Abstract
Objective
To evaluate predictors for intervention dropout and successful reduction of metabolic syndrome risk factors among obese children enrolled in a short-term, clinic-based weight-loss intervention.
Design, Setting, Subjects
Retrospective database review of 1080 children 8 months-17 y.o. seen a a pediatric obesity clinic.
Interventions
Behavior modification counseling to induce change in dietary and exercise choices.
Main Outcome Measures
1). Pre-/post-intervention change in body mass index (BMI), waist circumference, blood pressure, glucose, insulin, and cholesterol (LDL, HDL, & total). 2) Predictors of successful decrease in BMI and clinic drop-out.
Analysis
Paired t-tests for pre-/post-intervention comparisons. Linear regression to assess predictors of success and predictors of drop-out, with adjustment for age, gender, race, insurance status, and service area.
Results
Among children evaluated, adolescent females were most likely to achieve successful decrease in BMI, insulin level, and LDL cholesterol post-intervention. Nearly 40% of children dropped out early in the intervention. Predictors of drop out included age <6y, public insurance status, follow-up scheduled during summer months, and residence in a tertiary service area.
Conclusions
Clinic-based weight loss interventions can lead to successful improvements in BMI and other metabolic parameters in pediatric populations and may be more likely among adolescent females than in younger children or males. Drop-out is common, particularly among younger children, children with public insurance and children scheduled for follow-up in the summer. Identification of these drop-out predictors in individual patients may help in targeting children likely to succeed in short-term, clinic-based, weight-loss interventions.
Keywords: obesity, weight loss, child, adolescent
INTRODUCTION
Childhood obesity and the metabolic syndrome are major risk factors for adult cardiovascular disease and have become a worldwide pandemic [1-3]. Features of the metabolic syndrome include obesity, elevated insulin levels, elevated plasma glucose, low HDL cholesterol, elevated triglycerides, and hypertension. Studies have shown that children who are obese and exhibit the metabolic syndrome are more likely to remain obese and have the metabolic syndrome in adulthood [4-6]. According to the Centers for Disease Control, childhood obesity has tripled over the last 3 decades with approximately 1 out of every 6 children considered either overweight or obese. In the state of Virginia, about 1 out of every 3 children are either overweight or obese making Virginia the 25th heaviest state in the US with respect to childhood obesity [7]. The deleterious effects of childhood obesity are well recognized [4,8,9]. While childhood obesity and the metabolic syndrome have been recognized around the world as urgent issues, weight loss intervention programs for children remain scarce [10-12].
Children have the advantage of vertical growth and faster metabolic rates when compared to adults, making childhood the ideal time to combat obesity issues. However, many overweight and obese children are unable to take advantage of these intrinsic benefits due to lack of access to weight loss intervention programs, parental denial, inadequate motivation, and lack of understanding of healthy diet and proper exercise [13-17]. The most effective models for and duration of weight loss interventions in children are unclear. Weight loss interventions have been studied in experimental, clinical, and school settings. Some studies have suggested that interventions as short as 4 months can have beneficial effects on weight loss and other metabolic syndrome components such as insulin resistance while other studies have recommended longer weight loss interventions for children [18-21]. Multidisciplinary approaches to weight loss involving a dietician, exercise physiologist, and psychologist targeting lifestyle intervention have been shown to be most effective for childhood obesity [22-24]. However, drop out from these interventions have been significant [25] and appear to be influenced by race/ethnicity [25-27] and SES [25,27,28]. The role of transportation—including the travel distance required—has not been evaluated. Moreover, controversy exists regarding predictors of successful weight loss, including the role of baseline weight [25,28,29], sex [25,28], and age ([28,30]. Further knowledge of these predictors may help to target children at higher risk for drop-out to maintain efforts and have a higher rate of success.
Given the time constraints for most primary care pediatricians, parents of overweight and obese children are often left alone to assist their children with weight loss [10,31]. The University of Virginia Children's Fitness Clinic (CFC) was created in 2003 as the first pediatric behavioral-weight-management program in Virginia. Since its inception, over 1600 children from central Virginia and surrounding areas have been enrolled into this program. The goal of this project was to evaluate outcomes from the UVa CFC short-term weight loss intervention to determine predictors of intervention dropout and successful reduction of metabolic syndrome risk factors. Conclusions from this study may lead to enhancements in the identification of pediatric patients most likely to benefit from a clinic-based weight loss intervention.
METHODS
After obtaining approval from the Institutional Review Board of UVa, we performed a retrospective database review of 1,080 children enrolled in the UVa CFC from 2003-2007. The CFC offers a 6-month weight loss intervention with a multidisciplinary approach involving a nurse practitioner, registered dietician, exercise physiologist, and behavioral psychologist. Overweight children and adolescents from 0-17 years diagnosed by their primary physicians with non-pathologic weight gain are referred to the CFC. Participants are seen by a practitioner, a physical therapist and a dietician. They are interviewed regarding their current practices with respect to food intake and activity. They then receive tailored instructions in lifestyle modification including healthy nutrition options and age-appropriate portion sizes as well as exercise goals at each clinic visit. Along with the treatment team and parents, children and adolescents seen in the program set personal goals related to nutrition and activity. They or their parents then maintain a physical activity calendar based upon goals negotiated with the child and family during each visit. Written nutritional goals are also negotiated and discussed during each monthly visit using a positive non-food, non-monetary reward system.
