Abstract
Hypertensive disorders occur in approximately 6–8% of all pregnancies and are a significant source of maternal and fetal morbidity. Little is known about the range of agents routinely used in practice. We used Medicaid claims from 2000–2007 to identify completed pregnancies. We included women who were Medicaid beneficiaries from at least 3 months prior to last menstrual period to 1 month post-delivery, and were successfully linked to infant records. Maternal exposure to antihypertensive medications was derived from Medicaid pharmacy claims files and duration of exposure was assigned based on the days supply dispensed. We identified 1,106,757 Medicaid patients in our cohort of whom 48,453 (4.4%) were exposed to antihypertensive medications during pregnancy. The prevalence of antihypertensive use increased from 3.5% to 4.9% during the study period. Antihypertensive medications users were older than non-users, more likely to be Caucasian or African-American, and more likely to have comorbid diabetes and/or renal disease. Overall, 1.9% of pregnant women were exposed during the 1st trimester, 1.7% during the 2nd trimester, and 3.2% during the 3rd trimester. The range of antihypertensive medications to which patients were exposed was highly heterogeneous and frequently included agents other than methyldopa or labetalol. ACE inhibitor exposure, which is contraindicated in late pregnancy, occurred in 928 (4.9%) antihypertensive medication users in the 2nd trimester and 383 (1.1%) in the 3rd trimester. Antihypertensive use during pregnancy is relatively common and increasing. The wide range of agents used during pregnancy includes medications considered contraindicated during pregnancy.
Keywords: Hypertension, Pregnancy, Epidemiology, Antihypertensives
Introduction
Hypertensive disorders, including chronic hypertension, gestational hypertension, preeclampsia/eclampsia, and chronic hypertension with superimposed preeclampsia, occur in approximately 6–8% of all pregnancies1–3 and are a significant source of maternal and fetal morbidity4, 5.
For severe hypertension, pharmacologic treatment is clearly indicated5, but for mild-to-moderate hypertension, limited data are available. Synthesis of this information suggests that while treatment with medication decreases the risk of progression to severe hypertension, it has little effect on pregnancy outcomes including development of preeclampsia, preterm delivery, or fetal/neonatal demise6. Antihypertensive exposure may confer some risk to the fetus by increasing rates of intrauterine growth restriction (although whether such associations are causal or confounded by indication or relative hypotension is unknown)7, 8 and, for some agents, congenital malformations—although data are conflicting and these associations are controversial9–15. Further, while methyldopa and labetalol are generally considered in guidelines as the first line/preferred agents for the treatment of hypertension in pregnancy3, 5, 16, experts suggest that other antihypertensives can also be safely used17–19.
Little is known about how physicians balance these considerations in practice, or about the range of antihypertensive agents that physicians routinely use. Previous studies examining outpatient antihypertensive utilization in pregnancy had few data on Medicaid patients20, which are important as Medicaid provides coverage for approximately 40% of all pregnancies in the US21. Earlier studies also did not examine patterns of management of patients taking antihypertensives prior to pregnancy or separately examine which agents are used in new initiators of antihypertensives in pregnancy20. These data are important in focusing research efforts aimed at establishing the optimal approach to the treatment of patients with hypertensive disorders.
To better understand these issues, we examined a cohort of pregnant patients enrolled in Medicaid from 2000 to 2007.
Methods
Definition of Cohort
Medicaid is the joint state and federal health insurance program for low-income individuals in the United States. The Medicaid Analytic eXtract (MAX) dataset contains individual-level Medicaid enrollment and Medicaid healthcare utilization claims, which includes inpatient, outpatient, and non-hospital pharmacy dispensing claims. MAX data were used to create a pregnancy cohort for studies of drug utilization and safety22. The pregnancy cohort was identified from 2000–2007 MAX data. Women with an inpatient or outpatient delivery-related International Classification of Diseases, Ninth Revision (ICD-9) or Current Procedural Terminology (CPT) codes were identified. These women were linked to infants by the Medicaid Case Number, which is typically shared by family members, and by matching an infant’s date of birth within a woman’s delivery date range. The delivery date range for women with inpatient deliveries was defined as the woman’s delivery admission and discharge dates and for women with outpatient deliveries it was defined as the 5 days before and after the delivery-related code date. After linkage and cleaning to eliminate apparent duplicate deliveries and incorrect linkages, 6,107,572 pregnancies were available. Because neither gestational length nor the date of the last menstrual period (LMP) are available in healthcare utilization data, the LMP was assigned to be 245 days before the delivery date for pregnancies that had ICD-9 codes indicative of preterm delivery, and as 270 days before the delivery date for all other pregnancies. This method has been validated and accurately classifies gestational length within 2 weeks for most deliveries.23
Next, a number of eligibility criteria were applied to ensure that claims for women with pregnancies in the cohort would be present in MAX data. Pregnancies in which women were not continuously enrolled in Medicaid from 3 months before the month of the estimated LMP until the month after the delivery month were excluded. Also, pregnancies were excluded in which women had private insurance, restricted Medicaid benefits, or were enrolled in certain managed care plans in which claims were missing or underreported in MAX; 1,106,757 pregnancies remained in the cohort. The cohort included women from all states except Arizona, Connecticut, Michigan, and Montana. Approximately 50% of pregnancies in the cohort came from 6 states (California, Illinois, New York, Ohio, Tennessee, and Wisconsin).
