Abstract
Arterial vasoconstriction is an important physiological process in regulating blood pressure, and is involved in pathologies. The isolation of arteries from rats and mice, as well as the measurement of vascular tension in an ex vivo preparation, are important methods in studying the physiology of arteries and the pathophysiology associated with arterials. Three major methods to measure vascular tension are organ bath, wire myograph, and pressurized arterial myograph. The major method to measure vascular remodeling is by observing the zero-stress state of an artery.
Introduction
Arterial tone, one of the principal parameters of arterial function and regulation of blood pressure, is determined by the function of smooth muscle and the endothelium. Measuring contraction and relaxation in isolated arteries utilizes a range of methods that include organ bath as well as wire myography and pressurized diameter measurement system. This allows investigators to study physiological and pharmacological properties of arteries. Therefore, the ability to measure contraction and relaxation in isolated arteries is of great importance. Commonly used materials in tension experiments include strips, rings, and cannulated perfused vessels isolated from mouse and rat arteries. Preparations of isolated arteries enable one to study vascular function independent of systemic influences, which are present in in vivo preparations.
The most common animal models used for pulmonary hypertension are rats and mice with chronic hypoxia-induced pulmonary hypertension or monocrotaline-mediated pulmonary hypertension. It has to be noted, however, that differences of hemodynamics, histological (or structural) and functional changes in the pulmonary vasculature and right ventricle and pharmacological responses exist between patients with pulmonary hypertension and the animal models (rats and mice) of pulmonary hypertension.. Chronic hypoxia-induced pulmonary hypertension in mice, for example, causes minimal vascular remodeling, although pulmonary arterial pressure and pulmonary vascular resistance are both increased. In humans, however, pulmonary vascular remodeling, characterized by the intimal and medial thickening, is the major caused for the increased pulmonary vascular resistance [1]. In humans, adventitial thickening and fibrosis occur in the distal pulmonary arteries. However, in a study by Estrada and Chesler [2], a mouse model revealed that adventitial thickening and fibrosis occured in the proximal pulmonary arteries. In spite of this discrepancy, however, the use of chronically hypoxic rats and genetically modified mice will still provide significant insights into the pathogenic mechanisms of pulmonary hypertension that can be utilized to guide research for developing more effective and efficient therapeutic approaches for patients with pulmonary hypertension.
To facilitate future studies, we will describe methodologies for the isolation of pulmonary arteries from mice and rats, measurement of tension in isolated arteries, pharmacological modulation of contractility in isolated arteries, and other factors influencing contraction.
Isolation of pulmonary artery
Identifying the pulmonary artery for isolation
The rat and mouse lung consist of a single left lobe and four right lobes, which are called the cranial, medial, caudal, and postcaval lobes. Generally, the pulmonary artery is isolated from the left lobe due to the relative ease of working with a single, large lobe. The main pulmonary artery, originating from the right ventricle, splits into the left and right lobes of the lung. It then further splits into progressively smaller diameter vessels such as arterioles and capillaries as they extend to the end of lobe. It should be noted that, since the pulmonary artery and bronchial arteries run in parallel with one another, one should be careful to distinguish between the two. In general, the bronchial arterial wall consists of a thicker layer of smooth muscle cells compared to the pulmonary arterial wall. The isolated pulmonary artery from a mouse lung is shown in Figure 1A and the isolated pulmonary artery from a rat lung is shown in Figure 1B [3].
Figure 1.
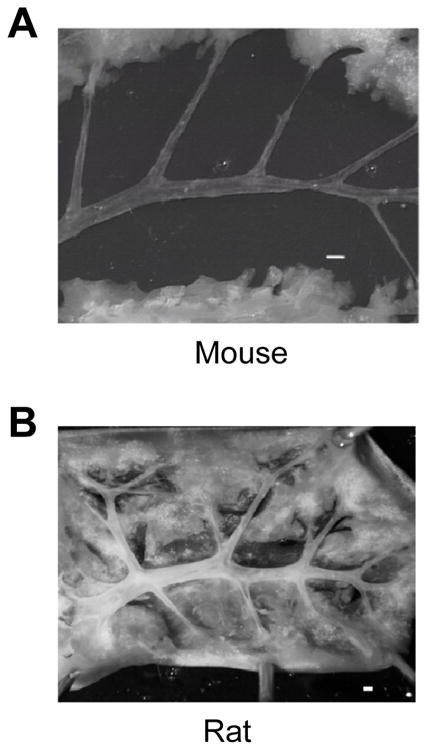
Image shows the isolated pulmonary artery. The pulmonary artery isolated from the left lobe in a 7–8 week old male, Balb C mouse; image from Nikon microscope (A). The pulmonary artery isolated from 8 week old male, SD rat; image from Nikon D-80 camera (B). Scale bar represents 500 μm in A and B.