Anthropometric measures of weight, height, and waist circumference were obtained at the initial visit and monthly follow-up visits throughout the six-month intervention. Waist circumference (WC) was measured by an experienced exercise physiologist using a tape measure and the following patient landmarks: anterior iliac spine and navel as described in the National Health and Nutrition Examination Survey Anthropometry Procedure Manual [32]. Fasting serum labs including insulin, blood glucose, and lipids were measured prior to the intervention. Follow-up labs were obtained for those participants who had abnormal baseline labs. Race, sex, insurance status, and service area (based on patient residence) were determined at the initial visit and entered into a clinical database. Service area was defined as primary, secondary, or tertiary depending on the distance of the county of residence from the clinic (Supplementary Figure 1). Patient drop out was defined as not returning for any follow-up visit after the initial visit. Examination of post-intervention outcomes was restricted to only those individuals who returned at least once after the initial intake visit and whose last follow-up visit was between three and seven months after the initial visit.
STATISTICAL ANALYSIS
Children were stratified by sex and age [0-5y, 6-11y (school age), and 12-17y (adolescent)]. Various factors were examined based on known geographic obstacles and on controversies from the literature to determine if they predicted patient retention, which was defined as attending at least one visit following the initial visit. These factors included age groups (0-5y, 6-11y, and 12-17y), sex, race (non-Hispanic white vs. non-white), insurance type (private vs. public), service area (primary, secondary, or tertiary), season of enrollment and baseline BMI z-score. For season of enrollment, “summer drop-out” was defined as children whose first failed return visit was scheduled from June-August, coinciding with school holidays (in this region, early June to late August/early September). The dropout analysis was performed on a univariate level via chi-square tests, followed by logistic regression to model odds of not returning for at least one follow-up visit. The impact of age (categorized as described above) and sex were allowed to interact in the model.
The same factors examined above, including the age x sex interaction, were then included in a linear model of change in raw BMI over time, for those children who attended the CFC for at least two visits separated by at least three months. We evaluated for change in raw BMI as opposed to change in BMI-z-score given the short time interval considered (6 months) and given instability of BMI z-score at the extremes of obesity [33]. Children five years and younger were excluded from this analysis due to small sample size. Paired t-tests, stratified by age and sex, were then performed to compare baseline to follow-up outcome parameters in this subset of children. To evaluate for a relationship between change-in-BMI and change-in-metabolic parameters over time, we performed Pearson correlation analyses. All statistical analyses were performed using SAS with a significance level of α=0.05. The age × sex interaction was deemed significant if the associated p-value from the model was less than 0.10.
RESULTS
SAMPLE CHARACTERISTICS
Sample characteristics are summarized in Table 1. The sample participants consisted of 1,080 whites (non-Hispanic), blacks (non-Hispanic), Hispanics, Asians, and other race children ages 0-17 years who underwent baseline and follow-up (if applicable) measures of weight, height, waist circumference, blood pressure, and fasting laboratory values (lipids, glucose, insulin). Racial and sex characteristics were similar among age groups. The preschool age group had a higher percentage of Hispanic ethnicity compared to school aged and adolescent children. The mean BMI z-score was significantly higher in the 0-5 year age group (3.73±1.19) compared to the 6-11y (2.48±0.33) and 12-17y (2.59±0.62) age groups (p<0.05).