Baseline characteristics of the cohort
Baseline characteristics of the cohort were determined and stratified by whether the patients were exposed to antihypertensives during pregnancy. The patients’ age at the time of LMP and self-reported race, as documented in the Medicaid individual enrollment file, were extracted from the database. The presence of chronic hypertension, gestational hypertension, chronic renal disease, and multiple gestations was identified by the recording of the appropriate ICD-9 CM codes one or more times during any visit from 90 days prior to the LMP through delivery. Diabetes mellitus was defined by ICD-9 CM codes indicating pre-gestational diabetes mellitus or gestational diabetes mellitus or filled prescriptions for alpha glucosidase inhibitors, glitazones, meglitinides, metformin, sulphonylureas, or insulin again from 90 days prior to the LMP through delivery. As a proxy for general health status and health care utilization, we also determined the number of physician visits for any reason and the number of distinct non-antihypertensive prescriptions drugs during the 90 days prior to the LMP. We also determined the mean age and distribution of race for the full, linked cohort.
Definition of pregnancy periods and exposure
The pre-pregnancy period was defined as 90 days prior to the LMP to the day prior to the LMP. The first trimester was defined as the LMP through day 90 of pregnancy, second trimester as day 91 to day 180, and third trimester as day 181 to delivery.
We examined the outpatient use of antihypertensive treatments by therapeutic class, as shown in the supplement, based on pharmacy dispensing claims. The period of exposure to a medication for each woman was defined by the day the prescription was dispensed and the duration of the prescription based on days supply. Antihypertensives dispensed prior to 90 days before the LMP were considered as an exposure if the days supply extended into the defined pre-pregnancy period. The number of days supplied was accumulated for consecutive prescriptions of the same medication. Because dihydropyridines can be used off-label for the prevention or treatment of pre-term labor and birth, we determined the proportion of patients with dihydropyridine exposure that had codes for preterm delivery or had diagnosis codes indicating preterm labor. We determined the number of women exposed to each class of antihypertensive in the pre-pregnancy period and each trimester, separately, as shown in Table 2.
Table 2.
Distribution of antihypertensive medications before and during pregnancy by class within users of antihypertensive medications.* The Medicaid Analytic Extract Pregnancy Cohort.
| Antihypertensive type | Pre- pregnancy, N (%) | 1st trimester, N (%) | 2nd trimester, N (%) | 3rd trimester, N (%) |
|---|---|---|---|---|
| Total | 22,653 | 20,641 | 19,000 | 35,571 |
| Diuretics | ||||
| Thiazides | 4,234 (18.7) | 3,278 (15.9) | 1,074 (5.7) | 665 (1.9) |
| Potassium-Sparing Agents | 404 (1.8) | 287 (1.4) | 83 (0.4) | 55 (0.2) |
| Acetazolamide | 169 (0.7) | 130 (0.6) | 84 (0.4) | 81 (0.2) |
| Adrenergic Inhibitors | ||||
| Central Alpha-Antagonists | 1,992 (8.8) | 5,839 (28.3) | 7,921 (41.7) | 9,914 (27.9) |
| Alpha Blockers | 89 (0.4) | 72 (0.3) | 31 (0.2) | 32 (0.1) |
| Beta Blockers | 7,354 (32.5) | 6,340 (30.7) | 4,238 (22.3) | 3,814 (10.7) |
| Combined Alpha And Beta Blockers | 941 (4.2) | 1,793 (8.7) | 2,879 (15.2) | 4,426 (12.4) |
| Direct Vasodilators | 98 (0.4) | 179 (0.9) | 268 (1.4) | 413 (1.2) |
| Calcium Channel Antagonists | ||||
| Nondihydropyridines | 1,322 (5.8) | 1,081 (5.2) | 591 (3.1) | 638 (1.8) |
| Dihydropyridines† | 3,192 (14.1) | 2,590 (12.5) | 3,766 (19.8) | 18,018 (50.7) |
| ACE Inhibitors | 3,979 (17.6) | 3,280 (15.9) | 928 (4.9) | 383 (1.1) |
| Angiotensin II Receptor Blockers | 798 (3.5) | 666 (3.2) | 189 (1) | 87 (0.2) |
| Combination Drugs | ||||
| Beta Blockers And Diuretics | 312 (1.4) | 270 (1.3) | 88 (0.5) | 48 (0.1) |
| ACE Inhibitors And Diuretics | 512 (2.3) | 424 (2.1) | 106 (0.6) | 48 (0.1) |
| Angiotensin II Receptor Antagonists And Diuretics | 647 (2.9) | 563 (2.7) | 157 (0.8) | 65 (0.2) |
| Calcium Antagonists And ACE Inhibitors | 462 (2.0) | 387 (1.9) | 107 (0.6) | 55 (0.2) |
| Other Combinations | 1,758 (7.8) | 1,324 (6.4) | 397 (2.1) | 316 (0.9) |
Pre-pregnancy is defined as exposure from 90 days prior to the LMP to LMP. Pregnancy is defined as the LMP to day 270 for term deliveries and 245 for pre-term deliveries. 1st trimester is defined as LMP to 90 days, 2nd trimester as 91 days to 180 days, 3rd trimester as 181 to delivery
Dihydropyridines are used off label for tocolysis and the prevention of preterm labor; 14,905 patients treated with these medications had preterm labor or preterm delivery.
Summed proportion is greater than 100% because women may receive treatment for more than one medication type.
Defining patterns of antihypertensive utilization in pre-pregnancy users
For patients exposed to antihypertensive medications during the 90 days prior to the estimated LMP, we examined patterns of antihypertensive dispensing during the first trimester and second trimester. We determined for each class of antihypertensive used during the pre-pregnancy period, in a hierarchical fashion, the proportion of patients who continued on the pre-pregnancy class of medication, changed to methyldopa, changed to labetalol, changed to a different class (other than methyldopa or labetalol), and were not dispensed any antihypertensive during the first trimester (discontinuers).
