > positive |
Repeated measures ANOVA analysis for the selected ROIs. Table displays main effects for protagonist and valence, and interaction effect.
NS = not significant.
Importantly, a significant valence by protagonist interaction effect was found in the MPFC, the bilateral insula and the right IFG, so that beta weights for negative events were higher than those for positive events only for the OTHER trials. Figure 2 displays these ROIs and the mean beta weights for each condition. As the right and the left IFG showed similar activities, an average of betas from the left and the right IFG was further calculated as the ‘bilateral IFG’. Similarly, betas from the left and the right insula have been merged into the ‘bilateral insula’. As shown in Figure 2, all of the ROIs selected showed sensitivity to the valence of the emotional event, so that negative events triggered higher activity than positive ones only for the OTHER trials.
Correlation between estimated activation in ROIs
The relationship between activations in the MPFC and the other ROIs was further examined. A correlation analysis with Bonferroni corrections between beta weights for the conditions of ‘OTHER negative’ and ‘OTHER positive’ was carried out. For the ‘OTHER negative’ condition, a significant correlation was found between the MPFC and the left insula (r = 0.651, P < 0.001), the left IFG (r = 0.628, P < 0.002) and the right IFG (r = 0.617, P < 0.003). For the ‘OTHER positive’ condition, a significant correlation was found between the MPFC and the left insula (r = 0.706, P < 0.0001) and the left IFG (r = 0.512, P < 0.01), indicating that a similar pattern of co-activation during positive and negative events occurring to other protagonists.
DISCUSSION
Traditionally, empathy is treated as an ability that is relevant to the negative spectrum of emotions, while empathy in response to positive events has been largely neglected. The current study sought to compare the process underlying the empathic reaction to individuals experiencing positive vs negative everyday life events. The behavioral results demonstrate for the first time that the level of emotional ratings for negative events, but not for positive events, predict empathic abilities, specifically the ability to imaging yourself in fictional situations. This implies that empathic abilities, as defined by self-reported empathy questionnaires, are more connected to negative than to positive emotional judgments.
The brain imaging demonstrates that in cases of thinking about the emotional state of oneself, the brain shows no preference for positive vs negative events. However, when making emotional judgments about the other protagonist, robust activity was found in several brain regions for negative as compared to positive emotional events. Remarkably, the regions that showed the most sensitivity to negative rather than positive events occurring to the other were regions that have been repeatedly related to empathy: the MPFC, the bilateral insula, the bilateral IFG and the STS. In particular, the role of the MPFC in the emotional judgment of the other has been reported in both neuroimaging (Fletcher et al., 1995; Goel et al., 1995; Castelli et al., 2000; Gallagher et al., 2000, 2002; Gallagher and Frith, 2003; Saxe and Kanwisher, 2003; Mitchell et al., 2005, 2006) and neuropsychological (Lough et al., 2001; Gregory et al., 2002) studies. The MPFC has also been shown to be a core region related to the self-other distinction (Lamm et al., 2010).
The insula was also found here to have more sensitivity to negative experiences occurring to the other. Indeed, it has been claimed that the insula plays a pivotal role in empathy to pain of others (Singer et al., 2004) and in sharing emotions such as disgust (Carr et al., 2003), further attesting to its necessity in empathy to negative emotions. In a recent review, the right and the left insula were reported to be a major area contributing to empathic processes (Fan et al., 2010). As for the involvement of the IFG in empathic processes, this region has been associated in particular with the mirror neuron system (Iacoboni, 2009) and with emotional empathy (Shamay-Tsoory et al., 2009). Chakrabarti et al. (2006) proposed that the IFG may constitute a biomarker for trait empathy across emotions, and Jabbi and Keysers (2008) claimed that activity in the IFG triggers anterior insula response to emotional facial expressions.