Dissection and preparation of pulmonary arteries
The lung is removed from the chest cavity and washed with a physiological salt solution (PSS, pH 7.4) containing 138 mM NaCl, 4.7 mM KCl, 1.2 mM NH2PO4, 1.2 mM MgSO4, 1.8 mM CaCl2, 5 mM HEPES, and 10 mM glucose. The solution is oxygenated with 100% O2. The lung is positioned toward the ventral view and carefully stretched and pinned onto a dissecting dish, coated with a layer of silicone plastic (Sylgard), and placed under a light microscope. During the dissection, the PSS should be replaced frequently in the dissecting dish to allow for a better visualization of the arteries since lung tissues contain many air bubbles. When the superficial tissue of the lung is dissected away with fine micro-scissors, the bronchial artery is first seen lying above the pulmonary artery. Therefore, the bronchus artery needs to be carefully removed without damaging the pulmonary artery. The main pulmonary artery branches into left and right branches (extrapulmonary artery) which further branch into resistance arteries known as intrapulmonary arteries. They are named based on the order that they branch from the main pulmonary artery. Fourth or fifth order intralobar (intrapulmonary) branches (150-300 μm) for rats and second or third order branches (<200 μm) for mice are used in contraction experiments and carefully dissected away from connective tissues. The adventitia is carefully removed from isolated arteries under a high-magnification microscope. Extreme care should be taken to avoid stretching of the artery as it is detrimental to the experiment.
To mount the vessel as a ring preparation, the cleaned pulmonary artery should be cut into 1.5-2 mm ring segments. For the vascular strip preparation, helically cut the vascular wall in a spiral orientation. The vascular strip preparation has smooth muscle cells whose long axis is parallel to the strip so vascular contraction can develop more fully [4]. One must be careful that the orientation of the smooth muscle cells within the different strips be identical between vessels.
Cannulation of the vessel requires a segment length long enough to allow this. One should use vessel segments free of branches. However, this is often difficult as the pulmonary artery contains many branches. For that reason, this method is not commonly used by most investigators to measure resistance pulmonary arterial tone. More common methods include the use of an organ bath or wire myograph to measure isometric tension in isolated pulmonary arteries.
Arteries are composed of three layers. The inner layer (intima) is composed of single, flattened cells called endothelial cells, which cover the inner surface of the artery. In order to use endothelium-denuded arteries, it is necessary to carefully remove endothelium while avoiding damaging the smooth muscle cells. Removal of the endothelium from isolated vessels is generally accomplished by mechanical rubbing or air perfusion.
The vessel is cut open longitudinally so the endothelial layer at the intimal surface can be gently rubbed with a stainless steel wire. Alternatively, endothelial cells are disrupted by perfusing an air bolus through the lumen of the vessels. However, Liu et al. showed the loss of the myogenic response following removal of the endothelium via air perfusion [5]. Thus, air perfusion should not be used to evaluate the myogenic response. A successful removal of the arterial endothelium is verified functionally by the loss of the relaxation response to acetylcholine.
To study an endothelium-intact artery, one needs to prepare the pulmonary artery more carefully from connective tissue because the endothelium is easy to damage from the mechanical stretch induced during the process of isolation, mounting wire, or cannulation. To study endothelium-dependent relaxation, isolated pulmonary arteries are preconstricted with the contractile agonist, phenylephrine, typically at a concentration of 10−6 M. Cumulative relaxation curves of acetylcholine (10−9 to 10−5 M) are then constructed. Besides the pharmacological challenge to determine the presence of endothelium, histological staining can show intact endothelium and smooth muscle at the end of an experiment. In a previous study, it showed stained endothelial cells and smooth muscle cells using lectin and α-actin to show presence of endothelial cells from mouse pulmonary artery [6]. The pulmonary artery rings were fixed overnight in 10% neutral buffered formalin after contraction experiments. The vessel tissues were embedded in paraffin blocks in an automatic tissue processor (Sakura Tissue-Tek VIP; Sakura Finetek). The paraffin-embedded rings were serially sectioned at a thickness of 5 μm for staining with either lectin, for endothelium, or α-actin, for smooth muscle. The cells were visualized with a fluorescent microscope. The image confirmed that the vessel wall was preserved and had a distinct intraluminal wall composed of an endothelial cell monolayer (Figure 2A). Extracellular application of bradykinin, an endothelium-dependent vasodilator, significantly reduced active tension in pulmonary arterial rings pre-constricted with 40 mM K+ (Figure 2B and C).
Figure 2.