Temporal patterns of utilization
For each year in the study period, we examined the proportion of women exposed to antihypertensives anytime during pregnancy and by trimester. We also examined trends in the exposure to the four most common antihypertensive classes at any time during pregnancy.
Results
There were 1,106,757 Medicaid patients included in our cohort of whom 48,453 (4.4%) were exposed to antihypertensive medications during pregnancy. Table 1 shows the baseline characteristics of the cohort, stratified by whether the woman was exposed to antihypertensives any time during pregnancy. In general, the antihypertensive exposed were older and more likely to be Caucasian or African-American. Over one-fifth of the antihypertensive exposed also had pre-gestational or gestational diabetes and 3% had co-morbid chronic renal disease. The antihypertensive exposed also had more non-antihypertensive medications prescribed prior to pregnancy and more pre-pregnancy physician visits. Compared to the full, linked cohort (n=6,107,572), the analytic cohort was slightly younger (23.2± 5.8 vs. 24.2 ± 5.5 years old) and had a higher proportion of non-white women (60.1% vs. 52.6%).
Table 1.
Baseline Characteristics of Patients in the Medicaid Analytic Extract Pregnancy Cohort Stratified by Whether the Patients Were Exposed to Antihypertensives During Pregnancy.
| Patient characteristics | Antihypertensive exposed, N (%) | Antihypertensive unexposed, N (%) |
|---|---|---|
| Total | 48,453 | 1,058,304 |
| Age at last menstrual period, years | ||
| 11 to 19 | 8,482 (17.5) | 318,052 (30.1) |
| 20 to 24 | 14,631 (30.2) | 376,424 (35.6) |
| 25 to 34 | 19,781 (40.8) | 311,849 (29.5) |
| 35 to 39 | 4,371 (9.0) | 42,725 (4.0) |
| 40 to 44 | 1,131 (2.3) | 8,800 (0.8) |
| > 44 | 57 (0.1) | 454 (<0.1) |
| Race | ||
| Caucasian | 21,598 (44.6) | 419,926 (39.7) |
| African-American | 17,842 (36.8) | 355,410 (33.6) |
| Hispanic/Latino | 6,252 (13.0) | 201,271 (19.1) |
| Native American | 718 (1.5) | 19,543 (1.9) |
| Asian/Pacific Islander | 994 (2.1) | 37,563 (3.5) |
| Other/Unknown | 1,049 (2.1) | 24,591 (2.3) |
| Multiple gestations | 2,162 (4.5) | 19,172 (1.8) |
| Comorbidities | ||
| Pre-gestational or gestational diabetes | 10,994 (22.7) | 103,023 (9.7) |
| Chronic renal disease | 1,439 (3.0) | 12,335 (1.2) |
| Chronic hypertension | 15,190 (31.4) | 19,821 (1.9) |
| Gestational hypertension | 3,569 (7.4) | 32,589 (3.1) |
| Number of distinct non- antihypertensive prescription drugs (from 90 days prior to the LMP to LMP) | ||
| 0 | 14,001 (28.9) | 507,889 (48.0) |
| 1 to 3 | 18,472 (38.1) | 382,994 (36.2) |
| Greater than 3 | 15,980 (33.0) | 167,421 (15.8) |
| Number of physician visits for any reason (from 90 days prior to the LMP to LMP) | ||
| 0 | 18,281 (37.7) | 563,312 (53.2) |
| 1 to 3 | 21,926 (45.3) | 411,868 (38.9) |
| Greater than 3 | 8,246 (17.0) | 83,124 (7.9) |
| Exposed to antihypertensives prior to pregnancy | 15,875 (32.8) | 6,778 (0.6) |
Table 2 shows the prevalence of exposure to antihypertensive medications in the 90 days before pregnancy and during each trimester. Overall, 2.0% (n=22,653) of the cohort was exposed prior to the estimated LMP, 1.9% (n=20,641) during the first trimester, 1.7% (n=19,000) during the second trimester, and 3.2% (n=35,571) during the third trimester. Prior to pregnancy, the most common medication classes were beta blockers, thiazides, angiotensin-converting enzyme (ACE) inhibitors, dihydropyridines, and central alpha-antagonists. These were also the most common classes of exposure during the first-trimester, although central alpha-antagonists accounted for a larger fraction of exposures in this group. By the second trimester, the proportion exposed to ACE inhibitors and thiazides declined substantially, while the proportion exposed to central-alpha antagonists, combined alpha and beta blockers, and dihydropyridines increased. In the third trimester, central-alpha antagonists, combined alpha and beta blockers, beta blockers, and dihydropyridines accounted for most of the antihypertensive exposures. Of the dihydropyridine exposed, 14,905 (82.7%) had a diagnosis code indicating preterm labor or preterm delivery. Such medications are used off-label as tocolytics to attenuate preterm contractions.
Table 3 shows the patterns of antihypertensive dispensing among initiators of antihypertensive therapy during each trimester; not exposed to antihypertensives at any point before or during pregnancy, prior to the period in question. The most commonly dispensed agents varied by trimester, but included central alpha-antagonists, beta blockers, thiazides, calcium channel blockers, and combined alpha and beta blockers.
Table 3.