Table 1.
Childrens Fitness Clinic Gender, Ethnicity and Anthropometric Characteristics *
| Characteristic | All Children | 0-5 Years | 6-11 Years | 12-17 years |
|---|---|---|---|---|
| N (%) | 1080 (100%) | 105 (9.7%) | 474 (43.9%) | 501 (46.4%) |
| Males | 456 (42.2%) | 48 (45.7%) | 204 (43.0%) | 204 (40.7%) |
| Ethnicity | ||||
| White, non-Hispanic | 602 (56.6%) | 49 (48.0%) | 267 (57.4%) | 286 (57.5%) |
| Black, non-Hispanic | 379 (35.6%) | 33 (32.4%) | 157 (33.8%) | 189 (38.0%) |
| Hispanic | 68 (6.4%) | 18 (17.6%) | 33 (7.1%) | 17 (3.4%) |
| Other, non-Hispanic | 15 (1.4%) | 2 (2.0%) | 8 (1.7%) | 5 (1.0%) |
| Private Insurance | 518 (54.3%) | 36 (39.1%) | 243 (57.2%) | 239 (54.7%) |
| Service Area | ||||
| Primary | 612 (57.7%) | 53 (51.5%) | 271 (58.2%) | 288 (58.7%) |
| Secondary | 261 (24.6%) | 27 (26.2%) | 122 (26.2%) | 112 (22.8%) |
| Tertiary | 187 (17.6%) | 23 (22.3%) | 73 (15.7%) | 91 (18.5%) |
| Drop-out during summer** | 215 (19.9%) | 22 (21.0%) | 94 (19.8%) | 99 (19.8%) |
| BMI Z-score at Enrollment (Mean (SD)) | 2.6 (0.6) | 3.7 (1.2) | 2.5 (0.3) | 2.5 (0.4) |
| Number of visits | ||||
| Initial only | 414 (38.3%) | 60 (57.1%) | 159 (33.5%) | 195 (38.9%) |
| 2-4 | 506 (46.9%) | 40 (38.1%) | 236 (49.8%) | 230 (45.9%) |
| 5 or more | 160 (14.8%) | 5 (4.8%) | 79 (16.7%) | 76 (15.2%) |
Unless otherwise noted, data are reported as frequencies (column percent)
Defined as first missed visit June-August
DROP-OUTS
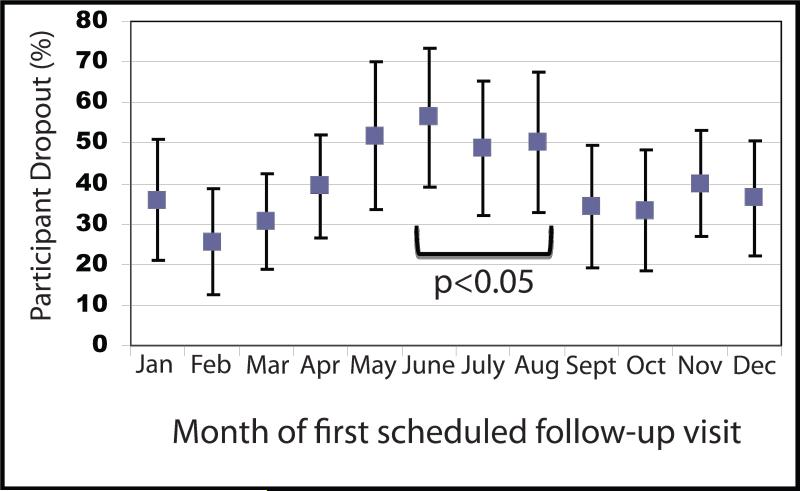
As shown in Table 1, 38% of children referred to the weight loss intervention did not return after the initial visit. The majority of participants (46.9%) returned for 2-4 visits out of a possible 6 visits. Logistic regression revealed that young males (<6 years), living in a tertiary service area, public insurance, and enrollment during the summer were independent predictors of drop out. (Table 2). Increased drop-out was observed when follow-up appointments were scheduled during the summer (June-August) compared to other times of the year (Figure 1; p<0.05). Only 80 subjects (8%) completed all 6 monthly visits in the program.
Table 2.
Logistic Regression Results: Odds of Drop-Out from Clinic*
| Model Covariate | Odds Ratio | 95% CI | p-value |
|---|---|---|---|
| Non-White (non-Hispanic) | 1.1 | (0.8, 1.4) | 0.73 |
| Private Insurance | 0.7 | (0.5, 1.0) | 0.02 |
| Service Area | |||
| Secondary vs. Primary | 0.9 | (0.6, 1.2) | 0.43 |
| Tertiary vs. Primary | 2.0 | (1.4, 3.0) | < 0.01 |
| Follow-up scheduled during Summer | 1.5 | (1.0, 2.0) | 0.03 |
| Baseline BMI Z-score ≥ 2.5 | 0.8 | (0.6, 1.0) | 0.08 |
| Gender × Age Interaction** | |||
| Females (0-5 years) | 0.4 | (0.2, 1.0) | 0.045 |
| Females (6-11 years) | 0.2 | (0.1, 0.4) | < 0.01 |
| Females (12+ years) | 0.2 | (0.1, 0.4) | < 0.01 |
| Males (6-11 years) | 0.2 | (0.1, 0.5) | < 0.01 |
| Males (12+ years) | 0.3 | (0.1, 0.6) | < 0.01 |
Not returning for at least one follow-up visit (i.e., initial visit only)
Males 0-5 years old is the referent category
Figure 1.
Percentage of drop-out after initial visit by calendar month among all UVA Children's Fitness Clinic (CFC) participants. Values shown reflect mean percentage of participant dropouts and the bars represent 95% confidence intervals. CFC participants with an initial follow-up visit during the summer (defined as May-July) were more likely to drop-out (p<0.05).
CLINICAL OUTCOMES
Anthropometric measures
BMI and WC were used as proxy measures of weight. Linear regression of change in BMI over time revealed that insurance status was the only significant predictor of change in BMI, with those who have private insurance losing almost one unit of BMI on average more than those with public insurance (p < 0.01; Table 3). A significant age × sex interaction (p = 0.084) existed with respect to change in BMI, with adolescent females tending to lose more BMI during this time period than other groups.
Table 3.