Collectively, these brain regions appear to serve as some of the building blocks of empathy. While these regions have been associated with empathy in response to negative events, a limited number of studies have addressed these regions in cases of positive emotions. Jabbi et al. (2007) reported that the IFG and the insula respond to both hunger and disgust. Hennenlotter et al. (2005) reported that a common neural basis for receptive and expressive pleasant face affect was revealed in the right premotor cortex, the IFG, the right operculum and the left insula. Moreover, the left IFG has been positively correlated with levels of empathic abilities across both positive and negative emotions (Chakrabarti et al., 2006). Here we extend the current knowledge of empathic processes and demonstrate that these regions are chiefly associated with negative, rather than positive, empathy-provoking events.
Nonetheless, the present results also demonstrate that although empathy-related regions are activated mainly for negative events, they also mediate empathy for positive events. These results support the assumption that both forms of empathy involve the same neural network. The finding that activations in the MPFC region correlate with activations in the insula and the IFG for both negative and positive events, confirms that the same set of brain regions mediates empathy in response to negative and positive emotional events. Interestingly, the present study did not find a lateralization effect with respect negative and positive emotions and does not support Davidson's model (1984) of emotional valence and brain asymmetry. It has been suggested that left prefrontal regions are particularly involved in approach-related, appetitive goals while right prefrontal regions, alternatively, are hypothesized to be particularly important in behavioral inhibition and vigilant attention that often accompanies certain aversive emotional states and traits. In the current study, both right and left regions were activated in response to positive and negative events.
Yet, despite the involvement of the same neural network in positive and negative events occurring to the other, the brain shows greater response, while empathizing with negative events experienced by the other. This remarkable reaction of the brain to negative events experienced by the other may be explained on an evolutionary level. It may be suggested that empathy has evolved in order to protect vulnerable individuals and to promote helping behaviors. For example, Panksepp (1996) has suggested that the mechanism by which a mother feels distress when perceiving the distress calls of her offspring will promote comforting behaviors that may alleviate the pain experienced by the offspring. Evolutionary evidence demonstrates that empathy carries survival value, as it could have evolved to deal with situations where there is a chance of being in a reverse situation when in need for help (Trivers, 1971). Sharing the happiness of the other has minimal implications for oneself, whereas a negative event experienced by the other may trigger helping behavior and as such has a more proximate implication for oneself. Indeed, according to the empathy-altruism hypothesis, pro-social motivation associated with feeling empathy for a person in need is directed toward the ultimate goal of benefiting that person (Batson, et al., 1991).
The overall effect of valence that was particularly evident in the judgment of the other protagonist might be also explained in terms of negativity bias. Negativity bias refers to the psychological phenomenon by which humans pay more attention to and give more weight to negative rather than positive information (Taylor, 1991). Numerous electrophysiological studies indicate that negative events elicit more rapid and more prominent responses than non-negative events and that people are especially sensitive to emotionally negative material (Huang and Luo, 2006). Nonetheless, the direct comparison between thinking about the self and about the other stresses the significance of this effect in empathic processes. Hence, the adaptive nature of negativity bias is such that empathy in response to negative events is likely to motivate specific helping behaviors, whereas there is little in the way of response warranted by the good fortune of others.
In conclusion, the present study sheds light on a very basic phenomenon of the human capacity to empathize, demonstrating that the brain reacts far more to the distress and sadness of others than to their joy.
FUNDING
Relevant research in S. Shamay-Tsoory's laboratory was supported by the Chief Scientist Office, Ministry of Health, Israel. Relevant research in T. Hendler's laboratory was supported by a grant from the University of Chicago's A New Science of Virtues Project and the John Templeton Foundation.
Conflict of Interest
None declared.