Patterns of antihypertensive dispensing among initiators during the first, second, and third trimesters. The Medicaid Analytic Extract Pregnancy Cohort.
| Antihypertensive type | First trimester N (%) | Second trimester N (%) | Third trimester N (%) |
|---|---|---|---|
| Total | 6,065 | 6,406 | 20,105 |
| Diuretics | |||
| Thiazides | 614 (10.1) | 119 (1.9) | 186 (0.9) |
| Potassium-Sparing Agents | 75 (1.2) | 21 (0.3) | 11 (0.1) |
| Acetazolamide | 41 (0.7) | 30 (0.5) | 26 (0.1) |
| Adrenergic Inhibitors | |||
| Central Alpha-Antagonists | 2,091 (34.5) | 2,070 (32.3) | 2,903 (14.4) |
| Alpha Blockers | 17 (0.3) | * | * |
| Beta Blockers | 1,685 (27.8) | 1,331 (20.8) | 981 (4.9) |
| Combined Alpha And Beta Blockers | 568 (9.4) | 793 (12.4) | 1,386 (6.9) |
| Direct Vasodilators | 54 (0.9) | 63 (1.0) | 138 (0.7) |
| Calcium Channel Antagonists | |||
| Nondihydropyridines | 214 (3.5) | 148 (2.3) | 197 (1) |
| Dihydropyridines | 556 (9.2) | 1,978 (30.9) | 14,052 (69.9) |
| ACE Inhibitors | 481 (7.9) | 88 (1.4) | 51 (0.3) |
| Angiotensin II Receptor Blockers | 80 (1.3) | * | * |
| Combination Drugs | |||
| Beta Blockers And Diuretics | 36 (0.6) | * | * |
| ACE Inhibitors And Diuretics | 52 (0.9) | 12 (0.2) | * |
| Angiotensin II Receptor Antagonists And Diuretics | 74 (1.2) | 14 (0.2) | * |
| Calcium Antagonists And ACE Inhibitors | 45 (0.7) | * | * |
| Other Combinations | 258 (4.3) | 63 (1) | 123 (0.6) |
Cannot be displayed due to restrictions regarding the publication of small cells in the data use agreement.
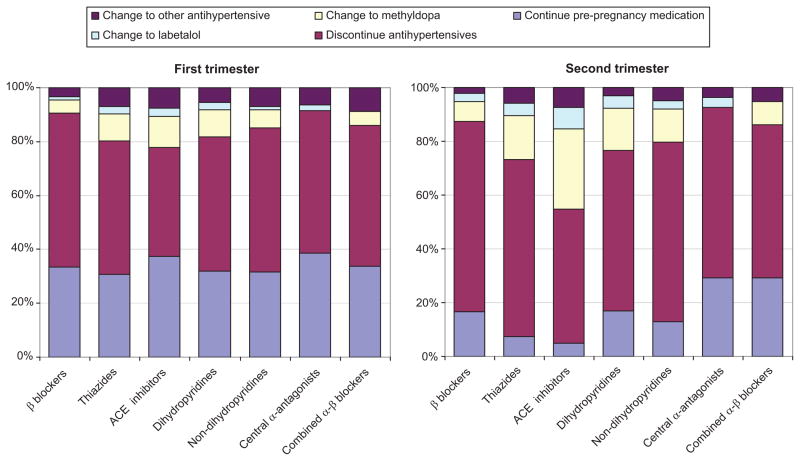
In Figures 1a and 1b we show patterns of antihypertensive dispensing during the first and second trimester, respectively, for patients taking antihypertensives pre-pregnancy stratified by class of pre-pregnancy therapy. For all classes of antihypertensives, during the first trimester, about half discontinued therapy (meaning no dispensing of medication during the trimester), about a third continued their pre-pregnancy medication, and the balance switched to methyldopa, labetalol, or another antihypertensive. By the second trimester between 50 to 70%, depending on the pre-pregnancy antihypertensive class, discontinued therapy. Also by this point in pregnancy, the majority of patients taking thiazides or ACE inhibitors pre-pregnancy, either discontinued therapy or were switched to methyldopa or labetalol. For those on beta blockers or calcium channel blockers, an approximately equal proportion continued the pre-pregnancy class and switched to methyldopa or labetalol. Additional information on this analysis is available in the supplement. We also examined the overall proportion of women taking antihypertensives prior to pregnancy and found that 12,373 (54.6%) received no antihypertensive during the first trimester and 15,692 (69.3%) received no antihypertensive during the second trimester. When we restricted the analysis to patients that were coded as having a diagnosis of chronic hypertension, only 2722 (31.6%) received no antihypertensive in the first trimester and 3991 (46.4%) received no antihypertensive during the second trimester.
Figure 1.
Figure 1a and 1b. Among patients taking certain classes of antihypertensives prior to pregnancy, patterns of antihypertensive dispensing during (a) the first trimester and (b) the second trimester. The Medicaid Analytic Extract Pregnancy Cohort.