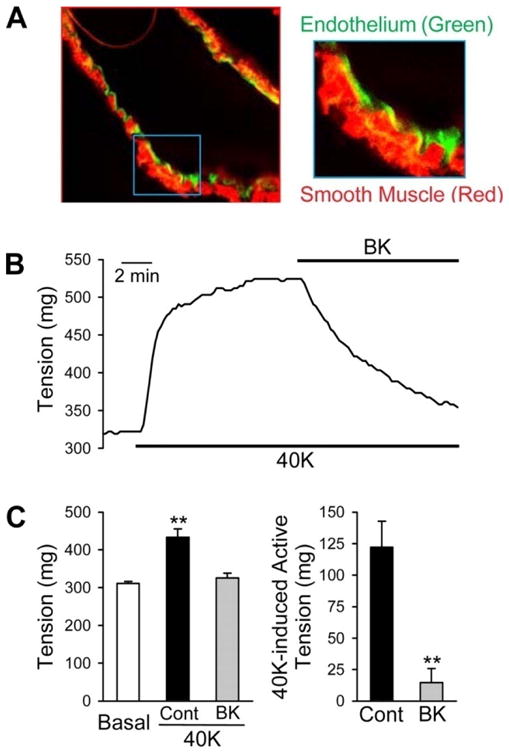
Bradykinin (BK) causes vasodilation in an endothelium-intact mouse pulmonary artery. A fluorescence image of a pulmonary artery segment used for the contraction experiments (A). Pulmonary arterial smooth muscle was stained in red with smooth muscle α-actin, while pulmonary artery endothelium was stained in green with lectin. Representative tension record showing 40K-induced active tension before and during the application of 20 μM BK (B). Summarized data (mean ± SE) showing absolute tension before (basal) and after application of 40K in the absence and presence (BK) of BK (left; n = 4), and 40K-induced active tension with or without BK treatment (right; n = 4) (C). **P < 0.01 vs. basal or 40K-BK.
Measurement of tension
Organ bath (ring or strip-mounted), wire (ring-mounted) myograph, and pressure myograph (pressurized vessel-mounted) are three common methods for studying functional responses and vascular reactivity in isolated arteries. In addition, the measurement of zero-stress in a pulmonary artery may be a useful tool to investigate the remodeling of the arterial wall. One should choose a particular method for studying vascular function based on the diameter of arteries, types of arteries, and purpose of experiments. Next, we will describe the technique of tension measurement and its application.
Organ bath
The organ bath system is a traditional method used to investigate the physiology and pharmacology of isolated vessels. Isotonic or isometric measurements can be made with appropriate transducers. An isometric measurement is most commonly used to measure vascular contractility as the vessel length remains constant.
In an isotonic measurement, the vessel length is shortened by force changes and is thus suitable to measure shortening velocities in vessels [7]. The organ bath, with isometric transducers, is commonly used to monitor tension in small tissue strips and rings (100 μm diameter) to large preparations.
Appropriate hooks or clips are selected depending on the diameter of the vessel. Ring segments are cut and two tungsten hooks are carefully passed through the lumen of pulmonary arterial rings in an organ bath containing PSS (Figure 3A). The bathing media is maintained at 37°C and is continuously aerated with 100% O2. One hook is attached to a displacement device, which allows the investigator to manually adjust the tension of the vessel, and the other hook is connected to an isometric force transducer [6]. The degree of adjustment of resting tension can affect vascular reactivity in response to a pharmacological agonist. Therefore, one needs to set the optimal resting tension. This is accomplished through a preliminary experiment to determine the maximum contraction induced by contractile agents such as high potassium chloride or norepinephrine. To set the optimal resting tension, the pulmonary arterial ring is stretched to different tensions and a contraction is elicited by pharmacological treatment at each tension. The tension which exhibits the largest contractile response is the optimal resting tension. In a previous study, the optimal resting (or basal) tension (300 mg) was determined, showing that active tension was significantly developed with exposure to the 40 mM K+ solution as the increasing the basal tension from 100 to 300 mg in isolated mouse pulmonary arterial rings [8]. For the rat pulmonary artery, the maximal tension developed the resting passive tension of 600-625 mg when the pulmonary arterial rings were exposed to the 40 mM K+ solution [9] with use of an isometric force transducer (Harvard Apparatus). Once at their optimal tension, the pulmonary arterial rings are allowed to further equilibrate for 30 minutes before the experiment. In all experiments, changes in the isometric force are measured with a force-displacement transducer connected to a carrier amplifier and then recorded on a pen recorder. After each experiment, the rings are weighed, and the active tension (mg) induced by agonists is normalized by wet tissue weight and expressed as milligram tension per milligram wet tissue weight (mg/mg).
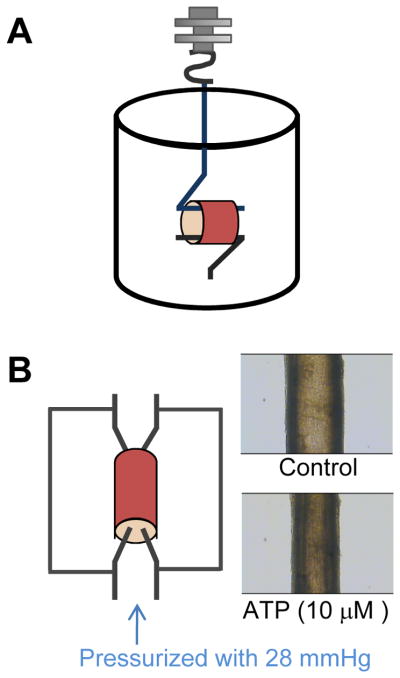
Figure 3.