Linear Regression Results: Estimated changes in BMI*
| Model Covariate | Estimate | 95% CI | p-value |
|---|---|---|---|
| Intercept** | 0.31 | (-0.47, 1.10) | 0.43 |
| Not (White (non-Hispanic)) | 0.01 | (-0.50, 0.52) | 0.97 |
| Private Insurance | -0.90 | (-1.42, -0.38) | < 0.01 |
| Service Area | |||
| Secondary | -0.22 | (-0.80, 0.36) | 0.46 |
| Tertiary | -0.39 | (-1.09, 0.31) | 0.27 |
| Summer Enrollment | -0.53 | (-1.16, 0.09) | 0.09 |
| Baseline BMI Z-score ≥ 2.5 | 0.06 | (-0.43, 0.56) | 0.81 |
| Male: 12 + years old† | 0.11 | (-0.68, 0.89) | 0.79 |
| Female: 6-11 years old† | 0.10 | (-0.58, 0.79) | 0.76 |
| Female: 12 + years old† | -0.66 | (-1.34, 0.02) | 0.06 |
For those with at least one follow-up three months after the initial visit (excluding those 0-5 years old)
Estimated change in BMI for those in the referent categories (white (non-Hispanic, other than private insurance, primary service area, non-summer enrollment, baseline BMI Z < 2.5, male 6-11 years old)
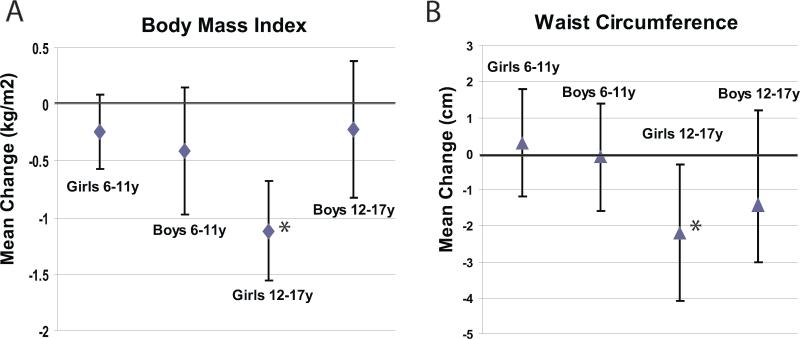
Post-intervention BMI and WC for each age group were then compared to baseline measures (Figure 2). Adolescent girls were the only group to have a significant reduction in BMI and WC [-1.12, 95% CI (-1.55, -0.68); -2.2 cm, 95% CI (-4.1, -0.3) respectively]. Although there was no significant weight loss in school age girls and all boys, there was also no significant increase in BMI or WC post-intervention.
Figure 2.
Comparison of pre- and post-intervention mean change in BMI (A) and WC (B) stratified by age and gender. Bars represent 95% confidence intervals. * p<0.05 for change from baseline.
Laboratory measures
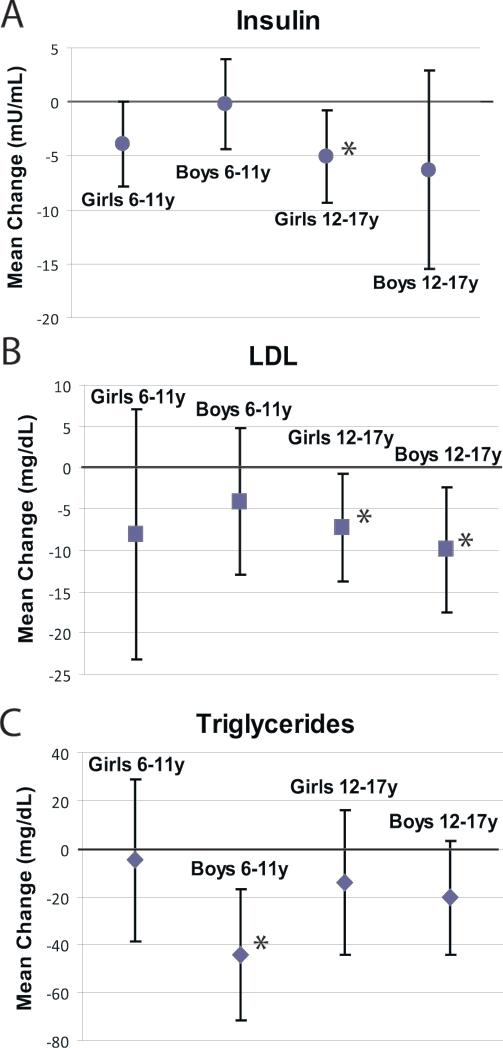
Post-intervention mean insulin, total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, and glucose values for each age/gender group were compared to baseline measures. School age and adolescent girls had a significant reduction in insulin [-3.9 μU/mL, 95% CI (-7.7, 0.0); -5.1 μU/mL (-9.4, -0.8) respectively] (Figure 3). Adolescent boys and adolescent girls had significant reductions in LDL cholesterol observed during the intervention [-9.9 mg/dL, 95% CI (-17.5, -2.3); -7.2 mg/dL, 95% CI (-13.7, -0.7) respectively] (Figure 3). School age boys had a significant reduction in triglyceride levels [-44.5 mg/dL, 95% CI (-71.9, -17.1)] (Figure 3). Among groups with significant changes in metabolic parameters there was no correlation between change in BMI and change in the given parameter (data not shown). Among all age/sex sub-groups there were no statistically significant differences in total cholesterol, HDL cholesterol, or glucose (data not shown).
Figure 3.
Comparison of pre- and post-intervention mean change in Insulin (A), LDL (B), and Triglycerides (C) stratified by age and gender. Bars represent 95% confidence intervals. Adolescent girls (ages 12-17y) were the only group with a significant change in either BMI (A) or WC (B). * p<0.05 for change from baseline.
DISCUSSION
Multidisciplinary weight loss interventions for overweight children have been previously reported with varied results [22-24]. While some of these interventions were performed in a “real world” setting such as the CFC at UVa, many studies report results from interventions conducted under well-funded experimental conditions, which have limited applicability for clinical pediatricians. We evaluated outcomes from the UVa CFC to identify predictors of successful improvement in BMI among overweight and obese children enrolled in a short-term weight loss intervention. In addition to assessing effects on BMI, we also focused on other aspects of the metabolic syndrome including effects on blood pressure, lipids, and insulin resistance. Knowledge of these predictors of success may help alter expectations among treating physicians and identify groups of children that may need additional efforts. These efforts may include additional contact with the family via phone calls or internet contact between visits, which while more time intensive has been shown to improve weight loss overall [34,35].