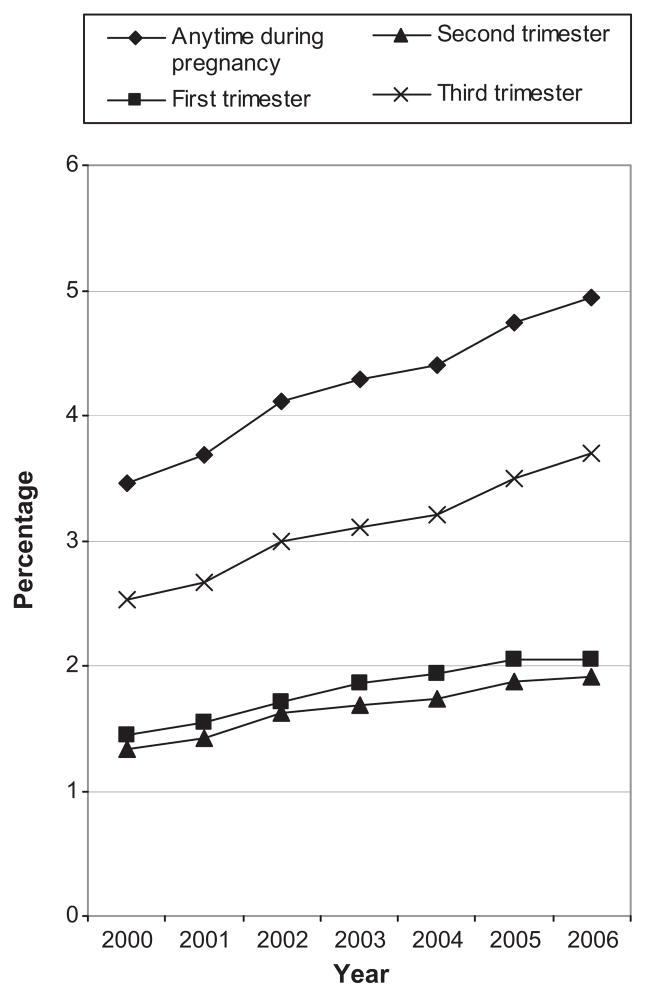
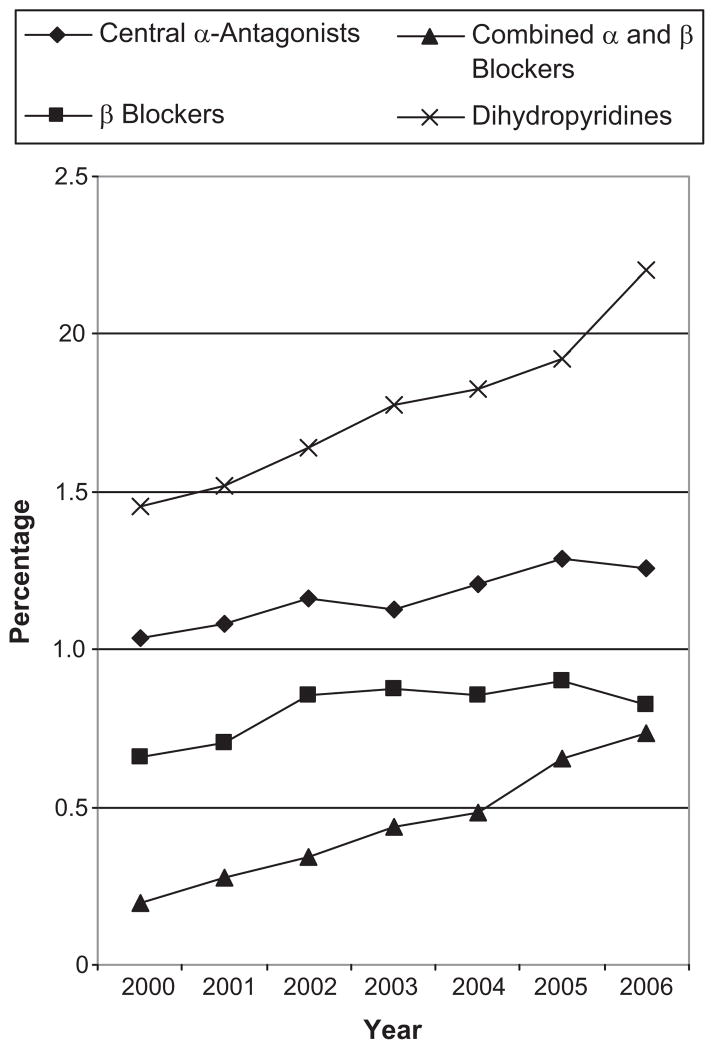
Figure 2 shows temporal trends in the exposure prevalence for antihypertensives anytime during pregnancy and by trimester from 2000 to 2006. Observations from deliveries in 2007 were not included due to concern that the small sample size from that year (n=29,255) may generate unstable estimates. The prevalence of exposure at anytime during pregnancy increased from 3.5% in 2000 to 4.9% in 2006 and during the first trimester from 1.4% to 2.0%. Figure 3 shows trends in the prevalence of exposure at any point during pregnancy to the four most common classes. Increases were seen for all classes, but were most substantial for combined alpha and beta blockers and dihydropyridines.
Figure 2.
Temporal trends in antihypertensive utilization during pregnancy (overall) and by trimester. The Medicaid Analytic Extract Pregnancy Cohort.
Figure 3.
Temporal trends in antihypertensive utilization during pregnancy (overall) by medication class. The Medicaid Analytic Extract Pregnancy Cohort.
Discussion
In this study of over 1.1 million Medicaid pregnancies, we found antihypertensive medications to be a common pregnancy exposure whose prevalence is consistently increasing. From 2000 to 2006 alone, the prevalence of antihypertensive use both in the first trimester and in pregnancy overall increased approximately 50%; by 2006 nearly 5% of all pregnancies were exposed to antihypertensives. We also found that there is substantial heterogeneity in the range of antihypertensive agents used across all trimesters of pregnancy and in the approach to the management of patients entering pregnancy on antihypertensives.