REFERENCES
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Batson CD, Batson JG, Slingsby JK, Harrell KL, Peekna HM, Todd RM. Empathic joy and the empathy-altruism hypothesis. Journal of Personality and Social Psychology. 1991;61(3):413–26. doi: 10.1037//0022-3514.61.3.413. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Dissociable systems for empathy. Novartis Foundation Symposium. 2007;278:134–41. doi: 10.1002/9780470030585.ch10. discussion 141–35, 216–121. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanisms of empathy in humans: a relay from neural systems for imitation to limbic areas. Proceedings of the National Academy of Sciences USA. 2003;100(9):5497–502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington SJ, Bailey AJ. Are there theory of mind regions in the brain? A review of the neuroimaging literature. Human Brain Mapping. 2009;30(8):2313–35. doi: 10.1002/hbm.20671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelli F, Happe F, Frith U, Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12(3):314–25. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- Chakrabarti B, Bullmore E, Baron-Cohen S. Empathizing with basic emotions: common and discrete neural substrates. Society for Neuroscience. 2006;1(3–4):364–84. doi: 10.1080/17470910601041317. [DOI] [PubMed] [Google Scholar]
- Cheon BK, Im DM, Harada T, et al. Cultural influences on neural basis of intergroup empathy. Neuroimage. 2011;57(2):642–50. doi: 10.1016/j.neuroimage.2011.04.031. [DOI] [PubMed] [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK. Implicit and explicit evaluation: FMRI correlates of valence, emotional intensity, and control in the processing of attitudes. Journal of Cognitive Neuroscience. 2004;16(10):1717–29. doi: 10.1162/0898929042947919. [DOI] [PubMed] [Google Scholar]
- Davidson RJ. Affect, Cognition and Hemispheric Specialization. New York: Cambridge University Press; 1984. [Google Scholar]
- Davis MH. Measuring individual differences in empathy: evidence for a multidimensional approach. Journal of Personality and Social Psychology. 1980;44:113–26. [Google Scholar]
- De Waal FB. Putting the altruism back into altruism: the evolution of emapthy. The Annual Review of Psychology. 2007;59(4):1–22. doi: 10.1146/annurev.psych.59.103006.093625. [DOI] [PubMed] [Google Scholar]
- de Waal FB, Leimgruber K, Greenberg AR. Giving is self-rewarding for monkeys. Proceedings of the National Academy of Sciences USA. 2008;105(36):13685–9. doi: 10.1073/pnas.0807060105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvash J, Gilam G, Ben-Ze'ev A, Hendler T, Shamay-Tsoory SG. The envious brain: the neural basis of social comparison. Human Brain Mapping. 2010;31(11):1741–50. doi: 10.1002/hbm.20972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neuroscience and Biobehavioral Reviews. 2010;35(3):903–11. doi: 10.1016/j.neubiorev.2010.10.009. [DOI] [PubMed] [Google Scholar]
- Farrow TF, Zheng Y, Wilkinson ID, et al. Investigating the functional anatomy of empathy and forgiveness. Neuroreport. 2001;12(11):2433–8. doi: 10.1097/00001756-200108080-00029. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Happe F, Frith U, et al. Other minds in the brain: a functional imaging study of "theory of mind" in story comprehension. Cognition. 1995;57(2):109–28. doi: 10.1016/0010-0277(95)00692-r. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of ‘theory of mind’. Trends in Cognitive Sciences. 2003;7(2):77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Happe F, Brunswick N, Fletcher PC, Frith U, Frith CD. Reading the mind in cartoons and stories: an fMRI study of 'theory of mind' in verbal and nonverbal tasks. Neuropsychologia. 2000;38(1):11–21. doi: 10.1016/s0028-3932(99)00053-6. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Jack AI, Roepstorff A, Frith CD. Imaging the intentional stance in a competitive game. Neuroimage. 2002;16(3 Pt 1):814–21. doi: 10.1006/nimg.2002.1117. [DOI] [PubMed] [Google Scholar]
- Goel V, Grafman J, Sadato N, Hallett M. Modeling other minds. Neuroreport. 1995;6(13):1741–6. doi: 10.1097/00001756-199509000-00009. [DOI] [PubMed] [Google Scholar]
- Gregory C, Lough S, Stone V, et al. Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer's disease: theoretical and practical implications. Brain. 2002;125(Pt 4):752–64. doi: 10.1093/brain/awf079. [DOI] [PubMed] [Google Scholar]
- Grenier F, Luthi A. Mouse brains wired for empathy? Nature Neurosciences. 2010;13(4):406–8. doi: 10.1038/nn0410-406. [DOI] [PubMed] [Google Scholar]