Our data show that adolescent girls are the age and sex group most likely to exhibit improvements in BMI and waist circumference by the end of the intervention. Although boys and school-age girls did not exhibit any significant reduction in BMI, importantly, there was no significant gain in BMI post-intervention in any age group compared to an expected tendency to gain BMI over time [36]. A study by Sinaiko et. al. suggested that excessive weight gain in childhood as opposed to the absolute weight is a predictor of adult cardiovascular risk [37]. Since children have the advantage of vertical growth, preventing weight gain may be just as advantageous as promotion of weight loss in this population of children.
Prior studies have shown that adolescent girls are particularly concerned about weight gain and appearing “fat” to their peers [38]. Unfortunately, overweight, normal weight, and, even some underweight girls frequently diet in an attempt to conform to an unhealthy body image promoted by media. It is plausible that self-imposed societal pressures to be thin, body dissatisfaction, and lower self-esteem may have contributed to the weight loss seen in adolescent females. While attempts at becoming “skinny” may result in eating disorders, the CFC weight loss intervention promotes healthy lifestyle modifications to achieve weight loss [39-41].
The optimal duration for pediatric weight loss interventions is unknown with prior studies publishing different recommendations based on study outcomes. While some studies have suggested that a minimum of a year is necessary for any significant weight loss, other studies have been conducted in as little as 16 weeks with positive outcomes [18-21]. We have shown that a weight loss intervention as short as 6 months can lead to a decrease in BMI for some children and perhaps, just as importantly, halt the progression of increasing BMI in others.
The effect of the CFC UVa short-term weight loss intervention on metabolic lab measures varied. Girls in each of the age groups analyzed had a significant reduction in insulin levels compared to boys who did not exhibit changes. Adolescent boys and adolescent girls had a significant reduction in LDL cholesterol while school age boys had a significant decrease in triglyceride levels post-intervention. It is unclear why these differences existed, though this may be aided by a physiologic decrease in LDL cholesterol levels that occurs during puberty [42]. There was no correlation between decreases in these metabolic parameters and decreases in BMI, suggesting the possibility that other components of the CFC program besides weight loss (such as exercise in the absence of weight loss) contribute to these changes—though low subject numbers limit the conclusions we can make from this lack of association.
The analyses of laboratory measures were limited by the small sample size of pre- and post-intervention labs. Many of the children in our study either never returned for post-intervention labs or were not referred for post-intervention labs if their pre-intervention laboratory measurements were normal. Thus, these differences in laboratory changes may be more reflective of higher percentages of abnormal baseline laboratory values in a particular group as opposed to a direct result of the weight loss intervention. It also introduces a selection bias toward evaluating those who completed the study. It is possible that if all children had received follow-up laboratory measurements, regardless of whether the baseline value was abnormal, we may have increased our power to detect significant reductions in these laboratory measures. Studies have shown that lifestyle modifications including exercise and healthy diet improve insulin sensitivity, glucose tolerance, and cholesterol metabolism in addition to weight loss [43-45]. These lifestyle modifications are routinely used in adults as first-line therapy.
The dropout of study participants was considerable. Only 14.8% of all children and adolescents enrolled in the CFC during the first 4 years of the program returned for at least 5 out of the 6 scheduled visits to the program and only 8% of patients completed all 6 monthly visits. Nearly 40% of children never returned after the initial intake visit. This finding is similar to other published reports of clinic-based weight loss intervention programs.[46,47] The low numbers of completers of the program left us with insufficient power to analyze predictors of which subjects were most likely to complete the treatment. Clinic-based weight loss programs such as the CFC are heavily supported by external sources of funding since insurance reimbursement has been historically poor. Thus, it is of extreme importance to target those children who are most likely to complete and benefit from a clinic-based short-term weight loss intervention. From a cost-benefit perspective, it is conceivable that allocating money to fund programs for children considered to be most likely to succeed in weight loss intervention programs is ideal. Also, enrollment of children with the characteristics of those most likely to drop out hinders money allocation for participation of children who would otherwise benefit from enrollment [48].
We determined that younger males (<6 years), those who lived further away (tertiary service area) and children scheduled for follow-up in the summer (June-August) were more likely to drop out of the program. It is uncertain why this relationship would exist, and potential explanations include family vacations during this time or other changes in the family's schedule. Children with private insurance appeared to have a benefit in that they were less likely to drop out compared to children with public insurance. Since it was less common for children <6 years to be referred to a weight loss intervention, outcome analyses in this age group were limited by the small sample size. Participation of children <6 years are most likely driven by direct parental involvement, so it is likely that parental barriers contributed the most to drop-out in this group. Studies have shown that parents of obese children have a tendency to minimize their child's weight and frequently are in denial of obesity-related health concerns [13-15]. Physicians may also be guilty of under-emphasizing the consequences of morbid obesity in infants and toddlers to parents [16].