While professional guidelines generally recommend methyldopa and labetalol as the first-line treatments for hypertension in pregnancy3, 5, 16, our data suggest that in actual practice the use of other agents is very common. A significant proportion of patients taking antihypertensives prior to pregnancy are maintained on their pre-pregnancy agent, and not switched to one of the preferred agents. Even for new initiators of antihypertensive therapy, beta blockers, thiazides, and calcium channel blockers were frequently chosen. With the exception of methyldopa (which is category B), the Food and Drug Administration categorizes most antihypertensives as category C, which means that animal studies either show an adverse effect or are lacking and no well-controlled human studies exist24. Concerns have been raised that beta blockers can predispose to intrauterine growth restriction25 and/or neonatal hypoglycemia14. Calcium channel blockers have recently been linked to increased rates of neonatal seizures14. Diuretics have been shown to prevent normal plasma volume expansion in pregnancy26, although it is unclear if this is detrimental. While experts interpret the limited data available to suggest the general safety of these agents during pregnancy5, 17, 24, more work is needed to establish that this is the case and to verify and establish the magnitude of any risks that do exist. Further, there is virtually no data on the comparative effectiveness and safety of the different treatment options for hypertension. The rapid increase in the use of these medications, and the heterogeneous nature of the agents used, suggest these data are urgently needed.
Another important finding in our study is that approximately half of all patients taking antihypertensive agents prior to pregnancy discontinue treatment during the first or second trimester (Figure 1). Meta-analysis of the available data suggests that pharmacologic therapy for mild-to-moderate hypertension decreases the incidence of severe hypertension, but does not reduce the risks of placental abruption, fetal demise, superimposed preeclampsia, or preterm birth (but the available data are underpowered to show even moderate reductions in these outcomes)6. Whether the decrease in severe maternal hypertension translates into decreased maternal morbidity also remains unclear. Again, further research is needed.
It is notable that in our large sample, several hundred women were exposed to ACE inhibitors or ARBs during late pregnancy. These agents are known to be fetotoxic in the second or third trimester24. Although the circumstances of their use cannot be discerned in this administrative dataset, automatic refills in patients with late registration to prenatal care or prescribing physicians’ failure to ask about the possibility of pregnancy are two plausible explanations. This underscores the caution with which physicians must use fetotoxic agents in women of reproductive age. Unplanned pregnancy and late registration to pre-natal care are not uncommon, particularly in disadvantaged populations such as those served by Medicaid. Automatic refills of potentially fetotoxic medications may be hazardous in these patients.
While data are conflicting, there is also substantial concern about the teratogenic potential of ACE inhibitors and ARBs during the first trimester10, 15, 24. In our sample, these agents accounted for nearly one-fifth of antihypertensive exposures during the first-trimester. As noted in the paragraph above, the precise circumstances of the use of these agents cannot be determined from claims data, but the prevalence of this exposure raises important safety concerns. Resolution of this controversy with larger and better controlled studies is necessary.
Consistent with what has been observed in other populations20, we report a marked increase in utilization of antihypertensives in pregnancy during our study period. While clearly some of this increase is due to greater off-label use of dihydropyridines for the prevention and treatment of preterm labor, we also observe increases in other classes of medications, including centrally acting alpha antagonists, beta blockers, and mixed alpha and beta blockers. First trimester utilization of antihypertensives also rose dramatically. These findings are consistent with the rising rates of chronic hypertension and gestational hypertension observed in population-based studies2, 27, which in turn may reflect rising rates of obesity28 and advanced maternal age29 in U.S. parturients.
It is notable that less than half of the patients exposed to antihypertensives in pregnancy had an inpatient or outpatient ICD-9 code for chronic or gestational hypertension. While this may be partially explained by the prescription of antihypertensives for other conditions, it likely primarily reflects the imperfect sensitivity of ICD-9 codes for hypertensive conditions in claims data 30–32. This suggests that utilization of medications along with diagnostic codes may provide a better approach to defining comorbidities in surveillance or epidemiologic studies using claims data, than algorithms that rely on ICD 9 codes alone.
Our study is subject to certain limitations. To assure accurate estimation of gestational age and continuous enrollment from 3 months prior to pregnancy through delivery, we only included those women in the MAX cohort for whom a linkage to neonatal record is possible and who met our strict inclusion criteria. This resulted in a substantial reduction in the size of the cohort which may decrease its representativeness of the general Medicaid population. Indeed, our cohort is younger and has a larger proportion of non-white women than the cohort before exclusion based on eligibility. Our data only include those pregnancies that result in livebirths, such that antihypertensive exposures in pregnancies that result in other outcomes (stillbirths, elective terminations, and miscarriages) are not captured in the present analysis. Also, while the use of Medicaid pharmacy data provides information on medication dispensing which is not subject to recall bias, it does not provide information on whether the medication was actually taken, which may result in misclassification. However, most Medicaid patients face at least nominal copayments for their prescriptions and these charges often represent a financial pressure for these low-income patients, suggesting that if the prescription is filled, it is likely taken. Additionally, as we only require 3 months of enrollment prior to pregnancy and examine this period to determine pre-pregnancy exposure to antihypertensives, this may result in a slight underestimate, as some patients may discontinue therapy >3 months prior to the LMP in anticipation of pregnancy. Likewise, the number of women exposed to antihypertensives during the pre-pregnancy window will be slightly underestimated due to women who were not Medicaid eligible >3 months before the LMP and were still taking antihypertensives dispensed prior to the window but did not refill their prescription during the window. Further, we do not have accurate information on the indications for the prescription of an antihypertensive medication, a large proportion of the dihydropyridine exposures in the third trimester are likely for off-label use for tocolysis33 and beta blockers and calcium channel blockers may be used as anti-arrhythmics or for migraine prophylaxis34. Last, while we used a validation algorithm to estimate LMP and gestational age, as we do not have a direct measure of these variables, some degree of misclassification on the gestational timing of drug dispensing is likely.
Supplementary Material
Novelty and Significance.
What Is New
This analysis includes over 1 million women enrolled in Medicaid to fill a gap in knowledge of population-level patterns of antihypertensive medication use during pregnancy.