- Hennenlotter A, Schroeder U, Erhard P, et al. A common neural basis for receptive and expressive communication of pleasant facial affect. Neuroimage. 2005;26(2):581–91. doi: 10.1016/j.neuroimage.2005.01.057. [DOI] [PubMed] [Google Scholar]
- Huang YX, Luo YJ. Temporal course of emotional negativity bias: an ERP study. Neuroscience Letters. 2006;398(1–2):91–6. doi: 10.1016/j.neulet.2005.12.074. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Imitation, empathy, and mirror neurons. Annual Review of Psychology. 2009;60:653–70. doi: 10.1146/annurev.psych.60.110707.163604. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Keysers C. Inferior frontal gyrus activity triggers anterior insula response to emotional facial expressions. Emotion. 2008;8(6):775–80. doi: 10.1037/a0014194. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Swart M, Keysers C. Empathy for positive and negative emotions in the gustatory cortex. Neuroimage. 2007;34(4):1744–53. doi: 10.1016/j.neuroimage.2006.10.032. [DOI] [PubMed] [Google Scholar]
- Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54(3):2492–502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- Lamm C, Meltzoff AN, Decety J. How do we empathize with someone who is not like us? A functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2010;22(2):362–76. doi: 10.1162/jocn.2009.21186. [DOI] [PubMed] [Google Scholar]
- Langford DJ, Crager SE, Shehzad Z, Smith SB, Sotocinal SG, Levenstadt JS, et al. Social modulation of pain as evidence for empathy in mice. Science. 2006;312(5782):1967–70. doi: 10.1126/science.1128322. [DOI] [PubMed] [Google Scholar]
- Lee KH, Siegle GJ. Common and distinct brain networks underlying explicit emotional evaluation: a meta-analytic study. Social Cognitive and Affective Neuroscience. 2009 doi: 10.1093/scan/nsp001. doi:10.1093/scan/nsp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lough S, Gregory C, Hodges JR. Dissociation of social cognition and executive function in frontal variant frontotemporal dementia. Neurocase. 2001;7(2):123–30. doi: 10.1093/neucas/7.2.123. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. The link between social cognition and self-referential thought in the medial prefrontal cortex. Journal of Cognitive Neuroscience. 2005;17(8):1306–15. doi: 10.1162/0898929055002418. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50(4):655–63. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Panksepp J. Advances in Biological Psychiatry. Vol. 2. Greenwich CT: JAI Press; 1996. [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Rozin P, Royzman EB. Negativity bias, negativity dominance, and contagion. Personality and Social Psychology Review. 2001;5(4):296–320. [Google Scholar]
- Saxe R. Uniquely human social cognition. Current Opinion in Neurobiology. 2006;16(2):235–9. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage. 2003;19(4):1835–42. doi: 10.1016/s1053-8119(03)00230-1. [DOI] [PubMed] [Google Scholar]
- Schulte-Ruther M, Markowitsch HJ, Fink GR, Piefke M. Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: a functional magnetic resonance imaging approach to empathy. Journal of Cognitive Neuroscience. 2007;19(8):1354–72. doi: 10.1162/jocn.2007.19.8.1354. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132(Pt 3):617–27. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- Singer T, Lamm C. The social neuroscience of empathy. Annals of the New York Academy of Sciences. 2009;1156:81–96. doi: 10.1111/j.1749-6632.2009.04418.x. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303(5661):1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Singer T. The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neurosci Biobehav Rev. 2006;30(6):855–63. doi: 10.1016/j.neubiorev.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Tamir DI, Mitchell JP. Neural correlates of anchoring-and-adjustment during mentalizing. Proceedings of the National Academy of Sciences USA. 2010;107(24):10827–32. doi: 10.1073/pnas.1003242107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE. Asymmetrical effects of positive and negative events: The mobilization-minimization hypothesis. Psychological Bulletin. 1991;110:67–85. doi: 10.1037/0033-2909.110.1.67. [DOI] [PubMed] [Google Scholar]
- Trivers RL. The Evolution of Reciprocal Altruism. The Quarterly Review of Biology. 1971;46(1):35–57. [Google Scholar]
- Xu X, Zuo X, Wang X, Han S. Do you feel my pain? Racial group membership modulates empathic neural responses. Journal of Neuroscience. 2009;29(26):8525–9. doi: 10.1523/JNEUROSCI.2418-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]