A schematic diagram of a tension measurement. Organ chamber(A) and pressurized arterial myograph (B). The rabbit pulmonary artery was pressurized with 28 mmHg. ATP (10 μM) caused vasoconstriction in rabbit pulmonary artery.
Wire myograph
Wire myograph is a useful technique to examine vascular function and the reactivity of resistance arteries with an internal diameter as small as 60 μm, based on isometric tension recordings [10]. Ring preparations of small arteries with internal diameters between 100–500 μm can be mounted on 40 μm stainless steel wires. However, one should use 25 3m tungsten wires for arteries with an internal diameter around 60 3m (Danish Myotechnology). Two mounting wires are threaded through the isolated vessel lumen (ring preparation) and secured to two supports. One support is attached to a micrometer for the adjustment of vessel circumference and application of tension. The other support is attached to an isometric transducer. Following mounting and equilibration, the ring is normalized to an initial active wall tension. The normalization of wire-mounted arteries makes it important to set the preparation to an internal circumference that gives a maximal response and standardizes experiments. To determine the passive exponential wall tension-internal circumference (L) relationship, the artery is allowed to stretch at 1 minute intervals. The method involved calculation of an equivalent radial distending pressure from the measured arterial circumference and the radial force derived from the Laplace equation: Effective pressure=wall tension/internal radius. According to the normalization procedure in [11], the maximum force was developed when the vessel was set at 90% of the internal circumference it would achieve under an intraluminal pressure of 100 mmHg. The vessel set to L0, where L0 = 0.9 × L100. In order to standardize the lumen diameter, the cross sectional area of the media of the mounted arteries (A) is calculated using the media thickness (m) and the circumference of vessels (L) based on the following equation: . Then, by using L0 and A, and assuming a constant media volume, the standardized media thickness of blood vessels (at L0) is calculated. The lumen diameter is defined as L0/π [12].
Pressurized arterial myograph
The pressurized arterial system allows the vessels to be closer to their in vivo condition and is used to study vascular function in physiology and patho-physiology states. It has been developed for use with resistant arteries (diameter >60 3m), and contractility is assessed by the changes in luminal diameter [13]. The isolated arteries are connected with perfusing cannulae which allow for the maintenance of intraluminal pressure that more closely models the physiology. The perfusing cannula (glass pipette) can be made to match the vessel lumen diameter using a model PP-83 vertical puller (Narishige, Tokyo, Japan). One cannula is in a fixed position while the other cannula is movable for the fine adjustment of longitudinal artery tension. Before the other side of artery is connected to the movable cannula, the artery is gently flushed to remove any blood and air bubbles from inside the vessel. All the side branches of pulmonary arteries are tied off using sutures to prevent leakage of intra-perfusing solution. A pressure transducer is used to maintain the appropriate pressure. In a previous report, they measured vessel diameter, pressurized to 12 mmHg, from the fourth order intrapulmonary artery of a rat with an inner diameter of 150 to 300 μm [14]. Outer flow in the bath chamber is maintained using a peristaltic pump. The temperature of the vessel environment is adjusted to 37°C and bubbled with oxygen. Internal lumen diameter and medial thickness of the vessel can be measured by a video camera attached to an imaging system (Figure 3B).
Zero-stress state of pulmonary artery
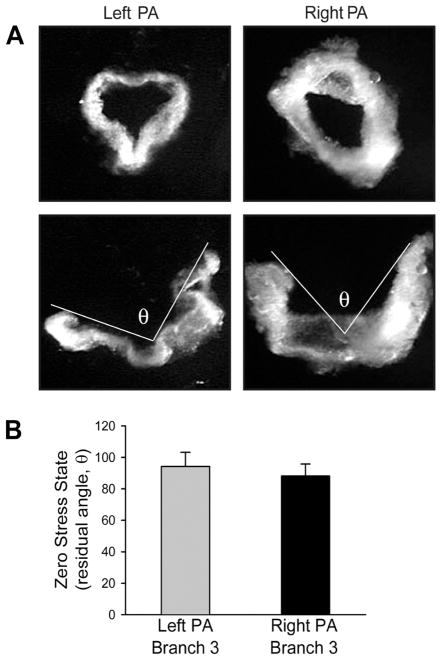
Vascular remodeling is mainly caused by excessive growth and proliferation of smooth muscle cells in the media and fibroblasts in the extracellular matrix, which also results in changes in the zero-stress state of the vessel. Therefore, to observe and measure changes in the zero-stress state is a valuable tool in determining vascular remodeling [15,16]. The zero-stress state is the stress-free configuration obtained by cutting vessel rings. When the rings are cut radially, the arterial wall will open at a specific angle [17]. The opening angle (θ) is the angle defined by two radii drawn from the midpoint of the inner wall to the tips of the inner wall of the open vessel ring. The physiological meaning of the opening angle can be defined as the residual strains in an artery at the no-load state and homeostatic strains at normal blood pressure [15]. Previous studies have demonstrated that the opening angle at the zero-stress state varies with non-uniform remodeling of the artery and arterial wall in hypertension, smoke exposure, diabetes, and aging [15,16,18-20]. Xu et al. compared pulmonary arterial rings (third- to fourth-order) isolated from left and right lungs with zero-stress state [6]. It was seen that left and right pulmonary arterial ring-shaped segments opened up into a sector (Figure 4). They found that the opening angles at the zero-stress states did not show a statistically significant difference between the left and right pulmonary artery. Conversely, the other study reported that the opening angles at the zero-stress states vary with the location and size of the pulmonary artery from a human [21].