Antihypertensive exposure occurs in nearly 5% of pregnant women and is increasing in frequency. The range of treatments used is highly heterogeneous.
Antihypertensives prescribed prior to pregnancy are frequently still being refilled into the 1st trimester and beyond, suggesting that exposure to potentially fetotoxic medications is not uncommon.
2) What Is Relevant?
With increasing exposure to antihypertensive medications during pregnancy, this study identifies areas of concern for clinicians caring for these patients and establishes the need for further research to define the safety and efficacy of the available agents.
3) Summary
Antihypertensive use during pregnancy is relatively common and increasing. The wide range of agents used during pregnancy includes medications considered contraindicated during pregnancy. Data on the comparative safety and efficacy of specific antihypertensives in pregnant women are urgently needed.
Perspectives.
In conclusion, our data suggest that the exposure to antihypertensive medications in pregnancy is relatively common and increasing. There is significant heterogeneity in the range of agents used and the management of patients taking antihypertensives prior to pregnancy. Research investigating the comparative safety and efficacy of antihypertensive therapy in pregnancy is urgently needed to define the optimal approach to therapy.
Acknowledgments
Sources of funding: Supported by the NIH (Grant GM007592 (BTB)) and by the Agency for Healthcare Research and Quality (AHRQ) (Grant R01HS018533 to SHD).. Kristin Palmsten is supported by Training Grant T32HD060454 in Reproductive, Perinatal and Pediatric Epidemiology from the National Institute of Child Health and Human Development (NICHD), National Institutes of Health.
Footnotes
Conflicts of interest: None
Contributor Information
Brian T. Bateman, Division of Pharmacoepidemiology and Pharmacoeconomics, Brigham and Women’s Hospital, Department of Anesthesia, Critical Care, and Pain Medicine, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts
Sonia Hernandez-Diaz, Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts.
Krista F. Huybrechts, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts.
Kristin Palmsten, Department of Epidemiology, Harvard School of Public Health, Boston, Massachusetts.
Helen Mogun, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts.
Jeffrey L. Ecker, Department of Obstetrics and Gynecology, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts.
Michael A. Fischer, Division of Pharmacoepidemiology and Pharmacoeconomics, Department of Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, Massachusetts.
References
- 1.Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: Statement from the international society for the study of hypertension in pregnancy (ISSHP) Hypertens Pregnancy. 2001;20:IX–XIV. doi: 10.1081/PRG-100104165. [DOI] [PubMed] [Google Scholar]
- 2.Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the united states. Obstet Gynecol. 2009;113:1299–1306. doi: 10.1097/AOG.0b013e3181a45b25. [DOI] [PubMed] [Google Scholar]
- 3.Report of the national high blood pressure education program working group on high blood pressure in pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- 4.Acog practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, january 2002. Obstet Gynecol. 2002;99:159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 5.Acog practice bulletin. Chronic hypertension in pregnancy. Acog committee on practice bulletins. Obstet Gynecol. 2001;98(suppl):177–185. doi: 10.1016/s0029-7844(01)01471-5. [DOI] [PubMed] [Google Scholar]
- 6.Abalos E, Duley L, Steyn DW, Henderson-Smart DJ. Antihypertensive drug therapy for mild to moderate hypertension during pregnancy. Cochrane Database Syst Rev. 2007:CD002252. doi: 10.1002/14651858.CD002252.pub2. [DOI] [PubMed] [Google Scholar]
- 7.de Swiet M. Maternal blood pressure and birthweight. Lancet. 2000;355:81–82. doi: 10.1016/S0140-6736(99)00288-3. [DOI] [PubMed] [Google Scholar]
- 8.Tabacova S, Kimmel CA, Wall K, Hansen D. Atenolol developmental toxicity: Animal-to-human comparisons. Birth Defects Res A Clin Mol Teratol. 2003;67:181–192. doi: 10.1002/bdra.10011. [DOI] [PubMed] [Google Scholar]
- 9.Kallen BA, Otterblad Olausson P. Maternal drug use in early pregnancy and infant cardiovascular defect. Reprod Toxicol. 2003;17:255–261. doi: 10.1016/s0890-6238(03)00012-1. [DOI] [PubMed] [Google Scholar]
- 10.Cooper WO, Hernandez-Diaz S, Arbogast PG, Dudley JA, Dyer S, Gideon PS, Hall K, Ray WA. Major congenital malformations after first-trimester exposure to ace inhibitors. N Engl J Med. 2006;354:2443–2451. doi: 10.1056/NEJMoa055202. [DOI] [PubMed] [Google Scholar]
- 11.Lennestal R, Otterblad Olausson P, Kallen B. Maternal use of antihypertensive drugs in early pregnancy and delivery outcome, notably the presence of congenital heart defects in the infants. Eur J Clin Pharmacol. 2009;65:615–625. doi: 10.1007/s00228-009-0620-0. [DOI] [PubMed] [Google Scholar]