Other barriers such as travel distance to our clinic and low socioeconomic status may have also contributed to the drop out rate. Since the UVa CFC is the only pediatric weight loss clinic in central Virginia, children from as far away as southwest Virginia are referred to the UVa CFC. The expectation of monthly clinic visits for 6 months may impose a considerable burden on some families, including time missed from work and/or school in addition to the cost of transportation. Recognition of barriers to participation in weight loss interventions may help to better promote recidivism in clinically based weight loss interventions [17]. It is unclear why children who had follow-up scheduled in the summer were more likely to drop out of the program. One might assume that it would be easier for children to participate given that most are on summer vacation from school. However, it is also plausible that these children prioritize other activities during this period over a clinic visit.
This study had limitations due to its retrospective nature, with multiple types of potential bias. All study participants to the CFC were referred by either a primary care physician or pediatrician, which may have led to referral bias. Participants who decided to return to the CFC were probably different from the nearly 40% of children who dropped out after the initial visit suggesting withdrawal bias. These differences may include participant motivation prior to and during the intervention as well as variable family support of participants. In our database we did not keep track of sibling pairs, which account for up to 10% of our subjects and thus may have over-estimated predictors from those families. Also, since the implementation of this intervention primarily occurs in the homes of study participants, there was no direct way to observe which participants were compliant with the treatment program. Finally, there was unfortunately a lack of control subjects, though prior studies of overweight children have demonstrated an increase in BMI percentiles over time [36].
In conclusion, we have shown that the UVa CFC short-term weight-loss intervention is effective for the prevention of weight gain in children ages 6-17 years and for weight loss in adolescent girls. The fact that younger age groups and adolescent boys were not as successful in weight loss could signal that these groups may require additional efforts such as a greater degree of contact (phone or email) between clinic visits. While drop-out from the clinic was striking, the rate of drop-out worsened significantly when follow-up visits were scheduled during the summer months—which may imply that families with summer follow-up would benefit from a greater effort at scheduling a follow-up appointment that the family is able to attend. However, further research is needed to determine whether targeting less-successful groups with added interventions or emphasizing compliance with follow-up during the summer may improve outcomes further. In addition, further research is needed to determine long-term predictors of weight loss success. The ultimate goal of any weight loss intervention in childhood should be to teach lifelong lifestyle modifications to children and parents. The incorporation of lifelong healthy living habits at an early age may help to decrease risk of future cardiovascular events, which has important public health implications.
Supplementary Material
Supplementary Figure 1. UVA Health System Service Area Map represents geographic regions of Virginia served by the UVA Health System.
PSA = Primary Service Area.
SSA = Secondary Service Area.
Tertiary Service areas are shown in blue, purple, green, and brown.
Acknowledgements
NIH HD060739-01 (to MDD), NIH 1R21DK085363 (to MDD and MJG), NIH T32H207956 (to SEW)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. The relation of childhood BMI to adult adiposity: the Bogalusa Heart Study. Pediatrics. 2005;115(1):22–7. doi: 10.1542/peds.2004-0220. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Curtin LR, Lamb MM, Flegal KM. Prevalence of high body mass index in US children and adolescents, 2007-2008. Jama. 2010;303(3):242–9. doi: 10.1001/jama.2009.2012. [DOI] [PubMed] [Google Scholar]
- 3.Popkin BM, Doak CM. The obesity epidemic is a worldwide phenomenon. Nutr Rev. 1998;56(4 Pt 1):106–14. doi: 10.1111/j.1753-4887.1998.tb01722.x. [DOI] [PubMed] [Google Scholar]
- 4.Sun SS, Liang R, Huang TT, Daniels SR, Arslanian S, Liu K, et al. Childhood obesity predicts adult metabolic syndrome: the Fels Longitudinal Study. J Pediatr. 2008;152(2):191–200. doi: 10.1016/j.jpeds.2007.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freedman DS, Khan LK, Serdula MK, Dietz WH, Srinivasan SR, Berenson GS. Interrelationships among childhood BMI, childhood height, and adult obesity: the Bogalusa Heart Study. Int J Obes Relat Metab Disord. 2004;28(1):10–6. doi: 10.1038/sj.ijo.0802544. [DOI] [PubMed] [Google Scholar]
- 6.Walker SE, Gurka MJ, Oliver MN, Johns DW, DeBoer MD. Racial/ethnic discrepancies in the metabolic syndrome begin in childhood and persist after adjustment for environmental factors. Nutr Metab Cardiovasc Dis. 2010 doi: 10.1016/j.numecd.2010.05.006. e-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.VonKutzleben W. [August 17, 2011];The Childhood and Adolescent Obesity Epidemic: Confronting Virginia Schools. 2010 www.cdc.gov/nceh/ehs/ephli/Reports/VonKutzeleben.doc.