Figure 4.
Comparative residual angle or zero-stress state in pulmonary artery (PA) rings isolated from left and right lungs. At no-load state (top), the internal pressure, external pressure, and longitudinal stress in a short-ring-shaped segment of the left and right PA (A). When the rings are cut longitudinally, the zero-stress state (represented as the residual angle θ subtended between two lines originating from midpoint tips of the inner wall) is increased. Summarized θ values (n = 6) obtained for third branches of left and right PA (B). The residual angles are not statistically different between the left and right PA.
Vessel viability
After an equilibration period, it is necessary to test vascular contractibility and obtain a stable contractile response using various vasoconstrictors such as an increased concentration of potassium (NaCl replaced by KCl on a molar basis) or norepinephrine (10−6 M). This is done in order to have a baseline measurement of the viability of each individual artery so that one can later normalize experimental data as a percent of norepinephrine induced contraction, since the contractile responses may vary with vessel size and across species. Isolated vessels are considered viable if they contract more than 40% of their resting tone, in response to a contractile agent. If the artery does not contract appropriately, then either the optimal basal tension/lumen pressure has not been properly adjusted or the artery may have been damaged during isolation or mounting of the vessel.
Pharmacological characteristic of vascular tension
Endothelium-denuded vascular contraction
Contraction in vascular smooth muscle can be initiated by stretch/intraluminal pressure, membrane potential changes, or a pharmacological stimulus. Active force development can be caused by the passive stretch of smooth muscle and is called a myogenic response. A myogenic response keeps the vascular tension constant during an increase in intraluminal pressure [5].
Membrane depolarization of vascular smooth muscle cells can also elicit a contraction by activating voltage-dependent calcium channels (L-type calcium channels) in smooth muscle cells. This causes an increase of Ca2+ sensitivity on the contractile machinery or Ca2+ release from the sarcoplasmic reticulum [22,23]. For example, exposure of smooth muscle cells to high potassium causes membrane depolarization, thus stimulating Ca2+ influx though Ca2+ channels and resulting in contraction.
Regulation of vascular smooth muscle contraction can be modified by various pharmacological agents such as norepinepherine, angiotensin II, vasopressin, endothelin-1, serotonin, and thromboxane A2 6,24-28]. Each of these substances bind to their own receptors on the vascular smooth muscle cell or endothelium. These agents cause elevation in cytoplasmic calcium concentration through extracellular Ca2+ entry or Ca2+ release from intracellular stores, resulting in vascular contraction.
Endothelium-intact vascular contraction
Endothelial dysfunction is regarded as an early, key event in the development of vascular pathology [29]. A vital and quantifiable feature of endothelial dysfunction is the inability of vasodilation to occur in response to an appropriate stimulus, such as acetylcholine. The vascular endothelium releases endothelium-derived relaxing factors such as nitric oxide (NO), which promotes vascular relaxation by stimulating the production of guanylate cyclase and increasing the production of cGMP in smooth muscle, resulting in smooth muscle relaxation [30]. To evaluate NO viability, acetylcholine concentration-response curves are repeated after 30 minutes of incubation with the nitric oxide synthase inhibitor, N-nitro-L-arginine methyl ester (L-NAME, 10−4 M) [31], or the guanylyl cyclase inhibitor, 1H-[1, 2, 4]oxadiazolo[4, 3-alpha]quinoxalin-1-one (ODQ, 10−5 M) [32].
Factors influencing contractile activity
Many elements are important in improving the contractile vascular activity in one’s tension experiments. These elements include: 1) dissection skills, 2) optimal resting tension, 3) resistant artery, 4) age of animals, 5) buffer solution, 6) temperature of dissecting medium, and 7) isolation time (Table 1).
Table 1.