- 12.Caton AR, Bell EM, Druschel CM, Werler MM, Lin AE, Browne ML, McNutt LA, Romitti PA, Mitchell AA, Olney RS, Correa A. Antihypertensive medication use during pregnancy and the risk of cardiovascular malformations. Hypertension. 2009;54:63–70. doi: 10.1161/HYPERTENSIONAHA.109.129098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakhai-Pour HR, Rey E, Berard A. Antihypertensive medication use during pregnancy and the risk of major congenital malformations or small-for-gestational-age newborns. Birth Defects Res B Dev Reprod Toxicol. 2010;89:147–154. doi: 10.1002/bdrb.20238. [DOI] [PubMed] [Google Scholar]
- 14.Davis RL, Eastman D, McPhillips H, Raebel MA, Andrade SE, Smith D, Yood MU, Dublin S, Platt R. Risks of congenital malformations and perinatal events among infants exposed to calcium channel and beta-blockers during pregnancy. Pharmacoepidemiol Drug Saf. 2011;20:138–145. doi: 10.1002/pds.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li DK, Yang C, Andrade S, Tavares V, Ferber JR. Maternal exposure to angiotensin converting enzyme inhibitors in the first trimester and risk of malformations in offspring: A retrospective cohort study. BMJ. 2011;343:d5931. doi: 10.1136/bmj.d5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The jnc 7 report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 17.Seely EW, Ecker J. Clinical practice. Chronic hypertension in pregnancy. N Engl J Med. 2011;365:439–446. doi: 10.1056/NEJMcp0804872. [DOI] [PubMed] [Google Scholar]
- 18.Magee LA, Helewa M, Moutquin JM, von Dadelszen P. Diagnosis, evaluation, and management of the hypertensive disorders of pregnancy. J Obstet Gynaecol Can. 2008;30:S1–48. [Google Scholar]
- 19.Brown MA, Hague WM, Higgins J, Lowe S, McCowan L, Oats J, Peek MJ, Rowan JA, Walters BN. The detection, investigation and management of hypertension in pregnancy: Full consensus statement. Aust N Z J Obstet Gynaecol. 2000;40:139–155. doi: 10.1111/j.1479-828x.2000.tb01137.x. [DOI] [PubMed] [Google Scholar]
- 20.Andrade SE, Raebel MA, Brown J, Lane K, Livingston J, Boudreau D, Rolnick SJ, Roblin D, Smith DH, Dal Pan GJ, Scott PE, Platt R. Outpatient use of cardiovascular drugs during pregnancy. Pharmacoepidemiol Drug Saf. 2008;17:240–247. doi: 10.1002/pds.1550. [DOI] [PubMed] [Google Scholar]
- 21.Markus AR, Rosenbaum S. The role of medicaid in promoting access to high-quality, high-value maternity care. Womens Health Issues. 2010;20:S67–78. doi: 10.1016/j.whi.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 22.Palmsten K, Huybrechts K, Mogun H, Setoguchi S, Hernandez-Diaz S. Medicaid analytic extract for studies of drug safety during pregnancy [abstract] Pharmacoepidemiol & Drug Safety. 2011;20:S266. [Google Scholar]
- 23.Margulis AV, Setoguchi S, Mittleman MA, Glynn RJ, Dormuth CR, Hernandez-Diaz S. Algorithms to estimate the beginning of pregnancy in administrative databases. Pharmacoepidemiol Drug Saf. 2012 doi: 10.1002/pds.3284. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Podymow T, August P. Update on the use of antihypertensive drugs in pregnancy. Hypertension. 2008;51:960–969. doi: 10.1161/HYPERTENSIONAHA.106.075895. [DOI] [PubMed] [Google Scholar]
- 25.Tabacova S, Kimmel CA, Wall K, Hansen D. Atenolol developmental toxicity: Animal-to-human comparisons. Birth Defects Res Part A-Clin Mol Teratol. 2003;67:181–192. doi: 10.1002/bdra.10011. [DOI] [PubMed] [Google Scholar]
- 26.Sibai BM, Grossman RA, Grossman HG. Effects of diuretics on plasma volume in pregnancies with long-term hypertension. Am J Obstet Gynecol. 1984;150:831–835. doi: 10.1016/0002-9378(84)90458-7. [DOI] [PubMed] [Google Scholar]
- 27.Bateman BT, Bansil P, Hernandez-Diaz S, Mhyre JM, Callaghan WM, Kuklina EV. Prevalence, trends, and outcomes of chronic hypertension: A nationwide sample of delivery admissions. Am J Obstet Gynecol. 2012;206:134, e131–138. doi: 10.1016/j.ajog.2011.10.878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SY, Dietz PM, England L, Morrow B, Callaghan WM. Trends in pre-pregnancy obesity in nine states, 1993–2003. Obesity (Silver Spring) 2007;15:986–993. doi: 10.1038/oby.2007.621. [DOI] [PubMed] [Google Scholar]
- 29.Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Mathews TJ, Kirmeyer S, Osterman MJ. Births: Final data for 2007. Natl Vital Stat Rep. 2010;58:1–85. [PubMed] [Google Scholar]
- 30.Klemmensen AK, Olsen SF, Osterdal ML, Tabor A. Validity of preeclampsia-related diagnoses recorded in a national hospital registry and in a postpartum interview of the women. Am J Epidemiol. 2007;166:117–124. doi: 10.1093/aje/kwm139. [DOI] [PubMed] [Google Scholar]
- 31.Bullano MF, Kamat S, Willey VJ, Barlas S, Watson DJ, Brenneman SK. Agreement between administrative claims and the medical record in identifying patients with a diagnosis of hypertension. Med Care. 2006;44:486–490. doi: 10.1097/01.mlr.0000207482.02503.55. [DOI] [PubMed] [Google Scholar]
- 32.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of icd-9-cm codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 33.Nassar AH, Aoun J, Usta IM. Calcium channel blockers for the management of preterm birth: A review. Am J Perinatol. 2011;28:57–66. doi: 10.1055/s-0030-1262512. [DOI] [PubMed] [Google Scholar]
- 34.Fenstermacher N, Levin M, Ward T. Pharmacological prevention of migraine. BMJ. 2011;342:d583. doi: 10.1136/bmj.d583. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.