- 8.Must A, Strauss RS. Risks and consequences of childhood and adolescent obesity. Int J Obes Relat Metab Disord. 1999;23(Suppl 2):S2–11. doi: 10.1038/sj.ijo.0800852. [DOI] [PubMed] [Google Scholar]
- 9.Must A. Morbidity and mortality associated with elevated body weight in children and adolescents. Am J Clin Nutr. 1996;63(3 Suppl):445S–447S. doi: 10.1093/ajcn/63.3.445. [DOI] [PubMed] [Google Scholar]
- 10.Caprio S. Treating child obesity and associated medical conditions. Future Child. 2006;16(1):209–24. doi: 10.1353/foc.2006.0002. [DOI] [PubMed] [Google Scholar]
- 11.CDC CDC Grand Rounds: Childhood Obesity in the United States. Morbidity and Mortality Weekly Report. 2011;60(2):42–46. [PubMed] [Google Scholar]
- 12.Lieb DC, Snow RE, DeBoer MD. Socioeconomic factors in the development of childhood obesity and diabetes. Clin Sports Med. 2009;28(3):349–78. doi: 10.1016/j.csm.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golan M, Weizman A, Apter A, Fainaru M. Parents as the exclusive agents of change in the treatment of childhood obesity. Am J Clin Nutr. 1998;67(6):1130–5. doi: 10.1093/ajcn/67.6.1130. [DOI] [PubMed] [Google Scholar]
- 14.Bossink-Tuna HN, L'Hoir MP, Beltman M, Boere-Boonekamp MM. Parental perception of weight and weight-related behaviour in 2- to 4-year-old children in the eastern part of the Netherlands. Eur J Pediatr. 2009;168(3):333–9. doi: 10.1007/s00431-008-0787-x. [DOI] [PubMed] [Google Scholar]
- 15.Jain A, Sherman SN, Chamberlin LA, Carter Y, Powers SW, Whitaker RC. Why don't low-income mothers worry about their preschoolers being overweight? Pediatrics. 2001;107(5):1138–46. doi: 10.1542/peds.107.5.1138. [DOI] [PubMed] [Google Scholar]
- 16.Mikhailovich K, Morrison P. Discussing childhood overweight and obesity with parents: a health communication dilemma. J Child Health Care. 2007;11(4):311–22. doi: 10.1177/1367493507082757. [DOI] [PubMed] [Google Scholar]
- 17.Rice J, Thombs D, Leach R, Rehm R. Successes and barriers for a youth weight-management program. Clin Pediatr (Phila) 2008;47(2):143–7. doi: 10.1177/0009922807306168. [DOI] [PubMed] [Google Scholar]
- 18.Wilfley DE, Tibbs TL, Van Buren DJ, Reach KP, Walker MS, Epstein LH. Lifestyle interventions in the treatment of childhood overweight: a meta-analytic review of randomized controlled trials. Health Psychol. 2007;26(5):521–32. doi: 10.1037/0278-6133.26.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snethen JA, Broome ME, Cashin SE. Effective weight loss for overweight children: a meta-analysis of intervention studies. J Pediatr Nurs. 2006;21(1):45–56. doi: 10.1016/j.pedn.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 20.Reinehr T, de Sousa G, Toschke AM, Andler W. Long-term follow-up of cardiovascular disease risk factors in children after an obesity intervention. Am J Clin Nutr. 2006;84(3):490–6. doi: 10.1093/ajcn/84.3.490. [DOI] [PubMed] [Google Scholar]
- 21.Saelens BE, Sallis JF, Wilfley DE, Patrick K, Cella JA, Buchta R. Behavioral weight control for overweight adolescents initiated in primary care. Obes Res. 2002;10(1):22–32. doi: 10.1038/oby.2002.4. [DOI] [PubMed] [Google Scholar]
- 22.Caranti DA, de Mello MT, Prado WL, Tock L, Siqueira KO, de Piano A, et al. Short- and long-term beneficial effects of a multidisciplinary therapy for the control of metabolic syndrome in obese adolescents. Metabolism. 2007;56(9):1293–300. doi: 10.1016/j.metabol.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 23.Chen AK, Roberts CK, Barnard RJ. Effect of a short-term diet and exercise intervention on metabolic syndrome in overweight children. Metabolism. 2006;55(7):871–8. doi: 10.1016/j.metabol.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Dao HH, Frelut ML, Oberlin F, Peres G, Bourgeois P, Navarro J. Effects of a multidisciplinary weight loss intervention on body composition in obese adolescents. Int J Obes Relat Metab Disord. 2004;28(2):290–9. doi: 10.1038/sj.ijo.0802542. [DOI] [PubMed] [Google Scholar]
- 25.Williams NA, Coday M, Somes G, Tylavsky FA, Richey PA, Hare M. Risk factors for poor attendance in a family-based pediatric obesity intervention program for young children. J Dev Behav Pediatr. 2010;31(9):705–12. doi: 10.1097/DBP.0b013e3181f17b1c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeBoer MD. Ethnicity, obesity and the metabolic syndrome: implications on assessing risk and targeting intervention. Expert Rev Endocrinol Metab. 2011;6(2):279–289. doi: 10.1586/eem.11.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tershakovec AM, Kuppler K. Ethnicity, insurance type, and follow-up in a pediatric weight management program. Obes Res. 2003;11(1):17–20. doi: 10.1038/oby.2003.4. [DOI] [PubMed] [Google Scholar]
- 28.Braet C. Patient characteristics as predictors of weight loss after an obesity treatment for children. Obesity (Silver Spring) 2006;14(1):148–55. doi: 10.1038/oby.2006.18. [DOI] [PubMed] [Google Scholar]
- 29.Goossens L, Braet C, Van Vlierberghe L, Mels S. Weight parameters and pathological eating as predictors of obesity treatment outcome in children and adolescents. Eat Behav. 2009;10(1):71–3. doi: 10.1016/j.eatbeh.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 30.Moens E, Braet C, Van Winckel M. An 8-year follow-up of treated obese children: children's, process and parental predictors of successful outcome. Behav Res Ther. 2010;48(7):626–33. doi: 10.1016/j.brat.2010.03.015. [DOI] [PubMed] [Google Scholar]
- 31.Holt N, Schetzina KE, Dalton WT, 3rd, Tudiver F, Fulton-Robinson H, Wu T. Primary care practice addressing child overweight and obesity: a survey of primary care physicians at four clinics in southern Appalachia. South Med J. 2011;104(1):14–9. doi: 10.1097/SMJ.0b013e3181fc968a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.CDC [August 17, 2011];National Health and Nutrition Examination Survey III Body Measurements (Anthropometry) 1988 http://www.cdc.gov/nchs/data/nhanes/nhanes3/cdrom/nchs/manuals/anthro.pdf.