Factors influencing contractile activity
| Methodological Parameters | Conditions to Improve Vascular Activity | |
|---|---|---|
| 1 | Dissection skills | Grab/hold the connective tissue with forceps and avoid stretching the vessel |
| 2 | Optimal resting tension | Preliminary experiment required to determine optimal resting tension |
| 3 | Using resistance arteries | 2nd or 3rd branch for mouse and 4th or 5th branch for rat |
| 4 | Age of animals | Adult age (7–8 weeks for mouse and rat) |
| 5 | PSS buffer solution | Continuous aeration with 100% O2 and adjust the pH of solutions at the experimental temperature |
| 6 | Temperature of dissection medium | Dissecting in chilled solution or in a container of ice to slow down metabolic processes |
| 7 | Isolation time | As fast as you can (within 30 minutes) |
The first methodological parameter to consider is a way to dissect the artery from its connective tissues. Proper care should be taken when using fine forceps to grasp only the connective tissue surrounding the vessel to avoid damaging and stretching the smooth muscle.
Secondly, the optimal resting tension must be determined accurately. Since the optimal resting tension depends on the vessel size, species, and type of artery, it should be determined before the experiment. The degree of initial preload applied to the vessel is the most critical factor in affecting vascular responses during the experiment. Regarding this aspect, previous studies have been reported that agonist-stimulated release of EDRF [33] and endothelium-dependent relaxation influenced by the initial stretch [34].
Thirdly, the size of isolated arteries is important because large conducting arteries contribute little to pulmonary vascular resistance. Resistance arteries should be isolated since they regulate pressure and flow. For instance, primary pulmonary hypertension occurs due to the constriction of small arteries, which then increases the resistance to blood flow through the lung [35]. Even though it is technically difficult to isolate resistance arteries, it is important to use them to study vascular function.
Next, the age of the animal can influence vascular function. It has been revealed that aging is associated with structural changes such as an increase in smooth muscle cell myofilament volume density [36]. In addition, the functional response of arteries can be affected by the age of animals. For example, the β adrenoceptor-mediated maximal relaxation has declined progressively as the age of the rats increased [37]. A previous report also suggested that the endothelium-mediated dilator response to acetylcholine was negligible at birth, but develops rapidly after birth, with a maximal effect at 3–10 days, and then declines gradually as one approaches adulthood. Also, it was observed that endothelium-independent dilator responses to sodium nitroprusside (SNP) were increased progressively with age in pulmonary artery [38]. Therefore, the age of the animals should be considered when designing experiments. In general, adult animals (7–8 weeks), weighing 300–500 grams for rats and 20–40 grams for mice, are used.
The fifth point to consider is the buffer solution chosen for the dissection and perfusing bath during the experiments as it affects the viability of the arteries. Alkalosis and acidosis, both, have a critical influence on pulmonary artery viability. Bicarbonate buffer is effective in buffering acids and bases, but it requires aeration with carbon dioxide (95% O2, 5% CO2) to maintain its pH. Alternatively, the artificial buffer, PSS, may be used as a buffer solution instead of bicarbonate solution by replacing the NaCO3 with HEPES, neutralized to pH 7.4 with NaOH, and aerated with 100% O2 [39]. For PSS, the pH should be adjusted at the experimental temperature because the pKa value of the artificial buffer is decreased with increases in temperature [40].
The sixth methodological parameter to consider is the temperature of the dissecting media. Low temperatures help to preserve the isolated tissue by slowing down degradation. Therefore, the dissection should be undertaken using chilled media (4°C).
Lastly, dissection and preparation of an isolated pulmonary artery should be undertaken as rapidly as possible.
Summary
Measurement of vascular contraction is an extremely useful tool for investigating physiological responses in normal conditions or abnormalities in pulmonary vascular disease. Several methods, with different scientific meanings and criteria for use, are available to measure vascular contraction and determine vascular remodeling. These methods include organ bath, wire myograph, pressurized artery myograph, and the zero-stress state of an artery. Pressurized artery myograph is more physiologically-based, but requires use of a non-branched pulmonary artery or, in the case of branches, the seal of all branches. When using the wire myograph technique, vessel leakage is not a concern. This review highlights the methodologies used for the preparation of the pulmonary artery and determination of its vascular function.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (DK083506, HL066012 and HL098053).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dempsey EC, et al. Neprilysin null mice develop exaggerated pulmonary vascular remodeling in response to chronic hypoxia. Am J Pathol. 2009;174:782–796. doi: 10.2353/ajpath.2009.080345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Estrada KD, Chesler NC. Collagen-related gene and protein expression changes in the lung in response to chronic hypoxia. Biomech Model Mechanobiol. 2009;8:263–272. doi: 10.1007/s10237-008-0133-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ko EA, et al. Functional characterization of voltage-gated K+ channels in mouse pulmonary artery smooth muscle cells. Am J Physiol Cell Physiol. 2007;293:928–937. doi: 10.1152/ajpcell.00101.2007. [DOI] [PubMed] [Google Scholar]
- 4.Herlihy JT. Helically cut vascular strip preparation: geometrical considerations. Am J Physiol. 1980;238:107–109. doi: 10.1152/ajpheart.1980.238.1.H107. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, et al. Myogenic activation of canine small renal arteries after nonchemical removal of the endothelium. Am J Physiol. 1994;267:302–307. doi: 10.1152/ajpheart.1994.267.1.H302. [DOI] [PubMed] [Google Scholar]
- 6.Xu M, et al. Characterization of agonist-induced vasoconstriction in mouse pulmonary artery. Am J Physiol Heart Circ Physiol. 2008;294:220–228. doi: 10.1152/ajpheart.00968.2007. [DOI] [PubMed] [Google Scholar]
- 7.Kong SK, Stephens NL. Mechanical properties of pulmonary arteries from sensitized dogs. J Appl Physiol. 1983;55:1669–1673. doi: 10.1152/jappl.1983.55.6.1669. [DOI] [PubMed] [Google Scholar]
- 8.Xu M, et al. p75 neurotrophin receptor regulates agonist-induced pulmonary vasoconstriction. Am J Physiol Heart Circ Physiol. 2008;295:1529–1538. doi: 10.1152/ajpheart.00115.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunichika N, et al. Overexpression of TRPC1 enhances pulmonary vasoconstriction induced by capacitative Ca2+ entry. Am J Physiol Lung Cell Mol Physiol. 2004;287:962–969. doi: 10.1152/ajplung.00452.2003. [DOI] [PubMed] [Google Scholar]
- 10.Mulvany MJ, Halpern W. Mechanical properties of vascular smooth muscle cells in situ. Nature. 1976;260:617–619. doi: 10.1038/260617a0. [DOI] [PubMed] [Google Scholar]
- 11.Mulvany MJ, Halpern W. Contractile properties of small arterial resistance vessels in spontaneously hypertensive and normotensive rats. Circ Res. 1977;41:19–26. doi: 10.1161/01.res.41.1.19. [DOI] [PubMed] [Google Scholar]
- 12.Li JS, Schiffrin EL. Effect of chronic treatment of adult spontaneously hypertensive rats with an endothelin receptor antagonist. Hypertension. 1995;25:495–500. doi: 10.1161/01.hyp.25.4.495. [DOI] [PubMed] [Google Scholar]
- 13.Duling BR, et al. Methods for isolation, cannulation, and in vitro study of single microvessels. Am J Physiol. 1981;241:108–116. doi: 10.1152/ajpheart.1981.241.1.H108. [DOI] [PubMed] [Google Scholar]
- 14.Jernigan NL, et al. Chronic hypoxia augments protein kinase G-mediated Ca2+ desensitization in pulmonary vascular smooth muscle through inhibition of RhoA/Rho kinase signaling. Am J Physiol Lung Cell Mol Physiol. 2004;287:1220–1229. doi: 10.1152/ajplung.00196.2004. [DOI] [PubMed] [Google Scholar]
- 15.Fung YC, Liu SQ. Change of residual strains in arteries due to hypertrophy caused by aortic constriction. Circ Res. 1989;65:1340–1349. doi: 10.1161/01.res.65.5.1340. [DOI] [PubMed] [Google Scholar]
- 16.Fung YC, Liu SQ. Changes of zero-stress state of rat pulmonary arteries in hypoxic hypertension. J Appl Physiol. 1991;70:2455–2470. doi: 10.1152/jappl.1991.70.6.2455. [DOI] [PubMed] [Google Scholar]
- 17.Liu SQ, Fung YC. Zero-stress states of arteries. J Biomech Eng. 1988;110:82–84. doi: 10.1115/1.3108410. [DOI] [PubMed] [Google Scholar]
- 18.Liu SQ, Fung YC. Influence of STZ-induced diabetes on zero-stress states of rat pulmonary and systemic arteries. Diabetes. 1992;41:136–146. doi: 10.2337/diab.41.2.136. [DOI] [PubMed] [Google Scholar]
- 19.Liu SQ, Fung YC. Material coefficients of the strain energy function of pulmonary arteries in normal and cigarette smoke-exposed rats. J Biomech. 1993;26:1261–1269. doi: 10.1016/0021-9290(93)90350-n. [DOI] [PubMed] [Google Scholar]
- 20.Saini A, et al. Effect of age and sex on residual stress in the aorta. J Vasc Res. 1995;32:398–405. doi: 10.1159/000159115. [DOI] [PubMed] [Google Scholar]