- 33.Woo JG. Using body mass index Z-score among severely obese adolescents: a cautionary note. Int J Pediatr Obes. 2009;4(4):405–10. doi: 10.3109/17477160902957133. [DOI] [PubMed] [Google Scholar]
- 34.Deforche B, De Bourdeaudhuij I, Tanghe A, Debode P, Hills AP, Bouckaert J. Post-treatment phone contact: a weight maintenance strategy in obese youngsters. Int J Obes (Lond) 2005;29(5):543–6. doi: 10.1038/sj.ijo.0802924. [DOI] [PubMed] [Google Scholar]
- 35.Kirk SF, Harvey EL, McConnon A, Pollard JE, Greenwood DC, Thomas JD, et al. A randomised trial of an Internet weight control resource: the UK Weight Control Trial [ISRCTN58621669]. BMC Health Serv Res. 2003;3(1):19. doi: 10.1186/1472-6963-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rudolf MC, Greenwood DC, Cole TJ, Levine R, Sahota P, Walker J, et al. Rising obesity and expanding waistlines in schoolchildren: a cohort study. Arch Dis Child. 2004;89(3):235–7. doi: 10.1136/adc.2002.020552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sinaiko AR, Donahue RP, Jacobs DR, Jr., Prineas RJ. Relation of weight and rate of increase in weight during childhood and adolescence to body size, blood pressure, fasting insulin, and lipids in young adults. The Minneapolis Children's Blood Pressure Study. Circulation. 1999;99(11):1471–6. doi: 10.1161/01.cir.99.11.1471. [DOI] [PubMed] [Google Scholar]
- 38.Moses N, Banilivy MM, Lifshitz F. Fear of obesity among adolescent girls. Pediatrics. 1989;83(3):393–8. [PubMed] [Google Scholar]
- 39.Field AE, Cheung L, Wolf AM, Herzog DB, Gortmaker SL, Colditz GA. Exposure to the mass media and weight concerns among girls. Pediatrics. 1999;103(3):E36. doi: 10.1542/peds.103.3.e36. [DOI] [PubMed] [Google Scholar]
- 40.Furnham A, Badmin N, Sneade I. Body image dissatisfaction: gender differences in eating attitudes, self-esteem, and reasons for exercise. J Psychol. 2002;136(6):581–96. doi: 10.1080/00223980209604820. [DOI] [PubMed] [Google Scholar]
- 41.Nowak M. The weight-conscious adolescent: body image, food intake, and weight-related behavior. J Adolesc Health. 1998;23(6):389–98. doi: 10.1016/s1054-139x(97)00263-2. [DOI] [PubMed] [Google Scholar]
- 42.Cook S, Auinger P, Huang TT. Growth curves for cardio-metabolic risk factors in children and adolescents. J Pediatr. 2009;155(3):S6, e15–26. doi: 10.1016/j.jpeds.2009.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rutledge JC, Hyson DA, Garduno D, Cort DA, Paumer L, Kappagoda CT. Lifestyle modification program in management of patients with coronary artery disease: the clinical experience in a tertiary care hospital. J Cardiopulm Rehabil. 1999;19(4):226–34. doi: 10.1097/00008483-199907000-00003. [DOI] [PubMed] [Google Scholar]
- 44.Franz MJ. Lifestyle modifications for diabetes management. Endocrinol Metab Clin North Am. 1997;26(3):499–510. doi: 10.1016/s0889-8529(05)70263-2. [DOI] [PubMed] [Google Scholar]
- 45.He J, Bazzano LA. Effects of lifestyle modification on treatment and prevention of hypertension. Curr Opin Nephrol Hypertens. 2000;9(3):267–71. doi: 10.1097/00041552-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 46.Honas JJ, Early JL, Frederickson DD, O'Brien MS. Predictors of attrition in a large clinic-based weight-loss program. Obes Res. 2003;11(7):888–94. doi: 10.1038/oby.2003.122. [DOI] [PubMed] [Google Scholar]
- 47.Zeller M, Kirk S, Claytor R, Khoury P, Grieme J, Santangelo M, et al. Predictors of attrition from a pediatric weight management program. J Pediatr. 2004;144(4):466–70. doi: 10.1016/j.jpeds.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 48.Yates J, Murphy C. A cost benefit analysis of weight management strategies. Asia Pac J Clin Nutr. 2006;15(Suppl):74–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. UVA Health System Service Area Map represents geographic regions of Virginia served by the UVA Health System.
PSA = Primary Service Area.
SSA = Secondary Service Area.
Tertiary Service areas are shown in blue, purple, green, and brown.