- 21.Huang W, Yen RT. Zero-stress states of human pulmonary arteries and veins. J Appl Physiol. 1998;85:867–873. doi: 10.1152/jappl.1998.85.3.867. [DOI] [PubMed] [Google Scholar]
- 22.Yuan XJ. Voltage-gated K+ currents regulate resting membrane potential and [Ca2+]i in pulmonary arterial myocytes. Circ Res. 1995;77:370–378. doi: 10.1161/01.res.77.2.370. [DOI] [PubMed] [Google Scholar]
- 23.Nelson MT, Quayle JM. Physiological roles and properties of potassium channels in arterial smooth muscle. Am J Physiol. 1995;268:799–822. doi: 10.1152/ajpcell.1995.268.4.C799. [DOI] [PubMed] [Google Scholar]
- 24.Cogolludo A, et al. Thromboxane A2-induced inhibition of voltage-gated K+ channels and pulmonary vasoconstriction: role of protein kinase Czeta. Circ Res. 2003;93:656–663. doi: 10.1161/01.RES.0000095245.97945.FE. [DOI] [PubMed] [Google Scholar]
- 25.Ko EA, et al. Pathophysiology of voltage-gated K+ channels in vascular smooth muscle cells: modulation by protein kinases. Prog Biophys Mol Biol. 103:95–101. doi: 10.1016/j.pbiomolbio.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 26.Ko EA, et al. Endothelin-1 increases intracellular Ca2+ in rabbit pulmonary artery smooth muscle cells through phospholipase C. Am J Physiol Heart Circ Physiol. 2005;289:1551–1559. doi: 10.1152/ajpheart.00131.2005. [DOI] [PubMed] [Google Scholar]
- 27.Oriowo MA, et al. Alpha 1-adrenoceptor subtypes mediating noradrenaline-induced contraction of pulmonary artery from pulmonary hypertensive rats. Eur J Pharmacol. 2003;482:255–263. doi: 10.1016/j.ejphar.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Tamaoki J, et al. Angiotensin II 1 receptor-mediated contraction of pulmonary artery and its modulation by prolylcarboxypeptidase. J Appl Physiol. 1994;76:1439–1444. doi: 10.1152/jappl.1994.76.4.1439. [DOI] [PubMed] [Google Scholar]
- 29.Vapaatalo H, et al. Role of endothelium and nitric oxide in experimental hypertension. Physiol Res. 2000;49:1–10. [PubMed] [Google Scholar]
- 30.Furchgott RF, Vanhoutte PM. Endothelium-derived relaxing and contracting factors. FASEB J. 1989;3:2007–2018. [PubMed] [Google Scholar]
- 31.Gruetter CA, et al. Relationship between cyclic guanosine 3':5'-monophosphate formation and relaxation of coronary arterial smooth muscle by glyceryl trinitrate, nitroprusside, nitrite and nitric oxide: effects of methylene blue and methemoglobin. J Pharmacol Exp Ther. 1981;219:181–186. [PubMed] [Google Scholar]
- 32.Hussain AS, et al. The soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3-alpha]quinoxalin-1-one (ODQ) inhibits relaxation of rabbit aortic rings induced by carbon monoxide, nitric oxide, and glyceryl trinitrate. Can J Physiol Pharmacol. 1997;75:1034–1037. [PubMed] [Google Scholar]
- 33.Dainty IA, et al. The influence of the initial stretch and the agonist-induced tone on the effect of basal and stimulated release of EDRF. Br J Pharmacol. 1990;100:767–773. doi: 10.1111/j.1476-5381.1990.tb14090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sekiguchi F, et al. Spontaneous and agonist-induced contractions and endothelium-dependent relaxation in aortae from SHRSP and WKY rats under various levels of passive force. Clin Exp Pharmacol Physiol. 1996;23:483–489. doi: 10.1111/j.1440-1681.1996.tb02765.x. [DOI] [PubMed] [Google Scholar]
- 35.McLaughlin VV, et al. Reduction in pulmonary vascular resistance with long-term epoprostenol (prostacyclin) therapy in primary pulmonary hypertension. N Engl J Med. 1998;338:273–277. doi: 10.1056/NEJM199801293380501. [DOI] [PubMed] [Google Scholar]
- 36.Hall SM, Haworth SG. Conducting pulmonary arteries: structural adaptation to extrauterine life in the pig. Cardiovasc Res. 1987;21:208–216. doi: 10.1093/cvr/21.3.208. [DOI] [PubMed] [Google Scholar]
- 37.O'Donnell SR, Wanstall JC. Thyroxine treatment of aged or young rats demonstrates that vascular responses mediated by beta-adrenoceptor subtypes can be differentially regulated. Br J Pharmacol. 1986;88:41–49. doi: 10.1111/j.1476-5381.1986.tb09469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu SF, et al. Developmental changes in endothelium-dependent pulmonary vasodilatation in pigs. Br J Pharmacol. 1992;106:324–330. doi: 10.1111/j.1476-5381.1992.tb14335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karaki H, et al. Artificial buffers do not inhibit contractile responses in the smooth muscle of rat portal vein and guinea pig taenia coli. Jpn J Pharmacol. 1981;31:979–983. doi: 10.1254/jjp.31.979. [DOI] [PubMed] [Google Scholar]
- 40.Farrukh IS, et al. Effect of intracellular pH on ferret pulmonary arterial smooth muscle cell calcium homeostasis and pressure. J Appl Physiol. 1996;80:496–505. doi: 10.1152/jappl.1996.80.2.496. [DOI] [PubMed] [Google Scholar]